Abstract
Aims
With the present study, we sought to determine the safety of three different endomyocardial biopsy (EMB) access routes in 514 patients admitted for diagnostic workup of heart failure of unknown aetiology.
Methods and results
In this retrospective monocentric cohort study, we analysed 514 consecutive patients with heart failure without evidence of significant coronary artery disease or valvular disease undergoing EMB between November 2013 and December 2018, stratified in three access route groups: transradial arterial left ventricular (LV‐)EMB (323 patients), transfemoral LV‐EMB (138 patients), and transfemoral right ventricular (RV‐)EMB (53 patients). Patients undergoing selective transradial LV‐EMB were older compared with patients undergoing selective transfemoral LV‐EMB or RV‐EMB [transradial LV‐EMB: 56.0 (45.0/64.0) vs. transfemoral LV‐EMB: 53 (42.5/64.5), P = 0.455; transradial LV‐EMB: 56 (45.0/64.0) vs. RV‐EMB: 53 (42.5/64), P = 0.695] and presented more often in New York Heart Association‐functional class III and IV. A total of eight major complications including permanent atrioventricular block requiring pacemaker implantation, pericardial tamponade necessitating pericardiocentesis, stroke and transient cerebral ischaemic attack as well as severe valvular damage, vascular access site complications, and ventricular fibrillation were documented with no significant differences between the groups (8/514, 1.5%). Minor complications such as transient chest pain, non‐sustained electrocardiogram abnormalities, and transient atrioventricular block were rare and equally distributed between groups.
Conclusions
Transradial LV‐EMB is a safe procedure for experienced radial operators and non‐inferior compared with transfemoral LV‐EMB and RV‐EMB. An accurate peri‐procedural and post‐procedural monitoring and follow‐up care should be recommended for all patients undergoing this procedure in order to identify potential complications.
Keywords: Heart failure, Endomyocardial biopsy, Access site, Transradial artery access, Complication rate
Introduction
Although improvements in diagnostics and treatment of heart failure (HF) over the past decades have increased survival and reduced hospitalization rates, HF is still a major cause of morbidity and mortality worldwide. 1 The aetiology of HF is likely to be multifactorial, and the underlying pathology cannot always be detected by established non‐invasive diagnostic procedures (i.e. echocardiography, electrocardiogram, and cardiac magnetic resonance imaging), even though an accurate diagnosis is mandatory in order to provide the most appropriate therapy for the patient. In this context, endomyocardial biopsy (EMB) has been demonstrated to improve diagnostics of unexplained HF in the majority of cases 2 as it provides important information concerning the degree of tissue damage and the presence of inflammation.
Based on a position paper of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases, EMB is recommended in all patients to identify the presence of inflammatory infiltrates and subsequently to provide an aetiology‐based treatment strategy. 3 EMB guidelines recommend EMB in any patient with HF that does not adequately respond to optimal medical therapy within 3 months of continued therapy, or that presents with haemodynamic or electrophysiologic instability. 4 The safety of EMB has significantly improved in the past years. This is mainly due to the use of smaller and more flexible bioptomes resulting in low complication rates comparable with those observed during diagnostic coronary angiography 5 without any differences compared with regard to major and minor complications between left ventricular (LV) biopsy and right ventricular (RV) biopsy. 6 No differences have been reported in terms of diagnostic accuracy, except one study that indicated a diagnostic superiority of biventricular EMB compared with selective LV‐EMB or RV‐EMB. 6 In general, the femoral artery is the preferred access site when LV‐EMB is performed, usually requiring an 8F sheath to allow passage of the biopsy forceps. The transfemoral access, however, is associated with an increased risk of bleeding due to the need of large diameter sheaths, and strict post‐procedural immobilization for several hours is mandatory. For diagnostic coronary angiography and percutaneous coronary interventions, the radial artery has emerged as the preferred access site in experienced centres and is also recommended by the guidelines of the European Society of Cardiology for the interventional therapy of coronary artery disease. 7 , 8 , 9 Compared with the femoral access, the transradial approach has been demonstrated to cause less major bleeding complications and vascular access site complications. 10 , 11 Establishing improved and sheathless guide catheters, LV‐EMB is nowadays feasible via the transradial access. To date, only a few small‐scale studies have reported about the safety 12 , 13 and non‐inferiority of the transradial procedure with regard to complications compared with the transfemoral access for LV‐EMB. 14 With the present study, we sought to analyse the safety of transradial LV‐EMB compared with both transfemoral LV‐EMB and transfemoral RV‐EMB in a large sample of 514 consecutive patients of an all‐comer population with HF of unknown aetiology.
Methods
Study population
In the present retrospective monocentre study, we analysed 514 consecutive patients of an all‐comer population with HF of unknown aetiology without evidence of significant coronary artery disease or valvular disease undergoing EMB between November 2013 and December 2018 at the Department of Cardiology of the University Medical Center Mainz. The patients were stratified in three groups according to access site (i.e. transradial LV‐EMB vs. transfemoral LV‐EMB vs. RV‐EMB). Selective LV‐EMB via transradial access was conducted in 323 patients, selective LV‐EMB via transfemoral access in 138 patients, and selective RV‐EMB in 53 patients. Based on the patients' medical reports, the following data were included in the analysis: personal history, clinical presentation, medication, laboratory results, electrocardiogram, echocardiography, and results of EMB. In order to screen for complications associated with EMB, medical reports and reports of the EMB procedure were thoroughly screened by a board‐certified cardiologist.
Echocardiography
A comprehensive transthoracic echocardiography was performed in all patients in the echocardiographic laboratory of the University Medical Center Mainz using a Philips ie33, a GE E95, or a Siemens Acuson S 2000 machine to examine cardiac function. To assess LV ejection fraction, LV chamber size, diastolic function, valvular disease, the thickness of the inferolateral and interventricular septal wall, the pressure gradient across the tricuspid wall, and pericardial effusion, the examination included two‐dimensional and M‐mode imaging, as well as colour‐flow Doppler, continuous‐wave Doppler, and pulsed‐wave Doppler. Within the examination, patients were also screened for obstacles for LV‐EMB such as a diameter of the lateral LV wall <8 mm or non‐compaction cardiomyopathy (which are both associated with an increased risk of myocardial perforation). Additionally, patients were also screened for relevant aortic valve stenosis.
Laboratory analyses and patient preparation
Routine laboratory tests were performed in all patients before EMB to assess coagulation and/or platelet disorders. In case of permanent oral anticoagulation therapy, an international normalized ratio value of <3.0 was required prior to all aforementioned procedures. Routine laboratory also included detection of biomarkers (i.e. levels of troponin and BNP) as well as a blood count. An Allen's test was performed in all patients undergoing LV‐EMB via the transradial access to assure the patency of the ulnar artery.
Sheaths and sheathless guiding catheter system
The 7.5F Eaucath system (Asahi Intecc) was originally designed as a sheathless guiding catheter for the use in transradial coronary interventions. 15 , 16 It has an outer hydrophilic coating in order to facilitate its introduction, to reduce pain, and to prevent radial artery spasm. Two different braiding patterns provide better torque stability and flexibility, while helping to maintain the lumen. Its outer diameter is 2.49 mm and smaller than that of a regular 6F introduction sheath (2.62 mm). With an inner diameter of 2.05 mm (0.081″), these guiding catheters are suitable for the passage of a 1.8 mm biopsy forceps without any significant increase in friction. For RV‐EMB, we used a 9F FastCath system. For transfemoral LV‐EMB, we used either an 8F MP GC system or a 7.5F Eaucath system (Asahi Intecc; sheathless).
Left ventricular and right ventricular endomyocardial biopsy
All procedures were performed by experienced interventional cardiologists trained in transradial artery and transfemoral artery access for percutaneous coronary interventions for several years in our department. In case of LV‐EMB, biopsies were taken from the lateral wall of the LV, whereas in case of RV‐EMB, biopsies were taken from the RV septum. Patients undergoing LV‐EMB through the radial access received 5000 IU unfractionated heparin and 0.2 mg nitroglycerin intra‐arterial to prevent radial artery occlusion or spasms. Patients undergoing LV‐EMB through the femoral access only received 5000 IU intra‐arterial, whereas patients undergoing RV‐EMB did not routinely receive heparin nor nitroglycerin. No heparin was administered intra‐procedurally in both patients undergoing LV‐EMB through the radial artery and patients undergoing LV‐EMB through the femoral artery if they were treated with vitamin‐K antagonists and laboratory results documented an international normalized ratio >2.5. In case of elective EMB procedures and concomitant direct oral anticoagulant therapy, the drug was paused 24 h before the procedures. Use of heparin was monitored intra‐procedurally by measuring the activated clotting time (target activated clotting time: >250 s). In all patients irrespective of the access site, the same type of biopsy forceps (Medwork bioptom, 180 cm, 1.8 mm, Cat. No. BIO‐C4‐18‐180) was used. The technical aspects of EMB through the radial artery have been described in detail previously. 13
Intra‐procedural and post‐procedural patient monitoring
During the EMB procedure, heart rhythm and invasive blood pressure were continuously monitored, and the patient was connected to self‐adhesive defibrillator patches. Each patient was monitored for at least 30 min after the procedure (including non‐invasive blood pressure measurement, heart rate, and oxygen saturation) within the cathlaboratory facility. A transthoracic echocardiogram was performed immediately after the procedure to rule out relevant pericardial effusion. Upon arrival at the cardiology ward, heart rhythm monitoring was continued if desired by the operator. In all patients, a transthoracic echocardiography was repeated the next day in order to re‐evaluate pericardial effusion and mitral regurgitation. For all patients, independent of access site and targeted ventricle (i.e. RV‐EMB and LV‐EMB), aspirin of 100 mg (once daily) was prescribed for 4 weeks to prevent thrombus formation/arterial embolism originating from the biopsy sites except for patients being under permanent oral anticoagulation.
Major and minor complications
Major complications included permanent atrioventricular (AV) block requiring pacemaker implantation, pericardial tamponade necessitating pericardiocentesis, stroke and transient cerebral ischaemic attack as well as severe valvular damage, dissection of the radial artery, access site bleeding, and ventricular fibrillation/death. Transient chest pain, non‐sustained electrocardiogram abnormalities, transient AV block, and small pericardial effusion were defined as minor complications.
Ethical aspects
Studies in Germany involving retrospective analysis of diagnostic standard data do not require an ethics statement.
Statistical analysis
Descriptive statistics for relevant baseline comparisons of symptomatic HF with reduced ejection fraction patients, who underwent EMB, stratified according to different EMB access routes were compared. The group of patients with transradial LV‐EMB, as the reference group, was compared with both access routes: transfemoral LV‐EMB and transfemoral RV‐EMB. Statistic comparison is provided as median and interquartile range or absolute numbers and corresponding percentages. We tested the continuous variables of the groups using the Mann–Whitney U test and categorical variables with the Fisher's exact test or the χ 2 test, as appropriate. IBM Statistical Package for the Social Sciences was used for computerized testing. A probability value <0.05 was considered statistically significant.
Results
Patient characteristics, functional parameters, and laboratory results
Our study population consisted of 514 patients. Transradial artery access for LV‐EMB was conducted in 323 patients; in 138 patients, LV‐EMB was performed through the femoral access; and transvenous transfemoral RV‐EMB was carried out in 53 patients. On the average, seven samples were taken per patient in the transradial LV‐EMB group [7 (5/7)] and in the transfemoral LV‐EMB group (7 [6/7]), whereas only five samples were taken per patient in the RV‐EMB group [5 (3/7)]. Patients undergoing selective transradial LV‐EMB were older compared with patients undergoing selective transfemoral LV‐EMB or selective RV‐EMB [transradial LV‐EMB: 56.0 (45.0/64.0) vs. transfemoral LV‐EMB: 53 (42.5/64.5), P = 0.455; transradial LV‐EMB: 56 (45.0/64.0) vs. RV‐EMB: 53 (42.5/64), P = 0.695]. Furthermore, we documented a pronounced cardiovascular risk profile in patients considered for transradial access, especially a higher prevalence of arterial hypertension (transradial LV‐EMB: 53.2% vs. transfemoral LV‐EMB: 35.5%, P < 0.001; transradial LV‐EMB: 53.2% vs. RV‐EMB: 49.1%, P = 0.601) and a higher prevalence of smoking (transradial LV‐EMB: 44% vs. RV‐EMB: 37.7%, P = 0.39). Although patients in the transradial LV‐EMB group presented more often in higher New York Heart Association‐functional class (i.e. New York Heart Association‐functional class III and IV; transradial LV‐EMB: 48.5% vs. transfemoral LV‐EMB: 36.6%, P = 0.006; transradial LV‐EMB: 48.5% vs. RV‐EMB: 29.6%, P = 0.004), no significant differences were observed in terms of LV ejection fraction between the groups [transradial LV‐EMB: 30% (25/40) vs. transfemoral LV‐EMB: 30% (20/45), P = 0.464; transradial LV‐EMB: 30% (25/40) vs. RV‐EMB: 35% (20/55), P = 0.48]. Baseline characteristics of the study population stratified for access site are depicted in detail in Table 1 . Additionally, information on HF medication at discharge is provided in Supporting Information, Table S1 , and the diagnostic findings based on EMB are depicted in Supporting Information, Table S2 . While the total number of LV‐EMB through the transradial access increased significantly between 2013 and 2018 [β‐estimate 1.372 (95% confidence interval, CI, 1.1 to 1.645)], the total number of LV‐EMB through the femoral access [β‐estimate −1.275 (95% CI −1.58 to −0.971)] and RV‐EMB [β‐estimate −0.783 (95% CI −1.267 to −0.299)] accordingly decreased. Temporal trends with regard to the choice of access site are shown in Figure 1 .
TABLE 1.
Baseline characteristics, medical history, and clinical presentation of the study population stratified for access site
| Variables |
LV‐EMB (femoral) N = 138 |
P‐value for comparison between both LV‐EMB groups |
LV‐EMB (radial) N = 323 |
P‐value for comparison of radial LV‐EMB and RV‐EMB groups |
RV‐EMB (venous) N = 53 |
|---|---|---|---|---|---|
| Age (years) | 53.0 (42.5/64.5) | 0.455 | 56.0 (45.0/64.0) | 0.695 | 53.0 (42.5/64) |
| Height (cm) | 175 (165.75/180) | 0.177 | 176 (169/182) | 0.215 | 173 (165.5/180) |
| Weight (kg) | 82 (69/95) | 0.437 | 83 (73/97) | 0.063 | 77 (68/92) |
| BMI (kg/m2) | 27.3 (23.6/30.8) | 0.956 | 27 (24/30.85) | 0.258 | 26.1 (23/28.85) |
| Women, n (%) | 50 (36.2%) | 0.044 | 86 (26.5%) | 0.32 | 16 (30.2%) |
| Classical cardiovascular risk factors | |||||
| Arterial hypertension | 49 (35.5%) | <0.001 | 172 (53.2%) | 0.601 | 26 (49.1%) |
| Diabetes mellitus | 25 (18.1%) | 0.74 | 54 (16.6%) | 0.18 | 5 (9.4%) |
| Smoking (current or previous) | 67 (48.5%) | 0.29 | 142 (44%) | 0.39 | 20 (37.7%) |
| Dyslipidaemia | 28 (20%) | 0.38 | 54 (16.6%) | 0.018 | 16 (30.2%) |
| Family history of MI/stroke | 29 (21%) | 0.79 | 71 (21.8%) | 0.15 | 7 (13.2%) |
| Functional parameters | |||||
| Left ventricular ejection fraction (%) | 30% (20/45) | 0.464 | 30% (25/40) | 0.48 | 35% (20/55) |
| LVEDD (mm) | 5.9 (5.2/6.6) | 0.969 | 5.9 (5.2/6.6) | 0.126 | 5.5 (4.7/6.4) |
| LVEDP (mmHg) | 17 (11/23) | 0.12 | 19 (14/27) | 0.354 | 18.5 (11/23.75) |
| Biomarkers at presentation | |||||
| Troponin I pos. (ng/mL) | 23.3 (12.4/96.8) | 0.203 | 22.4 (7.5/75.5) | 0.931 | 28 (5.4/104.4) |
| BNP | 753 (113/1354) | 0.065 | 385 (124/1012) | 0.221 | 241 (73/775) |
| Symptoms at presentation | |||||
| Angina pectoris | 47 (34%) | 0.169 | 88 (27.2%) | 0.47 | 17 (32.1%) |
| NYHA I | 34 (27.7%) | 0.006 for all NYHA classes | 70 (29.5%) | 0.004 for all NYHA classes | 12 (27.3%) |
| NYHA II | 44 (35.8%) | 52 (21.9%) | 19 (43.2%) | ||
| NYHA III and IV | 45 (36.6%) | 115 (48.5%) | 13 (29.6%) | ||
| Palpitations | 18 (13%) | 0.894 | 43 (13.3%) | 0.69 | 6 (11.3%) |
| Heart rhythm at presentation | |||||
| Sinus rhythm | 121 (87.7%) | 255 (79%) | 46 (86.8%) | ||
| Atrial fibrillation | 17 (12.3%) | 0.27 | 68 (21%) | 0.201 | 7 (13.2%) |
| Oral anticoagulation at presentation | |||||
| Phenprocoumon | 10 (7.2%) | 0.92 | 24 (7.4%) | 0.407 | 6 (11.3%) |
| Rivaroxaban | 4 (2.9%) | 0.454 | 15 (4.6%) | 1.0 | 2 (3.8%) |
| Apixaban | 4 (2.9%) | 0.454 | 15 (4.6%) | 1.0 | 2 (3.8%) |
| Dabigatran | 1 (0.7%) | 1.0 | 3 (0.9%) | 1.0 | 0 (0%) |
| Edoxaban | 0 (0%) | — | 0 (0%) | — | 0 (0%) |
| Median INR at date of EMB | 1.9 (1.5/2.3) | 0.75 | 1.8 (1.6/2.0) | 0.1 | 1.4 (1.0/1.8) |
| Antithrombotic therapy | |||||
| Aspirin | 32 (23.2%) | 0.547 | 66 (20.4%) | 0.027 | 18 (34%) |
| Clopidogrel | 2 (1.4%) | 0.73 | 7 (2.2%) | 1.0 | 1 (1.9%) |
| Ticagrelor | 0 (0%) | 0.557 | 3 (0.9%) | 1.0 | 0 (0%) |
| Prasugrel | 1 (0.7%) | 0.302 | 0 (0%) | — | 0 (0%) |
| Combined anticoagulation regimens | 0 (0%) | — | 0 (0%) | — | 0 (0%) |
| Biopsy samples | |||||
| Number of biopsy samples | 7 (6/7) | 7 (5/7) | 5 (3/7) | ||
BMI, body mass index; EMB, endomyocardial biopsy; INR, international normalized ratio; LVEDD, left ventricular end‐diastolic diameter; LVEDP, left ventricular end‐diastolic pressure; LV‐EMB, left ventricular‐endomyocardial biopsy; MI, myocardial infarction; NYHA, New York Heart Association; RV‐EMB, right ventricular‐endomyocardial biopsy.
Both femoral access EMB groups were compared with the reference group of radial LV‐EMB.
Bold was used in order to highlight statistical significant differences between the access site groups.
FIGURE 1.
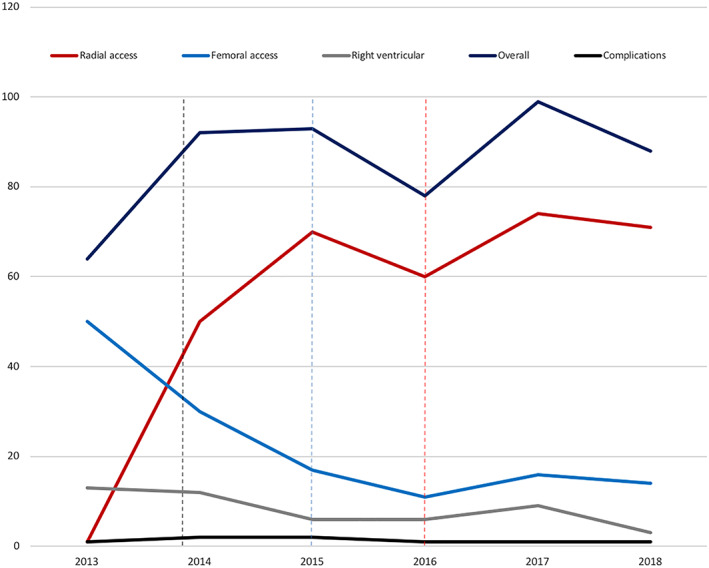
Temporal trends in the choice of access site from 2013 until 2018. Total numbers of endomyocardial biopsies stratified for access site are outlined for each year between 2013 and 2018. Additionally, a total number of annually major complications (i.e. permanent atrioventricular block requiring pacemaker implantation, pericardial tamponade necessitating pericardiocentesis, stroke and transient cerebral ischaemic attack as well as severe valvular damage, dissection of the radial artery, access site bleeding, and ventricular fibrillation/death) are illustrated. The black dashed line indicates the time of implementation of the in‐house feasibility study, the blue dashed line indicates the date of publication of the feasibility study by Schulz et al., and the red dashed line indicates the time of publication of the European Society of Cardiology guidelines on acute coronary syndrome.
Peri‐procedural and post‐procedural complications
We observed only a few minor and major complications in our study population with no significant differences between the three access site groups. Transient pericardial effusion without haemodynamic relevance was the most frequently documented minor complication in all three access site groups (transradial LV‐EMB: 11.2% vs. transfemoral LV‐EMB: 7.4% vs. RV‐EMB: 8.5%; P for difference). Non‐sustained ventricular tachycardia during the procedure was documented in three patients of the transradial LV‐EMB group and in two patients of the transfemoral LV‐EMB‐group, respectively, whereas one patient of the RV‐EMB group developed a transient high‐degree AV block with no pacing being required.
A total number of eight major complications was documented. In one patient who was primarily considered for transradial artery access, we had to switch to the femoral access due to a dissection of the radial artery. In the transradial LV‐EMB group, two patients developed a procedure‐related stroke, and ventricular fibrillation was documented in one patient during the insertion of the sheathless guiding catheter, which could be immediately terminated by external defibrillation with no impact on the patient's medical condition.
In the transfemoral LV‐EMB group, severe mitral regurgitation due to chordae rupture was documented in one patient during the follow‐up echocardiographic examination requiring surgery. Another patient of this group developed a procedure‐related stroke, and one patient required permanent pacemaker implantation due to a high‐degree AV block.
In the RV‐EMB group, we documented only one major complication (i.e. permanent pacemaker implantation due to a high‐degree AV block). All complications stratified for access site are depicted in detail in Table 2 .
TABLE 2.
Major and minor complications stratified for access site
| Variables |
LV‐EMB (femoral) N = 138 |
P‐value for comparisonbetween both LV‐EMB groups |
LV‐EMB (radial) N = 323 |
P‐value for comparison of radial LV‐EMB and RV‐EMB groups |
RV‐EMB (venous) N = 53 |
|---|---|---|---|---|---|
| Minor complications | |||||
| Transient chest pain | 0 (0%) | 1.0 | 1 (0.3%) | 0.7 | 0 (0%) |
| Non‐sustained VT | 2 (1.5%) | 0.63 | 3 (0.9%) | 1.0 | 0 (0%) |
| Transient AV block | 0 (0%) | — | 0 (0%) | 0.131 | 1 (2%) |
| Pericardial effusion | 10 (7.4%) | 0.21 | 36 (11.2%) | 0.802 | 4 (8.5%) |
| Major complications | |||||
| Permanent AV block | 1 (0.7%) | 0.295 | 0 (0%) | 0.133 | 1 (2%) |
| Pericardial tamponade | 0 (0%) | — | 0 (0%) | — | 0 (0%) |
| Stroke/TIA | 1 (0.7%) | 0.884 | 2 (0.6%) | 0.345 | 0 (0%) |
| Vascular access site complications | 0 (0%) | 0.653 | 1 a (0.3%) | 1.0 | 0 (0%) |
| Ventricular fibrillation | 0 (0%) | 0.517 | 1 b (0.3%) | 0.697 | 0 (0%) |
| Mitral regurgitation c | 1 d (0.7%) | 0.277 | 0 (0%) | — | 0 (0%) |
| Death | 0 (0%) | — | 0 (0%) | — | 0 (0%) |
AV, atrioventricular; LV‐EMB, left ventricular‐endomyocardial biopsy; RV‐EMB, right ventricular‐endomyocardial biopsy; TIA, transient ischaemic attack; VT, ventricular tachycardia.
Both femoral access EMB groups were compared with the reference group of radial LV‐EMB.
Radial artery dissection, conservative management.
Defibrillation, no resuscitation
More than moderate.
Chordae rupture.
Discussion
To our knowledge, this is the first study to investigate safety of the transradial artery access for LV‐EMB compared with established procedures of transfemoral LV‐EMB and RV‐EMB in one study centre. With the present study, we provide evidence that the transradial artery access for LV‐EMB is a safe procedure in high‐volume percutaneous coronary intervention centres, which is non‐inferior compared with the transfemoral artery access and RV‐EMB.
Several studies have already demonstrated safety and feasibility of LV‐EMB, although these studies predominantly investigated the transfemoral approach for LV‐EMB compared with RV‐EMB. 6 , 17 , 18 In a study by Chimenti and Frustaci, a total of 4221 patients underwent diagnostic EMB between 1983 and 2010 performed by only two experienced interventional cardiologists, with 2396 patients undergoing biventricular EMB, 1153 patients undergoing selective LV‐EMB, and 672 patients undergoing selective RV‐EMB. In line with the results of our study, the authors reported very low rates of major complications (i.e. 0.08% perforation with cardiac tamponade and 0.22% brain embolization with transient cerebral ischaemia) with no significant differences between LV‐EMB and RV‐EMB. 18 The fact that no perforation with cardiac tamponade was documented in our study population can probably be attributed to a detailed screening programme performed prior to EMB. Thus, a diameter of the lateral LV wall <8 mm documented by an echocardiographic examination was considered a contraindication for LV‐EMB. 13 In the study by Chimenti and Frustaci, all perforations were observed in patients with a thin LV wall (7.5 ± 1.3 mm), LV dilatation (72.5 ± 8.5 mm), and severe systolic dysfunction (20.3 ± 3.3%). Perforations with cardiac tamponade as a major complication of EMB have also been reported in a prospective study investigating complications in 546 patients undergoing RV‐EMB. 19 In this particular study, ventricle perforations were documented in 0.5% of patients (n = 3) with two deaths being reported. As mentioned by Chimenti and Frustaci, EMB should only be performed by experienced interventional cardiologists in order to reduce the risk of procedure‐related complications. Although safety of the femoral artery access for LV‐EMB has been proven, the radial artery access has gained increasing interest even for LV‐EMB. This is primarily based on the results of several studies providing robust evidence in favour of the transradial artery access in patients presenting with acute coronary syndrome undergoing primary percutaneous coronary intervention by experienced interventional cardiologists. For example, the Minimizing Adverse Haemorrhagic Events by TRansradial Access Site and Systemic Implementation of angioX (MATRIX) trial compared radial artery with the femoral artery access in 8404 patients presenting with acute coronary syndrome. 20 The results of the trial revealed that the radial artery access was associated with a significantly lower risks of access site bleeding (including need for transfusion) and vascular complications. Moreover, the authors reported a significant mortality benefit in patients allocated to the radial artery access site, which was in line with two previously published trials. 10 , 21 Besides reduced complication rates associated with the radial artery access, the radial approach also enables an earlier mobilization of the patients and a potentially earlier discharge. 22
Feasibility and safety of the transradial artery access for LV‐EMB have at first been described in two small‐scale studies investigating this approach in 37 patients 13 and 42 patients, 12 respectively. In the present study, we demonstrate a transradial artery access success of 99% (322 out of 323), which matches the success rates observed in the aforementioned study by Schäufele et al. (success rate of 98%) 12 and a study investigating the transradial approach compared with the transfemoral approach in a study population consisting of 264 patients (success rate also 99%). 14 In both studies, the switch to the transfemoral access was mandatory in case of a severe spasm of the radial artery, whereas in our study, a dissection of the radial artery necessitated the switch to the transfemoral access site. Although associated with relevant clinical consequences, access‐site vascular complications in patients undergoing transradial coronary procedures are rare 23 and include diverse pathologies such as spasm, occlusion, or perforation of the radial artery, haematoma, pseudoaneurysm, arteriovenous fistula, and nerve injury. 24 Besides intra‐procedural access site complications, a recent study revealed a high number of post‐procedural asymptomatic radial occlusions (50%) documented by duplex sonography in patients undergoing LV‐EMB using a sheathless catheter. 25 Interestingly, 66% of patients with documented radial artery occlusion had a palpable ‘radial artery pulse’ on clinical examination. The authors declared that the palpable pulse was not a radial pulse but rather an ulnar pulse fed by the palmar arch. In summary, these data indicate the necessity of a structured examination prior to EMB through the radial artery (confirmation of the patency of the ulnar artery).
A recently published study reported no major complications in conjunction with the transradial and transfemoral artery access but a spectrum of minor complications that was similar to that observed in our study. 14 Additionally, the authors provide evidence of a significantly higher prevalence of access site haematoma in patients undergoing transfemoral LV‐EMB compared with patients undergoing transradial LV‐EMB. Although Choudhury et al. documented no major complications in their study, the major complication rate of 1.5% (8/514) reported in our study is in line with the results of a study investigating LV‐EMB through the femoral artery and RV‐EMB with a major complication rate of 1% including stroke, haemopericardium necessitating pericardiocentesis, and AV block temporarily requiring pacemaker. 6
Conductance abnormalities after RV‐EMB have been analysed in a study, consisting of a retrospective and a prospective part. 5 Similar to our study, analysis of the retrospective part revealed low incidence rates of AV block with no patients experiencing other conductance abnormalities (i.e. atrial fibrillation). Especially, patients with a pre‐existing left bundle branch block (LBBB) were at significantly increased risk for experiencing total AV block, which is interesting, as the patient in the RV‐EMB group of our study who developed total AV block also had LBBB prior to intervention. Therefore, indication for RV‐EMB should be evaluated thoroughly in patients with pre‐existing LBBB. As the aforementioned study by Holzmann et al. outlined significantly higher complication rates in the prospective part of the study (probably attributable to a more detailed worksheet for each patient covering all minor complications in the prospective trial), an underreporting of especially conductance abnormalities might therefore also be true for our study.
In the present study, we have additionally documented severe mitral regurgitation due to chordae rupture in one patient undergoing LV‐EMB (transfemoral artery access), a complication that was not yet reported in studies systematically investigating LV‐EMB. However, chordae rupture leading to relevant regurgitation of an AV valve is a complication not solely attributed to LV‐EMB but has also been reported in patients undergoing RV‐EMB. 26
Finally, the results of our study indicate a significant sex difference between the amount of transradial artery access compared with transfemoral artery access (Table 1 ). The exact reason for the sex differences between different access sites remains elusive. It might be related to smaller anatomy and vessel diameters in women who had lower body height and body mass index as compared with men. As a consequence, radial access site should not be the preferred route in small or underweight patients, or in individuals with haemodialysis shunts or prior surgery in the forearm or wrist.
However, the present study has limitations. The study was conducted as a retrospective single‐centre cohort study. Due to the single‐centre design, it is questionable whether the results of our study (i.e. low major and minor complication rates in patients undergoing EMB) have a universal validity or whether they are only valid for high‐volume centres like our institution. Additionally, the reported complication rate might be underestimated due to selection bias and other confounders related to the non‐prospective study design.
Taken together, the results of the present study indicate that transradial LV‐EMB is a safe procedure with high success rate for experienced radial operators and non‐inferior to transfemoral LV‐EMB and RV‐EMB. An accurate pre‐procedural, peri‐procedural, and post‐procedural monitoring and follow‐up care should be recommended for all patients undergoing this procedure as well as for all patients undergoing LV‐EMB through the femoral artery and RV‐EMB in order to prevent potential (severe) complications.
Conflict of interest
None declared.
Funding
P.W. was supported by grants from the German Federal Ministry for Education and Research [Bundesministerium für Bildung und Forschung (BMBF) 01EO1503]. T.M. and P.W. are Principal Investigators of the German Center for Cardiovascular Research (DZHK).
Supporting information
Table S1. Heart failure medication at discharge stratified for access site
Table S2. Diagnosis based on endomyocardial biopsy findings
Acknowledgements
This manuscript contains results that are part of the medical doctoral thesis work of Finja Knies.
Göbel, S. , Schwuchow‐Thonke, S. , Jansen, T. , Karbach, S. , Emrich, T. , Gori, T. , Knies, F. , Schulz, E. , Münzel, T. , Keller, K. , and Wenzel, P. (2020) Safety of transradial and transfemoral left ventricular compared with transfemoral right ventricular endomyocardial biopsy. ESC Heart Failure, 7: 4015–4023. 10.1002/ehf2.13006.
References
- 1. Maggioni AP, Dahlstrom U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Nielsen OW, Zannad F, Tavazzi L, Heart Failure Association of the European Society of C . EURObservational Research Programme: regional differences and 1‐year follow‐up results of the Heart Failure Pilot Survey (ESC‐HF Pilot). Eur J Heart Fail 2013; 15: 808–817. [DOI] [PubMed] [Google Scholar]
- 2. Sotiriou E, Heiner S, Jansen T, Brandt M, Schmidt KH, Kreitner KF, Emrich T, Schultheiss HP, Schulz E, Munzel T, Wenzel P. Therapeutic implications of a combined diagnostic workup including endomyocardial biopsy in an all‐comer population of patients with heart failure: a retrospective analysis. ESC Heart Fail 2018; 5: 630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM, European Society of Cardiology Working Group on M, Pericardial D . Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34: 2636–2648 2648a‐2648d. [DOI] [PubMed] [Google Scholar]
- 4. Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, Virmani R, American Heart A, American College of C, European Society of C . The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation 2007; 116: 2216–2233. [DOI] [PubMed] [Google Scholar]
- 5. Holzmann M, Nicko A, Kuhl U, Noutsias M, Poller W, Hoffmann W, Morguet A, Witzenbichler B, Tschope C, Schultheiss HP, Pauschinger M. Complication rate of right ventricular endomyocardial biopsy via the femoral approach: a retrospective and prospective study analyzing 3048 diagnostic procedures over an 11‐year period. Circulation 2008; 118: 1722–1728. [DOI] [PubMed] [Google Scholar]
- 6. Yilmaz A, Kindermann I, Kindermann M, Mahfoud F, Ukena C, Athanasiadis A, Hill S, Mahrholdt H, Voehringer M, Schieber M, Klingel K, Kandolf R, Bohm M, Sechtem U. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation 2010; 122: 900–909. [DOI] [PubMed] [Google Scholar]
- 7. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Group ESCSD . 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 267–315. [DOI] [PubMed] [Google Scholar]
- 8. Neumann FJ, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO, Group ESCSD . 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019; 40: 87–165. [DOI] [PubMed] [Google Scholar]
- 9. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck‐Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ, Group ESCSD . 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020; 41: 407–477. [DOI] [PubMed] [Google Scholar]
- 10. Jolly SS, Yusuf S, Cairns J, Niemela K, Xavier D, Widimsky P, Budaj A, Niemela M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR, group Rt . Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet 2011; 377: 1409–1420. [DOI] [PubMed] [Google Scholar]
- 11. Noble S. Radial access in patients invasively treated for acute coronary syndromes: a lifesaving approach. JACC Cardiovasc Interv 2016; 9: 671–673. [DOI] [PubMed] [Google Scholar]
- 12. Schäufele TG, Spittler R, Karagianni A, Ong P, Klingel K, Kandolf R, Mahrholdt H, Sechtem U. Transradial left ventricular endomyocardial biopsy: assessment of safety and efficacy. Clin Res Cardiol 2015; 104: 773–781. [DOI] [PubMed] [Google Scholar]
- 13. Schulz E, Jabs A, Gori T, Hink U, Sotiriou E, Tschope C, Schultheiss HP, Munzel T, Wenzel P. Feasibility and safety of left ventricular endomyocardial biopsy via transradial access: technique and initial experience. Catheter Cardiovasc Interv 2015; 86: 761–765. [DOI] [PubMed] [Google Scholar]
- 14. Choudhury T, Lurz P, Schäufele TG, Menezes MN, Lavi S, Tzemos N, Hartung P, Stiermaier T, Makino K, Bertrand OF, Gilchrist IC, Mamas MA, Bagur R. Radial versus femoral approach for left ventricular endomyocardial biopsy. EuroIntervention 2019; 15: 678–684. [DOI] [PubMed] [Google Scholar]
- 15. Liang M, Puri A, Linder R. Transradial simultaneous kissing stenting (SKS) with SheathLess access. Catheter Cardiovasc Interv 2010; 75: 222–224. [DOI] [PubMed] [Google Scholar]
- 16. Mamas MA, Fath‐Ordoubadi F, Fraser DG. Atraumatic complex transradial intervention using large bore sheathless guide catheter. Catheter Cardiovasc Interv 2008; 72: 357–364. [DOI] [PubMed] [Google Scholar]
- 17. Stiermaier T, Fohrenbach F, Klingel K, Kandolf R, Boudriot E, Sandri M, Linke A, Rommel KP, Desch S, Schuler G, Thiele H, Lurz P. Biventricular endomyocardial biopsy in patients with suspected myocarditis: feasibility, complication rate and additional diagnostic value. Int J Cardiol 2017; 230: 364–370. [DOI] [PubMed] [Google Scholar]
- 18. Chimenti C, Frustaci A. Contribution and risks of left ventricular endomyocardial biopsy in patients with cardiomyopathies: a retrospective study over a 28‐year period. Circulation 2013; 128: 1531–1541. [DOI] [PubMed] [Google Scholar]
- 19. Deckers JW, Hare JM, Baughman KL. Complications of transvenous right ventricular endomyocardial biopsy in adult patients with cardiomyopathy: a seven‐year survey of 546 consecutive diagnostic procedures in a tertiary referral center. J Am Coll Cardiol 1992; 19: 43–47. [DOI] [PubMed] [Google Scholar]
- 20. Valgimigli M, Gagnor A, Calabro P, Frigoli E, Leonardi S, Zaro T, Rubartelli P, Briguori C, Ando G, Repetto A, Limbruno U, Cortese B, Sganzerla P, Lupi A, Galli M, Colangelo S, Ierna S, Ausiello A, Presbitero P, Sardella G, Varbella F, Esposito G, Santarelli A, Tresoldi S, Nazzaro M, Zingarelli A, de Cesare N, Rigattieri S, Tosi P, Palmieri C, Brugaletta S, Rao SV, Heg D, Rothenbuhler M, Vranckx P, Juni P, Investigators M . Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet 2015; 385: 2465–2476. [DOI] [PubMed] [Google Scholar]
- 21. Romagnoli E, Biondi‐Zoccai G, Sciahbasi A, Politi L, Rigattieri S, Pendenza G, Summaria F, Patrizi R, Borghi A, Di Russo C, Moretti C, Agostoni P, Loschiavo P, Lioy E, Sheiban I, Sangiorgi G. Radial versus femoral randomized investigation in ST‐segment elevation acute coronary syndrome: the RIFLE‐STEACS (Radial Versus Femoral Randomized Investigation in ST‐Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol 2012; 60: 2481–2489. [DOI] [PubMed] [Google Scholar]
- 22. Israeli Z, Lavi S, Bertand OF, Mamas MA, Bagur R. Radial versus femoral approach for same‐day inter‐facility transfer for percutaneous coronary intervention. J Interv Cardiol 2018; 31: 230–235. [DOI] [PubMed] [Google Scholar]
- 23. Burzotta F, Mariani L, Trani C, Coluccia V, Brancati MF, Porto I, Leone AM, Niccoli G, Tommasino A, Tinelli G, Mazzari MA, Mongiardo R, Snider F, Schiavoni G, Crea F. Management and timing of access‐site vascular complications occurring after trans‐radial percutaneous coronary procedures. Int J Cardiol 2013; 167: 1973–1978. [DOI] [PubMed] [Google Scholar]
- 24. Kanei Y, Kwan T, Nakra NC, Liou M, Huang Y, Vales LL, Fox JT, Chen JP, Saito S. Transradial cardiac catheterization: a review of access site complications. Catheter Cardiovasc Interv 2011; 78: 840–846. [DOI] [PubMed] [Google Scholar]
- 25. Kherad B, Kohncke C, Spillmann F, Post H, Noutsias M, Pieske B, Krackhardt F, Tschope C. Postprocedural radial artery occlusion rate using a sheathless guiding catheter for left ventricular endomyocardial biopsy performed by transradial approach. BMC Cardiovasc Disord 2016; 16: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lo CY, Chang HH, Hsu CP, Lai ST, Shih CC. Endomyocardial biopsy‐related tricuspid regurgitation after orthotopic heart transplantation: single‐center experience. J Chin Med Assoc 2007; 70: 185–192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Heart failure medication at discharge stratified for access site
Table S2. Diagnosis based on endomyocardial biopsy findings


