Abstract
Aims
Heart failure (HF) is associated with several metabolic changes, but it is unknown whether distinct components of the circulating metabolome may be related to cardiac structure and function, and with incident HF in the community.
Methods and results
We assayed 217 circulating metabolites in 2336 Framingham Study participants (mean age 55 ± 10 years, 53% women) without HF at baseline. We used linear and Cox regression to relate concentrations of metabolites to left ventricular (LV) diastolic dimension, LV wall thickness, LV ejection fraction, left atrial dimension, LV ventricular mass, and aortic root size cross‐sectionally and to incident HF prospectively. Bonferroni‐adjusted P‐values <0.05 denoted statistical significance. Circulating concentrations of kynurenine [β = −0.12 cm per standard deviation (SD) increment in normalized residual of metabolite, P = 7.3 × 10−8] and aminoadipate (−0.11 cm per SD increment, P = 2.61 × 10−5) were associated with left ventricular diastolic dimension, phosphatidylcholine (carbon:double bound = 38:6) with left atrial dimension (0.10 cm per SD increment, P = 9.7 × 10−6), and cholesterol ester (carbon:double bound = 20:5) with left atrial dimension (0.10 cm per SD increment, P = 1.4 × 10−5) in multivariable‐adjusted models. During an average follow‐up of 15.8 (range 0.02–23.2) years, 113 participants (5%) were diagnosed with HF with reduced ejection fraction and 106 individuals (5%) with HF with preserved ejection fraction. In multivariable analyses, concentrations of phosphatidylcholine (hazard ratio 0.63, P = 1.3 × 10−5) and ornithine (hazard ratio 1.44, P = 0.00014) were associated with HF with reduced ejection fraction.
Conclusions
Several metabolites, including the vasoactive metabolite kynurenine, were related to cardiac structure and function in our sample. Additional research is warranted to confirm our observations and investigate if these metabolites can risk stratify ambulatory individuals.
Keywords: Metabolomics, Echocardiography, Heart failure risk
Introduction
The heart is the most energy‐consuming organ of the human body (per gram of tissue). Consequently, the myocardium is very sensitive to the influence of metabolic alterations, but the relation between the circulating metabolome and heart failure (HF) risk in the community remains unknown. The impact of altered myocardial metabolism on the cardiac function is evidenced, for instance, in echocardiographic studies of patients with diabetes mellitus, thyroid disorders, and thiamine deficiency. 1 , 2 , 3 , 4 Several metabolic disturbances including obesity, 5 mitochondrial genetic disorders, 6 and lipid abnormalities 7 are also closely linked to the HF risk, but even subtle metabolic variations (e.g. high‐normal blood glucose levels) may antedate overt HF by years, underscoring the potential relevance of metabolomics for long‐term HF risk prediction. 8 , 9 , 10 , 11 In the setting of prevalent HF, emerging metabolomic profiling has begun to elucidate specific molecular signatures relating to phenotypic characterization and survival. 12 , 13 , 14 In the present investigation, we explored the associations of a targeted circulating metabolomic profile with select echocardiographic measures of subclinical cardiac remodelling (cross‐sectionally) and with the risk of incident HF in a community‐based sample of individuals who were free of overt HF at baseline.
Methods
For the present investigation, we analysed data of the Framingham Offspring cohort. 15 The Framingham Offspring cohort participants were enrolled in 1971 and have been followed up for the onset of cardiovascular disease in examination cycles at approximately 4–6 year intervals. As previously detailed, all participants from Framingham Offspring had blood drawn after an overnight fast at the fifth examination cycle (1991–1995) that was later used for metabolomic profiling. 16 The blood was collected in an ethylendiaminetetraacetic acid medium and was immediately stored at −80°C until assayed. The metabolomic profile of participants was measured between 2009 and 2011 using liquid chromatography with mass spectrometry techniques as detailed previously. 16 , 17 , 18 , 19 In brief, three distinct platforms were applied: the first to measure a variety of amino acids and amines, 16 the second to measure organic acids, nucleotides, and other intermediary metabolites, 19 and the third to measure lipids of varying classes. 18 In the present investigation, we related the measured circulating metabolites to echocardiographic variables in cross‐sectional analyses and to the risk of developing HF during follow‐up through 31 December 2016.
Echocardiographic measures
During the fifth examination cycle, all participants also underwent standard transthoracic echocardiography with Doppler colour flow imaging. The left ventricular diastolic dimension (LVDD) and systolic dimension (LVSD) were measured from the parasternal short‐axis view at the level just above the papillary muscles. Left ventricular fractional shortening was calculated as (LVDD − LVSD)∕LVDD and may be used as a proxy for left ventricular ejection fraction (LVEF). Aortic root dimension, left atrial dimension (LAD), and left ventricular wall thickness (the sum of septum and lateral wall thickness in end diastole) were also measured from M‐mode frames. LAD was measured in the end systole. Left ventricular mass was calculated based on the formula of Devereux and Reichek. 20
Heart failure definition
Heart failure was defined according to the established Framingham Heart Study (FHS) epidemiological criteria, which require at least two major, or one major plus two minor criteria, as outlined elsewhere. 21 All participants have been under continuous surveillance for incident HF that is adjudicated by reviewing hospitalization charts and by participant history and physical examinations during the FHS clinic visits. For all HF events, the final adjudication was undertaken by an expert study committee comprising at least three FHS physicians, of whom at least two were cardiologists. We defined HF with preserved ejection fraction (HFpEF) as HF with LVEF ≥ 50% and HF with reduced ejection fraction (HFrEF) as HF with LVEF < 50% at the time of HF diagnosis.
Statistical analyses
Both echocardiographic variables and metabolites were regressed first on age, sex, height, and weight to obtain residuals, which were then rank‐based inverse normal transformed for all subsequent analyses. Echocardiography variables (dependent variables) were related to metabolites (independent variables) using age‐adjusted and sex‐adjusted linear regression models. In secondary models, we additionally adjusted for weight, height, prevalent myocardial infarction, current smoking, diabetes, total and high‐density lipoprotein cholesterol levels, treatment for hypertension, and systolic blood pressure values. As sensitivity, we included estimated glomerular filtration rates (eGFR) in the models; this metabolite was not included in primary analyses because it may be along the causal pathway of some metabolites. 22
To model associations of metabolites with prospective risk of HFpEF and HFrEF, we used Cox regression after confirming that the assumption of proportionality of hazards was met. Two separate models were run, one for each of HFpEF and HFrEF. First, we adjusted for age and sex. Second, we additionally adjusted for weight, height, prevalent myocardial infarction, current smoking, diabetes, total and high‐density lipoprotein cholesterol levels, treatment for hypertension, and systolic blood pressure values. As sensitivity, we also adjusted for eGFR. Participants were censored when they died or at the end of follow‐up. For HFrEF models, participants were censored if they developed HFpEF (and vice versa for HFrEF). We used sandwich estimators to obtain robust standard errors accounting for familial relatedness among participants.
All statistical analyses were performed using R software Version 3.4.3 (R Foundation, Vienna, Austria). Two‐sided P‐values <0.05 were considered statistically significant after Bonferroni correction. For the echocardiographic variables, this corresponded to P = 3.8 × 10−5 (217 metabolites × 6 echocardiography variables, i.e. 1302 tests, α = 0.05/1302 = 3.8 × 10−5). For HFpEF and HFrEF, this corresponded to P = 2.3 × 10−4 (i.e. α = 0.05/217 tests). In addition, we used a less stringent false discovery rate (FDR) of q ≤ 0.10, as an exploratory hypothesis‐generating analysis because a Bonferroni may be overly conservative, especially when correlated variables are analysed.
Results
Baseline characteristics of our study sample are shown in Table 1 . In total, 2343 participants (mean age 55 ± 10 years, 53% women) had available metabolomics data. Of these, 7 (0.3%) were excluded from further analyses because of prevalent HF. The remaining 2336 individuals were followed up for an average of 15.8 years (min–max 0.02–23.2 years). During this period, 219 (9.4%) participants were diagnosed with HF: 113 (5%) of whom had HFrEF and 106 (5%) had HFpEF. The average time to HF onset was 12.6 years (p25–p75 8.9–17.4 years). Characteristics of the HF cases are available in Supporting Information, Table S1 .
TABLE 1.
Baseline characteristics of the study sample
| Men (n = 1109) | Women (n = 1227) | |
|---|---|---|
| Age (years) | 55 (10) | 55 (10) |
| Weight (kg) | 87 (14) | 70 (14) |
| Height (m) | 175 (7) | 161 (6) |
| Total cholesterol concentration (mg/dL) | 202 (35) | 209 (38) |
| High‐density lipoprotein cholesterol concentration (mg/dL) | 43 (11) | 56 (15) |
| Low‐density lipoprotein cholesterol concentration (mg/dL) | 129 (31) | 126 (34) |
| Very‐low‐density lipoprotein cholesterol concentration (mg/dL) | 26 (12) | 25 (11) |
| Systolic blood pressure (mmHg) | 129 (18) | 124 (20) |
| Prevalent myocardial infarction (N) | 44 (4%) | 6 (0.5%) |
| Current smoking (N) | 196 (18%) | 218 (18%) |
| Diabetes (N) | 93 (8%) | 63 (5%) |
| Use of antihypertensive medications (N) | 237 (21%) | 206 (17%) |
| Education | ||
| No high school degree | 57 (5%) | 71 (6%) |
| High school degree | 378 (31%) | 299 (27%) |
| Some college degree | 394 (32%) | 252 (23%) |
| College degree | 348 (28%) | 447 (40%) |
| Echocardiographic variables | ||
| Left atrial dimension (cm) | 4.0 (0.5) | 3.6 (0.5) |
| Left ventricular diastolic dimension (cm) | 5.0 (0.4) | 4.6 (0.4) |
| Left ventricular systolic dimension (cm) | 3.2 (0.5) | 2.9 (0.4) |
| Fractional shortening | 0.36 (0.07) | 0.38 (0.06) |
| Left ventricular wall thickness (cm) | 2.0 (0.3) | 1.8 (0.2) |
| Aortic root dimension (cm) | 3.4 (0.3) | 3.0 (0.3) |
| Left ventricular mass (g) | 184 (37) | 140 (29) |
Numbers in parentheses refer to standard deviations unless specified.
Echocardiographic traits
At an FDR q < 0.10, 36 metabolites were associated with echocardiographic variables in age‐adjusted and sex‐adjusted models (Figure 1 ). Of these, 24 metabolites remained statistically significant after multivariable adjustment. Using the more stringent Bonferroni‐adjusted threshold of P < 3.8 × 10−5, three metabolites were significantly associated with echocardiographic traits in sex‐adjusted and age‐adjusted models: kynurenine with LVDD [β estimate −0.11 cm for each 1 standard deviation (SD) increment of normalized residual, P = 1.3 × 10−5], diacylglycerol (carbon number:double bond number = 36:1) with LVDD (β estimate −0.11 cm per SD increment, P = 1.9 × 10−5), and leucine with LVDD (β estimate −0.09 cm per SD increment, P = 3.2 × 10−5). After multivariable adjustment, the association of kynurenine with LVDD remained statistically significant per the Bonferroni‐corrected threshold (−0.12 cm per SD increment, P = 7.3 × 10−8). Upon additional adjustment for eGFR, estimate remained similar [β estimate −0.10, standard error (SE) 0.028, P = 0.00030].
Figure 1.
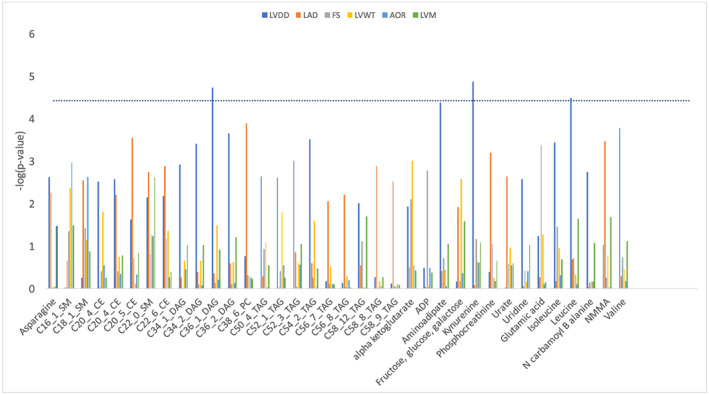
Association of selected metabolites and echocardiographic measures. Only variables that were significant at a false discovery rate level of ≤0.10 in one or more of the echocardiographic measures are presented. The y axis presents −log(P‐value). Dotted line represents a Bonferroni‐corrected P‐value of <0.05. AOR, aortic root; FS, fractional shortening; LAD, left atrial dimension; LVDD, left ventricular diastolic dimension; LVM, left ventricular mass; LVWT, left ventricular wall thickness. Metabolite abbreviations: SM, sphingomyelin; CE, cholesterol ester; DAG, diacylglycerol; TAG, triacylglycerol. C stands for carbon:double bond; for example, C16_1_SM is sphingomyelin (carbon:double bond = 16:1).
In addition, in multivariable models, aminoadipate was associated with LVDD (β estimate −0.11 cm per SD increment, P = 2.61 × 10−5), phosphatidylcholine (carbon:double bound = 38:6) with LAD (β estimate 0.10 cm per SD increment, P = 9.7 × 10−6), and cholesterol ester (carbon:double bound = 20:5) with LAD (0.10 cm per SD increment, P = 1.4 × 10−5). After additional adjustment for eGFR, estimates were similar: β −0.10, SE 0.028, P = 0.00037, for aminoadipate and LVDD; β 0.11, SE 0.025, P = 1.7e − 5, for phosphatidylcholine (carbon:double bound = 38:6) and LAD; and β 0.094, SE 0.025, P = 0.00017, for cholesterol ester (carbon:double bound = 20:5) and LAD.
Heart failure
In age‐adjusted and sex‐adjusted models, cotinine (hazard ratio 1.61 per SD increment normalized residual, P = 8.4 × 10−5) and hydroxybutyrate (hazard ratio 1.49 per SD increment, P = 0.00017) were significantly associated with incident HFpEF after Bonferroni correction. Upon multivariable adjustment, none of these (or other) metabolites remained, however, significantly associated with HFpEF risk (Table 2 ).
TABLE 2.
Associations of various metabolites with HFpEF and HFrEF risks: top hits
| Metabolite | Estimated β | SE | Robust SE | Hazard ratio | P‐value | FDR q |
|---|---|---|---|---|---|---|
| HFpEF, age‐adjusted and sex‐adjusted | ||||||
| Cotinine | 0.48 | 0.14 | 0.12 | 1.61 | 8.43e − 05 | 0.02 |
| β‐Hydroxybutyrate | 0.40 | 0.11 | 0.11 | 1.49 | 0.00017 | 0.02 |
| HFrEF, age‐adjusted and sex‐adjusted | ||||||
| C36_4_PC_A | −0.44 | 0.12 | 0.10 | 0.64 | 1.44e − 05 | 0.003 |
| Isocitrate | 0.45 | 0.10 | 0.11 | 1.57 | 2.93e − 05 | 0.003 |
| C18_2_LPC | −0.40 | 0.10 | 0.10 | 0.67 | 6.61e − 05 | 0.005 |
| Cotinine | 0.40 | 0.12 | 0.11 | 1.49 | 0.00023 | 0.01 |
| Ornithine | 0.27 | 0.10 | 0.09 | 1.32 | 0.0017 | 0.07 |
| ADMA | 0.29 | 0.09 | 0.09 | 1.34 | 0.0020 | 0.07 |
| Choline | 0.25 | 0.09 | 0.08 | 1.28 | 0.0023 | 0.07 |
| 5‐HIAA | 0.23 | 0.09 | 0.08 | 1.26 | 0.0027 | 0.07 |
| N‐carbamoyl‐β‐alanine | 0.30 | 0.09 | 0.10 | 1.34 | 0.0032 | 0.07 |
| Aconitate | 0.32 | 0.10 | 0.11 | 1.38 | 0.0032 | 0.07 |
| Lactate | 0.34 | 0.10 | 0.12 | 1.40 | 0.0036 | 0.07 |
| C18_2_CE | −0.26 | 0.10 | 0.09 | 0.77 | 0.0046 | 0.08 |
| Dimethylglycine | 0.28 | 0.09 | 0.10 | 1.32 | 0.0054 | 0.09 |
| Sucrose | 0.35 | 0.12 | 0.13 | 1.42 | 0.0056 | 0.09 |
| C14_0_SM | −0.26 | 0.11 | 0.09 | 0.77 | 0.0062 | 0.09 |
| C56_4_TAG | 0.28 | 0.10 | 0.11 | 1.33 | 0.0072 | 0.10 |
| HFrEF, multivariable‐adjusted | ||||||
| C36_4_PC_A | −0.45 | 0.12 | 0.10 | 0.63 | 1.29e − 05 | 0.003 |
| Ornithine | 0.36 | 0.10 | 0.10 | 1.44 | 0.00014 | 0.01 |
| C18_2_LPC | −0.40 | 0.11 | 0.11 | 0.67 | 0.00027 | 0.02 |
| ADMA | 0.37 | 0.09 | 0.11 | 1.45 | 0.00048 | 0.03 |
| Isocitrate | 0.36 | 0.11 | 0.10 | 1.43 | 0.00068 | 0.03 |
| Quinolinate | 0.33 | 0.10 | 0.10 | 1.39 | 0.00093 | 0.03 |
| Cotinine | 0.57 | 0.20 | 0.17 | 1.78 | 0.00099 | 0.03 |
| Dimethylglycine | 0.31 | 0.09 | 0.10 | 1.36 | 0.0020 | 0.06 |
| Trimethylamine N‐oxide | 0.27 | 0.09 | 0.09 | 1.31 | 0.0038 | 0.09 |
5‐HIAA, 5‐hydroxyindoleacetic acid; ADMA, asymmetric dimethylarginine; FDR, false discovery rate; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; SE, standard error. Metabolite abbreviations: SM, sphingomyelin; CE, cholesterol ester; DAG, diacylglycerol; TAG, triacylglycerol. C stands for carbon:double bond; for example, C16_1_SM is sphingomyelin (carbon:double bond = 16:1).
Sixteen metabolites were associated with HFrEF at FDR q ≤ 0.10, and nine remained statistically significant in multivariable‐adjusted models (Table 2 ). Phosphatidylcholine (carbon:double bond = 36:4; hazard ratio 0.64 per SD increment, P = 1.4 × 10−5), isocitrate (hazard ratio 1.57 per SD increment, P = 2.9 × 10−5), and lysophosphatidylcholine (carbon:double bond = 18:2; hazard ratio 0.67 per SD increment, P = 6.6 × 10−5) were associated with incident HFrEF in age‐adjusted and sex‐adjusted models, and cotinine was borderline statistically significant in the same models (hazard ratio 1.49 per SD increment, P = 0.00023). After additional adjustment, phosphatidylcholine (carbon:double bond = 36:4; hazard ratio 0.63 per SD increment, P = 1.3 × 10−5) and ornithine (hazard ratio 1.44 per SD increment, P = 0.00014) were observed to be significantly associated with incident HFrEF. Additional adjustment for eGFR did not change these associations [hazard ratio 0.56 per SD increment, P = 7.8 × 10−7, for phosphatidylcholine (carbon:double bond = 36:4), and hazard ratio 1.47 per SD increment, P = 0.00032, for ornithine].
Validation of echocardiographic associations
During their first examination cycle (2002–2005), the FHS Third Generation cohort (N = 4095; mean age 40 years ± standard deviation 9 years; 53% women) 23 underwent echocardiography and had certain metabolites measured in a similar platform. We sought to replicate our observations with FDR q < 0.10 based on a common set of metabolites across the two cohorts. Levels of α‐ketoglutarate, asparagine, carnosine, dimethylglycine, glutamic acid, glutamine, hydroxybutyrate, isoleucine, kynurenine, lactate, leucine, niacinamide, N G‐monomethyl‐l‐arginine, ornithine, phosphocreatine, quinolate, uridine, uric acid, valine, and xanthurenate were available in both generations. Of these, nine metabolites were associated with at least one of the echocardiographic traits in age‐adjusted and sex‐adjusted models (Table 3 ). Of note, we confirmed a strong association between kynurenine and LVDD in the FHS Third Generation sample.
TABLE 3.
Significant associations of metabolites and various echocardiographic variables (at age‐adjusted and sex‐adjusted P < 0.05) in the Third Generation cohort
| Metabolite | Significant echocardiographic outcomes | |
|---|---|---|
| α‐Ketoglutarate | LV wall thickness (β estimate 0.089 cm, P = 0.0055) | LVDD (β estimate −0.080, P = 0.014) |
| Asparagine | LVDD (β estimate 0.10 cm, P = 0.0018) | LV mass (β estimate 0.081 g, P = 0.032) |
| Dimethylglycine | LV wall thickness (β estimate 0.085 cm, P = 0.0076) | |
| Glutamic acid | LV thickness (β estimate 0.099 g, P = 0.0020) | |
| Hydroxybutyrate | Fractional shortening (β estimate 0.068%‐units, P = 0.036) | |
| Kynurenine | LVDD (β estimate −0.10 cm, P = 0.0020) | Fractional shortening (β estimate −0.064%‐units, P = 0.047) |
| Lactate | LVDD (β estimate −0.12 cm, P = 0.00067) | LV wall thickness (β estimate 0.099 cm, P = 0.0033) |
| Quinolate | LVDD (β estimate −0.082 cm, P = 0.012) | |
| Uric acid | LV wall thickness (β estimate 0.11 cm, P = 0.00081) | LVDD (β estimate −0.080 cm, P = 0.014) |
LV, left ventricular; LVDD, left ventricular diastolic dimension.
Estimates are per 1 SD increment of normalized residual metabolite.
Discussion
In this discovery effort using a high‐throughput mass spectrometry‐based targeted metabolomics panel in the Framingham Offspring cohort, we identified a set of metabolites associated with adverse cardiac remodelling and antedating the onset of HF by over a decade.
Metabolites associated with echocardiographic indices
Higher levels of circulating kynurenine were associated with lower LVDD (an endophenotype of HF risk) 24 in both the FHS Offspring and Third Generation cohorts. 25 Kynurenine is derived from the amino acid tryptophan after it has been metabolized via the kynurenine pathway. Kynurenine is a neuroactive molecule that is implicated in many mental disorders (partly through its effects on the N‐methyl‐d‐aspartate receptor signalling system). 26 It is also important in the setting of cytokine signalling and inflammation and has been associated with atherosclerosis. 27 Kynurenine acid levels, the downstream product of tryptophan metabolism in the kynurenine pathway, have previously shown to be increased in various emergencies such as cardiac arrest, sepsis, and stroke. 28 Higher ratios of kynurenine to tryptophan levels were recently reported to be associated with increased cardiovascular and all‐cause mortality in patients with stable coronary artery disease. 29 At the molecular level, kynurenine can also cause vascular relaxation via the Kv7 ion channels mediated by the adenylate and soluble guanylate cyclase pathways. 25 , 30 Kv7 potassium channels are present in both the myocardium (Kv7.1 subtype) and the arteries (Kv7.4 and Kv7.5), and kynurenine has just emerged as a potentially important HF biomarker. For instance, blood kynurenine levels have been reported to be inversely correlated with functional capacity, renal and liver function, and the risk of death in HF patients. 31 In our study, blood levels of kynurenine were inversely correlated with LVDD, which may be contraintuitive given its association to worsened status in HF patients. However, higher kynurenine levels were also associated with lower fractional shortening in the Third Generation cohort. These observations may both be consistent with a negative impact of kynurenine on the Frank–Starling curve (i.e. lowered preload from peripheral vasodilation can result in lower end‐diastolic dimensions and a lower fractional shortening). In agreement with such a hypothesis, kynurenine has been suggested to be contributing to hypotension in patients with sepsis. 32 The mechanisms by which kynurenine is associated to LVDD, fractional shortening, and potentially HF warrant more investigations, however (because it may relate to lifestyle‐related factors as well as be markers of adverse subclinical processes).
We observed that higher blood levels of diacylglycerol were associated with lower LVDD. Biological underpinnings of this association are unclear. Diacylglycerol is the backbone of phosphatidic acid, which can be converted to phosphatidylinositol, an important metabolite in several signalling pathways regulating cardiac contractility. It is also a major building block for phosphatidylcholine, which may be implicated in cardiac remodelling and HF. 33 Finally, we observed that circulating leucine levels were inversely associated with LVDD. This observation may be consistent with at least one prior study reporting that greater leucine intake was associated with lower blood pressures and lower carotid–femoral pulse wave velocities in women. 34 A leucine‐rich diet was also recently shown to attenuate HF risk in rats with cancer‐associated cachexia, but the mechanisms remain unclear. 35
Metabolites associated with heart failure risk
In our study, lower levels of circulating phosphatidylcholine (carbon:double bound = 36:4) and lysophosphatidylcholine (carbon:double bond = 18:2) were associated with greater risk of HFrEF. Much research has suggested that, in some individuals, the gut microbiome can metabolize phosphatidylcholine into trimethylamine N‐oxide before it is absorbed in the intestines. 36 High levels of trimethylamine N‐oxide are known to promote atherosclerosis and thrombosis. 37 , 38 Recent research has also suggested that higher trimethylamine N‐oxide levels may contribute to increased HF risk. For instance, mice fed with a diet rich in trimethylamine N‐oxide developed greater cardiac dilation, cardiac hypertrophy, lower LVEF, and more cardiac fibrosis after transaortic binding than mice fed with a control diet. 39 In humans with systolic HF, higher levels of trimethylamine N‐oxide have been associated with diabetes, greater New York Heart Association class, higher levels of pro‐BNP, worse diastolic function, and greater mortality risk. 40 In our analyses, higher trimethylamine N‐oxide levels were indeed associated with greater HFrEF risk, although it did not reach our Bonferroni‐adjusted statistically significance level (P = 0.0038 in multivariable‐adjusted models). Because the correlation coefficient between phosphatidylcholine (carbon:double bound = 36:4) and trimethylamine N‐oxide levels was negligible (data not shown), the observed association of higher levels of phosphatidylcholine with lowered risk of HFrEF is, however, unlikely to be explained by altered gut intestinal metabolism. To the best of our knowledge, an association of circulating blood levels of phosphatidylcholine (carbon:double bound = 36:4) with cardiovascular disease has not been reported previously, and further studies are warranted to confirm our observations. Other possible mechanistic explanations may include a direct effect of lysophosphatidylcholine on the microvascular endothelial signalling and environment, 41 its influence on the vasculature and thrombosis as a component of oxidized low‐density lipoprotein, 42 stimulation of cholesterol synthesis in the liver, and an effect on macrophages, B‐cells, and other inflammatory signalling pathways. 43
The amino acid ornithine was positively associated with incident HFrEF in multivariable‐adjusted models. Ornithine is not proteinogenic but has an important role in the urea cycle; l‐arginine is degraded to ornithine and urea, and ornithine can be recycled to form new l‐arginine. l‐Arginine is the substrate for endothelial nitric oxide synthesis, which may explain the observed association. In this context, dysregulated arginine metabolism and consequent elevated levels of asymmetric dimethylarginine have been noted in advanced systolic HF previously. 44 In our study, higher asymmetric dimethylarginine levels were associated with greater HFrEF, but not HFpEF risk in multivariable‐adjusted models, although the association did not meet the Bonferroni‐corrected P‐value (P = 0.00048).
A few metabolites that are directly implicated in myocardial energetics were also identified in our investigation as associated with the long‐term risk of HF: higher concentrations of isocitrate with increased HFrEF risk and higher concentrations of hydroxybutyrate with increased HFpEF risk. Isocitrate is an important metabolite of the mitochondrial citric acid cycle and of major importance in the production of ATP molecules, but the exact mechanism underlying our observations between isocitrate concentrations and HFrEF is not known. Hydroxybutyrate is an important ketone body for energy production in humans. A recent interventional study demonstrated that infusion of hydroxybutyrate enhanced cardiac blood flow and lowered the myocardial glucose uptake when infused in healthy individuals. 45 At the same time, however, the heart rate was increased by 25%, which may lead to increased long‐term risk of HF. 46 One of the more common subphenotypes of HFpEF includes insulin resistance and the metabolic syndrome, in which ketone body levels are increased 47 , 48 ; this could also be one of the reasons underlying our observed associations. In addition, high levels of ketone bodies may be a marker of subclinical myocardial dysfunction, because HF patients have well‐recognized higher levels of circulating ketone bodies than controls and greater myocardial utilization of ketone bodies for energy production. 49 , 50 , 51 Yet this latter hypothesis may not be fully supported by our data, where higher levels of hydroxybutyrate were associated with greater fractional shortening in the FHS Third Generation sample (Table 3 ).
Cotinine, a metabolite of nicotine, was also associated with HF risk in our community‐based sample. This association may reflect concomitant smoking and was attenuated upon adjustment for the same. 52 Current smokers were recently shown to have greater left ventricular mass and higher risk of incident HF, compared with never smokers, while prior smokers had a risk intermediate between the two other groups in the Jackson Heart Study. 53 Also, smokeless tobacco has also been associated with accelerated atherosclerosis and HF. 54 , 55 , 56
Strengths and limitations
The major strengths of our investigation include the well‐phenotyped population of the FHS and the agnostic strategy relating a large panel of metabolites to both echocardiographic indices and HF risk. Limitations include the echocardiographic measures, which did not include the most sensitive measures of left ventricular function (including tissue Doppler, speckle tracking, and strain). The diagnostic criteria for HFpEF relied on a clinical examination rather than echocardiographic variables, which may have influenced the sensitivity and specificity of the diagnosis. 57 The sample was moderate sized, and the number of incident HF events was limited and occurred on average more than 10 years later than the obtained metabolites, which may have weakened the associations between metabolites and HF risk; it may also explain why plasma kynurenine concentrations were associated with echocardiographical traits cross‐sectionally (with LVDD and fractional shortening), but not with incident HF prospectively. Blood metabolites are to some extent dependent on dietary habits and possibly other environmental factors, which can vary widely between different populations and countries. In this context, most participants in our study were of European ancestry, and the majority belonged to the middle class, which may have influenced the measured metabolite levels and potentially limited variability in the circulating metabolome. More studies, including studies of ethnically diverse populations, as well as independent replication, are warranted.
Conclusions and clinical implications
In summary, we identified several metabolites (cotinine, hydroxybutyrate, phosphatidylcholine, lysophophatidylcholine, isocitrate, ornithine, kynurenine, diacylglycerol, and leucine) that were associated with cardiac function cross‐sectionally or HF risk prospectively. Additional research is needed to confirm if these metabolites can be used as risk stratification tools in ambulatory patients (especially HFrEF risk, as fewer significant metabolites were associated with HFpEF risk). Further, mechanistic studies are warranted to determine if associations are causal and, if so, whether dietary interventions can modify the long‐term risk of HF.
Conflict of interest
None declared.
Funding
The Framingham Heart Study (FHS) was supported by Grants NO1‐HC‐25195, HHSN268201500001I, and 75N92019D00031 from the National Heart, Lung, and Blood Institute of the National Institutes of Health. The present work was also supported by the Foundation for the National Institutes of Health R01‐DK‐HL081572, R01‐HL‐080124, R01‐DK‐080739, R01‐DK‐081572, R01‐HL‐093328, and R01‐HL‐70100 grant, the Leducq Foundation (Fondation Leducq), and the American Heart Association. R.S.V. is supported in part by Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, School of Medicine, Boston University. The sponsors had no influence on study design, manuscript preparation, or decision to submit.
Supporting information
Table S1. Characteristics of HF cases.
Andersson, C. , Liu, C. , Cheng, S. , Wang, T. J. , Gerszten, R. E. , Larson, M. G. , and Vasan, R. S. (2020) Metabolomic signatures of cardiac remodelling and heart failure risk in the community. ESC Heart Failure, 7: 3707–3715. 10.1002/ehf2.12923.
References
- 1. Mariotti S, Zoncu S, Pigliaru F, Putzu C, Cambuli VM, Vargiu S, Deidda M, Mercuro G. Cardiac effects of l‐thyroxine administration in borderline hypothyroidism. Int J Cardiol 2008; 126: 190–195. [DOI] [PubMed] [Google Scholar]
- 2. Pearce EN, Yang Q, Benjamin EJ, Aragam J, Vasan RS. Thyroid function and left ventricular structure and function in the Framingham Heart Study. Thyroid 2010; 20: 369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andersson C, Gislason GH, Weeke P, Hoffmann S, Hansen PR, Torp‐Pedersen C, Sogaard P. Diabetes is associated with impaired myocardial performance in patients without significant coronary artery disease. Cardiovasc Diabetol 2010; 9: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dinicolantonio JJ, Lavie CJ, Niazi AK, O'Keefe JH, Hu T. Effects of thiamine on cardiac function in patients with systolic heart failure: systematic review and metaanalysis of randomized, double‐blind, placebo‐controlled trials. Ochsner J 2013. Winter; 13: 495–499. [PMC free article] [PubMed] [Google Scholar]
- 5. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med 2002; 347: 305–313. [DOI] [PubMed] [Google Scholar]
- 6. Meyers DE, Basha HI, Koenig MK. Mitochondrial cardiomyopathy: pathophysiology, diagnosis, and management. Tex Heart Inst J 2013; 40: 385–394. [PMC free article] [PubMed] [Google Scholar]
- 7. Velagaleti RS, Massaro J, Vasan RS, Robins SJ, Kannel WB, Levy D. Relations of lipid concentrations to heart failure incidence: the Framingham Heart Study. Circulation 2009; 120: 2345–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Held C, Gerstein HC, Yusuf S, Zhao F, Hilbrich L, Anderson C, Sleight P, Teo K, Investigators OT. Glucose levels predict hospitalization for congestive heart failure in patients at high cardiovascular risk. Circulation 2007; 115: 1371–1375. [DOI] [PubMed] [Google Scholar]
- 9. Frankel DS, Vasan RS, D'Agostino RB Sr, Benjamin EJ, Levy D, Wang TJ, Meigs JB. Resistin, adiponectin, and risk of heart failure: the Framingham Offspring Study. J Am Coll Cardiol 2009; 53: 754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ingelsson E, Arnlov J, Sundstrom J, Zethelius B, Vessby B, Lind L. Novel metabolic risk factors for heart failure. J Am Coll Cardiol 2005; 46: 2054–2060. [DOI] [PubMed] [Google Scholar]
- 11. Zheng Y, Yu B, Alexander D, Manolio TA, Aguilar D, Coresh J, Heiss G, Boerwinkle E, Nettleton JA. Associations between metabolomic compounds and incident heart failure among African Americans: the ARIC Study. Am J Epidemiol 2013; 178: 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hunter WG, Kelly JP, McGarrah RW 3rd, Khouri MG, Craig D, Haynes C, Ilkayeva O, Stevens RD, Bain JR, Muehlbauer MJ, Newgard CB, Felker GM, Hernandez AF, Velazquez EJ, Kraus WE, Shah SH. Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: evidence for shared metabolic impairments in clinical heart failure. J Am Heart Assoc 2016; 5: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shah SH, Hunter WG. Realizing the potential of metabolomics in heart failure: signposts on the path to clinical utility. JACC Heart Fail 2017; 5: 833–836. [DOI] [PubMed] [Google Scholar]
- 14. Lanfear DE, Gibbs JJ, Li J, She R, Petucci C, Culver JA, Tang WHW, Pinto YM, Williams LK, Sabbah HN, Gardell SJ. Targeted metabolomic profiling of plasma and survival in heart failure patients. JACC Heart Fail 2017; 5: 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med 1975; 4: 518–525. [DOI] [PubMed] [Google Scholar]
- 16. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med 2011; 17: 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho JE, Larson MG, Ghorbani A, Cheng S, Chen MH, Keyes M, Rhee EP, Clish CB, Vasan RS, Gerszten RE, Wang TJ. Metabolomic profiles of body mass index in the Framingham Heart Study reveal distinct cardiometabolic phenotypes. PLoS ONE 2016; 11: e0148361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, Yang E, Farrell L, Fox CS, O'Donnell CJ, Carr SA, Vasan RS, Florez JC, Clish CB, Wang TJ, Gerszten RE. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 2011; 121: 1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang TJ, Ngo D, Psychogios N, Dejam A, Larson MG, Vasan RS, Ghorbani A, O'Sullivan J, Cheng S, Rhee EP, Sinha S, McCabe E, Fox CS, O'Donnell CJ, Ho JE, Florez JC, Magnusson M, Pierce KA, Souza AL, Yu Y, Carter C, Light PE, Melander O, Clish CB, Gerszten RE. 2‐Aminoadipic acid is a biomarker for diabetes risk. J Clin Invest 2013; 123: 4309–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977; 55: 613–618. [DOI] [PubMed] [Google Scholar]
- 21. Andersson C, Vasan RS. Epidemiology of heart failure with preserved ejection fraction. Heart Fail Clin 2014; 10: 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rhee EP, Clish CB, Ghorbani A, Larson MG, Elmariah S, McCabe E, Yang Q, Cheng S, Pierce K, Deik A, Souza AL, Farrell L, Domos C, Yeh RW, Palacios I, Rosenfield K, Vasan RS, Florez JC, Wang TJ, Fox CS, Gerszten RE. A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol 2013; 24: 1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB Sr, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 2007; 165: 1328–1335. [DOI] [PubMed] [Google Scholar]
- 24. Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med 1997; 336: 1350–1355. [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Liu H, McKenzie G, Witting PK, Stasch JP, Hahn M, Changsirivathanathamrong D, Wu BJ, Ball HJ, Thomas SR, Kapoor V, Celermajer DS, Mellor AL, Keaney JF Jr, Hunt NH, Stocker R. Kynurenine is an endothelium‐derived relaxing factor produced during inflammation. Nat Med 2010; 16: 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Savitz J. The kynurenine pathway: a finger in every pie. Mol Psychiatry 2020; 25: 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baumgartner R, Forteza MJ, Ketelhuth DFJ. The interplay between cytokines and the kynurenine pathway in inflammation and atherosclerosis. Cytokine 2019; 122: 154148. [DOI] [PubMed] [Google Scholar]
- 28. Brouns R, Verkerk R, Aerts T, De Surgeloose D, Wauters A, Scharpe S, De Deyn PP. The role of tryptophan catabolism along the kynurenine pathway in acute ischemic stroke. Neurochem Res 2010; 35: 1315–1322. [DOI] [PubMed] [Google Scholar]
- 29. Pedersen ER, Svingen GF, Schartum‐Hansen H, Ueland PM, Ebbing M, Nordrehaug JE, Igland J, Seifert R, Nilsen RM, Nygard O. Urinary excretion of kynurenine and tryptophan, cardiovascular events, and mortality after elective coronary angiography. Eur Heart J 2013; 34: 2689–2696. [DOI] [PubMed] [Google Scholar]
- 30. Sakakibara K, Feng GG, Li J, Akahori T, Yasuda Y, Nakamura E, Hatakeyama N, Fujiwara Y, Kinoshita H. Kynurenine causes vasodilation and hypotension induced by activation of KCNQ‐encoded voltage‐dependent K+ channels. J Pharmacol Sci 2015; 129: 31–37. [DOI] [PubMed] [Google Scholar]
- 31. Konishi M, Ebner N, Springer J, Schefold JC, Doehner W, Dschietzig TB, Anker SD, von Haehling S. Impact of plasma kynurenine level on functional capacity and outcome in heart failure—results from Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). Circ J 2016; 81: 52–61. [DOI] [PubMed] [Google Scholar]
- 32. Changsirivathanathamrong D, Wang Y, Rajbhandari D, Maghzal GJ, Mak WM, Woolfe C, Duflou J, Gebski V, dos Remedios CG, Celermajer DS, Stocker R. Tryptophan metabolism to kynurenine is a potential novel contributor to hypotension in human sepsis. Crit Care Med 2011; 39: 2678–2683. [DOI] [PubMed] [Google Scholar]
- 33. Ecker J, Liebisch G. Application of stable isotopes to investigate the metabolism of fatty acids, glycerophospholipid and sphingolipid species. Prog Lipid Res 2014; 54: 14–31. [DOI] [PubMed] [Google Scholar]
- 34. Jennings A, MacGregor A, Welch A, Chowienczyk P, Spector T, Cassidy A. Amino acid intakes are inversely associated with arterial stiffness and central blood pressure in women. J Nutr 2015; 145: 2130–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toneto AT, Ferreira Ramos LA, Salomao EM, Tomasin R, Aereas MA, Gomes‐Marcondes MC. Nutritional leucine supplementation attenuates cardiac failure in tumour‐bearing cachectic animals. J Cachexia Sarcopenia Muscle 2016; 7: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013; 368: 1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011; 472: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu W, Wang Z, Tang WHW, Hazen SL. Gut microbe‐generated trimethylamine N‐oxide from dietary choline is prothrombotic in subjects. Circulation 2017; 135: 1671–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, Polhemus DJ, Tang WH, Wu Y, Hazen SL, Lefer DJ. Choline diet and its gut microbe‐derived metabolite, trimethylamine N‐oxide, exacerbate pressure overload‐induced heart failure. Circ Heart Fail 2016; 9: e002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tang WH, Wang Z, Shrestha K, Borowski AG, Wu Y, Troughton RW, Klein AL, Hazen SL. Intestinal microbiota‐dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail 2015; 21: 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murugesan G, Sandhya Rani MR, Gerber CE, Mukhopadhyay C, Ransohoff RM, Chisolm GM, Kottke‐Marchant K. Lysophosphatidylcholine regulates human microvascular endothelial cell expression of chemokines. J Mol Cell Cardiol 2003; 35: 1375–1384. [DOI] [PubMed] [Google Scholar]
- 42. Wu R, Huang YH, Elinder LS, Frostegard J. Lysophosphatidylcholine is involved in the antigenicity of oxidized LDL. Arterioscler Thromb Vasc Biol. 1998; 18: 626–630. [DOI] [PubMed] [Google Scholar]
- 43. Law SH, Chan ML, Marathe GK, Parveen F, Chen CH, Ke YL. An Updated Review of Lysophosphatidylcholine Metabolism in Human. Int J Mol Sci. 2019; 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shao Z, Wang Z, Shrestha K, Thakur A, Borowski AG, Sweet W, Thomas JD, Moravec CS, Hazen SL, Tang WH. Pulmonary hypertension associated with advanced systolic heart failure: dysregulated arginine metabolism and importance of compensatory dimethylarginine dimethylaminohydrolase‐1. J Am Coll Cardiol 2012; 59: 1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gormsen LC, Svart M, Thomsen HH, Sondergaard E, Vendelbo MH, Christensen N, Tolbod LP, Harms HJ, Nielsen R, Wiggers H, Jessen N, Hansen J, Botker HE, Moller N. Ketone body infusion with 3‐hydroxybutyrate reduces myocardial glucose uptake and increases blood flow in humans: a positron emission tomography study. J Am Heart Assoc 2017; 6: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nanchen D, Leening MJ, Locatelli I, Cornuz J, Kors JA, Heeringa J, Deckers JW, Hofman A, Franco OH, Stricker BH, Witteman JC, Dehghan A. Resting heart rate and the risk of heart failure in healthy adults: the Rotterdam Study. Circ Heart Fail 2013; 6: 403–410. [DOI] [PubMed] [Google Scholar]
- 47. Mahendran Y, Vangipurapu J, Cederberg H, Stancakova A, Pihlajamaki J, Soininen P, Kangas AJ, Paananen J, Civelek M, Saleem NK, Pajukanta P, Lusis AJ, Bonnycastle LL, Morken MA, Collins FS, Mohlke KL, Boehnke M, Ala‐Korpela M, Kuusisto J, Laakso M. Association of ketone body levels with hyperglycemia and type 2 diabetes in 9,398 Finnish men. Diabetes 2013; 62: 3618–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype‐specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 2016; 134: 73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bedi KC Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 2016; 133: 706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Janardhan A, Chen J, Crawford PA. Altered systemic ketone body metabolism in advanced heart failure. Tex Heart Inst J 2011; 38: 533–538. [PMC free article] [PubMed] [Google Scholar]
- 51. Lommi J, Kupari M, Koskinen P, Naveri H, Leinonen H, Pulkki K, Harkonen M. Blood ketone bodies in congestive heart failure. J Am Coll Cardiol 1996; 28: 665–672. [DOI] [PubMed] [Google Scholar]
- 52. Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, Smith AL, Bauer DC, Newman AB, Kim L, Bibbins‐Domingo K, Tindle H, Harris TB, Tang WW, Kritchevsky SB, Butler J. Cigarette smoking exposure and heart failure risk in older adults: the Health, Aging, and Body Composition Study. Am Heart J 2012; 164: 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kamimura D, Cain LR, Mentz RJ, White WB, Blaha MJ, de Filippis AP, Fox ER, Rodriguez CJ, Keith RJ, Benjamin EJ, Butler J, Bhatnagar A, Robertson RM, Winniford MD, Correa A, Hall ME. Cigarette smoking and incident heart failure: insights from the Jackson Heart Study. Circulation 2018; 137: 2572–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gupta R, Gupta N, Khedar RS. Smokeless tobacco and cardiovascular disease in low and middle income countries. Indian Heart J 2013; 65: 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boffetta P, Straif K. Use of smokeless tobacco and risk of myocardial infarction and stroke: systematic review with meta‐analysis. BMJ 2009; 339: b3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Arefalk G, Hergens MP, Ingelsson E, Arnlov J, Michaelsson K, Lind L, Ye W, Nyren O, Lambe M, Sundstrom J. Smokeless tobacco (snus) and risk of heart failure: results from two Swedish cohorts. Eur J Prev Cardiol 2012; 19: 1120–1127. [DOI] [PubMed] [Google Scholar]
- 57. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske‐Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA‐PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019; 40: 3297–3317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of HF cases.


