Abstract
The initiating mutations that contribute to cancer development are sometimes present in pre-malignant cells. Whether therapies targeting these mutations can eradicate pre-malignant cells is unclear. Acute myeloid leukemia (AML) is an attractive system for investigating the effect of preventative treatment as this disease is often preceded by a pre-malignant state (clonal hematopoiesis or myelodysplastic syndrome). In Npm1c/Dnmt3a mutant knock-in mice, a model of AML development, leukemia is preceded by a period of extended myeloid progenitor cell proliferation/self-renewal. We found that this self-renewal can be reversed by oral administration of a small molecule (VTP-50469) that targets the MLL1-Menin chromatin complex. These preclinical results support the hypothesis that individuals at high risk of developing AML might benefit from targeted epigenetic therapy in a preventative setting.
Main Text:
Nucleophosmin (NPM1) mutant actute myeloid leukemia (AML) is one of the most common types of AML (1–3). Despite its high prevalence, the mechanism of leukemogenesis is still poorly understood, and targeted therapy options are lacking (4). NPM1 gene mutations (NPM1c) induce cytoplasmic localization of NPM1 and often co-occur with other mutations in genes such as DNA methyltransferase 3A (DNMT3AR882H). NPM1c leukemias express a distinctive stem cell like gene expression pattern which includes homeobox cluster A and B (HOXA/B) genes and their DNA-binding co-factor MEIS1 (5–8). In humans DNMT3A mutations are detected in the most primitive hematopoietic stem cell compartment, often long before the development of leukemia, a condition often referred to as clonal hematopoiesis of indeterminate potential (CHIP) (9). NPM1 mutations are found in committed progenitors and differentiated myeloid cells in AML, but are absent from the stem cell and lymphoid compartments (9, 10). This suggests that NPM1c may induce self-renewal in myeloid progenitors as a critical step in the development of AML, and that this aberrant progenitor self-renewal may represent a critical step in the progression from CHIP to AML.
To identify the leukemia-initiating cellular population in NPM1c AML, we used previously developed mouse models with an inducible Cre recombinase (MxCre) and heterozygous conditional knock-in of the humanized Npm1 mutation (Npm1flox-cA/+; hererafter called Npm1c mutant mice), alone or in combination with Dnmt3aR878H mutation (Dnmt3aR878H/+; hererafter called Dnmt3a mutant mice) (5, 11). We confirmed Hox gene upregulation in different hematopoietic stem and progenitor populations of Npm1c single and Npm1c/Dnmt3a double mutant mice 16 weeks after induction of the knock-in allele by polyinosinic:polycytidylic acid (pIpC) injection (Fig. 1A). At this time, mutant mice showed no signs of leukemia, had normal blood counts, and only the double mutant showed a slight increase in granulocyte and macrophage progenitor (GMP) frequencies (Fig. S1A and B). Sorted wildtype (WT) and Dnmt3a single mutant cells showed a stepwise decrease of Hoxa9 mRNA expression from long term hematopoietic stem cells (LT-HSCs) to GMPs which coincides with their loss of self-renewal properties (Fig. 1A). Npm1c or Npm1c/Dnmt3a mutant cells maintained inappropriately high levels of Hoxa9 across the different progenitor cell types (Fig. 1A). RNAseq analysis 4 weeks after activation of the Npm1c allele, revealed that half of the top 20 upregulated genes in Npm1c GMPs were Hoxa/b genes. The HSC-enriched Lin−, Sca1+, Kit+ population (LSK) showed much lower fold changes due to their high baseline expression of Hoxa/b genes (Fig. 1B and table S1). The gene expression programs induced in Npm1c mutant GMPs were also enriched for LT-HSC and human Npm1c mutated AML signatures which include Hoxa/b genes and Meis1 (Fig. S1, C to I). Based on these gene expression data, we conclude that Npm1c supports the inappropriate expression of genes associated with normal stem cell self-renewal such as Hoxa/b cluster genes, throughout myeloid differentiation.
Fig. 1. Npm1c induces self–renewal properties in myeloid progenitor cells.
(A) Hoxa9 gene expression in Npm1c, Dnmt3a and Npm1c/Dnmt3a mutant LT-HSCs,multipotent progenitors (MPPs), common myeloid progenitors (CMPs) and GMPs 16 weeks after pIpC injection (n≥3 mice per group; error bars indicate mean ± SD). Rel., relative;GAPDH, glyceraldehyde 3-phosphate dehydrogenase. (B) Heatmap showing top 25 up-regulated genes in Npm1c versus WT LSK and GMPs, 4 weeks after pIpC treatment (n=3 mice per group).(C) Relative expression of Hoxa9 3 days after in vitro Cre transduction (n ≥ 4 mice per group;error bars indicate mean ± SEM). The dotted lines indicate Hoxa9 expression level from freshly isolated LSK cells and GMPs. (D) Peripheral blood percentage CD45.2 engraftment of WT and Npm1c, Dnmt3a, and Npm1c/Dnmt3a mutant GMPs sorted 4 weeks after pIpC induction, transplanted into lethally irradiated recipients. Error bars indicate mean ± SEM. (E) Summary table of GMP-transplanted mouse numbers and percentages engrafted ≥1% for >12 weeks. MIGCRE,MSCV-CRE-IRES-GFP retrovirus. ns, not significant; *P < 0.05; **P < 0.01; ***P <0.001.
We next investigated whether Npm1c can induce stem-cell associated gene expression de novo in committed progenitor cells, which lack self-renewal and have low levels of Hox/Meis1 expression. For this, we sorted Cre-negative Npm1c/Dnmt3a single or double mutant GMPs and LSK cells and then used retroviral Cre overexpression to induce the mutant knock-in alleles in vitro (Fig. 1C). Npm1c expression induced Hoxa9 expression in GMPs in vitro, suggesting that the Npm1c-driven stem cell-associated program can be turned on at different stages of myeloid differentiation (Fig. 1C). Induction of Dnmt3aR878H knock-in alone did not induce or enhance the Hoxa9 induction activated by Npm1c in progenitors, indicating that mutant Npm1c and not Dnmt3a is responsible for the observed upregulation of stem-cell associated genes.
Our gene expression data suggest that Npm1c induces stem cell properties in non-stem cells. To examine whether these transcriptional changes coincide with functional self-renewal properties in Npm1c progenitors, we first performed colony forming (CFU) assays. Npm1c mutant GMPs displayed increased in vitro self-renewal capacity as shown by their ability to replate up to 4 rounds in CFU assays (Fig. S2, A and B). In vivo transplantation experiments performed using in vivo pIpC-induced and in vitro Cre- transduced mutant GMPs demonstrated that Npm1c enhances engraftment and self-renewal of GMPs (Fig.1D, and fig. S2C). While some of the initially engrafted GMPs were depleted over time, about half of the mice retained self-renewing GMPs for >12 weeks (Fig. 1E). These long-term engrafting GMPs (LT-GMPs) showed CD11b+Gr1+ peripheral blood engraftment, and recipient mice showed no signs of leukemia for over 6 months (Fig. S2D). These experiments demonstrate that self-renewal properties induced by Npm1c in myeloid progenitors are sufficient to give rise to a preleukemic population that stably engrafts long-term.
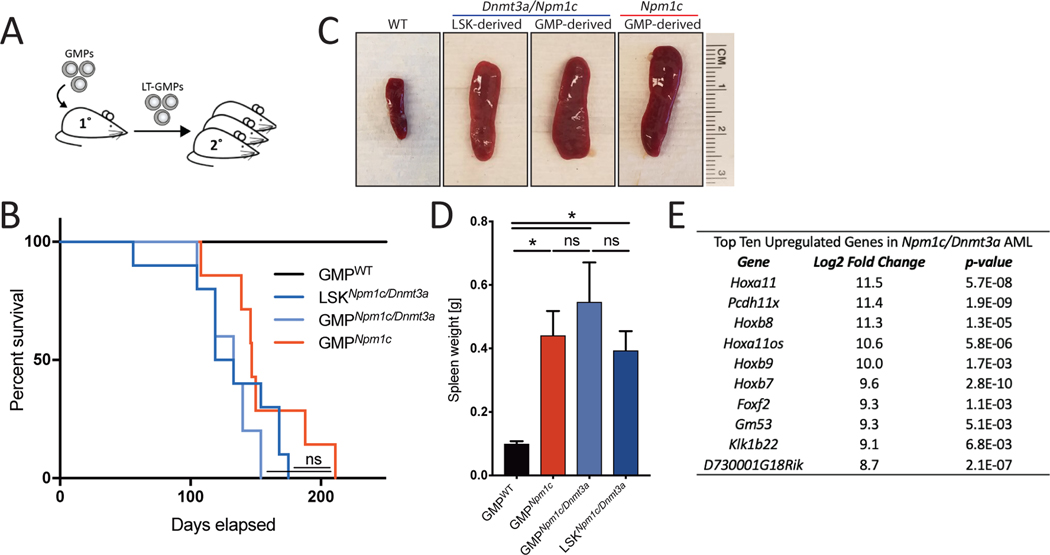
To determine whether these preleukemic Npm1c mutant clones would progress to leukemia, we performed secondary transplants of LT-GMPs (Fig. 2A). Secondary recipients of Npm1c mutant or Npm1c/Dnmt3a double mutant LT-GMPs developed AML 3–5 months post-secondary transplant similar to mice that received mutant LSK cells (Fig. 2B). LSK and GMP-derived secondary mice presented with high WBC counts, enlarged spleens, and extramedullary hematopoiesis suggesting that Npm1c is sufficient to give GMPs enough self-renewal capacity to ultimately generate AML. (Fig. 2, C and D, and fig. S3, A and B). The long latency indicates that Npm1c mutant LT-GMPs may acquire further mutations over time, which has been shown to occur in this and other Npm1c knock-in mouse models (7, 12). Furthermore, mouse Npm1c/Dnmt3a mutant leukemia cells presented with highly upregulated Hoxa/b expression resembling expression patterns observed in human NPM1c AML and other HOX-associated AMLs such as MLL-AF9 AML (Fig. 2E, and S3, C and D). These results confirm that preleukemic Npm1c mutant LT-GMP eventually give rise to leukemia.
Fig. 2. Myeloid progenitors are leukemia-initiating cells in Npm1c AML.
(A) Experimental overview of secondary transplantation of long-term engrafted mutant GMPs. (B to D) Kaplan-Meyer survival analysis (B), representative spleen images (C), and spleen weights (D) of secondary transplants of MIG-CRENpm1c/Dnmt3a LT-GMPs or LSK-derived cells and MxCreNpm1c LT-GMPs (n ≥ 4 mice per group). One-way analysis of variance (ANOVA) was performed. Error bars indicate mean ± SD. *P < 0.05; ns, not significant. (E) Top 10 up-regulated genes in MIG-CRENpm1c/Dnmt3a mutant leukemic GMPs compared with WT GMPs (n = 3 mice per group).
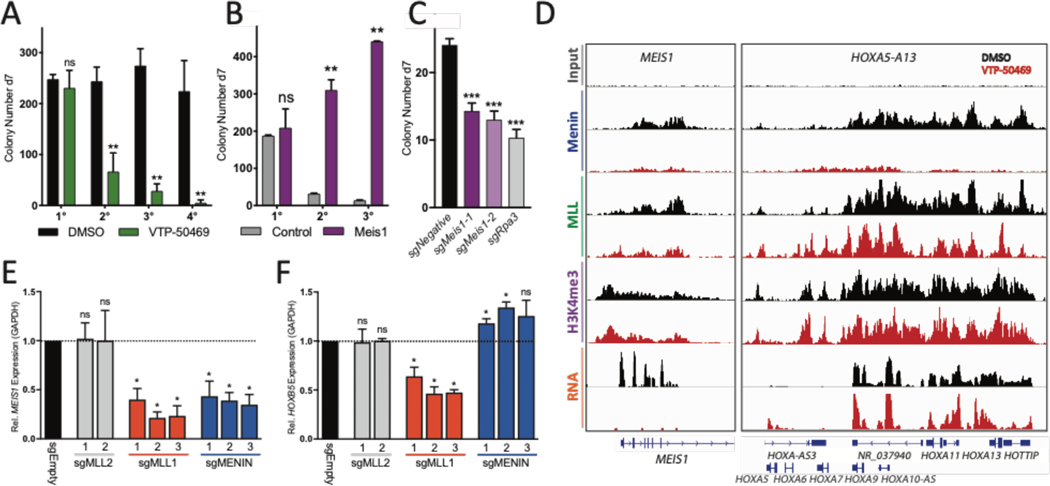
We have previously shown that inhibition of the interaction between the histone methyltransferase MLL1 and adaptor protein Menin reverses leukemogenic gene expression in NPM1c AML cell line OCI-AML3 (13). Menin-MLL interaction inhibitors were originally developed to target the oncogenic MLL-fusion complexes, by directly disrupting the oncogene complex from assembling on chromatin. Our findings however, suggests that the wildtype MLL1-Menin interaction is essential to maintain NPM1c-driven leukemia. To test this, we used an orally bioavailable inhibitor of the Menin-MLL1 interaction (VTP-50469). This compound has been used to treat established disease in models of MLL-rearranged AML and B-ALL [see (14) for details on the chemical synthesis of VTP-50469]. We assessed whether Npm1c mutant mouse cells respond to Menin inhibition in serial CFU replating assays of double and single mutant cell lines (Fig. 3A, and fig. S4A). Menin inhibition led to a rapid loss of replating capacity and upregulation of myeloid differentiation marker CD11b with no significant increase in apoptosis (Fig. S4, B and C). Gene expression analysis of Npm1c/Dnmt3a mouse cells after Menin inhibition revealed a rapid repression of stem cell genes including Meis1 and Pbx3 (Fig. S5A, left panel). Meis1/Pbx3 are important co-factors of Hoxa/b transcription factors and play essential roles in Hoxa9 driven leukemogenesis and maintenance of leukemic stem cell gene expression programs (15–17). Even though many Npm1c-induced genes, including Hoxa/b, remained highly expressed, the loss of essential co-factors such as Meis1 could account for the loss of self-renewal observed upon Menin inhibition.
Fig. 3. Meis1, Menin and MLL1 are essential for maintaining self-renewal program.
(A) CFU serial replating assay of mouse MIG-CRENpm1c/Dnmt3a mutant cell line (SIIIL12) with dimethyl sulfoxide (DMSO) or VTP-50469. Data represent the mean of three independent experiments. d7, day 7. (B) CFU assay of mouse SIIIL12 cells transduced with MSCV-PURO control (left) or Meis1-PURO (right) virus and grown in the presence of 10 nM VTP-50469. Data represent the mean of three independent experiments. (C) CFU assay of SIIIL12 cells electroporated with control or Meis1- or Rpa3-targeting single guide RNAs (sgRNAs) for Cas9-mediated KO. Data represent the mean of three independent experiments. (D) Chromatin immuno- precipitation sequencing (ChIP-seq) density plots showing changes in chromatin occupancy of Menin, MLL, and H3K4me3 and changes in mRNA expression in response to VTP-50469 in OCI-AML3 cells at the MEIS1 and HOXA loci. (E and F) MEIS1 (E) and HOXB5 (F) gene expression in OCI-AML3 cells transduced with control, sgMLL1, sgMLL2, and sgMenin. Data represent the mean of three independent experiments. One-way ANOVA was performed. Error bars indicate mean ± SD. ns, not significant; *P < 0.05; **P < 0.01; ***P <0.001.
To confirm that reduced Meis1 expression is crucial for the drastic differentiation of Npm1c/Dnmt3a mutant cells observed after VTP-50469 treatment, we first attempted to rescue the VTP-50469 induced loss of leukemic stem cell gene expression by retroviral overexpression of Meis1. Maintaining Meis1 expression rescued the replating capacity of Npm1c/Dnmt3a mutant cells in the presence of Menin inhibitor and increased the IC50 values significantly (Fig 3B, and fig. S5B). While control cells lost essential components of their self-renewal program in response to VTP-50469, Meis1-expressing cells showed increased expression of a group of stem-cell associated genes including Mecom and Pbx3 and retained them in the presence of Menin inhibitor (Fig S5, B to G). Conversely, Cas9 mediated knock-out of Meis1 led to a rapid loss of out-of-frame edited cells in culture as well as a reduction in CFU replating capacity confirming Meis1 as a dependency in NPM1c mutated AML (Fig 3C and fig S5H). These data confirm the essential role of Meis1 in maintaining leukemic stem cell programs.
Next, we confirmed that human NPM1c mutant leukemia cells line, OCI-AML3 also respond to VTP-50469. OCI-AML3 were highly sensitive to Menin-MLL inhibition, as demonstrated by their low IC50 value (3nM on day 6) and rapid downregulation of MEIS1 and PBX3 upon VTP-50469 treatment (Fig. S6, A to C). In contrast to previously published Menin inhibitor molecules such as MI-2–2 and MI-503 that were shown to reduce expression of HOXA/B cluster genes as well as MEIS1, HOX genes were not repressed in response to VTP-50469 in OCI-AML3 cells (S6, B and C). In mouse cells, a modest repressive effect on some Hox genes was observed while others were upregulated (S7, D and E and S10, A to D)(18, 19). Furthermore, we observed a reduction of Menin and MLL1 chromatin occupancy at the MEIS1 and PBX3 transcriptional start sites (TSS), while MLL1 binding at HOXA/B TSSs was retained in regions where Menin was depleted (Fig. 3D, and fig. S6E, and table S2). Globally, Menin chromatin occupancy was decreased, while MLL1 and H3K4me3 were lost only at specific sites which were highly enriched for genes downregulated in response to Menin inhibition (Fig. S6, F to H, S7, A to C). To verify that MLL1 loss is responsible for the observed loss of stem cell associated gene expression, we generated CRISPR Cas9 mediated OCI-AML3 knock-out (KO) cell lines of MLL1, MLL2 and Menin (Fig S8, A to C, Table S4). Menin KO mimicked the expression changes observed upon VTP-50469 treatment, with reduced MEIS1/PBX3 expression and upregulation of HOXB5 and HOXA5 (Fig. 3, E and F, and fig. S8, D to E). Loss of MLL1, however, also resulted in a reduction in HOX expression, while MLL2 disruption showed no or only minor effects on HOX/MEIS1 (Fig. 3F and fig. S8E). In agreement with this, Menin and MLL1 KO cells were rapidly depleted in competition assays while MLL2 KO cells were not (Fig S8F). Our findings confirm MLL1 as the main driver of oncogenic HOX/MEIS1 gene expression in NPM1c mutated AML and show that only a subset of MLL1 target genes are also Menin dependent.
DNMT3A mutations are frequently found in patients with CHIP and are associated with increased risk for hematologic malignancies (9, 20–23). By contrast, NPM1c mutations have not been reported in CHIP suggesting that their acquisition is rapidly followed by leukemic progression. This was demonstrated in at least one patient with IDH2 mutant CHIP that developed AML shortly after NPM1c was detected (8, 24). Our mouse model of preleukemic Npm1c/Dnmt3a LT-GMPs allowed us to test whether we can interrupt leukemia progression via eradication of Npm1c mutant preleukemic clones. We evaluated the in vivo efficacy of VTP-50469 using secondary transplants of Npm1c single and Npm1c/Dnmt3a double mutant LT-GMPs (Fig. S9A). Engraftment was confirmed 3 weeks post-transplant and mice were treated with 0.1% VTP-50469 spiked chow for 9 weeks (Fig. S9, B and C). In control animals we observed an expansion of the LT-GMP engraftment and eventually mice succumbed to AML (Fig. 4, A and B, and fig. S9, D and E). After three weeks of Menin inhibitor-treated preleukemic mice showed a rapid decrease in engraftment (<1%)(Fig 4B, and fig. S9, D and E). Strikingly, no relapse of LT-GMPs was observed more than 6 months after the treatment was discontinued and VTP-50469 treated groups showed prolonged survival of over 9 months versus and average of 5 month in the untreated groups. Furthermore, when VTP-50469 treated mice were sacrificed 300 days post-transplant, no Npm1c mutant cells were detected in bone marrow spleen or liver (Fig. S9, F to H). Wildtype stem cell self-renewal was not affected by VTP-50469 treatment as demonstrated by stable engraftment of WT HSCs (Fig. S9I). Repression of Meis1/Pbx3 and other stem cell factors was validated by RNAseq analysis of sorted Npm1c/Dnmt3a LT-GMPs after 5 days in vivo treatment (Fig S10, A to D). VTP-50469 was well tolerated even when administered for long periods (9 weeks continuously), which could potentially be extended to ensure complete clearance of NPM1c mutant cells if needed. These data indicate that we can specifically eradicate preleukemic Npm1c mutant self-renewing myeloid progenitor cells using targeted epigenetic therapy without having detrimental effects on either normal HSCs or hematopoiesis.
Fig. 4. Preleukemic Npm1c LT-GMPs and human AML cells can be eradicated by Menin inhibition.
(A and B) Percent engraftment of CD45.2 in peripheral blood (A) and Kaplan-Meier survival analysis (B) of mice transplanted with Npm1c/Dnmt3a LT-GMPs receiving control or 0.1% VTP-50469–spiked chow for 9 weeks (one-way ANOVA; n = 3 mice per group; error bars indicate mean ± SD). (C and D) Percent engraftment of hCD45 in peripheral blood (C) and aplan-Meier survival analysis (D) of NPM1c,FLT3ITD,FLT3TKD-transplanted PDX mice receiving control or 0.1% VTP-50469–spiked chow for 129 days (patient 1, table S5; n = 5 mice per group; error bars indicate mean ± SD). (E) Mutational screening of 49 paired MDS and sAML patient samples for RUNX1, TP53, NPM1, FLT3, ASXL1, DNMT3A, IDH1, and IDH2 mutations revealed six patients with persistent NPM1 mutations detected in MDS samples before AML development. IPSS, International Prognostic Scoring System; SNP, single- nucleotide polymorphism; CN-AML, cytogenetically normal AML; CNAs, copy number alterations. ns, not significant; **P < 0.01; ***P < 0.001.
We next wanted to investigate whether NPM1c mutant cells remained sensitive to Menin-MLL inhibition after progression to AML. Menin-MLL inhibitors have been shown to be effective targeting MLL-fusion leukemias in vivo, but whether they will be similarly effective in the more common NPM1c-mutant AML was less clear. To this end we used patient derived xenograft (PDX) assays of untreated and relapsed NPM1c AML harboring FLT3, DNMT3a and IDH1 co-mutations (Table S5). Inhibiting MLL1-Menin dramatically reduced tumor burden in blood, spleen, and BM of three different PDX models treated for 30–43 days (Fig. S11, A to I). The few detectable human cells expressed high levels of differentiation marker CD11b (Fig. S11, J and K). VTP-50469 treatment significantly prolonged survival in two independent NPM1c PDX models (Fig. 4, C and D, fig. S12 A and B). Gene expression analysis of NPM1c PDX cells isolated 10 days post in vivo Menin inhibitor treatment confirmed reduced expression of MEIS1/PBX3 as observed in our mouse model, while HOX genes were slightly increased (Fig. S12C). Furthermore, Menin inhibition was effective in PDX mice with high tumor burden (40–80% hCD45) (Fig. S13A). A reduction of blood leukemia burden was observed after 3 weeks of VTP-50469 treatment. Except for one mouse that expired after 10 days of treatment, the three remaining VTP-50469 treated mice survived over 150 days post-transplant with hCD45 engraftment <1% (Fig. S13B and C). Our data suggests that Menin-MLL inhibition is highly effective not just in the preleukemic setting, but also in fully developed aggressive human NPM1c mutant AMLs.
To determine the feasibility of detecting preleukemic NPM1 mutant clones in patients, we screened 49 paired MDS and secondary AML (sAML) samples for AML-associated mutations (NPM1, DNMT3A, RUNX1, TP53, NF1, ASXL1, IDH1 and IDH2). NPM1c was detected in six (12%) MDS and paired sAML samples, while co-occurring signaling mutations NF1 and FLT3 were mostly acquired during progression to sAML in these samples (Fig. 4E). Half of these NPM1c mutant MDS patient rapidly developed leukemia within 1–2 months, whereas the other group of patients progressed more slowly (5–6.5 month) (Table S6). NPM1c can therefore be detected in a preleukemic setting and may act as a marker for progression to AML, making it an ideal target for preventative therapy. In the context of screening and monitoring, this may plausibly be extended to individuals with large DNMT3A or IDH1/2 mutant CHIP clones, which is predictive of high AML risk (9).
In summary, this study shows that eliminating preleukemic cells with targeted therapy is a potentially promising approach; specifically, we present evidence in a mouse model of AML that early intervention is possible with molecules that target chromatin regulators. Combined with improved long-term monitoring of patients with high-risk CHIP or MDS for appearance of an NPM1c preleukemic clone, disease prevention could become a realistic possibility in the future.
Supplementary Material
Acknowledgments
We thank Z. Feng and all members of the Armstrong Lab for their help; A. Cremer and J. Perry for critically reading the manuscript; F. Perner for the Menin sgRNA constructs and Y. Soto-Feliciano for the ipUSEPR sgRNA expression plasmid.
Funding: S.A.A was supported by NIH grants CA176745, CA204639, CA066996, CA206963 and by grants from Wicked Good Cause and Cookies for Kids Cancer grants. K.D. and L.B. were supported by SFB 1074 project B3. HU was supported by the German Research Foundation (DFG, UC77/1–1). RLL was supported by NIH grants P30 CA008748 and by U54 OD020355–04. GSV is funded by a Cancer Research UK Senior Fellowship (C22324/A23015).
Footnotes
Competing interests: SAA has been a consultant and/or shareholder for Vitae/Allergan Pharmaceuticals, Epizyme Inc, Imago Biosciences, Cyteir Therapeutics, C4 Therapeutics, Syros Pharmaceuticals, OxStem Oncology, Accent Therapeutics and Mana Therapeutics. SAA has received research support from Janssen, Novartis, and AstraZeneca. RLL is on the supervisory board of Qiagen and is a scientific advisor to Loxo, Imago, C4 Therapeutics and Isoplexis, which each include an equity interest. He receives research support from and consulted for Celgene and Roche, he has received research support from Prelude Therapeutics, and consulted for Incyte, Novartis, Astellas, Morphosys and Janssen. He has received honoraria from Lilly and Amgen for invited lectures and from Gilead for grant reviews. GSV is a consultant for Oxstem and a consultant for and minor stockholder in Kyma. H.G. owns stock in Theravance Biopharma.
Data and materials availability: VTP50469 can be obtained via MTA from GMM/Syndax. All data of this study are deposited in the NCBI Gene Expression Omnibus (GEO) under accession number GSE129638.
References and Notes:
- 1.Falini B. et al. Cytoplasmic Nucleophosmin in Acute Myelogenous Leukemia with a Normal Karyotype. N. Engl. J. Med 352, 254–266 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Schlenk RF et al. Mutations and Treatment Outcome in Cytogenetically Normal Acute Myeloid Leukemia. N. Engl. J. Med 358, 1909–1918 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Papaemmanuil E. et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med 374, 2209–2221 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Döhner H, Weisdorf DJ & Bloomfield CD Acute Myeloid Leukemia. N. Engl. J. Med 373, 1136–1152 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Vassiliou GS et al. Mutant nucleophosmin and cooperating pathways drive leukemia initiation and progression in mice. Nat Genet 43, 470–475 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunetti L. et al. Mutant NPM1 Maintains the Leukemic State through HOX Expression. Cancer Cell 34, 499–512.e9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dovey OM et al. Molecular synergy underlies the co-occurrence patterns and phenotype of NPM1 -mutant acute myeloid leukemia. Blood 130, 1911–1922 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med 368, 2059–74 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaiswal S. et al. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N. Engl. J. Med 371, 2488–2498 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shlush LI et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 506, 328–33 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guryanova OA et al. DNMT3A mutations promote anthracycline resistance in acute myeloid leukemia via impaired nucleosome remodeling. Nat. Med 22, 1488–1495 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loberg MA et al. Sequentially inducible mouse models reveal that Npm1 mutation causes malignant transformation of Dnmt3a-mutant clonal hematopoiesis. Leukemia 1 (2019) doi: 10.1038/s41375-018-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kühn MWM et al. Targeting Chromatin Regulators Inhibits Leukemogenic Gene Expression in NPM1 Mutant Leukemia. Cancer Discov. 6, 1166–1181 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krivtsov AV et al. A Menin-MLL Inhibitor Induces Specific Chromatin Changes and Eradicates Disease in Models of MLL-Rearranged Leukemia. Cancer Cell 36, 660–673.e11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z. et al. PBX3 and MEIS1 Cooperate in Hematopoietic Cells to Drive Acute Myeloid Leukemias Characterized by a Core Transcriptome of the MLL-Rearranged Disease. Cancer Res. 76, 619–29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang GG, Pasillas MP & Kamps MP Persistent Transactivation by Meis1 Replaces Hox Function in Myeloid Leukemogenesis Models: Evidence for Co-Occupancy of Meis1-Pbx and Hox-Pbx Complexes on Promoters of Leukemia-Associated Genes. Mol. Cell. Biol 26, 3902–3916 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z. et al. PBX3 is an important cofactor of HOXA9 in leukemogenesis. Blood 121, 1422–1431 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grembecka J. et al. Menin-MLL inhibitors reverse oncogenic activity of MLL fusion proteins in leukemia. Nat. Chem. Biol 8, 277–284 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borkin D. et al. Pharmacologic inhibition of the Menin-MLL interaction blocks progression of MLL leukemia in vivo. Cancer Cell 27, 589–602 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKerrell T. et al. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell Rep. 10, 1239–45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie M. et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med 20, 1472–1478 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genovese G. et al. Clonal Hematopoiesis and Blood-Cancer Risk Inferred from Blood DNA Sequence. N. Engl. J. Med 371, 2477–2487 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abelson S. et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature 559, 400–404 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desai P. et al. Somatic mutations precede acute myeloid leukemia years before diagnosis. Nat. Med 24, 1015–1023 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.