Abstract
Background:
Subjects undergoing coronary stenting with complex lesion anatomy may experience different risks and benefits with prolonged dual antiplatelet therapy.
Objective:
The authors assessed the effect of 30 versus 12 months of dual antiplatelet therapy after percutaneous coronary intervention (PCI) based on the presence or absence of anatomically-complex target lesions.
Methods:
In the Dual Antiplatelet Therapy (DAPT) Study, combined myocardial infarction (MI) or stent thrombosis and moderate/severe bleeding were assessed in enrolled (N=25416) and randomized (N=11554) subjects. Complex lesions had any of the following characteristics: unprotected left main, >2 lesions per vessel, length ≥30 mm, bifurcation with side branch ≥2.5 mm, vein bypass graft, or thrombus-containing lesion. Events were evaluated according to increasing number of complexity characteristics and compared according to DAPT score.
Results:
Enrolled subjects with more complex target lesions had higher rates of MI or stent thrombosis in the first 12 months after PCI (3.9% vs. 2.4%, P<0.001). Among those event-free at 12 months, rates of MI or stent thrombosis between 12–30 months were similar between those with versus without complex anatomy (3.5% vs. 2.9%, P=0.07). Reduction of MI or stent thrombosis with continued thienopyridine beyond 12 months vs. placebo was similar for subjects with (2.5% vs. 4.5%, HR 0.55, 95% CI 0.38–0.79; P=0.001) and without (2.0% vs. 3.8%, HR 0.52, 95% CI 0.39–0.69; P<0.001) anatomic complexity (Pinteraction=0.81), as was increase in moderate/severe bleeding (Pinteraction=0.44). Among subjects with anatomic complexity, those with DAPT scores ≥2 randomized to continued thienopyridine had greater reductions in MI or stent thrombosis (3.0% vs. 6.1%; P<0.001) compared with subjects with scores <2 (1.7%= vs. 2.3%, P=0.42, P comparing risk differences=0.03).
Conclusions:
Complex target-lesion anatomy is associated with increased ischemic events, particularly within the first year after PCI. Among those without events in the first 12 months, the benefits of extending dual antiplatelet therapy were similar in subjects with and without complex lesions. High DAPT score identified those experiencing the most benefit from extended treatment among patients with and without complex anatomy.
Keywords: Dual antiplatelet therapy, Percutaneous coronary intervention, Complex Lesions, Dual Antiplatelet Therapy Score
CONDENSED ABSTRACT
Subjects undergoing coronary stenting with complex lesion anatomy may experience different risks and benefits with prolonged dual antiplatelet therapy. In this subgroup analysis of the Dual Antiplatelet Therapy (DAPT) Study comparing the effect of 30 versus 12 months of dual antiplatelet therapy after percutaneous coronary intervention, complex target-lesion anatomy was associated with increased ischemic events, particularly within the first year. Among those without events after 12 months, the benefits of extending therapy were similar in subjects with and without complex lesions. High DAPT score identified those experiencing the most benefit from extended treatment among patients with and without complex anatomy.
INTRODUCTION
The Dual Antiplatelet Therapy (DAPT) Study found that continuing thienopyridine plus aspirin beyond one year after coronary stenting was associated with decreased rates of stent thrombosis and major adverse cardiovascular and cerebrovascular events (MACCE), but increased rates of moderate or severe bleeding compared with aspirin alone.(1) The population included over 11 000 randomized patients who were compliant with thienopyridine therapy and who were free from bleeding and ischemic events during the 12 month-period post-coronary stenting, during which they received open label thienopyridine plus aspirin. Based on data from this and other studies of dual antiplatelet therapy duration, the American College of Cardiology/American Heart Association recent 2016 guidelines gave a Class I recommendation for at least 6 months of dual antiplatelet therapy post-stenting with drug-eluting stents. The guidelines also emphasized the need to individualize treatment duration, and consider longer therapy in those patients at lower bleeding and potentially higher ischemic risk.(2)
Features of coronary anatomical and procedural complexity, such as the number, caliber and length of lesions treated, as well as pre- and post-procedural flow grades, are known risk factors for stent thrombosis.(3) In a recent pooled analysis of trials, longer dual antiplatelet therapy (≥ 12 months compared to 3 or 6 months) was found to be more beneficial in patients with complex coronary anatomy, with significant reductions in major adverse cardiac events in the complex PCI group (adjusted HR 0.56, 95% CI 0.35–0.89) versus the noncomplex group (adjusted HR 1.01, 95% CI 0.75 −1.35; interaction P=0.01).(4) We sought to examine the relationship between anatomical/procedural complexity and optimal duration of dual antiplatelet therapy among those patients reaching 1 year without an ischemic or bleeding event in the DAPT Study, the largest randomized trial comparing thienopyridine duration after PCI.
METHODS
Design
The DAPT Study, previously described(1,2), was a double-blind, international, multicenter, randomized, placebo-controlled trial that compared the benefits and risks of 30 versus 12 months of thienopyridine therapy (clopidogrel or prasugrel) when prescribed in addition to aspirin following coronary stenting with bare metal stents (BMS) or drug-eluting stents (DES) in subjects who tolerated dual antiplatelet therapy for 12 months (ClinicalTrials.gov # NCT00977938). Five individual component studies were incorporated into this single, uniform randomized trial, with enrollment of subjects either by the Baim Institute for Clinical Research (formerly known as the Harvard Clinical Research Institute), or through one of four post-marketing surveillance studies. The results comparing randomized treatments in DES-treated(1) and BMS-treated(3,4) cohorts have been reported previously, as have results in various subpopulations of the study.(5,6)
The institutional review board at each participating institution approved the study. The purpose of the present post hoc analysis was to examine whether the ischemic benefits and bleeding risks associated with 30 versus 12 months of dual antiplatelet therapy are consistent among subjects presenting with versus without complex coronary lesion anatomy.
Study Population and Procedures
Briefly, subjects with coronary artery disease who were candidates for dual antiplatelet therapy and who received treatment with Food and Drug Administration (FDA)-approved DES and BMS devices provided written informed consent and were enrolled within 3 days of stent placement. Stent treatment was performed according to site standards of care. DES types included sirolimus-eluting stent (Cypher, Cordis), zotarolimus-eluting stent (Endeavor, Medtronic), paclitaxel-eluting stent (TAXUS, Boston Scientific), and everolimus-eluting stents (Xience, Abbott Vascular; PROMUS, Boston Scientific).
All subjects received open-label aspirin plus thienopyridine for the first 12 months after stent implantation. In one contributing study, all subjects received prasugrel under an Investigational Device Exemption from the FDA; in the remaining four studies, the selection of clopidogrel or prasugrel was left to the discretion of the treating physician. At 12 months, subjects who were treatment compliant (defined as having taken 80%−120% of thienopyridine therapy without an interruption of longer than 14 days) and who had not experienced a MACCE, repeat revascularization, or moderate or severe bleeding were randomized to continued thienopyridine or placebo for an additional 18 months. Both groups continued aspirin therapy.
A computer-generated randomization schedule stratified subjects according to the type of stent they had received (DES vs BMS), hospital site, thienopyridine type, and presence or absence of at least 1 prespecified clinical or anatomical characteristic for complexity. At 30 months, subjects discontinued the randomized treatment, remained on aspirin, and were followed for another 3 months.
In this current analysis, we assessed outcomes and treatment effect differences in the subgroups of subjects with and without complex lesion anatomy, using the pre-specified variable in the DAPT Study protocol (randomization stratification variable). A treated lesion was considered anatomically complex if it had any of the following characteristics: unprotected left main, >2 lesions per vessel, lesion length ≥30 mm, bifurcation lesion with side branch ≥ 2.5 mm, vein bypass graft (segment or anastomosis), or thrombus-containing lesion.
Endpoints
For this analysis, the primary ischemic endpoints were the incidence of combined myocardial infarction or stent thrombosis, according to the Academic Research Consortium definitions(7) and the incidence of MACCE in all randomized subjects at 12–30 months post-index procedure. The primary safety endpoint was moderate or severe bleeding at 12–30 months, assessed according to the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries (GUSTO) classification.(8) Bleeding was also ascertained according to the Bleeding Academic Research Consortium (BARC) definitions.(9)
All endpoint events were adjudicated by an independent Clinical Events Committee blinded to treatment assignment. An unblinded independent central data monitoring committee oversaw the safety of all subjects by reviewing data from all subjects at regular intervals.
Statistical Analysis
The intent of the analysis was to examine whether the presence of anatomical lesion complexity could be used by clinicians to identify patients at high risk for events within 0–12, and 12–30 months after PCI, and further whether its presence effectively stratified the magnitude of ischemic benefit for continuation of thienopyridine therapy beyond 12 months. We compared crude proportions of endpoint events between subjects with and without anatomically complex lesions in the 0–12 month period among all enrolled patients using the chi-square test. Kaplan-Meier estimates of endpoint events between 12 and 30 months after PCI were compared between randomized subjects with and without anatomically complex lesions using the log-rank test, irrespective of treatment arm. Next, Kaplan-Meier estimates of endpoint events were generated for each treatment group for subjects with and without anatomic complexity. The effects of continued thienopyridine vs. placebo for subjects with and without anatomic complexity were assessed using Cox-proportional hazards regression models, and are expressed as hazard ratios (HR) and associated two-sided 95% confidence intervals (CI). The consistency of the treatment effect between subjects with and without anatomic complexity was evaluated through the inclusion of randomized treatment-by-anatomic complexity status interaction terms (multiplicative interaction) in a Cox model. Because clinically meaningful differences in treatment effect can exist on the absolute scale (e.g. number needed to treat) even in the absence of differences in relative treatment effect, we further compared absolute risk differences between randomized treatment groups for those patients without versus without anatomically complex lesions using the Z test (additive interaction). These analyses were repeated, stratifying patients based on the number of anatomic lesion complexity characteristics (0, 1 or 2 or more characteristics). Randomized treatment effect was additionally examined after stratifying patients with and without anatomic lesion complexity by DAPT Score(10) in the overall randomized population (DAPT score <2 vs. ≥2). The unadjusted association between individual complexity characteristics and the primary ischemic and bleeding outcomes between 0–12 and 12–30 months was assessed using logistic and Cox regression, respectively.
As sensitivity analyses, we repeated these analyses in subgroups of subjects with and without complex lesion anatomy according to a previously published alternative definition of coronary complexity(11), which included any of the following characteristics: 3 vessels treated, ≥3 stents implanted, ≥3 lesions treated, bifurcation with 2 stents implanted, total stent length >60 mm, or chronic total occlusion. In addition, we repeated analyses after 1) excluding patients who received paclitaxel-eluting stents and 2) including only everolimus-eluting stents.
All analyses were performed on randomized subjects for whom lesion complexity status was available. All statistical analyses were conducted at the Baim Institute for Clinical Research with the use of SAS software, version 9.2. (SAS Institute Inc., Cary, NC, USA). A two-sided p value of 0.05 or less was considered statistically significant for all analyses.
RESULTS
Study Population
Of 25 416 subjects enrolled in the DAPT study and with anatomic lesion complexity information available, 8381 (33.0%) were found to have complex coronary lesions at the index procedure. Of the 25 416 subjects, one year after the index procedure, 4651 (55.5%) with and 9211 (54.1%) without anatomic lesion complexity were either not eligible for randomization or were eligible but not randomized, and 3730 (44.5%) complex and 7824 (45.9%) non-complex patients were randomized at 12 months. Of the 8381 enrolled patients with lesion complexity, 7044 had 1 complex characteristic and 1337 had 2 or more characteristics for lesion complexity. Of 3730 randomized patients with lesion complexity, 3182 had 1 characteristic and 548 had 2 or more characteristics for lesion complexity. At 30 months, 3364 (90.2%) complex patients and 7093 (90.7%) non-complex patients had clinical follow-up data available (Figure 1).
Figure 1. Enrollment, randomization and follow-up of subjects in the DAPT Study stratified by lesion complexity status.
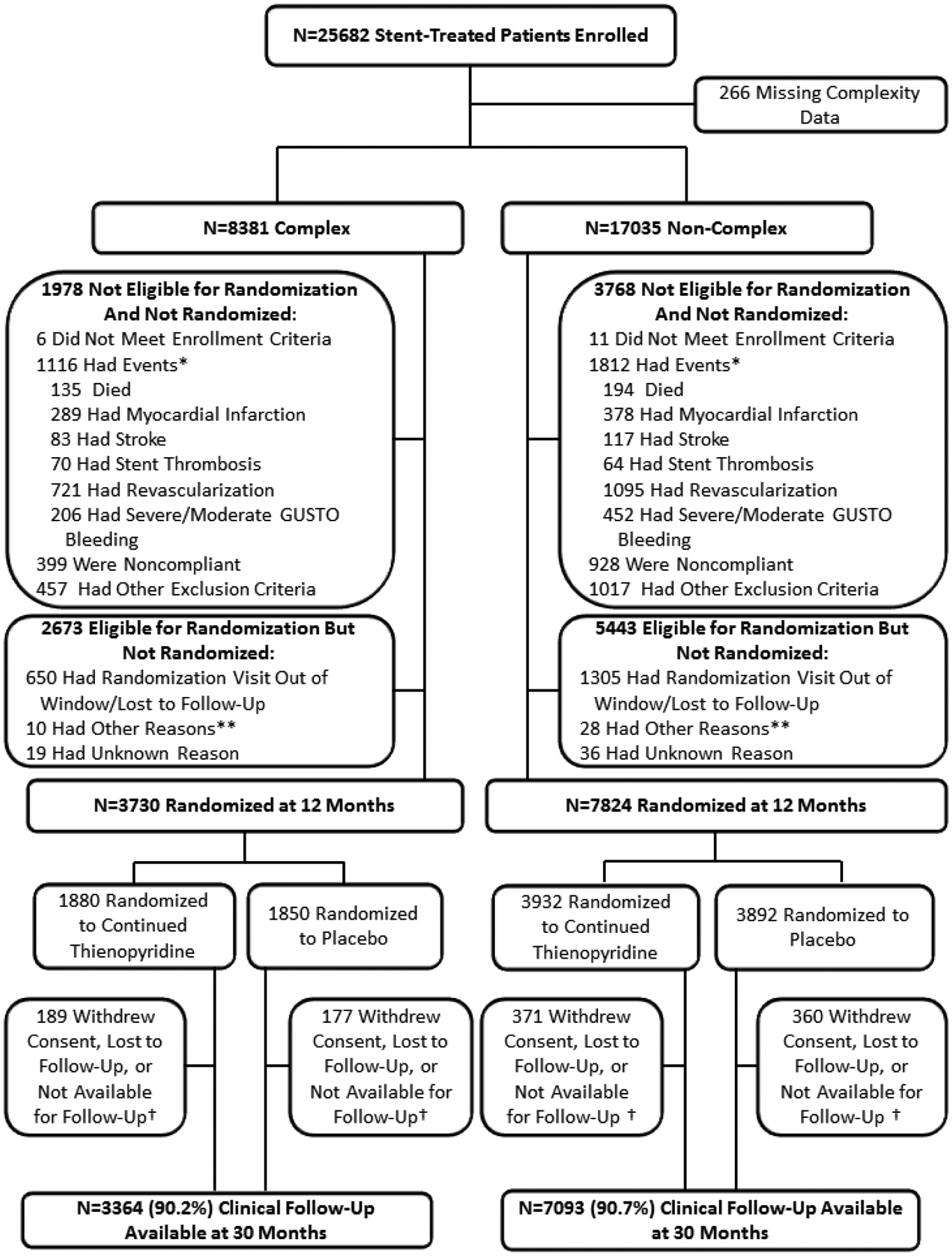
Of 25,416 subjects enrolled in the DAPT study and with anatomic lesion complexity information available, 8,381 (33.0%) were found to have complex coronary lesions at the index procedure. *Subjects may have >1 event. **Site terminated participation, randomization target met prior to subject follow-up, or subject not recognized to be eligible by site. †Subjects moved, were incarcerated, or were prematurely exited from the study.
Randomized subjects with anatomic lesion complexity were younger, more likely to be male, less likely to have diabetes mellitus and hypertension, but more likely to be a cigarette smoker, to have previous MI, and to have undergone coronary artery bypass grafting (Table 1).
Table 1.
Baseline characteristics of randomized subjects in the DAPT study, with versus without complex coronary anatomy.*
| Measure | Complex Anatomy (N=3730 Subjects) | Non-Complex Anatomy (N=7824 Subjects) | P-value |
|---|---|---|---|
| Age (years) | 60.4±10.5 (3730) | 61.8±10.2 (7824) | <.001 |
| Male | 78.7% (2934/3730) | 73.2% (5727/7824) | <.001 |
| Hispanic or Latino | 3.8% (137/3654) | 3.5% (267/7687) | 0.48 |
| Race – Non-white | 7.8% (286/3653) | 9.0% (694/7688) | 0.04 |
| Body mass index (kg/m2) | 30.2±5.6 (3705) | 30.5±5.8 (7752) | 0.003 |
| Diabetes mellitus | 26.4% (982/3717) | 30.5% (2376/7796) | <.001 |
| Hypertension | 67.8% (2517/3714) | 75.9% (5929/7808) | <.001 |
| Current cigarette smoker or within past year | 31.6% (1165/3689) | 25.5% (1962/7697) | <.001 |
| Stroke/Transient ischemic attack | 3.4% (127/3723) | 3.4% (267/7810) | 1.00 |
| Congestive heart failure | 4.3% (159/3717) | 4.5% (354/7804) | 0.56 |
| Left ventricular ejection fraction < 30% | 2.4% (82/3478) | 1.7% (121/7157) | 0.02 |
| Renal insufficiency/failure | 4.3% (160/3719) | 3.9% (304/7801) | 0.31 |
| Peripheral arterial disease | 5.7% (212/3730) | 5.5% (428/7824) | 0.63 |
| Previous percutaneous coronary intervention | 28.4% (1057/3717) | 29.0% (2262/7802) | 0.55 |
| Coronary artery bypass graft | 15.0% (556/3719) | 8.7% (682/7816) | <.001 |
| Atrial fibrillation | 2.7% (99/3717) | 3.0% (230/7798) | 0.40 |
| Previous MI | 22.6% (832/3684) | 20.7% (1593/7709) | 0.02 |
| History of cancer | 9.4% (348/3699) | 9.0% (702/7774) | 0.51 |
| Indication for Index Procedure | |||
| STEMI | 26.3% (979/3730) | 8.9% (693/7824) | <.001 |
| Non-STEMI | 19.0% (711/3730) | 14.9% (1167/7824) | <.001 |
| Stable Angina | 27.2% (1014/3730) | 39.7% (3102/7824) | <.001 |
| Unstable Angina** | 14.0% (521/3730) | 16.5% (1288/7824) | <.001 |
| Other | 13.5% (505/3730) | 20.1% (1574/7824) | <.001 |
| Number of Treated Vessels (per patient) | <.001 | ||
| 1 | 87.8% (3261/3716) | 90.8% (7099/7821) | |
| 2 | 11.0% (408/3716) | 9.2% (721/7821) | |
| 3 | 1.3% (47/3716) | 0.0% (1/7821) | |
| Mean±SD (N) | 1.1±0.4 (3716) | 1.1±0.3 (7821) | <.001 |
| Number of Stents (per patient) | 1.7±0.9 (3730) | 1.3±0.6 (7824) | <.001 |
| Prasugrel | 34.4% (1284/3730) | 30.6% (2393/7824) | <.001 |
| Clopidogrel | 65.6% (2446/3730) | 69.4% (5431/7824) | |
| Anatomic Complexity Characteristics | |||
| Unprotected left main stented | 1.2% (43/3730) | 0.0% (0/7824) | <.001 |
| >2 lesions per vessel | 5.5% (204/3730) | 0.0% (0/7824) | <.001 |
| Lesion length ≥30 mm | 29.9% (1115/3729) | 0.0% (0/7824) | <.001 |
| Bifurcation lesion with side branch ≥2.5 mm stented | 19.3% (719/3728) | 0.0% (0/7824) | <.001 |
| Vein bypass graft stented | 8.6% (322/3730) | 0.0% (0/7824) | <.001 |
| Thrombus-containing lesion | 43.2% (1532/3550) | 0.0% (0/7192) | <.001 |
| Prior brachytherapy | 0.8% (28/3716) | 0.0% (0/7824) | <.001 |
| Stent Type | <.001 | ||
| Sirolimus-Eluting | 9.6% (357/3730) | 9.7% (758/7824) | |
| Zotarolimus-Eluting | 11.1% (414/3730) | 10.8% (848/7824) | |
| Paclitaxel-Eluting | 24.5% (915/3730) | 22.3% (1747/7824) | |
| Everolimus-Eluting | 35.1% (1308/3730) | 42.4% (3316/7824) | |
| >1 DES type | 2.6% (95/3730) | 1.5% (115/7824) | |
| BMS | 17.2% (641/3730) | 13.3% (1040/7824) | |
| Stent Diameter <3 mm | 43.1% (1606/3730) | 43.4% (3399/7824) | 0.70 |
| Stent Diameter ≥3mm | 56.9% (2124/3730) | 56.6% (4425/7824) | |
| Total stent lengths (mm) | 35.3±20.8 (3730) | 23.1±12.1 (7824) | <.001 |
| BMS | 17.2% (641/3730) | 13.3% (1040/7824) | <.001 |
| DES | 82.8% (3089/3730) | 86.7% (6784/7824) | |
| Treated Vessel | |||
| Left Main | 1.5% (56/3730) | 0.7% (55/7822) | <.001 |
| Left anterior descending | 41.7% (1554/3730) | 46.0% (3596/7822) | <.001 |
| Right coronary artery | 39.7% (1480/3730) | 34.2% (2677/7822) | <.001 |
| Circumflex | 22.3% (833/3730) | 28.0% (2188/7822) | <.001 |
| Venous graft | 8.6% (320/3730) | 0.0% (0/7824) | <.001 |
| Arterial graft | 0.4% (15/3730) | 0.6% (47/7824) | 0.220 |
Abbreviations: BMS, bare metal stent; DES, drug-eluting stent; MI, myocardial infarction; STEMI, ST-elevation myocardial infarction.
Values are % (N/D), except as noted. Plus–minus values are means ±SD.
This category included unstable angina without reported elevation of cardiac enzymes.
Outcomes Comparing Subjects With vs. Without Anatomically Complex Lesions
When assessing outcomes in enrolled subjects with or without anatomic complexity from 0–12 months, subjects with complex anatomy experienced increased rates of MACCE (5.3% vs. 3.5%, P<0.001) and MI or stent thrombosis (3.9% vs. 2.4%, P<0.001) compared with patients without complex anatomy, and no difference in moderate or severe bleeding (2.2% vs. 2.4%, P=0.40) (Appendix Table 1).
During the 12–30 month period, randomized subjects with or without complex anatomy experienced similar rates of MI or stent thrombosis (3.5% vs. 2.9%, respectively, P=0.07) and MACCE (5.5% vs. 4.8%, respectively, P=0.11), as well as similar rates of GUSTO moderate/severe bleeding (1.9% vs. 1.9%, P=0.91). (Table 2) The individual components comprising the complexity definition were more strongly associated with ischemic events in the 0–12 month period, compared with the 12–30 month period. (Appendix Tables 2 and 3)
Table 2.
Kaplan-Meier rates of outcomes in randomized subjects with vs. without anatomic lesion complexity between 12–30 months after percutaneous coronary intervention
| Event | Complex Anatomy (N =3730) | Non-Complex Anatomy (N =7824) | Log Rank P Value |
|---|---|---|---|
| MI or Stent Thrombosis | 127 (3.5%) | 219 (2.9%) | 0.07 |
| MACCE | 199 (5.5%) | 364 (4.8%) | 0.11 |
| Bleeding (moderate/severe) | 68 (1.9%) | 145 (1.9%) | 0.91 |
| Stent Thrombosis (definite/probable) | 36 (1.0%) | 61 (0.8%) | 0.31 |
| MI | 127 (3.5%) | 215 (2.8%) | 0.051 |
| Death | 67 (1.9%) | 121 (1.6%) | 0.32 |
Abbreviations: BARC, Bleeding Academic Research Consortium; MACCE, major adverse cardiovascular and cerebrovascular events; MI, myocardial infarction.
A trend for increasing event rates associated with increasing number of complexity characteristics was seen for ischemic outcomes both at 0–12 months after enrollment (enrolled patients N=25416, Appendix Table 4) and 12–30 months after enrollment (randomized patients N=11554, Appendix Table 5). The rates of MI or stent thrombosis increased from 2.4% in patients with 0 complexity characteristics to 3.4% in patients with 1 complexity characteristic, to 6.4% in patients with 2 or more complexity characteristics in the 0–12 months study period (P value for trend <0.001). The same was true for rates of MI or stent thrombosis in randomized patients in the 12–30 month period, with rates of 2.9%, 3.2%, and 5.5% for patients with 0, 1, or 2 or more complexity characteristics, respectively (P value for trend = 0.01).
Consistency of Treatment Effect of Continued Thienopyridine in Subjects With and Without Anatomically Complex Lesions
The relative reduction of MI or stent thrombosis associated with continued thienopyridine was similar for subjects with and without anatomic complexity (Pinteraction comparing relative risk reduction= 0.81, P comparing absolute risk differences = 0.75). In subjects with anatomic complexity, the rate of MI or stent thrombosis was 2.5% for continued thienopyridine vs. 4.5% for placebo (HR 0.55, 95% CI 0.38–0.79; P=0.001), whereas for subjects without anatomic complexity, the corresponding rates were 2.0% vs. 3.8%, respectively (HR 0.52; 95% CI 0.39–0.69; P<0.001). Similarly, the relative reduction in MACCE was comparable for patients with (4.7% vs. 6.3%, HR 0.72, 95% CI 0.55–0.96, P=0.02) and without anatomic complexity (4.1% vs. 5.5%, HR 0.74, 95% CI 0.60–0.91, P=0.01, Pinteraction =0.88, P comparing absolute risk differences =0.71) (Table 3, Figure 2). No significant interactions were observed when the population excluded those treated with paclitaxel-eluting stents (Appendix Table 6), or was limited to only those subjects receiving everolimus-eluting stents (Appendix Table 7).
Table 3.
Treatment comparison of continued thienopyridine vs. placebo in randomized subjects with vs. without anatomical complexity, according to intention-to-treat analysis with subjects who had anatomic lesion complexity information available.
| Event | Continued Thienopyridine (N=5812) | Placebo (N=5742) | Hazard Ratio [95% CI] | Risk Difference [95% CI] Continued Thienopyridine - Placebo | Log Rank P-value | P Value for Risk Difference | P Value for Interaction |
|---|---|---|---|---|---|---|---|
| MI or Stent thrombosis | 0.75 | 0.81 | |||||
| Complex (N=3730) | 46 (2.5%) | 81 (4.5%) | 0.55 [0.38, 0.79] | −2.0% [−3.2%,−0.8%] | 0.001 | ||
| Not Complex (N=7824) | 76 (2.0%) | 143 (3.8%) | 0.52 [0.39, 0.69] | −1.8% [−2.5%,−1.0%] | <.001 | ||
| MACCE | 0.71 | 0.88 | |||||
| Complex (N=3730) | 85 (4.7%) | 114 (6.3%) | 0.72 [0.55, 0.96] | −1.7% [−3.2%,−0.2%] | 0.02 | ||
| Not Complex (N=7824) | 157 (4.1%) | 207 (5.5%) | 0.74 [0.60, 0.91] | −1.4% [−2.3%,−0.4%] | 0.01 | ||
| Bleeding (moderate/severe) | 0.41 | 0.44 | |||||
| Complex (N=3730) | 40 (2.2%) | 28 (1.6%) | 1.41 [0.87, 2.28] | 0.6% [−0.3%,1.5%] | 0.16 | ||
| Not Complex (N=7824) | 93 (2.5%) | 52 (1.4%) | 1.78 [1.27, 2.50] | 1.1% [0.4%,1.7%] | <.001 |
Figure 2. Cumulative incidence of myocardial infarction or stent thrombosis (Panel A), major adverse cardiovascular or cerebrovascular events (MACCE) (Panel B), and GUSTO moderate/severe bleeding (Panel C) from 12–30 months after randomization, according to randomization group in subjects with and without anatomically complex lesions.
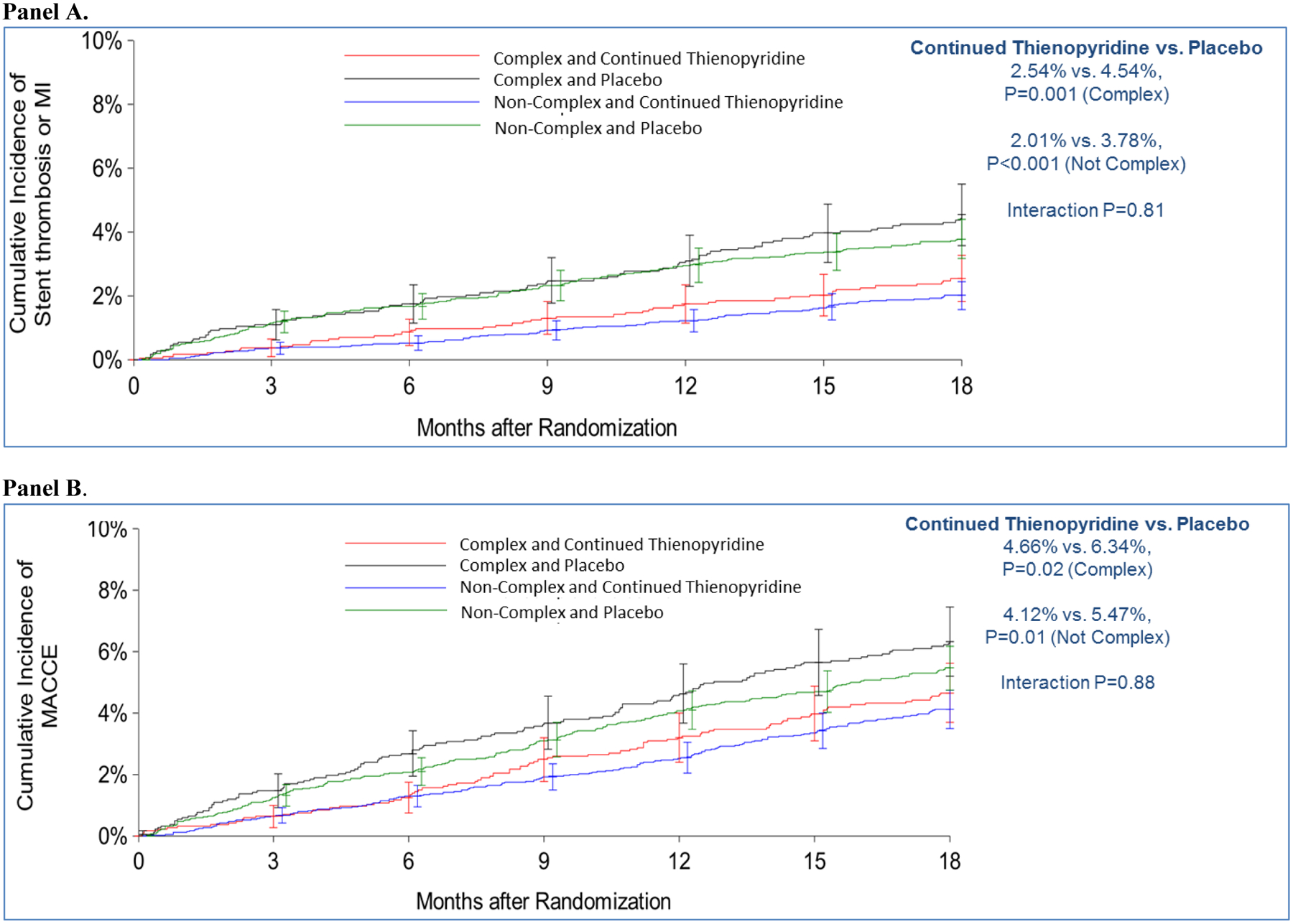

Consistency in treatment effect of continued thienopyridine was observed in subjects with and without anatomically complex lesions in the 12–30 month time period for all major endpoints.
For the endpoint of GUSTO moderate/severe bleeding, continued thienopyridine was associated with a similar increase for subjects with anatomic complexity (2.2% vs. 1.6%, HR 1.41, 95% CI 0.87–2.28, P=0.16) vs. subjects without anatomic complexity (2.5% vs. 1.4%, HR 1.78, 95% CI 1.27–2.50, P<0.001, Pinteraction=0.44, P comparing risk differences=0.41).
In order to assess whether treatment effects differed depending on the number of complexity factors, outcomes were also analyzed comparing randomized treatments among subgroups of subjects with 0 (N=7824), 1 (N=3182), or 2 or more (N=548) anatomic complexity characteristics. Relative treatment effects were consistent for the outcomes of MI or stent thrombosis and MACCE independent of the number of complexity characteristics (Figure 3).
Figure 3. Cumulative incidence of endpoint events from 12–30 months after randomization, stratified by randomized treatment arm and number of anatomical complexity characteristics. MI, myocardial infarction; MACCE, major adverse cardiovascular or cerebrovascular events.

Outcomes were analyzed comparing randomized treatments among subgroups of subjects with 0 (N=7824), 1 (N=3182), or 2 or more (N=548) anatomic complexity characteristics in order to assess whether treatment effects differed depending on the number of complexity factors. Relative treatment effects were consistent for the outcomes of MI or stent thrombosis and MACCE independent of the number of complexity characteristics. MI, myocardial infarction; MACCE, major adverse cardiovascular or cerebrovascular events.
Treatment Effect Among Complex and Non-Complex Anatomy Subjects by DAPT Score
Among the 3730 randomized subjects with anatomic complexity, those with DAPT scores ≥2 (n=2267) randomized to continued thienopyridine had greater absolute reductions in MI or stent thrombosis (3.0% for continued thienopyridine vs. 6.1% for placebo; risk difference [RD], −3.1%, 95% CI −4.9% to −1.3%, P<0.001) compared with subjects with DAPT scores <2 (n=1463; 1.7% for continued thienopyridine vs. 2.3% for placebo; RD −0.6%, 95% CI −2.1% to 0.9%, P=0.42; Pinteraction=0.34, P comparing risk differences=0.03). Patients with DAPT scores ≥2 and with complex lesions also had significantly greater reductions in MACCE after randomization to continued thienopyridine (5.1% for continued thienopyridine vs. 8.4% for placebo; RD −3.3%, 95% CI −5.4% to −1.2%, P=0.002) compared with patients with DAPT scores <2 and with complex lesions (4.0% for continued thienopyridine vs. 3.4% for placebo; RD 0.6%, 95% CI −1.4% to 2.6%, P=0.56, Pinteraction=0.03, P comparing risk differences=0.01).
Similar results were observed among subjects without complex anatomy and DAPT scores ≥2, with greater absolute risk reductions in MI or stent thrombosis (Pinteraction=0.11, P comparing risk differences=0.003) and MACCE (Pinteraction=0.12, P comparing risk differences=0.03) compared to patients with DAPT scores <2. (Figure 4)
Figure 4. Cumulative incidence of endpoint events from 12–30 months after randomization, stratified by randomized treatment arm and presence of anatomical complexity, for patients with DAPT score ≥ 2 vs. < 2. MI, myocardial infarction; MACCE, major adverse cardiovascular or cerebrovascular events.

High DAPT score identified those experiencing the most benefit from extended treatment in the 12–30 month time period among patients with and without complex anatomy. MI, myocardial infarction; MACCE, major adverse cardiovascular or cerebrovascular events.
Among subjects with complex anatomy, randomization to continued thienopyridine had a numerically greater increase in GUSTO moderate or severe bleeding among those with DAPT score <2 (3.0% for continued thienopyridine vs. 1.8% for placebo; RD 1.2%, P=0.12) compared with those with DAPT score ≥2 (1.7% for continued thienopyridine vs. 1.4% for placebo; RD 0.3%, P=0.61), although the P values comparing risk differences (P=0.32) and for interaction (Pinteraction=0.46) were not significant. Similar results were observed for those without complex anatomy (2.9% for continued thienopyridine vs. 1.3% for placebo; RD 1.6%, P=<0.001 for DAPT score <2; 1.9% for continued thienopyridine vs. 1.5% for placebo; RD, 0.4%, P=0.31 for DAPT score ≥2; Pinteraction=0.14, P comparing risk differences=0.07).
Sensitivity Analyses
A sensitivity analysis was performed to determine whether the treatment effect of continued thienopyridine was consistent when anatomic lesion complexity was defined according to an alternative definition.(11) Results similar to those seen in the DAPT protocol-classified definition of anatomic complexity analyses were observed, with consistent reductions in MI or stent thrombosis among subjects with and without complex coronary anatomy (Pinteraction =0.66, P for risk difference =0.91), MACCE (Pinteraction =0.31, P for risk difference =0.35) and GUSTO moderate/severe bleeding (Pinteraction=0.29; P for risk difference =0.53; Appendix Table 8). In addition, no difference in the treatment effect between complex and non-complex subjects (protocol definition) for ischemic or bleeding events was observed when examining either the 12–33 month or 30–33 month time periods. (Appendix Table 9)
DISCUSSION
Among patients enrolled and randomized in the DAPT Study, we found that those undergoing PCI with more complex coronary artery target lesions had a higher rate of subsequent ischemic events particularly within the first year after PCI compared with patients without complex lesions. After the first year, this association was attenuated. Consistent with this observation, among patients reaching one year after PCI without a major ischemic or bleeding event, the magnitude of ischemic benefit associated with continuing thienopyridine for an additional 18 months was not greater among patients with complex coronary lesion characteristics compared to those without. Independent of anatomical complexity of the index lesion, those with DAPT scores ≥2 derived greater ischemic reductions with numerically lesser impact on bleeding than those with DAPT scores <2. These findings suggest that complex target lesion anatomy may be a more useful discriminator for predicting the benefit of dual antiplatelet therapy within the first year after PCI, but less useful for predicting benefit of longer durations after 12 months.
These findings may be explained by several hypotheses. First, the majority of ischemic benefit of continued thienopyridine has been found to be due to the reduction in myocardial infarction in territories distinct from the target lesion that was stented during the index procedure. As such, complex characteristics of the index lesion may not adequately capture a patient’s risk of future ischemic events, particularly in non-stented vessels. Next, patients who sustained an ischemic event within the first 12 months after enrollment were not randomized in the DAPT Study, such that some patients with complex anatomy and high potential benefit of thienopyridine continuation were not randomized. Third, although the DAPT study collected some features of coronary lesion complexity using a prespecified definition, not all factors defining complex lesion anatomy could be accounted for.
In a pooled analysis of 6 randomized trials assessing long-term (≥12 months) versus short-term (3 or 6 months) dual antiplatelet therapy in 9577 patients undergoing PCI, Giustino et al.(11) found that at median 392 days, the 1680 patients who underwent complex PCI had a higher risk of major adverse cardiac events (adjusted HR 1.98, 95% CI 1.50–2.60). Additionally, long-term dual antiplatelet therapy (vs. short-term) was associated with reductions in major adverse cardiac events in the complex PCI group (4.1% vs. 6.8%, adjusted HR 0.56, 95% CI 0.35–0.89) compared to the noncomplex group (adjusted HR 1.01, 95% CI 0.75–1.35; Pinteraction=0.01), and increased risk for major bleeding that was similar between groups (Pinteraction=0.96). The study also found that the benefit of long-term dual antiplatelet therapy increased additively with each increase in procedural complexity.
In order to assess whether these differences in study findings were due to different definitions for coronary lesion complexity, we performed a sensitivity analysis employing the definition of complexity used in the Giustino et al. analysis.(11) We continued to observe consistent reductions in ischemic events across levels of coronary lesion complexity. The differences observed between studies may be due primarily to the different time intervals examined after initial PCI. Within the 1st year after PCI, it is conceivable that stent and lesion-related factors have a stronger influence on potential benefit of continued dual antiplatelet therapy, as observed in the prior study. In contrast, some patients at highest lesion risk were likely not randomized in the DAPT Study due to the occurrence of early events that were exclusionary, consistent with the high rates of ischemic events we observed within the first year for enrolled patients with complex anatomy. A unifying conclusion from these studies may be that while lesion-related factors may be important for determining which patients might extend therapy past a minimum period of 3 or 6 months, for those patients reaching 1 year without an ischemic event, patient factors, rather than lesion factors, may become more important in determining expected benefit of thienopyridine continuation.
The 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease(12) recommended a minimum duration of 6 months of dual antiplatelet therapy after DES placement, but emphasized the need to individualize therapy on the basis of ischemic and bleeding risk. The DAPT Score is a decision tool that stratifies patients on the basis of expected benefit versus harm from extending dual antiplatelet treatment.(10,13) In the creation of this score, a large number of candidate variables were considered, including factors related to anatomical complexity: minimum stent diameter, number of stents and total stent length, presence of severe coronary calcification, bifurcation stenting, complex lesion class, and unprotected left main stenting, among others. These factors, however, were ultimately found not to be predictive of events in the 12–30 month period and were thus not retained. Among randomized patients with anatomically complex coronary lesions in this analysis, those with DAPT scores ≥ 2 had significantly greater reductions in MI/stent thrombosis and MACCE as compared with those with DAPT scores < 2, and similar (and numerically smaller) increases in bleeding. These results suggest that among patients similar to those randomized in the DAPT Study, this score may be a useful decision aid, even among the subgroup of patients with known high-risk anatomical characteristics.
Various limitations of this study are worth noting. The DAPT study population included only patients who had taken 12 months of dual antiplatelet therapy without experiencing a major bleeding or ischemic event, and therefore findings may not be applicable to patients who have not reached that time point. Coronary anatomy lesion complexity characteristics were site-reported and not reviewed by an angiographic core laboratory, and may not have included all angiographic markers of lesion complexity or risk. Finally, individual lesion complexity factors may modify the treatment effects of prolonged dual antiplatelet therapy to different degrees. However, the study was not sufficiently powered to understand this type of heterogeneity in very small subgroups.
CONCLUSIONS
In conclusion, complex target-lesion anatomy is associated with increased ischemic events, particularly within the first year after PCI, but less strongly influences ischemic events thereafter. Among those without events in the first 12 months, the benefits of extending dual antiplatelet therapy were similar in subjects with and without complex lesions. High DAPT score identified those experiencing the most benefit from extended treatment among patients with and without complex anatomy.
Supplementary Material
PERSPECTIVES.
Competency in Patient Care:
Patients undergoing percutaneous coronary intervention (PCI) of complex target lesions are at elevated risk of subsequent ischemic events particularly within the first year. However, after 1 year, this risk diminishes such that the benefits of prolonged dual antiplatelet therapy are similar irrespective of index lesion complexity. The DAPT score remains a useful tool for helping determine the benefits and risks of continuation of thienopyridine therapy beyond 1 year for patients with and without complex lesions at index PCI.
Translational Outlook:
Additional investigation is needed to understand the influence of complex target lesion anatomy for populations not included or less well represented in the DAPT Study, including patients on chronic oral anticoagulation or those undergoing highly complex PCI (e.g. complex chronic total occlusion intervention).
Acknowledgments
Sponsored by the Baim Institute for Clinical Research. Funded by Abbott, Boston Scientific Corporation, Cordis Corporation, Medtronic, Inc., Bristol-Myers Squibb Company/Sanofi Pharmaceuticals Partnership, Eli Lilly and Company, and Daiichi Sankyo Company Limited and the US Department of Health and Human Services (1RO1FD003870-01).
Disclosures:
Dr. Yeh reports research funding and consulting income from Boston Scientific, and consulting for Abbott Vascular.
Dr. Kereiakes reports consultancy for and research funding from Boston Scientific Corporation and Abbott Vascular.
Dr. Steg discloses the following relationships: research grant from Merck, Sanofi, and Servier; speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers-Squibb, Daiichi-Sankyo, Janssen, Lilly, Merck Novartis, Pfizer, Regeneron, Sanofi, Servier.
Dr. Cutlip reports contracted research support from Medtronic and Boston Scientific.
Dr. Croce reports no conflicts.
Dr. Massaro reports no conflicts.
Dr. Mauri reports grant funding from Amgen, Abbott, Boston Scientific, Boehringer Ingelheim, Biotronik, Corvia, and Recor and consultancy for Amgen, Boehringer Ingelheim, Corvia, and Recor
Abbreviations:
- ARC
Academic Research Consortium
- BARC
Bleeding Academic Research Consortium
- BMS
Bare metal stent
- DAPT Study
Dual Antiplatelet Therapy Study
- DES
Drug-eluting stent
- FDA
Food and Drug Administration
- GUSTO
Global Utilization of Streptokinase and TPA for Occluded Arteries
- MACCE
Major adverse cardiovascular and cerebrovascular events
- MI
Myocardial Infarction
- PCI
Percutaneous Coronary Intervention
Footnotes
ClinicalTrials.gov number NCT00977938
REFERENCES:
- 1.Mauri L, Kereiakes DJ, Yeh RW et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. The New England journal of medicine 2014;371:2155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mauri L, Kereiakes DJ, Normand SL et al. Rationale and design of the dual antiplatelet therapy study, a prospective, multicenter, randomized, double-blind trial to assess the effectiveness and safety of 12 versus 30 months of dual antiplatelet therapy in subjects undergoing percutaneous coronary intervention with either drug-eluting stent or bare metal stent placement for the treatment of coronary artery lesions. Am Heart J 2010;160:1035–41, 1041 e1. [DOI] [PubMed] [Google Scholar]
- 3.Kereiakes DJ, Yeh RW, Massaro JM et al. Comparison of ischemic events after drug-eluting stents or bare metal stents in subjects receiving dual antiplatelet therapy: results from the randomized Dual Antiplatelet Therapy Study. Circulation 2014;130. [Google Scholar]
- 4.Kereiakes DJ, Yeh RW, Massaro JM et al. Antiplatelet therapy duration following bare metal or drug-eluting coronary stents: the dual antiplatelet therapy randomized clinical trial. Jama 2015;313:1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meredith IT, Tanguay JF, Kereiakes DJ et al. Diabetes Mellitus and Prevention of Late Myocardial Infarction After Coronary Stenting in the Randomized Dual Antiplatelet Therapy Study. Circulation 2016;133:1772–82. [DOI] [PubMed] [Google Scholar]
- 6.Hermiller JB, Krucoff MW, Kereiakes DJ et al. Benefits and Risks of Extended Dual Antiplatelet Therapy After Everolimus-Eluting Stents. JACC Cardiovascular interventions 2016;9:138–47. [DOI] [PubMed] [Google Scholar]
- 7.Mehran R, Rao SV, Bhatt DL et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011;123:2736–47. [DOI] [PubMed] [Google Scholar]
- 8.An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The GUSTO investigators. The New England journal of medicine 1993;329:673–82. [DOI] [PubMed] [Google Scholar]
- 9.Cutlip DE, Windecker S, Mehran R et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–51. [DOI] [PubMed] [Google Scholar]
- 10.Yeh RW, Secemsky EA, Kereiakes DJ et al. Development and Validation of a Prediction Rule for Benefit and Harm of Dual Antiplatelet Therapy Beyond 1 Year After Percutaneous Coronary Intervention. Jama 2016;315:1735–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giustino G, Chieffo A, Palmerini T et al. Efficacy and Safety of Dual Antiplatelet Therapy After Complex PCI. Journal of the American College of Cardiology 2016;68:1851–1864. [DOI] [PubMed] [Google Scholar]
- 12.Levine GN, Bates ER, Bittl JA et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. The Journal of thoracic and cardiovascular surgery 2016;152:1243–1275. [DOI] [PubMed] [Google Scholar]
- 13.Kereiakes DJ, Yeh RW, Massaro JM et al. DAPT Score Utility for Risk Prediction in Patients With or Without Previous Myocardial Infarction. Journal of the American College of Cardiology 2016;67:2492–502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


