Abstract
This 24-mo randomized controlled trial was based on a double-blind parallel design, and it compared the effectiveness of 2 fluoride application protocols in arresting dentine caries in primary teeth. Three-year-old children with active dentine caries were recruited and randomly allocated to 2 treatment groups. Children in group A received a semiannual application of a 25% silver nitrate (AgNO3) solution followed by a commercially available varnish with 5% sodium fluoride (NaF) on the carious tooth surfaces. Children in group B received a semiannual application of a 25% AgNO3 solution followed by another commercially available varnish with 5% NaF containing functionalized tricalcium phosphate (fTCP). Carious tooth surfaces that were hard when probing were classified as arrested. Intention-to-treat analysis and a hierarchical generalized linear model were undertaken. A total of 408 children with 1,831 tooth surfaces with active dentine caries were recruited at baseline, and 356 children (87%) with 1,607 tooth surfaces (88%) were assessed after 24 mo. At the 24-mo evaluation, the mean (SD) number of arrested carious tooth surfaces per child were 1.8 (2.2) and 2.6 (3.3) for group A (without fTCP) and group B (with fTCP), respectively (P = 0.003). The arrest rates at the tooth surface level were 42% for group A and 57% for group B (P < 0.001). Results of the hierarchical generalized linear model indicated that protocol B (with fTCP) had a higher predicted probability (PP = 0.656) in arresting dentine caries than protocol A (without fTCP; PP = 0.500) when the carious lesions were on buccal/lingual surfaces, were on anterior teeth, had dental plaque coverage, and were in children from low-income families (P = 0.046). In conclusion, protocol B, which applied a 25% AgNO3 solution followed by a commercially available 5% NaF varnish with fTCP semiannually, is more effective in arresting dentine caries in primary teeth as compared with protocol A, which applied a 25% AgNO3 solution followed by another commercially available 5% NaF varnish without fTCP semiannually (ClinicalTrials.gov NCT03423797).
Keywords: child, dental care, dental caries, dentin, silver, fluorides
Introduction
Dental caries in the primary dentition is prevalent around the world (El Tantawi et al. 2018). Untreated caries in the primary dentition was ranked as the 10th-most prevalent oral disease in 2015 (Kassebaum et al. 2017). Teeth with dentine caries involving the dental pulp cause discomfort and dental pain, and they can possibly lead to difficulty with sleeping and eating. Poor dentition may also affect children’s school performance, growth, and even their quality of life (Neves et al. 2016). However, conventional restorative treatment is insufficient for managing this oral epidemic disease because it is often inaccessible and unaffordable. In addition, many young children cannot cope with the lengthy and complicated treatment procedures. Thus, a simple, low-cost, and minimally invasive approach that includes the use of topical fluoride and antimicrobial agents is introduced for caries control (Chen et al. 2018a).
Topical fluoride agents were shown to be effective in slowing down or halting the progression of early childhood caries (Gao, Zhang, et al. 2016). A recent systematic review concluded that sodium fluoride (NaF) varnish had a significant caries-inhibiting effect on noncavitated buccal and lingual carious lesions (Urquhart et al. 2019). However, NaF varnish alone was ineffective in arresting cavitated dentine caries (Chu et al. 2002). A silver nitrate (AgNO3) solution, which has strong antibacterial properties, was introduced as an adjunct to the fluoride varnish application for arresting cavitated dentine caries (Gao et al. 2018). A laboratory study indicated that the application of NaF varnish and AgNO3 solution was effective in arresting dentine caries (Zhao et al. 2017). A subsequent randomized clinical trial corroborated the use of AgNO3 and NaF varnish as an alternative for managing early childhood caries (Gao et al. 2019).
Functionalized tricalcium phosphate (fTCP), which works with fluoride synergistically, has recently been incorporated into NaF varnish to promote the remineralization process (Karlinsey 2016). The fTCP is designed to prevent premature fluoride-calcium interactions and to enhance fluoride-based nucleation activity (Karlinsey et al. 2010; Karlinsey and Pfarrer 2012). A laboratory study showed that fluoride varnish with fTCP enhanced remineralization and increased resistance of teeth against the acid challenge (Elkassas and Arafa 2014). Another study reported that the application of NaF varnish containing fTCP in adjunct with AgNO3 reduced dentine damage by cariogenic biofilm (Yu et al. 2018). Although these laboratory studies revealed promising results, no published clinical trial warrants the caries-arresting effectiveness of NaF varnish with fTCP in complement with AgNO3 solution.
The objective of this 24-mo randomized clinical trial was to compare the effectiveness of a 25% AgNO3 solution followed by a 5% NaF varnish with fTCP, with that of a 25% AgNO3 solution followed by a 5% NaF varnish, in arresting dentine caries in preschool children when applied semiannually. The null hypothesis was that among preschool children, there would be no difference in the caries-arresting effectiveness in the semiannual application of a 25% AgNO3 solution followed by a 5% NaF varnish with and without fTCP.
Methods
This randomized controlled trial was based on a double-blind 2-arm parallel design, and it received approval from the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW17-176). An information sheet and consent form was distributed, and approval from parents or legal guardians was obtained. Children’s individual data were not contained in this article, and publication consent is not applicable. The study protocol was published (Chen et al. 2018b) and registered in ClinicalTrials.gov (NCT03423797). This study was reported following the 2010 CONSORT statement (Consolidated Standards of Reporting Trials; Schulz et al. 2010).
Sample Size Calculation
Following the anticipated caries-arresting rate of 70% in group A (Gao et al. 2019) and with a 10% absolute difference as a clinical significance, at least 820 active carious tooth surfaces (410 in each treatment group) were needed with a power set at 90% (β = 0.1) and a level of statistical significance at 5% (α = 0.05) for a 2-tailed test (calculated by G*Power; Franz Faul). According to our previous study, the mean number of active carious tooth surfaces among 3-y-old children was 3, and the intraclass correlation coefficient at the tooth surface level within the individual was 0.13 (Fung et al. 2016). Thus, the design effect was calculated to be 1.26. With the estimated dropout rate of 15%, at least 408 children (204 in each group) needed to be recruited at baseline.
Recruitment, Randomization, and Allocation
This study was conducted in Hong Kong, where the drinking water was fluoridated (0.5 ppm). The inclusion criteria were that the kindergarten children 1) were 3 y old and had written parental consent, 2) were generally healthy, and 3) had at least 1 tooth surface with active dentine caries. Children who were uncooperative during the dental examination process, had major systemic diseases, and/or were taking long-term medication were excluded. This study adopted a stratified block randomization method (block size of 6). After the oral examination, eligible children were categorized into 2 strata according to the number of decayed tooth surfaces that they had (1 to 3 surfaces and >3 surfaces). An independent researcher generated the allocation sequences of 2 strata. Allocation numbers were sealed in a pile of opaque envelopes, and a field assistant implemented the treatment allocation.
Oral Examination and Treatment
A dentist (K.J.C) was trained and calibrated with an expert (C.H.C) before and during the study in the field, and they maintained excellent interexaminer reliability. The same calibrated dentist performed oral examinations at baseline and follow-ups without the adoption of a radiographic examination. A visual-tactile assessment was adopted to assess the status of carious tooth surfaces, by using a World Health Organization CPI probe (405/WHO probe; Otto Leibinger) and a disposable dental mirror with an attached light-emitting diode (MirrorLite; Kudos Crown Limited). A tooth surface was diagnosed as active if any softened area was detected on the recruited lesion via gentle probing, or it was recorded as arrested if the entire recruited lesion was hard upon probing (Chu et al. 2002; Ekstrand et al. 2009). If a carious lesion involved >1 tooth surface and affected more than one-third of the extended surface, each affected tooth surface was recorded individually. All tooth surfaces with active dentine caries were included at baseline. Nonvital teeth with signs of discoloration, hypermobility, and dental abscess were excluded. Dental caries experience (decayed, missing, and filled teeth/surfaces [dmft/dmfs] score) and oral hygiene status (visible plaque index) were recorded. Approximately 10% of the children were randomly selected to assess the intraexaminer agreement at baseline and follow-ups.
The decayed tooth was isolated with cotton rolls. Food debris and plaque were removed with a probe and cotton bud before examination and treatment. The tooth was not dried before intervention. An independent operator provided treatment on carious tooth surfaces. The examiner, the study children, and their caregivers were blinded to the treatment group. Each recruited participant received one of the following interventions:
Group A: application of a 25% AgNO3 solution (Gordon Labs) followed by a 5% NaF varnish (Nupro White Varnish; Dentsply Sirona) semiannually
Group B: application of a 25% AgNO3 solution (Gordon Labs) followed by a 5% NaF varnish with fTCP (Clinpro White Varnish; 3M ESPE) semiannually
The operator used a microbrush to apply the AgNO3 to each carious tooth surface for approximately 5 s. Afterward, the surface was left undisturbed for another 5 s. The operator then used another microbrush to apply the assigned varnish for another 5 s before treating the next tooth surface. After the treatment for caries arrest was provided, the assigned varnish (0.25 mL per child in total) was applied to all tooth surfaces with a brush according to the study protocol (Chen et al. 2018b). Children were instructed not to eat or drink for 30 min after the treatment. Study children had the right to receive any treatment from other dentists or to withdraw from the study.
Questionnaires
At baseline, a self-reported parental questionnaire was distributed to collect the information regarding the children’s sociodemographic backgrounds (sex, age, family monthly income, father’s and mother’s education levels) and oral health–related behaviors (bottle-feeding habit, oral hygiene practice habit, sugary snacking habit). At the 24-mo follow-up, information on the children’s oral health–related behaviors was collected by phone calls.
Statistical Analysis
Data were analyzed with SPSS 25.0 (IBM) and SAS OnDemand for Academics (SAS Institute). This study employed intention-to-treat analysis (Gupta 2011). The recruited carious tooth surfaces were recorded as treatment failures (active) if the decay was restored, if the tooth was lost, or if the child dropped out of the study. The primary outcome was the statuses of the carious tooth surfaces (arrested or active). The intraexaminer agreement was assessed by Cohen’s κ statistic. Univariate analysis, including the χ2 test and t test, was performed to compare the differences between groups regarding the baseline information, dropout rates, number of arrested carious tooth surfaces, and caries arrest rate, as well as to investigate the independent variables’ effects on caries arrest.
This study adopted a 2-level hierarchical generalized linear model to address the correlation effect, as multiple carious tooth surfaces would be recruited in a child, and to analyze the occurrence of individual outcomes by the intercept (Burnside et al. 2007; O’Connell et al. 2008). The random intercept was used with logit link: level 1, within-subject level (tooth surface); level 2, subject level (child; O’Connell et al. 2008). Independent variables with a P value <0.10 from the univariate analysis were studied as potential risk factors in the models and categorized into 2 levels: within-subject level and subject level. Three models were built to obtain the best-fit model: model 0, an unconditional model without any risk factor; model 1, with within-subject-level risk factors; and model 2, with both within-subject-level and subject-level risk factors. A model with the smallest −2 log likelihood value was chosen as the best-fit model and is presented in the results.
Results
Baseline
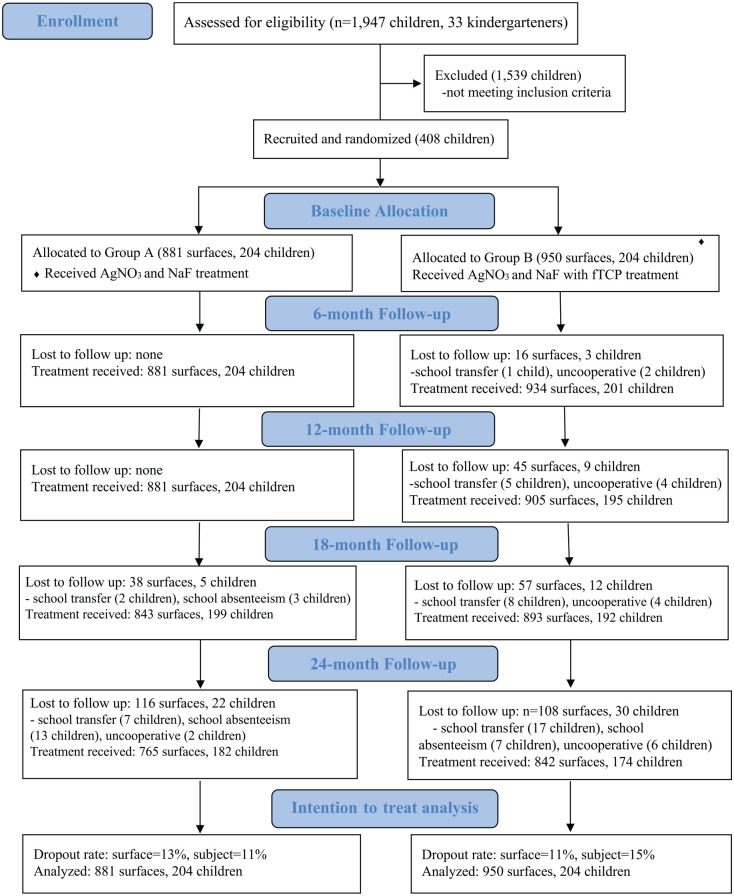
The Figure is a flow diagram of the recruitment and follow-up. A total of 408 3-y-old children (198 boys, 49%) were recruited, with 204 placed in each treatment group. The mean (SD) number of active carious tooth surfaces per child was 4.3 (3.9) in group A (without fTCP) and 4.7 (4.6) in group B (with fTCP; t test, P = 0.420). Group A had 881 tooth surfaces with active caries, and group B had 950. All parents of the participants returned their questionnaires. No significant differences were found between the groups regarding children’s sociodemographic backgrounds, oral health–related behaviors, and oral characteristics (χ2 test and t test, P > 0.05; Table 1).
Figure.
CONSORT 2010 flow diagram. AgNO3, silver nitrate; fTCP, functionalized tricalcium phosphate; NaF, sodium fluoride.
Table 1.
Children’s Sociodemographic Background, Oral Health–Related Behaviors, and Oral Characteristics at Baseline (N = 408).
| Individual Variables | Group A (n = 204) | Group B (n = 204) | P Value |
|---|---|---|---|
| Sociodemographic background | |||
| Sex | |||
| Male | 100 (49) | 98 (48) | 0.843 |
| Female | 104 (51) | 106 (52) | |
| Father’s educational level | 0.915 | ||
| Compulsory education | 64 (31) | 65 (32) | |
| Post–compulsory education | 140 (69) | 139 (68) | |
| Mother’s educational level | 0.329 | ||
| Compulsory education | 56 (28) | 65 (32) | |
| Post–compulsory education | 148 (72) | 139 (68) | |
| Family monthly income, HKD | 0.457 | ||
| Low: ≤20,000 | 100 (49) | 111 (54) | |
| Middle: 20,001 to 40,000 | 80 (39) | 68 (33) | |
| High: >40,000 | 24 (12) | 25 (12) | |
| Oral health-related behaviors | |||
| Bottle feeding at night | 0.545 | ||
| Yes | 85 (42) | 79 (39) | |
| No | 119 (58) | 125 (61) | |
| Sugary snacking habit | 0.426 | ||
| Less than twice daily | 109 (53) | 117 (57) | |
| At least twice daily | 95 (47) | 87 (43) | |
| Toothbrushing habit | 0.842 | ||
| Less than twice daily | 114 (56) | 112 (55) | |
| At least twice daily | 90 (44) | 92 (45) | |
| Use of toothpaste | 0.892 | ||
| Yes | 171 (84) | 172 (84) | |
| No | 33 (16) | 32 (16) | |
| Oral characteristics | |||
| Scorea | |||
| dmft | 3.4 (2.6) | 3.5 (2.7) | 0.764 |
| dmfs | 4.8 (4.8) | 4.8 (5.0) | 0.911 |
| VPI | 0.59 (0.19) | 0.58 (0.20) | 0.432 |
| Tooth locationb | 0.508 | ||
| Anterior | 638 (72) | 701 (74) | |
| Posterior | 243 (28) | 249 (26) | |
| Tooth surfaceb | 0.072 | ||
| Buccal/lingual | 249 (28) | 312 (33) | |
| Proximal | 478 (54) | 469 (49) | |
| Occlusal | 154 (18) | 169 (18) | |
Values are presented as n (%), and P values are based on a χ2 test, unless noted otherwise. VPI, visible plaque index.
Mean (SD), t test.
Group A, 881 surfaces; group B, 950 surfaces.
At the 24-mo Follow-up
A total of 356 children with 1,607 tooth surfaces remained in the study at the 24-mo follow-up. The participant dropout rate was 12.7%, and the tooth surface dropout rate was 12.2%. The primary reason for the loss of follow-up was school transfer (46.1%). No significant difference between groups was found for the participant dropout rate (χ2 test, P = 0.235) and surface dropout rate (χ2 test, P = 0.241). Upon the 24-mo examination, the children had received planned treatments 4 times (baseline and 6-, 12-, and 18-mo follow-up). The intraexaminer reliability of caries arrest assessments at baseline and 6-, 12-, 18-, and 24-mo follow-up was 1, 0.97, 0.88, 0.97, and 0.86, respectively (Cohen’s κ statistics).
At the 24-mo evaluation, the mean (SD) number of arrested carious tooth surfaces per child in group A (without fTCP) was 1.8 (2.2), whereas the number in group B (with fTCP) was 2.6 (3.3). Group B had a significantly higher mean number of arrested carious tooth surfaces per child than group A at the 12-, 18-, and 24-mo follow-up (t test, P = 0.049, 0.008, and 0.003, respectively; Table 2). At the tooth surface level, the caries arrest rate was 42.1% for group A (without fTCP) and 56.7% for group B (with fTCP) at the 24-mo examination. Group B also presented a significantly higher caries arrest rate than group A at 12-, 18-, and 24-mo follow-up (χ2 test, P = 0.001, P < 0.001, and P < 0.001). The proportions of arrested carious tooth surfaces in different tooth locations and tooth surface types according to the treatment groups are shown in Table 2. Group B generally had significantly higher caries arrest rates than group A in different tooth locations and tooth surface types (χ2 test, P < 0.05).
Table 2.
Number of Arrested Carious Surfaces and Caries Arrest Rates (Surface Level) at 6-, 12-, 18-, and 24-mo Examinations According to the Study Groups.
| Group A | Group B | P Value | |
|---|---|---|---|
| No. of arrested carious surfacesa | |||
| Baseline: active | 4.3 | 4.7 | |
| 6 mo | 1.1 (1.8) | 1.1 (1.6) | 0.774 |
| 12 mo | 1.7 (2.2) | 2.2 (2.6) | 0.049b |
| 18 mo | 1.8 (2.3) | 2.5 (3.0) | 0.008b |
| 24 mo | 1.8 (2.2) | 2.6 (3.3) | 0.003b |
| Caries arrest rates for | |||
| All tooth surfaces | |||
| Baseline, n | 881 | 950 | |
| 6 mo | 23.2 | 22.7 | 0.831 |
| 12 mo | 38.8 | 46.2 | 0.001b |
| 18 mo | 41.4 | 53.5 | <0.001b |
| 24 mo | 42.1 | 56.7 | <0.001b |
| Surfaces on anterior teeth | |||
| Baseline, n | 638 | 701 | |
| 6 mo | 24.8 | 24.0 | 0.734 |
| 12 mo | 44.2 | 49.8 | 0.041b |
| 18 mo | 48.4 | 60.5 | <0.001b |
| 24 mo | 49.1 | 63.5 | <0.001b |
| Surfaces on posterior teeth | |||
| Baseline, n | 243 | 249 | |
| 6 mo | 18.9 | 19.3 | 0.922 |
| 12 mo | 24.7 | 36.1 | 0.006b |
| 18 mo | 23.0 | 33.7 | 0.009b |
| 24 mo | 23.9 | 37.8 | 0.001b |
| Buccal/lingual surfaces | |||
| Baseline, n | 249 | 312 | |
| 6 mo | 34.1 | 30.4 | 0.353 |
| 12 mo | 53.8 | 58.0 | 0.320 |
| 18 mo | 50.2 | 62.5 | 0.003b |
| 24 mo | 48.6 | 68.3 | <0.001b |
| Proximal surfaces | |||
| Baseline, n | 478 | 469 | |
| 6 mo | 19.5 | 19.0 | 0.851 |
| 12 mo | 36.4 | 44.6 | 0.011b |
| 18 mo | 43.5 | 56.9 | <0.001b |
| 24 mo | 45.8 | 58.4 | <0.001b |
| Occlusal surfaces | |||
| Baseline, n | 154 | 169 | |
| 6 mo | 16.9 | 18.9 | 0.631 |
| 12 mo | 22.1 | 29.0 | 0.155 |
| 18 mo | 20.8 | 27.2 | 0.177 |
| 24 mo | 20.1 | 30.8 | 0.029b |
Values are presented as percentages, and P values are based on a χ2 test, unless noted otherwise.
Mean (SD), t test.
Statistically significant difference between groups.
Eleven potential variables, including treatment protocol, that had a P value <0.10 in the univariate analysis were studied as potential risk factors (Table 3). Among them, dental plaque on the carious tooth surface, the tooth location, and the tooth surface type were categorized as within-subject-level risk factors. Treatment protocol, mother’s education level, family monthly income, dmft and dmfs scores at baseline, visible plaque index score at 24 mo, and oral health–related behaviors at 24 mo, including toothbrushing frequency and bottle-feeding habits, were subject-level risk factors. The results of the unconditional model showed significant variance in caries arrest among participants (P < 0.001), whereas the variance in teeth or kindergartens was not significant (P = 0.613 and P = 0.907, respectively). Thus, this study adopted a 2-level model. Model 2 with the smallest −2 log likelihood value was presented as a final model (Table 4).
Table 3.
Independent Variables for Caries Arrest Rate at 24-mo Follow-up: Univariate Analysis (N = 1,831).
| Arrested Surface (n = 910) | Active Surface (n = 921) | P Value | |
|---|---|---|---|
| Sociodemographic background | |||
| Sex | 0.936 | ||
| Male | 473 (52) | 477 (52) | |
| Female | 437 (48) | 444 (48) | |
| Father’s educational level | 0.626 | ||
| Compulsory education | 340 (37) | 334 (36) | |
| Post–compulsory education | 570 (63) | 587 (64) | |
| Mother’s educational level | 0.002a | ||
| Compulsory education | 319 (35) | 261 (28) | |
| Post–compulsory education | 591 (65) | 660 (72) | |
| Family monthly income, HKD | <0.001a | ||
| Low: ≤20,000 | 558 (65) | 440 (48) | |
| Middle: 20,001 to 40,000 | 255 (28) | 415 (45) | |
| High: >40,000 | 67 (7) | 66 (7) | |
| Oral health–related behaviors at 24 mo | |||
| Bottle feeding at night | |||
| Yes | 128 (14) | 210 (23) | <0.001a |
| No | 782 (86) | 711 (77) | |
| Sugary snacking habit | 0.509 | ||
| Less than twice daily | 345 (38) | 363 (39) | |
| At least twice daily | 565 (62) | 558 (61) | |
| Toothbrushing habit | 0.038a | ||
| Less than twice daily | 205 (23) | 246 (27) | |
| At least twice daily | 705 (77) | 675 (73) | |
| Use of toothpaste | 0.121 | ||
| Yes | 891 (98) | 891 (97) | |
| No | 19 (2) | 30 (3) | |
| Oral characteristics | |||
| Dental plaque on carious surface | <0.001a | ||
| Yes | 766 (84) | 915 (99) | |
| No | 144 (15) | 6 (1) | |
| Scoreb | |||
| VPI at 24 mo | 0.65 (0.15) | 0.67 (0.17) | 0.001a |
| dmft at baseline | 5.5 (3.3) | 6.0 (3.6) | 0.004a |
| dmfs at baseline | 8.6 (7.2) | 9.6 (7.8) | 0.004a |
| Tooth location | <0.001a | ||
| Anterior | 758 (83) | 581 (63) | |
| Posterior | 152 (17) | 340 (37) | |
| Tooth surface type | <0.001a | ||
| Buccal/palatal | 334 (37) | 227 (25) | |
| Proximal | 493 (54) | 454 (49) | |
| Occlusal | 83 (9) | 240 (26) | |
Values are presented as n (%), and P values are based on a χ2 test, unless noted otherwise. VPI, visible plaque index.
Statistically significant difference between groups.
Mean (SD), t test.
Table 4.
Hierarchical Generalized Linear Model (Random Intercept Only) for the Association Between Caries Arrest Rate and Risk Factors (N = 1,831).
| Estimates | Predicted Probabilitya,b | Odds Ratioa (95% CI) | ||||
|---|---|---|---|---|---|---|
| Model 0 | Model 1 | Model 2a | P Value | |||
| Intercept | 0.030 (0.152) | −0.254 (0.318)c | −0.002 (0.384)c | 0.500 | ||
| Within-subject level | ||||||
| Tooth location | ||||||
| Anterior | 0.958 (0.275)c | 0.966 (0.274)c | 0.724 | 2.63 (1.54 to 4.49)c | <0.001c | |
| Posteriord | 0.500 | |||||
| Tooth surface type | ||||||
| Proximal | −0.650 (0.187)c | −0.639 (0.186)c | 0.345 | 0.53 (0.37 to 0.76)c | <0.001c | |
| Occlusal | −1.970 (0.353)c | −1.950 (0.351)c | 0.124 | 0.14 (0.07 to 0.28)c | <0.001c | |
| Buccal/linguald | 0.500 | |||||
| Dental plaque on carious surface | ||||||
| No | 4.759 (0.590)c | 4.692 (0.588)c | 0.991 | 109.11 (34.44 to 345.66)c | <0.001c | |
| Yesd | 0.500 | |||||
| Subject level | ||||||
| Treatment protocol | ||||||
| Group B (with fTCP) | 0.646 (0.323)c | 0.656 | 1.91 (1.01 to 3.60)c | 0.046c | ||
| Group A (without fTCP)d | 0.500 | |||||
| Family monthly income, HKD | ||||||
| High: >40,000 | −0.809 (0.539) | 0.308 | 0.45 (0.15 to 1.28) | 0.133 | ||
| Middle: 20,001 to 40,000 | −1.393 (0.351)c | 0.199 | 0.25 (0.13 to 0.49)c | <0.001c | ||
| Low: ≤20,000d | 0.500 | |||||
| Error variance: between subjects | 5.717 (0.957)c | 6.719 (1.147)c | 6.169 (1.078)c | |||
| Model fit: −2 log likelihood, n | 2,078 | 1,793 | 1,772 | |||
Model 0, unconditional model; model 1, adjust with within-subject-level risk factors; model 2, adjust with both within-subject- and subject-level risk factors. fTCP, functionalized tricalcium phosphate.
Best-fitting model.
Predicted probability of arrested caries evaluated with other variables set at the reference category.
P < .05. Intraclass correlation coefficient: 0.63. Values based on SAS PROC GLIMMIX. Estimation method: Laplace.
Reference group.
The final model found 5 risk factors that have an effect on caries arrest. The active carious tooth surfaces treated in group B (with fTCP) had a slightly higher chance of being arrested than those treated in group A (without fTCP; odds ratio, 1.91; 95% CI, 1.01 to 3.60; P = 0.046). Lesions without dental plaque coverage, on buccal/lingual surfaces, and on anterior teeth had a comparatively higher chance of being arrested (P < 0.001). Children who came from middle-income families had a comparatively lower chance of having their caries arrested (P < 0.001). When other risk factors were held constant, the predicted probabilities of caries arrest for group A and group B were 0.656 and 0.500, respectively (P = 0.046). The mean (SD) number of recruited teeth that became nonvital per child at the 24-mo evaluation were 0.35 (0.87) in group A and 0.25 (0.80) in group B (t test, P = 0.238). This study also found no significant adverse effects, including nausea, vomiting, hospitalization, and systemic illnesses.
Discussion
The present study is the first published clinical trial on NaF with fTCP. It provides scientific evidence of the potential benefit of complementing AgNO3 with the use of NaF with fTCP on caries arrest in young children. Our clinical results are consistent with previous laboratory results (Yu et al. 2018). Still, caution should be exercised for the recommendation of NaF with fTCP for caries arrest. Although both NaF varnishes contained 22,600 ppm fluoride, their other ingredients may not be the same, thus possibly affecting the outcome. In addition, the number of tooth surface types among the 2 groups were not distributed equally. In this study, the random allocation was based on the subject level, yielding a higher number of buccal surfaces in group B than in group A. The treatment effect remained statistically significant after the tooth surface type was adjusted in the final model. Clinicians may have divided views regarding its clinical significance, although a statistically significant difference exists in the predicted probability between groups.
This parallel-design randomized controlled trial directly compared the effectiveness of the 2 treatments. This study design can make causal inferences, provide evidence of a treatment’s efficacy, and minimize bias. The strengths of this study include a relatively large sample size, a high retention rate, and good intraexaminer reliability. We adopted several strategies for reducing possible bias, including randomization, allocation concealment, double-blinding, and intention-to-treat analysis, which is recommended in the CONSORT guidelines for reporting on randomized clinical trials (Moher et al. 2001). Intention to treat allows the analysis to include all children who were randomized into the treatment groups for data analysis, as it ignores noncompliance and dropouts after randomization (Gupta 2011). In this study, all extracted and restored teeth were regarded as treatment failures to maintain a conservative result. This study used the visual-tactile assessment reported in previous clinical trials (Chu et al. 2002; Fung et al. 2018; Gao et al. 2019). The same assessment criteria enabled comparisons of the caries-arresting results among these studies. We applied individually packed varnish so that all children received the same amount of fluoride (Chen et al. 2018b). The 0.25-mL varnish was adequate to cover all tooth surfaces. In addition, we monitored the caries activity at 6-mo intervals to avoid interrupting classes and school activities, but changes in caries activities within the periods that were <6 mo could not be detected.
Except for treatment protocol, 4 other risk factors influenced caries arrest, including dental plaque, tooth location, tooth surface type, and family monthly income. Dental plaque contains cariogenic bacteria, which produce acid that promotes demineralization (Hick et al. 2004). With this, dental plaque tends to accumulate in hard-to-clean areas, such as pits and fissures on occlusal surfaces, and tight contact areas on proximal surfaces. This makes the occlusal surfaces of the posterior teeth and proximal surfaces vulnerable sites for dental caries (König 1963; Ferro et al. 2009). Correspondingly, the results of the present study support the use of NaF with fTCP for the control of dentine caries on the buccal/lingual surfaces of the anterior teeth. In addition, this study found that socioeconomic status was related to caries arrest. Moreover, the application time per child varied per the number of decayed teeth. However, we found no correlation effect between caries experience and caries arrest. When contemplating which caries arrest treatment should be adopted, one should bear in mind that the treatment success depends on several related factors, such as the location of the decayed lesions, plaque accumulation on lesions, and socioeconomic factors.
In this study, we did not request the participating children to adopt any specific oral care practices. This study design could better generalize the research findings to the general population. However, it should be noted that children in this study lived in an area in which drinking water was fluoridated at 0.5 ppm. Caution should be taken when interpreting these findings for children who reside in nonfluoridated areas. Although the carious lesions were discolored after AgNO3 was applied (i.e., became black or dark brown), we successfully recruited the children within the timeline of the study period, and the study could maintain a satisfactory retention rate over 24 mo. Thus, the aesthetic concern is unlikely to be a significant barrier for this caries arrest treatment in our study population. Nevertheless, further research in other places with differences in cultures and parenting practices is required to warrant or refute the use of a medical model in caries management in young children. Apart from the combined use of AgNO3 solution and fluoride varnish, silver diamine fluoride (SDF) can be used to arrest caries. SDF use is effective and simple; thus, it should be considered in managing caries in children (Gao, Zhao, et al. 2016). However, SDF is not available in some countries, whereas NaF with fTCP and AgNO3 are generally available. Therefore, the caries arrest protocol in this study can be adopted as an alternative to arrest active dentine caries in young children. The results of the present study provide clinical evidence regarding the effectiveness of adopting a medical approach to caries management in young children.
Conclusion
The topical application of a 25% AgNO3 solution followed by a commercially available 5% NaF varnish with fTCP is more effective in arresting dentine caries in primary teeth as compared with the application of a 25% AgNO3 solution followed by another commercially available 5% NaF varnish without fTCP. The location of carious lesions, family income, and dental plaque on lesions also influence caries arrest.
Author Contributions
K.J. Chen, contributed to design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; S.S. Gao, contributed to design, data acquisition, analysis, and interpretation, critically revised the manuscript; D. Duangthip, C.H. Chu, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; E.C.M. Lo, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank Samantha K.Y. Li for her statistical advice. They also thank the kindergarten teachers, children, and parents for their support in this study.
Footnotes
This research received support from the Seed Fund for Basic Research (201810159024).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iDs: E.C.M. Lo  https://orcid.org/0000-0002-3618-0619
https://orcid.org/0000-0002-3618-0619
Research Data: The data set used and/or analyzed during the current study is available from the corresponding author upon reasonable request.
References
- Burnside G, Pine C, Williamson PR. 2007. The application of multilevel modelling to dental caries data. Stat Med. 26(22):4139–4149. [DOI] [PubMed] [Google Scholar]
- Chen KJ, Gao SS, Duangthip D, Lo ECM, Chu CH. 2018. a. Managing early childhood caries for young children in China. Healthcare. 6(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KJ, Gao SS, Duangthip D, Lo ECM, Chu CH. 2018. b. The caries-arresting effect of incorporating functionalized tricalcium phosphate into fluoride varnish applied following application of silver nitrate solution in preschool children: study protocol for a randomized, double-blind clinical trial. Trials. 19(1):352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CH, Lo EC, Lin HC. 2002. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentin caries in Chinese pre-school children. J Dent Res. 81(11):767–770. [DOI] [PubMed] [Google Scholar]
- Ekstrand KR, Zero DT, Martignon S, Pitts NB. 2009. Lesion activity assessment. In: Pitts N, Lussi A, Whitford GM, editors. Detection, assessment, diagnosis and monitoring of caries. Basel (Switzerland): Karger; p. 63–90. [Google Scholar]
- El Tantawi M, Folayan MO, Mehaina M, Vukovic A, Castillo JL, Gaffar BO, Arheiam A, Al-Batayneh OB, Kemoli AM, Schroth RJ, et al. 2018. Prevalence and data availability of early childhood caries in 193 United Nations countries, 2007–2017. Am J Public Health. 108(8):1066–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkassas D, Arafa A. 2014. Remineralizing efficacy of different calcium-phosphate and fluoride based delivery vehicles on artificial caries like enamel lesions. J Dent. 42(4):466–474. [DOI] [PubMed] [Google Scholar]
- Ferro R, Besostri A, Olivieri A. 2009. Caries prevalence and tooth surface distribution in a group of 5-year-old Italian children. Eur Arch Paediatr Dent. 10(1):33–37. [DOI] [PubMed] [Google Scholar]
- Fung MHT, Duangthip D, Wong MCM, Lo ECM, Chu CH. 2016. Arresting dentine caries with different concentration and periodicity of silver diamine fluoride. JDR Clin Trans Res. 1(2):143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung MHT, Duangthip D, Wong MCM, Lo ECM, Chu CH. 2018. Randomized clinical trial of 12% and 38% silver diamine fluoride treatment. J Dent Res. 97(2):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SS, Duangthip D, Wong MCM, Lo ECM, Chu CH. 2019. Randomized trial of silver nitrate with sodium fluoride for caries arrest. JDR Clin Trans Res. 4(2):126–134. [DOI] [PubMed] [Google Scholar]
- Gao SS, Zhang S, Mei ML, Lo ECM, Chu CH. 2016. Caries remineralisation and arresting effect in children by professionally applied fluoride treatment—a systematic review. BMC Oral Health. 16:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SS, Zhao IS, Duffin S, Duangthip D, Lo ECM, Chu CH. 2018. Revitalising silver nitrate for caries management. Int J Environ Res Public Health. 15(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SS, Zhao IS, Hiraishi N, Duangthip D, Mei ML, Lo ECM, Chu CH. 2016. Clinical trials of silver diamine fluoride in arresting caries among children: a systematic review. JDR Clin Trans Res. 1(3):201–210. [DOI] [PubMed] [Google Scholar]
- Gupta SK. 2011. Intention-to-treat concept: a review. Perspect Clin Res. 2(3):109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hick J, Garcia-Godoy F, Flaitz C. 2004. Biological factors in dental caries: role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 1). Int J Clin Pediatr Dent. 28(1):47–52. [DOI] [PubMed] [Google Scholar]
- Karlinsey RL. 2016. Benefits of functionalized tricalcium phosphate. EC Dent Sci. 7:41–42. [Google Scholar]
- Karlinsey RL, Mackey AC, Walker ER, Frederick KE. 2010. Preparation, characterization and in vitro efficacy of an acid-modified beta-TCP material for dental hard-tissue remineralization. Acta Biomater. 6(3):969–978. [DOI] [PubMed] [Google Scholar]
- Karlinsey RL, Pfarrer AM. 2012. Fluoride plus functionalized β-TCP: a promising combination for robust remineralization. Adv Dent Res. 24(2):48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassebaum NJ, Smith AGC, Bernabe E, Fleming TD, Reynolds AE, Vos T, Murray CJL, Marcenes W; GBD 2015 Oral Health Collaborators. 2017. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. J Dent Res. 96(4):380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König KG. 1963. Dental morphology in relation to caries resistance with special reference to fissures as susceptible areas. J Dent Res. 42(1):461–476. [DOI] [PubMed] [Google Scholar]
- Moher D, Schulz KF, Altman D; CONSORT Group. 2001. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. JAMA. 285(15):1987–1991. [DOI] [PubMed] [Google Scholar]
- Neves ÉTB, Firmino RT, de França Perazzo M, Gomes MC, Martins CC, Paiva SM, Granville-Garcia AF. 2016. Absenteeism among preschool children due to oral problems. J Public Health. 24(1):65–72. [Google Scholar]
- O’Connell A, Goldstein J, Rogers H, Peng C. 2008. Multilevel logistic models for dichotomous and ordinal data. Charlotte (NC): Information Age Publishing, Inc. [Google Scholar]
- Schulz KF, Altman DG, Moher D. 2010. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart O, Tampi MP, Pilcher L, Slayton RL, Araujo MWB, Fontana M, Guzman-Armstrong S, Nascimento MM, Novy BB, Tinanoff N, et al. 2019. Nonrestorative treatments for caries: systematic review and network meta-analysis. J Dent Res. 98(1):14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu OY, Zhao IS, Mei ML, Lo ECM, Chu CH. 2018. Effect of silver nitrate and sodium fluoride with tri-calcium phosphate on Streptococcus mutans and demineralised dentine. Int J Mol. 19(5):1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao IS, Mei ML, Li QL, Lo ECM, Chu CH. 2017. Arresting simulated dentine caries with adjunctive application of silver nitrate solution and sodium fluoride varnish: an in vitro study. Int Dent J. 67(4):206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]