Abstract
Objective:
The opioid crisis has increased risks for injection drug use (IDU)–associated HIV outbreaks throughout the United States. Polysubstance use and syringe sharing are common among rural people who inject drugs (PWID). However, little is known about how polysubstance IDU affects engagement in HIV prevention efforts among non-urban PWID. This study assesses the associations between profiles of polysubstance injection, injection-related HIV risk, acquiring syringes from a syringe services program (SSP), HIV testing, and pre-exposure prophylaxis (PrEP) awareness and interest among PWID in rural Appalachia.
Method:
We used survey data from 392 respondents in Cabell County, West Virginia who had injected drugs in the past 6 months. We conducted a latent class analysis using seven measures of IDU and tested for associations with injection-related HIV risk, receiving syringes from an SSP, having been tested for HIV, and PrEP awareness and interest.
Results:
We identified three classes of polysubstance IDU in our sample: polysubstance use, heroin and crystal methamphetamine use, and crystal methamphetamine and buprenorphine/suboxone use. The polysubstance use class had the highest injection-related HIV risk (81.8% at risk), high syringe acquisition at an SSP (67.7%), and highest rate of HIV testing (60.0%). PrEP awareness was low across the sample (30.0%), but most PWID expressed interest in using PrEP (57.7%).
Conclusions:
Patterns of polysubstance IDU have unique relationships with key HIV risk factors and protective behaviors. The expansion of harm reduction services in rural settings is warranted to prevent incident HIV infections.
The opioid crisis has increased risks for HIV outbreaks throughout rural America as rising opioid use has led to more injection drug use (IDU; Jones et al., 2015; Wejnert et al., 2016). The sudden rise in IDU threatens to reverse previous public health gains in curbing incident HIV infections among people who inject drugs (PWID; Wejnert et al., 2016; Zibbell et al., 2015, 2018). In 2015, Scott County, Indiana experienced an opioid injection-related HIV outbreak, resulting in a total of 215 incident HIV infections, contrasting with the community’s typical 5 incident HIV cases annually (Conrad et al., 2015; Gonsalves & Crawford, 2018). In response, researchers identified 220 counties vulnerable to similar outbreaks, primarily in rural Appalachia (Van Handel et al., 2016). Of note, West Virginia bears a disproportionate burden of HIV risk vulnerability, as 28 of its 55 counties were identified as vulnerable to IDU-associated HIV outbreak.
Previous research has identified poverty, a lack of health services, and geographical isolation as barriers to injection-related infectious disease prevention in rural areas (Cloud et al., 2019). Stigmatization of PWID can disincentivize persons from engaging in positive health-seeking behaviors (Biancarelli et al., 2019; Paquette et al., 2018). In addition, in rural communities it may be difficult for PWID to remain anonymous when accessing health services, thus potentially obstructing healthcare and HIV risk reduction services utilization (Townsend, 2009; Warner et al., 2005).
Many rural communities have few harm reduction services, including syringe services programs (SSPs; i.e., needle/syringe exchanges). SSPs are among the most effective HIV prevention strategies for PWID (Des Jarlais et al., 2015; Schranz et al., 2018). SSPs offer a variety of services beyond providing sterile injection equipment, including syringe disposal, HIV/sexually transmitted infection (STI) testing, naloxone provision, referrals to primary care, and linkage to substance use disorder treatment (Centers for Disease Control and Prevention [CDC], 2016a). SSP utilization is associated with reductions in overdose and HIV incidence, as well as reduced drug use through referrals to substance use treatment and reduced needlestick injuries through proper syringe disposal (CDC, 2016b; Hagan et al., 2000; Hoffman, 2017; Kidorf et al., 2011; Lorentz et al., 2000; Strathdee et al., 1999; Tobin et al., 2009; Wodak & Cooney, 2006). SSP implementation is associated with significant cost savings (Ruiz et al., 2016; Wodak & Cooney, 2006). For example, a recent study estimated that SSPs saved the city of Philadelphia in excess of $2.4 billion over 10 years, through the prevention of new HIV infections among PWID (Ruiz et al., 2019). Despite the benefits, many rural communities remain reluctant to implement SSPs because of fears that they will increase drug use, crime, and discarded syringes, outcomes that are not supported by more than 30 years of research (CDC, 2016b; Kim & Harley, 2019). In West Virginia, for example, there are only 18 operational SSPs across the state despite most counties being at high risk for IDU-associated HIV outbreaks (Office of Epidemiology and Prevention Services, 2020b; Van Handel et al., 2016). Despite the strong evidence base supporting SSP implementation, such programs remain controversial in Appalachia. For example, in January 2020, a bill was filed in West Virginia that would have prohibited SSP operations throughout the state (Prohibition of Syringe Exchange Programs, 2020).
HIV testing services are also often lacking in rural settings, with as many as 14% of rural counties having no local testing services available (Sutton et al., 2010). Among counties with testing services, testing sites per county are limited (Mdn = 2), many of which charge fees for testing and do not provide outreach and transportation services (Sutton et al., 2010). In West Virginia, only 5,403 individuals were tested annually for HIV between 2012 and 2016, and an estimated 12.1% of people who are living with HIV in the state are unaware of their status (Hoffman, 2017). Because of limited HIV testing services, rural individuals are often diagnosed with HIV later than their urban counterparts (Ohl & Perencevich, 2011). Early diagnosis is essential for ensuring that persons living with HIV are able to live healthy lives. Persons diagnosed early in their infection have lower mortality, better treatment responses, lower medical costs, and reduced progression to AIDS (Castilla et al., 2002; Girardi et al., 2007; Krentz et al., 2004; Valdiserri et al., 1999). HIV testing is the first step in the process toward early identification and treatment of incident HIV infections, and it is essential that testing be routinely offered and at a variety of venues that are accessible to persons at high risk of infection, such as PWID.
The vulnerability of rural areas to HIV outbreaks requires innovative and multifaceted solutions to avert a new epidemic. Pre-exposure prophylaxis (PrEP) is one under-used tool that can prevent new HIV infections among PWID. PrEP is a medication that can be taken daily to prevent HIV acquisition and has been studied amongst a variety of vulnerable populations (e.g., PWID, men who have sex with men, sex workers; Bekker et al., 2015; Choopanya et al., 2013; Food and Drug Administration, 2014; Grant et al., 2010; Spinner et al., 2016). According to a 2013 randomized placebo-controlled trial among PWID, PrEP was effective in preventing HIV for both male and female PWID (Choopanya et al., 2013). The CDC issued recommendations that persons who may be exposed to HIV (e.g., via shared injection equipment, unprotected sex) consider using PrEP for HIV prevention (CDC, 2018). Recently, the U.S. Preventative Services Task Force (Owens et al., 2019) issued a recommendation that clinicians offer PrEP to individuals at high risk for HIV. Despite the CDC and United States Preventative Services Task Force recommendations, programs designed to increase PrEP use among rural PWID have not been broadly implemented. Given the lack of SSPs and routine HIV testing services in rural settings, PrEP could carry significant HIV prevention utility for PWID in these areas.
Existing research on PrEP interest among PWID is limited to primarily urban environments. In Vancouver, a study found that increased risks for HIV acquisition among PWID was associated with greater willingness to use PrEP (Escudero et al., 2015). Relatedly, a study in New York City found that PWID were less likely to be aware of PrEP than groups with high sexual risks for HIV acquisition (Walters et al., 2017). A recent study of PWID in Baltimore found that PrEP awareness was low (24%) and interest was reasonably high (63%) once PrEP was described to participants (Sherman et al., 2019). This study also found that being eligible for PrEP, based on HIV risk behaviors, was associated with increased interest in PrEP. Overall, PrEP awareness is low among PWID, whereas interest varies. Research is needed to better understand PrEP awareness and interest among rural PWID and how interest may relate to HIV risk behaviors.
Sharing injection equipment with someone of unknown or positive HIV status continues to be a core driver of HIV transmission among PWID (Abelson et al., 2006; Des Jarlais et al., 1988; McCoy et al., 2004; Van Ameijden et al., 1992). Research has shown that polysubstance use, or the use of multiple drugs over a given period, is one factor in sharing injection equipment (Harrell et al., 2012; Morley et al., 2015). Although polysubstance use has been linked to high-risk injection behaviors, little research has connected specific profiles of polysubstance use to HIV risk behaviors. Of the few studies, a common finding was that persons who use more drugs tend to engage in more HIV risk behaviors, including sharing injection equipment and having unprotected sex (Harrell et al., 2012; Roth et al., 2015; Wu et al., 2011). These studies considered different substances and different routes of administration when measuring polysubstance use profiles, resulting in no clear consensus on what constitutes a “high-risk” polysubstance use profile for HIV. To our knowledge, no comparable studies have been conducted in rural settings. Therefore, research is needed to better understand how polysubstance use may contribute to potential HIV risks in rural settings.
Historically, the majority of incident HIV infections in predominantly rural states have not been IDU related. In 2017, there were 78 new infections in West Virginia, and only 14.1% of new infections among men and 38.5% of new infections among women were associated with IDU (Center for AIDS Research at Emory University, 2019). However, epidemiological data surrounding HIV in rural communities is not static. In 2019, an HIV cluster linked to IDU was identified in Cabell County, WV, and, to date, has been associated with 80 new infections among PWID (Office of Epidemiology and Prevention Services, 2020a). The number of new HIV infections in Cabell County is especially alarming because it exceeds the total number of incident HIV infections for the entirety of West Virginia in 2018 (Hoffman, 2017). An estimated 1,857 PWID live in Cabell County and an estimated 41% share syringes, making them vulnerable to HIV infection (Allen et al., 2019b). Given the rapidly evolving HIV landscape in rural America, this study aims to better understand how polysubstance use is related to HIV risk, service utilization, and interest in PrEP among PWID in Cabell County.
Method
Study design
This analysis used data from the West Virginia COUNTS! Study, which aimed to calculate a PWID population estimate in Cabell County, West Virginia. The parent study has been previously described (Allen et al., 2019a, 2019b, 2020). Participants were required to be at last 18 years old and to have previously used drugs of any form. Survey data were collected in two phases in June and July 2018. During the first phase, we recruited PWID at the Cabell-Huntington Harm Reduction Program. The program is housed at the Cabell-Huntington Health Department and offers a variety of services to PWID, including HIV and hepatitis testing, naloxone overdose reversal trainings, referrals to drug treatment, recovery coaching, and contraceptive services. In the second phase, we recruited PWID in areas throughout Cabell County where PWID congregate (as identified via geospatial analyses of overdose fatality data, locations of syringe disposal, and key informant interviews). All data were collected anonymously and via audio computer-assisted self-interview. Participants received a snack bag or $10 grocery gift card as incentives. For this analysis, we restricted the sample to individuals who reported IDU in the previous 6 months (n = 421). Given our interest in HIV prevention (including PrEP), we excluded individuals who had previously received an HIV diagnosis (n = 13) as well as those who reported having ever taken PrEP (n = 15). We also excluded data from one transgender participant to preserve their confidentiality. This procedure resulted in a final analytic sample of 392 participants. This study was approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Measures
Sociodemographic characteristics.
Participants reported their age, gender (male/female), current homelessness (yes/no), and education level (less than high school graduate, high school graduate or equivalent, some college or more). Participants reported their race and whether they considered themselves Hispanic. As our sample was predominantly non-Hispanic White, we dichotomized these categories to non-Hispanic White and other. Participants also reported their sexual orientation, which we dichotomized as sexual minority (i.e., lesbian, gay, bisexual, or “other”) and heterosexual.
Injection-related HIV risk.
We asked participants about whether they had used any injection equipment they knew had been previously used by someone else in the previous 6 months, including syringes/needles, cottons, cookers, and rinse water. We used these data to create an aggregate, dichotomous variable that indicated whether participants reported sharing any injection equipment in the past 6 months.
Syringe acquisition from an SSP.
Participants were asked to indicate if they had received syringes from a SSP in the past 6 months.
Past-6-month HIV testing.
We first asked participants if they had ever been tested for HIV. For participants who had been tested, we asked them when they were last tested (within last 3 months, 4–6 months ago, 7–12 months ago, more than a year ago). We then generated a binary variable indicating any HIV test in the past 6 months (yes/no). Responses of “don’t know” to this question were recoded as missing data.
PrEP awareness and interest.
PrEP was described to participants as “a way for people who do not have HIV to prevent HIV infection by taking a pill every day.” Participants were asked if they had ever heard of PrEP before taking the survey (yes/no). Participants were then asked how interested they would be in taking a pill every day to prevent HIV infection (very interested, somewhat interested, somewhat disinterested, very disinterested). We dichotomized responses, indicating any PrEP interest (very or somewhat interested/very or somewhat disinterested).
Past-6-month injection drug use.
Participants were asked to report which of the following drugs they had injected in the past 6 months: crystal methamphetamine, cocaine, heroin, speedball (heroin & cocaine together), fentanyl, pain-killers (Oxycontin, Percocet, Codeine, Darvon, Percodan, Dilaudid, and Demerol), and buprenorphine/suboxone.
Analysis
The first analytical step was to conduct a latent class analysis (LCA) using the IDU variables to identify profiles of polysubstance injection. This method identifies homogenous subgroups based on the manifest indicators, in this case, IDU measures (Goodman, 1974; Lazarsfeld & Henry, 1968). We estimated models with one through six classes using a full information maximum likelihood estimator. Only one individual had missing data on the IDU measures, and differential missingness in the model indicators was not of concern. We considered the model fit statistics (Akaike Information Criterion [AIC], Bayesian Information Criterion [BIC], and Lo–Mendell–Rubin Likelihood Ratio Tests [LRT] and substantive interpretations of the classes to determine the final number of classes (Nylund et al., 2007). To test whether the identified latent classes were associated with our outcomes (injection-related HIV risk, syringe acquisition at an SSP, HIV testing, PrEP awareness, and PrEP interest), we used a manual three-step approach to adjust for potential misclassification of participants into classes (Vermunt, 2010). We controlled for gender, race/ethnicity, age, education, homelessness, and sexual minority status when testing associations between the latent classes and outcomes. We used Wald Tests to identify any overall differences in each outcome by latent classes and performed follow-up pairwise tests to identify where differences between classes occur. We conducted the analyses for this study using Mplus Version 8.2 (Muthén & Muthén, 1998–2017).
Results
The sample characteristics are reported in Table 1. Our sample was primarily male (61.0%) and non-Hispanic White (84.3%). The average age was 36.0 years (SD = 8.6). One quarter (26.9%) of the sample had less than a 12th grade education, 35.5% had a 12th grade equivalent education, and 37.6% had attended some college or more. Approximately half (54.6%) of participants were homeless. Only 16.2% identified as a sexual minority. In the past 6 months, participants most commonly reported injecting heroin (81.1%) and crystal methamphetamine (70.3%), whereas painkillers (22.2%) and buprenorphine/suboxone (29.3%) were the least common. Sixty-one percent of our sample reported sharing injection equipment in the past 6 months. Most (65.1%) had received syringes from an SSP in the past 6 months. Half (51.0%) had been tested for HIV in the past 6 months. Only 30% had previously heard of PrEP. However, 57.7% were interested in taking PrEP once it was described.
Table 1.
Prevalences of sociodemographic characteristics, injection drug use indicators, and outcomes (N = 392)

| Variable | Prevalence (%) |
| Demographic characteristics | |
| Age, M (SD) | 36.0 (8.6) |
| Gender | |
| Male | 61.0% |
| Female | 39.0% |
| Race/ethnicity | |
| Non-Hispanic White | 84.3% |
| Other | 15.7% |
| Education | |
| Less than high school | 26.9% |
| 12th grade or GED | 35.5% |
| Some college or more | 37.6% |
| Homeless | 54.6% |
| Sexual minority | 16.2% |
| Past-6-month injection drug use | |
| Crystal methamphetamine | 70.3% |
| Cocaine | 33.2% |
| Heroin | 81.1% |
| Speedball | 36.2% |
| Fentanyl | 54.3% |
| Painkillers | 22.2% |
| Buprenorphine/suboxone | 29.3% |
| HIV and PrEP outcomes | |
| Injection-related HIV risk | 61.0% |
| Syringe acquisition from an SSP | 65.1% |
| Tested for HIV in past 6 months | 51.0% |
| Previously aware of PrEP | 30.0% |
| Interested in taking PrEP | 57.7% |
Notes: GED = General Educational Development credential; HIV = human immunodeficiency virus; PrEP = pre-exposure prophylaxis; SSP = syringe services program.
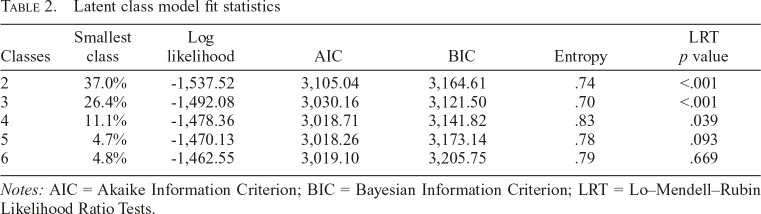
Latent classes of polysubstance injection
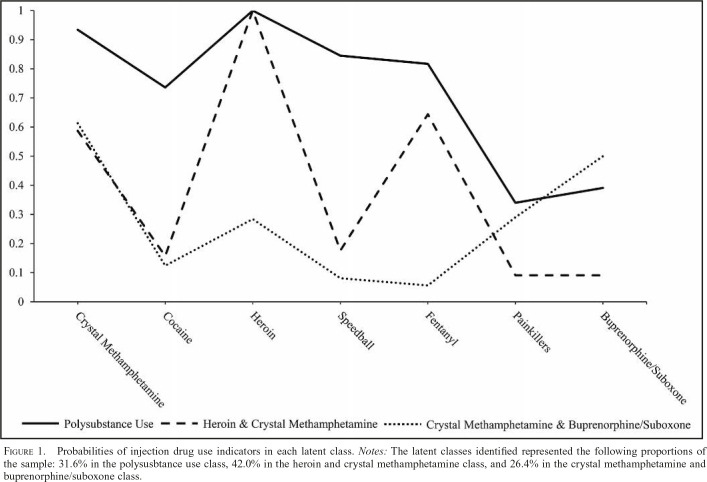
We fit models with up to six classes (Table 2). Based on the BIC and the substantive interpretation of the classes, we selected a three-class model of polysubstance injection. The three-class model did have the lowest entropy. Entropy is generally regarded as a model characteristic rather than a meaningful model selection criterion; therefore, this did not deter our selection of the three-class model (Masyn, 2013). The largest class contained 42.0% of the sample based on the posterior probabilities of class membership. This class was primarily categorized by high heroin and crystal methamphetamine injection (heroin and crystal methamphetamine [HCM] class). This class also had a high probability of endorsing fentanyl injection. The next class represented 31.6% of the sample and was characterized by overall high levels of drugs use (polysubstance use [PU] class). The smallest class contained 26.4% of the sample and was characterized by moderate crystal methamphetamine and buprenorphine/suboxone use and low levels of other drug use (crystal methamphetamine and buprenorphine/suboxone [CMBS] class). The probabilities of endorsing use of each drug for each of the three classes are displayed in Figure 1.
Table 2.
Latent class model fit statistics
| Classes | Smallest class | Log likelihood | AIC | BIC | Entropy | LRT p value |
| 2 | 37.0% | -1,537.52 | 3,105.04 | 3,164.61 | .74 | <.001 |
| 3 | 26.4% | -1,492.08 | 3,030.16 | 3,121.50 | .70 | <.001 |
| 4 | 11.1% | -1,478.36 | 3,018.71 | 3,141.82 | .83 | .039 |
| 5 | 4.7% | -1,470.13 | 3,018.26 | 3,173.14 | .78 | .093 |
| 6 | 4.8% | -1,462.55 | 3,019.10 | 3,205.75 | .79 | .669 |
Notes: AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion; LRT = Lo–Mendell–Rubin Likelihood Ratio Tests.
Figure 1.
Probabilities of injection drug use indicators in each latent class. Notes: The latent classes identified represented the following proportions of the sample: 31.6% in the polysusbtance use class, 42.0% in the heroin and crystal methamphetamine class, and 26.4% in the crystal methamphetamine and buprenorphine/suboxone class.
Sociodemographic correlates of latent class membership
The distribution of sociodemographic correlates within each polysubstance injection class is summarized in Table 3. The PU class did not differ in age from the other classes. The CMBS class was marginally younger than the HCM class (β = -0.048, p = .072). The CMBS class was significantly more likely to be male than were both the PU (β = 0.894, p = .042) and HCM (β = 1.299, p = .004) classes. The PU and HCM classes did not differ in terms of gender (β = -0.405, p = .248). There were no differences by race/ethnicity across the classes. In terms of education, the classes had similar proportions of members who had a high school equivalent education, but the PU class had more members who had less than a high school education than the HCM class (β = 1.111, p = .018) and marginally more than the CMBS class (β = 0.807, p = .091). The CMBS class was significantly less likely to be homeless than both the PU (β = -0.787, p = .025) and HCM (β = -1.031, p = .004) classes. The PU and HCM classes were similar in terms of homelessness (β = 0.244, p = .479). The CMBS class had a higher proportion of sexual minority participants than the PU (β = 1.521, p = .013) and HCM (β = 1.126, p = .012) classes. The PU and HCM classes were not significantly different in terms of sexual minority status (β = 0.395, p = .569).
Table 3.
Distribution of demographic characteristics by latent class membership
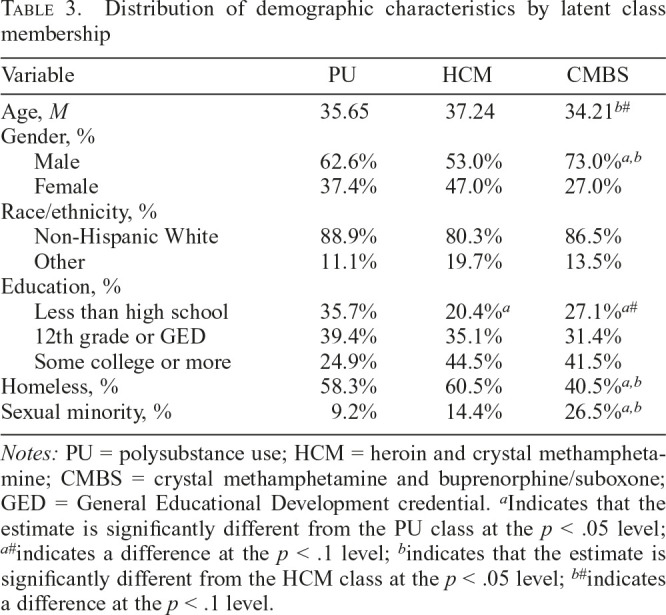
| Variable | PU | HCM | CMBS |
| Age, M | 35.65 | 37.24 | 34.21b# |
| Gender, % | |||
| Male | 62.6% | 53.0% | 73.0%a,b |
| Female | 37.4% | 47.0% | 27.0% |
| Race/ethnicity, % | |||
| Non-Hispanic White | 88.9% | 80.3% | 86.5% |
| Other | 11.1% | 19.7% | 13.5% |
| Education, % | |||
| Less than high school | 35.7% | 20.4%a | 27.1%a# |
| 12th grade or GED | 39.4% | 35.1% | 31.4% |
| Some college or more | 24.9% | 44.5% | 41.5% |
| Homeless, % | 58.3% | 60.5% | 40.5%a,b |
| Sexual minority, % | 9.2% | 14.4% | 26.5%a,b |
Notes: PU = polysubstance use; HCM = heroin and crystal methamphetamine; CMBS = crystal methamphetamine and buprenorphine/suboxone; GED = General Educational Development credential.
Indicates that the estimate is significantly different from the PU class at the p < .05 level;
indicates a difference at the p < .1 level;
indicates that the estimate is significantly different from the HCM class at the p < .05 level;
indicates a difference at the p < .1 level.
Injection-related HIV risk, syringe acquisition, and HIV testing by latent class
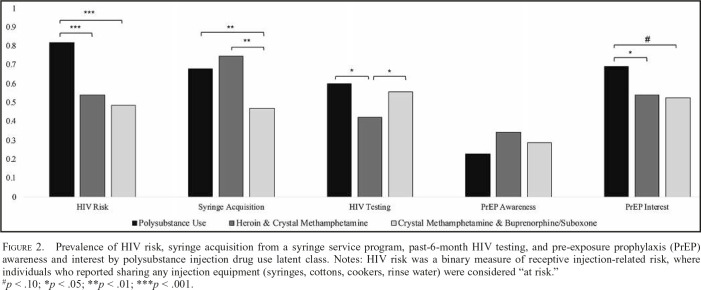
Figure 2 displays the prevalences for each latent class of recent injection-related HIV risk, syringe acquisition from an SSP, and HIV testing. The classes differed significantly in injection-related HIV risk (χ2 = 26.00, p < .001). A total of 81.8% of the PU class members were at risk for HIV based on their injection practices, which was significantly higher than those in the HCM (54.1%; p < .001) and CMBS (48.4%; p < .001) classes. The HCM and CMBS classes did not differ on injection-related HIV risk (p = .68). Recent syringe acquisition at an SSP varied by class (χ2 = 9.22, p = .010). The PU (67.7%; p = .010) and HCM (74.7%; p = .004) classes were more likely to have received syringes from an SSP than the CMBS class (46.9%). The PU and HCM classes did not differ on receiving syringes (p = .622). The classes further varied in terms of their recent HIV testing history (χ2 = 7.26, p = .027). The PU (60.0%; p = .015) and CMBS (55.7%; p = .024) classes had higher prevalences of past-6-month HIV testing than the HCM class (42.2%). The PU and CMBS classes did not significantly differ on HIV testing (p = .993).
Figure 2.
Prevalence of HIV risk, syringe acquisition from a syringe service program, past-6-month HIV testing, and pre-exposure prophylaxis (PrEP) awareness and interest by polysubstance injection drug use latent class. Notes: HIV risk was a binary measure of receptive injection-related risk, where individuals who reported sharing any injection equipment (syringes, cottons, cookers, rinse water) were considered “at risk.”
#p < .10; *p < .05; **p < .01; ***p < .001.
PrEP awareness and interest by latent class
The prevalences of PrEP awareness and interest for each latent class are presented in Figure 2. PrEP awareness did not differ by class (χ2 = 1.99, p = .37). Awareness was low overall, ranging from 23.0% in the PU class to 34.4% in the HCM class. The classes did marginally differ in PrEP interest (χ2 = 4.93, p = .085). The PU class has more interest in PrEP (68.9%) than the HCM class (54.1%; p = .044) and had marginally more PrEP interest than the CMBS class (52.6%; p = .086). The HCM and CMBS classes did not differ in terms of PrEP interest (p = .838).
Discussion
This study explored the complex relationships between polysubstance use, injection-related HIV risk, syringe acquisition from an SSP, HIV testing, and PrEP awareness and interest among a population of rural PWID in Appalachia. We found that more than 60% of PWID in our study reported having recently shared injection equipment and 51% had been tested for HIV in the past 6 months. Further, 65% of our sample reported having recently accessed sterile syringes at an SSP. These findings demonstrate that existing HIV prevention services are reaching a large proportion of the PWID population, but expanded services are still needed given the prevalence of sharing injection equipment. Currently, only 18 SSPs operate in West Virginia and few offer mobile services (Office of Epidemiology and Prevention Services, 2020b). Further, the majority of these SSPs operate on a one-for-one exchange basis, rather than a needs-based distribution strategy, which is considered best practice (National Harm Reduction Coalition, 2009). Expanding harm reduction services is essential to mitigate community risks for HIV outbreaks among PWID.
Paralleling PrEP studies conducted in urban areas which typically find that 7%–24% of PWID are aware of PrEP and 47%–71% would be interested in using it, we found that 30% of rural PWID in our sample were aware of PrEP (Kuo et al., 2016; Sherman et al., 2019; Shrestha et al., 2017; Stein et al., 2014; Walters et al., 2017). After we informed people about how using PrEP may lower risks of HIV acquisition, 58% reported being interested in using it. Our findings suggest that addressing the HIV prevention needs of rural PWID warrants a multipronged approach in which people have increased access to harm reduction, HIV/STI testing, and PrEP services. A multipronged HIV prevention strategy is of particular importance given our finding that there are distinct profiles of polysubstance injection drug use among rural PWID and that each class had unique relationships with HIV prevention services. Potential strategies to improve the HIV prevention landscape in rural Appalachia include offering mobile harm reduction services, supporting secondary syringe exchange, integrating PrEP education into existing programs, and offering HIV/STI testing services at venues frequented by PWID. Given that an HIV cluster among PWID was identified in the months following data collection, expanding HIV prevention efforts are now more important than ever (West Virginia Department of Health & Human Resources, 2019).
We identified three classes of polysubstance use, which had different relationships with our HIV prevention outcomes. For example, the two drug use classes with the greatest injection-related HIV risks (i.e., the PU and HCM classes) had the highest prevalences of recently accessing sterile syringes at an SSP. That these two highest-risk classes of PWID are most likely to use SSPs underscores the value of implementing harm reduction services. In the absence of harm reduction programs, members of these high-risk classes would have decreased access to sterile syringes and be at elevated risk for HIV acquisition. These findings are also not indicative of SSP utilization driving high-risk injection practices, but rather that these programs are reaching people with complex profiles of IDU that place them at increased risk for HIV. Although SSP implementation may be controversial, their sustained operations carries widespread community-level benefits, especially for PWID with complex profiles of polysubstance use. Our findings also provide support for the integration of PrEP services at venues frequented by high-risk PWID. By locating PrEP services within existing programs, PWID may be more likely to initiate PrEP utilization as well as receive myriad other HIV prevention services.
The polysubstance injection classes differed in terms of sociodemographic characteristics. The PU class was more structurally vulnerable than the other classes because of the class’s comparatively high rate of homelessness and lower education level. This class also had the highest injection-related HIV risk. These findings suggest that integrating syringe services and HIV testing within existing shelters and other homeless service providers may improve HIV prevention services utilization and decrease risks for HIV acquisition among highly vulnerable PWID. Research has shown that co-locating different types of health and social services can improve both access and outcomes for homeless individuals (Jego et al., 2018; Pirraglia et al., 2012; U.S. Department of Housing and Urban Development, 2010; Zlotnick et al., 2013). To further improve vulnerable PWIDs’ access to HIV prevention services, programs should be as accessible as possible and without restrictions that stipulate who can access them. In addition, mobile SSP and HIV testing services may be warranted in Cabell County in order to reach geographically isolated PWID. Mobile SSPs have been shown to reach high-risk PWID who are often missed by other service models (Strike & Miskovic, 2018). Mobile harm reduction programs may help meet the unique needs of rural clients, as they can cover large geographic areas (Fisher et al., 2017; Strike & Miskovic, 2018). Currently, few West Virginia counties have mobile SSPs that provide syringes, HIV and hepatitis C testing, and referrals to family planning and substance use treatment services (Office of Epidemiology and Prevention Services, 2020b). Making services easily accessible to the PWID at highest risk for HIV should be a core element of prevention strategies in the current high-risk era.
The crystal methamphetamine and buprenorphine class was relatively unique within our sample. This class had comparatively low rates of homelessness, a higher proportion of males, and more sexual minority individuals than the other classes. Given these sociodemographic characteristics, it is likely that the CMBS polysubstance use class has a different relationship to HIV than the other classes in this study. First, the comparatively low rate of homelessness suggests that this class is more structurally stable than the other classes in our study, and structural stability is associated with less risky injection practices (Metraux et al., 2004; Salazar et al., 2007; Wolitski et al., 2007). One interpretation of the high rates of HIV testing despite the low injection-related HIV risk among this class is that these individuals potentially have high sexual risk for HIV. Tailored interventions may be warranted to address the unique HIV prevention needs of the CMBS class; however, more research is necessary to fully understand the needs of this class.
This study has limitations and strengths to consider. Our measure of HIV risk is relatively simple, as we characterized individuals as having some versus no risk based on their injection practices. Total injection-related behavioral risk for HIV would be better measured by including the frequency of injecting with shared equipment and the relative differences in risk each type of shared equipment carries. Future studies should use more nuanced measures of injection-related HIV risk. Future studies should also include sexual risk measures for HIV, which was not measured in this study. In addition, research is needed to explore systems-level factors that influence HIV risk, such as involvement in the criminal justice system. Last, our findings may not generalize to locales outside of Appalachia. This study’s strengths outweigh these limitations. By using latent class analysis, we were able to utilize a nuanced measurement of polysubstance use in this study. Furthermore, we provide insight into rural PWIDs’ awareness of and interest in PrEP, which has not previously been well researched. Overall, this study provides an important contribution to our understanding of the relationship between polysubstance use, injection-related HIV risk, and HIV prevention strategies.
Conclusions
Our study explored the relationship between polysubstance use and HIV-related behaviors among rural PWID. Various profiles of polysubstance IDU each have different associations with HIV risk and prevention behaviors. The PWID who have the highest behavioral risk for HIV also experience the highest levels of structural vulnerability, which can make accessing harm reduction services more difficult. Expanding the availability of harm reduction services is essential in rural settings at risk for HIV outbreaks. Effective public health strategies to prevent future HIV outbreaks will need to consider the challenges rural PWID face in accessing services and adapt to meet the needs of this population.
Acknowledgments
The authors are grateful for the collaboration of the Cabell Huntington Health Department, without which this project would not have been possible. We are especially grateful to Tim Hazelett, Thommy Hill, Tyler Deering, Kathleen Napier, Jeff Keatley, Michelle Perdue, Chad Heilig, and Charles “CK” Babcock for all their support throughout the study implementation. We are also grateful for the hard work of the West Virginia COUNTS! research team: Megan Keith, Anne Maynard, Aspen McCorkle, Terrance Purnell, Ronaldo Ramirez, Kayla Rodriguez, Lauren Shappell, Brad Silberzahn, Dominic Thomas, Kevin Williams, and Hayat Yusuf. We gratefully acknowledge the West Virginia Department of Health and Human Resources. We also wish to acknowledge Josh Sharfstein, Michelle Spencer, Dori Henry, and Akola Francis for their support throughout each phase of the study. Most importantly, we are grateful to our study participants.
Conflict-of-Interest Statement
Susan G. Sherman is an expert witness for plaintiffs in opioid litigation. No other authors have competing interests to disclose.
Footnotes
This research was supported by a grant from the Bloomberg American Health Initiative at the Johns Hopkins Bloomberg School of Public Health to Dr. Sean T. Allen. Dr. Allen is also supported by National Institutes of Health (NIH) Grant K01DA046234. Dr. Schneider is supported by National Institute on Drug Abuse Grant 5T32DA007292. This research has been facilitated by the infrastructure and resources provided by the Johns Hopkins University Center for AIDS Research, an NIH funded program (P30AI094189) and the District of Columbia Center for AIDS Research, an NIH funded program (AI117970). The content is solely the responsibility of the authors and does not necessarily represent the views of the funders.
References
- Abelson J., Treloar C., Crawford J., Kippax S., van Beek I., Howard J. Some characteristics of early-onset injection drug users prior to and at the time of their first injection. Addiction. 2006;101:548–555. doi: 10.1111/j.1360-0443.2006.01379.x. doi:10.1111/j.1360-0443.2006.01379.x. [DOI] [PubMed] [Google Scholar]
- Allen S. T., O’Rourke A., White R. H., Schneider K. E., Hazelett T., Kilkenny M., Sherman S. G. Applying population estimation methods in rural America. 2019a. Retrieved from https://americanhealth.jhu.edu/themes/bahi_stable/assets/pdfs/Opioid_Services_Tool-kit_012419.pdf. [Google Scholar]
- Allen S. T., O’Rourke A., White R. H., Schneider K. E., Kilkenny M., Sherman S. G. Estimating the number of people who inject drugs in a rural county in Appalachia. American Journal of Public Health. 2019b;109:445–450. doi: 10.2105/AJPH.2018.304873. doi:10.2105/AJPH.2018.304873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S. T., White R. H., O’Rourke A., Ahmad N. J., Hazelett T., Kilkenny M. E., Sherman S. G. Correlates of transactional sex among a rural population of people who inject drugs. AIDS and Behavior. 2020;24:775–781. doi: 10.1007/s10461-019-02612-7. doi:10.1007/s10461-019-02612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker L.-G., Johnson L., Cowan F., Overs C., Besada D., Hillier S., Cates W., Jr. Combination HIV prevention for female sex workers: what is the evidence? The Lancet. 2015;385:72–87. doi: 10.1016/S0140-6736(14)60974-0. doi:10.1016/S0140-6736(14)60974-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancarelli D. L., Biello K. B., Childs E., Drainoni M., Salhaney P., Edeza A.. . .Bazzi A. R. Strategies used by people who inject drugs to avoid stigma in healthcare settings. Drug and Alcohol Dependence. 2019;198:80–86. doi: 10.1016/j.drugalcdep.2019.01.037. doi:10.1016/j.drugalcdep.2019.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla J., Sobrino P., De La Fuente L., Noguer I., Guerra L., Parras F. Late diagnosis of HIV infection in the era of highly active antiretroviral therapy: Consequences for AIDS incidence. AIDS. 2002;16:1945–1951. doi: 10.1097/00002030-200209270-00012. doi:10.1097/00002030-200209270-00012. [DOI] [PubMed] [Google Scholar]
- Center for AIDS Research at Emory University. AIDSVu Local Data: West Virginia. 2019. Retrieved from https://aidsvu.org/state/west-virginia/ [Google Scholar]
- Centers for Disease Control and Prevention. Department of Health and Human Services implementation guidance to support certain components of syringe service programs. 2016a. Retrieved from https://www.cdc.gov/hiv/pdf/risk/hhs-ssp-guidance.pdf. [Google Scholar]
- Centers for Disease Control and Prevention. 2016b. HIV and injection drug use: Syringe services programs for HIV prevention [fact sheet] Retrieved from https://www.cdc.gov/vitalsigns/pdf/2016-12-vitalsigns.pdf. [Google Scholar]
- Centers for Disease Control and Prevention. US Public Health Service: Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 Update: Clinical providers’ supplement. 2018. Retrieved from https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-provider-supplement-2017.pdf. [Google Scholar]
- Choopanya K., Martin M., Suntharasamai P., Sangkum U., Mock P. A., Leethochawalit M., Vanichseni S. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): A randomised, double-blind, placebo-controlled phase 3 trial. The Lancet. 2013;381:2083–2090. doi: 10.1016/S0140-6736(13)61127-7. doi:10.1016/ S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- Cloud D. H., Ibragimov U., Prood N., Young A. M., Cooper H. L. F. Rural risk environments for hepatitis C among young adults in Appalachian Kentucky. International Journal on Drug Policy. 2019;72:47–54. doi: 10.1016/j.drugpo.2019.05.006. doi:10.1016/j.drugpo.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C., Bradley H. M., Broz D., Buddha S., Chapman E. L., Galang R. R., Duwve J. M. Community outbreak of HIV infection linked to injection drug use of oxymorphone—Indiana, 2015. Morbidity and Mortality Weekly Report. 2015;64:443–444. Retrieved from https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6416a4.htm. [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais D. C., Friedman S. R., Stoneburner R. L. HIV infection and intravenous drug use: Critical issues in transmission dynamics, infection outcomes, and prevention. Reviews of Infectious Diseases. 1988;10:151–158. doi: 10.1093/clinids/10.1.151. doi:10.1093/clinids/10.1.151. [DOI] [PubMed] [Google Scholar]
- Des Jarlais D. C., Nugent A., Solberg A., Feelemyer J., Mermin J., Holtzman D. Syringe service programs for persons who inject drugs in urban, suburban, and rural areas—United States, 2013. Morbidity and Mortality Weekly Report. 2015;64:1337–1341. doi: 10.15585/mmwr.mm6448a3. doi:10.15585/mmwr.mm6448a3. [DOI] [PubMed] [Google Scholar]
- Escudero D. J., Kerr T., Wood E., Nguyen P., Lurie M. N., Sued O., Marshall B. D. Acceptability of HIV pre-exposure prophylaxis (PrEP) among people who inject drugs (PWID) in a Canadian setting. AIDS and Behavior. 2015;19:752–757. doi: 10.1007/s10461-014-0867-z. doi:10.1007/s10461-014-0867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K., Smith T., Nairn K., Anderson D. Rural people who inject drugs: A cross-sectional survey addressing the dimensions of access to secondary needle and syringe program outlets. Australian Journal of Rural Health. 2017;25:94–101. doi: 10.1111/ajr.12304. doi:10.1111/ajr.12304. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. Truvada for PrEP fact sheet: Ensuring safe and proper use. 2014. Retrieved from https://www.fda.gov/media/83586/download. [Google Scholar]
- Girardi E., Sabin C. A., Monforte A. D. Late diagnosis of HIV infection: Epidemiological features, consequences and strategies to encourage earlier testing. Journal of Acquired Immune Deficiency Syndromes. 2007;46(Supplement 1) doi: 10.1097/01.qai.0000286597.57066.2b. S3–S8. doi:10.1097/01.qai.0000286597.57066.2b. [DOI] [PubMed] [Google Scholar]
- Gonsalves G. S., Crawford F. W. Dynamics of the HIV outbreak and response in Scott County, IN, USA, 2011-15: A modelling study. The Lancet HIV. 2018;5:e569–e577. doi: 10.1016/S2352-3018(18)30176-0. doi:10.1016/S2352-3018(18)30176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman L. A. Exploratory latent structure analysis using both identifiable and unidentifiable models. Biometrika. 1974;61:215–231. doi:10.1093/biomet/61.2.215. [Google Scholar]
- Grant R. M., Lama J. R., Anderson P. L., McMahan V., Liu A. Y, Vargas L, Glidden D. V. & the iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England Journal of Medicine. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. doi:10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan H., McGough J. P., Thiede H., Hopkins S., Duchin J., Alexander E. R. Reduced injection frequency and increased entry and retention in drug treatment associated with needle-exchange participation in Seattle drug injectors. Journal of Substance Abuse Treatment. 2000;19:247–252. doi: 10.1016/s0740-5472(00)00104-5. doi:10.1016/S0740-5472(00)00104-5. [DOI] [PubMed] [Google Scholar]
- Harrell P. T., Mancha B. E., Petras H., Trenz R. C., Latimer W. W. Latent classes of heroin and cocaine users predict unique HIV/HCV risk factors. Drug and Alcohol Dependence. 2012;122:220–227. doi: 10.1016/j.drugalcdep.2011.10.001. doi:10.1016/j.drugalcdep.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman W. C. HIV epidemiologic profile West Virginia. 2017. Retrieved from https://dhhr.wv.gov/oeps/std-hiv-hep/HIV_AIDS/Documents/WV_HIV_Epi_Profile.pdf. [Google Scholar]
- Jego M., Abcaya J., Ştefan D.-E., Calvet-Montredon C., Gentile S. Improving health care management in primary care for homeless people: A literature review. International Journal of Environmental Research and Public Health. 2018;15:309. doi: 10.3390/ijerph15020309. doi:10.3390/ijerph15020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. M., Logan J., Gladden R. M., Bohm M. K. Vital signs: Demographic and substance use trends among heroin users—United States, 2002–2013. Morbidity and Mortality Weekly Report. 2015;64:719–725. [PMC free article] [PubMed] [Google Scholar]
- Kidorf M., King V. L., Peirce J., Kolodner K., Brooner R. K. Benefits of concurrent syringe exchange and substance abuse treatment participation. Journal of Substance Abuse Treatment. 2011;40:265–271. doi: 10.1016/j.jsat.2010.11.011. doi:10.1016/j.jsat.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. J., Harley D. A. Needle and syringe programs in rural areas: Addressing the intravenous drug use epidemic. Rehabilitation Research, Policy, and Education. 2019;33:56–64. doi:10.1891/2168-6653.33.1.56. [Google Scholar]
- Krentz H. B., Auld M. C., Gill M. J. The high cost of medical care for patients who present late (CD4 <200 cells/microL) with HIV infection. HIV Medicine. 2004;5:93–98. doi: 10.1111/j.1468-1293.2004.00193.x. doi:10.1111/j.1468-1293.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- Kuo I., Olsen H., Patrick R., Phillips G., II Magnus, M., Opoku J., Greenberg A. Willingness to use HIV pre-exposure prophylaxis among community-recruited, older people who inject drugs in Washington, DC. Drug and Alcohol Dependence. 2016;164:8–13. doi: 10.1016/j.drugalcdep.2016.02.044. doi:10.1016/j.drugalcdep.2016.02.044. [DOI] [PubMed] [Google Scholar]
- Lazarsfeld P., Henry N. Latent structure analysis. Boston, MA: Houghton Mifflin Company; 1968. [Google Scholar]
- Lorentz J., Hill L., Samimi B. Occupational needlestick injuries in a metropolitan police force. American Journal of Preventive Medicine. 2000;18:146–150. doi: 10.1016/s0749-3797(99)00137-3. doi:10.1016/S0749-3797(99)00137-3. [DOI] [PubMed] [Google Scholar]
- Masyn K. E. Latent class analysis and finite mixture modeling. In: Little T. E., editor. The Oxford Handbook of Quantitative Methods in Psychology. Volume 2. 2013. doi:10.1093/oxfordhb/9780199934898.013.0025. [Google Scholar]
- McCoy C. B., Lai S., Metsch L. R., Messiah S. E., Zhao W. Injection drug use and crack cocaine smoking: Independent and dual risk behaviors for HIV infection. Annals of Epidemiology. 2004;14:535–542. doi: 10.1016/j.annepidem.2003.10.001. doi:10.1016/j.annepidem.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Metraux S., Metzger D. S., Culhane D. P. Homelessness and HIV risk behaviors among injection drug users. Journal of Urban Health. 2004;81:618–629. doi: 10.1093/jurban/jth145. doi:10.1093/jurban/jth145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley K. I., Lynskey M. T., Moran P., Borschmann R., Winstock A. R. Polysubstance use, mental health and high-risk behaviours: Results from the 2012 Global Drug Survey. Drug and Alcohol Review. 2015;34:427–437. doi: 10.1111/dar.12263. doi:10.1111/dar.12263. [DOI] [PubMed] [Google Scholar]
- Muthén L. K., Muthén B. O. Mplus user’s guide. Los Angeles, CA: Authors; 1998–2017. [Google Scholar]
- National Harm Reduction Coalition. Recommended best practices for effective syringe exchange programs in the United States. New York, NY: 2009. [Google Scholar]
- Nylund K. L., Asparouhov T., Muthén B. O. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling. 2007;14:535–569. doi:10.1080/10705510701575396. [Google Scholar]
- Office of Epidemiology and Prevention Services. Increase in HIV: Outbreak of human immunodeficiency virus (HIV) linked to injection drug use. 2020a Retrieved from https://oeps.wv.gov/hiv-aids/pages/default.aspx.
- Office of Epidemiology and Prevention Services. West Virginia Harm Reduction Programs At-A-Glance. 2020b Retrieved from https://oeps.wv.gov/harm_reduction/documents/about/wv_hrp.pdf.
- Ohl M. E., Perencevich E. Frequency of human immunodeficiency virus (HIV) testing in urban vs. rural areas of the United States: Results from a nationally-representative sample. BMC Public Health. 2011;11:681. doi: 10.1186/1471-2458-11-681. doi:10.1186/1471-2458-11-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens D. K., Davidson K. W., Krist A. H., Barry M. J., Cabana M., Caughey A. B., Wong J. B. & the US Preventive Services Task Force. Preexposure prophylaxis for the prevention of HIV infection: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321:2203–2213. doi: 10.1001/jama.2019.6390. doi:10.1001/jama.2019.6390. [DOI] [PubMed] [Google Scholar]
- Paquette C. E., Syvertsen J. L., Pollini R. A. Stigma at every turn: Health services experiences among people who inject drugs. International Journal on Drug Policy. 2018;57:104–110. doi: 10.1016/j.drugpo.2018.04.004. doi:10.1016/j.drugpo.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirraglia P. A., Rowland E., Wu W.-C., Friedmann P. D., O’Toole T. P., Cohen L. B., Taveira T. H. Benefits of a primary care clinic co-located and integrated in a mental health setting for veterans with serious mental illness. Preventing Chronic Disease. 2012;9:E51. doi:10.5888/pcd9.110113. [PMC free article] [PubMed] [Google Scholar]
- Prohibition of Syringe Exchange Programs. Senate Bill 286, West Virginia Legislature. 2020 [Google Scholar]
- Roth A. M., Armenta R. A., Wagner K. D., Roesch S. C., Bluthenthal R. N., Cuevas-Mota J., Garfein R. S. Patterns of drug use, risky behavior, and health status among persons who inject drugs living in San Diego, California: A latent class analysis. Substance Use & Misuse. 2015;50:205–214. doi: 10.3109/10826084.2014.962661. doi:10.3109/10826084.2014.962661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz M. S., O’Rourke A., Allen S. T. Impact evaluation of a policy intervention for HIV prevention in Washington, DC. AIDS and Behavior. 2016;20:22–28. doi: 10.1007/s10461-015-1143-6. doi:10.1007/s10461-015-1143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz M. S., O Rourke A., Allen S. T., Holtgrave D. R., Metzger D., Benitez J., Wen L. D. Using interrupted time series analysis to measure the impact of legalized syringe exchange on HIV diagnoses in Baltimore and Philadelphia. Journal of Acquired Immune Deficiency Syndromes. 2019;82(Supplement 2):S148–S154. doi: 10.1097/QAI.0000000000002176. doi:10.1097/ QAI.0000000000002176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar L. F., Crosby R. A., Holtgrave D. R., Head S., Hadsock B., Todd J., Shouse R. L. Homelessness and HIV-associated risk behavior among African American men who inject drugs and reside in the urban south of the United States. AIDS and Behavior. 2007;11:70–77. doi: 10.1007/s10461-007-9239-2. doi:10.1007/s10461-007-9239-2. [DOI] [PubMed] [Google Scholar]
- Schranz A. J., Barrett J., Hurt C. B., Malvestutto C., Miller W. C. Challenges facing a rural opioid epidemic: Treatment and prevention of HIV and hepatitis C. Current HIV/AIDS Reports. 2018;15:245–254. doi: 10.1007/s11904-018-0393-0. doi:10.1007/s11904-018-0393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S. G., Schneider K. E., Park J. N., Allen S. T., Hunt D., Chaulk C. P., Weir B. W. PrEP awareness, eligibility, and interest among people who inject drugs in Baltimore, Maryland. Drug and Alcohol Dependence. 2019;195:148–155. doi: 10.1016/j.drugalcdep.2018.08.014. doi:10.1016/j.drugalcdep.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha R., Karki P., Altice F. L., Huedo-Medina T. B., Meyer J. P., Madden L., Copenhaver M. Correlates of willingness to initiate pre-exposure prophylaxis and anticipation of practicing safer drug- and sex-related behaviors among high-risk drug users on methadone treatment. Drug and Alcohol Dependence. 2017;173:107–116. doi: 10.1016/j.drugalcdep.2016.12.023. doi:10.1016/j. drugalcdep.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinner C. D., Boesecke C., Zink A., Jessen H., Stellbrink H.-J., Rockstroh J. K., Esser S. HIV pre-exposure prophylaxis (PrEP): A review of current knowledge of oral systemic HIV PrEP in humans. Infection. 2016;44:151–158. doi: 10.1007/s15010-015-0850-2. doi:10.1007/s15010-015-0850-2. [DOI] [PubMed] [Google Scholar]
- Stein M., Thurmond P., Bailey G. Willingness to use HIV pre-exposure prophylaxis among opiate users. AIDS and Behavior. 2014;18:1694–1700. doi: 10.1007/s10461-014-0778-z. doi:10.1007/s10461-014-0778-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee S. A., Celentano D. D., Shah N., Lyles C., Stambolis V. A., Macalino G., Vlahov D. Needle-exchange attendance and health care utilization promote entry into detoxification. Journal of Urban Health. 1999;76:448–460. doi: 10.1007/BF02351502. doi:10.1007/BF02351502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strike C., Miskovic M. Scoping out the literature on mobile needle and syringe programs-review of service delivery and client characteristics, operation, utilization, referrals, and impact. Harm Reduction Journal. 2018;15:6. doi: 10.1186/s12954-018-0212-3. doi:10.1186/s12954-018-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton M., Anthony M. N., Vila C., McLellan-Lemal E., Weidle P. J. HIV testing and HIV/AIDS treatment services in rural counties in 10 southern states: Service provider perspectives. Journal of Rural Health. 2010;26:240–247. doi: 10.1111/j.1748-0361.2010.00284.x. doi:10.1111/j.1748-0361.2010.00284.x. [DOI] [PubMed] [Google Scholar]
- Tobin K. E., Sherman S. G., Beilenson P., Welsh C., Latkin C. A. Evaluation of the Staying Alive programme: Training injection drug users to properly administer naloxone and save lives. International Journal on Drug Policy. 2009;20:131–136. doi: 10.1016/j.drugpo.2008.03.002. doi:10.1016/j.drugpo.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Townsend T. Ethics conflicts in rural communities: Privacy and confidentiality. In: Nelson W. A., editor. Handbook for rural health care ethics: A practical guide for professionals (pp. 126–141) Lebanon, NH: Dartmouth College Press; 2009. [Google Scholar]
- U.S. Department of Housing and Urban Development. Strategies for improving homeless people’s access to mainstream benefits and services. 2010. Retrieved from https://www.urban.org/sites/default/files/publication/28626/412089-Strategies-for-Improving-Homeless-People-s-Access-to-Mainstream-Benefits-and-Services.PDF. [Google Scholar]
- Valdiserri R. O., Holtgrave D. R., West G. R. Promoting early HIV diagnosis and entry into care. AIDS. 1999;13:2317–2330. doi: 10.1097/00002030-199912030-00003. doi:10.1097/00002030-199912030-00003. [DOI] [PubMed] [Google Scholar]
- van Ameijden E. J., van den Hoek J. A., van Haastrecht H. J., Coutinho R. A. The harm reduction approach and risk factors for human immunodeficiency virus (HIV) seroconversion in injecting drug users, Amsterdam. American Journal of Epidemiology. 1992;136:236–243. doi: 10.1093/oxfordjournals.aje.a116489. doi:10.1093/oxfordjournals.aje.a116489. [DOI] [PubMed] [Google Scholar]
- Van Handel M. M., Rose C. E., Hallisey E. J., Kolling J. L., Zibbell J. E., Lewis B., Brooks J. T. County-level vulnerability assessment for rapid dissemination of HIV or HCV infections among persons who inject drugs, United States. Journal of Acquired Immune Deficiency Syndromes. 2016;73:323–331. doi: 10.1097/QAI.0000000000001098. doi:10.1097/QAI.0000000000001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermunt J. K. Latent class modeling with covariates: Two improved three-step approaches. Political Analysis. 2010;18:450–469. doi:10.1093/pan/mpq025. [Google Scholar]
- Walters S. M., Rivera A. V., Starbuck L., Reilly K. H., Boldon N., Anderson B. J., Braunstein S. Differences in awareness of pre-exposure prophylaxis and post-exposure prophylaxis among groups at-risk for HIV in New York State: New York City and Long Island, NY, 2011–2013. Journal of Acquired Immune Deficiency Syndromes. 2017;75(Supplement 3):S383–S391. doi: 10.1097/QAI.0000000000001415. doi:10.1097/QAI.0000000000001415. [DOI] [PubMed] [Google Scholar]
- Warner T. D., Monaghan-Geernaert P., Battaglia J., Brems C., Johnson M. E., Roberts L. W. Ethical considerations in rural health care: A pilot study of clinicians in Alaska and New Mexico. Community Mental Health Journal. 2005;41:21–33. doi: 10.1007/s10597-006-2597-1. doi:10.1007/s10597-005-2597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wejnert C., Hess K. L, Hall H. I., Van Handel M., Hayes D., Jr., Fulton P., Jr.. .Valleroy L. A. Vital signs: Trends in HIV diagnoses, risk behaviors, and prevention among persons who inject drugs—United States. Morbidity and Mortality Weekly Report. 2016;65:1336–1342. doi: 10.15585/mmwr.mm6547e1. doi:10.15585/mmwr.mm6547e1. [DOI] [PubMed] [Google Scholar]
- West Virginia Department of Health & Human Resources. 2019. Health Advisory #155: Increase in New HIV Infections Among Persons Who Inject Drugs. (WV155-03-22-19) [Google Scholar]
- Wodak A., Cooney A. Do needle syringe programs reduce HIV infection among injecting drug users: A comprehensive review of the international evidence. Substance Use & Misuse. 2006;41:777–813. doi: 10.1080/10826080600669579. doi:10.1080/10826080600669579. [DOI] [PubMed] [Google Scholar]
- Wolitski R. J., Kidder D. P., Fenton K. A. HIV, homelessness, and public health: Critical issues and a call for increased action. AIDS and Behavior. 2007;11(Supplement):167–171. doi: 10.1007/s10461-007-9277-9. doi:10.1007/s10461-007-9277-9. [DOI] [PubMed] [Google Scholar]
- Wu L.-T., Ling W., Burchett B., Blazer D. G., Yang C., Pan J.-J., Woody G. E. Use of item response theory and latent class analysis to link poly-substance use disorders with addiction severity, HIV risk, and quality of life among opioid-dependent patients in the Clinical Trials Network. Drug and Alcohol Dependence. 2011;118:186–193. doi: 10.1016/j.drugalcdep.2011.03.018. doi:10.1016/j.drugalcdep.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibbell J. E., Asher A. K., Patel R. C., Kupronis B., Iqbal K., Ward J. W., Holtzman D. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. American Journal of Public Health. 2018;108:175–181. doi: 10.2105/AJPH.2017.304132. doi:10.2105/AJPH.2017.304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibbell J. E., Iqbal K., Patel R. C., Suryaprasad A., Sanders K. J., Moore-Moravian L., Holtzman D. the Centers for Disease Control and Prevention (CDC) Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. Morbidity and Mortality Weekly Report. 2015;64:453–458. [PMC free article] [PubMed] [Google Scholar]
- Zlotnick C., Zerger S., Wolfe P. B. Health care for the homeless: What we have learned in the past 30 years and what’s next. American Journal of Public Health. 2013;103(Supplement 2) doi: 10.2105/AJPH.2013.301586. S199–S205. doi:10.2105/AJPH.2013.301586. [DOI] [PMC free article] [PubMed] [Google Scholar]