Abstract
Endothelial cell dysfunction proceeding with increased inflammation and monocyte increase is one of the main causes of vessel injury in CAD. SIRT1 (Sirtuin 1) protein plays an important role in the regulation of cellular physiological mechanisms. SIRT1 has roles in regulating angiogenesis and preventing endothelial dysfunction and reperfusion injury due to ischemia. Suppression of SIRT1 causes monocyte affinity due to endothelial dysfunction. Sirtuins activators are involved in pathologies of many diseases with promising treatments. The objective of this review is to summarize the current progress and future directions of sirtuin protein in the field of CAD.
Keywords: Coronary artery disease, endothelial cell dysfunction, inflammation, Sirtuin 1
Coronary artery disease (CAD) is the most frequent cardiovascular disease worldwide. CAD arises from the interaction of genetic factors as other complex hereditary diseases. The Genome-wide analysis predicted that the role of genetic factors in CAD is about 40–50% [1, 2]. Sirtuins (SIRTs) are NAD + - dependent class 3 histone deacetylases (HDAC’s) containing various biological activities, such as transcription, recombination and genome stability by modifying histones, transcription factors and epigenetic enzymes [3]. Genetic code is highly preserved for SIRTs and translated into seven sirtuin proteins (SIRT1-SIRT7) in mammalians (Table 1) [4]. SIRT1 gene is located on 10q21.3 chromosome and consists of 11 exons [5]. SIRT1 is a cardioprotective protein taking serious roles in the regulation of angiogenesis and the prevention of endothelial dysfunction and malicious effects of ischemia-reperfusion injury [6, 7]. It enhances the DNA stability by binding and deactivating several substrates at a molecular level; regulates the expression of endothelial nitric oxide synthase (eNOS) and manganese superoxide dismutase (MnSOD) and activates the FoxO1 dependent pathways [8, 9]. Increased levels of SIRT1 in patients with prior myocardial infarction or CAD is a result of a compensatory mechanism for eliminating hazardous effects of oxidative stress and hypoxia [10]. SIRT1 inhibits ischemia-induced endothelial dysfunction by regulating nitric oxide synthase (eNOS) expression and acts as a cardioprotective molecule. Treatment with SIRT1 activators is tested as the treatment of coronary artery disease. Sirtuins provide additional benefits in many diseases by regulating physiological signals. Shortly, sirtuin modulation promises to help overcome several diseases. The objective of this review is to summarize the current progress and future directions of sirtuin protein in the field of CAD.
TABLE 1.
Mammal sirtuins
| Sirt | Class | Size (kDa) | Localization | Enzymatic activity |
|---|---|---|---|---|
| 1 | I | 62 | Nucleus | Lysine deacetylase |
| 2 | I | 41 | Cytoplasm | Lysine deacetylase |
| 3 | I | 44 | Mitochondrium | Lysine deacetylase |
| 4 | II | 35 | Mitochondrium | ADP-ribosyltransferase |
| 5 | III | 34 | Mitochondrium | Succinyltransferase, malonyltransferase |
| 6 | IV | 39 | Nucleus | Demyristoylase, depalmitoylase, ADP-ribosyltransferase, deacetylase |
| 7 | IV | 45 | Nucleus | Demyristoylase, depalmitoylase, ADP-ribosyltransferase, deacetylase |
The Cardioprotective Role of SIRT1 in CAD
SIRT1 participates in the biological processes associated with the development of heart failure (HF), including regulation of energy consumption, autophagy and cell death and survival [11]. Insulin-like growth factor 1 (IGF-1) propeptide (mIGF-1) protects the heart against hypertrophy and oxidative stress by preventing infarction using induction of SIRT1 expression in cardiomyocytes [12]. Oxidative stress and tissue hypoxia are among the most important triggering factors for the development and exacerbation of cardiovascular diseases. SIRT1 is known to be induced in cardiac diseases to manage adverse events associated with diseases driven by hypoxia and oxidative stress [10, 13]. The role of SIRT1 in some age-related diseases like diabetes, neurological disorders, renal and cardiovascular diseases is proven [14–16]. In detail, SIRT1 regulation causes hyperacetylation of chromatin and increases the eNOS expression, which enhances endothelial cell injury [8]. Besides, SIRT1 mediated hyperacetylation causes increased SREBP1C gene transcription, which leads to the accumulation of fatty acids and cholesterol [17]. Moreover, at the very beginning of the cardiac stress, the nucleus and its cytoplasmic location are remodeled [18]. Remodeling of the SIRT1 gene is a natural phenomenon during the growth of blood vessels, so changes in transcription, translation and/or post-translation modifications of this gene give rise to the generation of protein products disrupting vessel formation mechanisms [19]. Endothelial SIRT1 is assumed to be an atherosclerotic factor and might be associated with more reasonable mechanisms, such as inhibition of apoptosis by oxLDL, regulation of eNOS expression and improving endothelial relaxation function [19].
The functional role of monocytes in CAD must be investigated more to provide a novel therapeutic approach in patients with CAD. In another study performed by Chan et al. [20], by suppressing SIRT1 in patients with CAD, monocytes show a greater affinity to endothelial cells. Increasing oxidative stress also causes upregulation in endothelial adhesion molecules of monocytes and these lead to more deterioration in endothelial dysfunction. Inhibition of SIRT1 causes increased apoptosis and oxidative stress. However, there are not enough data coming from studies confirming that inhibition of SIRT1 in monocytes is increasing or not in CAD yet.
To increase SIRT1 expression and/or activity may lead to an increase in histone deacetylation and consequent DNA methylation [21]. SIRT1 mediated epigenetic changes may increase the risk of CAD. These studies suggest that oxidative stress may cause a pathological increase in SIRT1 expression in patients with CAD. Increasing SIRT1 levels as a therapeutic approach may be suggested as a compensatory mechanism to increase antioxidants against oxidative stress in CAD. Besides, some medications used in CAD treatment may increase SIRT1 levels [22].
Some other disease markers, such as endothelial dysfunction and oxidative stress and their association with SIRT1 protein levels, are also investigated to explain the effects of SIRT1 protein in CAD more explicitly. The common feature of the most cardiovascular risk factors, such as hypertension and obesity, is originated from endothelial dysfunction, which is increasing atherosclerosis. Endothelial dysfunction may be due to a decrease in eNOS expression. eNOS protects the cardiovascular system from atherosclerosis using regulating vascular relaxation. eNOS deficiency is associated with several risks for CAD, such as hypertension, ventricular hypertrophy and dietary dependent atherosclerosis [23].
SIRT1 hyperphosphorylation is another mechanism that reduces SIRT1 activity. Specifically, the serine/threonine kinase cyclin-dependent Kinase 5 (CDK5) phosphorylates SIRT1 in serine 47 increases with aging (Fig. 1). Hyperphosphorylated SIRT1 is distributed outside the nucleoles to the nucleoplasm. SIRT1 delocalization has an inhibitory effect on cytosolic Liver Kinase B1 (LKB1) and Aging-Aging Nuclear Telomeric Recombinant Factor 2-Interfering Protein 1 (TERF2IP) [24]. Specifically, they show an increase in the production of ROS on chronic stress, resulting in loss of cathepsin and consequent loss of lysosomal integrity leading to the decomposition and inactivation of SIRT1 [25]. Ito et al. [26] showed that increased miR-34a expression in endothelial cells caused inhibition of SIRT1 protein translation in CAD (Fig. 1). SIRT1, which has an important role in endothelial physiology, protects against endothelial aging and vascular homeostatic changes. Specifically, it regulates p53 acetylation, Jun NH2-terminal kinase (JNK) phosphorylation, and endothelial NO synthase (eNOS) expression and activity. These mechanisms reduce the vascular pathologies associated with diabetes and atherosclerosis (Fig. 1) [27–29].
FIGURE 1.
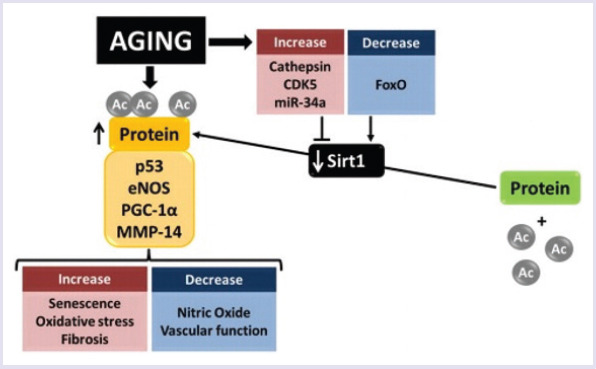
SIRT1-mediated anti-aging effects of endothelium.
The Cardioprotective Role of SIRT1 in Other Situations
SIRT1 acts a pivotal role in mediating calorie restrictions. Activating SIRT1 can deescalate metabolic syndrome via its protective effects against oxidative and genotoxic stress by deacetylation of some substrates, such as the p53 transcription factor [30]. SIRT1 decreases cholesterol accumulation in macrophages by activating the liver X receptor (LXR), functioning as a cholesterol sensor [31]. SIRT1 activation preserves cardiac and renal functions under stressful circumstances. Lack of SIRT1 is associated with pathophysiological changes in cardiovascular and renal diseases [32, 33].
Recent studies suggested that long-lived SIRT1 acts a part in metabolic regulation of AMP-activated protein kinase (AMPK) in skeletal muscles, liver, fatty tissue, and pancreas beta cells. Moreover, it is enounced that the aging process itself is associated with a decrease in both SIRT1 and AMPK activities [34–36]. Wang et al. [37] provided evidence about the important role of AMPK in cardiovascular prevention against ischemia and reperfusion injury. SIRT1 emerges as a critical regulator in the heart in the course of ischemia/reperfusion [38]. SIRT1 activates the LKB1 gene leading to the activation of AMPK, which is acting a role in glucose homeostasis and being the central energy regulator in maintaining cellular ATP levels [8]. AMPK, fulfill SIRT1 activation via increasing intracellular NAD+ levels [35].
SIRT1-AMPK signaling disrupted by ischemia makes mitochondria become more susceptible to ischemic stress and triggers the formation of reactive oxygen species causing inflamatuar response during ischemia and reperfusion. Studies show that AMPK is the potential pharmacological target to prevent adverse events due to aging. Metformin, biguanide derivative as an anti-diabetic agent, can induce AMPK activation. Administration of metformin during myocardial ischemia is shown to reduce the ischemia-reperfusion injury and left ventricular reverse remodeling [39]. Cardiac SIRT1 activity decreases with age; therefore, ischemia-reperfusion injury is more frequent in elderly people than younger adults [38].
Activation of SIRT1 not only suppresses apoptosis but also balances the oxidative stress in the heart such that lack of SIRT1 causes chronic inflammation, oxidative stress and arrest of the cellular cycle [40]. SIRT1 operates as a cardioprotective molecule protecting against aging and inducing the resistance against the hypertrophic and oxidative stress, inhibiting apoptosis of cardiomyocytes and regulating cardiac energy metabolism [41].
Chan et al. [20] discovered that activation of SIRT1 is protective against homocysteine derived endothelial apoptosis and oxidative stress. Exercise training is shown to increase the SIRT1 activity and antioxidant capacity in elder animals [42].
Most of the studies observe increased SIRT1 expression in both tissues and blood circulation after losing weight and reports diminished SIRT1 levels in obese people [43]. In a previous transgenic rat study, researchers discovered that SIRT1 is acting as a dose-dependent way in the heart. For example, the moderate elevation of SIRT1 preserves cardiomyocytes, and an extreme increase of SIRT1 decreases cardiac function and causes cardiomyopathy [9]. In another study, Sun et al. [44] observed an increase in SIRT1 protein expression in patients with atrial fibrillation. Alcendor et al. [9] demonstrate that increased SIRT1 expression of 2,5 to 7,5 fold decreases age-dependent cardiac hypertrophy, apoptosis, cardiac dysfunction and expression of aging markers; however, 12,5 fold increase in SIRT1 expression causes oxidative stress, apoptosis and increased cardiac hypertrophy.
CONCLUSION
Preventing endothelial dysfunction and reperfusion injury due to ischemia, SIRT1 operates as a cardioprotective molecule via regulating the expression of endothelial nitric oxide synthase (eNOS), which improves endothelial relaxation function and inducing the resistance against the hypertrophic and oxidative stress, inhibiting apoptosis of cardiomyocytes and regulating cardiac energy metabolism. Endothelial SIRT1 is assumed to be an atherosclerotic factor, so suppression of SIRT1 causes increased monocyte affinity to endothelial cells, which is leading an increase in endothelial dysfunction. However, there are not enough data confirming that inhibition of SIRT1 in monocytes is increasing or not in CAD yet; thus, the functional role of monocytes in CAD should be investigated more in future studies to provide a novel therapeutic approach in patients with CAD.
Increasing SIRT1 levels as a therapeutic approach may be suggested as a compensatory mechanism to increase antioxidants, but the appropriate level needed to do so should be evaluated in further prospective studies. Diminished SIRT1 levels in obese people may also be a therapeutic target to be considered in future studies.
SIRT1 participates in the biological processes associated with the development of heart failure (HF), including regulation of energy consumption, autophagy and cell death and survival. Thus, HF is also in the scope of the therapeutic approach depending on the SIRT1 regulation.
Footnotes
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
Authorship Contributions: Concept – LA, OT, ST; Design – LA, HT; Supervision – ST, OT; Analysis and/or interpretation – LA, ST; Literature review – LA, OT, ST; Writing – LA, HT; Critical review – ST.
REFERENCES
- 1.Dai X, Wiernek S, Evans JP, Runge MS. Genetics of coronary artery disease and myocardial infarction. World J Cardiol. 2016;8:1–23. doi: 10.4330/wjc.v8.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS. The epidemic of the 20(th) century:coronary heart disease. Am J Med. 2014;127:807–12. doi: 10.1016/j.amjmed.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–38. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sauve AA, Wolberger C, Schramm VL, Boeke JD. The biochemistry of sirtuins. Annu Rev Biochem. 2006;75:435–65. doi: 10.1146/annurev.biochem.74.082803.133500. [DOI] [PubMed] [Google Scholar]
- 5.Voelter-Mahlknecht S, Mahlknecht U. Cloning chromosomal characterization and mapping of the NAD-dependent histone deacetylases gene sirtuin 1. Int J Mol Med. 2006;17:59–67. [PubMed] [Google Scholar]
- 6.Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, et al. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem. 2010;285:8375–82. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–82. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–60. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–21. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 10.Kilic U, Gok O, Bacaksiz A, Izmirli M, Elibol-Can B, Uysal O. SIRT1 gene polymorphisms affect the protein expression in cardiovascular diseases. PLoS One. 2014;9:e90428. doi: 10.1371/journal.pone.0090428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanno M, Kuno A, Horio Y, Miura T. Emerging beneficial roles of sirtuins in heart failure. Basic Res Cardiol. 2012;107:273. doi: 10.1007/s00395-012-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinciguerra M, Santini MP, Martinez C, Pazienza V, Claycomb WC, Giuliani A, et al. mIGF-1/JNK1/SirT1 signaling confers protection against oxidative stress in the heart. Aging Cell. 2012;11:139–49. doi: 10.1111/j.1474-9726.2011.00766.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamaç AH, Kılı çÜ. Effect of statins on sirtuin 1 and endothelial nitric oxide synthase expression in young patients with a history of premature myocardial infarction. Turk Kardiyol Dern Ars. 2018;46:205–15. doi: 10.5543/tkda.2018.32724. [DOI] [PubMed] [Google Scholar]
- 14.Han J, Wei M, Wang Q, Li X, Zhu C, Mao Y, et al. Association of Genetic Variants of SIRT1 With Type 2 Diabetes Mellitus. Gene Expr. 2015;16:177–85. doi: 10.3727/105221615X14399878166195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donmez G, Outeiro TF. SIRT1 and SIRT2:emerging targets in neurodegeneration. EMBO Mol Med. 2013;5:344–52. doi: 10.1002/emmm.201302451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Ni J, Guo R, Li W. In Patients with Coronary Artery Disease and Type 2 Diabetes SIRT1 Expression in Circulating Mononuclear Cells Is Associated with Levels of Inflammatory Cytokines but Not with Coronary Lesions. Biomed Res Int. 2016;2016 doi: 10.1155/2016/8734827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponugoti B, Kim DH, Xiao Z, Smith Z, Miao J, Zang M, et al. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem. 2010;285:33959–70. doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–32. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- 19.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–58. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan SH, Hung CH, Shih JY, Chu PM, Cheng YH, Lin HC, et al. Exercise intervention attenuates hyperhomocysteinemia-induced aortic endothelial oxidative injury by regulating SIRT1 through mitigating NADPH oxidase/LOX-1 signaling. Redox Biol. 2018;14:116–25. doi: 10.1016/j.redox.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakeling LA, Ions LJ, Ford D. Could Sirt1-mediated epigenetic effects contribute to the longevity response to dietary restriction and be mimicked by other dietary interventions? Age (Dordr) 2009;31:327–41. doi: 10.1007/s11357-009-9104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Höppner S, Schänzer W, Thevis M. Fragmentation studies of SIRT1-activating drugs and their detection in human plasma for doping control purposes. Rapid Commun Mass Spectrom. 2013;27:35–50. doi: 10.1002/rcm.6421. [DOI] [PubMed] [Google Scholar]
- 23.Durand JL, Nawrocki AR, Scherer PE, Jelicks LA. Gender differences in adiponectin modulation of cardiac remodeling in mice deficient in endothelial nitric oxide synthase. J Cell Biochem. 2012;113:3276–87. doi: 10.1002/jcb.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai B, Liang Y, Xu C, Lee MY, Xu A, Wu D, et al. Cyclin-dependent kinase 5-mediated hyperphosphorylation of sirtuin-1 contributes to the development of endothelial senescence and atherosclerosis. Circulation. 2012;126:729–40. doi: 10.1161/CIRCULATIONAHA.112.118778. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Xavier S, Moskowitz-Kassai E, Chen R, Lu CY, Sanduski K, et al. Cathepsin cleavage of sirtuin 1 in endothelial progenitor cells mediates stress-induced premature senescence. Am J Pathol. 2012;180:973–83. doi: 10.1016/j.ajpath.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito T, Yagi S, Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochem Biophys Res Commun. 2010;398:735–40. doi: 10.1016/j.bbrc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Ota H, Akishita M, Eto M, Iijima K, Kaneki M, Ouchi Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol. 2007;43:571–9. doi: 10.1016/j.yjmcc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Orimo M, Minamino T, Miyauchi H, Tateno K, Okada S, Moriya J, et al. Protective role of SIRT1 in diabetic vascular dysfunction. Arterioscler Thromb Vasc Biol. 2009;29:889–94. doi: 10.1161/ATVBAHA.109.185694. [DOI] [PubMed] [Google Scholar]
- 29.Cencioni C, Spallotta F, Mai A, Martelli F, Farsetti A, Zeiher AM, et al. Sirtuin function in aging heart and vessels. J Mol Cell Cardiol. 2015;83:55–61. doi: 10.1016/j.yjmcc.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Yuan J, Luo K, Liu T, Lou Z. Regulation of SIRT1 activity by genotoxic stress. Genes Dev. 2012;26:791–6. doi: 10.1101/gad.188482.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 32.He W, Wang Y, Zhang MZ, You L, Davis LS, Fan H, et al. Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest. 2010;120:1056–68. doi: 10.1172/JCI41563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Planavila A, Dominguez E, Navarro M, Vinciguerra M, Iglesias R, Giralt M, et al. Dilated cardiomyopathy and mitochondrial dysfunction in Sirt1-deficient mice:a role for Sirt1-Mef2 in adult heart. J Mol Cell Cardiol. 2012;53:521–31. doi: 10.1016/j.yjmcc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 34.Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, et al. AMPK and SIRT1:a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298:E751–60. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+metabolism and SIRT1 activity. Nature. 2009;458:1056–60. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulco M, Sartorelli V. Comparing and contrasting the roles of AMPK and SIRT1 in metabolic tissues. Cell Cycle. 2008;7:3669–79. doi: 10.4161/cc.7.23.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Quan N, Sun W, Chen X, Cates C, Rousselle T, et al. Cardiomyocyte-specific deletion of Sirt1 gene sensitizes myocardium to ischaemia and reperfusion injury. Cardiovasc Res. 2018;114:805–21. doi: 10.1093/cvr/cvy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong C, Morrison A, Mattison S, Qian S, Bryniarski M, Rankin B, et al. Impaired SIRT1 nucleocytoplasmic shuttling in the senescent heart during ischemic stress. FASEB J. 2013;27:4332–42. doi: 10.1096/fj.12-216473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Messaoudi S, Rongen GA, de Boer RA, Riksen NP. The cardioprotective effects of metformin. Curr Opin Lipidol. 2011;22:445–53. doi: 10.1097/MOL.0b013e32834ae1a7. [DOI] [PubMed] [Google Scholar]
- 40.Gu C, Xing Y, Jiang L, Chen M, Xu M, Yin Y, et al. Impaired cardiac SIRT1 activity by carbonyl stress contributes to aging-related ischemic intolerance. PLoS One. 2013;8:e74050. doi: 10.1371/journal.pone.0074050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo XY, Qu SL, Tang ZH, Zhang Y, Liu MH, Peng J, et al. SIRT1 in cardiovascular aging. Clin Chim Acta. 2014;437:106–14. doi: 10.1016/j.cca.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 42.Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, et al. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11:139–50. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- 43.Mariani S, Fiore D, Persichetti A, Basciani S, Lubrano C, Poggiogalle E, et al. Circulating SIRT1 Increases After Intragastric Balloon Fat Loss in Obese Patients. Obes Surg. 2016;26:1215–20. doi: 10.1007/s11695-015-1859-4. [DOI] [PubMed] [Google Scholar]
- 44.Sun XL, Bu PL, Liu JN, Wang X, Wu XN, Zhao LX. Expression of SIRT1 in right auricle tissues and the relationship with oxidative stress in patients with atrial fibrillation [Article in Chinese] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2012;28:972–4. [PubMed] [Google Scholar]


