Abstract
Nitric oxide (NO) is a free radical playing an important pathophysiological role in cardiovascular and immune systems. Recent studies reported that NO levels were significantly lower in patients with COVID-19, which was suggested to be closely related to vascular dysfunction and immune inflammation among them. In this review, we examine the potential role of NO during SARS-CoV-2 infection from the perspective of the unique physical, chemical and biological properties and potential mechanisms of NO in COVID-19, as well as possible therapeutic strategies using inhaled NO. We also discuss the limits of NO treatment, and the future application of this approach in prevention and therapy of COVID-19.
Keywords: COVID-19, Nitric oxide, Vasodilation, Anti-viral, Anti-inflammation
Graphical abstract
1. Introduction
Coronaviruses (CoVs) are a family of single-stranded positive-sense RNA viruses that mainly infect mammals and birds. They usually contain a ~30 kilobase (kb) genome and are named after their protruding crown-like spikes on the virus surface [1]. Outside of the human coronaviruses (e.g., types 229E, NL63, OC43, and HKU1) [2,3], CoVs that are found in other species do not infect humans directly from their natural hosts, but they trigger acute respiratory syndromes in humans after overcoming species barriers, such as the severe acute respiratory syndrome (SARS or SARS-CoV-1) in 2002, the Middle East respiratory syndrome (MERS or MERS-CoV) in 2012 and COVID-19 caused by SARS-CoV-2 in 2019 [4]. As of December 1, 2020, the total number of confirmed infected cases worldwide stood at 63.3 million, with a death toll of 1.47 million (from Johns Hopkins Center for Systems Science and Engineering) [5]. The fatality rate has varied greatly by region and age groups. Although many COVID-19 patients may be asymptomatic, those with symptoms include fever, dry cough, shortness of breath and myalgia. Death is mostly due to acute lung injury (ALI), acute respiratory distress syndrome (ARDS) and sepsis, which are caused by viral infection and very similar to the pathological features of SARS and MERS [6]. According to genomic and proteomic analyses, the similarity between total nucleoside sequences of SARS-CoV-2 and SARS-CoV-1 is about 79.5%, and the similarity of amino acid sequences between seven conserved replicate domains in the open reading frame 1ab (ORF1ab) is as high as 94.4% [7,8]. These indicate that SARS-CoV-2 belongs to the β-line coronavirus family and is a member of the SARS-CoV species [7,8]. It has been confirmed that SARS-CoV-2 and SARS-CoV-1 invade cells via a similar mechanism, i.e. binding to human type I trans-membrane receptor angiotensin converting enzyme 2 (ACE-2) through the S-protein. The receptor-binding ability of SARS-CoV-2 is about 4 times that of SARS-CoV-1 [9], which explains the higher infectivity of SARS-CoV-2. Due to the overlap of the genetic structures and pathological features between them, known facts about SARS-CoV-1 indeed have provided hints for our understanding of SARS-CoV-2.
Nitric oxide (NO) is a key player in both the cardiopulmonary and immune systems. The role of NO depends on its site of production and concentration. Abnormal levels of NO in vivo are usually closely related to the occurrence and development of diseases, such as viral infection. To date, there is no comprehensive report on the role, potential mechanism and therapeutic application of NO in COVID-19. In this review, NO in COVID-19 was systematically examined from the perspectives of its general features, six known pathways in lungs, possible roles in COVID-19 etiology, and clinical use in COVID-19 prevention and therapy.
2. NO level and bioavailability in COVID-19 patients
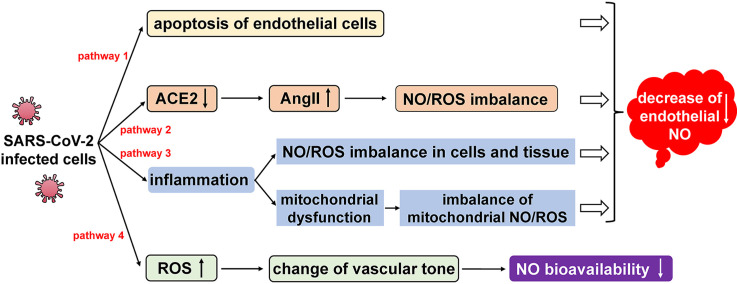
Determination of nitrate and nitrite (metabolites of NO, abbreviated as NOx) by the Griess assay in the blood of patients with severe COVID-19 revealed that the production of NO was significantly higher than that of healthy individuals (as a control group) [10]. This may be compatible with macrophage activation, which is common during inflammatory immune responses. Inducible nitric oxide synthase (iNOS) in macrophages can be 2–3 fold higher following inflammation, which releases a large amount of NO leading to local and systemic increases of nitrate or nitrite [11]. However, one original clinical report from Canada showed that, through thrombotic factor profiling with immunoassays and in vitro experiments on human pulmonary microvascular endothelial cells, exaggerated and persistent injury to endothelium in severe COVID-19 patients was clearly found [12]. In addition, diffuse lymphocytic endotheliitis and apoptotic bodies were also observed in autopsy and surgical tissue specimens [13], which showed significantly decreased endothelial NO in patients with COVID-19 or related complications [14], and was suggested to be closely related to lung injury and an imbalance of NO and ROS [15]. Different findings about iNOS and eNOS suggested multiple pathways of NO during infection. Four potential pathways during SARS-CoV-2 infection have been described as below (Fig. 1 ).
Fig. 1.
Regulation of NO level and bioavailability via four potential pathways in COVID-19 etiology.
Although SARS-CoV-2 mainly infects bronchial ciliated epithelial cells and pulmonary type II cells, residual viral particles in endothelial cells were also found by electron microscopy. This observation confirmed that SARS-CoV-2, like SARS-CoV-1 and MERS-CoV, could directly infect endothelial cells, leading to cell apoptosis and a decrease of endothelial NO (Fig. 1, Pathway 1) [16]. In addition, decreased NO production is also along the progression of viral infection. SARS-CoV-2 invades host cells through its surface stimulating glycoprotein-S protein binding to angiotensin converting enzyme 2 (ACE2), and then down-regulates expression of ACE2 [17]. It is known that ACE converts angiotensin I (AngI) into the pro-inflammatory peptide angiotensin II (AngII), and ACE2 metabolizes AngII to produce angiotensin-(1–7) ((Ang-(1–7))) and Ang-(1–7) promotes endothelial cells to produce NO. Due to down-regulation of ACE2, suppression of ACE and its downstream product AngII is alleviated. ACE inhibits NO production, and promotes ROS production and inflammation. Furthermore, as a pro-inflammatory peptide, AngII itself activates macrophages to produce pro-inflammatory cytokines and ROS [18,19], leading to excessive inflammatory responses and NO/ROS imbalance (Fig. 1, Pathway 2). Inflammation is the normal response of the human immune system to injuries and attacks. Analysis of peripheral blood indicated that viral infection often caused a significant increase in pro-inflammatory cytokines and chemokines, and developed into a strong cytokine storm [20,21]. When high inflammation persists for a long time, it causes damage to multiple tissues and organs. In addition, high inflammation causes a severe imbalance of NO/ROS in body, which in turn leads to oxidative stress (Fig. 1, Pathway 3) [22]. A large number of activated pro-inflammatory cytokines and chemokines were found in serum of patients with severe COVID-19, including IL-2, IL-6, IL-10, TNF-α, GSCF and MCP-1 [23]. By blocking mitochondrial oxidative phosphorylation and adenosine triphosphate production, pro-inflammatory cytokines promote production of excessive ROS in the mitochondria, and lead to increased membrane permeability and changed dynamics, resulting in mitochondrial dysfunction and apoptosis [24]. Mitochondria are the main source of ROS. When excessive ROS are present, endothelial injury is aggravated by cell apoptosis, activation of transcription factors (NF-kB, AP-1), and overexpression of inflammatory cytokines and adhesion molecules (ICAM-1, VCAM-1, E-selectin), which then significantly reduce the production of NO (Fig. 1, Pathway 3). In addition, ROS changes vascular tones by increasing intracellular calcium concentration and reducing the bioavailability of NO (Fig. 1, Pathway 4) [25].
How NO level and bioavailability impacted COVID-19 patients is closely related to its unique features. In the following section, the general biochemical properties of NO would be described, in order to understand its role in COVID-19.
3. General biochemical features of NO
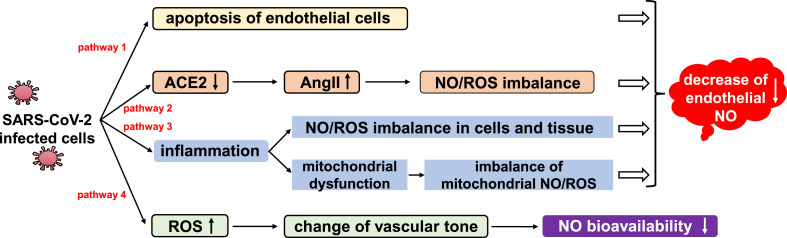
NO is a highly reactive heteronuclear diatomic gas molecule with one single electron in the 2p-π anti-bonding orbital (π*2p). It reacts with various reagents and regulates multiple signaling pathways. NO can directly bind to target molecules including metal centers, DNA and lipid free radicals, and also reacts with oxygen (O2) or superoxide free radicals (O2 -) to form corresponding nitrogen oxide compounds, which then attack target molecules. There are two main synthetic pathways of NO in vivo: the aerobic oxidation of l-arginine (L-Arg) catalyzed by nitric oxide synthase (NOS), and the anaerobic or hypoxic reduction of nitrite catalyzed by nitrite reductase. NO also gets produced through nitrite disproportionation, or via release from dinitrosyl iron complex (DNIC) in vivo (Fig. 2 ).
Fig. 2.
General biochemical features of NO.
Under normoxia, NO can be generated from three types of NOSs encoded by different genes: endothelial nitric oxide synthase (eNOS), neuronal nitric oxide synthase (nNOS) and iNOS (Fig. 2). Among them, eNOS and nNOS are constitutive nitric oxide synthases that are found in endothelial, epithelial, smooth muscle and nerve cells. eNOS and nNOS are regulated by intracellular calcium and calmodulin. iNOS is mainly found in macrophages, monocytes and muscle cells, and does not depend on the regulation of calcium ions [26,27]. Depending on its source and concentration, NO has a Janus face in a variety of pathophysiological processes, participating in the regulation of blood circulation, immune response and nervous system signaling [28].
4. Role of NO in lungs
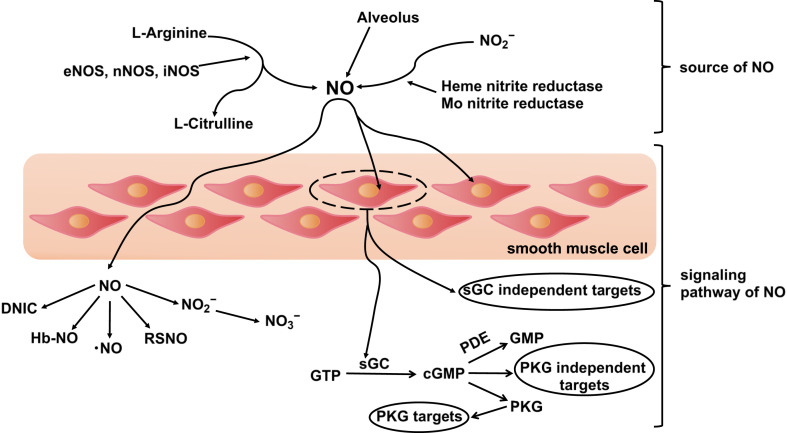
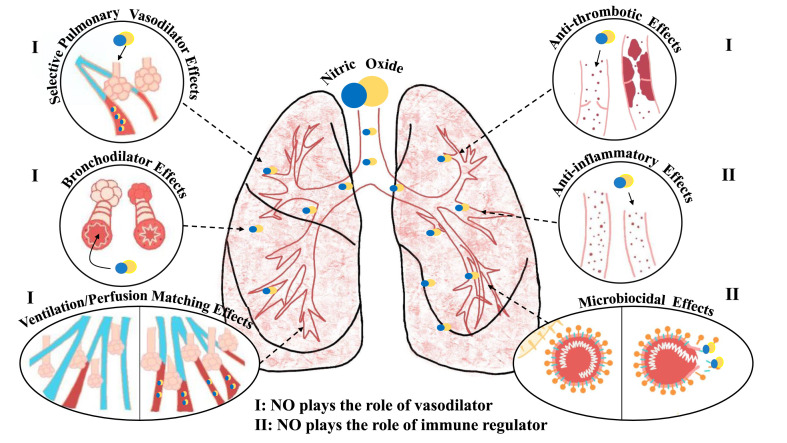
NO can serve as a vasodilator or immune regulator (Fig. 3 ) [[29], [30], [31], [32]]. As a vasodilator, NO functions as a selective pulmonary vasodilator to improve oxygenation and reduce pulmonary vascular resistance (Fig. 3, upper left), as a bronchial/airway dilator to promote oxygen inhalation (Fig. 3, middle left), to increase the blood flow of capillaries that exchange gas with alveoli and accelerate oxygen circulation of body (Fig. 3, lower left), and as a vascular anticoagulant that inhibits blood clotting and excessive platelet activation (Fig. 3, upper right). The effects of NO as an immune regulator include: as an anti-inflammatory molecule, preventing excessive inflammation through early non-specific immunity, and later regulation of vascular inflammation and immune cell proliferation (Fig. 3, middle right), and as an antiviral effector, inhibiting the replication cycle of SARS-CoV-1/SARS-CoV-2 (Fig. 3, bottom right). In COVID-19, NO was reported to function from one of these four potential mechanisms: vasodilation, anticoagulation, anti-inflammation and anti-viral [33].
Fig. 3.
Six pathways of NO to function in the lungs. (I) NO plays the role of a vasodilator, including selective pulmonary vasodilator effects, bronchodilator effects, increased blood flow to the alveoli and anti-thrombotic effects. (II) NO plays the role of an immune regulator, including anti-inflammatory effects and microbiocidal effects (reference 33, reprinted with permission of the American Thoracic Society. Copyright © 2020 American Thoracic Society. Cite: Roger A. Alvarez, Lorenzo Berra, and Mark T. Gladwin. 2020, Home Nitric Oxide Therapy for COVID-19, Am J Respir Crit Care Med, 202, 16–20. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society).
4.1. NO as a vasodilator
Around three decades ago, researchers found that NO promoted vasodilation, improving oxygenation and reducing pulmonary vascular resistance. Ever since, NO has been used to treat pulmonary dysfunction [34]. At vasoconstriction (general physiological contraction, inflammation or vascular injury), NO effectively relaxes smooth muscle cells and dilates blood vessels, therefore modulating vascular tension and local blood flow.
It has been generally accepted that NO binds to the heme center of its typical receptor, soluble guanylate cyclase (sGC), to form a NO-ferrous heme adduct (Heme-NO), thus activating sGC, and resulting in tremendous production of intracellular cyclic guanosine monophosphate (cGMP). cGMP is a second messenger that activates Ca2+ATPase, a P-type ATPase enzyme, which reduces intracellular Ca2+ concentration and relaxes smooth muscle cells. Kinase-G also promotes phosphorylation of protein G in smooth muscle cells, which connects seven regions of transmembrane receptors and phospholipases. It then thereby inhibits ligand-receptor interaction and contractile signal transmission, enhancing relaxation of smooth muscle cells and promoting local blood flow [35,36]. In addition, NO is also involved in the metabolic pathways of nitrosothiol (RSNO). RSNO is relatively stable, with a half-life of 40 min in an oxygen-rich environment (anaerobic half-life of NO is only 1–5 s, oxygen-rich <3 s). It has a strong bronchiectasis effect independent of the cGMP pathway, which effectively improves airway tension and increases oxygen intake [37].
Hypertension, diabetes, and cardiovascular diseases often associate with vascular dysfunction and decreased endothelial NO production or bioavailability [38]. Compared to other chronic conditions, these three diseases have become the most frequent comorbidities in COVID-19 patients who require hospitalization. In a meta-analysis that included six Chinese studies and 1527 COVID-19 patients, the overall prevalence of hypertension, cardiovascular and cerebrovascular diseases and diabetes were 17.1%, 16.4% and 9.7%, respectively. Another pooled analysis included seven studies published from Jan 1, 2019 to Feb 25, 2020 in PubMed, EMBASE, and the Web of Science databases, and showed that hypertension and a history of cardiovascular disease conferred 2.4 (95% confidence interval [CI] 1.5–3.8) and 3.4 (95% CI 1.9–6.2) times higher risk of severe COVID-19 disease [39], which suggest an important role for NO in COVID-19 etiology.
4.2. NO as an anticoagulant
Endothelial cells produce anticoagulants, including heparin, prostaglandin and NO [40]. NO inhibits blood clotting and excessive activation of platelets. Under normoxia, NO is produced by l-arginine catalyzed by calmodulin-dependent NOS. It maintains physiological vascular homeostasis in tissue and protects blood vessels from damage by platelets and circulating cells. Pathological changes start following endothelial cell dysfunction, including decreased or ceased release of NO depending on the degree of injury. It leads to accumulation of free Ca2+ in vascular smooth muscle cells, continuous vasoconstriction, and subsequently a blood hypercoagulable state. Platelets contain vascular growth factors and release a variety of pro-inflammatory mediators. When blood vessels are damaged, platelets quickly gather to the injured site to form platelet clots and a complex with plasma factor VIIa, whose subsequent interaction with extravascular tissue factor initiates the action of thrombin (via conversion of inactive protease factor X into the active protease factor Xa). Thrombin then converts soluble fibrin into insoluble fibrin, which makes the platelet clot entangled with blood cells to form a thrombus. Therefore, a decrease of endothelial NO production is a sign of endothelial dysfunction and thrombotic events [41].
Abnormal coagulation has been reported in critically ill patients with SARS and MERS, and is closely related to poor prognosis. Autopsy reports on SARS patients indicated thrombosis in lungs, bronchi and small pulmonary veins [42,43]. Recent studies showed that a hypercoagulable state was one of the main pathological events in COVID-1944. Pulmonary and distal thrombotic complications were one important reason for high mortality in COVID-19 patients [44]. Early coagulation disorders in patients with COVID-19 were characterized by a significant increase in D-dimer and fibrin/fibrinogen degradation products, which developed into disseminated intravascular coagulation (DIC) in severe cases [45]. A clinical report on 99 patients diagnosed with COVID-19 at Jinyintan Hospital in Wuhan showed that about 36% patients had a significant increase of D-dimer during hospitalization (>1.0 μg/mL) [[46], [47], [48]]. Activated partial thromboplastin time (APTT) (21–37 s) and prothrombin time (PT) (9.2–15 s) also increased significantly [[46], [47], [48]], suggesting that these infected patients had the risk of thrombosis. Excessive platelet activation and platelet-monocyte aggregation caused by high blood coagulation were observed in 49 patients with severe COVID-19 treated by the Oswaldo Cruz Institute in Brazil, which was not observed in patients with mild cases [44], and suggested the pathological inflammatory activation of thrombus formation in severe patients.
4.3. NO as an anti-inflammatory molecule
Inflammation is one major defensive mechanism to inactivate pathogens, remove irritants, and lay the foundation for tissue repairs [49]. However, excessive inflammation causes injury. Studies have shown that NO promotes or inhibits almost every stage of inflammation [50]. Low concentrations of NO produced by eNOS reduce vascular permeability and inhibit inflammatory exudation, but high NO produced by iNOS has the opposite effect [51]. When pathogens invade the body, immune cells recognize them and their pattern molecules through germline-encoded pattern recognition receptors (PRRs). When PRRs sense the presence of pathogen-associated molecular patterns (PAMPs), including exogenous pathogen cell wall components, flagellin, and nucleic acid, they immediately activate the NF-κB and MAPK pathways. The transcription factors NF-κB and activator protein (AP-1) induces expression of iNOS, leading to an increase in NO concentration. The bactericidal effect of NO involves DNA damage, protein modification, inhibition of the mitochondrial electron transport chain or other metabolic pathway enzymes. These biochemical reactions produce cytotoxicity to pathogens and inhibit their further invasion. Because immune cells are the main effector cells of iNOS expression, the bactericidal effect of NO produced by iNOS is mainly aimed at microorganisms in the cytoplasm. Additionally, due to the membrane permeability of NO, it can also kill extracellular pathogens through diffusion [52].
Moreover, NO is also involved in the regulation of vascular inflammation through ameliorating vascular dysfunction and preventing complications, such as tissue edema caused by vascular leakage and respiratory failure. NO reduces vascular damage caused by inflammation [53]. Furthermore, NO inhibits proliferation of immune cells in inflammatory responses. It inhibits production of a large number of cytokines in lymphocytes, eosinophils, monocytes and other immune cells, including key cytokines in the inflammatory response [26]. Therefore, extreme inflammatory effects, such as the cytokine storm, are diminished, and uncontrolled body injury is avoided by NO [54].
4.4. NO as an antiviral effector
Non-specific antiviral effects of NO have been reported in a variety of viral infections, including HIV, vaccinia virus, enterovirus and coronavirus [[55], [56], [57]]. For example, expression of iNOS was found to significantly increase in mouse heart infected with coxsackievirus B3, a common enterovirus. The viral load increased significantly after NG-monomethyl-l-arginine acetate (L-NMMA) or nitro-l-arginine methyl ester (l-NAME) was used to inhibit iNOS [58]. In addition, it was found that the NO donor S-nitroso-N-acetylpenicillamine (SNAP) significantly inhibited the 3C protease of coxsackievirus by acting on the viral replication process [59]. During the SARS outbreak, researchers found that exogenous NO donors effectively suppressed SARS-CoV-1. The mechanisms will be described as below.
4.4.1. Inhibition of NO on the SARS-CoV-1 replication cycle
In 2004, a Swedish group studied the inhibitory effect of NO donors on SARS-CoV-1 infection in VeroE6 cells [60]. Cells were treated with SNAP and another non-NO donor compound (as control) at various concentrations (0, 50, 100, 200, 400 μM) at 1 h after infection. The 50% tissue culture infection dose (TCID50) was determined after 24 h. The results showed that SNAP significantly inhibited the replication cycle of SARS-CoV-1 at both RNA and cellular levels in a dose-dependent manner. Besides, iNOS expression was found to accompany decreased offspring viruses by 82% [60]. Two NO-mediated antiviral mechanisms were proposed, which were later experimentally verified: (1) NO affected one or two replication-related cysteine proteases encoded by SARS-CoV-1 ORF1a, which directly inhibited viral RNA replication, and (2) NO decreased the palmitoylation level of S protein and inhibited the membrane fusion of offspring virus S protein binding to host cell receptor ACE261.
Replication of SARS-CoV-1 was mediated by the nonstructural proteins nsp1-nsp16, and the later contain two cysteine proteases, which would be discussed in further details below [62,63]. Cysteine protease cleaves pp1ab replicase polyproteins with varied efficiency. Upon treatment with SNAP, two new high-molecular weight peptides were found. It was suggested that NO changed the original cutting mode of cysteine proteases, thereby affecting production of the non-structural proteins, and terminating the replication process of viral RNA [61,64].
The effect of NO on S protein was also investigated. VeroE6 cells infected with recombinant vaccinia virus carrying S gene (rVV-L-S) were treated with 400 μM SNAP, which was labeled with [3H] palmitic acid. After immune-precipitation of the S protein, it was found that SNAP treatment significantly reduced the number of palmitoylated S protein. The intercellular fusion was significantly decreased due to the low expression of S protein after SNAP-treated rVV-L-S was mixed with CHO-ACE2 cells (a cell model that stably expresses ACE2 on the cell surface). The results showed that the entry efficiency of the pseudotyped virus was significantly lower after SNAP treatment, and the virus infection rate decreased by about 70% [61]. O2 - is also produced during viral infection, which reacts with NO readily to produce peroxynitrite (ONOO-) [65], a viral inhibitor. In order to clarify whether ONOO- contributed to this effect, the ONOO- donor SIN-1 was used to treat cells infected with SARS-CoV-1. ONOO- did not show inhibitory effect on SARS-CoV-1 replication, which ruled out the contribution of peroxynitrite in this case [61].
4.4.2. Potential mechanism of NO in COVID-19
SARS-CoV-2 and SARS-CoV-1 share a similar infection process: they both rely on the membrane fusion mediated by the viral S protein with the host cell receptor ACE2 to promote the injection of viral genetic material [66]. At present, the 3D atomic map of the SARS-CoV-2 S protein has been successfully constructed by cryogenic electron microscopy. The SARS-CoV-2 S protein is a trimer protein with a large number of glycosylation modifications, and its protein sequence is very similar to the S protein of SARS-CoV-1 [67]: although the S2 region (mediated membrane fusion) is highly homologous (99%), there is a difference in amino acid residues of the S protein receptor region (RBD). This difference has been shown to promote the cell entry mechanism of SARS-CoV-2 [68,69]. In addition, the SARS-CoV-2 genome is divided into six main functional open reading frames, including ORF1ab, spinous (S), envelope (E), membrane glycoprotein (M), nucleocapsid (N) and helper gene [70]. The replicase protein pp1ab, formed by ORF1ab, is cleaved into 16 non-structural proteins (nsp) involved in virus replication by papain-like (PLPro) and 3C-like (3CLPro) proteases encoded by SARS-CoV-2. In addition, the homology between the SARS-CoV-2-encoded 3CLPro and that of SARS-CoV-1 is as high as 96%, and their structures are basically similar [71,72].
Therefore, the inhibition of SARS-CoV-2 by NO may be similar to that of SARS-CoV-1. NO also inhibits viral replication by affecting one or two cysteine proteases encoded by the SARS-CoV-2 ORF1a and reducing the palmitoylation level of the S protein [73]. However, the mechanism of NO in SARS-CoV-2 remains unclear. Researchers have recommended NO together with clinically recommended antiviral drugs as an effective strategy for the treatment of COVID-19 [71,74].
5. Application of NO in clinical treatment
5.1. Use of NO in pulmonary and cardiovascular diseases
The main format of NO used in therapy is its precursors, such as sodium nitroprusside (SNP). SNP is usually administered intravenously and releases NO immediately after entering the bloodstream. It is widely prescribed as a vasodilator for the treatment of acute hypertension. However, intravenous administration of this type of drugs may lead to systemic vasodilation and arterial hypotension [75], and alternative medicines have therefore attracted much interest.
NOS is expressed in healthy paranasal sinus epithelial cells and produces high-level NO gas continuously [76]. Through respiration, NO then reaches deep regions of lungs at a lower concentration, promoting dilation of bronchi and blood vessels, as well as increasing oxygen intake in the lungs [77,78]. Upon reaching the bloodstream, NO may immediately get scavenged by hemoglobin (Hb), thereby preventing systemic vasodilatation [79]. Therefore, researchers have tested the effect of inhaled NO (iNO) as a selective pulmonary vasodilator as a potential treatment for respiratory failure caused by lung diseases [80]. Currently, iNO has been used in the treatment of ARDS, pulmonary bacterial infections and persistent pulmonary hypertension of the newborn (PPHN), and was approved by the FDA as a clinical adjuvant therapy for PPHN in 1999 [81,82]. iNO was also known to benefit therapy in other diseases, including myocardial or cerebral ischemia, sickle cell disease, cerebral malaria and ischemia-reperfusion injury. iNO may be used in surgery or organ transplants according to animal experiments. Although there were no obvious side effects after iNO treatment, it is necessary to continuously monitor the levels of met-myoglobin and nitrogen dioxide (NO2), as well as changes in blood clotting [81,83].
Coronavirus mainly infects humans through the respiratory tract. In severe cases, it causes complications, such as respiratory failure and irreversible damage to the lungs. Based on the above therapeutic features, iNO had been studied by researchers as an adjuvant therapy for respiratory failure caused by coronaviruses.
5.2. Use of NO in SARS and MERS
The global mortality rate of SARS patients was 10.5%, with around 20% patients developed ARDS, accompanied by severe lung infiltration and extensive consolidation [[84], [85], [86]]. Corticosteroids and ribavirin were widely used to reduce pulmonary infection in patients, but extensive use of these drugs had serious side effects, including osteonecrosis of femoral heads [87]. The optimal treatment strategy for SARS had not been released yet. Appropriate preventive measures were certainly needed to slow down aggravation of this disease.
During May–July 2003, one rescue trial of SARS suggested that exogenous inhaled NO could effectively improve arterial oxygenation in severe patients and inhibit the virus [85]. This trial involved 14 SARS patients (8 females and 6 males, ages 19–63) who were treated in the intensive care units (ICU) of Chao Yang Hospital and China-Japan Friendship Hospital. Patients were divided into two groups, one treatment group (6 subjects) and one control group (8 subjects). These six subjects received iNO for at least 3 days, decreasing dose day by day from 30 ppm, 20 ppm–10 ppm, and then to 0 ppm on the 4th day. After that, the subjects continued to inhale 10 ppm NO if arterial oxygenation deteriorated. Blood oxygen saturation (SpO2) was continuously monitored and the inhaled oxygen fraction (FiO2) was measured intermittently in 4 patients in the treatment group and 5 in the control group. Results showed that after iNO treatment, the average SpO2 increased from 93% to 99%, and the oxygen supply decreased from 6 L/min to 2 L/min. The ratio of PaO2 (the partial pressure of oxygen)/FiO2 monitored (4 subjects) increased from 97 mmHg to 260 mmHg. Also, continuous positive airway pressure (CPAP) and bilevel mask positive airway pressure (BiPAP) ventilation were reduced and even discontinued in all 4 subjects. In addition, SpO2 remained at a high level after stopping NO inhalation. The density of pulmonary infiltration in 5 patients also decreased significantly, and chest radiography showed a decreased spread or decreased density of the lung infiltrates in 5 of the 6 patients. In the control group, two patients died, with the other six patients recovering and leaving the hospital within 8 weeks after the end of the study. Taken together, these results showed the great potential of iNO for SARS treatment [85].
During the MERS outbreak in 2012, noninvasive ventilation (NIV) was commonly used for patients who had related acute hypoxemic respiratory failure (AHRF), although the overall effectiveness remained controversial [88]. Analysis on a multicenter retrospective cohort of severe MERS patients from 14 participating tertiary care hospitals in 5 cities in Saudi Arabia admitted between September 2012 and October 2015 were reported on August 9, 2018. This analysis included 302 MERS patients. 105 (35%) patients initially used NIV, whereas 197 (65%) patients were only managed with invasive mechanical ventilation (MV). Through the Mann-Whitney U or Student's t tests, the main interventions for patients initially managed with NIV were compared to those managed only with invasive MV. Results showed that patients managed initially with NIV were more likely to subsequently require iNO compared to invasive MV patients [20.0% vs 11.7%, P = 0.05], and suggested the important role of iNO as an adjunctive therapy in the treatment of MERS [89].
According to the above promising outcomes among SARS and MERS patients, investigations of iNO therapy are warranted for the treatment of COVID-19, and have been put into practice for a number of clinical trials as discussed below.
5.3. Use of NO in the treatment of COVID-19
5.3.1. Use of NO in moderate and severe COVID-19 patients
Studies have shown that about 26% of COVID-19 patients needed ICU treatment, out of whom 61% developed ARDS [90,91]. Once ARDS occurs in patients with hypoxemia, invasive therapy has been shown to be indispensable [92]. Moreover, there is no other treatment that could replace oxygen ventilation support in critically ill patients. As such, iNO has been studied as an alternative rescue method in COVID-1993.
In March 2020, one multicenter clinical trial was organized by Harvard University and Air Force Medical University (China) to investigate whether continuous inhalation of NO could be used as a rescue therapy to effectively improve oxygenation and the survival rate of COVID-19 patients. Exclusion criteria included a history of intubation less than 72 h before treatment, lung malignant tumors, brain function damages, etc. 200 patients were recruited and randomly divided into the iNO-treatment and control groups. The treatment group first received 80 ppm NO, and then 40 ppm until symptoms of hypoxia completely disappeared. Once PaO2/FiO2 was >300 mmHg within 24 h, patients could be weaned from iNO step by step. Levels of NO2 (<2 ppm) and methemoglobin (non-invasive combined oximeter, <5%) were closely monitored throughout the study to ensure the steady progress of the experiment (Table 1 , NCT04306393) [94]. Outcomes of this trial have not been released yet.
Table 1.
Clinical regimens of iNO for prevention or treatment of COVID-19.
| Subjects (patients) | Dose (ppm) | Number/time of action | Total days | Existing/possible results | Ref/NCT/EUCTR |
|---|---|---|---|---|---|
| Moderate/severe (200) | 80 → 40 | All day | 2 | Arterial oxygen saturation increased about 20% | 04306393 87a |
| ICU (20) | 160 | 6 h/day once a day | 2 | High dose iNO could have beneficial effects | 04383002 |
| Severe (100) | 1–80 | / | / | Reduced organ damage, mechanical ventilation, death | 2020-001329-30-AT |
| Moderate/severe (42) | 20 | 1.5 times/hour | 14 | Reduced progressive systemic deoxygenation, intubation, or death | 04388683b |
| Moderate/severe (20) | 160 | / | 26 | Reduced oxygen therapy, BIPAP, CPAP, intubation, mechanical ventilationc | 03331445a |
| Severe pregnant (6) | 160 → 200 | twice a day | <17 | Improved cardiopulmonary function, newborns were in good condition | 90a |
| Mild (260) | 140 → 300 | 20–30 min/time | / | Improved short term respiratory status, prevent hospitalization | 04338828 |
| Mild/moderate (67) | 140 → 180 | 20–30 min/time twice a day | 14 | iNO has beneficial effects | 04305457 |
| Mild (300) | / | Nasal Spray + Nasal Irrigation/day | 14 | Treat and prevent exacerbation | 04443868 |
| Mild (240) | 140 → 180 | 20–30min/time twice a day | 28 | Safely slow down or stop deterioration | 91a |
| Mild (20) | 200/200 + 20 | 30 min/time twice a day (20 ppm all day) | 14 | Improved oxygenation | 04476992a |
| Mild (39) | 30(start) | Up or down through the course of disease | 2.1 | Reduced endotracheal intubation (53.9%), improved oxygenation | 92 |
| Mild (female) | 20/day 10 → 0/night |
12–14 h/day (2 h/night) | 10 | Progressive dyspnea and fatigue etc. Completely disappeared | 93a |
| Close contacts (470) | 160 | 30 min/times twice a day | 14 | Reduce the incidence to 5% | 04312243 95a |
| Close contacts (200) | / | NOG/morning NONI/evening NONS<5 times/dayb |
14 | Inactivate SARS-CoV-2 | 04337918 |
| Mild (50) | 80/150 | 40 min/time four times/day | <14 | / | 04456088 |
| All (20) | 80 | 40 min/time four times/day | / | 04397692 | |
| Moderate/severe (500) | 20 | All day | <14 | INOpulse is safe and effective | 04421508 |
| Mild/moderate | 20 | 8–24 h/day | / | INOpulse is safe and effective | 2020-002394-94-BE |
Levels of NO2 (<2 ppm) or methemoglobin (<5%) are closely monitored throughout the experiment.
Nitric Oxide Gargle (NOG), Nitric Oxide Nasophyaryngeal Irrigation (NONI), Nitric Oxide Nasal Spray (NONS).
Biphasic intermittent positive airway pressure (BIPAP), continuous positive airway pressure (CPAP).
University Health Network submitted a clinical trial in May 2020 on whether the use of high dose iNO (≥160 ppm, high medical dose) was safe and could reverse virus burden and respiratory failure in COVID-19 patients on mechanical ventilation. It was expected to recruit 20 patients, each of whom would be given 160 ppm iNO for 6 h, once per day for two days. The primary outcome measured COVID-19 PCR status at completion of treatment (day 7) from tracheal aspirate (Table 1, NCT04383002)95. Similar trials were carried out, including EUCTR2020-001329-30-AT [96], which focused on iNO therapy in patients with pulmonary failure (Table 1, all underway).
In the same month, Tufts Medical Center carried out a pilot randomized-controlled (2:1) open label investigation of iNO to prevent progression to more advanced disease among 42 COVID-19 patients with dyspnea. Subjects received NO using one an iNO pulse device with a dose of 125 mcg/kg IBW/hr (equivalent to approximately 20 ppm). The primary outcome of this study is to determine whether iNO treatment slows down progressive systemic hypoxia within 28 days (Table 1, NCT04388683) [95]. A similar trial including NCT03331445, which focuses on patients with respiratory distress, is being carried out by Nitric Solutions-Mobile Unit (Table 1, all underway) [95].
On August 26, 2020, a clinical trial of iNO in severe pregnant patients with COVID-19 in Massachusetts General Hospital was reported. Six pregnant women with severe COVID-19 from April to June 2020 were admitted and received iNO therapy. They were treated with high-dose NO (160–200 ppm) via mask twice a day, and a total of 39 treatments was administered. An improvement in cardiopulmonary function was observed after commencing NO gas, as evidenced by an increase in systemic oxygenation in each administration session among those with evidence of baseline hypoxemia, and reduction of tachypnea in all patients in each session. Three patients delivered a total of four neonates during hospitalization and they were home after 28 days with their newborns in good condition. Five of six patients had two negative test results by nasopharyngeal swab for SARS-CoV-2 within 28 days from admission. The result of the study showed that 160–200 ppm iNO appeared to be well tolerated, and might benefit pregnant patients with hypoxic respiratory failure (Table 1) [97].
5.3.2. Use of NO in mild patients
In addition to the treatment of moderate or severe COVID-19 patients, iNO was also applied to treat patients with a mild form of the disease.
In April 2020, a clinical trial on mild COVID-19 patients (defined by a positive RT-PCR test for SARS-CoV-2) was carried out at Massachusetts General Hospital. This trial was expected to recruit 420 patients with mild COVID-19 (210 subjects, and the other 210 as a control group) and the iNO treatment would be administered at 140–300 ppm for 20–30 min. The primary goal was to prevent the deterioration of mild COVID-19 patients, which was defined as whether patients needed to return to the emergency department or be intubated (Table 1, NCT04338828)95. Similar trials include NCT04305457 (Massachusetts General Hospital) and NCT04443868 (Sanotize Research and Development Corp) (Table 1, all underway) [95].
In May 2020, Xijing Hospital and Massachusetts General Hospital also designed a joint trial on early-stage COVID-19 patients, who still breathed on their own, in order to determine whether high-dose iNO treatment could safely slow down or prevent the deterioration of this disease. 240 patients with mild symptoms such as fever and cough were recruited. They were treated with 140 ppm iNO twice daily and the number of patients who needed endotracheal intubation within 28 days was recorded (Table 1, underway) [98]. Similar trials include NCT04476992 (Safety Study on the Use of Intermittent Versus Continuous Inhalation of NO in Spontaneous Breathing COVID-19 Patients) carried out in Research Institute of Cardiology (Table 1, underway) [95].
The combined use of NO and other medicines were also studied. Boston University conducted a small clinical trial on the therapeutic effect of iNO in patients with COVID-19 spontaneous respiration. In this study, 39 COVID-19 patients with an average age of 61 years, who could breathe on their own, were treated with iNO for an average course of 2.1 days. The initial dose of iNO was 30 ppm (increased or decreased according to the course of disease in the later stage), and SpO2/FiO2 was used to evaluate the oxygenation status during ventilation. During this period, patients were treated with IL-6 receptor antagonists (34 cases, 87.2%), hydroxychloroquine (24 cases, 61.5%), azithromycin (21 cases, 53.9%) and autologous immunomodulators (23 cases, 59%). After SASv9.4 analysis, the results showed that a total of 21 patients (53.9%) did not need follow-up invasive mechanical ventilation after iNO treatment, of which 20 cases were successfully discharged from the hospital, with 1 case resulting in death. The median ratio of SpO2/FiO2 (IQR) decreased from 108 (96–118) to 54.9 (30–86.1) (Table 1) [99].
Inhaled NO has not been widely promoted for the treatment of COVID-19. To date, no outcomes from large-scale clinical trials have been released. Besides the trials mentioned above, there was a single case involving one patient who significantly improved after treatment with only iNO. This female patient had idiopathic pulmonary artery hypertension (IPAH) before being diagnosed with COVID-19, and had symptoms such as progressive dyspnea and fatigue that might have aggravated into pulmonary hypertension before treatment. She was evaluated with a non-canister iNO system, GENOSYL, which is commonly used to treat PPHN in newborns. The patient was first treated with 20 ppm NO plus 2 LPM intranasal supplementation of O2 for 12–14 h during the daytime, and the dose was gradually reduced at night (10, 5, 0 ppm) for 2–3 h. After 6 days of inhalation treatment, the above symptoms were improved, and completely disappeared within only 10 days after the dose was gradually reduced (Table 1) [100]. Although it was unclear whether she was completely recovered from COVID-19, her treatment was carried out entirely at home and did not require any emergency care or hospitalization. Certainly this single case is not representative of most COVID-19 patients, or even similar co-infection patients. However, it does indicate the potential of a portable NO inhalation system for treating mild COVID-19 patients at home to improve dyspnea and other symptoms [33], in order to achieve the effect of shunt treatment.
5.4. Use of NO in COVID-19 prevention
With the COVID-19 pandemic infecting tens of millions worldwide, identifying safe and effective preventive measures to stop the spread of the disease has become paramount. NO has been known to prevent viral transmission, and promote viral clearance and host recovery. Therefore, researchers speculated that exogenous NO might be effective for SARS-CoV-2 infection prevention, and recently iNO has been put into clinical trials for this purpose [101].
One randomized clinical trial of iNO (160 ppm) was conducted to prevent COVID-19 infection among healthcare workers at Massachusetts General Hospital. It was aimed at helping healthcare workers who were in close contact with COVID-19 patients. 470 people (235 in the iNO group) were expected to be recruited and given 160 ppm NO inhalation administration for 30 min twice daily. The incidence of COVID-19 was compared between the iNO and control groups. Based on the clinical data available in China and Italy, iNO inhalation was expected to reduce the incidence to 5% (previously 15%). In addition, a portable NO inhalation device was invented for this study. The device was small in size and easy to carry, with a gas storage capacity of 3 L. It also minimized the production of NO2, which facilitates wide clinical application of iNO (Table 1, NCT04312243, underway) [101]. A similar trial, NCT04337918, is also underway (Sanotize Research and Development Corp, Table 1) [95].
During the SARS outbreak in China, an interesting phenomenon observed was that only 8% of smokers (mostly male) have been infected with SARS-CoV-1 [102]. In this COVID-19 pandemic, less than 10% of smokers have been infected, much lower than the percentage of male smokers among the whole male population (~52%) [103]. In addition, there were similar reports in France, the United States and Italy, where smokers infected with SARS-CoV-2 were only 5–6% when the reference number was 25.4%, 13.7% and 14.9%, respectively [93]. With a range of 250–1350 ppm, NO is the main nitrogen oxide in cigarette smoke (the concentration of NO2 is very low or undetectable), and is much higher than the medical use of iNO, which is generally no more than 80–160 ppm. It appears that high concentrations of NO may have a preventative effect on COVID-19. In view of existing iNO experiments, intermittent high concentrations of NO administration may be tested using a NO inhalation system independent of gas tank supply [93]. A similar medical device (GENOSYL DS®) has been approved by the US FDA, and has been used for the treatment of PPHN with good outcomes.
6. Limits of NO in the treatment of COVID-19
Inhaled NO has been suggested as an alternative rescue method before invasive treatment, especially for the relief of hypoxemia. However, according to recent clinical trials in Italy, NO appears unable to reverse oxygenation in patients with extensive mechanical ventilation who had developed persistent hypoxemia [104,105]. Since the targeted effectors of NO in the vascular system and immune system were usually cells or viruses, the inefficacy of NO in the treatment of patients with severe mechanical ventilation might be explained by damage from effectors and excessive viral infection. It also suggests that oxygenation treatment for critically ill COVID-19 patients should instead focus on more effective antiviral drugs, instead of ordinary ventilation support. Therefore, the treatment of iNO in critically ill patients should be evaluated comprehensively with multiple factors.
Although a large number of studies have been carried out focusing on the therapeutic effects of iNO in COVID-19, the safe and effective dose for iNO is still uncertain. Therapeutic doses for COVID-19 patients have ranged from 20 to 300 ppm. Only a few investigations examined the safety and effectiveness of 80,150 and 160 ppm iNO (Table 1, NCT04456088, NCT04397692, NCT04383002) [95]. The results of these trials have not been published yet. Among those clinical devices available for iNO use, including GENOSYL, LungFit Delivery System and INOpulse, only few trials have verified their feasibility or safety in the treatment of COVID-19 (Table 1, NCT04421508, EUCTR2020-002394-94-BE) [95].
7. Conclusion
NO level and bioavailability decreased in patients with COVID-19, which indicated exogenous supplementation of NO might help prevent infection or treat patients. Here, we covered the general features and potential mechanisms of how NO functions in COVID-19 etiology, as well as its potential clinical applications. Inhaled NO might participate in multiple stages of COVID-19 prevention or therapy, including prevention of infection, intervention of mild patients, alternative rescue treatment of moderate and severe patients, and adjuvant treatment of mechanically ventilated patients. Although promising, the safety and effectiveness of iNO needs comprehensive and careful evaluation.
Funding source
Department of Education of Hubei Province, China, 337/370, 6101/12267.
References
- 1.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dijkman R., van der Hoek L. Human coronaviruses 229E and NL63: close yet still so far. J. Formos. Med. Assoc. 2009;108:270–279. doi: 10.1016/S0929-6646(09)60066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmermann P., Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr. Infect. Dis. J. 2020;39:355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adusumilli N.C., Zhang D., Friedman J.M., Friedman A.J. Harnessing nitric oxide for preventing, limiting and treating the severe pulmonary consequences of COVID-19. Nitric Oxide. 2020;103:4–8. doi: 10.1016/j.niox.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.2020.12.01. https://coronavirus.jhu.edu/map.html
- 6.Takahashi N., et al. Clinical course of a critically ill patient with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) J. Artif. Organs. 2020;23:397–400. doi: 10.1007/s10047-020-01183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu R., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/s0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou P., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q., et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904. doi: 10.1016/j.cell.2020.03.045. e899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alamdari D.H., et al. Application of methylene blue -vitamin C -N-acetyl cysteine for treatment of critically ill COVID-19 patients, report of a phase-I clinical trial. Eur. J. Pharmacol. 2020;885:173494. doi: 10.1016/j.ejphar.2020.173494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleinbongard P., et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic. Biol. Med. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 12.Fraser D.D., et al. Endothelial injury and glycocalyx degradation in critically ill coronavirus disease 2019 patients: implications for microvascular platelet aggregation. Crit Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker R.C. COVID-19 update: covid-19-associated coagulopathy. J. Thromb. Thrombolysis. 2020;50:54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozdemir B., Yazici A. Could the decrease in the endothelial nitric oxide (NO) production and NO bioavailability be the crucial cause of COVID-19 related deaths? Med. Hypotheses. 2020;144:109970. doi: 10.1016/j.mehy.2020.109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amraei R., Rahimi N. COVID-19, renin-angiotensin system and endothelial dysfunction. Cells. 2020;9:1652. doi: 10.3390/cells9071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varga Z., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H., Liu Z., Ge J. Scientific research progress of COVID-19/SARS-CoV-2 in the first five months. J. Cell Mol. Med. 2020;24:6558–6570. doi: 10.1111/jcmm.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banu N., Panikar S.S., Leal L.R., Leal A.R. Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to Macrophage Activation Syndrome: therapeutic implications. Life Sci. 2020;256:117905. doi: 10.1016/j.lfs.2020.117905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosca L., Zeini M., Traves P.G., Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology. 2005;208:249–258. doi: 10.1016/j.tox.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 20.England J.T., et al. Weathering the COVID-19 storm: lessons from hematologic cytokine syndromes. Blood Rev. 2020:100707. doi: 10.1016/j.blre.2020.100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark I.A. The advent of the cytokine storm. Immunol. Cell Biol. 2007;85:271–273. doi: 10.1038/sj.icb.7100062. [DOI] [PubMed] [Google Scholar]
- 22.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song P., Li W., Xie J., Hou Y., You C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta. 2020;509:280–287. doi: 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shenoy S. Coronavirus (Covid-19) sepsis: revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflamm. Res. 2020;69:1077–1085. doi: 10.1007/s00011-020-01389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urso C., Caimi G. Oxidative stress and endothelial dysfunction. Minerva Med. 2011;102:59–77. [PubMed] [Google Scholar]
- 26.Guzik T.J., Korbut R., Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- 27.Uehara E.U. Shida Bde, S. & de Brito, C. A. Role of nitric oxide in immune responses against viruses: beyond microbicidal activity. Inflamm. Res. 2015;64:845–852. doi: 10.1007/s00011-015-0857-2. [DOI] [PubMed] [Google Scholar]
- 28.Gibaldi M. What is nitric oxide and why are so many people studying it? J. Clin. Pharmacol. 1993;33:488–496. doi: 10.1002/j.1552-4604.1993.tb04694.x. [DOI] [PubMed] [Google Scholar]
- 29.Star R.A. Nitric oxide. Am. J. Med. Sci. 1993;306:348–358. doi: 10.1097/00000441-199311000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Tripathi P. Nitric oxide and immune response. Indian J. Biochem. Biophys. 2007;44:310–319. [PubMed] [Google Scholar]
- 31.Susswein A.J., Katzoff A., Miller N., Hurwitz I. Nitric oxide and memory. Neuroscientist. 2004;10:153–162. doi: 10.1177/1073858403261226. [DOI] [PubMed] [Google Scholar]
- 32.Robbins R.A., Grisham M.B. Nitric oxide. Int. J. Biochem. Cell Biol. 1997;29:857–860. doi: 10.1016/s1357-2725(96)00167-7. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez R.A., Berra L., Gladwin M.T. Home nitric oxide therapy for COVID-19. Am. J. Respir. Crit. Care Med. 2020;202:16–20. doi: 10.1164/rccm.202005-1906ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee A., Butt W. Nitric oxide: a new role in intensive care. Crit Care Resusc. 2020;22:72–79. doi: 10.51893/2020.1.sr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michel J.B. Role of endothelial nitric oxide in the regulation of the vasomotor system. Pathol. Biol. 1998;46:181–189. [PubMed] [Google Scholar]
- 36.Friebe A., Sandner P., Schmidtko A. cGMP: a unique 2nd messenger molecule - recent developments in cGMP research and development. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020;393:287–302. doi: 10.1007/s00210-019-01779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ricciardolo F.L. Multiple roles of nitric oxide in the airways. Thorax. 2003;58:175–182. doi: 10.1136/thorax.58.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bohlen H.G. Nitric oxide and the cardiovascular system. Comp. Physiol. 2015;5:808–823. doi: 10.1002/cphy.c140052. [DOI] [PubMed] [Google Scholar]
- 39.Teixeira R., Santos M., Gil V. COVID-19 and cardiovascular comorbidities: an update. Rev. Port. Cardiol. 2020;39:417–419. doi: 10.1016/j.repc.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goeijenbier M., et al. Review: viral infections and mechanisms of thrombosis and bleeding. J. Med. Virol. 2012;84:1680–1696. doi: 10.1002/jmv.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martel J., Ko Y.F., Young J.D., Ojcius D.M. Covid-19 accelerates endothelial dysfunction and nitric oxide deficiency. Microb. Infect. 2020;22:168–171. doi: 10.1016/j.micinf.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng K.H., et al. Pulmonary artery thrombosis in a patient with severe acute respiratory syndrome. Postgrad. Med. 2005;81:e3. doi: 10.1136/pgmj.2004.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hottz E.D., et al. Platelet activation and platelet-monocyte aggregates formation trigger tissue factor expression in severe COVID-19 patients. Blood. 2020;136:1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miesbach W., Makris M. COVID-19: coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin. Appl. Thromb. Hemost. 2020;26 doi: 10.1177/1076029620938149. 1076029620938149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen N., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Long H., et al. D-dimer and prothrombin time are the significant indicators of severe COVID-19 and poor prognosis. BioMed Res. Int. 2020;2020:6159720. doi: 10.1155/2020/6159720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuprash D.V., Nedospasov S.A. Molecular and cellular mechanisms of inflammation. Biochemistry (Mosc.) 2016;81:1237–1239. doi: 10.1134/S0006297916110018. [DOI] [PubMed] [Google Scholar]
- 50.Korhonen R., Lahti A., Kankaanranta H., Moilanen E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets - Inflamm. Allergy. 2005;4:471–479. doi: 10.2174/1568010054526359. [DOI] [PubMed] [Google Scholar]
- 51.Moilanen E., Vapaatalo H. Nitric oxide in inflammation and immune response. Ann. Med. 1995;27:359–367. doi: 10.3109/07853899509002589. [DOI] [PubMed] [Google Scholar]
- 52.Ramachandran R.A., Lupfer C., Zaki H. The inflammasome: regulation of nitric oxide and antimicrobial host defence. Adv. Microb. Physiol. 2018;72:65–115. doi: 10.1016/bs.ampbs.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Kvietys P.R., Granger D.N. Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic. Biol. Med. 2012;52:556–592. doi: 10.1016/j.freeradbiomed.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo X.J., Thomas P.G. New fronts emerge in the influenza cytokine storm. Semin. Immunopathol. 2017;39:541–550. doi: 10.1007/s00281-017-0636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hermann E., et al. Role of nitric oxide in the regulation of lymphocyte apoptosis and HIV-1 replication. Int. J. Immunopharm. 1997;19:387–397. doi: 10.1016/s0192-0561(97)00060-x. [DOI] [PubMed] [Google Scholar]
- 56.Mĕlková Z., Esteban M. Inhibition of vaccinia virus DNA replication by inducible expression of nitric oxide synthase. J. Immunol. 1995;155:5711–5718. [PubMed] [Google Scholar]
- 57.Haagmans B.L., Osterhaus A.D. Coronaviruses and their therapy. Antivir. Res. 2006;71:397–403. doi: 10.1016/j.antiviral.2006.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lowenstein C.J., et al. Nitric oxide inhibits viral replication in murine myocarditis. J. Clin. Invest. 1996;97:1837–1843. doi: 10.1172/JCI118613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saura M., et al. An antiviral mechanism of nitric oxide: inhibition of a viral protease. Immunity. 1999;10:21–28. doi: 10.1016/s1074-7613(00)80003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akerstrom S., et al. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79:1966–1969. doi: 10.1128/JVI.79.3.1966-1969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akerstrom S., Gunalan V., Keng C.T., Tan Y.J., Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology. 2009;395:1–9. doi: 10.1016/j.virol.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baez-Santos Y.M., St John S.E., Mesecar A.D. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antivir. Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snijder E.J., Decroly E., Ziebuhr J. The nonstructural proteins directing coronavirus RNA synthesis and processing. Adv. Virus Res. 2016;96:59–126. doi: 10.1016/bs.aivir.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mannick J.B. The antiviral role of nitric oxide. Res. Immunol. 1995;146:693–697. doi: 10.1016/0923-2494(96)84920-0. [DOI] [PubMed] [Google Scholar]
- 65.Ahmad R., Hussain A., Ahsan H. Peroxynitrite: cellular pathology and implications in autoimmunity. J. Immunoassay Immunochem. 2019;40:123–138. doi: 10.1080/15321819.2019.1583109. [DOI] [PubMed] [Google Scholar]
- 66.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shang J., et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoffmann M., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Phan T. Genetic diversity and evolution of SARS-CoV-2. Infect. Genet. Evol. 2020;81:104260. doi: 10.1016/j.meegid.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He J., et al. Potential of coronavirus 3C-like protease inhibitors for the development of new anti-SARS-CoV-2 drugs: insights from structures of protease and inhibitors. Int. J. Antimicrob. Agents. 2020;56:106055. doi: 10.1016/j.ijantimicag.2020.106055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tahir Ul Qamar M., Alqahtani S.M., Alamri M.A., Chen L.L. Structural basis of SARS-CoV-2 3CL(pro) and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal. 2020;10:313–319. doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stefano G.B., Esch T., Kream R.M. Potential immunoregulatory and antiviral/SARS-CoV-2 activities of nitric oxide. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2020;26 doi: 10.12659/MSM.925679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andreou A., Trantza S., Filippou D., Sipsas N., Tsiodras S. COVID-19: the potential role of copper and N-acetylcysteine (NAC) in a combination of candidate antiviral treatments against SARS-CoV-2. In Vivo. 2020;34:1567–1588. doi: 10.21873/invivo.11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tinker J.H., Michenfelder J.D. Sodium nitroprusside: pharmacology, toxicology and therapeutics. Anesthesiology. 1976;45:340–354. [PubMed] [Google Scholar]
- 76.Lundberg J.O. Nitric oxide and the paranasal sinuses. Anat. Rec. 2008;291:1479–1484. doi: 10.1002/ar.20782. [DOI] [PubMed] [Google Scholar]
- 77.Scadding G. Nitric oxide in the airways. Curr. Opin. Otolaryngol. Head Neck Surg. 2007;15:258–263. doi: 10.1097/MOO.0b013e32825b0763. [DOI] [PubMed] [Google Scholar]
- 78.Martel J., Ko Y.F., Young J.D., Ojcius D.M. Could nasal nitric oxide help to mitigate the severity of COVID-19? Microb. Infect. 2020;22:168–171. doi: 10.1016/j.micinf.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frostell C.G., Blomqvist H., Hedenstierna G., Lundberg J., Zapol W.M. Inhaled nitric oxide selectively reverses human hypoxic pulmonary vasoconstriction without causing systemic vasodilation. Anesthesiology. 1993;78:427–435. doi: 10.1097/00000542-199303000-00005. [DOI] [PubMed] [Google Scholar]
- 80.Ichinose F., Roberts J.D., Jr., Zapol W.M. Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation. 2004;109:3106–3111. doi: 10.1161/01.CIR.0000134595.80170.62. [DOI] [PubMed] [Google Scholar]
- 81.Barnes M., Brisbois E.J. Clinical use of inhaled nitric oxide: local and systemic applications. Free Radic. Biol. Med. 2020;152:422–431. doi: 10.1016/j.freeradbiomed.2019.11.029. [DOI] [PubMed] [Google Scholar]
- 82.Pedersen J., et al. Current and future treatments for persistent pulmonary hypertension in the newborn. Basic Clin. Pharmacol. Toxicol. 2018;123:392–406. doi: 10.1111/bcpt.13051. [DOI] [PubMed] [Google Scholar]
- 83.Yu B., Ichinose F., Bloch D.B., Zapol W.M. Inhaled nitric oxide. Br. J. Pharmacol. 2019;176:246–255. doi: 10.1111/bph.14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manocha S., Walley K.R., Russell J.A. Severe acute respiratory distress syndrome (SARS): a critical care perspective. Crit. Care Med. 2003;31:2684–2692. doi: 10.1097/01.CCM.0000091929.51288.5F. [DOI] [PubMed] [Google Scholar]
- 85.Chen L., et al. Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: a rescue trial in Beijing. Clin. Infect. Dis. 2004;39:1531–1535. doi: 10.1086/425357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peiris J.S., et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/s0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lai S.T. Treatment of severe acute respiratory syndrome. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24:583–591. doi: 10.1007/s10096-005-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arabi Y.M., et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann. Intern. Med. 2014;160:389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 89.Alraddadi B.M., et al. Noninvasive ventilation in critically ill patients with the Middle East respiratory syndrome. Influenza Other Respir Viruses. 2019;13:382–390. doi: 10.1111/irv.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang X., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine. 2020;8:475–481. doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang D., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J. Am. Med. Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hedenstierna G., Chen L., Hedenstierna M., Lieberman R., Fine D.H. Nitric oxide dosed in short bursts at high concentrations may protect against Covid 19. Nitric Oxide. 2020;103:1–3. doi: 10.1016/j.niox.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lei C., et al. Protocol of a randomized controlled trial testing inhaled Nitric Oxide in mechanically ventilated patients with severe acute respiratory syndrome in COVID-19 (SARS-CoV-2) medRxiv. 2020 doi: 10.1101/2020.03.09.20033530. [DOI] [Google Scholar]
- 95.2020 September 20. https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=nitric+oxide&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=
- 96.2020 October 1. https://clinicaltrials.gov/ct2/who_table
- 97.Safaee Fakhr B., et al. High concentrations of nitric oxide inhalation therapy in pregnant patients with severe coronavirus disease 2019 (COVID-19) Obstet. Gynecol. 2020 doi: 10.1097/AOG.0000000000004128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lei C., et al. Protocol for a randomized controlled trial testing inhaled nitric oxide therapy in spontaneously breathing patients with COVID-19. medRxiv. 2020 doi: 10.1101/2020.03.10.20033522. [DOI] [Google Scholar]
- 99.Parikh R., et al. Inhaled nitric oxide treatment in spontaneously breathing COVID-19 patients. Ther. Adv. Respir. Dis. 2020;14 doi: 10.1177/1753466620933510. 1753466620933510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zamanian R.T., et al. Outpatient inhaled nitric oxide in a patient with vasoreactive idiopathic pulmonary arterial hypertension and COVID-19 infection. Am. J. Respir. Crit. Care Med. 2020;202:130–132. doi: 10.1164/rccm.202004-0937LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gianni S., et al. Nitric oxide gas inhalation to prevent COVID-2019 in healthcare providers. medRxiv. 2020 doi: 10.1101/2020.04.05.20054544. [DOI] [Google Scholar]
- 102.Tsui P.T., Kwok M.L., Yuen H., Lai S.T. Severe acute respiratory syndrome: clinical outcome and prognostic correlates. Emerg. Infect. Dis. 2003;9:1064–1069. doi: 10.3201/eid0909.030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berlin I., Thomas D., Le Faou A.L., Cornuz J. COVID-19 and smoking. Nicotine Tob. Res. 2020;22:1650–1652. doi: 10.1093/ntr/ntaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tavazzi G., et al. Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia. Crit. Care. 2020;24:508. doi: 10.1186/s13054-020-03222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ferrari M., et al. Inhaled nitric oxide in mechanically ventilated patients with COVID-19. J. Crit. Care. 2020;60:159–160. doi: 10.1016/j.jcrc.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]