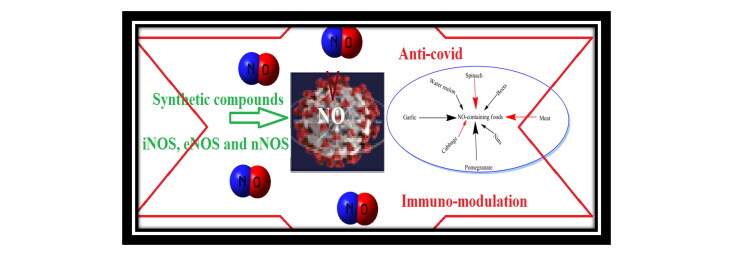
Abstract
In the prevailing covid times, scientific community is busy in developing vaccine against COVID-19. Under such fascination this article describes the possible role of nitric oxide (NO) releasers in aiding the immune system of a human body against this dreadful pandemic disease. Despite some prodrug antiviral compounds are in practice to recover the patients suffering from covid-19, however, co-morbidity deaths are highest among the total deaths happened so far. This concurrence of a number of diseases in a patient along with this viral infection is indicative of the poor immunity. Literature background supports the use of NO as immunity boosting agent and hence, the nitric oxide releasing compounds could act as lucrative in this context. Some dietary suggestions of NO-containing food items have also been introduced in this article. Also, the profound effect of NO in relieving symptomatic severity of covid-19 has been opined in this work.
Communicated by Ramaswamy H. Sarma
Keywords: NO, Immunity, NO-release, food, synthetic moieties
Graphical Abstract
Introduction
Nitric oxide (NO) is considered as a biologically important free radical that is produced during the metabolic pathway of L-arginine (Mir et al., 2019). The molecule has been proven of its physiological role in maintaining vascular tone, neuronal functionality, tumor-suppressing ability and more importantly profound implicative in human immune system as well as a microbicide (Maurya & Mir, 2014; Mir et al., 2017; Palmer et al., 1987). Moreover, NO generated naturally is expressive in so many immune functions, viz, T-cell regulation. Due to the fact that this molecule possesses its physiological impacts almost in every system of a human body, scientists are busy in developing NO-releasers for the beneficial applicability in case of the requirement wherein its production is too low to maintain homeostasis (Hibbs et al., 1987). In due course so many inorganic and organic NO-donors have been proposed by the scientific community. Some of them are even consumed by well defined commercial names generally called as NO-boosters (NO-supplements) (Mir et al., 2019).
As of now the world is suffering from the deadly viral pandemic generally known as corona virus-19 (COVID-19). Till now no treatment is available for this disease. However, some known viral drugs and social distancing have decreased the effect and spread of this calamity. Such declining infection rates and lockdown strategies have shown a ray of hope. But, these precautionary measures alone are not sufficient to lessen the increasing peak of the covid-19 affected statistical graph. Even the developed countries like USA are hopeless in this combat. The increasing trend of this catastrophe thus cannot be halted completely. For every field of scientific research it is hence a first and foremost question trending in the current era.
The bio-medicinal chemistry is trying all by leaps and bounds to bring forth a successful vaccine against this viral menace. As a contributory step, before a successful vaccine is developed, one of the routine food intake habits could be modified by the meticulous consumption of some natural and artificial items having NO-boosting capability. Advancements made so far in knowing the immunomodulting effects of NO and the role as mitigating the viral pathogenesis is thought to contribute in the development of antiviral vaccines. Also, the possible use of nitrate/nitrite metabolites together with the enzyme iNOS could act as prognostic viral infection markers (Uehara et al., 2015). In our conspicuous interest towards NO and NO-like molecules associated investigation (Mir et al., 2017; 2019; Mir & Maurya, 2018a, 2018b, 2018c, 2018d, 2018e) this article describes some of the medically proven NO-releasers and some of the available NO-boosters that could be suggested as heath supplements to tackle the covid-19 effects. Also, their inclusion in the COVID-19 vaccination has also been suggested with suitable reasons.
Possible relation between NO and COVID infection
In medicine, concurrence of additional conditions with a primary health-worsening condition is termed as. Such a status is usually considered as fatal to defend covid infection because of poor immunity. Very recently Guan et al reported that patients having comorbidity yield poorer results for clinically and number of associated deaths is the highest as compared to other groups (Guan et al., 2020). Therefore it is suggested that the immune response must be enhanced among the entire set of populations worldwide to defend the covid-19 infection. COVID-19 drug development involves the development of preventative vaccination or therapeutic drugs that would mitigate the severity of Coronavirus disease 2019 (COVID-19). Very recently, so many research institutes, drug companies and health organizations put forth 115 vaccine candidates and 249 potential therapies for COVID-19 disease in various stages of preclinical or clinical research. By late April, some 330 clinical trials were in progress worldwide to evaluate potential therapies against COVID-19.
Possible role of nitric oxide in pathogenesis of COVID-19: immunomodulation and antioxidative stress
This is a well known fact that during glycolysis of aerobic type mitochondrial ATP generation in the presence of O2 involvement of mitochondrial respiratory chain (DiMauro & Schon, 2003). However, when a tissue gets injured, the low levels of O2 and glucose are characteristic feature of the spot, together with an elevated concentration of reductive metabolic species (Saadi et al., 2002). As a result, these areas show inflammation and vasodilation maintained oftenly by NO synthase (specifically iNOsynthase). Exposure to NO ensures the occurence of the S-nitrosylation/inhibition of cytochromecoxidase, hence, bioenergetic functioning switch becomes operational to anaerobic milieu. Therefore, it becomes clearly stemming the assumption of NO-mediated immune response (Fiorucci et al., 2004). NO thus exerts a regulatory impression upon lymphocyte and monocytes functioning providing a potent signal transduction between the innate and acquired immunity. Thus, A key regulator of endothelial function is endothelium-derived nitric oxide (NO) generated by endothelial NO synthase (eNOS) [28]. Vascular NO relaxes blood vessels, prevents platelet aggregation and adhesion, limits oxidation of low density lipoprotein (LDL) cholesterol, inhibits proliferation of vascular smooth muscle cells, and decreases the expression of pro-inflammatory genes that advance atherogenesis.
The positive results shown by NO against SARS-1, raises hope to expect NO similar active against COVID-19, whether in treating symptomatic severity or in curtailing corona viral load (Klingström et al., 2006; Martel et al., 2020). To treat SARS-CoV-2 it has been that suggested to optimize the nitric oxide level within a human body. In this disease nitric oxide deficiency elicited by dysfunction in endothelial tissue eventually suppresses thrombotic event, restoration of NO may prove highly beneficial. Based on the NO-mediated positive results for SARS-1, nitric oxide gas is under clinical phase second to find its use in the treatment of COVID-19 (Martel et al., 2020).
Four main potent post-infection therapies–favipiravir, remdesivir, lopinavir and hydroxychloroquine (or chloroquine) – have been found in the final stage of human testing and yet a lot is to be done to win this combat. Among severe symptoms associated with covid-19 infection include difficulty in breathing or breathlessness, paining chest and difficulty in body movements. The enhancement of respiratory response and relief from chest pain could be achieved using NO-releasing compounds therapeutically as has been initiated to investigate by some research groups (https://in.mobile.reuters.com/article/amp/idINFWN2D80HB; LSU Health, 2020). It is worthy to mention here that the fact of this molecule in serving to protect lungs from physiological aging has been well documented (Boe et al., 2015). Earlier, it was noted that the due to vasodialtion effect of NO may induce relaxation of the smooth muscle confined to airway, activates guanylatecyclase, and consequently provokes bronchodilation effect in. Therefore, the lung physiological severity caused by COVID-19 infection could be mitigated using NO at the target. However, the responsiveness of a selected NO-donor needs to be carefully monitored (Ricciardolo, 2003).
Nitric oxide as antiviral agent with special reference to corona virus
There are three isoforms of nitric oxide synthase (NOS) enzyme that is responsible for natural production of NO inside a human body. These include neural (nNOS), inducible (iNOS) and endothelial (eNOS) that are widely known. In general host immune mechanism, NO generated via iNOS is sufficient to mitigate an infection (Knowles & Moncada, 1994; MacMicking et al., 1997). Advancements made so far in the study of its role in immunomodulation and pathogenesis of viral infections in particular has led to the repurposing in the development of vaccines/therapeutics (Uehara et al., 2015). From previous studies the role of NO in inhibiting protease activity helps in understanding the mechanism of action of this molecule as an anti-viral drug (Saura et al., 1999). Moreover, literature survey reveals that NO halts the replication of genetic material of various viruses including Coxsackievirus, Picornaviruses, hantavirus, herpesvirus, rhinovirus, Japanese encephalitis, vaccinia, retrovirus, etc (Croen, 1993; Kaul et al., 1999; Klingstrom et al., 2006; Zaragoza et al., 1997; 1998).
In the current times whole world is combating with the severity caused by corona virus. As far as the identification of this virus is concerned, it was in 2002, in China, when the first family member of Coronaviridae was detected and identified (Akarid et al., 1995; Lin et al., 1997). In due course of finding the treatment of this dreadful virus, it has been reported that NO donor S-nitroso-N-acetyl inhibits the virus progeny and hence halts the respective RNA-replication (Harris et al., 1995; Resh, 2006). As mentioned above the viral conquer results in oxidative stress and in the meantime NO and O2 rapidly forms peroxynitrite to maintain the balance. This bio-intermediate is also efficient antiviral agent (Tan et al., 2006) and has been specially found active against Hantaviruses (Akerstrom et al., 2005). Therefore it becomes logical here to state the anticorona property in three ways; by halting the RNA-replication, by deactivating oxidation stress and performing antiviral action even if NO changes to nitrite form. The severity caused by corona infection is now read under two heads, severe acute respiratory syndrome corona virus disease-1 (SARS-COVID-1) and (SARAS-COVID-2). The second one is also referred as COVID-19 or nCOVID. In reference to the activity against pulmonary infections shown by earlier reports suggest that higher levels of NO-exhalation exhibits less symptoms of common cold and refer nasally NO production as defense to invasion by corona through airways (Akaike & Maeda, 2000; Akerstrom et al., 2005).
Hence, from the positive results shown by NO against SARS-1 (Jung et al., 2010; Klingstrom et al., 2006), similar results are expected from NO against covid-19 (Ritz et al., 2018), whether in treating symptomatic severity or in curtailing corona activity. To treat SARS-CoV-2 it has been that suggested strategic to elevate the nitric oxide airway (Keyaerts et al., 2004). Since, in this disease remarkably nitric oxide deficiency elicited by dysfunction in endothelial tissue and eventually concurrence of suppressed thrombotic events, restoration of NO may prove highly beneficial (Akerstrom et al., 2005). Also, NO-sensitivity of proteases of the virus causing the Covid-19 has caught considerable attention in fighting this disease (Martel, et al., 2020). Based on a the discussion made above in terms of positive results for improving lung function during SARS-1 outbreak, nitric oxide gas is under clinical phase second to find its use in the treatment of Covid-19 (Martel et al., 2020).
Clinical trials of nitric oxide for the treatment of SARS CoV-2
On June 29, 2020, Kalytera Therapeutics Inc. (TSX-V.KALY, OTCQB:KALTF, Forum) made an important announcement that it has entered into a binding Letter of Intent (LOI) to license R-107 from Salzman Group for treatment of coronavirus and COVID-19 infection. This prodrug injection is non-gaseous form nitric oxide and releases nitric oxide into lung tissues over 48 h (https://stockhouse.com/news/newswire/2020/07/15/introducing-prodrug-that-s-anti-covid-19). Similarly, COViNOX another NO-releasing drug is under third phase of clinical trial (https://www.clinicaltrials.gov/ct2/show/NCT04421508).
Natural food items as NO-boosters (home-made concoctions to boost immunity)
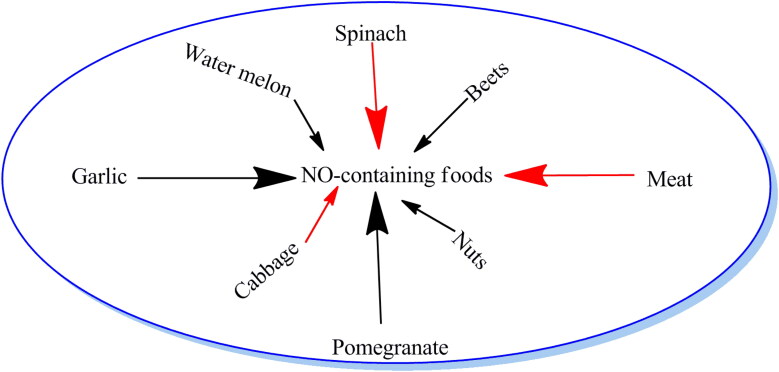
In the sudden outbreak of pandemic corona virus disease, it is necessary to be cautious in maintaining hygiene. In addition, some kitchen based inventions may prove helpful in boosting immunity power. The liquid intake and naturally NO-boosting agents may enter a human body by this way. These self-made items include gingery drink, lemon grass tonic, turmeric, honey and garlic preparations. Beets represent rich source of dietary nitrates, which can get converted to nitric oxide (Figure 1). Consuming garlic activates enzymatic action to produce nitric oxide from the L-arginine (Maurya & Mir, 2014; Mir et al., 2019). Similarly meat, poultry, seafood, spinach, cabbage, arugula and citrus fruits enhance nitric oxide level. Pomegranate is loaded with potent antioxidants that can protect your cells against damage and preserve nitric oxide. Nuts and seeds are high in arginine, a type of amino acid that is involved in the production of nitric oxide. Watermelon is one of the best sources of citrulline, an amino acid that’s converted to arginine and, ultimately to nitric oxide (www.healthline.com/nutrition/nitric-oxide-foods).
Figure 1.
NO-containing foods.
Synthetic NO donors
Despite the fact that NO is an endogenously generated molecule having free radical nature, linked with diversified physiological functions, an optimal level of NO concentration is essential for normal functioning of a human body (Maurya & Mir, 2014; Mir et al., 2019). However, due to high reactivity of NO, direct administration of NO-gas cylinders is generally avoided (Cheng et al., 2019). Under such quest of developing efficient NO donors capable of storing and releasing NO reversibly under specific conditions has attained tremendous fascination (Jin et al., 2018). The synthesis and applications of such molecular scaffolds e.g. metal nitrosyls, N-diazeniumdiolates, organic nitrites and S-nitrosothiols have been given special attention towards NO exposure and look out for its meticulous release (Dong et al., 2015; Troncy et al., 1997).
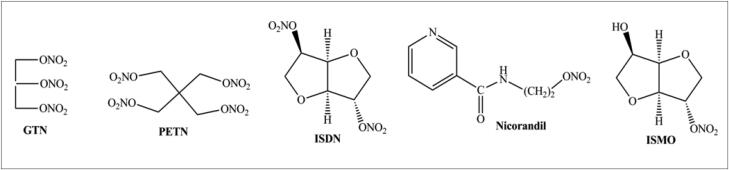
Organic nitrates (RONO2) represent the oldest class of NO donors that have been clinically applied. Representative organic nitrates include glyceryl trinitrate (GTN), pentaerythrityl tetranitrate (PETN), isosorbide dinitrate (ISDN), isosorbide 5-mononitrate (ISMO), and nicorandil (Figure 2). The partially denitrated metabolites of GTN, glyceryl dinitrates (GDN) and mononitrates (GMN), are still pharmacologically active but considerably less potent than GTN.
Figure 2.
Examples of some NORMS.
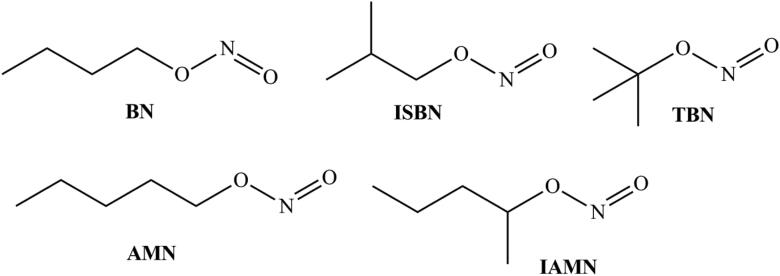
Organic nitrates due to three-electron reduction forming NO have long been used vascular relaxant (Cederqvist et al., 1994; Hinz et al., 1998; Jugdutt, 2004; Leier et al., 1981; Thatcher & Weldon, 1998; Torfgard & Ahlner, 1994). Among organic nitrites e.g. isobutyl nitrite (ISBN), butyl nitrite (BN), tert-butyl nitrite (TBN), isoamyl nitrite (IAMN) and amyl nitrite (AMN) have been clinically used as vasodilators for a long time (Patel & Williams, 1990) (Figure 3). Also, the nitrosyl moiety of nitrites can be readily transferred to a sulfhydryl group (Kowaluk & Fung, 1991; Meloche & O'Brien, 1993), rendering the significance of S-nitrosothiols in vivo [60] showing the respective NO release as an enzymatic process (Chung and Fung, 1990; Ji et al., 1996; Meyer et al., 1994). Enzymatically, xanthine oxidase (XO) has also been reported to catalyze the reduction of organic nitrites to NO under anaerobic conditions (Doel et al., 2000).
Figure 3.
Some organic nitrites.
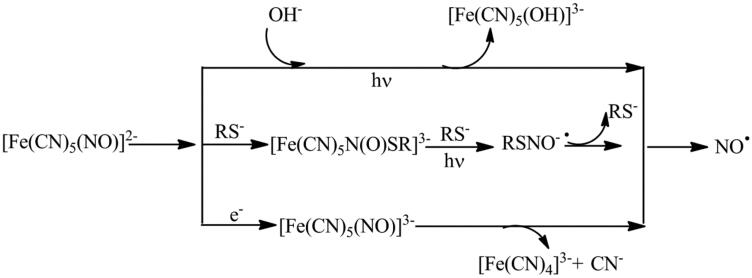
Among metal nitrosyls, Sodium nitro prusside (SNP) is the oldest NO-donor clinically applied for over 70 years to treat hypertension because of producing vasodilation effect (Marks et al., 1995). When kept dry and stored away from light its crystalline form can retain stability at room temperature for years (Zhelyaskov & Godwin, 1999). This clearly shows the photosensitivity and oxidation prone nature of SNP (Feelisch, 1991). Although the mechanism of NO release from SNP is not fully understood, it is clear that NO release requires either irradiation with light or one-electron reduction and is usually enhanced by thiols (Scheme 1).
Scheme 1.
Mechanism of NO release from SNP.
In human body, release of NO from SNP may be triggered both enzymatically and non-enzymatically, under the presence of vascular tissue or a reducing catalyst is required (Bates et al., 1991). Photolytic cleavage of NO in physiological conditions is not always significant (Butler & Glidewell, 1987). The use of nonenzymatic reduction procedure (using thiols, hemoproteins, ascorbate) are abundantly found in most of the biological tissues help in releasing significant amounts of NO. A membrane-bound enzyme may be involved in the generation of NO from SNP in biological tissues, and either NADH or NADPH appears to be required as the cofactor (Kowaluk et al., 1992; Mohazzab-H et al., 1992; Rao et al., 1991). Some concerns regarding the decomposition of SNP accompanied by cyanide release (a maximum of 5 equiv of CN- per mole SNP), has lead to cellular toxicity (Arnold et al., 1984). This has limited the use of SNP for the said purpose. Also, it has been found that spontaneous NO-release of SNP may form cytotoxic peroxynitrite which may harm a healthy tissue (Lamarque & Whittle, 1995; Wink et al., 1996).
To seek for low molecular weight, long-duration stable, efficient storing capacity and ease of NO-release so molecular systems have been tested (e.g. N-diazeniumdiolates; NONOates) (Riccio & Schoenfisch, 2012). Unfortunately, due to the toxic biological response in mammalian tissues/cells macromolecular-NO-storage systems have been proposed (Park et al., 2016; Zhang et al., 2003). To increase NO payloads metal organic frame-have also been used for this purpose (Lowe et al., 2013). Cyclodextrins based NO-releasing molecular vehicles and other sugar-like biopolymers have also been fabricated to favor this necessity (Deniz et al., 2012; Piras et al., 2013).
Concluding remarks
Social distancing (physical distancing) alone can’t help in halting the spread of covid-19 infection. Though prevention is better than cure, but human body has to always be ready to combat infections. At least a dietary routine could be modified to allow inclusion of those foods which can help in boosting immune system. The article hence describes the significant use of NO as immune-modulator and agent to attain relief from the infection severity including breathlessness and chest pain. Due to some distinctive NO-releasing differences among inorganic and organic species, attention must be paid towards the design of efficient NO-releasing compounds.
Disclosure statement
No conflict of interest was declared by the authors for this work
References
- Akaike, T., & Maeda, H. (2000). Nitric oxide and virus infection. Immunology, 101(3), 300–308. 10.1046/j.1365-2567.2000.00142.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akarid, K., Sinet, M., Desforges, B., & Gougerot-Pocidalo, M. A. (1995). Inhibitory effect of nitric oxide on the replication of a murine retrovirus in vitro and in vivo. Journal of Virology, 69(11), 7001–7005. 10.1128/JVI.69.11.7001-7005.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerstrom, S., Mousavi-Jazi, M., Klingstrom, J., Leijon, M., Lundkvist, A., & Mirazimi, A. (2005). Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. Journal of Virology, 79(3), 1966–1969. 10.1128/JVI.79.3.1966-1969.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold, W. P., Longnecker, D. E., & Epstein, R. M. (1984). Photodegradation of sodium nitroprusside: Biologic activity and cyanide release. Anesthesiology, 61(3), 254–260. 10.1097/00000542-198409000-00004 [DOI] [PubMed] [Google Scholar]
- Bates, J. N., Baker, M. T., Guerra, R., Jr., & Harrison, D. G. (1991). Nitric oxide generation from nitroprusside by vascular tissue: Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochemical Pharmacology, 42, S157–S165. 10.1016/0006-2952(91)90406-U [DOI] [PubMed] [Google Scholar]
- Boe, A. E., Eren, M., Morales-Nebreda, L., Murphy, S. B., Budinger, G. R. S., Mutlu, G. M., Miyata, T., & Vaughan, D. E. (2015). Nitric oxide prevents alveolar senescence and emphysema in a mouse model. PLoS One, 10(3), e0116504. 10.1371/journal.pone.0116504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIEF-Beyond Air Receives Approval From Health Canada To Study Nitric Oxide Generated And Delivered By The Lungfit™ In Covid-19 Patients (2020). https://in.mobile.reuters.com/article/amp/idINFWN2D80HB.
- Butler, A. R., & Glidewell, C. (1987). Recent chemical studies of sodium nitroprusside relevant to its hypotensive action. Chemical Society Reviews, 16, 361–380. 10.1039/cs9871600361 [DOI] [Google Scholar]
- Cederqvist, B., Persson, M. G., & Gustafsson, L. E. (1994). Direct demonstration of NO formation in vivo from organic nitrites and nitrates, and correlation to effects on blood pressure and to in vitro effects. Biochemical Pharmacology, 47(6), 1047–1053. 10.1016/0006-2952(94)90416-2 [DOI] [PubMed] [Google Scholar]
- Cheng, J., He, K., Shen, Z., Zhang, G., Yu, Y., & Hu, J. (2019). Applications of nitric oxide (NO)-releasing macromolecules: rational design and biomedical applications. Frontiers in Chemistry, 7, 530. 10.3389/fchem.2019.00530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, S. J., & Fung, H. L. (1990). Identification of the subcellular site for nitroglycerin metabolism to nitric oxide in bovine coronary smooth muscle cells. The Journal of Pharmacology and Experimental Therapeutics, 253(2), 614–619. [PubMed] [Google Scholar]
- Croen, K. D. (1993). Evidence for antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. The Journal of clinical investigation, 91(6), 2446–2452. 10.1172/JCI116479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniz, E., Kandoth, N., Fraix, A., Cardile, V., Graziano, A. C. E., Lo Furno, D., Gref, R., Raymo, F. M., & Sortino, S. (2012). Photoinduced fluorescence activation and nitric oxide release with biocompatible polymer nanoparticles. Chemistry (Weinheim an der Bergstrasse, Germany), 18(49), 15782–15787. ‒ 10.1002/chem.201202845 [DOI] [PubMed] [Google Scholar]
- DiMauro, S., & Schon, E. A. (2003). Mitochondrial respiratory-chain diseases. The New England Journal of Medicine, 348(26), 2656–2668. 10.1056/NEJMra022567 [DOI] [PubMed] [Google Scholar]
- Doel, J. J., Godber, B. L. J., Goult, T. A., Eisenthal, R., & Harrison, R. (2000). Reduction of organic nitrites to nitric oxide catalyzed by xanthine oxidase: Possible role in metabolism of nitrovasodilators. Biochemical and Biophysical Research Communications, 270(3), 880–885. 10.1006/bbrc.2000.2534 [DOI] [PubMed] [Google Scholar]
- Dong, R., Zhou, Y., Huang, X., Zhu, X., Lu, Y., & Shen, J. (2015). Functional supramolecular polymers for biomedical applications . Advanced Materials (Deerfield Beach, Fla.).), 27(3), 498–526. 10.1002/adma.201402975 [DOI] [PubMed] [Google Scholar]
- Feelisch, M. J. (1991). The biochemical pathways of nitric oxide formation from nitrovasodilators. Journal of Cardiovascular Pharmacology, 17(Supplement 3), S25–S33. 10.1097/00005344-199117003-00006 [DOI] [Google Scholar]
- Fiorucci, S., Mencarelli, A., Distrutti, E., Baldoni, M., del Soldato, P., & Morelli, A. (2004). Nitric oxide regulates immune cell bioenergetic: A mechanism to understand immunomodulatory functions of nitric oxide-releasing anti-inflammatory drugs. Journal of Immunology (Baltimore, Md. : 1950)), 173(2), 874–882. 10.4049/jimmunol.173.2.874 [DOI] [PubMed] [Google Scholar]
- Guan, W-j., Liang, W-h., Zhao, Y., Liang, H-r., Chen, Z-s., Li, Y-m., Liu, X-q., Chen, R-c., Tang, C-l., Wang, T., Ou, C-q., Li, L., Chen, P-y., Sang, L., Wang, W., Li, J-f., Li, C-c., Ou, L-m., Cheng, B., … He, J-x. (2020). Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. European Respiratory Journal, 55(5), 2000547. ) 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, N., Buller, R. M., & Karupiah, G. (1995). Gamma interferon-induced, nitric oxide-mediated inhibition of vaccinia virus replication. Journal of Virology, 69(2), 910–915. 10.1128/JVI.69.2.910-915.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs, J. B., Taintor, R. R., & Vavrin, Z. (1987). Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite . Science (New York, N.Y.), 235(4787), 473–476. 10.1126/science.2432665 [DOI] [PubMed] [Google Scholar]
- Hinz, B., Kuntze, U., & Schröder, H. (1998). Pentaerithrityl tetranitrate and its phase I metabolites are potent activators of cellular cyclic GMP accumulation. Biochemical and Biophysical Research Communications, 253(3), 658–661. 10.1006/bbrc.1998.9845https://www.healthline.com/nutrition/nitric-oxide-foods. [DOI] [PubMed] [Google Scholar]
- Ji, Y., Akerboom, T. P. M., & Sies, H. (1996). Microsomal formation of S-nitrosoglutathione from organic nitrites: Possible role of membrane-bound glutathione transferase. Biochemical Journal, 313(2), 377–380. 10.1042/bj3130377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, H., Yang, L., Jasmine, M., Ahonen, R., & Schoenfisch, M. H. (2018). Nitric oxide-releasing cyclodextrins. Journal of the American Chemical Society, 140(43), 14178–14184. 10.1021/jacs.8b07661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugdutt, B. I. (2004). Nitric oxide and cardioprotection during ischemia-reperfusion. In I. B., Jugdutt (Ed.), The role of nitric oxide in heart failure. Springer. 10.1007/1-4020-7960-5_20. [DOI] [PubMed] [Google Scholar]
- Jung, K., Gurnani, A., Renukaradhya, G. J., & Saif, L. J. (2010). Nitric oxide is elicited and inhibits viral replication in pigs infected with porcine respiratory coronavirus but not porcine reproductive and respiratory syndrome virus. Veterinary Immunology and Immunopathology, 136(3-4), 335–339. 10.1016/j.vetimm.2010.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul, P., Singh, I., & Turner, R. B. (1999). Effect of nitric oxide on rhinovirus replication and virus-induced interleukin-8 elaboration. American Journal of Respiratory and Critical Care Medicine, 159(4 Pt 1), 1193–1198. 10.1164/ajrccm.159.4.9808043 [DOI] [PubMed] [Google Scholar]
- Keyaerts, E., Vijgen, L., Chen, L., Maes, P., Hedenstierna, G., & Van Ranst, M. (2004). Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. International Journal of Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases, 8(4), 223–226. 10.1016/j.ijid.2004.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingström, J., Akerström, S., Hardestam, J., Stoltz, M., Simon, M., Falk, K. I., Mirazimi, A., Rottenberg, M., & Lundkvist, A. (2006). Nitric oxide and peroxynitrite have different antiviral effects against hantavirus replication and free mature virions. European Journal of Immunology, 36(10), 2649–2657. 10.1002/eji.200535587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingstrom, J., Akerstrom, S., Hardestam, J., Stoltz, M., Simon, M., Falk, K. I., Mirazimi, A., Rottenberg, M., & Lundkvist, A. (2006). Nitric oxide and peroxynitrite have different antiviral effects against hantavirus replication and free mature virions. European Journal of Immunology, 36(10), 2649–2657. 10.1002/eji.200535587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles, R. G., & Moncada, S. (1994). Nitric oxide synthases in mammals. Biochemical Journal, 298(2), 249–258. (Pt 10.1042/bj2980249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowaluk, E. A., & Fung, H. L. (1991). Vascular nitric oxide-generating activities for organic nitrites and organic nitrates are distinct. The Journal of Pharmacology and Experimental Therapeutics, 259(2), 519–525. [PubMed] [Google Scholar]
- Kowaluk, E. A., Seth, P., & Fung, H. L. (1992). Metabolic activation of sodium nitroprusside to nitric oxide in vascular smooth muscle. The Journal of Pharmacology and Experimental Therapeutics, 262(3), 916–922. [PubMed] [Google Scholar]
- Lamarque, D., & Whittle, B. J. (1995). Role of oxygen-derived metabolites in the rat gastric mucosal injury induced by nitric oxide donors. European Journal of Pharmacology, 277(2-3), 187–194. 10.1016/0014-2999(95)00075-V [DOI] [PubMed] [Google Scholar]
- Leier, C. V., Bambach, D., Thompson, M. J., Cattaneo, S. M., Goldberg, R. J., & Unverferth, D. V. (1981). Central and regional hemodynamic effects of intravenous isosorbide dinitrate, nitroglycerin and nitroprusside in patients with congestive heart failure. The American Journal of Cardiology, 48(6), 1115–1123. 10.1016/0002-9149(81)90329-5 [DOI] [PubMed] [Google Scholar]
- Lin, Y. L., Huang, Y. L., Ma, S. H., Yeh, C. T., Chiou, S. Y., Chen, L. K., & Liao, C. L. (1997). Inhibition of Japanese encephalitis virus infection by nitric oxide: Antiviral effect of nitric oxide on RNA virus replication. Journal of Virology, 71(7), 5227–5235. 10.1128/JVI.71.7.5227-5235.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, A., Chittajallu, P., Gong, Q., Li, J., & Balkus, K. J. (2013). Storage and delivery of nitric oxide via diazeniumdiolated metal organic framework. Microporous and Mesoporous Materials, 181, 17–‒22. 10.1016/j.micromeso.2013.07.017 [DOI] [Google Scholar]
- LSU Health . (2020). Shreveport trailblazing research into using nitric oxide as a COVID-19 treatment . https://www.knoe.com/content/news/Possible-game-changer-LSU-Health-Shreveport-trailblazing-research-into-using-nitric-oxide-as-a-COVID-19-treatment-570806781.html
- MacMicking, J., Xie, Q. W., & Nathan, C. (1997). Nitric oxide and macrophage function. Annual Review of Immunology, 15, 323–350. 10.1146/annurev.immunol.15.1.323 [DOI] [PubMed] [Google Scholar]
- Marks, G. S., McLaughlin, B. E., Jimmo, S. L., Poklewska-Koziell, M., Bien, J. F., & Nakatsu, K. (1995). Time-dependent increase in nitric oxide formation concurrent with vasodilation induced by sodium nitroprusside, 3-morpholinosydnonimine, and S-nitroso-N-acetylpenicillamine but not by glyceryl trinitrate. Drug Metabolism and Disposition, 23, 1248–1252. [PubMed] [Google Scholar]
- Martel, J., Ko, Y.-F., Young, J. D., & Ojcius, D. M. (2020). Could nitric oxide help to prevent or treat COVID-19? Microbes Infect, 22(4-5), 168-171. 10.1016/j.micinf.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel, J., Ko, Y. F., Young, J. D., & Ojcius, D. M. (2020). Could nasal nitric oxide help to mitigate the severity of COVID-19? A commentary. Microbes and Infection, 22(4-5), 168–171. 10.1016/j.micinf.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya, R. C., & Mir, J. M. (2014). Medicinal industrial & environmental relevance of metal nitrosyl complexes: A review. International Journal of Scientific and Engineering Research, 5, 305–320. [Google Scholar]
- Meloche, B. A., & O'Brien, P. J. (1993). S-nitrosyl glutathione-mediated hepatocyte cytotoxicity. Xenobiotica; the Fate of Foreign Compounds in Biological Systems, 23(8), 863–871. 10.3109/00498259309059414 [DOI] [PubMed] [Google Scholar]
- Meyer, D. J., Kramer, H., & Ketterer, B. (1994). Human glutathione transferase catalysis of the formation of S‐nitrosoglutathione from organic nitrites plus glutathione. FEBS Letters, 351(3), 427–428. 10.1016/0014-5793(94)00904-X [DOI] [PubMed] [Google Scholar]
- Mir, J. M., & Maurya, R. C. (2018. a). A gentle introduction to gasotransmitters with special reference to nitric oxide: Biological and chemical implications. Reviews in Inorganic Chemistry, 38(4), 193–220. [Google Scholar]
- Mir, J. M., & Maurya, R. C. (2018. b). A new Ru(II) carbonyl complex of 2-benzoylpyridine: Medicinal and material evaluation at the computational–experimental convergence. Journal of Chinese Advanced Materials Society, 36, 156–168. [Google Scholar]
- Mir, J. M., & Maurya, R. C. (2018. c). Nitric oxide functionalized molybdenum(0) pyrazolone Schiff base complexes: Thermal and biochemical study. RSC Advances, 8(61), 35102–35130. 10.1039/C8RA05956J [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir, J. M., & Maurya, R. C. (2018. d). NO news is good news for eyes: A mini review. Annals of Ophthalmology and Visual Sciences, 1003, 1–4. [Google Scholar]
- Mir, J. M., & Maurya, R. C. (2018. e). Physiological and pathophysiological implications of hydrogen sulfide: A persuasion to change the fate of the dangerous molecule. Journal of the Chinese Advanced Materials Society, 6(4), 434–458. 10.1080/22243682.2018.1493951 [DOI] [Google Scholar]
- Mir, J. M., Jain, N., Jaget, P. S., & Maurya, R. C. (2017). Density functionalized [RuII(NO)(Salen)(Cl)] complex: Computational photodynamics and in vitro anticancer facets. Photodiagnosis and Photodynamic Therapy, 19, 363–374. 10.1016/j.pdpdt.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Mir, J. M., Jain, N., Jaget, P. S., Khan, W., Vishwakarma, P. K., Rajak, D. K., Malik, B. A., & Maurya, R. C. (2019). Urinary tract anti-infectious potential of DFT-experimental composite analyzed ruthenium nitrosyl complex of N-dehydroacetic acid-thiosemicarbazide. Journal of King Saud University-Science, 31, 89–100. [Google Scholar]
- Mir, J. M., Jain, N., Malik, B. A., Chourasia, R., Vishwakarma, P. K., Rajak, D. K., & Maurya, R. C. (2017). Urinary tract infection fighting potential of newly synthesized ruthenium carbonyl complex of N-dehydroacetic acid-N′-o-vanillin-ethylenediamine. Inorganica Chimica Acta, 467, 80–92. 10.1016/j.ica.2017.07.051 [DOI] [Google Scholar]
- Mir, J. M., Malik, B. A., & Maurya, R. C. (2019). Nitric oxide-releasing molecules at the interface of inorganic chemistry and biology: A concise overview. Reviews in Inorganic Chemistry, 39(2), 91–112. 10.1515/revic-2018-0017 [DOI] [Google Scholar]
- Mohazzab-H, K. M., Gurrant, C. E., & Wolin, M. S. (1992). Microsomal NADH-oxidoreductase mediates nitric oxide release and relaxation to nitroprusside in the calf pulmonary artery. Circulation, 86, 1–489.1617762 [Google Scholar]
- Palmer, R. M., Ferrige, A. G., & Moncada, S. (1987). Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature, 327(6122), 524–526. [DOI] [PubMed] [Google Scholar]
- Park, D., Kim, J., Lee, Y. M., Park, J., & Kim, W. J. (2016). Polydopamine hollow nanoparticle functionalized with n-diazeniumdiolates as a nitric oxide delivery carrier for antibacterial therapy. Advanced Healthcare Materials, 5(16), 2019–2024. ‒ 10.1002/adhm.201600150 [DOI] [PubMed] [Google Scholar]
- Patel, H. M. S., & Williams, D. L. H. (1990). Nitrosation by alkyl nitrites. Part 6. Thiolate nitrosation. Journal of the Chemical Society, Perkin Transactions 2, 2(1), 37–42. 10.1039/p29900000037 [DOI] [Google Scholar]
- Piras, L., Theodossiou, T. A., Manouilidou, M. D., Lazarou, Y. G., Sortino, S., & Yannakopoulou, K. (2013). S-nitroso-β-cyclodextrins as new bimodal carriers: preparation, detailed characterization, nitric-oxide release, and molecular encapsulation. Chemistry, An Asian Journal, 8(11), 2768–2778. 10.1002/asia.201300543 [DOI] [PubMed] [Google Scholar]
- Rao, D. N., Elguindi, S., & O'Brien, P. J. (1991). Reductive metabolism of nitroprusside in rat hepatocytes and human erythrocytes. Archives of Biochemistry and Biophysics, 286(1), 30–37. 10.1016/0003-9861(91)90005-4 [DOI] [PubMed] [Google Scholar]
- Resh, M. D. (2006). Palmitoylation of ligands, receptors, and intracellular signaling molecules. Science's STKE: Signal Transduction Knowledge Environment, 2006(359), re14 10.1126/stke.3592006re14 [DOI] [PubMed] [Google Scholar]
- Ricciardolo, F. L. M. (2003). Multiple roles of nitric oxide in the airways. Thorax, 58(2), 175–182. 10.1136/thorax.58.2.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio, D. A., & Schoenfisch, M. H. (2012). Nitric oxide release: Part I. Macromolecular scaffolds. Chemical Society Reviews, 41(10), 3731–3741. 10.1039/c2cs15272j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz, T., Trueba, A. F., Vogel, P. D., Auchus, R. J., & Rosenfield, D. (2018). Exhaled nitric oxide and vascular endothelial growth factor as predictors of cold symptoms after stress. Biological Psychology, 132, 116–124. 10.1016/j.biopsycho.2017.11.006 [DOI] [PubMed] [Google Scholar]
- Saadi, S., Wrenshall, L. E., & Platt, J. L. (2002). Regional manifestations and control of the immune system. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 16(8), 849–856. 10.1096/fj.01-0690hyp [DOI] [PubMed] [Google Scholar]
- Saura, M., Zaragoza, C., McMillan, A., Quick, R. A., Hohenadl, C., Lowenstein, J. M., & Lowenstein, C. J. (1999). An antiviral mechanism of nitric oxide: Inhibition of a viral protease. Immunity, 10(1), 21–28. 10.1016/S1074-7613(00)80003-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, Y. J., Lim, S. G., & Hong, W. (2006). Understanding the accessory viral proteins unique to the severe acute respiratory syndrome (SARS) coronavirus. Antiviral Research, 72(2), 78–88. 10.1016/j.antiviral.2006.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher, G. R. J., & Weldon, H. (1998). NO problem for nitroglycerin: Organic nitrate chemistry and therapy. Chemical Society Reviews, 27, 331–337. [Google Scholar]
- Torfgard, K. E., & Ahlner, J. (1994). Mechanisms of action of nitrates. Cardiovascular Drugs and Therapy, 8(5), 701–717. 10.1007/BF00877117 [DOI] [PubMed] [Google Scholar]
- Troncy, E., Francoeur, M., & Blaise, G. (1997). Inhaled nitric oxide: Clinical applications, indications, and toxicology. Canadian Journal of Anaesthesia = Journal Canadien D'anesthesie, 44(9), 973–988. ‒ 10.1007/BF03011970 [DOI] [PubMed] [Google Scholar]
- Uehara, E. C., Shida, B., de, S., & de Brito, C. A. (2015). Role of nitric oxide in immune responses against viruses: Beyond microbicidal activity. Inflammation Research: Official Journal of the European Histamine Research Society, 64(11), 845–852. 10.1007/s00011-015-0857-2 [DOI] [PubMed] [Google Scholar]
- Wink, D. A., Cook, J., Pacelli, R., DeGraff, W., Gamson, J., Liebmann, J., Krishna, M., & Mitchell, J. B. (1996). The effect of various nitric oxide-donor agents on hydrogen peroxide-mediated toxicity: A direct correlation between nitric oxide formation and protection. Archives of Biochemistry and Biophysics, 331(2), 241–248. 10.1006/abbi.1996.0304 [DOI] [PubMed] [Google Scholar]
- Zaragoza, C., Ocampo, C. J., Saura, M., McMillan, A., & Lowenstein, C. J. (1997). Nitric oxide inhibition of coxsackievirus replication in vitro. The Journal of Clinical Investigation, 100(7), 1760–1767. 10.1172/JCI119702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza, C., Ocampo, C., Saura, M., Leppo, M., Wei, X. Q., Quick, R., Moncada, S., Liew, F. Y., & Lowenstein, C. J. (1998). The role of inducible nitric oxide synthase in the host response to Coxsackievirus myocarditis. Proceedings of the National Academy of Sciences of the United States of America, 95(5), 2469–2474. 10.1073/pnas.95.5.2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Annich, G. M., Miskulin, J., Stankiewicz, K., Oster-Holzer, K., Merz, S. I., Bartlett, R. H., & Meyerhoff, M. E. (2003). Nitric oxide-releasing fumed silica particles: Synthesis, characterization, and biomedical application. Journal of the American Chemical Society, 125(17), 5015–5024. 10.1021/ja0291538 [DOI] [PubMed] [Google Scholar]
- Zhelyaskov, V. R., & Godwin, D. W. (1999). A nitric oxide concentration clamp. Nitric Oxide: Biology and Chemistry, 3(5), 419–425. 10.1006/niox.1999.0253 [DOI] [PubMed] [Google Scholar]