Abstract
Aims
Heart failure (HF) and chronic obstructive pulmonary disease (COPD) are main causes of dyspnoea, and echocardiography and spirometry are essential investigations for these diagnoses. Our aim was to determine the prevalence of HF and COPD in a general population, also how the diseases may be identified, and to what extent their clinical characteristics differ.
Methods and results
In the seventh survey of Tromsø study (2015–16), subjects aged 40 years or more were examined with echocardiography, spirometry, lung sound recordings, questionnaires, including the modified Medical Research Council (mMRC) questionnaire on dyspnoea, and N‐terminal pro‐brain natriuretic peptide analysis. A diagnosis of HF (HF with reduced ejection fraction, HF with mid‐range ejection fraction, or HF with preserved ejection fraction) or COPD was established according to current guidelines. Predictors of HF and COPD were evaluated by logistic regression and receiver operating characteristic curve analysis. A total of 7110 participants could be evaluated for COPD, 1624 for HF, and 1538 for both diseases. Age‐standardized prevalence of HF was 6.8% for women and 6.1% for men; the respective figures for COPD were 5.2% and 5.1%. Among the 1538 evaluated for both diseases, 139 subjects fulfilled the HF criteria, but only 17.1% reported to have the disease. Of those fulfilling the COPD criteria, 31.6% reported to have the disease. Shortness of breath at exertion was a frequent finding in HF; 59% of those with mMRC ≥2 had HF, while such shortness of breath was found in 24% among those with COPD. Reporting mMRC ≥2 had an odds ratio for HF of 19.5 (95% confidence interval 11.3–33.7), whereas the odds ratio for COPD was 6.3 (95% confidence interval 3.5–11.6). Current smoking was the strongest predictor of COPD but did not predict HF. Basal inspiratory crackles were significant predictors of HF in multivariable analysis. Among the subtypes of HF, an age <70 years was most frequently found in HF with reduced ejection fraction, in 51.7%. Clinical scores based on the predictive value in multivariable analysis of history, symptoms, and signs predicted HF and COPD with areas under the curve of 0.833 and 0.829, respectively.
Conclusions
Study participants with HF and COPD were in most cases not aware of their condition. In general practice, when an elderly patient present with shortness of breath, both diseases should be considered. Previous cardiovascular disease points at HF, while a history of smoking points at COPD. The threshold should be low for ordering echocardiography or spirometry for verifying the suspected cause of dyspnoea.
Keywords: Epidemiology, Heart failure, Chronic obstructive pulmonary disease, Spirometry, Echocardiography, Symptoms, Identification, Biomarkers
Introduction
Heart failure (HF) and chronic obstructive pulmonary disease (COPD) are two of the most common chronic diseases and are leading causes of morbidity and mortality worldwide with high social and economic negative impact. Exacerbations of HF and COPD are major causes of admittance to emergency departments. 1 , 2 HF and COPD are not curable diseases, but early diagnosis can lead to preventive measures, including smoking cessation, 3 that can prolong life and reduce the number of acute exacerbations. 2 , 4
Heart failure and COPD might be underdiagnosed at an early stage partly due to unspecific early symptoms. 5 A cardinal symptom for HF, exertional dyspnoea, 6 is also shared by COPD, and also, cough may be present in both diseases. 7 HF and COPD may overlap and increasingly by age. 8 , 9
Clinicians take chest signs like crackles and wheezes into account when HF or COPD is suspected, but the sensitivity and specificity of these findings are regarded to be low. 6 , 10 This study is based on the seventh survey of the Tromsø study where recording of lung sounds was included among the clinical investigations. 11
The aim of this study was to determine how abnormal lung sounds and respiratory symptoms, shortness of breath in particular, may predict HF and COPD, as identified by current guidelines and to what extent the occurrence of these diseases overlap in a general population.
Methods
Study population
The Tromsø study was established in 1974 with the main aim of understanding the role of modifiable cardiovascular disease risk factors. Seven waves of the study have been carried out with the last health survey performed in 2015–16. Main features of the methodology and study design have been described previously. 12 In this cross‐sectional study, our sample consists of randomly selected participants attending the second visit of the seventh survey of the Tromsø study (Tromsø 7), between May 2015 and October 2016. All Tromsø residents 40 years or older (n = 32 591) received a postal invitation to participate in the first visit of Tromsø 7. A random sample was selected for the second visit including 20% of those aged 40–59 years and 60% of those aged 60–84 years, and, among these, those who attended the first visit were invited. In addition, individuals who had participated in echocardiography in previous surveys of the study were invited to obtain repeated measurements.
Data collection
The following information on participant's diseases and risk factors was retrieved from self‐administered questionnaires at the first visit: myocardial infarctions, angina, atrial fibrillation, arterial hypertension, diabetes, COPD, and asthma. Smoking was categorized as never smoked, previous, and current smokers, and the participants answered the question ‘Do you cough about daily for some periods of the year?’. At the second visit, before spirometry, the participants answered modified Medical Research Council (mMRC) questionnaire on dyspnoea. 13 Dyspnoea was further characterized using the question: ‘How is your breathing today compared to normal?’. Serum levels of N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) and C‐reactive protein were measured with reagents from Roche Diagnostics, Norway. The age‐related reference values of NT‐proBNP (97.5 percentile in a healthy population) were provided by Roche Diagnostics (Appendix A).
Echocardiographic assessment was performed by an experienced echo technician using a GE Vivid E9 scanner (GE Medical, Horten, Norway). Echopac version 113 (GE Medical) was used for offline image reading performed by M. S.
Ultrasound examination was performed according to guidelines 14 in the left lateral decubitus position. Left ventricular myocardial mass index was estimated by normalizing left ventricular mass and to height raised to allometric power of 2.7.
Echo markers of diastolic dysfunction in individuals with normal left ventricular ejection fraction (LVEF) were as follows: average E/e′ >14; septal e′ velocity <7 cm/s or lateral e′ velocity <10 cm/s; tricuspid regurgitation velocity >2.8 m/s; and left atrial volume index >34 mL/m2.
Spirometry was performed using SensorMedics Vmax 20c Encore (VIASYS Healthcare Respiratory Technologies, Yorba Linda, CA, USA). Calibration was performed daily. We followed the standards of the American Thoracic Society/European Respiratory Society. 15 Tests with forced expiratory volume in 1 s (FEV1) <0.3 L and with expiration lasting less than 3 s were regarded invalid. Post‐bronchodilator measurements were not carried out. We used the Global Lung Function Initiative (GLI 2012) as a reference with the fifth percentile among healthy never smokers as lower limit of normal (LLN). 16 We registered arterial oxygen saturation (Sp02) with a pulse oximeter Onyx II model 9550 (Nonin Medical, Inc., Plymouth, MN, USA) after resting 15 min. The highest value after three measurements was registered. Medication for asthma and COPD should be taken as usual.
Lung sounds were recorded at six locations of the chest, 15 s at each site, with a Sennheiser microphone inserted in the tube of a Littmann Classic II stethoscope. Presence of wheezes and crackles (also called rales or crepitations) during inspiration and expiration was determined by clinicians independently classifying the recordings blinded for other information; details on recording sites and classification of the sounds have recently been published. 11 Basal inspiratory crackles heard bilaterally were a category used in the analysis. 17
Definition of heart failure and chronic obstructive pulmonary disease
Heart failure was defined according to the latest European Society of Cardiology (ESC) guidelines 2 into three types: heart failure with reduced ejection fraction (HFrEF) characterized by LVEF <40% and presence of dyspnoea (mMRC ≥1); heart failure with mid‐range ejection fraction (HFmrEF) with LVEF 40–49%, dyspnoea, serum NT‐proBNP >125 pg/mL, and any of the following: structural heart disease/diastolic dysfunction; and heart failure with preserved ejection fraction (HFpEF) with LVEF ≥50%, dyspnoea, serum NT‐proBNP >125 pg/mL, and any of the following: structural heart disease/diastolic dysfunction.
Structural heart disease included left ventricular hypertrophy and/or left atrial enlargement. Diastolic dysfunction was defined by joint European Society of Cardiovascular Imaging and American Society of Echocardiography guidelines. 18
A diagnosis of COPD was established when FEV1/forced ventilator capacity (FVC) was lower than normal (LLN, the 5% percentile in a healthy non‐smoking population), and the participants had answered yes to the question ‘Do you get short of breath when hurrying on a level surface or walking up a slight hill?’ (mMRC = 1 or higher) or to the question ‘Do you cough about daily for some periods of the year?’. 19
Sensitivity analysis
Evaluation of predictors of HF and COPD was mainly performed in subjects classified for both diagnoses. In addition, we evaluated HF predictors in all subjects that could be classified according to the HF criteria and likewise COPD predictors in all subjects that could be classified according to COPD criteria.
Statistical methods
Crude prevalences were standardized for age and gender using the population distribution from the Tromsø municipality of 1 January 2019. Frequency of characteristics was determined according to HF and COPD status in the subjects evaluated for both diseases. When describing the types of HF, all subjects with HF were included, also those not evaluated for COPD. Differences between groups were analysed with χ 2 statistics. Predictors of HF (irrespective of COPD co‐morbidity) and COPD (irrespective of HF co‐morbidity) were evaluated by logistic regression, adjusted for age, and relevant explanatory variables associated with outcome with a P‐value <0.1 were entered in the multivariable analysis. Receiver operating characteristic (ROC) curves evaluating predictive value of variable from history, symptoms, signs, and biomarkers were produced based on output (β) from multivariable logistic regression with backward elimination, with age more or less than 70 years as one of the explanatory variables. SPSS statistical software version 26 (IBN, Armonk, NY, USA) was used. Visual assessment of overlap between HF, COPD, and mMRC ≥2 was made using a Venn diagram (R package version 6.0.0.).
Ethical considerations
The Tromsø study protocol was performed in accordance with the Declaration of Helsinki and was approved by the Regional Committee for Medical and Health Research Ethics, North Norway (2014/940/REK Nord). Written consent was provided by all study participants.
Results
Inclusion of participants in the analyses is shown in Figure 1 . Out of 7316 attending the spirometry, valid measures were obtained in 7247 with a mean age of 63.0 years (range 40–84 years). Among these, 7110 answered the mMRC questionnaire and the question on cough, and a COPD diagnosis could be made in 432 (6.1%). Age‐standardized prevalence of COPD was 5.1% for men and 5.2% for women. Echocardiography was performed in 2340 participants, 2106 of these also attended spirometry. Evaluation of EF could be performed in 2007 subjects (Figure 1 ), and among these, the mMRC questionnaire was answered, and NT‐proBNP was analysed in 1624 (mean age 64.0 years). A diagnosis of HF was established in 155 (9.5%). The age‐standardized prevalence of HF was 6.1% for men and 6.8% for women. Presence of both HF and COPD could be evaluated in 1538 participants (mean age 63.7 years), 51.5% were women, and 48.5% were men. A diagnosis of COPD was established in 84 and HF in 139 participants, and 14 had both (Table 1 , Figure 2 ). The prevalence of the diseases did not differ by sex but significantly by age (P < 0.001); 64.8% of the 125 subjects with HF and without COPD were aged 70 years or more compared with 28.8% of those with COPD (Table 1 ). Of those with COPD, 23% had mMRC = 0 with the diagnosis based solely on spirometry and periods of daily cough. Among those with mMRC ≥2, 59% had HF, while 23.9% had COPD (Figure 2 ). Crackles were heard in one out of five subjects with HF or COPD, but basal bilateral crackles in only one out of 20. Wheezes were heard more frequently in COPD than in HF (Table 1 ). High frequency of FEV1 < LLN was found in both COPD and HF and reached 64.3% in subjects with both COPD and HF. Increased levels of NT‐proBNP were only found in the HF groups. SpO2 values between 92% and 100% were found, and an increased occurrence of SpO2 ≤95% was mainly found among subjects with COPD (9%, P = 0.02).
Figure 1.
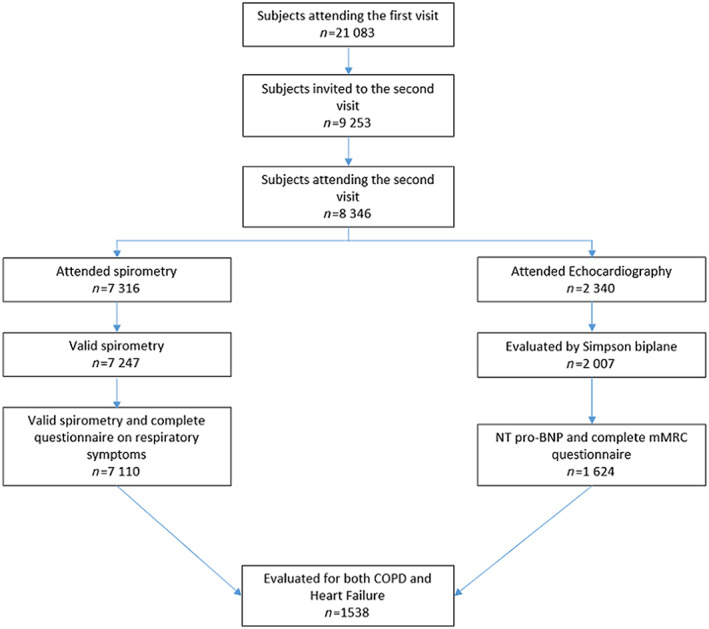
Flow chart of the participants of the seventh Tromsø study. COPD, chronic obstructive pulmonary disease; mMRC, modified Medical Research Council; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
TABLE 1.
Characteristics of 1538 participants of the seventh Tromsø study by established diagnoses of heart failure and chronic obstructive pulmonary disease
|
Heart failure, no COPD N = 125 |
Heart failure and COPD N = 14 |
COPD, no heart failure N = 70 |
No COPD, no heart failure N = 1329 |
All N = 1538 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | P‐value | |
| Men | 62 | 49.6 | 9 | 64.3 | 30 | 42.9 | 645 | 48.5 | 746 | 48.5 | 0.5 |
| Women | 63 | 50.4 | 5 | 35.7 | 40 | 57.1 | 684 | 51.5 | 792 | 51.5 | |
| Age (years) | |||||||||||
| 40–59 | 12 | 9.6 | 2 | 14.3 | 19 | 27.1 | 437 | 32.9 | 470 | 30.6 | <0.001 |
| 60–69 | 32 | 25.6 | 4 | 28.6 | 25 | 35.7 | 509 | 38.3 | 570 | 37.1 | |
| 70–84 | 81 | 64.8 | 8 | 57.1 | 26 | 37.1 | 383 | 28.8 | 498 | 32.4 | |
| Smoking (15 missing) | |||||||||||
| Never | 47 | 38.2 | 0 | 0 | 7 | 10.0 | 537 | 40.8 | 591 | 38.8 | <0.001 |
| Previous | 68 | 55.3 | 10 | 71.4 | 41 | 58.6 | 641 | 48.7 | 760 | 49.9 | |
| Current | 8 | 6.5 | 4 | 28.6 | 22 | 31.4 | 138 | 10.5 | 172 | 11.3 | |
| Respiratory symptoms | |||||||||||
| mMRC | |||||||||||
| 0 | 0 | 0 | 0 | 0 | 16 | 22.9 | 1045 | 78.6 | 1061 | 69.0 | <0.001 |
| 1 | 91 | 72.8 | 6 | 42.9 | 45 | 64.3 | 264 | 19.9 | 406 | 26.4 | |
| 2 | 21 | 16.8 | 7 | 50.0 | 5 | 7.1 | 15 | 1.1 | 48 | 3.1 | |
| 3–4 | 13 | 10.4 | 1 | 7.1 | 4 | 5.7 | 5 | 0.4 | 23 | 1.5 | |
| Daily cough in periods of the year | 27 | 21.6 | 7 | 50.0 | 31 | 44.3 | 199 | 15.0 | 264 | 17.2 | <0.001 |
| More shortness of breath than normal the examination day | 20 | 16.0 | 3 | 21.4 | 19 | 27.1 | 111 | 8.4 | 153 | 9.9 | <0.001 |
| Crackles and wheezes (103 missing) | |||||||||||
| Any crackle | 23 | 20.0 | 3 | 23.1 | 15 | 22.1 | 162 | 13.1 | 203 | 14.1 | 0.03 |
| Basal bilateral inspiratory crackles | 6 | 5.2 | 1 | 7.7 | 4 | 5.9 | 21 | 1.7 | 32 | 2.2 | 0.007 |
| Any wheeze | 24 | 20.9 | 3 | 23.1 | 24 | 35.3 | 240 | 19.4 | 291 | 20.3 | 0.02 |
| Ordinary or long expiratory wheezes | 10 | 8.7 | 1 | 7.7 | 14 | 19.1 | 107 | 8.6 | 131 | 9.1 | 0.04 |
| Self‐reported diseases | |||||||||||
| Myocardial infarction (59 missing) | 25 | 21.6 | 5 | 50.0 | 2 | 3.0 | 52 | 4.1 | 86 | 5.8 | <0.001 |
| Angina pectoris (64 missing) | 15 | 13.4 | 1 | 8.3 | 3 | 4.6 | 41 | 3.2 | 60 | 4.1 | <0.001 |
| Atrial fibrillation (63 missing) | 30 | 26.5 | 3 | 23.1 | 7 | 10.6 | 87 | 6.8 | 127 | 8.6 | <0.001 |
| Heart failure (61 missing) | 19 | 17.3 | 2 | 15.4 | 0 | 0 | 26 | 2.0 | 47 | 3.2 | <0.001 |
| Hypertension (39 missing) | 85 | 70.8 | 3 | 23.1 | 17 | 25.4 | 417 | 32.1 | 522 | 34.8 | <0.001 |
| Diabetes (46 missing) | 20 | 16.9 | 1 | 7.7 | 3 | 4.4 | 71 | 5.5 | 95 | 6.4 | <0.001 |
| COPD (50 missing) | 5 | 4.4 | 6 | 46.2 | 19 | 28.8 | 32 | 2.5 | 64 | 4.2 | <0.001 |
| Asthma (45 missing) | 16 | 13.9 | 5 | 38.5 | 28 | 41.2 | 126 | 9.7 | 175 | 11.7 | <0.001 |
| FEV1 < LLN | 12 | 9.6 | 9 | 64.3 | 33 | 47.1 | 60 | 4.5 | 114 | 7.4 | <0.001 |
| NT‐proBNP >125 pg/mL | 101 | 80.8 | 14 | 100 | 12 | 17.1 | 224 | 16.9 | 351 | 22.8 | <0.001 |
| NT‐proBNP ≥97.5 percentile | 41 | 32.8 | 4 | 28.6 | 1 | 1.4 | 44 | 3.3 | 90 | 5.9 | <0.001 |
| C‐reactive protein ≥5 mg/L (9 missing) | 13 | 10.5 | 2 | 14.3 | 8 | 11.4 | 83 | 6.3 | 106 | 6.9 | 0.08 |
| SpO2 ≤95% (1 missing) | 6 | 4.8 | 3 | 21.4 | 5 | 7.2 | 54 | 4.1 | 68 | 4.4 | 0.01 |
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; LLN, lower limit of normal; mMRC, modified Medical Research Council; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Figure 2.
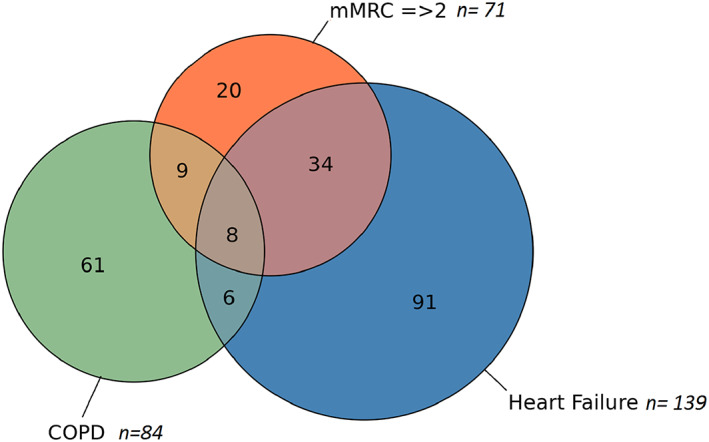
Venn diagram showing associations between heart failure, chronic obstructive pulmonary disease (COPD), and modified Medical Research Council (mMRC) ≥2 in 1538 participants.
Prediction of heart failure and chronic obstructive pulmonary disease
Age‐adjusted odds ratios (ORs) in predicting HF and COPD are shown in Table 2 . Current smoking predicted COPD with an OR of 15.8 but was not associated with HF. Reporting mMRC ≥2 was a particular strong predictor of HF, with an OR of 19.5 [95% confidence interval (CI) 11.3–33.7]. The corresponding OR for COPD was 6.3 (95% CI 3.5–11.6). Reporting more shortness of breath than usual on the examination day predicted both HF and COPD. Basal bilateral inspiratory crackles were associated with both diseases but significantly only with COPD (in the univariable analysis), whereas hearing wheezes was a significant predictor of COPD only. Self‐reported hypertension, atrial fibrillation, and myocardial infarction had ORs for HF between 3.2 and 5.4 but did not predict COPD. Self‐reported HF was a strong predictor of HF but was registered in only 17.1% of participants with an established HF. HF could be established in only 21 of the 47 participants with self‐reported HF. Among those with an established COPD, 31.6% reported to have the disease (Table 1 ), but overdiagnosis was as common as for HF. COPD predicted HF with an OR of 1.97. NT‐proBNP was a strong predictor of HF and not associated with COPD.
TABLE 2.
Age‐adjusted odds ratios for heart failure (n = 139) and chronic obstructive pulmonary disease (n = 84) in univariable and multivariable analysis of clinical characteristics in 1538 participants
| Odds ratio (OR) for heart failure | Odds ratio (OR) for COPD | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||
| OR | 95% CI | OR | OR | 95% CI | OR | |||
| Male gender | 1.1 | 0.8–1.5 | 0.9 | 0.6–1.4 | ||||
| Smoking | ||||||||
| Never | Ref | Ref | ||||||
| Previous | 1.2 | 0.8–1.7 | 5.7 | 2.6–12.7 | 3.2 | 1.3–7.5 | ||
| Current | 1.1 | 0.6–2.2 | 15.7 | 6.7–37.1 | 6.5 | 2.5–16.9 | ||
| Respiratory symptoms | ||||||||
| mMRC = 0–1 | Ref | |||||||
| mMRC = 2 | 21.6 | 11.2–41.7 | 20.3 | 9.3–43.9 | 6.8 | 3.4–13.7 | 2.8 | 1.0–8.3 |
| mMRC = 3–4 | 15.0 | 6.2–36.7 | 10.6 | 3.2–34.5 | 5.3 | 1. – 15.0 | 0.6 | 0.1–3.4 |
| Daily cough in periods | 1.5 | 1.0–2.4 | 1.4 | 0.8–2.4 | 4.4 | 2.8–6.7 | 3.4 | 1.9–6.3 |
| More shortness of breath than normal the examination day | 2.2 | 1.4–3.7 | 1.4 | 0.7–2.8 | 3.7 | 2.2–6.2 | 2.2 | 1.2–4.3 |
| Crackles | ||||||||
| Any crackles | 1.2 | 0.7–1.9 | 1.7 | 1.0–3.0 | 0.9 | 0.4–2.0 | ||
| Basal bilateral inspiratory crackles | 2.2 | 0.9–5.2 | 2.8 | 1.0–8.8 | 3.0 | 1.1–8.1 | 2.1 | 0.6–7.6 |
| Wheezes | ||||||||
| Any wheezes | 1.0 | 0.6–1.5 | 2.0 | 1.3–3.3 | 1.3 | 0.6–2.9 | ||
| Ordinary or long expiratory wheezes | 0.8 | 0.4–1.6 | 2.2 | 1.2–4.0 | 1.5 | 0.5–4.1 | ||
| Self‐reported diseases | ||||||||
| Myocardial infarction ever | 5.4 | 3.3–8.8 | 2.6 | 1.4–5.2 | 2.0 | 0.9–4.2 | 1.6 | 0.6–4.7 |
| Angina pectoris ever | 3.0 | 1.6–5.6 | 1.4 | 0.6–3.3 | 1.2 | 0.4–3.5 | ||
| Atrial fibrillation ever | 3.9 | 2.4–6.2 | 2.6 | 1.5–4.8 | 1.5 | 0.8–3.0 | ||
| Heart failure | 7.7 | 4.1–14.4 | 0.7 | 0.2–2.9 | ||||
| Hypertension ever | 3.2 | 2.2 – 4.7 | 3.2 | 2.0–5.2 | 0.6 | 0.3–0.9 | 0.5 | 0.2–0.9 |
| Diabetes ever | 2.7 | 1.6–4.7 | 1.2 | 0.6–2.5 | 0.7 | 0.3–2.0 | ||
| COPD ever | 1.9 | 1.0–3.9 | 0.5 | 0.2–1.4 | 16.7 | 9.3–29.9 | ||
| Asthma ever | 1.7 | 1.0–2.8 | 1.0 | 0.5–2.0 | 6.3 | 3.9–10.1 | 3.5 | 1.9–6.4 |
| COPD detected | 2.0 | 1.1–3.7 | 1.5 | 0.6–4.1 | ||||
| Heart failure detected | 1.9 | 1.0–3.6 | 1.6 | 0.6–4.7 | ||||
| FEV1 < LLN | 2.5 | 1.5–4.2 | 1.6 | 0.7–3.4 | 19.0 | 11.6–31.0 | 13.6 | 7.4–25.0 |
| NT‐proBNP >125 pg/mL | 21.0 | 12.7–34.6 | 1.4 | 0.8–2.4 | ||||
| NT‐proBNP ≥97.5 percentile | 11.2 | 6.9–18.1 | 0.9 | 0.4–2.4 | ||||
| C‐reactive protein ≥5 mg/L | 1.5 | 0.8–2.7 | 1.8 | 0.9–3.6 | 1.3 | 0.5–3.2 | ||
| SpO2 ≤95% | 1.1 | 0.55–2.4 | 2.2 | 1.0–5.0 | 0.6 | 0.2–1.8 | ||
CI, confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; LLN, lower limit of normal; mMRC, modified Medical Research Council; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Missing values are shown in Table 1 . Clinical variables associated with the heart failure and COPD with a P < 0.1 are selected for the multivariable analysis, excluding self‐reported heart failure and self‐reported COPD, respectively. NT‐proBNP is also excluded from the multivariable analysis.
Multivariable analysis
In the multivariable analysis, basal bilateral inspiratory crackles became significant predictors of HF, but not of COPD. ROC curves based on the multivariable analyses and the relative weight of the included predictors in the scores applied are shown in Figure 3 . When assessing prediction of HF, self‐reported HF was excluded, and an area under the curve (AUC) of 0.833 (95% CI 0.790–0.875) was obtained. Including this variable gave similar ROC curve (AUC = 0.829). When instead three levels of raised NT‐proBNP (<125 pg/mL, ≥125 pg/mL: <97.5 percentile and ≥97.5 percentile) were included in the analysis, an AUC of 0.909 (95% CI 0.877–0.940) was found. When assessing predictors of COPD, self‐reported COPD was excluded, and an AUC of 0.829 (95% CI 0.783–0.875) was found. When the variable self‐reported COPD was included, a nominally higher AUC of 0.840 was found. SpO2 ≤95% and C‐reactive protein ≥ 5 mg/L were not significant predictors of COPD in the multivariable analysis.
Figure 3.
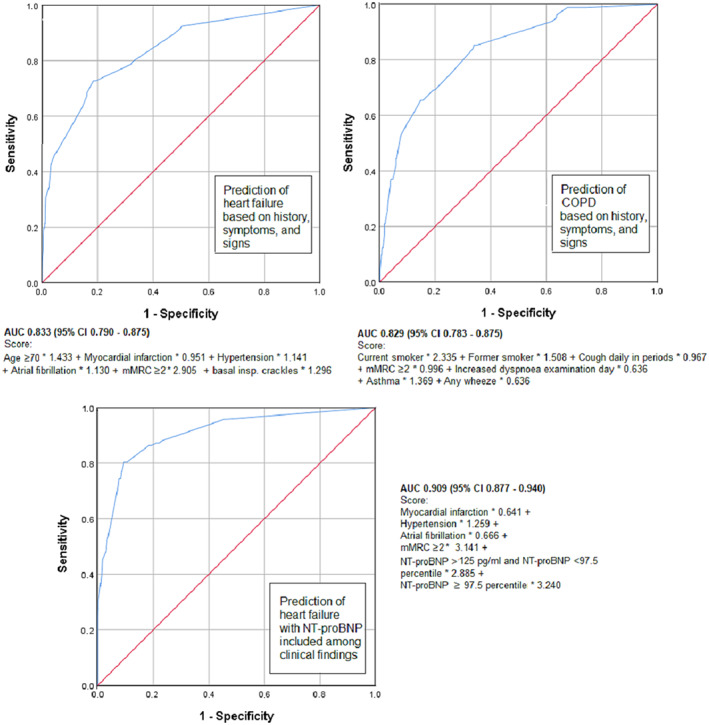
Receiver operating characteristic curves showing predictive value of smoking, self‐reported diseases, symptoms, and signs + N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) for heart failure, n = 1381, and chronic obstructive pulmonary disease (COPD), n = 1370. AUC, area under the curve; CI, confidence interval; mMRC, modified Medical Research Council.
Subtypes of heart failure
The frequency of establishing the three categories of HF was 38.7% for HFrEF, 21.3% for HFmrEF, and 40% for HFpEF. The occurrence of HF type varied significantly with age (P = 0.003, Table 3 ), HFrEF was the most frequent type in participants younger than 70 years, while HFpEF was most frequent in those older than 70 years (Figure 4 ). For COPD without concomitant HF, only a slight, and statistically insignificant, increase in prevalence by age was observed (Table 1 , Figure 4 ).
TABLE 3.
Characteristics of 155 participants with heart failure in the seventh Tromsø study by type of heart failure
| HFrEF | HFmrEF | HFpEF | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | P‐value | |
| Men | 37 | 61.7 | 16 | 48.5 | 22 | 35.5 | 0.02 |
| Women | 23 | 38.3 | 17 | 51.5 | 40 | 64.5 | |
| Age (years) | |||||||
| 40–59 | 10 | 16.7 | 2 | 6.1 | 2 | 3.2 | 0.003 |
| 60–69 | 21 | 35.0 | 5 | 15.2 | 12 | 19.4 | |
| 70–84 | 29 | 48.3 | 26 | 78.8 | 48 | 77.4 | |
| Smoking (2 missing) | |||||||
| Never | 20 | 26.7 | 12 | 36.4 | 21 | 35.0 | 0.6 |
| Previous | 47 | 62.7 | 19 | 57.6 | 31 | 51.7 | |
| Current | 8 | 10.7 | 2 | 6.1 | 8 | 13.3 | |
| Respiratory symptoms | |||||||
| mMRC | |||||||
| 1 | 41 | 68.3 | 16 | 48.5 | 47 | 75.8 | 0.1 |
| 2 | 14 | 23.3 | 10 | 30.3 | 7 | 11.3 | |
| 3–4 | 5 | 8.3 | 7 | 21.2 | 8 | 13.0 | |
| Daily cough in periods of the year (7 missing) | 17 | 28.3 | 8 | 25.8 | 10 | 17.5 | 0.4 |
| More shortness of breath than normal the examination day | 10 | 16.7 | 6 | 18.2 | 11 | 17.7 | 1.0 |
| Crackles and wheezes (11 missing) | |||||||
| Any crackle | 12 | 21.1 | 6 | 20.0 | 13 | 22.8 | 0.9 |
| Basal bilateral inspiratory crackles | 3 | 5.3 | 2 | 6.7 | 4 | 7.0 | 0.9 |
| Any wheezes | 16 | 28.1 | 3 | 10.0 | 14 | 24.6 | 0.2 |
| Ordinary or long expiratory wheezes | 6 | 10.5 | 2 | 6.7 | 6 | 10.5 | 0.8 |
| Self‐reported diseases | |||||||
| Myocardial infarction (11 missing) | 13 | 22.8 | 9 | 30.0 | 14 | 24.6 | 0.8 |
| Angina pectoris (15 missing) | 7 | 13.0 | 5 | 17.9 | 7 | 12.1 | 0.8 |
| Atrial fibrillation (15 missing) | 15 | 27.8 | 11 | 39.3 | 12 | 20.7 | 0.2 |
| Heart failure (18 missing) | 11 | 20.0 | 7 | 25.9 | 4 | 7.3 | 0.057 |
| Hypertension (9 missing) | 32 | 57.1 | 24 | 77.4 | 39 | 66.1 | 0.2 |
| Diabetes (8 missing) | 13 | 22.8 | 6 | 20.7 | 3 | 4.9 | 0.015 |
| COPD (12 missing) | 5 | 8.9 | 3 | 10.3 | 9 | 15.5 | 0.5 |
| Asthma (12 missing) | 9 | 16.1 | 6 | 20.7 | 13 | 22.4 | 0.7 |
| FEV1 < LLN (10 missing) | 14 | 23.7 | 4 | 12.9 | 8 | 14.5 | 0.3 |
| COPD, defined by spirometry and symptoms (16 missing) | 6 | 10.3 | 2 | 6.9 | 6 | 11.5 | 0.8 |
| NT‐proBNP >125 pg/mL | 35 | 58.3 | 33 | 100 | 62 | 100 | <0.001 |
| NT‐proBNP ≥97.5 percentile | 18 | 30.0 | 17 | 51.5 | 16 | 25.8 | 0.03 |
| C‐reactive protein ≥5 mg/L (1 missing) | 8 | 13.6 | 5 | 15.2 | 4 | 6.5 | 0.3 |
| SpO2 ≤95% | 4 | 6.7 | 1 | 3.0 | 7 | 11.3 | 0.3 |
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LLN, lower limit of normal; mMRC, modified Medical Research Council; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Figure 4.
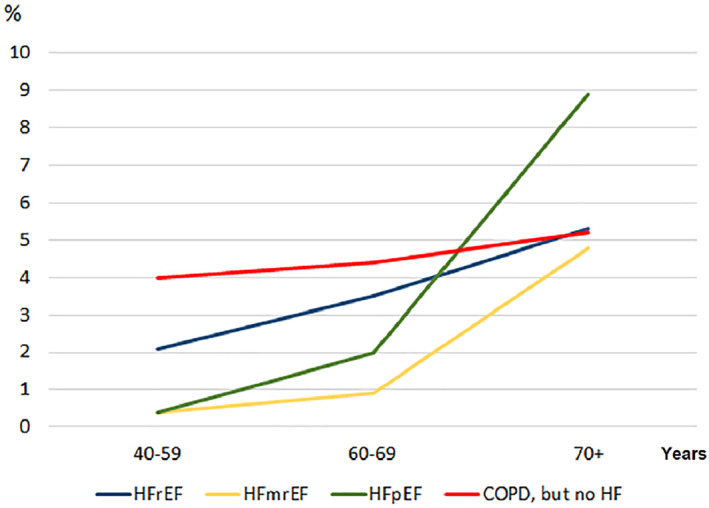
Frequency of chronic obstructive pulmonary disease (COPD) [COPD without concomitant heart failure (HF), based on 7110 participants] and three types of HF (based on 1624 participants) by age in the seventh Tromsø study. HFmrEF, HF with mid‐range ejection fraction; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction.
NT‐proBNP >125 pg/mL was found in 58.3% of the subjects with HFrEF.
Sensitivity analysis
Only small and insignificant changes were found when predictors of HF were evaluated by univariable and multivariable logistic regression in a somewhat extended sample of 1624 subjects. When predictors of COPD were evaluated in a sample of 7110 subjects, we found that statistical associations became stronger for crackles and wheezes, but self‐reported cardiovascular diseases and NT‐proBNP were still not associated with COPD. SpO2 ≤95% became more strongly associated with COPD with an OR of 3.5 (95% CI 2.3–5.4). ROC curves based on multivariable analysis in the extended samples did not change the AUC.
Discussion
Main findings
Our study indicates that HF should always be considered in patients reporting exertional dyspnoea when presenting in primary care and also in the presence of COPD. We found HF in as many as 59% of the participants who reported to ‘walk slower than people of the same age on the level because of breathlessness or has to stop for breath when walking at own pace on the level’ (mMRC = 2). The content of mMRC = 2 corresponds rather closely to New York Heart Association grade 3, 20 a scale mainly used for grading HF patients. We found a history of smoking to be the strongest predictor of COPD, but smoking status is already recognized as the most important information in early diagnosis of this disease. 19 We could confirm that crackles are a useful finding in the diagnosis of HF but also frequently found in COPD. Hearing crackles (any or basal) was not a significant independent predictor of COPD in the multivariable analysis, which may partly be explained by frequent occurrence of crackles among current smokers. 11 Wheezes were confirmed to be independent predictors of COPD. 21 ROC analyses showed that HF and COPD can be identified with similar ease based on history, symptoms, and signs. HF was most strongly predicted when information on NT‐proBNP was included. However, NT‐proBNP ≥125 pg/mL had a low sensitivity for HFrEF (58%).
Comparison with previous studies
To our knowledge, the 2016 ESC guidelines have not previously been applied in population‐based studies. The distribution of the HF subtypes was similar to what found in a recent Danish study. 22 In a Swedish register‐based study, including a population aged 18 years or more, the prevalence of age‐standardized HF was less than 2%. 23 As only 40% had the diagnosis based on echocardiography, this might have caused a significant underdiagnosis, especially in patients not requiring hospitalization where they are more likely to receive echocardiography. The Swedish figure is more comparable with the rate of self‐reported HF in our study than the actual prevalence of HF, which is more than twice as high. We found a considerable underdiagnosis of HF, and one may question whether the 2016 ESC diagnostic criteria are sufficiently strict. Because the criteria for diastolic dysfunction have been sharpened, decreasing the prevalence in a general population from 30% to 3%, this is unlikely. 2 The prevalence of COPD (with FEV1/FVC < LLN instead of <0.7 as diagnostic criterion, like in our study) was 7.3% in a Norwegian study from 2006 to 2008. 24 The lower prevalence in our study (6.2%) may be partly explained by the inclusion of respiratory symptoms as diagnostic criterion. Improved lung health due to drop in smoking after 2008 may also have played a role.
A combination of HF and COPD seems to be less frequent than previously reported. We found HF in 16.7% of those with COPD, while the corresponding frequency in a register‐based study from Taiwan was 28.9%. 9 Among the subjects with HF in our study, we found COPD co‐morbidity in 10% only. In a Danish study, COPD was found in 35% of patients admitted with HF. 25 This discrepancy may be partly explained by selection of patients with severe disease in the Danish study. The use of a fixed FEV1/FVC ratio of 0.7 as criterion for COPD in the Danish study, instead of LLN, which we used, has probably also contributed to a higher prevalence of COPD. 24 Several authors have warned against overdiagnosis of COPD in HF, because pulmonary congestion can lead to bronchial obstruction and a decreased FEV1/FVC ratio. 5 , 26 , 27 The strict criteria for COPD in our study (FEV1/FVC < LLN and symptoms) have probably made overdiagnosis less likely. The high frequency of coughing, smoking, FEV1 < LLN, and self‐reported COPD in the group with both diseases compared with those with HF only (Table 1 ) indicates that overdiagnosis of COPD in subjects with HF has been a minor problem.
Predictors of HF have often been evaluated in selected populations, like in emergency departments. Shortness of breath has been found to be of great importance in such settings. 6 , 28 Roalfe and co‐workers were not able to evaluate shortness of breath as a diagnostic clue in their study, because almost all patients referred from primary care for cardiologic evaluation had this symptom, and the symptom was not graded. 29 Crackles (crepitations) were found to be sufficiently discriminating to be included in a clinical prediction rule for HF, together with a history of myocardial infarction, leg oedema, and a natriuretic peptide test. In a larger study by Fonseca and co‐workers from 2004, 1058 primary care patients with history, symptoms, or signs indicating HF were examined with echocardiography. 17 Shortness of breath was a strong predictor of HF, together with cardiovascular disease, like in our study. Crackles (basal rales) were more frequently registered and were more strongly associated with HF than in our study. One may question whether selection bias has led to overrepresentation of patients with crackles in this study. Crackles tend to be more frequent when the HF gets more severe, 30 and registration of crackles in the study by Fonseca and co‐workers might have been influenced by the degree of suspicion of HF.
In a recent study of 1088 patients with HFrEF or HFmrEF, 19% had NT‐proBNP <125 pg/mL. 31 This is probably comparable with our results, with a frequency of 42% in the HFrEF group and, by definition, 0% in the HFmrEF group. Finding NT‐proBNP <125 pg/mL does not exclude HFrEF, but with such low values, the prognosis is good. 31
Strengths and limitations
Tromsø 7 had a 65% response rate. The Tromsø study has a high external validity for the Norwegian population, 12 but people with poor health might have found it difficult to participate and are probably underrepresented. The added inclusion of subject who had been participating in previous waves of the Tromsø study was probably only a minor source of error, because they had been randomly included in the previous surveys. They probably raised the mean age of the study sample, but this was taken care of by applying age‐standardized prevalence and age‐adjusted analysis. We applied the new diagnostic criteria for HF, and reading of echocardiography was blinded for other information about the participants. Regarding the diagnosis of COPD, we included respiratory symptoms among the diagnostic criteria, which is now recommended in GOLD guidelines. 19 However, post‐bronchodilator spirometry was not carried out, which might have led to overdiagnosis in some participants. 32 Recorded lung sounds have not been applied in previous studies on prediction of HF and COPD. The classification of the sounds was also blinded, and an objective identification of lung sounds was thus made possible. We were not able to evaluate other clinical signs, such as oedema and neck–vein distension. Relying on self‐report for previous diseases introduces the limitation of recall bias. Further, self‐reported diseases do not accurately reflect the diseases diagnosed in health care. In a study from the USA, only 38% of participants with a diagnosis of HF found in medical records reported to have the disease. 33 Although such discordance might be less pronounced in other diseases, the significance of self‐reported diseases should always be questioned.
Conclusions
The study indicates that a doctor with considerable certainty may identify HF and COPD and also differentiate the diseases from each other, based on history, symptoms, and signs. In general practice, when an elderly patient present with shortness of breath, both diseases should be considered. Abnormal lung sounds may be found in both diseases, previous cardiovascular disease points at HF, while a history of smoking points at COPD. Wheezes were independent predictors of COPD, while NT‐proBNP was not associated with COPD. The threshold should be low for ordering echocardiography or spirometry to verify the suspected cause of dyspnoea.
Conflict of interest
Dr Schirmer reports grants from Novartis, grants and personal fees from Astra Zeneca, and personal fees from MSD, all outside the submitted work. No other conflict of interest is reported.
Funding
H.S. received research grants from Simon Fougner Hartmann's Family Foundation and from the Health Authorities North Norway (#SFP1272‐16).
Supporting information
Data S1. Abstract HF and COPD paper.
Data S2. HF and COPD_ESC_HF_CoverLetter.
Data S3. Permission statement.
Appendix A.
The 97.5 percentile of N‐terminal pro‐brain natriuretic peptide by age based on 1981 blood donors aged 18–65 years and 283 patients aged between 50 and 90 years without symptoms or history indicating increased risk of heart disease, table found in Instructions for Use from Roche Diagnostics.
| Age (years) | Women (pg/mL) | Men (pg/mL) |
|---|---|---|
| 18–44 | 130 | 86 |
| 45–54 | 249 | 121 |
| 55–64 | 287 | 210 |
| 65–74 | 301 | 376 |
| ≥75 | 738 | 486 |
Melbye, H. , Stylidis, M. , Solis, J. C. A. , Averina, M. , and Schirmer, H. (2020) Prediction of chronic heart failure and chronic obstructive pulmonary disease in a general population: the Tromsø study. ESC Heart Failure, 7: 4139–4150. 10.1002/ehf2.13035.
References
- 1. DALYs GBD , Collaborators H . Global, regional, and national disability‐adjusted life‐years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1859–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group ESCSD . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 3. Danielsen SE, Lochen ML, Medbo A, Vold ML, Melbye H. A new diagnosis of asthma or COPD is linked to smoking cessation—the Tromsø study. Int J Chron Obstruct Pulmon Dis 2016; 11: 1453–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collaborators GBDCRD . Global, regional, and national deaths, prevalence, disability‐adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2017; 5: 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rutten FH, Broekhuizen BDL. Misclassification of both chronic obstructive pulmonary disease and heart failure. JAMA Netw Open 2018; 1: e185486. [DOI] [PubMed] [Google Scholar]
- 6. Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G, European Society of C , European Society of Intensive Care M . Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 2010. May; 12: 423–433. [DOI] [PubMed] [Google Scholar]
- 7. Theander K, Hasselgren M, Luhr K, Eckerblad J, Unosson M, Karlsson I. Symptoms and impact of symptoms on function and health in patients with chronic obstructive pulmonary disease and chronic heart failure in primary health care. Int J Chron Obstruct Pulmon Dis 2014; 9: 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rutten FH, Cramer MJ, Lammers JW, Grobbee DE, Hoes AW. Heart failure and chronic obstructive pulmonary disease: an ignored combination? Eur J Heart Fail 2006; 8: 706–711. [DOI] [PubMed] [Google Scholar]
- 9. Su VY, Yang YH, Perng DW, Tsai YH, Chou KT, Su KC, Su WJ, Chen PC, Yang KY. Real‐world effectiveness of medications on survival in patients with COPD‐heart failure overlap. Aging (Albany N Y) 2019; 11: 3650–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Broekhuizen BD, Sachs AP, Hoes AW, Verheij TJ, Moons KG. Diagnostic management of chronic obstructive pulmonary disease. Neth J Med 2012; 70: 6–11. [PubMed] [Google Scholar]
- 11. Aviles‐Solis JC, Jacome C, Davidsen A, Einarsen R, Vanbelle S, Pasterkamp H, Melbye H. Prevalence and clinical associations of wheezes and crackles in the general population: the Tromsø study. BMC Pulm Med 2019; 19: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacobsen BK, Eggen AE, Mathiesen EB, Wilsgaard T, Njolstad I. Cohort profile: the Tromsø study. Int J Epidemiol 2012; 41: 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahler DA, Ward J, Waterman LA, McCusker C, ZuWallack R, Baird JC. Patient‐reported dyspnea in COPD reliability and association with stage of disease. Chest 2009; 136: 1473–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J‐U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 15. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, Force AET. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 16. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, Stocks J, Initiative ERSGLF . Multi‐ethnic reference values for spirometry for the 3–95‐yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fonseca C, Morais H, Mota T, Matias F, Costa C, Gouveia‐Oliveira A, Ceia F, Investigators E. The diagnosis of heart failure in primary care: value of symptoms and signs. Eur J Heart Fail 2004; 6: 795–800 821‐792. [DOI] [PubMed] [Google Scholar]
- 18. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277–314. [DOI] [PubMed] [Google Scholar]
- 19. Global Initiative for Chronic Obstructive Lung Disease . GOLD‐2019‐POCKET‐GUIDE. 2020. www.goldCOPD.org
- 20. Miller‐Davis C, Marden S, Leidy NK. The New York Heart Association classes and functional status: what are we really measuring? Heart Lung 2006. ‐Aug; 35: 217–224. [DOI] [PubMed] [Google Scholar]
- 21. Oshaug K, Halvorsen PA, Melbye H. Should chest examination be reinstated in the early diagnosis of chronic obstructive pulmonary disease? Int J Chron Obstruct Pulmon Dis 2013; 8: 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nielsen OW, Valeur N, Sajadieh A, Fabricius‐Bjerre A, Carlsen CM, Kober L. Echocardiographic subtypes of heart failure in consecutive hospitalised patients with dyspnoea. Open Heart 2019; 6: e000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lindmark K, Boman K, Olofsson M, Tornblom M, Levine A, Castelo‐Branco A, Schlienger R, Bruce Wirta S, Stalhammar J, Wikstrom G. Epidemiology of heart failure and trends in diagnostic work‐up: a retrospective, population‐based cohort study in Sweden. Clin Epidemiol 2019; 11: 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhatta L, Leivseth L, Mai XM, Chen Y, Henriksen AH, Langhammer A, Brumpton BM. Prevalence and trend of COPD from 1995–1997 to 2006–2008: the HUNT study, Norway. Respir Med 2018; 138: 50–56. [DOI] [PubMed] [Google Scholar]
- 25. Iversen KK, Kjaergaard J, Akkan D, Kober L, Torp‐Pedersen C, Hassager C, Vestbo J, Kjoller E, Group EC‐LFS . Chronic obstructive pulmonary disease in patients admitted with heart failure. J Intern Med 2008; 264: 361–369. [DOI] [PubMed] [Google Scholar]
- 26. Hawkins NM, Petrie MC, Jhund PS, Chalmers GW, Dunn FG, McMurray JJ. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail 2009; 11: 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guder G, Brenner S, Stork S, Hoes A, Rutten FH. Chronic obstructive pulmonary disease in heart failure: accurate diagnosis and treatment. Eur J Heart Fail 2014; 16: 1273–1282. [DOI] [PubMed] [Google Scholar]
- 28. Basset A, Nowak E, Castellant P, Gut‐Gobert C, Le Gal G, L'Her E. Development of a clinical prediction score for congestive heart failure diagnosis in the emergency care setting: the Brest score. Am J Emerg Med 2016; 34: 2277–2283. [DOI] [PubMed] [Google Scholar]
- 29. Roalfe AK, Mant J, Doust JA, Barton P, Cowie MR, Glasziou P, Mant D, McManus RJ, Holder R, Deeks JJ, Doughty RN, Hoes AW, Fletcher K, Hobbs FD. Development and initial validation of a simple clinical decision tool to predict the presence of heart failure in primary care: the MICE (Male, Infarction, Crepitations, Edema) rule. Eur J Heart Fail 2012; 14: 1000–1008. [DOI] [PubMed] [Google Scholar]
- 30. Platz E, Lewis EF, Uno H, Peck J, Pivetta E, Merz AA, Hempel D, Wilson C, Frasure SE, Jhund PS, Cheng S, Solomon SD. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur Heart J 2016; 37: 1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spinar J, Spinarova L, Malek F, Ludka O, Krejci J, Ostadal P, Vondrakova D, Labr K, Spinarova M, Pavkova Goldbergova M, Benesova K, Jarkovsky J, Parenica J. Prognostic value of NT‐proBNP added to clinical parameters to predict two‐year prognosis of chronic heart failure patients with mid‐range and reduced ejection fraction—a report from FAR NHL prospective registry. PLoS ONE 2019; 14: e0214363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johannessen A, Omenaas ER, Bakke PS, Gulsvik A. Implications of reversibility testing on prevalence and risk factors for chronic obstructive pulmonary disease: a community study. Thorax 2005; 60: 842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Camplain R, Kucharska‐Newton A, Loehr L, Keyserling TC, Layton JB, Wruck L, Folsom AR, Bertoni AG, Heiss G. Accuracy of self‐reported heart failure. The atherosclerosis risk in communities (ARIC) study. J Card Fail 2017; 23: 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Abstract HF and COPD paper.
Data S2. HF and COPD_ESC_HF_CoverLetter.
Data S3. Permission statement.


