Abstract
Aims
This study was to determine the cost‐effectiveness of dapagliflozin in heart failure with reduced ejection fraction (HFrEF) patients in China from a perspective of health care payers.
Methods and results
We built a Markov model to perform a cost‐effectiveness analysis comparing standard treatment + dapagliflozin (10 mg, q.d.) with standard treatment for HFrEF. The base case in our simulation was a 65‐year‐old HFrEF patient and was modelled over 15 years. Inputs of the model were derived from the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial and other relevant data from China. Costs, quality‐adjusted life year (QALY), and incremental cost‐effectiveness ratio (ICER) were estimated for adding dapagliflozin relative to standard treatment. Costs and QALY were discounted at a 4.2% rate annually. All costs are presented in 2017 US dollars. Dapagliflozin would be considered very cost‐effective if the ICER was lower than a willingness‐to‐pay (WTP) threshold of $8573.4. Uncertainty was assessed in our model using one‐way, two‐way, and probabilistic sensitivity analysis (PSA). In our base case, compared with standard treatment, adding dapagliflozin was more expensive ($5829.4 vs. $4377.1) but more effective (4.82 vs. 4.44 QALYs). The respondent ICER was $3827.6 per QALY gained at 15‐year follow‐up. When the simulated horizon was longer than 3.5 years, the respondent ICER became lower than the WTP threshold. The inputs with the largest impact on ICER were the cost of dapagliflozin, the cardiovascular mortality in both groups, and the cost of hospitalization for heart failure. Most results of sensitivity analysis were robust. PSA showed a similar result as the base case (ICER = $4412.5 per QALY gained). In Monte Carlo simulation, at a WTP threshold of $8573.4 per QALY, dapagliflozin was considered very cost‐effective in 53.10% of the simulations.
Conclusions
Dapagliflozin was a very cost‐effective treatment option for HFrEF patients in China according to the result of our model. Our findings will help doctors and health care payers to make decisions in clinical practice. Future real‐world studies of cost‐effectiveness of dapagliflozin based on Chinese population were also needed.
Keywords: Dapagliflozin, HFrEF, Cost‐effectiveness, China
Introduction
Heart failure (HF) is a severe public health problem with heavy economic burden worldwide. A recent study reported that the prevalence of HF was about 1.3% and estimated that there were more than 10 million HF patients in China. 1 The estimated HF cost for China was $5.42 billion, accounting for 5.01% of total health care costs in 2012. 2 Heidenreich et al. estimated that hospitalization for HF would account for 80% of the cost for care of HF patients. 3 In China, the inpatient cost among urban HF patients accounted for 66% of their total cost. 4 Therefore, a large amount of money for HF management will be saved if we are able to reduce HF admission rate.
Several large‐sample‐sized clinical trials have shown that inhibitors of sodium–glucose cotransporter 2 reduce the risk of HF hospitalization in patients with type 2 diabetes mellitus. 5 , 6 , 7 , 8 Recently, the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA‐HF) trial indicated that adding dapagliflozin, compared with placebo, resulted in a significant 26% reduction in the primary outcome of a composite of HF worsening (hospitalization or an urgent visit resulting in intravenous therapy for HF) or cardiovascular (CV) death in HF with reduced ejection fraction (HFrEF) patients. 9 Thus, it is reasonable to initiate dapagliflozin for HFrEF patients who have been treated by standard treatment to further improve their outcomes.
Besides therapy effectiveness, economic benefit is also an important consideration in medical decision and medical policymaking. Cost‐effectiveness (CE) analysis (CEA) is a useful approach to assess medication value by quantifying and comparing the therapy cost and effectiveness of different treatment strategies. The CE of dapagliflozin (compared with different treatment strategies) for patients with type 2 diabetes mellitus was not consistent in China. 10 , 11 Additionally, there is no study about the CE of dapagliflozin among HFrEF patients in China. With the goal of aiding decision making, we estimated the CE of adding dapagliflozin to standard treatment compared with not adding dapagliflozin for HFrEF patients in China's medical circumstance from a perspective of health care payers.
Material and method
Rational and construction of model
We used a Markov model (Model 1) to perform a CEA comparing two treatment strategies for HFrEF patients: dapagliflozin (10 mg, q.d.) + standard treatment (dapagliflozin group) versus standard treatment (control group). Standard treatment was defined as the therapy for HFrEF based on the DAPA‐HF trial. Unless contraindicated or not tolerated, treatment should include (i) an angiotensin‐converting‐enzyme inhibitor (ACEI), an angiotensin receptor blocker (ARB), or sacubitril–valsartan; (ii) a beta‐blocker; and, if appropriate, (iii) a mineralocorticoid receptor antagonist. 9 Detailed structures of Model 1 were shown in Figure 1 . The base case was a 65‐year‐old patient from a hypothetical cohort of 2370 patients based on the characteristics observed in the DAPA‐HF trial. 9 Additional model inputs were derived from other published reports. We assumed that this cohort received fixed treatments and kept the doses throughout the model horizon. There were five Markov states in our model: New York Heart Association (NYHA) functional classification I, II, III, and IV and death. The initial distribution of NYHA class of our hypothetical cohort was based on the baseline characteristics of the DAPA‐HF trial (0% I, 67.5% II, 31.6% III, and 0.9% IV). 9 We set the cycle length to 3 months and the total number of cycles to 60 (equal to 15 years), which was able to not only compare the results of our model with the results observed in the DAPA‐HF trial at the end of the sixth cycle (18 months), but also explore the potential long‐term effect. Because the risk of readmission in vulnerable period was substantially higher than that in stable period, 12 we assumed that all HF readmission occurred in 2 months for patients who experienced an HF hospitalization in our model, and we arranged a fixed probability of HF readmission for each HF hospitalization. Patients would transition between different NYHA functions at the end of each cycle. Events included HF hospitalization, HF readmission, CV death, and non‐CV death. Our model used half‐cycle correction to prevent overestimation of expected survival. Only direct costs (hospitalization and prescription drugs for HF) were accounted for in our model. Inputs of cost in Markov model were converted into respondent values in 2017 using the consumer price indexes of health care. According to the National Bureau of Statistics of China, the consumer price indexes of health care in China from 2015 to 2018 were 102.7, 103.8, 106.0, and 104.3, respectively. 13 Future costs were discounted at a rate of 4.2%, which was the geometric mean based on these figures. Although there was an agreement on the need for discounting when it came to measuring costs or outcomes in monetary values, there was no universal agreement on whether or not to discount non‐monetary outcomes. In this study, both life year and quality‐adjusted life year (QALY) were discounted at the same rate of 4.2%, and a range of 0% to 8% was also tested in sensitivity analysis. Other non‐monetary outcomes (HF hospitalization, HF readmission, and death) were not discounted. In addition, we also built Model 2 where a single risk of HF‐associated hospitalization was used to replace the risk of HF hospitalization and the probability of HF readmission in Model 1, while other inputs were the same as those in Model 1.
Figure 1.
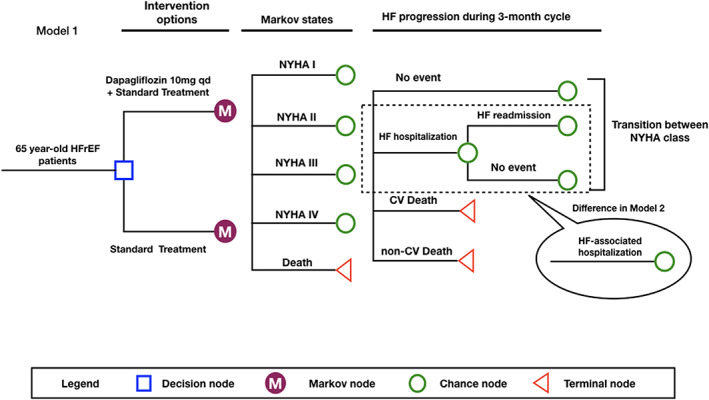
Markov model structure. HFrEF patients enter the model and are placed on either dapagliflozin + standard treatment or standard treatment. The initial distribution of NYHA classification is derived from the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial. Patients will experience no event, heart failure (HF) hospitalization, or death every 3 months. Those who do not die will transition their NYHA classification at the end of cycle. In Model 2, the risk of HF‐associated hospitalization replaces the risk of HF hospitalization and the fixed probability of HF readmission. CV, cardiovascular; HFrEF, heart failure with reduced ejection fraction; NYHA, New York Heart Association.
To enable readers to reproduce our simulation, the details of our model were described in Table 1 . Model building and analyses were performed with TreeAge Pro 2011 (Williamstown, MA. USA).
TABLE 1.
Inputs of the Markov model
| Input variable (3‐month probability) | Value | Range | Distribution | Distribution parameters | Source |
|---|---|---|---|---|---|
| Model 1 | |||||
| Event rate of the control group | |||||
| Risk of HF hospitalization | 2.346% | 2.085–2.606% | Beta | α = 2.346; ß = 97.654 | DAPA‐HF trial 9 |
| Probability of HF readmission | 47.359% | 43.170–51.549% | Beta | α = 47.359; ß = 52.641 | DAPA‐HF trial 9 |
| CV mortality | 1.996% | 1.758–2.232% | Beta | α = 1.996; ß = 98.004 | DAPA‐HF trial 9 |
| Non‐CV mortality | China CDC 14 | ||||
| 65~ | 0.244% | 0.236–0.253% | None | None | |
| 70~ | 0.312% | 0.301–0.323% | None | None | |
| 75~ | 0.450% | 0.435–0.464% | None | None | |
| Event rate of the dapagliflozin group | |||||
| Risk of HF hospitalization | 1.674% | 1.454–1.884% | Beta | α = 1.674; ß = 98.326 | DAPA‐HF trial 9 |
| Probability of HF readmission | Same as the probability for the control group | ||||
| CV mortality | 1.644% | 1.436–1.866% | Beta | α = 1.644; ß = 98.356 | DAPA‐HF trial 9 |
| Non‐CV mortality | Same as the probability for the control group | ||||
| Utility inputs | |||||
| NYHA I | 0.20375 | 0.19525–0.21250 | Beta | α = 20.375; ß = 79.625 | CARE‐HF trial 15 |
| NYHA II | 0.18000 | 0.17325–0.18725 | Beta | α = 18; ß = 82 | CARE‐HF trial 15 |
| NYHA III | 0.14750 | 0.13775–0.15725 | Beta | α = 14.75; ß = 85.25 | CARE‐HF trial 15 |
| NYHA IV | 0.12700 | 0.10300–0.15125 | Beta | α = 12.7; ß = 87.3 | CARE‐HF trial 15 |
| Disutility for hospitalization/readmission | 0.100 | 0.080–0.130 | Beta | α = 10; ß = 90 |
CARE‐HF trial 15 SENIORS trial 16 |
| Discounted rate | 4.2% | 0–8% | None | None | From the National Bureau of Statistics of China 13 |
| Cost | |||||
| Standard treatment | $95.9 | 95.9–566.7 | Gamma | Mean = 95.9; SD = 154 | From Huang et al. 4 |
| Dapagliflozin | $59.1 | 29.6–177.4 | None | None | Higher than the AstraZeneca's recommended retail price |
| Hospitalization for HF | $1993.7 | 996.9–5981.1 | Gamma | Mean = 1993.7; SD = 2772.9 | From Huang et al. 4 |
| Model 2 | |||||
| Risk of HF‐associated hospitalization (control group) | 3.568% | 3.257–3.891% | Beta | α = 3.568; ß = 96.432 | DAPA‐HF trial 9 |
| Risk of HF‐associated hospitalization (dapagliflozin group) | 2.517% | 2.251–2.776% | Beta | α = 2.517; ß = 97.483 | DAPA‐HF trial 9 |
CARE‐HF, Cardiac Resynchronization—Heart Failure; CV, cardiovascular; DAPA‐HF, Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; HF, heart failure; NYHA, New York Heart Association; SD, standard deviation; SENIORS, Study of the Effects of Nebivolol Intervention on Outcomes and Re‐hospitalization in Seniors with Heart Failure.
Model inputs
Inputs of mortality and heart failure‐associated hospitalization
An assumption of our model was that CV mortality and risks of hospitalization for HF were constant throughout the model horizon. During the 18.2‐month follow‐up of the DAPA‐HF trial, 9.6% of patients in the dapagliflozin group and 11.5% of patients in the placebo group died due to CV causes. 9 We converted these to 3‐month probabilities, which were 1.644% in the dapagliflozin group and 1.996% in the control group. We assumed that the risk of non‐CV death was similar across both groups. An age‐dependent risk of non‐CV death was derived from the Report on China's Cause of Death 2017 (published by the China Center for Disease Control and Prevention). 14 The risks of HF hospitalization were 9.7% in the dapagliflozin group and 13.4% of patients in the placebo group. 9 These risks were converted to 3‐month probabilities (1.674% for the dapagliflozin group and 2.346% for the control group). We used a fixed probability of experiencing an HF readmission after an HF hospitalization (47.359%) according to the result of the DAPA‐HF trial. 9 In Model 2, we used risks of HF‐associated hospitalization, and the parameters were 3.568% in the control group and 2.517% in the dapagliflozin group.
Inputs of New York Heart Association function classification
New York Heart Association classification reflected the activities of daily living and was regarded as a marker of health status. The utilities for NYHA functional classification were based on the result of the Cardiac Resynchronization—Heart Failure trial 15 and were converted to 3‐month utilities. HF‐associated hospitalization was assumed to cause the worsening of health status; thus, we applied a one‐time disutility each cycle, by subtracting patient's utility in each NYHA class by 0.1 if patients experienced a hospitalization for HF (including HF hospitalization, HF readmission, and HF‐associated hospitalization). 15 , 16 Transition between different NYHA classes at the end of every cycle was assumed to be similar for both arms because of lack of the effect of dapagliflozin on NYHA classification. Estimates of transition probabilities between NYHA classifications (Table 2 ) were derived from the Study of the Effects of Nebivolol Intervention on Outcomes and Re‐hospitalization in Seniors with Heart Failure trial. 16
TABLE 2.
New York Heart Association classification transition probabilities per cycle (3 months)
| To | I | II | III | IV | Distribution | |
|---|---|---|---|---|---|---|
| From | ||||||
| I | 0.977 | 0.019 | 0.004 | 0 | Dirichlet | |
| II | 0.008 | 0.981 | 0.010 | 0.001 | Dirichlet | |
| III | 0 | 0.034 | 0.960 | 0.006 | Dirichlet | |
| IV | 0 | 0 | 0.055 | 0.945 | Dirichlet | |
Inputs of costs
All inputs of costs were converted to US dollars (1 dollar = 7 Chinese Renminbi). The costs of hospitalization for HF and basic HF medications were derived from a real‐world study in 2014 that covered national urban employees and residents in China. 4 The costs in 2014 were inflated at rates of 2.7%, 3.8%, and 6.0% to respondent values in 2017. 13 Finally, the cost of per hospitalization for HF was $1993.7, and the cost for basic HF medications (including ACEI, ARB, beta‐blocker, spironolactone, diuretics, organic nitrates, phosphodiesterase inhibitor, digitalis, trimetazidine, statins, and anti‐arrhythmic drugs) was $95.9 every 3 months. Because the cost for basic HF medications included ACEI, ARB, beta‐blocker, spironolactone, and diuretics, we assumed that standard treatment (control group) cost was $95.9 per cycle. In the DAPA‐HF trial, 10.7% of patients were prescribed sacubitril–valsartan, which was a more expensive drug than traditional anti‐renin‐angiotensin‐aldosterone system drugs. The recommended cost of sacubitril–valsartan (target dose 200 mg, b.i.d.) was $511.7 every 3 months in 2019, 17 and we converted it into $470.9 in 2017. Accordingly, we tested a range of standard treatment cost from $95.9 to $566.7 in sensitivity analysis. The cost of dapagliflozin per cycle ($64.3) used by our model was higher than the AstraZeneca's recommended retail price in 2019 ($56.1) 17 , and we converted it to the value in 2017 ($59.1). Therefore, the drug cost in the dapagliflozin group was $155 per cycle. As mentioned before, future costs were discounted at a rate of 4.2%, and a range of 0% to 8% would also be tested in sensitivity analysis.
Outcome measures
We calculated all‐cause mortality, CV mortality, life years, QALYs, number of hospitalization for HF, mean total cost (HF drugs and hospitalization for HF), and incremental CE ratio (ICER, dapagliflozin group versus control group). QALY was an outcome measured as life years adjusted by patients' preferences for various health states. It incorporated both the quality (morbidity) and quantity (mortality) of life. ICER was the ratio of the difference in costs divided by the difference in outcomes. When one of the alternatives (dapagliflozin group) was both more expensive and more effective than another (control group), the ICER was used to determine the magnitude of the added cost for each unit in health improvement. There was no fixed willingness‐to‐pay (WTP) threshold to determine CE in China. The World Health Organization—Choosing Interventions That Are Cost‐Effective method recommended researchers to determine whether an intervention was cost‐effective by comparing the ICER to the per capita gross domestic product (GDP) for the country where the intervention occurred. 18 According to this method, intervention was considered cost‐effective if its ICER was less than three times the per capita GDP and not cost‐effective if it was greater than or equal to this threshold. If the ICER was less than the per capita GDP for the country, it was considered very cost‐effective. Data of the per capita GDP for China in 2017 ($8573.4) were derived from the National Bureau of Statistics of China. 19
Sensitivity analysis
Uncertainty was assessed in our model using one‐way, two‐way, and probabilistic sensitivity analysis (PSA). In one‐way sensitivity analysis, we tried a plausible range for a certain variable (Table 1 ) to determine its potential impact on ICER, while all other variables were held constant. In addition to varying model inputs, we also varied model horizon from 18 months to 15 years. Two‐way sensitivity analysis would be used between inputs that affected the ICER by more than $800.
As for PSA, one advantage was that any number of parameter uncertainties could be incorporated into an analysis. Results of PSA estimated the total impact of uncertainty on the model. In PSA of our model, statistical distributions were assigned to key parameters. A second‐order Monte Carlo simulation was performed at random 10 000 times based on the specific distributions of key parameters (Table 1 ). Results of the PSA were shown as scatterplots.
Results
Base case and cohort
According to Model 1, mean total costs for patients in the dapagliflozin group and the control group were $5829.4 and $4377.1 over a 15‐year time horizon, respectively. The dapagliflozin group yielded 4.82 QALYs with 7.11 life years, and the control group yielded 4.44 QALYs with 6.60 life years. These resulted in an ICER (dapagliflozin group versus control group) of $3827.6 per QALY gained, which was less than the per capita GDP for China in 2017 ($8573.4).
In the simulated cohort (N = 2370) analysis, 1656 patients (69.9%) in the dapagliflozin group and 1794 patients (75.7%) in the control group died during the 15‐year follow‐up. The total number of hospitalization for HF in the dapagliflozin group (2085) was 623 less than that in the control group (2708). Supporting Information, Table S1 showed relative events derived from the DAPA‐HF trial and our model. These results demonstrated that the calculated value of our model fitted well with the results of the DAPA‐HF trial.
As the model horizon varied from 18 months to 15 years (Figure 2 ), the respondent ICER decreased from $14 883.4 (less than three times per capita GDP for China in 2017) to $3827.6 per QALY gained. Notably, when the simulated horizon of Model 1 was longer than 3.5 years, the respondent ICER became lower than the per capita GDP for China in 2017 ($8573.4).
Figure 2.
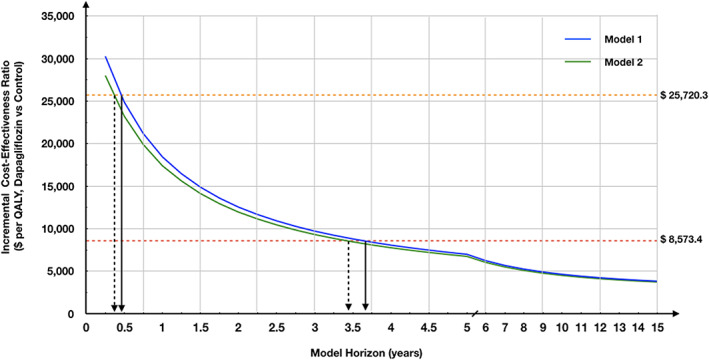
The incremental cost‐effectiveness ratio of Markov model by different simulated horizon. The orange dotted line represents three times the per capita gross domestic product (GDP) in 2017; the red dotted line represents the per capita GDP. QALY, quality‐adjusted life year.
The ICER derived from Model 2 was $3732.3 per QALY gained. Detailed results of Model 2 were shown in Table 3 .
TABLE 3.
Result of the base case and the simulated cohort
| Measurements | Model 1 | Model 2 | ||
|---|---|---|---|---|
| Time horizon = 15 years | Dapagliflozin | Control | Dapagliflozin | Control |
| Mean total cost ($) | 5829.4 | 4377.1 | 5858.4 | 4436.6 |
| Mean life year | 7.11 | 6.60 | 7.11 | 6.60 |
| Mean CE ($ per life year) | 819.6 | 663.7 | 823.7 | 672.7 |
| ICER ($ per life year) | 2809.4 | — | 2750.6 | — |
| Mean QALYs | 4.82 | 4.44 | 4.82 | 4.44 |
| Mean CE ($ per QALY) | 1209.0 | 985.4 | 1215.4 | 999.4 |
| ICER ($ per QALY) | 3827.6 | — | 3732.3 | — |
| Cohort analysis | N = 2370 | N = 2370 | N = 2370 | N = 2370 |
| Total number of HF hospitalization | 1415 | 1838 | — | — |
| Total number of HF readmission | 670 | 870 | — | — |
| Total number of HF‐associated hospitalization | 2085 | 2708 | 2138 | 2793 |
| Total death (n, %) | ||||
| Cardiovascular death | 1394 (58.8%) | 1557 (65.7%) | 1394 (58.8%) | 1557 (65.7%) |
| Non‐cardiovascular death | 1656 (69.9%) | 1794 (75.7%) | 1656 (69.9%) | 1795 (75.7%) |
CE, cost‐effectiveness; HF, heart failure; ICER, incremental cost‐effectiveness ratio; QALY, quality‐adjusted life year.
One‐way and two‐way sensitivity analysis
In one‐way analysis, we found that the cost of dapagliflozin had the biggest impact on the ICER of Model 1. At its upper limit, the respondent ICER was $12696.6 per QALY gained, which was higher than the per capita GDP for China in 2017 ($8573.4) but still lower than three times per capita GDP (Supporting Information, Table S2 ). Notably, most results of one‐way sensitivity analysis indicated that the ICERs were lower than $8573.4. A tornado diagram (Figure 3 ) was used to depict the variables that affected the ICER by more than $800.
Figure 3.
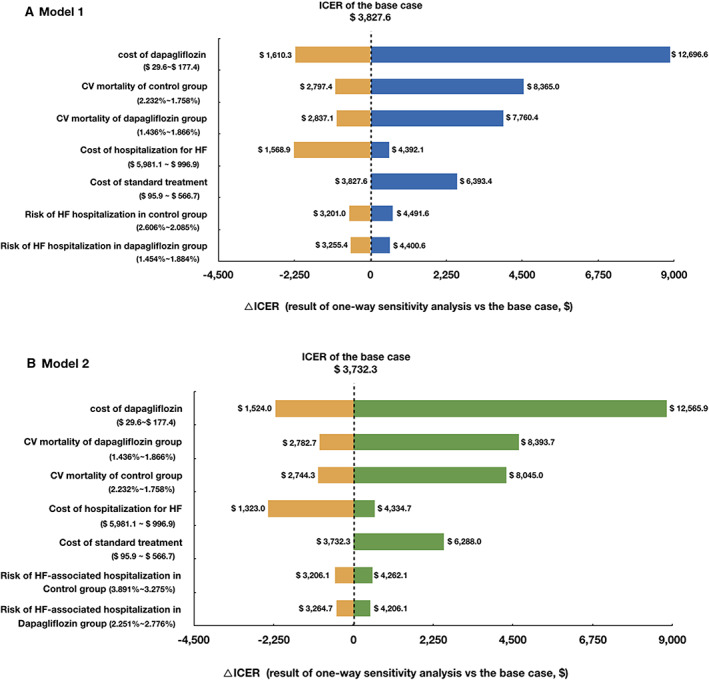
One‐way sensitivity analysis of Markov models (tornado diagram). Variables that affect the ICER by more than $800 are displayed. CV, cardiovascular; HF, heart failure; ICER, incremental cost‐effectiveness ratio.
Results of two‐way sensitivity analysis were presented in Supporting Information, Table S3 . Most results of two‐way sensitivity analysis were also robust, while the parameters with the largest impact on ICER were the CV mortality in both groups and the cost of dapagliflozin, which led the ICER higher than three times the per capita GDP ($25 720.3).
The results of sensitivity analysis for Model 2 (Supporting Information, Tables S2 and S4 ) were similar as those of Model 1.
Probabilistic sensitivity analysis
The results of PSA were presented by an incremental cost‐incremental QALYs scatterplot (Figure 4 ). For Model 1, mean total costs for patients were $6170.3 with 4.99 QALYs in the dapagliflozin group and $4639.8 with 4.64 QALYs in the control group over a 15‐year time horizon. These resulted in an ICER of $4412.5 per QALY gained, which was $584.9 higher than the base case. In Monte Carlo simulation, 81.30% of simulations indicated that adding dapagliflozin was more expensive and 59.15% of simulations showed that adding dapagliflozin was more effective than standard treatment. At a WTP threshold of $8573.4 per QALY, dapagliflozin was considered cost‐effective in 53.10% of the simulations. This acceptability only increased to 57.43% at a WTP threshold of $25 720.3 per QALY. The PSA result of Model 2 was presented in Figure 4 .
Figure 4.
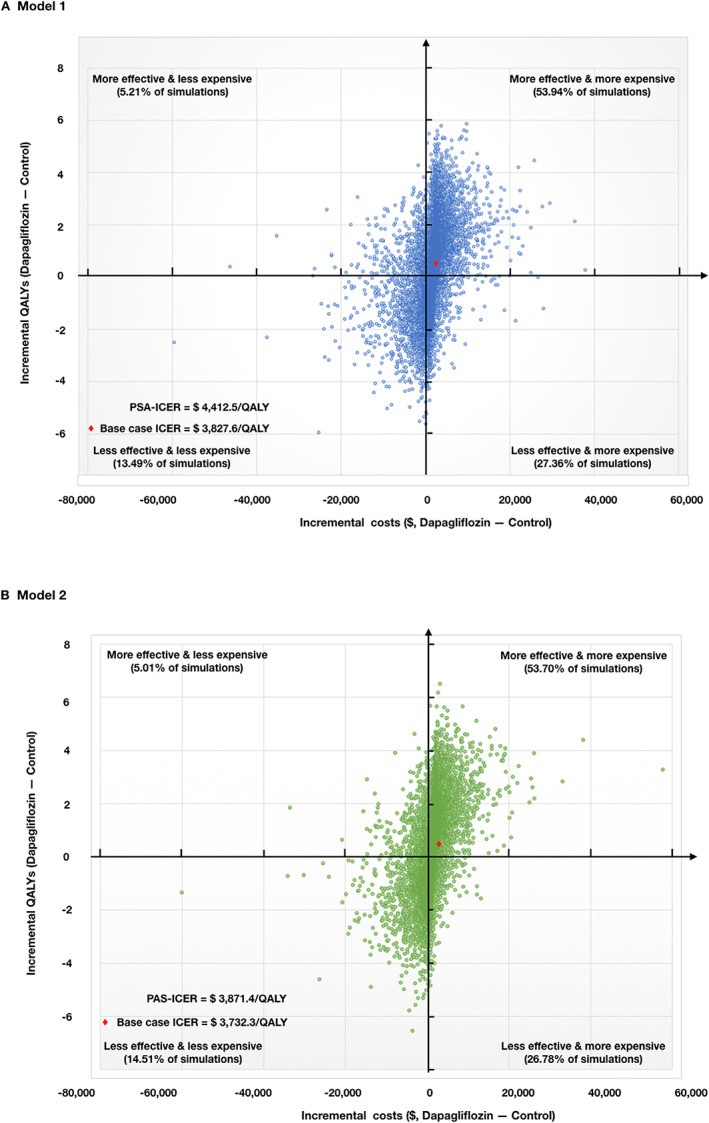
Scatterplot of incremental costs and incremental QALYs over a 15‐year follow‐up for adding dapagliflozin relative to standard treatment. Probabilistic sensitivity analysis results of 10 000 model simulations. (A) The effect of uncertainty of Model 1. At a WTP threshold of $8573.4 per QALY, dapagliflozin was considered cost‐effective in 53.10% of the simulations. This acceptability only increased to 57.43% at a WTP threshold of $25 720.3 per QALY. (B) The effect of uncertainty of Model 2. At a WTP threshold of $8573.4 per QALY, dapagliflozin was considered cost‐effective in 53.94% of the simulations. This acceptability only increased to 57.37% at a WTP threshold of $25 720.3 per QALY. ICER, incremental cost‐effectiveness ratio; PSA, probabilistic sensitivity analysis; QALY, quality‐adjusted life year.
Discussion
Several CE studies have tested whether dapagliflozin could be cost‐effective compared with other treatment strategies for diabetes patients in China, but their conclusions were not consistent. 10 , 11 However, there was no CE study for dapagliflozin in HFrEF patients. Given that the combination of dapagliflozin has potential for widespread use among HFrEF patients, decision makers must determine whether the extra benefit with dapagliflozin is worth the additional costs. This is the first CEA of dapagliflozin for HFrEF patients regardless of diabetes. The simulations (Models 1 and 2) showed that even though adding dapagliflozin to standard treatment for HFrEF was unlikely to be cost saving, dapagliflozin effectively reduced the total number of hospitalization for HF and CV death. The consequential ICERs ($3827.6 per QALY gained in Model 1 and $3732.3 in Model 2) were lower than the per capita GDP for China in 2017 ($8573.4), which indicated that adding dapagliflozin was a very cost‐effective strategy compared with standard treatment. The results of sensitivity analysis were robust to most inputs' uncertainty. Overall, our study provides a valuable, quantitative assessment of dapagliflozin for decision makers and health care payers.
Although the results of PSA also demonstrated that adding dapagliflozin was very cost‐effective, at a range of WTP from $8573.4 per QALY to $25 720.3 per QALY, the acceptability was very stable (nearly 60%, which was not high). The level of WTP just has a slight impact on the acceptability of adding dapagliflozin. This means that it is necessary for us to identify the potential HFrEF patients who may benefit more from adding dapagliflozin to standard treatment, because there may be some individuals with dapagliflozin who cannot reduce the risk of events or require high medical expense with just a slight benefit.
According to our models, the model horizon had a large impact on the ICER. When patients use dapagliflozin for half a year, adding dapagliflozin would be considered cost‐effective. If dapagliflozin treatment lasts for more than 3.5 years, it would be very cost‐effective. This indicated that if patients with HFrEF have a long life expectancy and good compliance, they may benefit more from dapagliflozin treatment. Additionally, low price of dapagliflozin and high expense on hospitalization for HF would make adding dapagliflozin more cost‐effective.
We also found that CV mortality had a great impact on the model results. If the gap of CV mortality between two groups was small enough, it was possible that adding dapagliflozin was not cost‐effective according to the results of our models. The range of variation of CV mortality was derived directly from the total cohort of the DAPA‐HF trial. It was noteworthy that the hazard ratio (dapagliflozin versus placebo) of Asian patient subgroup in the DAPA‐HF trial was smaller than the hazard ratio of the total cohort. 9 Therefore, the gap of a composite of worsening HF and CV mortality in Asian patients was probably larger between adding dapagliflozin and standard treatment than that in our models.
Although similar beneficial effects of dapagliflozin on outcomes for HFrEF were observed in different subgroups (age, background therapy, etc.), 20 , 21 the results of the DAPA‐HF trial also showed that HFrEF patients who were men, Asian, or with NYHA II, left ventricular ejection fraction ≤ median (unknown), N‐terminal pro‐B‐type natriuretic peptide ≤ median (around 1400 pg/mL), or without atrial fibrillation might benefit more from dapagliflozin treatment. 9 According to the results of our models, it is reasonable to be more positive to recommend dapagliflozin therapy to HFrEF patients if they have a long life expectancy (>3.5 years), or they can receive dapagliflozin therapy at a low price, or their cost of per hospitalization for HF was very expensive, because adding dapagliflozin in these patients would not only reduce their adverse events but also provide a lower cost of per QALY gained.
Besides dapagliflozin, the Prospective comparison of Angiotensin Receptor neprilysin inhibitors with Angiotensin converting enzyme inhibitors to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM‐HF) trial showed that another emerging drug, sacubitril–valsartan, effectively reduced the risk of a composite of CV mortality and HF hospitalization in HFrEF patients. 22 It was of interest which drug was appropriate to prescribe for HFrEF patients if their symptoms were not well controlled after standard HF treatment. However, there was no head‐to‐head trial to compare the effect between sacubitril–valsartan and dapagliflozin on outcomes in HFrEF patients. Additionally, there was no CE study of sacubitril–valsartan in China either. Conclusions of previous studies of CEA of sacubitril–valsartan were different by foreign health care circumstances. 23 , 24 , 25 , 26 In China, although both of dapagliflozin and sacubitril–valsartan are in the health insurance drug list, the cost of sacubitril–valsartan (200 mg, b.i.d., $5.7) was more expensive than the cost of dapagliflozin (10 mg, q.d., $0.6). Notably, when the cost of standard treatment increased (when considering the cost of sacubitril–valsartan) in our model, the ICER increased simultaneously, which meant that adding dapagliflozin to standard treatment that contained sacubitril–valsartan would be less cost‐effective. This suggests that it may be reasonable and acceptable for us to choose dapagliflozin first based on the economic consideration in some HFrEF patients who are economically challenged.
Because this was a mathematical model study, and the analysis was performed from the perspective of China's health care payers, the results were confined to Chinese circumstance and should be interpreted cautiously. Our results were also confined to those with the characteristics observed in the DAPA‐HF trial, and it was unclear whether it would be cost‐effective in a population with more extensive heterogeneity. The clinical inputs (event rate) were not derived from Chinese population because these data did not currently exist. Additionally, because we only use direct medical costs in our models, while direct non‐medical costs and indirect costs (unavailable) were not included in our analysis, we could not provide CE from a perspective of society, which was the most appropriate and comprehensive perspective. Finally, fixed inputs such as CV mortality and risks of HF‐associated hospitalization were also limitations because these parameters would be changed as patients become older or if treatment discontinued. However, results of sensitivity analysis showed that the CE of dapagliflozin was robust over a relative wide range of these inputs.
Our analysis provided an insight into the CE of adding dapagliflozin for HFrEF patients compared with standard treatment. Adding dapagliflozin was considered very cost‐effective, which was associated with reduced total number of hospitalization for HF and CV death, and improved effectiveness. Our findings will help doctors and health care payers to make decisions. Future real‐world studies of CE of dapagliflozin based on Chinese population were needed.
Conflict of interest
None declared.
Funding
This work was supported by the Key Projects in the National Science and Technology Pillar Program of the 13th Five‐Year Plan Period (grant 2017YFC1308300 to J.Z,).
Supporting information
Table S1. Results between the markov model and the DAPA‐HF trial
Table S2. Results of one‐way sensitivity analysis
Table S3. Two‐way sensitivity analysis of the Model 1
Table S4. Two‐way sensitivity analysis of the Model 2
Yao, Y. , Zhang, R. , An, T. , Zhao, X. , and Zhang, J. (2020) Cost‐effectiveness of adding dapagliflozin to standard treatment for heart failure with reduced ejection fraction patients in China. ESC Heart Failure, 7: 3582–3592. 10.1002/ehf2.12844.
References
- 1. Hao G, Wang X, Chen Z, Zhang L, Zhang Y, Wei B, Zheng C, Kang Y, Jiang L, Zhu Z, Zhang J, Wang Z, Gao R, China Hypertension Survey Investigators . Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey 2012‐2015. Eur J Heart Fail 2019; 21: 1329–1337. [DOI] [PubMed] [Google Scholar]
- 2. Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol 2014; 171: 368–376. [DOI] [PubMed] [Google Scholar]
- 3. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichlo G, Pham M, Pina IL, Trogdon JG. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013; 6: 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang J, Yin HJ, Zhang ML, Qian N, Xuan JW. Understanding the economic burden of heart failure in China: impact on disease management and resource utilization. J Med Econ 2017; 20: 549–553. [DOI] [PubMed] [Google Scholar]
- 5. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, EMPA‐REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 6. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 7. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, SAbatine MS. DECLARE‐TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 8. Perkovic V, Jardine MJ, Neal B, Bompoints S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW, CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 9. Mcmurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM. DAPA‐HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 10. Cai X, Shi L, Yang W, Gu S, Chen Y, Nie L, Ji L. Cost‐effectiveness analysis of dapagliflozin treatment versus metformin treatment in Chinese population with type 2 diabetes. J Med Econ 2019; 22: 336–343. [DOI] [PubMed] [Google Scholar]
- 11. Nian H, Wan X, Ma J, Jie F, Wu B. Economic evaluation of dapagliflozin versus metformin in Chinese patients whose diabetes is inadequately controlled with diet and exercise. Cost Eff Resour Alloc; Published online ahead of print; 18: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol 2015; 12: 220–229. [DOI] [PubMed] [Google Scholar]
- 13. National Bureau of Statistics of China . National data. 2019. http://data.stats.gov.cn/tablequery.htm?code=AD09&from=singlemessage. Accessed 31 Jan 2020.
- 14. National Center for Chronic and Noncommunicable Disease Control and Prevention; Chinese Center for Disease Control and Prevention In China mortality surveillance dataset 2017. Beijing: China Science and Technology Press; 2018. p 17–82. [Google Scholar]
- 15. Yao G, Freemantle N, Calvert MJ, Bryan S, Daubert JC, Cleland JG. The long‐term cost‐effectiveness of cardiac resynchronization therapy with or without an implantable cardioverter‐defibrillator. Eur Heart J 2007; 28: 42–51. [DOI] [PubMed] [Google Scholar]
- 16. Yao G, Freemantle N, Flather M, Tharmanathan P, Coats A, Poole‐Wilson PA, SENIORS Investigators . Long‐term cost‐effectiveness analysis of nebivolol compared with standard care in elderly patients with heart failure: an individual patient‐based simulation model. Pharmacoeconomics 2008; 26: 879–889. [DOI] [PubMed] [Google Scholar]
- 17. National Healthcare Security Administration . National basic drug list. 2019. (31 Jan 2020), http://www.nhsa.gov.cn/art/2019/8/20/art_37_1666.html
- 18. Edejer TT‐T, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans DB, Murray CJL. WHO guide to cost‐effectiveness analysis. (31 Jan 2019), https://www.who.int/choice/book/en/
- 19. National Bureau of Statistics of China . China Statistical Yearbook 2017. 2018. (31 Jan 2020), http://data.stats.gov.cn/easyquery.htm?cn=C01&zb=A0201&sj=2017
- 20. Docherty KF, Jhund PS, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, DeMets DL, Sabatine MS, Bengtsson O, Sjöstrand M, Langkilde AM, Desai AS, Diez M, Howlett JG, Katova T, Ljungman CEA, O'Meara E, Petrie MC, Schou M, Verma S, Vinh PN, Solomon SD, JJV MM. Effects of dapagliflozin in DAPA‐HF according to background heart failure therapy. Eur Heart J 2020. [Epub]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martinez FA, Serenelli M, Nicolau JC, Petrie MC, Chiang CE, Tereshchenko S, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Ponikowski P, Sabatine MS, DeMets DL, Dutkiewicz‐Piasecka M, Bengtsson O, Sjöstrand M, Langkilde AM, Jhund PS, McMurray JJV. Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age: insights from DAPA‐HF. Circulation 2020; 141: 100–111. [DOI] [PubMed] [Google Scholar]
- 22. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 23. King JB, Shah RU, Bress AP, Nelson RE, Bellows BK. Cost‐effectiveness of sacubitril–valsartan combination therapy compared with enalapril for the treatment of heart failure with reduced ejection fraction. JACC: Heart Failure 2016; 4: 392–402. [DOI] [PubMed] [Google Scholar]
- 24. Liang L, Bin‐Chia Wu D, Aziz MIA, Wong R, Sim D, Leong KTG, Wei YQ, Tan D, Ng K. Cost‐effectiveness of sacubitril/valsartan versus enalapril in patients with heart failure and reduced ejection fraction. J Med Econ 2018; 21: 174–181. [DOI] [PubMed] [Google Scholar]
- 25. McMurray JJV, Trueman D, Hancock E, Cowie MR, Briggs A, Taylor M, Mumby‐Croft J, Woodcock F, Lacey M, Haroun R, Deschaseaux C. Cost‐effectiveness of sacubitril/valsartan in the treatment of heart failure with reduced ejection fraction. Heart 2018; 104: 1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krittayaphong R, Permsuwan U. Cost‐effectiveness analysis of sacubitril–valsartan compared with enalapril in patients with heart failure with reduced ejection fraction in Thailand. Am J Cardiovasc Drugs 2018; 18: 405–413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Results between the markov model and the DAPA‐HF trial
Table S2. Results of one‐way sensitivity analysis
Table S3. Two‐way sensitivity analysis of the Model 1
Table S4. Two‐way sensitivity analysis of the Model 2


