Abstract
Aims
The purpose of this retrospective propensity score‐matched study was to evaluate the superiority of different application approaches [continuous diuretics use (CDU) vs. intermittent diuretics use (IDU)] and types [loop diuretics (LDs) vs. thiazide diuretics (TDs)] of diuretics on long‐term outcomes for rheumatic heart disease (RHD) patients with compensated chronic heart failure (CHF).
Methods and results
A total of 494 RHD patients with compensated CHF were analysed after propensity score matching. Cox proportional hazards regression model was used to investigate the associations of different diuretic application approaches and types with all‐cause mortality, cardiovascular death (CVD), and cerebrovascular death. Binary logistic regression analyses were used to evaluate the associations of different diuretic application approaches and types with 1‐, 3‐, and 5‐year heart failure (HF) re‐hospitalization as well as new‐onset atrial fibrillation (AF). In the comparison between IDU and CDU strategies for RHD patients with compensated CHF, CDU was associated with increased risks of all‐cause mortality [adjusted hazard ratio (HR) = 2.47, 95% confidence interval (CI): 1.54–3.97, P < 0.001] and CVD (adjusted HR = 3.67, 95% CI: 1.95–6.89, P < 0.001) except cerebrovascular death (adjusted HR = 1.07, 95% CI: 0.34–3.41, P = 0.905). CDU was also associated with increased risks of 3‐year [adjusted odds ratio (OR) = 1.80, 95% CI: 1.09–2.96, P = 0.022] and 5‐year (adjusted OR = 2.02, 95% CI: 1.18–3.45, P = 0.010) HF re‐hospitalization risk and new‐onset AF (adjusted OR = 2.34, 95% CI: 1.31–4.20, P = 0.004) except 1‐year HF re‐hospitalization risk (adjusted OR = 1.54, 95% CI: 0.88–2.70, P = 0.130). In the comparison between TDs and LDs among study participants receiving IDU strategy, LDs were only associated with decreased 1‐year HF re‐hospitalization risk (adjusted OR = 0.30, 95% CI: 0.12–0.77, P = 0.012) rather than all‐cause mortality, CVD, cerebrovascular death, 3‐ and 5‐year HF re‐hospitalization, and new‐onset AF (all adjusted P > 0.05). In the comparison between TDs and LDs among study participants receiving CDU strategy, LDs were not associated with cerebrovascular death and 1‐year HF re‐hospitalization (both adjusted P > 0.05) but with increased risks of all‐cause mortality (adjusted HR = 1.80, 95% CI: 1.09–2.99, P = 0.023), CVD (adjusted HR = 1.89, 95% CI: 1.04–3.44, P = 0.037), 3‐year (adjusted OR = 1.91, 95% CI: 1.06–3.43, P = 0.031) and 5‐year (adjusted OR = 2.16, 95% CI: 1.12–4.19, P = 0.022) HF re‐hospitalization, and new‐onset AF (adjusted OR = 2.66, 95% CI: 1.25–5.68, P = 0.012).
Conclusions
Continuous diuretics use (especially LDs) was associated with increased risks of all‐cause mortality, CVD, medium‐term/long‐term HF re‐hospitalization, and new‐onset AF in RHD patients with compensated CHF.
Keywords: Rheumatic heart disease, Diuretic, Outcomes, Heart failure, Atrial fibrillation
Introduction
Rheumatic heart disease (RHD) resulting from rheumatic fever is characterized by permanent heart valve damage associated with valvular stenosis and/or valvular insufficiency of mitral, tricuspid, and/or aortic valves. RHD is virtually eliminated in most developed regions, but the burden of morbidity and mortality still remains high in developing countries and under‐developed regions and is still a major health problem in these countries and regions based on accumulating data on subclinical RHD. 1 , 2 Chronic heart failure (CHF) is recognized as the leading cause of complication and re‐hospitalization in RHD patients, especially with advanced RHD that may not be amenable to surgery. CHF can be ‘compensated’ or ‘decompensated’. The classic clinical feature of RHD with decompensated CHF is congestion, 3 which is defined as signs and symptoms of extracellular fluid accumulation that result from increased left and/or right cardiac filling pressures. Removal of excess fluid overload with diuretics is one of the mainstays in volume management of CHF (Class I recommendation). 4 Diuretic therapy is generally continued indefinitely for fluid control in RHD patients with decompensated CHF unless cardiac function improves, but a significant proportion is still in state of persistent congestion (including subclinical hyperaemia) at the time of discharge or after discharge, associated with increased mortality. 5 Basing on which, a long‐standing perception has attributed a beneficial effect to continuous diuretics use (CDU) in the setting of CHF, even after congestion has been eliminated. However, few clinical trials have studied the impact of CDU on clinical outcomes in RHD patients with compensated CHF.
Conventional diuretics appear to reduce the risk of death and heart failure (HF) re‐hospitalization in patients with CHF compared with placebo, 6 although only small trials were available. However, observational data have raised safety concerns about CDU of CHF. Many cross‐sectional studies have linked diuretic use to increased mortality, 7 manifesting the characteristics in a dose‐dependent manner. 8 , 9 , 10 A small and exploratory study indicated that short‐term HF medication (e.g. diuretics) omission among outpatients with stable HF and a left ventricular ejection fraction <45% led to increasing congestion but it was good for increasing blood pressure and improving renal function, suggesting that short‐term discontinuation of diuretics may not cause HF progression. 11 Post hoc analysis of the HF‐ACTION study showed that the initiation or discontinuation of diuretics was not associated with a difference in mortality, HF readmission, and exercise capacity in a 6‐month timeframe. 7 Two small prospective pilot studies showed that long‐term maintenance of lower‐dose diuretics for stable CHF patients was feasible and did not worsen the clinical situation. 12 Further, in a prospective, randomized, and double‐blind study on the safety and tolerability of withdrawing low‐dose furosemide in stable HF outpatients in Brazil, Rohde et al. 13 recently found that short‐term diuretic withdrawal may be feasible in outpatients with compensated HF after receiving optimal medical therapy. These results suggested that the diuretics use in patients with CHF is dynamic and vary according to the state of congestion, which means that application approaches and dose of diuretics in CHF patients with euvolaemia should be adjusted as early as possible using a careful, de‐escalation therapy [defined as intermittent diuretics use (IDU)] after achieving successful decongestion with diuretic therapy. 5 The relationship between this strategy and outcome of CHF remains poorly defined.
Further, loop diuretics (LDs) are the cornerstone of decongestion therapy in decompensated HF; however, is this drug established as the best option? The majority of patients with CHF receive long‐term treatment with LDs, but LDs not only had no significant superiority with respect to all‐cause mortality and also increased the risk of death as mentioned earlier. On the other hand, thiazide diuretics (TDs) are not prescribed because of its weak diuretic effects, especially in the advanced stage of HF. Compared with long‐acting TDs (e.g. chlorthalidone), short‐acting TDs (e.g. hydrochlorothiazide) may be preferred in CHF patients with acute decompensation related to its better bioavailability, short half‐life, and lower risk of electrolyte imbalance. 14 In addition, a meta‐analysis showed a significant beneficial effect on TD in decreasing the risk for HF in hypertension patients. 15 These suggest that compensated CHF patients may benefit from using TDs for maintaining decongestion. Yet now there remains a lack of head‐to‐head comparison trials examining the effect of different diuretics (LDs vs. TDs) on long‐term prognosis in patients with compensated CHF.
Current guidelines state diuretics as a treatment for the clinical signs and symptoms of congestion mainly based on non‐valvular heart diseases. However, there is no evidence that it affects progression of RHD. RHD patients with CHF who are more symptomatic are likely to receive higher doses of diuretics and to receive LDs rather than TDs, and more symptomatic/congested patients have a worse prognosis. 16 In fact, in RHD patients with compensated CHF, prescription of diuretics remains, to a large extent, subjective and evidence free, 4 lacking of conclusive supporting data derived from large‐scale, randomized studies with ‘hard’ clinical endpoints. It is not clear whether the de‐escalation therapy can improve the prognosis of RHD. Here, we conducted a retrospective propensity score‐matched study to examine the effects of diuretic application approaches (CDU vs. IDU) on long‐term outcomes in RHD patients with compensated CHF as well as diuretics types (LDs vs. TDs).
Methods
Study participants
The retrospective propensity score‐matched study was reviewed and approved by the Ethics Committee of Guangzhou First People's Hospital, South China University of Technology, Guangzhou, China (K‐2018‐136‐1). RHD was diagnosed according to echocardiographic diagnostic criteria of the World Heart Federation. 17 Patients were entered into the study from the date of first diagnosis of RHD with different types of valve damage (any location and condition). Patients' missing/invalid clinical data and any contraindication to use of diuretics were excluded from the study. All RHD patients with first hospitalization for HF were followed up since the date of the first post‐discharge when euvolaemia has been achieved. Patient survival time was calculated from date of their first discharge for HF to date of death or last follow‐up. The final follow‐up date was 31 December 2018. Clinical data were collected from patient interviews, review of medical records, and contact with treating physicians. The CDU group was defined as the group that RHD patients with CHF had persistent prescription and administration of diuretics during compensated HF period. The IDU group was defined as the group that RHD patients with CHF were not prescribed any diuretics during compensated HF period. However, the types and modes (e.g. intravenous or oral) of diuretic administration were not limited when RHD patients were in acute decompensated HF during the follow‐up, regardless of whether in the CDU or IDU group. RHD patients in the CDU group were divided into two subgroups according to the type of diuretic used: (i) the LDs subgroup, which represented the patients who only receive furosemide, and (ii) the TDs subgroup, which represented the patients who only receive hydrochlorothiazide. In addition, RHD patients with combination therapy of LDs and TDs were excluded from the study.
Rheumatic heart disease surgical treatment was defined as surgical or percutaneous intervention for valve repair or replacement of any affected valve(s) (e.g. mitral, aortic, and tricuspid) using tissue or mechanical prosthesis according to the updated guidelines during the follow‐up. HF at baseline and during the study (recurrent HF) was diagnosed according to Framingham HF criteria. Atrial fibrillation (AF) was diagnosed according to prior history of AF or electrocardiographic findings at baseline plus follow‐up examinations. Coexisting medical conditions (e.g. hypertension, coronary heart disease, type 2 diabetes mellitus, and stroke) were evaluated according to relevant guidelines (details guidelines information was presented in Methods section of supporting information). Cardiac standard chamber quantification was determined by echocardiography according to recommendations from the European Association of Echocardiography. 18 Biochemical tests were performed using standard chemical lab methods.
Propensity score matching
Because the IDU group was much smaller than the CDU group, an imbalance in crucial covariates related to outcomes could have biased the estimation of the CDU effect. To adjust for other baseline factors, we performed a 1:2 propensity score matching (PSM) using R Statistical Software version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). The propensity score was generated through logistic regression to predict the probability of effectiveness of CDU as a function of baseline factors as follows: age, sex, course, smoking, drinking, waiting time for surgery, cardiac valve damage, surgical intervention, New York Heart Association (NYHA) functional classification, medical condition (e.g. hypertension, coronary heart disease, type 2 diabetes mellitus, AF, and stroke), combined medication [e.g. antiplatelet drugs, warfarin, digoxin, nitrates, renin‐angiotensin system inhibitors (RSIs), beta‐receptor blockers (BBs), mineralocorticoid receptor antagonists (MRAs), calcium channel blockers, and statin], and level of partial blood biochemical index (e.g. white blood cell count, haemoglobin, alanine aminotransferase, creatinine, C‐reactive protein, B‐type natriuretic peptide, and serum potassium). The calliper width for PSM was 0.1. After PSM, a total of 494 RHD patients finally entered the study.
Long‐term follow‐up endpoints
Primary endpoints were all‐cause mortality, cardiovascular death (CVD), and cerebrovascular death (details definition was presented in Methods section of supporting information). Secondary endpoints were the risks of HF re‐hospitalization and new‐onset AF. In particular, the cerebrovascular death was defined as death due to cerebral infarction or cerebral haemorrhage. HF re‐hospitalization was defined as a hospital re‐hospitalization (including at least one overnight admission to emergency) for which HF was the primary cause, requiring treatment with diuretics, intravenous inotropes, or nitrates. Three information sources were queried to identify primary and secondary endpoints: participants and their families, medical records, and the Center for Disease Control and Prevention.
Statistical analysis
All statistical analyses were performed using SPSS version 24 (SPSS, Chicago, IL), R Statistical Software version 3.6.1 (R Foundation for Statistical Computing), and PASS version 15 (Statistical Solution Ltd, Cork, Ireland). Categorical variables were presented as numbers and percentages. Continuous variables with non‐normal variables were reported as median (interquartile range). Significant differences among categorical variables were determined by χ 2 test or Fisher's test. Significant differences among continuous variables were analysed by Mann–Whitney U test, independent‐sample t‐test, or two‐way ANOVA. Patient survival time was determined based on the clinical information. The Cox proportional hazards regression model for survival analysis was fit to estimate the crude hazard ratios (HRs), adjusted HRs, and their 95% confidence intervals (CIs) with adjustments for potential confounders. Binary logistic regression analysis was used to evaluate the odds ratio (OR) of HF re‐hospitalization (1, 3, and 5 years) and new‐onset AF. P values of less than 0.05 were considered as statistically significant. All probabilities are two‐tailed estimated.
Results
Characteristics of study participants
Baseline clinical and echocardiographic characteristics of the study patients before PSM are listed in Table S1 . More than half of study participants had a combination of valve damage (54.4%, predominantly mitral valve), NYHA functional Classes II–IV (87.2%), surgical treatment (54.2%), and AF (65.0%). After PSM, there was no significant difference between the CDU and IDU groups on baseline clinical characteristic (Table 1 ). A total of 494 RHD patients were included in the study with median follow‐up of 5.1 years, a median age of 47 years, and 69.2% were female.
TABLE 1.
Baseline characteristics of study participants after propensity score matching
| IDU | CDU | P value | |
|---|---|---|---|
| N | 190 | 304 | — |
| Male:female | 59:131 | 93:211 | 0.914 |
| Age of onset (years) | 47.0 (37.0, 56.0) | 47.0 (34.0, 59.0) | 0.917 |
| Age of surgery (years) | 48.0 (41.0, 55.0) | 48.0 (40.0, 55.0) | 0.718 |
| Follow‐up (years) | 5.2 (2.6, 8.1) | 5.1 (2.7, 9.5) | 0.224 |
| Disease duration (years) | 10.0 (5.0, 18.0) | 11.5 (4.3, 20.0) | 0.716 |
| Smoking (%) | 19 (10.0) | 30 (9.9) | 0.962 |
| Drinking (%) | 21 (11.1) | 30 (9.9) | 0.674 |
| Types of diuretics (thiazine:loop) | 61:129 | 122:182 | 0.072 |
| Average daily dose of TD (mg) | 25 (12.5, 25) | 25 (25, 25) | 0.085 |
| Cumulative use time of TD (years) | 1.7 (0.9, 2.9) | 4.8 (1.8, 7.1) | <0.001 |
| Average daily dose of LD (mg) | 40 (20, 60) | 40 (20, 60) | 0.169 |
| Cumulative use time of LD (years) | 1.7 (1.2, 2.8) | 5.1 (2.0, 8.5) | <0.001 |
| SBP at initial diagnosis (mmHg) | 120 (115, 128) | 120 (114, 127) | 0.402 |
| DBP at initial diagnosis (mmHg) | 75 (68, 80) | 73 (67, 81) | 0.509 |
| Heart rate at initial diagnosis (b.p.m.) | 80 (75, 91) | 81 (76, 96) | 0.462 |
| Cardiac valve damage | |||
| (A‐I) MS | |||
| No | 56 (29.5) | 81 (26.6) | 0.494 |
| Yes | 134 (70.5) | 223 (73.4) | |
| (A‐II) MS degree | |||
| Mild | 85 (63.4) | 120 (53.8) | 0.094 |
| Moderate | 33 (24.6) | 58 (26.0) | |
| Severe | 16 (11.9) | 45 (20.2) | |
| (B‐I) MR | |||
| No | 84 (44.2) | 112 (36.8) | 0.103 |
| Yes | 106 (55.8) | 192 (63.2) | |
| (B‐II) MR degree | |||
| Mild | 42 (39.6) | 66 (34.4) | 0.654 |
| Moderate | 27 (25.5) | 55 (28.6) | |
| Severe | 37 (34.9) | 71 (37.0) | |
| (C‐I) AS | |||
| No | 146 (76.8) | 230 (75.7) | 0.764 |
| Yes | 44 (23.2) | 74 (24.3) | |
| (C‐II) AS degree | |||
| Mild | 26 (59.1) | 37 (50.0) | 0.627 |
| Moderate | 12 (27.3) | 24 (32.4) | |
| Severe | 6 (13.6) | 13 (17.6) | |
| (D‐I) AR | |||
| No | 122 (64.2) | 194 (63.8) | 0.929 |
| Yes | 68 (35.8) | 110 (36.2) | |
| (D‐II) AR degree | |||
| Mild | 29 (42.6) | 49 (44.5) | 0.840 |
| Moderate | 30 (44.1) | 44 (40.0) | |
| Severe | 9 (13.2) | 17 (15.5) | |
| (E‐I) TR | |||
| No | 146 (76.8) | 214 (70.4) | 0.117 |
| Yes | 44 (23.2) | 90 (29.6) | |
| (E‐II) TR degree | |||
| Mild | 21 (47.7) | 27 (30.0) | 0.078 |
| Moderate | 14 (31.8) | 30 (33.3) | |
| Severe | 9 (20.5) | 33 (36.7) | |
| (F‐I) PAH | |||
| No | 64 (33.7) | 80 (26.3) | 0.080 |
| Yes | 126 (66.3) | 224 (73.7) | |
| (F‐II) PAH degree | |||
| Mild | 83 (65.9) | 120 (53.6) | 0.076 |
| Moderate | 27 (21.4) | 69 (30.8) | |
| Severe | 16 (12.7) | 35 (15.6) | |
| Cardiac valve damage (stenosis or regurgitation) | |||
| MV | 76 (40.0) | 128 (42.1) | 0.155 |
| AV | 4 (2.1) | 8 (2.6) | |
| TV | 1 (0.5) | 2 (0.7) | |
| MV + AV | 66 (34.7) | 78 (25.7) | |
| MV + TV | 31 (16.3) | 50 (16.4) | |
| MV + AV + TV | 12 (6.3) | 38 (12.5) | |
| Surgical intervention | |||
| (A) Valve replacement (tissue or mechanical prosthesis) | |||
| MV | 64 (66.0) | 101 (68.2) | 0.835 |
| AV | 3 (3.1) | 7 (4.7) | |
| MV + AV | 29 (29.9) | 38 (25.7) | |
| MV + TV | 1 (1.0) | 2 (1.4) | |
| (B) Valve repair | |||
| MV | 9 (64.3) | 10 (45.5) | 0.468 |
| TV | 5 (35.7) | 9 (40.9) | |
| AV | 0 (0.0) | 2 (9.1) | |
| MV + TV | 0 (0.0) | 1 (4.5) | |
| NYHA | |||
| I | 40 (21.1) | 38 (12.5) | 0.089 |
| II | 58 (30.5) | 106 (34.9) | |
| III | 72 (37.9) | 124 (40.8) | |
| IV | 20 (10.5) | 36 (11.8) | |
| Medical condition | |||
| HT | 38 (20.0) | 81 (26.6) | 0.093 |
| CHD | 17 (8.9) | 18 (5.9) | 0.202 |
| T2D | 14 (7.4) | 28 (9.2) | 0.475 |
| AF | 79 (41.6) | 118 (38.8) | 0.542 |
| Stroke | 18 (9.5) | 31 (10.2) | 0.793 |
| Combined medication | |||
| Antiplatelet drugs | 27 (14.2) | 47 (15.5) | 0.705 |
| Warfarin | 120 (63.2) | 167 (54.9) | 0.072 |
| Digoxin | 93 (48.9) | 169 (55.6) | 0.150 |
| Nitrates | 17 (8.9) | 29 (9.5) | 0.826 |
| RSIs | 78 (41.1) | 130 (42.8) | 0.708 |
| BBs | 69 (36.3) | 105 (34.5) | 0.688 |
| MRAs | 97 (51.1) | 178 (58.6) | 0.103 |
| CCBs | 19 (10.0) | 33 (10.9) | 0.763 |
| Statins | 25 (13.2) | 24 (7.9) | 0.057 |
| Blood biochemical index | |||
| WBC (×109/L) | 6.94 (5.57, 9.26) | 7.15 (5.58, 9.24) | 0.598 |
| HGB (g/L) | 124.0 (113.0, 135.0) | 121.0 (108.0, 134.0) | 0.115 |
| PLT (×109/L) | 186.5 (139.5, 239.5) | 182.0 (143.0, 234.5) | 0.885 |
| FBG (mmol/L) | 4.98 (4.39, 5.98) | 5.10 (4.30, 6.20) | 0.849 |
| ALT (U/L) | 18.0 (12.0, 23.0) | 18.0 (11.0, 28.0) | 0.241 |
| AST (U/L) | 25.0 (20.0, 33.0) | 23.0 (18.0, 34.7) | 0.642 |
| Cr (μmol/L) | 77.5 (64.8, 94.0) | 82.0 (68.0, 96.0) | 0.234 |
| CRP (mg/L) | 3.7 (1.7, 10.7) | 5.9 (2.0, 15.9) | 0.144 |
| ASO (U/mL) | 50.3 (0.0, 101.7) | 40.0 (0.0, 78.4) | 0.445 |
| RF (U/mL) | 10.0 (0.0, 11.3) | 9.2 (0.0, 11.3) | 0.613 |
| ESR (mm/h) | 16.0 (9.0, 26.0) | 20.0 (10.0, 34.0) | 0.054 |
| BNP (pg/mL) | 218.3 (84.7, 914.9) | 209.5 (56.2, 817.0) | 0.322 |
| TRIG (mmol/L) | 1.07 (0.76, 1.40) | 0.96 (0.70, 1.36) | 0.169 |
| TC (mmol/L) | 4.30 (3.48, 5.05) | 4.25 (3.46, 5.02) | 0.671 |
| HDL‐C (mmol/L) | 1.10 (0.90, 1.33) | 1.09 (0.86, 1.35) | 0.717 |
| LDL‐C (mmol/L) | 2.37 (1.89, 2.97) | 2.30 (1.81, 2.90) | 0.411 |
| Na+ (mmol/L) | 140.4 (137.5, 142.1) | 139.3 (136.3, 142.1) | 0.157 |
| K+ (mmol/L) | 3.90 (3.65, 4.20) | 4.00 (3.68, 4.22) | 0.213 |
| Echocardiography | |||
| LVD (cm) | 4.50 (4.15, 4.94) | 4.57 (4.23, 4.99) | 0.466 |
| LAD (cm) | 4.58 (3.97, 5.09) | 4.69 (4.04, 5.09) | 0.676 |
| RVD (cm) | 1.90 (1.43, 2.39) | 2.00 (1.63, 2.42) | 0.066 |
| RAD (cm) | 3.46 (3.22, 3.97) | 3.40 (3.15, 3.96) | 0.097 |
| LVEF (%) | 54.0 (48.0, 59.0) | 53.0 (48.0, 58.0) | 0.660 |
AF, atrial fibrillation; ALT, alanine aminotransferase; AR, aortic regurgitation; AS, aortic stenosis; ASO, antistreptolysin O; AST, aspartate aminotransferase; AV, aortic valve; BBs, beta‐receptor blockers; BNP, B‐type natriuretic peptide; CCBs, calcium channel blockers; CDU, continuous diuretics use; CHD, coronary heart disease; Cr, creatinine; CRP, C‐reactive protein; DBP, diastolic blood pressure; ESR, erythrocyte sedimentation rate; FBG, fasting blood glucose; HDL‐C, high‐density lipoprotein cholesterol; HGB, haemoglobin; HT, hypertension; IDU, intermittent diuretics use; K+, serum potassium; LAD, left atrial end‐systolic diameter; LD, loop diuretic; LDL‐C, low‐density lipoprotein cholesterol; LVD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MRAs, mineralocorticoid receptor antagonists; MS, mitral stenosis; MV, mitral valve; Na+, serum sodium; NYHA, New York Heart Association; PAH, pulmonary arterial hypertension; PLT, platelet count; RAD, right atrial end‐systolic diameter; RF, rheumatoid factor; RSIs, renin‐angiotensin system inhibitors; RVD, right ventricular end‐diastolic diameter; SBP, systolic blood pressure; T2D, type 2 diabetes mellitus; TC, total cholesterol; TD, thiazide diuretic; TR, tricuspid regurgitation; TRIG, triglyceride; TV, tricuspid valve; WBC, white blood cell count.
Effect of different diuretic application approaches (intermittent diuretics use vs. continuous diuretics use) on all‐cause mortality, cardiovascular death, and cerebrovascular death
Compared with IDU, CDU was associated with increased risks of all‐cause mortality (adjusted HR = 2.47, 95% CI: 1.54–3.97, P < 0.001) and CVD (adjusted HR = 3.67, 95% CI: 1.95–6.89, P < 0.001) in RHD patients with compensated CHF except cerebrovascular death (adjusted HR = 1.07, 95% CI: 0.34–3.41, P = 0.905), as shown in Figure 1 .
Figure 1.
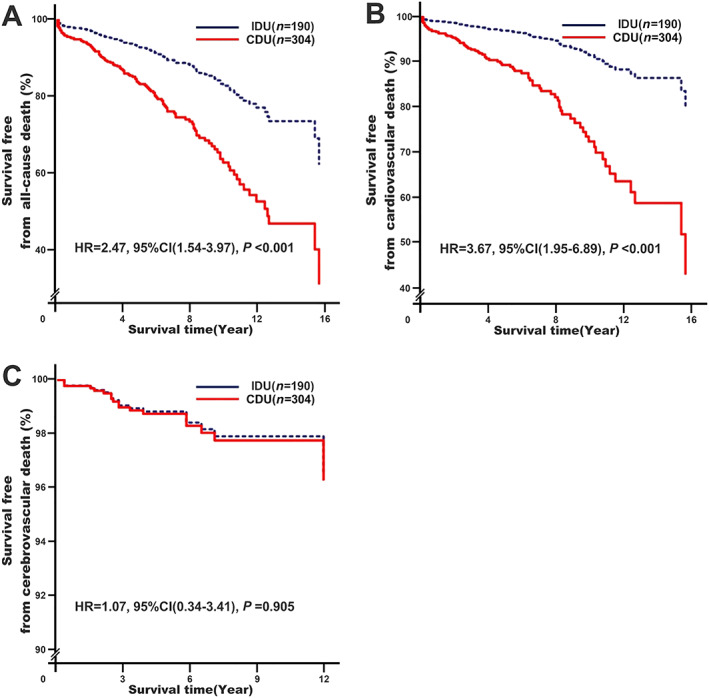
Effect of different diuretic application approaches (IDU vs. CDU) on all‐cause mortality, cardiovascular death, and cerebrovascular death. Model 1: adjusting for baseline adjustment covariates, including age, gender, smoking, drinking, rheumatic heart disease course, New York Heart Association functional classification, cardiac valve damage, surgical intervention, medical condition (hypertension, coronary heart disease, type 2 diabetes mellitus, atrial fibrillation, and stroke), combined medication (antiplatelet drugs, warfarin, digoxin, nitrates, renin‐angiotensin system inhibitors, beta‐receptor blockers, mineralocorticoid receptor antagonists, calcium channel blockers, and statins), types of diuretics, cumulative use time, blood biochemical index (white blood cell count, haemoglobin, serum sodium, serum potassium, creatinine, haemoglobin A1C, and C‐reactive protein), and echocardiography (left atrial end‐systolic diameter, left ventricular end‐diastolic diameter, right ventricular end‐diastolic diameter, and right atrial end‐systolic diameter). CI, confidence interval; CDU, continuous diuretics use; HR, hazard ratio; IDU, intermittent diuretics use.
Effects of different diuretic application approaches (intermittent diuretics use vs. continuous diuretics use) on the risks of heart failure re‐hospitalization and new‐onset atrial fibrillation
As shown in Table 2 , CDU was associated with increased risk of 3‐year (adjusted OR = 1.80, 95% CI: 1.09–2.96, P = 0.022) and 5‐year (adjusted OR = 2.02, 95% CI: 1.18–3.45, P = 0.010) HF re‐hospitalization with exception of 1 year (adjusted OR = 1.54, 95% CI: 0.88–2.70, P = 0.130). CDU was also associated with increased new‐onset AF risk (adjusted OR = 2.34, 95% CI: 1.31–4.20, P = 0.004).
TABLE 2.
Effects of different diuretic application approaches (intermittent diuretics use vs. continuous diuretics use) on the risks of heart failure re‐hospitalization a and new‐onset atrial fibrillation b in rheumatic heart disease patients
| Clinical outcomes | IDU (ref., N/%) | CDU (N/%) | Crude OR (95% CI) | Crude P value | Adjusted OR (95% CI) | Adjusted P value | |
|---|---|---|---|---|---|---|---|
| 1‐year HF re‐hospitalization | No | 154 (84.2) | 212 (73.9) | 1.88 (1.17–3.02) | 0.009 | 1.54 (0.88–2.70) | 0.130 a |
| Yes | 29 (15.8) | 75 (26.1) | |||||
| 3‐year HF re‐hospitalization | No | 104 (68.4) | 132 (52.6) | 1.95 (1.28–2.98) | 0.002 | 1.80 (1.09–2.96) | 0.022 a |
| Yes | 48 (31.6) | 119 (47.4) | |||||
| 5‐year HF re‐hospitalization | No | 69 (53.9) | 77 (33.3) | 2.33 (1.50–3.64) | <0.001 | 2.02 (1.18–3.45) | 0.010 a |
| Yes | 59 (46.1) | 154 (66.7) | |||||
| New‐onset AF | No | 80 (73.0) | 90 (48.4) | 2.88 (1.73–4.79) | <0.001 | 2.34 (1.31–4.20) | 0.004 b |
| Yes | 30 (27.0) | 96 (51.6) | |||||
AF, atrial fibrillation; CDU, continuous diuretics use; CI, confidence interval; HF, heart failure; IDU, intermittent diuretics use; OR, odds ratio.
Model 2: it is the same as Model 1, and also including new‐onset AF.
Model 3: it is the same as Model 2 with exception of those covariates, including new‐onset AF, left ventricular end‐diastolic diameter, right ventricular end‐diastolic diameter, and right atrial end‐systolic diameter.
Effects of different diuretic types (thiazide diuretics vs. loop diuretics) on the risks of all‐cause mortality, cardiovascular death, and cerebrovascular death
As shown in Table 3 , in the IDU group of RHD patients with compensated CHF, there was no significant difference between the LDs and TDs subgroups on all‐cause mortality (adjusted HR = 0.31, 95% CI: 0.09–1.13, P = 0.076), CVD (adjusted HR = 0.16, 95% CI: 0.02–1.57, P = 0.116), and cerebrovascular death (adjusted HR = 0.22, 95% CI: 0.03–1.95, P = 0.175). But in the CDU group, LDs were associated with higher risk of all‐cause mortality (adjusted HR = 1.80, 95% CI: 1.09–2.99, P = 0.023) and CVD (adjusted HR = 1.89, 95% CI: 1.04–3.44, P = 0.037) except cerebrovascular death (adjusted HR = 1.14, 95% CI: 0.16–8.02, P = 0.896).
TABLE 3.
Effects of different diuretic types (thiazide diuretics vs. loop diuretics) on the risks of all‐cause mortality, cardiovascular death, and cerebrovascular death in rheumatic heart disease patients with different diuretic application approaches (intermittent diuretics use or continuous diuretics use) a
| Clinical outcomes | Effect size for a change of | IDU | CDU | ||
|---|---|---|---|---|---|
| Effect size (95% CI) | P value | Effect size (95% CI) | P value | ||
| All‐cause mortality | TDs (ref.) vs. LDs | 0.31 (0.09–1.13) | 0.076 | 1.80 (1.09–2.99) | 0.023 |
| CVD | TDs (ref.) vs. LDs | 0.16 (0.02–1.57) | 0.116 | 1.89 (1.04–3.44) | 0.037 |
| Cerebrovascular death | TDs (ref.) vs. LDs | 0.22 (0.03–1.95) | 0.175 | 1.14 (0.16–8.02) | 0.896 |
CDU, continuous diuretics use; CI, confidence interval; CVD, cardiovascular death; IDU, intermittent diuretics use; LDs, loop diuretics; TDs, thiazide diuretics.
Model 4: it is the same as Model 1 with exception of types of diuretics.
Effects of different diuretic types (thiazide diuretics vs. loop diuretics) on the risks of heart failure re‐hospitalization and new‐onset atrial fibrillation
As shown in Table 4 , in the IDU group of RHD patients with compensated CHF, LDs were only associated with lower risk of 1‐year (adjusted OR = 0.30, 95% CI: 0.12–0.77, P = 0.012) HF re‐hospitalization rather than the risks of 3‐year (adjusted OR = 0.58, 95% CI: 0.25–1.33, P = 0.197) and 5‐year (adjusted OR = 0.57, 95% CI: 0.23–1.44, P = 0.233) HF re‐hospitalization and new‐onset AF (adjusted OR = 1.56, 95% CI: 0.48–5.09, P = 0.466). In contrast, in the CDU group, LDs were associated with higher risk of 3‐year (adjusted OR = 1.91, 95% CI: 1.06–3.43, P = 0.031) and 5‐year (adjusted OR = 2.16, 95% CI: 1.12–4.19, P = 0.022) HF re‐hospitalization and new‐onset AF (adjusted OR = 2.66, 95% CI: 1.25–5.68, P = 0.012) except 1‐year (adjusted OR = 1.24, 95% CI: 0.68–2.27, P = 0.481) HF re‐hospitalization.
TABLE 4.
Effects of different diuretic types (thiazide diuretics vs. loop diuretics) on the risks of heart failure re‐hospitalization a and new‐onset atrial fibrillation b in rheumatic heart disease patients with different diuretic application approaches (intermittent diuretics use or continuous diuretics use)
| Group | Clinical outcomes | TDs (ref., N/%) | LDs (N/%) | Crude OR (95% CI) | Crude P value | Adjusted OR (95% CI) | Adjusted P value | |
|---|---|---|---|---|---|---|---|---|
| IDU | 1‐year HF re‐hospitalization | No | 44 (75.9) | 110 (88.0) | 0.43 (0.19–0.96) | 0.040 | 0.30 (0.12–0.77) | 0.012 a |
| Yes | 14 (24.1) | 15 (12.0) | ||||||
| 3‐year HF re‐hospitalization | No | 31 (62.0) | 73 (71.6) | 0.65 (0.32–1.33) | 0.235 | 0.58 (0.25–1.33) | 0.197 a | |
| Yes | 19 (38.0) | 29 (28.4) | ||||||
| 5‐year HF re‐hospitalization | No | 18 (46.2) | 51 (57.3) | 0.64 (0.30–1.36) | 0.246 | 0.57 (0.23–1.44) | 0.233 a | |
| Yes | 21 (53.8) | 38 (42.7) | ||||||
| New‐onset AF | No | 23 (79.3) | 58 (70.7) | 1.59 (0.57–4.38) | 0.374 | 1.56 (0.48–5.09) | 0.466 b | |
| Yes | 6 (20.7) | 24 (29.3) | ||||||
| CDU | 1‐year HF re‐hospitalization | No | 86 (78.2) | 126 (71.2) | 1.45 (0.83–2.53) | 0.191 | 1.24 (0.68–2.27) | 0.481 a |
| Yes | 24 (21.8) | 51 (28.8) | ||||||
| 3‐year HF re‐hospitalization | No | 52 (59.1) | 80 (49.1) | 1.50 (0.89–2.53) | 0.130 | 1.91 (1.06–3.43) | 0.031 a | |
| Yes | 36 (40.9) | 83 (50.9) | ||||||
| 5‐year HF re‐hospitalization | No | 34 (43.0) | 43 (28.3) | 1.92 (1.09–3.38) | 0.025 | 2.16 (1.12–4.19) | 0.022 a | |
| Yes | 45 (57.0) | 109 (71.7) | ||||||
| New‐onset AF | No | 50 (62.5) | 40 (37.7) | 2.75 (1.51–5.01) | 0.001 | 2.66 (1.25–5.68) | 0.012 b | |
| Yes | 30 (37.5) | 66 (62.3) | ||||||
AF, atrial fibrillation; CDU, continuous diuretics use; CI, confidence interval; HF, heart failure; IDU, intermittent diuretics use; LDs, loop diuretics; OR, odds ratio; TDs, thiazide diuretics.
Model 5: it is the same as Model 2 with exception of types of diuretics.
Model 6: it is the same as Model 3 with exception of types of diuretics.
Discussion
Association of different diuretic application approaches with mortality, and heart failure re‐hospitalization and new‐onset atrial fibrillation
To the best of our knowledge, this is the only study relating diuretic application approaches to long‐term clinical hard outcomes in RHD patients with real and complex valve damage during compensated HF period. In this study, the difference between the two diuretic application approaches (IDU vs. CDU) was whether or not to receive diuretics treatment among RHD patients with CHF during their compensated HF period. The daily routine dose of diuretics was not different between the two treatment options, but the cumulative use time in patients with CDU was about three times higher than that with IDU, which was associated with worse prognosis. Similar results were found as follows: Ahmed et al. 19 reported that diuretics use increased all‐cause mortality and CVD by 36–51% while the risks reported by Hamaguchi et al. 20 are even higher, up to 50–72%. Interestingly, we also found that CDU had no effect on short‐term (within 1 year) HF re‐hospitalization risk but was significantly associated with increased risk of the medium‐term/long‐term (over 3 years) HF re‐hospitalization (approximately increased by at least 80%) and had tendency to longer diuretics use time and higher HF re‐hospitalization risk. This was consistent with the observation by Domanski et al., 21 who reported that diuretics use increased HF readmission risk by 31–66% in post hoc analysis of SOLVD and DIG study. Recent studies by Miura et al. 22 and Damman et al. 23 confirmed that diuretics use increased the compound endpoint (e.g. all‐cause mortality, CVD, and HF re‐hospitalization) by 28–63%. In addition, Schartum‐Hansen et al. 24 found that the use of diuretics in suspected coronary artery disease patients without systolic HF was associated with increased risk of all‐cause mortality. These results suggest that diuretic strategy of CDU should be discouraged when RHD patients are under decongested status. 25 In contrast, Grinstead et al. 26 found that the probability of remaining off diuretics at 6 weeks was 71% in a 12‐week clinical trial of 41 patients with stable HF. Damman et al. 23 insightfully evaluated the feasibility for trials randomizing euvolaemic patients with CHF to withdrawal or dose reduction of diuretics. It was shown that lower dose of diuretics (compared with continuous use or use of higher doses of diuretics) was associated with lower risk of CVD and HF readmission. Similar benefits on diuretic reduction were observed in patients with cardiac resynchronization therapy. 27 Studies have shown that the adverse effect of persistent diuretic use was associated with age, haemoglobin concentration, worsening renal function, imbalance of serum electrolytes (e.g. hypokalaemia, hyponatraemia, and hypomagnesaemia), and thiamine deficiency 12 , 16 , 19 , 28 , 29 and contributed to increased sympathetic tone 30 and renin‐angiotensin‐aldosterone system activation, 31 which is the potential mechanism for a poor prognosis triggered by long‐term CDU. It is well established that diuretics activate the sympathetic nervous system and the renin‐angiotensin‐aldosterone system in HF. This occurs as a response to diuretic treatment rather than as a result of the disease process itself, 7 manifesting the characteristics of a dose‐dependent manner. 28 Higher dose of diuretics use leads to a higher risk of cardiovascular events, 16 even after triple blockade with RSIs, BBs, and MRAs. 22 On the contrary, the activation of renin‐angiotensin‐aldosterone system (e.g. plasma renin levels) decreases with diuretic reduction. 32
In this study, our result also showed that CDU was associated with increased new‐onset AF risk in RHD patients with compensated CHF (approximately increased by 1.34‐fold), which was similar to the observation by Jong et al., 33 who reported that diuretics use increased non‐valvular AF risk by 39%, and an observation showed increased mortality in hypertensive patients with AF treated with diuretics. 24 This may explain that diuretics increase the risk of HF and mortality at least partially. The underlying mechanism may be related to persistent left atrial structural and electrical remodelling. 34 As expected, the size of left atrial end‐systolic diameter was enlarged more obviously in those RHD patients with CDU (Supporting Information, Table S2 ). In addition, diuretic‐induced hypokalaemia and hypomagnesaemia were also linked to the risk of AF, especially in the presence of severe myocardial fibrosis and digoxin use. 28
Valvular AF (especially caused by RHD) is associated with increased risk of stroke, which is the leading cause of cerebrovascular death. However, there are limited prospective data to assess the effect of diuretic use on risk of cerebrovascular death from RHD. In this study, we also found that there was no significant difference on cerebrovascular death risk between IDU and CDU in RHD patients with compensated CHF. This was inconsistent with previously reported association of diuretic use with hypertension‐related cerebrovascular death. In the HYVET and SHEP study, diuretics (e.g. indapamide and chlorthalidone) caused a 30–36% reduction in the incidence of stroke while in the PROGRESS study, the stroke risk was lowered by 43% in combination with diuretics (e.g. indapamide) on the basis of RSIs (e.g. perindopril) treatment. 35 , 36 This difference might result from hypertension and RHD‐related cerebrovascular death, which are mainly caused by stroke. The former is more common in large‐artery atherosclerosis stroke and small‐artery occlusion lacunar stroke. The latter, however, is mostly attributed to cardioembolism. 37 RHD‐related cardioembolism is associated with left atrial enlargement, which is an independent predictor of stroke even after adjustment by types of AF (paroxysmal or sustained) and valvular heart diseases. 38 Furthermore, our study showed that both IDU and CDU did not reverse left atrial remodelling but made it more serious (Supporting Information, Tables S2 and S3 ). The explanation of the effect could be partly attributed to this observation.
Effect of diuretic types on mortality, and heart failure re‐hospitalization and new‐onset atrial fibrillation
Rheumatic heart disease patients in the IDU group were not prescribed any diuretics during non‐congestion but still received LDs or TDs when acute decompensated HF occur, which was no difference on the treatment for RHD patients with acute decompensated HF in the CDU group. In this study, we found that there was no significant difference on all‐cause mortality, CVD, cerebrovascular death, medium‐term/long‐term HF readmission, and new‐onset AF in RHD patients with acute decompensated HF between the LDs and TDs subgroups (Table 3 ). These results suggested that the prognosis is related to diuretics use for eliminating congestion and has nothing to do with the choice of diuretic types. Our findings were similar to the observation by Trullas et al. 39 on different types of TDs on patients with acute compensated HF. Multicentre PSM analysis of different LDs use (e.g. bumetanide, furosemide, and torasemide) on mortality in CHF patients showed that mortality was not affected by the choice of individual LDs 40 as well as the mode of administration (oral vs. subcutaneous injection). 41 However, LDs treatment decreased the short‐term (within 1 year) HF readmission by 70% compared with TDs treatment (Table 4 ). This was consistent with a recent observation by Brisco‐Bacik et al., 29 who reported that LDs rather than early introduction of TDs (e.g. metolazone) may be the preferred strategy in clinical practice for acute decompensated HF. Compared with short‐acting LDs (e.g. furosemide), the long‐acting LDs (e.g. azosemide) significantly reduced CVD risk, 30 but there was no significant difference in HF‐associated arrhythmias between these two diuretics. 42 In the TORIC study, torasemide rather than furosemide was reported to improve NYHA functional classification, 43 and the effect of torasemide on improving the prognosis, however, depends on the upcoming results of the TRANSFORM‐HF study (a large‐scale, pragmatic, randomized, unblinded clinical effectiveness study comparing torasemide vs. furosemide as treatment for HF on clinical outcomes over 12 months, NCT03296813).
Importantly, not all RHD patients with CHF can maintain decongested status without diuretics. When diuretics have to be continuously used for maintaining optimal individualized decongestion, the choice of diuretics types (LDs vs. TDs) is still a dilemma. Compared with TDs, LDs were more likely to be prescribed to maintain euvolaemia due to clinical biases, and higher dose of LDs was generally prescribed for severe or advanced RHD patients with CHF. The difference, however, in efficacy of long‐term use of LDs and TDs on clinical outcomes in RHD patients with CHF remains unclear. In this study, we are the first to report that continuous LDs use was associated with increased risks of all‐cause mortality (by 80%), CVD (by 89%), medium‐term/long‐term HF readmission (by at least 91%), and new‐onset AF (by 1.66‐fold) compared with continuous TDs use. This is consistent with the meta‐analysis results obtained by Tager et al., who reported that there was no significant superiority of LDs with respect to mortality and safety endpoints, 44 and the death risk was positively correlated with the dose of LDs use. 23 As expected, we observed that sub‐participants with continuous LDs use had worse renal function, more severe anaemia, and imbalanced serum electrolytes (Supporting Information, Table S4 ). In contrast, continuous TDs use may be better from the prognostic perspective among RHD patients with CHF for maintaining decongestion status.
Furthermore, it is worth noting that at least 50% of RHD patients with CHF received MRAs treatment in this study, especially in the CDU group (up to 58.1%). MRAs, as one of the neuroendocrine blockers, have become an indispensable drug for HF treatment and are recommended in all patients with symptomatic (NYHA Classes II–IV) HF with reduced ejection fraction. On the other hand, MRAs are also as diuretics. Regular dose of MRAs has relatively weak diuretic effects in patients with HF, but early initiation of MRA therapy with a regular dose might be useful in offsetting the hypokalaemic effect of potassium‐wasting LDs and TDs and improving the prognosis of HF with reduced ejection fraction patients being discharged on an optimized disease‐modifying therapy regimen. 5 Larger doses of MRAs can assist LDs or (and) TDs to achieve natriuretic effects, especially when diuretic resistance has developed. 45 Butler et al. 46 found that adding treatment with high‐dose MRAs (e.g. 100 mg spironolactone daily) on the basis of standard LDs therapy for patients with acute HF was well tolerated compared with combined with usual care (e.g. 25 mg spironolactone daily) during 96‐h follow‐up, as it did not result in hyperkalaemia or worsening of renal function. However, due to the limited long‐term safety data of continuous triple diuretic therapy (LDs + TDs + MRAs), it is necessary to be very cautious when using it and closely monitor the volume status and electrolytes to avoid hyperkalaemia.
The findings of this study point to a clear correlation between CDU and increased risk of mortality in RHD patients with compensated CHF. Randomized clinical trials will be necessary to investigate whether CDU is actually a causative factor of this increase in mortality or merely a sign of compromised neurohormonal status.
Limitations
This study was hurdled by some limitations. Firstly, the design of our study was only a retrospective study that quite possibly limits the value all of its results. Study participants were not randomized to receive either diuretic application strategy. It is not possible to know how many patients in the CDU group would have been impossible to treat as IDU due to inability to discontinue diuretics without fluid retention. Thus, the results of this analysis contain a bias similar to ‘per‐protocol’ analyses present in clinical trials; the mere fact that patients could do without diuretics for extended periods of time may indicate a more favourable neurohumoral profile that may actually be the cause of better outcomes. Secondly, this was not a randomized study, and the decision to either continuous diuretic treatment or not was not study driven. PSM was performed to form comparable groups with similar a priori risks for mortality, and this matching procedure was obviously successful because the major differences between the unmatched populations vanished. However, the matching method utilized in this study was especially vulnerable to magnifying hidden confounding variables so that the bias may be exacerbated by the fact that there are still no guidelines regarding when to use and when to discontinue diuretics. What motivates the clinician to prescribe diuretics (and which diuretic) to any given RHD patient is a complete unknown. Thus, it is utterly incapable of eliminating the bias introduced by physician decision as well as a potentially favourable neurohormonal profile. Thirdly, because the sample size of this study is not big enough (N = 494), large‐scale prospective studies will help to further verify those findings. Finally, due to the lack of knowledge on optimal medical therapy among RHD patients with HF, basic HF medications (e.g. RSIs, BBs, and MRAs) were used not enough among participants in this retrospective study. Therefore, results must be interpreted carefully.
Conclusions
Continuous diuretics use was associated with higher risks of all‐cause mortality, CVD, re‐hospitalization, and new‐onset AF. Our analysis suggests that de‐escalating diuretic therapy is often feasible in euvolaemic patients with RHD and is associated with better outcomes. Whether this observed relationship is causal due to an unnecessary persistence with diuretic therapy or just reflects the expected association between greater severity of HF and more intensive diuretic therapy is uncertain. These alternative hypotheses should be investigated in randomized trials.
Conflict of interest
None declared.
Funding nm
This study was funded by the National Natural Science Foundation of China (81100235), the Natural Science Foundation of Guangdong Province (S2011040004458), the Science and Technology Planning Project of Guangdong Province (2014A020212372), and the Guangzhou Municipal Science and Technology Project (2012J4100035 and 201804010214).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethics guidelines of the institutional and with the principles of the Declaration of Helsinki.
Supporting information
Table S1. Baseline characteristics of study participants before PSM.
Table S2. Effect of different diuretic application approaches on heart remodeling in RHD participants with compensated CHF at the end of follow‐up.
Table S3. Effect of different application approaches and types of diuretics on heart remodeling in RHD participants with compensated CHF at the end of follow‐up.
Table S4. Effect of different types of diuretics on partial biochemical indicators in RHD participants with compensated CHF at the end of follow‐up.
Figure S1. Causes of death among study participants before PSM.
Acknowledgements
The authors wish to thank all the study participants from the South China Cardiovascular‐related Disease Cohort (SCCDC), research staff, and students who participated in this work.
Liu, C. , Lai, Y. , Guan, T. , Shen, Y. , Pan, Y. , and Wu, D. (2020) Outcomes of diuretics in rheumatic heart disease with compensated chronic heart failure: a retrospective study. ESC Heart Failure, 7: 3929–3941. 10.1002/ehf2.12987.
References
- 1. Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, Forouzanfar MH, Longenecker CT, Mayosi BM, Mensah GA, Nascimento BR, Ribeiro ALP, Sable CA, Steer AC, Naghavi M, Mokdad AH, Murray CJL, Vos T, Carapetis JR, Roth GA. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med 2017; 377: 713–722. [DOI] [PubMed] [Google Scholar]
- 2. Zuhlke L, Karthikeyan G, Engel ME, Rangarajan S, Mackie P, Cupido‐Katya Mauff B, Islam S, Daniels R, Francis V, Ogendo S, Gitura B, Mondo C, Okello E, Lwabi P, Al‐Kebsi MM, Hugo‐Hamman C, Sheta SS, Haileamlak A, Daniel W, Goshu DY, Abdissa SG, Desta AG, Shasho BA, Begna DM, ElSayed A, Ibrahim AS, Musuku J, Bode‐Thomas F, Yilgwan CC, Amusa GA, Ige O, Okeahialam B, Sutton C, Misra R, Abul Fadl A, Kennedy N, Damasceno A, Sani MU, Ogah OS, Elhassan TO, Mocumbi AO, Adeoye AM, Mntla P, Ojji D, Mucumbitsi J, Teo K, Yusuf S, Mayosi BM. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low‐ and middle‐income countries: two‐year follow‐up of the Global Rheumatic Heart Disease Registry (the REMEDY Study). Circulation 2016; 134: 1456–1466. [DOI] [PubMed] [Google Scholar]
- 3. Verma A, Kalman JM, Callans DJ. Treatment of patients with atrial fibrillation and heart failure with reduced ejection fraction. Circulation 2017; 135: 1547–1563. [DOI] [PubMed] [Google Scholar]
- 4. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136: e137–e161. [DOI] [PubMed] [Google Scholar]
- 5. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner‐La Rocca HP, Martens P, Testani JM, Tang WHW, Orso F, Rossignol P, Metra M, Filippatos G, Seferovic PM, Ruschitzka F, Coats AJ. The use of diuretics in heart failure with congestion—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 137–155. [DOI] [PubMed] [Google Scholar]
- 6. Faris RF, Flather M, Purcell H, Poole‐Wilson PA, Coats AJ. Diuretics for heart failure. Cochrane Database Syst Rev 2012; 2: CD003838. [DOI] [PubMed] [Google Scholar]
- 7. Fudim M, O'Connor CM, Mulder H, Coles A, Bhatt AS, Ambrosy AP, Kraus WE, Pina IL, Whellan DJ, Mentz RJ. Loop diuretic adjustments in patients with chronic heart failure: insights from HF‐ACTION. Am Heart J 2018; 205: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol 2006; 97: 1759–1764. [DOI] [PubMed] [Google Scholar]
- 9. Abdel‐Qadir HM, Tu JV, Yun L, Austin PC, Newton GE, Lee DS. Diuretic dose and long‐term outcomes in elderly patients with heart failure after hospitalization. Am Heart J 2010; 160: 264–271 e1. [DOI] [PubMed] [Google Scholar]
- 10. Martins J, Lourenco P, Araujo JP, Mascarenhas J, Lopes R, Azevedo A, Bettencourt P. Prognostic implications of diuretic dose in chronic heart failure. J Cardiovasc Pharmacol Ther 2011; 16: 185–191. [DOI] [PubMed] [Google Scholar]
- 11. Dovancescu S, Pellicori P, Mabote T, Torabi A, Clark AL, Cleland JGF. The effects of short‐term omission of daily medication on the pathophysiology of heart failure. Eur J Heart Fail 2017; 19: 643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kapelios CJ, Kaldara E, Ntalianis A, Nana E, Pantsios C, Repasos E, Margari Z, Sousonis V, Malliaras K, Nanas JN. Lowering furosemide dose in stable chronic heart failure patients with reduced ejection fraction is not accompanied by decompensation: a randomized study. Int J Cardiol 2014; 177: 690–692. [DOI] [PubMed] [Google Scholar]
- 13. Rohde LE, Rover MM, Figueiredo Neto JA, Danzmann LC, Bertoldi EG, Simoes MV, Silvestre OM, Ribeiro ALP, Moura LZ, Beck‐da‐Silva L, Prado D, Sant'Anna RT, Bridi LH, Zimerman A, Raupp da Rosa P, Biolo A. Short‐term diuretic withdrawal in stable outpatients with mild heart failure and no fluid retention receiving optimal therapy: a double‐blind, multicentre, randomized trial. Eur Heart J 2019; 40: 3605–3612. [DOI] [PubMed] [Google Scholar]
- 14. Patterson JH, Adams KF Jr. Investigating the role of thiazide‐like diuretics in acute heart failure: potential approach to an unmet need. J Card Fail 2016; 22: 537–538. [DOI] [PubMed] [Google Scholar]
- 15. Olde Engberink RH, Frenkel WJ, van den Bogaard B, Brewster LM, Vogt L, van den Born BJ. Effects of thiazide‐type and thiazide‐like diuretics on cardiovascular events and mortality: systematic review and meta‐analysis. Hypertension 2015; 65: 1033–1040. [DOI] [PubMed] [Google Scholar]
- 16. Pellicori P, Cleland JG, Zhang J, Kallvikbacka‐Bennett A, Urbinati A, Shah P, Kazmi S, Clark AL. Cardiac dysfunction, congestion and loop diuretics: their relationship to prognosis in heart failure. Cardiovasc Drugs Ther 2016; 30: 599–609. [DOI] [PubMed] [Google Scholar]
- 17. Remenyi B, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, Lawrenson J, Maguire G, Marijon E, Mirabel M, Mocumbi AO, Mota C, Paar J, Saxena A, Scheel J, Stirling J, Viali S, Balekundri VI, Wheaton G, Zuhlke L, Carapetis J. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence‐based guideline. Nat Rev Cardiol 2012; 9: 297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 19. Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J 2006; 27: 1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamaguchi S, Kinugawa S, Tsuchihashi‐Makaya M, Goto D, Yamada S, Yokoshiki H, Takeshita A, Tsutsui H, Investigators J‐C. Loop diuretic use at discharge is associated with adverse outcomes in hospitalized patients with heart failure: a report from the Japanese cardiac registry of heart failure in cardiology (JCARE‐CARD). Circ J 2012; 76: 1920–1927. [DOI] [PubMed] [Google Scholar]
- 21. Domanski M, Tian X, Haigney M, Pitt B. Diuretic use, progressive heart failure, and death in patients in the DIG study. J Card Fail 2006; 12: 327–332. [DOI] [PubMed] [Google Scholar]
- 22. Miura M, Sugimura K, Sakata Y, Miyata S, Tadaki S, Yamauchi T, Onose T, Tsuji K, Abe R, Oikawa T, Kasahara S, Nochioka K, Takahashi J, Shimokawa H, Investigators C . Prognostic impact of loop diuretics in patients with chronic heart failure—effects of addition of renin‐angiotensin‐aldosterone system inhibitors and beta‐blockers. Circ J 2016; 80: 1396–1403. [DOI] [PubMed] [Google Scholar]
- 23. Damman K, Kjekshus J, Wikstrand J, Cleland JG, Komajda M, Wedel H, Waagstein F, McMurray JJ. Loop diuretics, renal function and clinical outcome in patients with heart failure and reduced ejection fraction. Eur J Heart Fail 2016; 18: 328–336. [DOI] [PubMed] [Google Scholar]
- 24. Schartum‐Hansen H, Loland KH, Svingen GF, Seifert R, Pedersen ER, Nordrehaug JE, Bleie O, Ebbing M, Berge C, Nilsen DW, Nygard O. Use of loop diuretics is associated with increased mortality in patients with suspected coronary artery disease, but without systolic heart failure or renal impairment: an observational study using propensity score matching. PLoS ONE 2015; 10: e0124611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maggioni AP. Audits of clinical practice should be encouraged. Use of loop diuretics in patients without a diagnosis of heart failure should be discouraged. Eur J Prev Cardiol 2019; 26: 289–290. [DOI] [PubMed] [Google Scholar]
- 26. Grinstead WC, Francis MJ, Marks GF, Tawa CB, Zoghbi WA, Young JB. Discontinuation of chronic diuretic therapy in stable congestive heart failure secondary to coronary artery disease or to idiopathic dilated cardiomyopathy. Am J Cardiol 1994; 73: 881–886. [DOI] [PubMed] [Google Scholar]
- 27. Martens P, Verbrugge FH, Nijst P, Dupont M, Mullens W. Changes in loop diuretic dose and outcome after cardiac resynchronization therapy in patients with heart failure and reduced left ventricular ejection fractions. Am J Cardiol 2017; 120: 267–273. [DOI] [PubMed] [Google Scholar]
- 28. Simonavicius J, Knackstedt C, Brunner‐La Rocca HP. Loop diuretics in chronic heart failure: how to manage congestion? Heart Fail Rev 2019; 24: 17–30. [DOI] [PubMed] [Google Scholar]
- 29. Brisco‐Bacik MA, Ter Maaten JM, Houser SR, Vedage NA, Rao V, Ahmad T, Wilson FP, Testani JM. Outcomes associated with a strategy of adjuvant metolazone or high‐dose loop diuretics in acute decompensated heart failure: a propensity analysis. J Am Heart Assoc 2018; 7: e009149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kasama S, Toyama T, Kurabayashi M. Comparative effects of long and short‐acting loop diuretics on mortality in patients with chronic heart failure. Int J Cardiol 2017; 244: 242–244. [DOI] [PubMed] [Google Scholar]
- 31. Huang X, Dorhout Mees E, Vos P, Hamza S, Braam B. Everything we always wanted to know about furosemide but were afraid to ask. Am J Physiol Renal Physiol 2016; 310: F958–F971. [DOI] [PubMed] [Google Scholar]
- 32. McKie PM, Schirger JA, Benike SL, Harstad LK, Chen HH. The effects of dose reduction of furosemide on glomerular filtration rate in stable systolic heart failure. JACC Heart Fail 2014; 2: 675–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jong GP, Chen HY, Li SY, Liou YS. Long‐term effect of antihypertensive drugs on the risk of new‐onset atrial fibrillation: a longitudinal cohort study. Hypertens Res 2014; 37: 950–953. [DOI] [PubMed] [Google Scholar]
- 34. Yaghi S, Song C, Gray WA, Furie KL, Elkind MS, Kamel H. Left atrial appendage function and stroke risk. Stroke 2015; 46: 3554–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. PROGRESS Collaborative Group . Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358: 1033–1041. [DOI] [PubMed] [Google Scholar]
- 36. Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ, Group HS . Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358: 1887–1898. [DOI] [PubMed] [Google Scholar]
- 37. Arsava EM, Helenius J, Avery R, Sorgun MH, Kim GM, Pontes‐Neto OM, Park KY, Rosand J, Vangel M, Ay H. Assessment of the predictive validity of etiologic stroke classification. JAMA Neurol 2017; 74: 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hamatani Y, Ogawa H, Takabayashi K, Yamashita Y, Takagi D, Esato M, Chun YH, Tsuji H, Wada H, Hasegawa K, Abe M, Lip GY, Akao M. Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non‐valvular atrial fibrillation. Sci Rep 2016; 6: 31042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trullas JC, Morales‐Rull JL, Casado J, Freitas Ramirez A, Manzano L, Formiga F, Investigators C . Rationale and design of the “Safety and Efficacy of the Combination of Loop with Thiazide‐type Diuretics in Patients with Decompensated Heart Failure (CLOROTIC) Trial:” a double‐blind, randomized, placebo‐controlled study to determine the effect of combined diuretic therapy (loop diuretics with thiazide‐type diuretics) among patients with decompensated heart failure. J Card Fail 2016; 22: 529–536. [DOI] [PubMed] [Google Scholar]
- 40. Tager T, Frohlich H, Grundtvig M, Seiz M, Schellberg D, Goode K, Kazmi S, Hole T, Katus HA, Atar D, Cleland JGF, Agewall S, Clark AL, Frankenstein L. Comparative effectiveness of loop diuretics on mortality in the treatment of patients with chronic heart failure—a multicenter propensity score matched analysis. Int J Cardiol 2019; 289: 83–90. [DOI] [PubMed] [Google Scholar]
- 41. Gilotra NA, Princewill O, Marino B, Okwuosa IS, Chasler J, Almansa J, Cummings A, Rhodes P, Chambers J, Cuomo K, Russell SD. Efficacy of intravenous furosemide versus a novel, pH‐neutral furosemide formulation administered subcutaneously in outpatients with worsening heart failure. JACC Heart Fail 2018; 6: 65–70. [DOI] [PubMed] [Google Scholar]
- 42. Asami M, Aoki J, Tanimoto S, Horiuchi Y, Watanabe M, Furui K, Yasuhara K, Sato T, Tanabe K, Hara K. Effects of long‐acting loop diuretics in heart failure with reduced ejection fraction patients with cardiac resynchronization therapy. Int Heart J 2017; 58: 211–219. [DOI] [PubMed] [Google Scholar]
- 43. Cosin J, Diez J, Investigators T . Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail 2002; 4: 507–513. [DOI] [PubMed] [Google Scholar]
- 44. Tager T, Frohlich H, Seiz M, Katus HA, Frankenstein L. READY: relative efficacy of loop diuretics in patients with chronic systolic heart failure—a systematic review and network meta‐analysis of randomised trials. Heart Fail Rev 2019; 24: 461–472. [DOI] [PubMed] [Google Scholar]
- 45. Butler J, Hernandez AF, Anstrom KJ, Kalogeropoulos A, Redfield MM, Konstam MA, Tang WH, Felker GM, Shah MR, Braunwald E. Rationale and design of the ATHENA‐HF trial: aldosterone targeted neurohormonal combined with natriuresis therapy in heart failure. JACC Heart Fail 2016; 4: 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Butler J, Anstrom KJ, Felker GM, Givertz MM, Kalogeropoulos AP, Konstam MA, Mann DL, Margulies KB, McNulty SE, Mentz RJ, Redfield MM, Tang WHW, Whellan DJ, Shah M, Desvigne‐Nickens P, Hernandez AF, Braunwald E, National Heart L , Blood Institute Heart Failure Clinical Research N . Efficacy and safety of spironolactone in acute heart failure: the ATHENA‐HF randomized clinical trial. JAMA Cardiol 2017; 2: 950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of study participants before PSM.
Table S2. Effect of different diuretic application approaches on heart remodeling in RHD participants with compensated CHF at the end of follow‐up.
Table S3. Effect of different application approaches and types of diuretics on heart remodeling in RHD participants with compensated CHF at the end of follow‐up.
Table S4. Effect of different types of diuretics on partial biochemical indicators in RHD participants with compensated CHF at the end of follow‐up.
Figure S1. Causes of death among study participants before PSM.


