Abstract
Aims
Ceramides exert several biological activities that may contribute to the pathophysiology of cardiovascular disease and heart failure (HF). The association between plasma levels of distinct ceramides (that have been previously associated with increased cardiovascular risk) and cardiovascular mortality in patients with chronic HF has received little attention.
Methods and results
In a post hoc ancillary analysis of the Gruppo Italiano per lo Studio della Sopravvivenza nella Insufficienza Cardiaca‐Heart Failure (GISSI‐HF; NCT00336336) trial, we randomly selected a sample of 200 ambulatory patients with chronic HF who died due to cardiovascular causes and 200 patients who were alive at the end of the trial (after a median follow‐up period of 3.9 years). We measured baseline plasma concentrations of six previously identified high‐risk ceramide species [Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/20:0), Cer(d18:1/22:0), Cer(d18:1/24:0), and Cer(d18:1/24:1) and their individual plasma ratios with Cer(d18:1/24:0)]. Patients who died due to cardiovascular causes had significantly (P < 0.05 or less) higher levels of plasma Cer(d18:1/16:0) and Cer(d18:1/24:1), but lower levels of plasma Cer(d18:1/22:0) and Cer(d18:1/24:0) than had those who did not. All plasma ratios of each ceramide with Cer(d18:1/24:0) were significantly higher in patients who died due to cardiovascular causes. In Cox regression analyses, all five plasma ratios of each ceramide with Cer(d18:1/24:0) were significantly associated with a greater risk of cardiovascular mortality (with unadjusted hazard ratios ranging from 1.23 to 1.59; P < 0.001 or less). These significant associations were attenuated after adjustment for multiple established risk factors, New York Heart Association functional class, left ventricular ejection fraction, use of medications, plasma pentraxin‐3 levels, and, especially, plasma N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels. When we applied a Bonferroni correction for multiple comparisons (using a P‐threshold 0.05/5 ceramide ratios = 0.01), none of the five plasma ratios of each ceramide with Cer(d18:1/24:0) remained statistically associated with the risk of cardiovascular mortality (with adjusted hazard ratios ranging from 1.10 to 1.23).
Conclusions
Higher levels of specific plasma ceramides [especially when used in ratios with Cer(d18:1/24:0)] are associated with increased cardiovascular mortality in ambulatory patients with chronic HF. However, these associations are weakened after adjustment for established cardiovascular risk factors, medication use, and plasma NT‐proBNP concentrations.
Keywords: Ceramides, Heart failure, Cardiovascular mortality, Risk factors
Introduction
Ceramides are highly bioactive lipid molecules composed of a sphingosine backbone attached to a fatty acyl chain that are found in high concentrations within cell membranes and play important roles as both structural lipids and second messengers for intra‐cellular and inter‐cellular signalling pathways, such as cellular growth, proliferation, differentiation, and apoptosis. 1 , 2 Over the last decade, convincing evidence mainly derived from in vitro experiments and in vivo animal studies has supported the importance of specific ceramides as possible mediators or biomarkers of complex disease mechanisms, including atherosclerotic processes. 1 , 3 , 4
Large prospective studies have recently identified plasma ceramides with long and very‐long chains [mostly increased plasma levels of Cer(d18:1/16:0), Cer(d18:1/18:0), and Cer(d18:1/24:1) and their ratios with Cer(d18:1/24:0)] as significant predictors of cardiovascular morbidity and mortality both in the general population 5 and in patients with established coronary artery disease (CAD) or acute coronary syndrome, independent of traditional cardiovascular risk factors. 6 , 7 , 8 , 9 Additionally, higher levels of these plasma ceramides have been strongly associated with greater angiographic severity of coronary stenoses in patients with CAD, 10 as well as with lower post‐stress myocardial wall perfusion in patients with established, or suspected, CAD undergoing stress myocardial perfusion scintigraphy. 11
That said, in this hypothesis‐generating study, we have supposed that there is a significant association between higher plasma levels of specific ceramides (that have been previously associated with higher risk of adverse cardiovascular outcomes in patients with CAD) and the risk of cardiovascular mortality also in patients with chronic heart failure (HF), that is, a progressive, complex clinical syndrome characterized by high rates of cardiovascular morbidity and mortality. 12 , 13 However, to the best of our knowledge, no evidence is currently available for the existence of a significant association between the aforementioned plasma ceramides [and their plasma ratios with Cer(d18:1/24:0)] and the risk of cardiovascular mortality among patients with chronic HF. We believe that this topic is of clinical importance, because the observation of an association between circulating levels of specific plasma ceramides and risk of cardiovascular mortality in this patient population might lead to improved pathophysiologic understanding of the complex biological mechanisms promoting cardiovascular mortality/morbidity in chronic HF and might also provide new potential targets for management of CAD in this group of patients.
Therefore, in this post hoc ancillary analysis of the Gruppo Italiano per lo Studio della Sopravvivenza nella Insufficienza Cardiaca‐Heart Failure (GISSI‐HF) trial, we aimed at examining whether there were significant associations between circulating levels of previously identified high‐risk plasma ceramides [and their plasma ratios with Cer(d18:1/24:0)] and the risk of cardiovascular mortality among ambulatory patients with chronic HF.
Methods
Study population and design
The design and the main results of the GISSI‐HF trial have been thoroughly presented elsewhere in prior publications, 14 , 15 and the registered study protocol is available online at https://clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT00336336).
Briefly, the GISSI‐HF trial started in 2002 as a pragmatic, double‐blind, placebo‐controlled, nationwide multicentre study in 326 cardiology and 31 internal medicine centres with the specific aim of testing with a nested design the efficacy and safety of two different drugs [i.e. rosuvastatin 10 mg/day or n−3 polyunsaturated fatty acids (n−3 PUFAs) 1 g/day] in ambulatory patients with chronic HF. The trial enrolled 6975 patients with clinical evidence of chronic and stable HF [New York Heart Association (NYHA) functional class II–IV], irrespective of age, left ventricular (LV) ejection fraction, and HF aetiology. Major exclusion criteria included the presence of any non‐cardiac co‐morbidity that was unlikely to be compatible with sufficiently long follow‐up (e.g. active cancer); acute coronary syndrome or revascularization procedures within 1 month; planned cardiovascular surgery, expected to be performed within 3 months after randomization; significant liver diseases; serum creatinine concentrations ≥ 2.5 mg/dL; and serum aminotransferase concentrations > 1.5 times the upper normal limit. 14 , 15
Primary endpoint of the GISSI‐HF trial was time to death, and time to death or admission to hospital for cardiovascular causes. Over the follow‐up period of the trial (median duration of 3.9 years), there were 1969 total deaths. 14 , 15 Among the specific causes of death, ~75% were cardiovascular (mainly due to worsening HF or sudden cardiac death), ~21% were non‐cardiovascular (mainly due to malignancy or acute infections), and ~4% were unknown. All of these study endpoints were adjudicated blindly by an ad hoc committee on the basis of pre‐agreed definitions and procedures. 14 , 15
In the present post‐hoc ancillary analysis of the GISSI‐HF trial, using the stored blood samples of participants enrolled in the 51 clinical centres adhering to the biologic sub‐study, we have randomly selected a sample of 200 patients with chronic HF who died due to cardiovascular causes (i.e. cases) and 200 patients who were alive at the end of the trial (i.e. controls).
The GISSI‐HF trial was approved by each local Institutional Review Board of all the participating centres. A written informed consent was obtained from each study participant before the study enrolment.
Plasma ceramides
The methodology for measurement of plasma ceramides has been described previously. 11 , 17 Blood samples for ceramide measurements were stored at −80°C until analysis. Previous studies showed that no significant alterations were observed for plasma ceramides upon long‐term storage at −80°C. 16 An expert laboratory technician (G. L.), who was blinded to the clinical details of the participants, performed all measurements of plasma ceramides at the central Laboratory of the ‘IRCCS Sacro Cuore’ Hospital of Negrar. Ceramide standards were purchased from Avanti Polar Lipids Inc. (Alabaster, Alabama, USA). Plasma concentrations of Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/20:0, Cer(d18:1/22:0), Cer(d18:1/24:0), and Cer(d18:1/24:1) were measured by liquid–liquid extraction with 2‐propanol:ethyl acetate (4:1 v/v) and gradient reverse‐phased chromatography on an Agilent Poroshell 120 C18 column (4.6 × 50 mm, 2.7 μm). 11 , 17 Cer(d18:1/17:0) was used as internal standard. The apparatus consisted of an Agilent 1290 ultra‐high‐performance liquid chromatography system coupled with an Agilent 6495 Triple Quadrupole liquid chromatography/mass spectrometry (LC/MS) system. Mobile phases consisted in LC/MS grade water, acetonitrile with 0.1% formic acid, and 10 mM ammonium acetate in 2‐propanol. [M + H] + →264 MRM (multiple reaction monitoring) transition was selected to quantify each ceramide. Calibration standards (six points) were prepared each day in surrogate matrix (5% bovine serum albumin) at concentrations ranging from 1.0 to 0.031 μM/L for Cer(d18:1/16:0), Cer(d18:1/18:0), and Cer(d18:1/20:0) and from 10 to 0.31 μM/L for Cer(d18:1/22:0), Cer(d18:1/24:0), and Cer(d18:1/24:1), respectively. Linearity regression coefficients were R 2 > 0.99 for all ceramides. The inter‐assay and intra‐assay coefficients of variation for precision and accuracy for all measured ceramides were <15%. 11 , 17 Each plasma ceramide was expressed both as crude concentration and as ratio with Cer(d18:1/24:0). In particular, the ratios of each plasma ceramide with Cer(d18:1/24:0) have been demonstrated to be strongly associated with increased risk of incident cardiovascular events and mortality in previous follow‐up studies. 5 , 6
Clinical and laboratory data
Serum lipids, glucose, creatinine, and other biochemical blood measurements were determined in all participants after an overnight fasting using standard laboratory procedures. The estimated glomerular filtration rate (eGFR) was estimated by using the four‐variable Modification of Diet in Renal Disease (MDRD) study equation. 18
Plasma concentrations of high‐sensitivity C‐reactive protein (hs‐CRP), pentraxin‐3 (PTX3), and two biomarkers of myocardial damage [high‐sensitivity troponin T (hs‐TnT) and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP)] were also measured in a single core laboratory. 19
Body mass index (BMI) was calculated by dividing patients' weight in kilograms by their height in squared metres. Patients were considered to have hypertension if their blood pressure was ≥140/90 mmHg or if they were taking any anti‐hypertensive agents. The presence of known diabetes was defined as self‐reported physician diagnosis of diabetes or use of any antihyperglycaemic drugs. The LV diameter, wall thickness, and ejection fraction were assessed by trans‐thoracic echocardiography according to international standard criteria. More details about clinical and laboratory data have been published elsewhere. 14 , 15
Statistical analysis
Owing to the exploratory, hypothesis‐generating design of the study, we did not perform a priori sample size calculation. Data are expressed as means ± SD when normally distributed, as medians and inter‐quartile ranges (IQRs) when non‐normally distributed, or as frequencies for categorical variables. The Mann–Whitney U test and the Fisher exact test were used to examine differences in clinical and biochemical variables between patients deceased for cardiovascular causes and patients still alive at the end of follow‐up. Restricted cubic regression splines were used to formally test the assumption of a linear association between each plasma ceramide [or its ratio with Cer(d18:1/24:0)] and the outcome. Cox proportional hazards regression analysis was used to estimate the hazard ratios (and 95% confidence intervals) associated with the risk of cardiovascular mortality. Associations were presented for every 1‐SD increment in plasma ratios of each ceramide with Cer(d18:1/24:0). Four multivariable Cox regression models with pre‐specified adjustments were examined: a Model 1 adjusted for age and sex; Model 2 adjusted for age, sex, total cholesterol, and use of any lipid‐lowering drugs; Model 3 with further adjustment for heart rate, BMI, NYHA functional class, smoking history, hypertension, diabetes, stroke, atrial fibrillation/flutter, ischaemic HF aetiology, LV ejection fraction, log‐transformed serum creatinine, and drug study treatment during the trial (n−3 PUFA vs. rosuvastatin); and, finally, Model 4 with additional adjustment for log‐transformed plasma PTX3 and NT‐proBNP levels. Covariates included in these multivariable Cox regression models were selected based on their significance in univariable analyses or based on their biological plausibility as potential confounding factors. Statistical significance was set at two‐sided P‐value of 0.05. To further correct the analyses for multiple comparisons, we also applied a Bonferroni correction using a significance threshold of 0.01 (0.05/5 plasma ceramide ratios). All statistical analyses were performed using SPSS software (IBM SPSS Statistics for Windows, version 25.0, Armonk, NY) at the Department of Cardiovascular Research, ‘Istituto di Ricerche Farmacologiche Mario Negri IRCCS’ of Milan (Italy).
Results
By study design, the cohort comprised a randomly selected sample of 200 ambulatory patients with chronic HF who died due to cardiovascular causes during the follow‐up period of the trial (median duration, 3.9 years; IQR, 3.0–4.5 years) and 200 patients who did not (controls). This cohort of 400 patients with chronic HF was representative and well comparable with the respective two cohorts of the GISSI‐HF participants who died or did not for cardiovascular causes during the trial (data not shown).
The baseline characteristics of the study participants, stratified by cardiovascular deaths occurred during the trial, are listed in Table 1 . Compared with survivors, chronic HF patients who died due to cardiovascular reasons were more likely to be older; were less frequently currently smoking; and had higher heart rate, lower blood pressure, poorer NYHA functional classes, lower eGFRMDRD, lower total haemoglobin, lower plasma lipids, and higher prevalence of established diabetes and atrial fibrillation/flutter. These patients also had higher plasma biomarkers of inflammation (i.e. plasma hs‐CRP and PTX3) and myocardial damage (i.e. hs‐TnT and NT‐proBNP). Moreover, they were also more likely to be treated with diuretics, anticoagulants, and nitrates; whereas no significant differences were found in the use of statins, aldosterone antagonists, beta‐blockers, calcium channel blockers, digitalis, amiodarone, or anti‐platelet agents. Sex, BMI, ischaemic aetiology of HF, LV ejection fraction, and prior history of myocardial infarction, angina, stroke and pacemaker/implantable defibrillator(s) did not significantly differ between the two groups. Drug study treatments were also well balanced between the two patient groups (n−3 PUFAs, 50.5% vs. 47.5%, P = 0.629; rosuvastatin, 28.5% vs. 27.0%, P = 0.911; for survivors and deceased, respectively).
Table 1.
Baseline clinical and biochemical characteristics of ambulatory patients with chronic heart failure stratified by cardiovascular deaths at follow‐up
| Survivors (n = 200) | Deceased (n = 200) | P‐value | |
|---|---|---|---|
| Male sex, % | 163 (81.5%) | 169 (84.5%) | 0.505 |
| Age, years | 63.8 ± 10.2 | 70.1 ± 9.5 | 6.5 × 10 −10 |
| Age ≥70 years, % | 64 (32%) | 114 (57%) | 7.2 × 10 −7 |
| NYHA class, III–IV, % | 45 (22.5%) | 90 (45%) | 5.0 × 10 −6 |
| BMI, kg/m2 | 27.1 ± 4.4 | 26.6 ± 4.3 | 0.572 |
| BMI ≥ 30 kg/m2, % | 36 (18%) | 41 (20.5%) | 0.612 |
| Systolic blood pressure, mmHg | 131.5 ± 21.5 | 123.1 ± 18.0 | 1.4 × 10 −4 |
| Diastolic blood pressure, mmHg | 79.6 ± 10.5 | 75.3 ± 9.9 | 1.2 × 10 −4 |
| Heart rate, b.p.m. | 71.4 ± 12.6 | 75.3 ± 14.1 | 0.012 |
| Diabetes, % | 50 (25%) | 72 (36%) | 0.022 |
| Hypertension, % | 109 (54.5%) | 111 (55.5%) | 0.920 |
| Current smokers, % | 28 (14%) | 14 (7%) | 0.011 |
| Ischaemic HF aetiology, % | 95 (47.5%) | 109 (54.5%) | 0.193 |
| LV ejection fraction, % | 33 [26–39] | 31 [25–37] | 0.116 |
| LV ejection fraction > 40%, % | 26 (13%) | 22 (11%) | 0.645 |
| Atrial fibrillation/flutter, % | 35 (17.5%) | 54 (27%) | 0.030 |
| Pacemaker, % | 23 (11.5%) | 37 (18.5%) | 0.068 |
| Implantable defibrillator, % | 16 (8%) | 18 (9%) | 0.858 |
| Previous myocardial infarction, % | 85 (42.5%) | 99 (49.5%) | 0.192 |
| Angina pectoris, % | 25 (12.5%) | 37 (18.5%) | 0.128 |
| Previous stroke, % | 8 (4%) | 11 (5.5%) | 0.639 |
| Creatinine, mg/dL | 1.11 [0.9–1.2] | 1.30 [1.0–1.6] | 1.4 × 10 −8 |
| eGFRMDRD, mL/min/1.73 m2 | 71.2 [56.7–82.8] | 57.1 [43.3–70.5] | 2.1 × 10 −9 |
| Haemoglobin, g/dL | 14.0 [13.1–15.0] | 13.3 [12.2–14.4] | 3.1 × 10 −5 |
| Total cholesterol, mg/dL | 200 [171–227] | 173 [148–209] | 7.7 × 10 −7 |
| LDL cholesterol, mg/dL | 120 [93–141] | 100 [75–129] | 2.9 × 10 −4 |
| Triglycerides, mg/dL | 130 [89–185] | 113 [80–159] | 0.026 |
| Fasting glucose, mg/dL | 103 [92–123] | 104 [92–135] | 0.432 |
| hs‐CRP, mg/L | 1.58 [0.78–3.68] | 3.15 [1.21–7.51] | 1.0 × 10 −6 |
| PTX3, ng/mL | 3.90 [2.83–5.68] | 5.05 [3.73–8.26] | 3.0 × 10 −6 |
| hs‐TnT, ng/L | 15.0 [9.3–21.8] | 29.6 [18.9–46.0] | 6.8 × 10 −21 |
| NT‐proBNP, pg/mL | 699 [314–1245] | 2021 [1016–3850] | 1.3 × 10 −23 |
| Concomitant medications | |||
| Open label statins | 61 (30.5%) | 55 (27.5%) | 0.582 |
| ACE‐I or ARBs | 197 (98.5%) | 190 (95%) | 0.087 |
| Beta‐blockers | 133 (66.5%) | 115 (57.5%) | 0.080 |
| Calcium channel antagonists | 17 (8.5%) | 16 (8%) | 0.998 |
| Aldosterone antagonists | 79 (39.5%) | 90 (45%) | 0.311 |
| Diuretics | 182 (91%) | 197 (98.5%) | 0.001 |
| Digitalis | 82 (41%) | 99 (49.5%) | 0.108 |
| Antiplatelets | 118 (59%) | 99 (49.5%) | 0.071 |
| Anticoagulants | 55 (27.5%) | 84 (42%) | 0.003 |
| Nitrates | 62 (31%) | 84 (42%) | 0.029 |
| Amiodarone | 42 (21%) | 51 (25.5%) | 0.344 |
Abbreviations: ACE‐I, ACE‐inhibitors; ARBs, angiotensin II receptor blockers; BMI, body mass index; HF, heart failure; hs‐CRP, high‐sensitivity C‐reactive protein; hs‐TNT, high‐sensitivity troponin T; LV, left ventricular; eGFR, estimated glomerular filtration rate assessed by the Modification of Diet in Renal Disease study equation; LDL, low‐density lipoprotein; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; PTX3, pentraxin‐3.
Cohort size, n = 400. Data are presented as mean ± SD, median [IQR], or n (%), as appropriate. P‐value for the Mann–Whitney U test or the Fisher exact test, as appropriate. All deaths are due to cardiovascular causes. For the sake of clarity, significant P‐values are highlighted in bold. Hypertension was defined as blood pressure ≥ 140/90 mmHg or use of any anti‐hypertensive drugs. Diabetes was defined as self‐reported physician diagnosis of diabetes or use of any antihyperglycaemic agents.
Table 2 shows the baseline levels of plasma ceramides and their individual ratios with Cer(d18:1/24:0) in the two patient groups. Patients who died due to cardiovascular causes had significantly (P < 0.05 or less) higher levels of plasma Cer(d18:1/16:0) and Cer(d18:1/24:1), but lower levels of Cer(d18:1/22:0) and Cer(d18:1/24:0) than those who did not. Additionally, all plasma ratios of each ceramide with Cer(d18:1/24:0) were significantly higher in those who died due to cardiovascular causes than in those who did not.
Table 2.
Baseline plasma ceramide levels and their plasma ratios with Cer(d18:1/24:0) in ambulatory patients with chronic heart failure stratified by cardiovascular deaths at follow‐up
| Survivors (n = 200) | Deceased (n = 200) | P‐value a | |
|---|---|---|---|
| Each plasma ceramide | 0.179 [0.140–0.221] | 0.196 [0.141–0.248] | 0.021 |
| Cer(d18:1/16:0), μmol/L | |||
| Cer(d18:1/18:0), μmol/L | 0.094 [0.072–0.119] | 0.093 [0.074–0.121] | 0.868 |
| Cer(d18:1/20:0), μmol/L | 0.080 [0.064–0.095] | 0.081 [0.065–0.100] | 0.591 |
| Cer(d18:1/22:0), μmol/L | 0.529 [0.420–0.633] | 0.471 [0.372–0.614] | 0.007 |
| Cer(d18:1/24:0), μmol/L | 2.269 [1.784–2.622] | 1.930 [1.506–2.476] | 5.5 × 10−5 |
| Cer(d18:1/24:1), μmol/L | 0.794 [0.634–0.955] | 0.828 [0.694–1.021] | 0.028 |
| Ratios with Cer(18:1/24:0) | |||
| Cer(d18:1/16:0)/Cer(d18:1/24:0) | 0.077 [0.064–0.099] | 0.099 [0.074–0.125] | 4.4 × 10−9 |
| Cer(d18:1/18:0)/Cer(d18:1/24:0) | 0.042 [0.033–0.055] | 0.045 [0.038–0.065] | 0.001 |
| Cer(d18:1/20:0)/Cer(d18:1/24:0) | 0.036 [0.030–0.042] | 0.041 [0.035–0.053] | 1.0 × 10−6 |
| Cer(d18:1/22:0)/Cer(d18:1/24:0) | 0.233 [0.210–0.263] | 0.244 [0.226–0.272] | 0.003 |
| Cer(d18:1/24:1)/Cer(d18:1/24:0) | 0.361 [0.292–0.440] | 0.447 [0.359–0.568] | 6.8 × 10−10 |
Abbreviation: Cer, ceramide.
Cohort size, n = 400. Data are presented as median [IQR].
P‐value for the Mann–Whitney U test.
Figure 1 shows the unadjusted Kaplan–Meier curves for cardiovascular mortality rates among patients stratified by the median value of each ceramide ratio at baseline. Notably, patients who had the plasma ratios of each ceramide with Cer(d18:1/24:0) above the median had significantly higher rates of cardiovascular mortality than those with values below the median.
Figure 1.
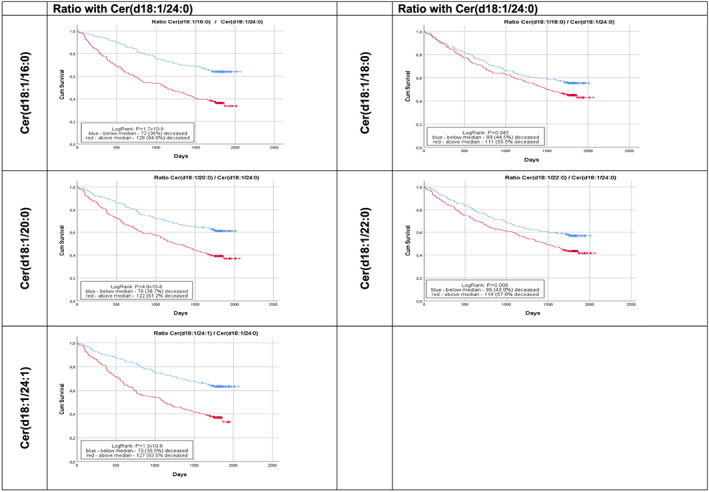
Kaplan–Meier curves for time to cardiovascular mortality in ambulatory patients with chronic heart failure stratified by baseline levels of each plasma ceramide ratio with Cer(18:1/24:0). In these plots, the red line indicates patients with baseline values of each plasma ceramide ratio above the median, whereas the blue line indicates those with baseline values of each ceramide below the median.
In Figure S1 are reported the restricted cubic spline analyses that we used to formally test the assumption of a linear association between each plasma ceramide [and its ratio with Cer(d18:1/24:0)] and the risk of cardiovascular mortality. Because there was a non‐linear association between the individual ceramide species and risk of cardiovascular mortality, a single hazard ratio cannot summarize the association over the range of ceramide values. Thus, for ease of interpretation, we included hazard ratios for the ceramide ratios and restricted cubic spline plots to display the associations between the individual ceramide species and mortality risk.
Table 3 shows the association between plasma ratios of each ceramide with Cer(d18:1/24:0) and the risk of cardiovascular mortality, as well as the effect of the progressive adjustment for multiple established risk factors for HF and potential confounders on these associations in the whole cohort of chronic HF patients. In unadjusted Cox regression analyses, higher plasma ratios of each ceramide with Cer(d18:1/24:0) were significantly associated with higher risk of cardiovascular mortality. Notably, in Cox regression analyses adjusted for multiple known cardiovascular risk factors, serum creatinine, medication use, NYHA functional class, ischaemic HF aetiology, LV ejection fraction, and also drug study treatment during the trial (Adjusted Model 3), we found that all five plasma ceramide ratios maintained a significant positive association with the risk of cardiovascular mortality. In contrast, the additional adjustment for plasma NT‐proBNP and PTX3 levels markedly attenuated the significant associations between all plasma ceramide ratios and cardiovascular mortality; in particular, none of these associations remained statistically significant when we also applied a Bonferroni correction for multiple comparisons (Adjusted Model 4).
Table 3.
Associations between plasma ratios of each ceramide with Cer(d18:1/24:0) and risk of cardiovascular mortality in ambulatory patients with chronic heart failure
| Ratios with Cer(18:1/24:0) | Unadjusted model | Adjusted Model 1 | Adjusted Model 2 | Adjusted Model 3 | Adjusted Model 4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Cer(d18:1/16:0)/Cer(d18:1/24:0) | 1.56 | 1.41–1.74 | 4.6 × 10 −17 | 1.43 | 1.28–1.60 | 3.4 × 10 −10 | 1.35 | 1.20–1.53 | 2.0 × 10 −6 | 1.30 | 1.13–1.48 | 1.7 × 10 −4 | 1.13 | 0.87–1.47 | 0.366 |
| Cer(d18:1/18:0)/Cer(d18:1/24:0) | 1.37 | 1.20–1.56 | 3.0 × 10 −6 | 1.34 | 1.18–1.54 | 1.5 × 10 −5 | 1.22 | 1.06–1.41 | 0.006 | 1.21 | 1.04–1.41 | 0.013 | 1.10 | 0.84–1.44 | 0.486 |
| Cer(d18:1/20:0)/Cer(d18:1/24:0) | 1.52 | 1.34–1.73 | 2.6 × 10 −10 | 1.46 | 1.28–1.67 | 2.5 × 10 −8 | 1.33 | 1.14–1.54 | 1.9 × 10 −4 | 1.32 | 1.13–1.54 | 3.9 × 10 −4 | 1.34 | 1.03–1.74 | 0.031 |
| Cer(d18:1/22:0)/Cer(d18:1/24:0) | 1.23 | 1.08–1.39 | 0.001 | 1.28 | 1.12–1.45 | 1.9 × 10 −4 | 1.22 | 1.07–1.39 | 0.003 | 1.16 | 1.02–1.33 | 0.028 | 1.22 | 0.98–1.52 | 0.076 |
| Cer(d18:1/24:1)/Cer(d18:1/24:0) | 1.59 | 1.23–1.78 | 1.0 × 10 −16 | 1.45 | 1.29–1.63 | 7.9 × 10 −10 | 1.33 | 1.15–1.53 | 8.0 × 10 −5 | 1.30 | 1.12–1.52 | 0.001 | 1.34 | 1.04–1.72 | 0.024 |
Cohort size, n = 400. Each plasma ceramide (Cer) ratio [expressed as ratio with Cer(18:1/24:0)] has been standardized (for every 1‐SD increment). Data are presented as hazard ratio (HR) and 95% confidence interval (95% CI) as assessed by Cox proportional hazards regression analysis. For the sake of clarity, significant P‐values under Bonferroni correction (P‐threshold 0.05/5 ceramide ratios = 0.01) are highlighted in bold. Adjusted Model 1: adjusted for age (years) and sex (male vs. female). Adjusted Model 2: adjusted for age (years), sex (male vs. female), serum total cholesterol (mg/dL) and current use of any lipid‐lowering drugs (yes vs. no). Adjusted Model 3: adjusted for age, sex, NYHA functional class (III–IV vs. I–II), heart rate (b.p.m.), BMI (kg/m2), smoking history (yes vs. no), hypertension (yes vs. no), diabetes (yes vs. no), previous stroke (yes vs. no), atrial fibrillation/flutter (yes vs. no), current use of any lipid‐lowering drugs (yes vs. no), ischaemic aetiology of HF (yes vs. no), LV ejection fraction (%), serum total cholesterol (mg/dL), log‐transformed serum creatinine (mg/dL), and drug study treatments during the trial (i.e. n−3 PUFAs vs. rosuvastatin). Adjusted Model 4: the same covariates included in Model 3 plus log‐transformed plasma PTX3 and log‐transformed plasma NT‐proBNP concentrations.
Discussion
The main findings of our hypothesis‐generating study are as follows: (i) higher levels of plasma ceramides with long and very‐long chains [especially when used in ratios with Cer(d18:1/24:0), which have been demonstrated to be more strongly associated with greater risk of adverse cardiovascular outcomes in previous prospective studies than individual ceramide species 5 , 6 ] were significantly associated with an increased risk of cardiovascular mortality in ambulatory patients with chronic HF; (ii) these significant associations persisted even after adjustment for multiple established risk factors, NYHA functional class, ischaemic HF aetiology, LV ejection fraction, and also drug study treatment during the trial (i.e. n−3 PUFA vs. rosuvastatin); and (iii) these associations were substantially weakened after additional adjustment for plasma NT‐proBNP and PTX3 levels (especially when we also applied a Bonferroni correction for multiple comparisons).
Recent prospective studies found that higher levels of specific plasma ceramides [especially higher plasma levels of Cer(d18:1/16:0), Cer(d18:1/18:0), and Cer(d18:1/24:1)] were significantly associated with an increased risk of major adverse cardiovascular events both in the general adult population and in cohorts of patients with CAD or acute coronary syndrome, independent of plasma lipids and other known cardiovascular risk factors. 5 , 6 , 7 , 8 , 9 , 20 More recently, some prospective studies have also examined the association between circulating levels of these plasma ceramides and the risk of incident HF, but they provided inconclusive findings. 21 , 22 Notably, in an individual participant data meta‐analysis of the Framingham Heart Study and the Study of Health in Pomerania, Peterson et al. found that higher plasma ratios of Cer(d18:1/24:0) or Cer(d18:1/22:0) with Cer(d18:1/16:0) were associated with a significantly lower risk of all‐cause and cardiovascular mortality, as well as with a marginally lower risk of incident HF over a mean follow‐up of ~6 years. 21 Moreover, in a biracial cohort of 4249 older individuals, Lemaitre et al. found that higher circulating levels of Cer(d18:1/16:0) were associated with higher risk of incident HF, whereas higher levels of Cer(d18:1/22:0) were associated with lower risk of incident HF over a median follow‐up of ~9.5 years. 22 Plasma levels of Cer(d18:1/20:0) and Cer(d18:1/24:0) were not significantly associated with risk of incident HF. 22
To our knowledge, this is the first study that has examined the association between plasma ceramides and cardiovascular mortality in a setting of a multicentre randomized controlled trial. Previously, in an observational study involving ~400 elderly Chinese individuals with chronic HF, Yu et al. reported that the total plasma ceramide concentrations (defined as the sum of plasma Cer18, Cer20, Cer22, and Cer24 concentrations) were associated with a higher risk of all‐cause mortality, independently of traditional cardiovascular risk factors, NT‐proBNP levels, and LV ejection fraction, over a mean follow‐up period of 4.4 years. 23 However, in this observational study, the authors did not perform separate statistical analyses for each distinct plasma ceramide, and no data were available on specific causes of mortality. 23
To date, the biochemical pathways responsible for greater ceramide synthesis in human HF are not fully understood. Accumulating experimental evidence has suggested that some ceramides, especially those with long and very‐long chains, exhibit multiple biological activities that may influence the pathophysiology of both HF and atherosclerotic cardiovascular disease, including effects on cellular apoptosis, oxidative stress, endothelial dysfunction, inflammation, lipotoxicity, insulin resistance, and cardiomyopathy. 1 , 2 , 3 , 4 , 24 , 25 , 26 Experimentally, it has been demonstrated that the myocardium may produce certain ceramides in response to acute ischaemia and reperfusion, leading to an increase of specific ceramides that activate mitochondrial autophagy and apoptosis. 27 , 28 In addition, Ji et al., analysing the myocardial tissue and serum from patients with advanced HF undergoing placement of LV assist devices, also showed significant increases in long‐chain and very long‐chain ceramides [mostly Cer(d18:1/16:0) and Cer(d18:1/24:1)] in the failing myocardium and serum of these patients. 29
Our study has important strengths, such as the prospective design of the study, the inclusion of a relatively large sample of patients with chronic HF belonging to a multicentre randomized controlled trial (i.e. the GISSI‐HF, which is essentially a pragmatic all‐comer‐oriented clinical trial), the use of a ‘hard’ cardiovascular endpoint that in accordance with the design of the trial was blindly adjudicated in all participants, the completeness of the database with baseline information on many potential confounders, the relatively long duration of the follow‐up (median of 3.9 years), and the exclusion of patients with significant liver and kidney diseases. We believe that the inclusion of patients with such co‐morbidities might have confounded the interpretation of data.
Among the study limitations, we mention that this is a post‐hoc ancillary analysis of the GISSI‐HF trial with a case–control design, and we had only a single measurement of plasma ceramides at baseline. Thus, the design of the study does not allow us to establish any causal association between plasma ceramide levels and the risk of cardiovascular mortality. Moreover, the GISSI‐HF participants were not necessarily representative of the variety of patients with chronic HF, as the results herein presented pertain to a population of ambulatory patients with chronic HF (mostly with depressed LV ejection fraction), who were followed up by cardiologists and specifically selected to be enrolled in a clinical trial. Given the case–control design of this analysis, it could be difficult to determine whether the associations we observed are specific to cardiovascular mortality or to mortality in general. Additional studies including also chronic HF patients with non‐cardiovascular causes of death will be needed to better elucidate this issue. Unfortunately, we did not measure plasma ceramide levels during the trial. However, it is reasonable to hypothesize that the two drug study treatments (i.e. n−3 PUFA vs. rosuvastatin) did not impact differently on the study outcome(s), because these two drug study treatments were well balanced at baseline between the randomly selected groups of surviving and deceased patients. Finally, we did not measure other biomarkers of the ceramide pathway (e.g. sphingomyelins).
In conclusion, our hypothesis‐generating study shows for the first time that higher levels of plasma ceramides with long and very‐long chains [especially when used in ratios with Cer(d18:1/24:0)] are significantly associated with an increased risk of cardiovascular mortality, over a median period of 3.9 years, in ambulatory patients with chronic HF. However, these associations are weakened after adjustment for established cardiovascular risk factors, use of medications, and, especially, for plasma NT‐proBNP levels (when we also applied a Bonferroni correction for multiple comparisons). Further studies are certainly needed to confirm these results in larger cohorts of ambulatory patients with chronic HF and to investigate pharmacological and nutritional factors that might favourably influence the levels of these plasma ceramides.
Conflict of Interest
None.
Funding
Pfizer, Sigma Tau, and AstraZeneca concurred to fund the study and provided the experimental treatments. The study was planned, conducted, and analysed by the GISSI‐HF group, which has full ownership of the data, in complete independence. All members of the Steering and Writing Committees had full access to the database. All members reviewed the paper and unanimously agreed to submit it to the journal. G.T. was supported by research grants from the University of Verona School of Medicine, Italy.
Author Contributions
G.T. and L.T. conceived and designed the study. G.T. wrote the draft of the manuscript. G.L., A.M., S.B., P.L.T., E.N., and D.N. analysed the data. J.M. performed the statistical analyses. L.T., A.P.M, and R.L. researched the data, contributed to discussion, and reviewed/edited the manuscript. All authors read and approved the final version of the manuscript. G.T. and R.L. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data.
Supporting information
Figure S1. Restricted cubic spline analyses between each plasma ceramide [and its ratio with Cer(d18:1/24:0)] and risk of cardiovascular mortality. Circulating levels of each ceramide (expressed as either crude values or plasma ratios) are reported on the x‐axis, whereas logarithmic hazard ratios for cardiovascular mortality are reported on the y‐axis.
Acknowledgements
The authors thank the participants, the physicians, and the staff of the GISSI‐HF participating centres.
Appendix A.
Full list of the GISSI‐HF participating members
Writing Committee
Luigi Tavazzi (Chairman), Gianni Tognoni (Co‐Chairman), Simona Barlera, Maria Grazia Franzosi, Roberto Latini, Donata Lucci, Aldo P. Maggioni, Roberto Marchioli, Gian Luigi Nicolosi, Maurizio Porcu.
Steering Committee
Luigi Tavazzi (Chairman), Gianni Tognoni (Co‐Chairman), Maria Grazia Franzosi, Roberto Latini, Aldo P Maggioni, Roberto Marchioli, Gian Luigi Nicolosi, Maurizio Porcu.
Data and Safety Monitoring Board
Salim Yusuf (Chairman), Fulvio Camerini, Jay N Cohn, Adriano Decarli, Bertram Pitt, Peter Sleight, Philip A Poole‐Wilson.
Primary Endpoint Committee
Enrico Geraci (Chairman), Marino Scherillo (Co‐Chairman), Gianna Fabbri (Coordinator), Barbara Bartolomei (Secretary), Daniele Bertoli, Franco Cobelli, Claudio Fresco, Antonietta Ledda, Giacomo Levantesi, Cristina Opasich, Franco Rusconi, Gianfranco Sinagra, Fabio Turazza, Alberto Volpi.
Clinical Monitoring
Martina Ceseri, Gianluca Alongi, Antonio Atzori, Filippo Bambi, Desiree Bastarolo, Francesca Bianchini, Iacopo Cangioli, Vittoriana Canu, Concetta Caporusso, Gabriele Cenni, Laura Cintelli, Michele Cocchio, Alessia Confente, Eva Fenicia, Giorgio Friso, Marco Gianfriddo, Gianluca Grilli, Beatrice Lazzaro, Giuseppe Lonardo, Alessia Luise, Rachele Nota, Mariaelena Orlando, Rosaria Petrolo, Chiara Pierattini, Valeria Pierota, Alessandro Provenzani, Velia Quartuccio, Anna Ragno, Chiara Serio, Alvise Spolaor, Arianna Tafi, Elisa Tellaroli.
Subprojects
Stefano Ghio, Elisa Ghizzardi (Ventricular Remodeling—Echo), Roberto Latini, Serge Masson (Biohumoral), Maria Grazia Franzosi, Lella Crociati (Genetic), Maria Teresa La Rovere (Arrhythmic and Autonomic Pattern—Holter monitoring), Ugo Corrà (Exercise Capacity), Paola Di Giulio (Quality of Life and Depression), Andrea Finzi (Implantable Cardiac Defibrillator).
Database Management and Statistics
Donata Lucci, Simona Barlera, Marco Gorini, Lucio Gonzini, Valentina Milani, Giampietro Orsini.
Regulatory, Administrative and Secretariat
Elisa Bianchini, Silvia Cabiddu, Ilaria Cangioli, Laura Cipressa, Maria Lucia Cipressa, Giuseppina Di Bitetto, Barbara Ferri, Luisa Galbiati, Andrea Lorimer, Carla Pera, Paola Priami, Antonella Vasamì.
Centres and Investigators participating to the GISSI‐HF biohumoral subproject
Switzerland—Lugano (T. Moccetti, M.G.R, A. Anesini). Italy—Piemonte Saluzzo (P. Allemano, S.G. Reynaud), Torino, Martini (R. Fenoil), Veruno (P. Giannuzzi, A. Mezzani, U. Corrà). Lombardia Bergamo (A. Gavazzi, A. Grosu), Giussano (A. Volpi, K.N. Jones), Menaggio (C. Lissi), Milano (F.M. Turazza, M. Frigerio), Montescano (O. Febo, F. Olmetti), Monza (A. Cirò, A. Vincenzi), Pavia, San Matteo (L.Tavazzi, L. Scelsi, C. Campana), Pavia, Salvatore Maugeri (C. Opasich, A. Gualco), Pieve di Coriano (M.A. Iannone), Sondrio (G. Cucchi). Veneto Belluno (G. Catania, L. Tarantini), Bovolone (G. Rigatelli, S. Boni), Cittadella (R. Carlon), Conegliano Veneto (A. Sacchetta, L. Borgese), Mirano (P. Sarto, S. Milan), Portogruaro (D. Milan), Rovigo (L. Roncon, M. Carraro), San Bonifacio (R. Rossi, E.C., A. Valentini), Villafranca di Verona (G. Brighetti). Liguria Sarzana—Loc. S. Caterina (A. Cantarelli). Emilia Romagna Ferrara (R. Ferrari, A. Fucili), Loiano (A. Bonfiglioli). Toscana Castelnuovo Garfagnana (P.R. Mariani). Umbria Gubbio (S. Martinelli, M. Buccolieri), Marche Ascoli Piceno (L.M., L. Partemi, G. Gregori). Lazio Marino (D. Testa), Roma, San Camillo (G. Pulignano), Roma, San Filippo Neri (M. Santini, A. Varveri), Roma, Santo Spirito (N. Aspromonte). Molise Termoli (D. Staniscia, E. Calgione). Campania Caserta (A. Vetrano), Napoli, Federico II (P. Perrone Filardi). Puglia Casarano (G. Pettinati, S.C., M.R. Gualtieri), San Giovanni Rotondo (M. Villella). Basilicata Lagonegro (R. Lauletta, E. Tagliamonte). Calabria Catanzaro (A. Scozzafava, S. Cassano), Cosenza (G. Misuraca, R. Caporale), Mormanno (G. Musca, C. Carpino), Sicilia Catania (G. Leonardi), Erice (G. Ledda), Messina (G. Di Tano), Palermo, Villa Sofia (V. Cirrincione, N. Sanfilippo), Palermo, Cervello (F. Enia, M. Floresta). Sardegna Cagliari (M. Porcu, P. Orrù).
Targher, G. , Lunardi, G. , Mantovani, A. , Meessen, J. , Bonapace, S. , Temporelli, P. L. , Nicolis, E. , Novelli, D. , Conti, A. , Tavazzi, L. , Maggioni, A. P. , and Latini, R. (2020) Relation between plasma ceramides and cardiovascular death in chronic heart failure: A subset analysis of the GISSI‐HF trial. ESC Heart Failure, 7: 3288–3297. 10.1002/ehf2.12885.
The full list of participating members is available at Appendix A.
References
- 1. Borodzicz S, Czarzasta K, Kuch M, Cudnoch‐Jedrzejewska A. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis 2015; 14: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature 2014; 510: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bismuth J, Lin P, Yao Q, Chen C. Ceramide: a common pathway for atherosclerosis? Atherosclerosis 2008; 196: 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weil BR, Canty JM Jr. Ceramide signaling in the coronary microcirculation: a double‐edged sword? Circ Res 2014; 115: 475–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Havulinna AS, Sysi‐Aho M, Hilvo M, Kauhanen D, Hurme R, Ekroos K, Salomaa V, Laaksonen R. Circulating ceramides predict cardiovascular outcomes in the population‐based FINRISK 2002 cohort. Arterioscler Thromb Vasc Biol 2016; 36: 2424–2430. [DOI] [PubMed] [Google Scholar]
- 6. Laaksonen R, Ekroos K, Sysi‐Aho M, Hilvo M, Vihervaara T, Kauhanen D, Suoniemi M, Hurme R, März W, Scharnagl H, Stojakovic T, Vlachopoulou E, Lokki ML, Nieminen MS, Klingenberg R, Matter CM, Hornemann T, Jüni P, Rodondi N, Räber L, Windecker S, Gencer B, Pedersen ER, Tell GS, Nygård O, Mach F, Sinisalo J, Lüscher TF. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL‐cholesterol. Eur Heart J 2016; 37: 1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meeusen JW, Donato LJ, Bryant SC, Baudhuin LM, Berger PB, Jaffe AS. Plasma ceramides. A novel predictor of major adverse cardiovascular events after coronary angiography. Arterioscler Thromb Vasc Biol 2018; 38: 1933–1939. [DOI] [PubMed] [Google Scholar]
- 8. Anroedh S, Hilvo M, Akkerhuis KM, Kauhanen D, Koistinen K, Oemrawsingh R, Serruys P, van Geuns RJ, Boersma E, Laaksonen R, Kardys I. Plasma concentrations of molecular lipid species predict long‐term clinical outcome in coronary artery disease patients. J Lipid Res 2018; 59: 1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang DD, Toledo E, Hruby A, Rosner BA, Willett WC, Sun Q, Razquin C, Zheng Y, Ruiz‐Canela M, Guasch‐Ferré M, Corella D, Gómez‐Gracia E, Fiol M, Estruch R, Ros E, Lapetra J, Fito M, Aros F, Serra‐Majem L, Lee CH, Clish CB, Liang L, Salas‐Salvadó J, Martínez‐González MA, Hu FB. Plasma ceramides, Mediterranean diet, and incident cardiovascular disease in the PREDIMED trial (Prevención con Dieta Mediterránea). Circulation 2017; 135: 2028–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mantovani A, Bonapace S, Lunardi G, Canali G, Dugo C, Vinco G, Calabria S, Barbieri E, Laaksonen R, Bonnet F, Byrne CD, Targher G. Associations between specific plasma ceramides and severity of coronary‐artery stenosis assessed by coronary angiography. Diabetes Metab 2020; 46: 150–157. [DOI] [PubMed] [Google Scholar]
- 11. Mantovani A, Bonapace S, Lunardi G, Salgarello M, Dugo C, Gori S, Barbieri E, Verlato G, Laaksonen R, Byrne CD, Targher G. Association of plasma ceramides with myocardial perfusion in patients with coronary artery disease undergoing stress myocardial perfusion scintigraphy. Arterioscler Thromb Vasc Biol 2018; 38: 2854–2861. [DOI] [PubMed] [Google Scholar]
- 12. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011; 8: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braunwald E. Heart failure. JACC Heart Fail 2013; 1: 1–20. [DOI] [PubMed] [Google Scholar]
- 14. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G, Investigators GISSI‐HF. Effect of n‐3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI‐HF trial): a randomised, double‐blind, placebo‐controlled trial. Lancet 2008; 372: 1223–1230. [DOI] [PubMed] [Google Scholar]
- 15. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G, GISSI‐HF Investigators . Effect of rosuvastatin in patients with chronic heart failure (the GISSI‐HF trial): a randomised, double‐blind, placebo‐controlled trial. Lancet 2008; 372: 1231–1239. [DOI] [PubMed] [Google Scholar]
- 16. Brunkhorst R, Pfeilschifter W, Patyna S, Büttner S, Eckes T, Trautmann S, Thomas D, Pfeilschifter J, Koch A. Preanalytical biases in the measurement of human blood sphingolipids. Int J Mol Sci 2018; 19: pii: E1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mantovani A, Bonapace S, Lunardi G, Salgarello M, Dugo C, Canali G, Byrne CD, Gori S, Barbieri E, Targher G. Association between plasma ceramides and inducible myocardial ischemia in patients with established or suspected coronary artery disease undergoing myocardial perfusion scintigraphy. Metabolism 2018; 85: 305–312. [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999; 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 19. Latini R, Gullestad L, Masson S, Nymo SH, Ueland T, Cuccovillo I, Vårdal M, Bottazzi B, Mantovani A, Lucci D, Masuda N, Sudo Y, Wikstrand J, Tognoni G, Aukrust P, Tavazzi L. Investigators of the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) and GISSI‐Heart Failure (GISSI‐HF) trials. Pentraxin‐3 in chronic heart failure: the CORONA and GISSI‐HF trials. Eur J Heart Fail 2012; 14: 992–999. [DOI] [PubMed] [Google Scholar]
- 20. Hilvo M, Meikle PJ, Pedersen ER, Tell GS, Dhar I, Brenner H, Schöttker B, Lääperi M, Kauhanen D, Koistinen KM, Jylhä A, Huynh K, Mellett NA, Tonkin AM, Sullivan DR, Simes J, Nestel P, Koenig W, Rothenbacher D, Nygård O, Laaksonen R. Development and validation of a ceramide‐ and phospholipid‐based cardiovascular risk estimation score for coronary artery disease patients. Eur Heart J 2020; 41: 371–380. [DOI] [PubMed] [Google Scholar]
- 21. Peterson LR, Xanthakis V, Duncan MS, Gross S, Friedrich N, Völzke H, Felix SB, Jiang H, Sidhu R, Nauck M, Jiang X, Ory DS, Dörr M, Vasan RS, Schaffer JE. Ceramide remodeling and risk of cardiovascular events and mortality. J Am Heart Assoc 2018; 7: pii: e007931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lemaitre RN, Jensen PN, Hoofnagle A, McKnight B, Fretts AM, King IB, Siscovick DS, Psaty BM, Heckbert SR, Mozaffarian D, Sotoodehnia N. Plasma ceramides and sphingomyelins in relation to heart failure risk. Circ Heart Fail 2019; 12: e005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu J, Pan W, Shi R, Yang T, Li Y, Yu G, Bai Y, Schuchman EH, He X, Zhang G. Ceramide is up‐regulated and associated with mortality in patients with chronic heart failure. Can J Cardiol 2015; 31: 357–363. [DOI] [PubMed] [Google Scholar]
- 24. Jernigan PL, Makley AT, Hoehn RS, Edwards MJ, Pritts TA. The role of sphingolipids in endothelial barrier function. Biol Chem 2015; 396: 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Law BA, Liao X, Moore KS, Southard A, Roddy P, Ji R, Szulc Z, Bielawska A, Schulze PC, Cowart LA. Lipotoxic very‐long‐chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB J 2018; 32: 1403–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kusminski CM, Scherer PE. Lowering ceramides to overcome diabetes. Science 2019; 365: 319–320. [DOI] [PubMed] [Google Scholar]
- 27. Beresewicz A, Dobrzyn A, Gorski J. Accumulation of specific ceramides in ischemic/reperfused rat heart; effect of ischemic preconditioning. J Physiol Pharmacol 2002; 53: 371–382. [PubMed] [Google Scholar]
- 28. de Carvalho LP, Tan SH, Ow GS, Tang Z, Ching J, Kovalik JP, Poh SC, Chin CT, Richards AM, Martinez EC, Troughton RW, Fong AY, Yan BP, Seneviratna A, Sorokin V, Summers SA, Kuznetsov VA, Chan MY. Plasma ceramides as prognostic biomarkers and their arterial and myocardial tissue correlates in acute myocardial infarction. JACC Basic Transl Sci 2018; 3: 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ji R, Akashi H, Drosatos K, Liao X, Jiang H, Kennel PJ, Brunjes DL, Castillero E, Zhang X, Deng LY, Homma S, George IJ, Takayama H, Naka Y, Goldberg IJ, Schulze PC. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight 2017; 2: pii: 82922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Restricted cubic spline analyses between each plasma ceramide [and its ratio with Cer(d18:1/24:0)] and risk of cardiovascular mortality. Circulating levels of each ceramide (expressed as either crude values or plasma ratios) are reported on the x‐axis, whereas logarithmic hazard ratios for cardiovascular mortality are reported on the y‐axis.


