Abstract
The European Commission submitted to the EFSA Panel on Plant Health a Dossier by USDA proposing a systems approach to mitigate the risk of entry of Bretziella fagacearum to the EU when trading oak logs with bark from the USA. Due to the forthcoming ban of methyl bromide (MB), the Dossier indicates sulfuryl fluoride (SF) as the substitute fumigant for this commodity. After collecting additional evidence from USDA,EU NPPOs, external experts and the published literature, the Panel performed a quantitative assessment on the likelihood of pest freedom for B. fagacearum at the point of entry in the EU, comparing the proposed systems approach with those already implemented by Commission Decision 2005/359/EC. The Panel provided also a non‐quantitative assessment for all risk reduction options (RROs) proposed to be undertaken in the EU, from the point of entry to processing at the sawmill. The quantitative assessment until the EU point of entry, based on experts’ judgement, indicated that: i) the most effective import option remains the current one with MB (95% certainty that between 9,573 and 10,000 containers per 10,000 would be free of B. fagacearum), followed by that with SF (95% certainty that between 8,639 and 10,000 containers per 10,000 would be free of B. fagacearum) and, last, by the other existing option based on delivering white oak logs in certain periods of the year to certain regions of the EU without fumigation (95% certainty, between 7,803 and 10,000 containers per 10,000). RROs proposed to be undertaken in the EU are expected to further reduce the risk of establishment of B. fagacearum, should these RROs be regulated, correctly implemented and checked by NPPOs. A wood pathway analysis is needed to quantitatively assess the importance of each measure and to optimise regulatory actions and risk management efforts.
Keywords: fumigant treatment, fungi, nitidulid beetle, pathway analysis, Quercus spp., veneer wood
1. Introduction
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
Council Directive 2000/29/EC1 laid down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the EU or to be moved within the EU. Under Directive 2000/29/EC, oak (Quercus L.) logs with bark attached, originating in the United States of America, were not allowed to be introduced into the EU because of the risk of introducing Bretziella fagacearum, the cause of oak wilt.
Council Directive 2000/29/EC has been repealed and the above‐mentioned prohibition has been maintained through Commission Implementing Regulation 2019/20722.
Commission Decision of 29 April 20053 (as amended in 20064 and 20105) provides for a derogation from this prohibition of Council Directive 2000/29/EC as regards oak (Quercus L.) logs with bark attached, originating in the United States. The above‐mentioned decision requires the use of fumigation with methyl bromide (MB) to mitigate the risk of spreading of Bretziella fagacearum in oak logs (Quercus species) with bark. Those measures are temporary and will expire on 31 December 2020.
The Commission has informed the US that the European Union (EU) will not renew the use of MB after expiration of EU Commission Decision 2005/359/EC for control of oak wilt in oak logs with bark before export from the US.
The US Department of Agriculture Animal and Plant Health Inspection Service (USDA APHIS) is proposing an integrated systems approach, which includes fumigation using sulfuryl fluoride (SF), to achieve equivalent risk mitigation when compared with existing required MB fumigation while maintaining wood quality for veneer processing in the EU.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 29 of Regulation (EC) No 178/20026, to provide a scientific opinion.
Taking into account the available scientific information, including the technical information provided by United States, EFSA is requested to provide a scientific opinion assessing the level of certainty of pest freedom from Bretziella fagacearum of oak logs with bark produced in US under an integrated systems approach, which includes fumigation using SF. When key weaknesses of this integrated systems approach are identified, they should be analysed and RROs which could lead to the increase of the level of pest freedom of the commodity should be described, where appropriate.
1.2. Interpretation of the Terms of Reference
The EFSA Panel on Plant Health (from this point forward referred to as ‘the Panel’) was requested:
to assess the level of certainty of pest freedom from Bretziella fagacearum of oak (Quercus sp.) logs with bark produced in the US under the integrated systems approach described in the Dossier (from this point forward referred to as ‘the Dossier’) provided by USDA APHIS (the Animal and Plant Health Inspection Service of United States Department of Agriculture);
to identify aspects that, managed differently, could reduce the level of risk, including alternative or additional risk reduction options (RROs) able to increase the level of protection for such a commodity.
In its evaluation, the Panel:
reviewed the information provided by USDA APHIS in the Dossier and by later exchanges;
evaluated the effectiveness of the proposed measures included in the systems approach described in the Dossier;
identified the critical aspects of the current system (regulated under Commission Decision 2005/359/EC) and of the alternative system proposed in the Dossier.
Risk management decisions are not within EFSA's remit. Therefore, the Panel: (i) provided a rating for the likelihood of pest freedom for B. fagacearum at the point of entry (i.e. where the EU phytosanitary inspections are performed: either at the port or at the first place of storage, as indicated in Art. 4 of Commission Decision 2005/359/EC) comparing the RROs of Commission Decision 2005/359/EC with the systems approach proposed in the Dossier in a quantitative manner, using expert judgement (Section 6.1), and (ii) conducted a non‐quantitative assessment of all the other steps (Section 6.2).
The level of certainty of pest freedom was evaluated exclusively for B. fagacearum and not for its insect vectors. The role of vectors is however mentioned in various sections of the opinion where relevant.
1.3. The derogation
The European Commission provided a derogation (2005/359/EC) from the Directive 2000/29/EC that forbids the import of oak (Quercus spp.) logs with bark. The derogation offers two options for importing oak logs with bark from the US. Both import options entail a systems approach to prevent the introduction of B. fagacearum. These two import options are summarised below.
Beginning 1 January 2021, the EU will not renew the use of MB for control of oak wilt in oak logs with bark before export from the US. Therefore, the import option 1 described in Table 1 will be no longer available.
Table 1.
Summary of the two options for importing oak logs with bark from the US as described in Commission Decision 2005/359/EC. The regulated actions are organised according to the phase (where and when) at which they have to be performed
| Where and when | Phytosanitary measures in import option 1 | Phytosanitary measures in import option 2 |
|---|---|---|
| Country of origin | Logs should be fumigated with methyl bromide according to the procedure prescribed in Annex I of Commission Decision 2005/359/EC |
Logs, of white oak only, cannot leave the port of shipment before 15 October and arrive at the destination port after 30 April A consignment can consist of white oak only |
|
Notes: Fumigation with MB at a minimum rate of 240 g/m3 for 72 h, with logs temperature of at least 5°C A non‐removable fumigation mark should be placed on the log. This mark is allotted to one shipper The fumigation procedure should be supervised by officials of national plant protection organisations After fumigation, the phytosanitary certificate is issued. The certificate includes the species name, the number of logs and the fumigation batch identification marks |
||
| Ports of unloading |
Logs can be unloaded at a selected set of 35 ports (as amended by Commission Decision 2006/750/EC) Phytosanitary inspection takes place either in port or at other authorised control points |
|
| Notes: Appropriate fumigation is to be tested by fumigation colour reaction test on an appropriate number of logs selected at random from the consignment | Notes: White oak log identification colour test is to be performed on at least 10% of the logs per consignment | |
| Transport | Logs are not allowed to enter areas below 45° latitude | |
| Notes: Greece, Spain, Italy, Cyprus, Malta and Portugal are excluded from this import option | ||
| Storage locations (ports and mills) | Logs can only be stored at certified locations |
Logs can only be stored at certified locations Continuous wet storage of oak logs is required, starting at the latest at time of flushing in the neighbouring oak stands |
| Notes: MS may exempt fumigated logs from continuous wet storage as from Art. 2.2 of Commission Decision 2005/359/EC | ||
| Mills |
Logs are only to be processed at plants that have been notified to and approved by the said responsible official bodies Bark and other waste arising from the processing should immediately be destroyed at the place of processing Neighbouring oak stands should regularly be inspected for symptoms of B. fagacearum at appropriate intervals by the responsible official bodies If symptoms that may have been caused by B. fagacearum are found, further official testing is to be carried out in accordance with appropriate methods to confirm whether or not the fungus is present. If the presence of B. fagacearum is confirmed, the Commission is to immediately be informed |
|
| Notes: MS may exempt the immediate bark and other waste destruction as from Art. 2.2. of Commission Decision 2005/359/EC | ||
For the assessment of the effectiveness of fumigation with MB, see Section 6.1.
2. Data and methodologies
2.1. Data
2.1.1. Data provided by USDA APHIS
The Panel considered all the data and information provided in the Dossier received together with the mandate letter, including the additional material provided by USDA APHIS during the hearing of the 16 September 2020 (EFSA, online) and in successive email exchanges. The Dossier and supplementary material are stored and accessible by EFSA.
The structure and overview of the Dossier are shown in Table 2.
Table 2.
Structure and overview of the information provided by USDA APHIS
| Dossier section | Overview of contents | Filename |
|---|---|---|
| 1 |
Dossier prepared by USDA APHIS and received from DG SANTE with the mandate. It provides: i) the description of the proposed systems approach ii) the survey of EU mills processing oak logs, imported from the US, to produce veneer: form and analysis of the received replies |
The Dossier |
| 2 | Minutes of the hearing of USDA APHIS representatives conducted by the EFSA working group | The hearing (EFSA, online) |
The data and supporting information provided by USDA APHIS formed the basis of this commodity risk assessment.
2.1.2. Literature searches performed by EFSA
Literature searches were undertaken by EFSA to supplement the knowledge gaps on: (i) the pest: B. fagacearum and its vectors; (ii) the commodity: US oak logs with bark; (iii) the RROs in the US and during oversea shipment; during transport, storage and processing in the EU.
One systematic literature review dedicated to the two fumigants SF and MB was performed applying an ad hoc search string, and periodically run between 31 July and 25 October 2020. In Appendix A, the search strategy, results and an extraction table summarising the main evidence are provided.
Additional searches, limited to retrieve documents, were run when developing the opinion. The available scientific information, including previous EFSA opinions on the relevant pests (e.g. EFSA PLH Panel 2018a) as well as the relevant grey literature (e.g. USDA guidelines on oak wilt) and legislation (e.g. Commission Decision 2005/359/EC) were considered.
2.1.3. Further information provided by experts and national authorities
To integrate information on log processing and the forest‐wood chain in veneer production, the Panel involved the hearing expert Roberto Zanuttini, professor of wood technology at the University of Turin.
In support of the non‐quantitative assessment of the steps after entry, the Panel consulted the EU National Plant Protection Organisations (NPPOs) with a questionnaire circulated via email on the 28 September 2020. Additional clarifications were asked to specific MSs with ad hoc emails in a second phase.
2.2. Methodologies
While developing the opinion, the Panel followed the EFSA Guidance on commodity risk assessment for the evaluation of high‐risk plant dossiers (EFSA PLH Panel, 2019a). In addition, given the specific context of the ‘systems approach’, also the ISPM 14 on ‘The use of integrated measures in a systems approach for pest risk management’ (FAO, 2019) was considered (Section 5.1).
The systems approach described in the Dossier includes a series of actions to be undertaken before and after shipment to the EU.
Part of the actions to be undertaken in the EU is currently not regulated and at the discretion of the individual mills, which could involve a large variety of scenarios, influenced by country and industry characteristics (e.g. location, size, product destination). The assessment of those actions, if included in a quantitative process, would result in a very high uncertainty. To reduce this uncertainty, the Panel would need to conduct a deep and resource‐consuming data collection and analysis, e.g. involving representatives of sawmill sector of all the EU importing countries.
For these reasons, and to comply with the mandate deadline, the Panel agreed to provide a quantitative assessment of the pest freedom till the point of entry in the EU (i.e. the port of arrival of the shipped containers or the first place of storage referred to in Article 5 of Commission Decision 2005/359/EC), by applying the same quantitative methodology described for commodity risk assessments conducted under the high‐risk plants’ mandates (EFSA PLH Panel, 2019a). Therefore, the Panel provided a rating based on expert judgement on the likelihood of pest freedom at the point of entry for oak logs with bark fumigated with SF under the systems approach proposed in the Dossier.
This rating is compared with that referred, again at the point of entry, to the two current import options (regulated under Commission Decision 2005/359/EC) (Section 6.1.2):
oak logs with bark fumigated with MB
white oak logs with bark non‐fumigated, under the conditions listed in Art. 8 of Commission Decision 2005/359/EC.
From the point of entry ahead, the Panel reviewed and assessed the actions listed in the systems approach in a non‐quantitative manner, by consulting experts and identifying specific critical points (Section 6.2).
To ensure the full understanding of the Dossier content and to clarify any further doubt, before the assessment the Panel organised an official hearing with USDA APHIS representatives (Section 2.1.1), whose outcomes are published on the EFSA website (EFSA, online).
2.2.1. Pest data
The pest categorisation on B. fagacearum (EFSA PLH Panel, 2018a) was the reference document used to assess the risk of entry of this fungus. Additional and more recent information was integrated, in particular about the range of potential vectors in the US and in the EU.
2.2.2. Commodity data
The characteristics of the commodity were summarised mainly based on the information provided in the Dossier and at the hearing (EFSA, online).
2.2.3. Listing and evaluation of risk reduction options proposed in a systems approach
All RROs proposed in the systems approach that could reduce the level of risk for this commodity are listed in two summary tables: Table 6 of Section 6.1.1 and Table 8 of Section 6.2. The first summary table (Table 6) lists those RROs performed up to the point of entry in the EU, while the second (Table 8) the RROs performed from the point of entry to the processing of logs. Table 6 represents also part of the evidence used to conduct the EKE, whose results are given in Section 6.1.2. The tables only include those actions expected to reduce the level of risk related to B. fagacearum. Therefore, all those steps mentioned in the Dossier not specific to the pest (e.g. tagging of logs) are not assessed.
Table 6.
List of the proposed RROs to be undertaken up to the entry point in the EU and evaluation of their effectiveness
| Step from Figure 2 | Risk reduction option (RRO) | Effect on pest/pathogen | RROs in the US | Regulated | Evaluation and uncertainties | |
|---|---|---|---|---|---|---|
| 0 | RRO1 | Forest certification | Yes | The forest management practices employed in a certified forest are assessed against a series of standards assuring that the wood products respect a series of independently verified ecological, economic and social sustainability principles (Gutierrez Garzon et al., 2020) | No certification is not compulsory. |
Certified forests are more likely to follow official guidelines on the pest management compared to non‐certified forests. Therefore, forest certification is expected to have some effects in reducing the likelihood of introduction of the pest in the EU. However, it cannot be excluded that logs exported to the EU come also from uncertified forests (EFSA, online) Uncertainties: The level of efficacy of forest certification in lowering the likelihood of introduction of the pest in the EU |
| 0 | RRO2 | Surveillance | Yes | The surveillance activity is organised according to state‐specific protocols. Oak wilt is officially surveyed, intensely along the edge of the known disease range and broadly surveyed in unaffected portions of forest land (EFSA, online) |
Yes Surveillance is regulated and compulsory only in some circumstances |
Surveillance in the US is carried out particularly at the edges of the range of expansion of pest. Information of presence, prevalence and incidence of the pest should be considered to modulate tree harvesting for export. This measure is expected to be more effective in reducing the likelihood of introduction of the pest in the EU on logs coming from stands located at the edge of the range of expansion of the pest in the US compared to stands that have been infested for a long time Uncertainties: The design and intensity of surveillance If data on the surveillance are used to decide whether stands might be harvested for export |
| 0 | RRO3 | Laboratory‐based confirmation | Yes | The standard polymerase chain reaction (PCR) methods currently applied are provided in Yang and Juzwik (2017). Both the molecular and the standard isolation test are conducted on samples, mainly coming from outside the known disease range (EFSA, online) | No |
The nested PCR protocol is the most accurate in detecting the pathogen from actively wilting trees and from 1‐year‐dead branches. These diagnostic methods are considered effective, especially if they are combined with sound sampling approaches Uncertainties: Sampling effectiveness, especially in asymptomatic plants How often PCR methods are applied in practice |
| 0 | RRO4 | Removal and disposal of infected trees | Yes | Wilted red oaks can be cut and treated or destroyed before development of the mats (Juzwik et al., 2011).Debarking the diseased red oaks facilitates drying and prevents mat formation (Juzwik et al., 2011) | No |
The removal and sanitation of the diseased trees in the right moment (i.e. not later than 1 April) prevents the development of sources of inoculum and can reduce the vectors population density. These measures may be effective in the reduction of the likelihood of introduction of the pest in the EU Uncertainties: The level of efficacy of this RRO in lowering the likelihood of introduction of the pest in the EU The effective moment of disposal and correct handling of logs from diseased oaks which are frequently used as firewood |
| 0 | RRO5 | Wound paints | Yes | When cutting or breaking of branches are caused in areas where oak wilt is present, wounds should be immediately painted (e.g. latex paint or a wound dressing) (Juzwik et al., 2011) | No |
Painting prevents nitidulid beetles from accessing the exposed wood. Therefore, this RRO is expected to have some effects in reducing the likelihood of introduction of the pest in the EU Uncertainties: Whether and in which extent wound painting can be effectively conducted in forests |
| 0 | RRO6 | Disruption of root grafting | Yes | Trenching separating healthy from infected trees may be implemented to lower the risk of pathogen transmission. | No |
Trenching can be highly effective in reducing the spread of the disease (Juzwik et al., 2011; Harrington, 2013), although the efficacy depends on the ability to identify disease centres Uncertainties: No uncertainties |
| 1 | RRO7 | Silvicultural system | Yes | Selection cutting (EFSA, online). Only the best trees are harvested for export to the EU | No | Trees with wilting symptoms are excluded from marking for export (EFSA, online). Therefore, this RRO is expected to be effective in reducing the likelihood of introduction of the pest in the EU, although asymptomatic yet infected trees may be marked.Uncertainties:Crown symptoms are not specific. The diagnosis is often based on the visual observation of the leaf vein necrosis symptom, but in some species, in particular white oak, laboratory confirmation is necessary (Juzwik et al., 2011) and infected white oaks can stay asymptomatic for many years |
| 1 | RRO8 | Marking season | Yes | Trees should be marked for cutting in a time when diseased plants are easier to recognise | No |
The visual selection of symptomless plants limits the risk of felling infected plants Uncertainties: Marking is not always carried out at the best moment The fungus might spread within trees during the time elapsing from marking to cutting Symptomless yet infected trees, particularly white oaks, may be marked |
| 1 | RRO9 | Harvest procedure | Yes | During harvesting, attention should be paid to limit injuries to standing trees | No |
This measure may be effective as wounds attract the vector beetles and are infection courts for the fungus Uncertainties: The extent to which this measure may be successful in preventing injuries to trees |
| 4 | RRO10 | Logs inspection before export | Yes | Logs are inspected before export (before loading them into containers) | Yes |
Phytosanitary inspection before export is conducted on a visual basis (EFSA, online). Therefore, this RRO is expected to be partially effective in reducing the likelihood of introduction of the pest in the EU. Inspection is conducted without standardised sampling and detection procedures, including the use of molecular tools, and this may be a shortcoming Uncertainties: Level of efficacy of visual inspection of logs |
| 6 | RRO11 | Fumigation | Yes | Oak logs are fumigated in containers (the Dossier) | Yes |
Fumigation with SF is expected to reduce viable inoculum of B. fagacearum, although not to eradicate the pathogen from logs (see extraction table in Appendix A and EKE reasoning in Appendix B) Fumigation with SF is expected to be more effective against vectors of B. fagacearum than against the pathogen itself Uncertainties: Level of efficacy of SF against the fungus on oak logs with bark Level of penetrability of oak logs with bark to SF General efficacy of the fumigation on logs (with bark) up to 91.5 cm in diameter |
Table 8.
List of RROs and potential RROs to be undertaken from the entry point till processing, and evaluation of their effectiveness
| Step from figure | Risk reduction option (RRO) | Effect on pest/pathogen | RROs in the EU | Regulated | Evaluation and uncertainties | |
|---|---|---|---|---|---|---|
| 7 | RRO12 | Limited number of ports of entry | Yes | Yes |
The limitation in the number of ports of entry allows handling of the commodity at place where specific inspection facilities are available and concentrate the entry points where the consignments have to be checked. This RRO is deemed effective (Robinet et al., 2016) Uncertainties: Based on replies obtained from NPPOs, a big part of the phytosanitary inspections is performed directly at the mill, where the container is frequently opened for the very first time after shipment, and where the likelihood of spread to new areas of establishment could be easier for forestry pests than at the port |
|
| 7 | RRO13 | Import inspection | Yes | Phytosanitary inspection consists of a fumigation colouring check to verify that the fumigation has been carried out properly. In case of non‐fumigated white oak, a white oak colour test on at least 10% of randomly selected logs has to take place. Additionally, the NPPO checks other non‐compliances | Yes | This RRO is considered at least partially effective. The border inspections most often take place at the mills where the logs are unloaded from the container and placed into wet storage. Spain reported that phytosanitary inspection takes place at the port of entry after which the logs are transported to the mills. Interception data indicate that incorrect fumigation occurred. Next, sometimes insects were found which is another indication that fumigation was not completely successful. Phytosanitary inspection is conducted without standardised sampling and detection procedures, including the use of molecular tools, and this may be a shortcoming.Uncertainties:As above, the location of the inspection (at the port or at the mill) can influence the level or risk.B. fagacearum was never intercepted and, given the wide distribution in the US, it is not clear whether it is the effect of fumigation or how frequently laboratory analysis is performed to test for the presence of B. fagacearum |
| 7 | RRO14 | EPPO standard | Yes | EPPO diagnostic protocol (EPPO, 2001) | No |
Not effective on the commodity as it suggests the use of small branches, which is a condition not applicable to logs for export. Furthermore, the standard does not include protocols hinging on molecular detection of the pest Uncertainties: No uncertainties |
| 8 | RRO15 | Transport | Yes | Wood logs are loaded into shipping containers in the US and do not leave the closed containers until receipt at the mills. (except for Spain). Additionally, sometimes containers are opened at the ports by the customs. The containers have a transit time of approximately 2 weeks from the port of shipment to the port of entry | No | Transport in closed containers seems to be an effective way of reducing the risk of the pest escaping during transportation (Robinet et al., 2016).Uncertainties:Transport in closed containers is not prescribed in the regulations and so it is not clear whether this always happens, in particular when phytosanitary inspection takes place at the ports |
| 9 | RRO16 | Storage at mills | Yes |
Logs unloaded at the mills are generally processed shortly after opening the container or compliantly stored under wet conditions (information from the NPPOs). When logs with bark are fumigated, wet storage (i.e. the storage of wood under a sprinkler system or a pond) is required from the moment oak trees in the surrounding start flushing, although MSs may exempt fumigated logs from wet storage. Non‐fumigated white oak with bark needs continuous wet storage. According to the Dossier survey to EU mills, most mills (six out of eight) store oak logs under a watering (sprinkler) system and two out of eight reported to store logs in ponds.Wet storage is applied to preserve the quality of the wood and prevent drying out of the wood, and discoloration of the wood (Simpson, 2001). Two NPPOs reported that they take advantage of the fact that sawmills are the locations where the phytosanitary inspections take place as this allows frequent inspection of the wet storage conditions The US systems approach proposes continuous wet storage, independently of the period of the year. This is a deviation from the current practice |
Yes |
Wet storage is considered effective because it is expected to reduce the risk of fungal mat development and uptake of inoculum of the pathogen by potential native insect vectors. It is not excluded that if the wet storage of logs under a sprinkler system fails or is not correctly applied, the likelihood of establishment of the pest may even increase as a result of fungal mat development and uptake of inoculum of the pathogen by potential native insect vectors Wet storage is needed to preserve the quality of the wood which increases the likelihood that this RRO is effectively put in place. Furthermore, it is an advantage that in many countries that site of inspection is at the mills, which makes easy inspection compliance with other RROs.Uncertainties:The effectiveness of wet storage in reducing the spread of the fungus. Furthermore, the end time of wet storage is not specified (i.e. wet storage is obligatory starting the latest when nearby oak stand start flushing).It is uncertain whether wet storage could enhance the likelihood of spread of insects imported with oak logs from US |
| 9 | RRO17 | Inspection of nearby stands on the presence of B. fagacearum | Yes | Regular inspection of nearby stands needs to take place at appropriate intervals | No |
Seven out of eight mills surveyed by USDA APHIS reported that the nearest oak trees are more than 1 km away Uncertainties: Interval of inspection is not prescribed, nor the survey method. Inspectors may not be experienced to detect oak wilt because of its absence in the EU The cryptic asymptomatic period of the disease may also hamper a prompt detection of the pest |
| 10 | RRO18 | Cooking logs in hot water | Before the logs are processed to veneer the wood is cooked in a hot water vat at temperature ranging from 75 to 90°C for 18 to 65 h. Cooking may be conducted for logs either with or without bark (Roberto Zanuttini, personal communication) | No |
Cooking logs in hot water is expected to kill the fungus (Jones, 1973). Therefore, this measure is effective in reducing the risk of spread of the fungus from the mill through processed wood. The fungus has been reported as viable in sawn lumber up to 24 weeks after sawing (Tainter et al., 1984) This RRO can be inappropriate for products other than veneers (e.g. barrels). Therefore, the absence of this step can result in a higher risk of establishment of the pest in the EU Uncertainties: No uncertainties |
|
| 11 | RRO19 | Destruction of residues | Yes |
After the logs have been processed in the mills, the residues are destroyed. Seven out of eight mills in the USDA survey reported that residues are burnt. This is confirmed by two NPPOs who reported that the residues are burnt (for energy production) Destruction of residues is not required in the case of fumigated logs (Art 2.2 of Commission Decision 2005/359/EC) |
Yes |
Destruction of residues is an effective way to reduce the risk that the pathogen disperses through residues Destruction of residues is not needed if the entire logs with bark underwent cooking in hot water (RRO 18) When it applies to non‐fumigated wood, destruction of residues is to take place at the production facility. Otherwise, it is not regulatedUncertainties:Even when it is commonly understood that destruction means ‘burning’ this is not clearly stated in the legislation and the risk of misinterpretations cannot be excluded |
Both tables indicate for each RRO whether it is regulated or not: this was considered by the Panel a relevant detail to identify aspects that, if regulated, could increase the level of protection for such a commodity.
To estimate the pest freedom of the commodity up to the point of entry in the EU, an EKE was performed following EFSA Guidance (Annex B.8 of EFSA Scientific Committee, 2018). The commodity exported to the EU is oak logs with bark, charged in shipping containers where they are submitted to fumigation and from which they are unloaded only when delivered at the final destination, i.e. the sawmill. For this reason, the selected unit is the container, where the conditions within can be considered uniform and can differ from another container even when treated in the same way. The whole container is considered infested when at least one of the transported logs is infested. Therefore, the specific question for the EKE was: ‘Taking into account: (i) the RROs proposed in the exporting country and (ii) other relevant information, how many of 10,000 containers of oak logs with bark will be infested with B. fagacearum when arriving in the EU?’. The EKE question was the same for the three scenarios for which the pest freedom of the commodity was estimated (Section 2.2).
The uncertainties associated with each EKE were taken into account and quantified in the probability distribution applying the semi‐formal method described in Section 3.5.2 of the EFSA Guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018b). Finally, the results were reported in terms of the likelihood of pest freedom. The lower 5% percentile of the uncertainty distribution reflects the opinion that pest freedom is with 95% certainty above this limit.
3. The pest
3.1. Biology of Bretziella fagacearum
Bretziella fagacearum (syn. Ceratocystis fagacearum) is the causal agent of oak wilt. The pathogen causes a vascular wilt by colonising the sapwood of the trees, which may result in a brownish discoloration of the xylem, visible in cross sections (branches, stem) of wilted trees (EFSA, online). The fungus develops mycelial mats under the bark of recently killed trees on which spores (i.e. conidia first and ascospores later) are produced. As the fungal mats grow, the bark is pushed away and cracks open. The fruit‐like odour emitted by the mats attracts nitidulid beetles, which may subsequently carry fungal spores of the pathogen to other oak trees (Harrington, 2013). Fresh, xylem‐penetrating wounds, leaking sap, generally less than 72 hours old, are required for successful infection mediated by nitidulid beetles (Kuntz and Drake, 1957). Wounds created by human activities (cut branch ends, fresh stump surfaces, stem wounds) or strong winds (broken branches and stems) may provide suitable infections courts, particularly in spring and early summer (Juzwik et al., 2011).
Sexual ascospores can stick to the exoskeleton of insects and are more effectively dispersed than conidia. B. fagacearum is heterothallic and can only reproduce sexually upon mating of two strains with opposite mating type. This may occur when nitidulid beetles are visiting different mats and cross‐fertilise the fungus (Harrington, 2013).
Sporulating mats are only produced when the bark/wood interface is moist (Gibbs and French, 1980). There is a fairly narrow range of sapwood moisture content (37–45% in spring, 44–52% in autumn) that allows for fungal mat formation (EFSA, online). Mats are produced in a temperature range of 8–25°C with faster and larger development in warmer conditions (EFSA, online). B. fagacearum is poorly competitive as a saprophyte and is rapidly replaced by many other organisms within 1 year after the death of the tree (EFSA, online). Sporulating mats are important for the spread of the pathogen by insect vectors. Mats may also form on firewood and logs, on which the disease can be transported to new areas (Juzwik et al., 2011). Spread of oak wilt within a forest stand mostly occurs through root grafts between trees of the same oak species (Bruhn et al., 1991; Appel, 1995a). Root grafting may also occur between trees of different oak species, but the importance of this type of grafting for disease transmission is unknown (Juzwik, 2009). Root graft transmission results in distinct disease foci, which can be observed in forest stands as clusters of symptomatic and killed oak trees. B. fagacearum can infect many oak species, which exhibit different levels of susceptibility or resistance. Members of the red oak group (e.g. Q. falcata,Q. rubra,Q. shumardii,Q. velutina) are highly susceptible and can be killed within months after infection (Juzwik et al., 2011; Harrington, 2013). In contrast, members of the white oak section (e.g. Q. alba,Q. fusiformis,Q. macrocarpa and Q. virginiana) display moderate to high levels of resistance. In Q. alba, infections by B. fagacearum may result in dieback of a few branches, but the trees can survive for many years (Juzwik et al., 2011). This is because the trees can produce new annual rings of sapwood and compartmentalise the fungus. Thus, the vascular staining associated with the fungus is observed deeper in the sapwood (EFSA, online).
The oak wilt symptoms are not visible during the dormant stage (EFSA, online). Identification of oak wilt‐infected trees is more reliable for red oaks, where symptoms develop rapidly. In the white oak (Q. alba), identification of infected trees can be more difficult because of the slow development of disease symptoms. In this species, the fungus can remain undetected for many years (e.g. 20‐year infection observed in a Q. alba tree) (EFSA, online).
Fungal mats are usually absent or rare on white oaks (Engelhard, 1955; Cones, 1967). Mats develop almost exclusively on red oak, with a proportion of one‐third of infected trees actually producing mats that rupture the bark, based on a mat survey of standing dead trees (EFSA, online).
3.2. Prevalence and incidence of Bretziella fagacearum
B. fagacearum is not present in the EU. It is regulated as a quarantine pest in Annex II/A of Commission Implementing Regulation (EU) 2019/2072 [CERAFA]. The pathogen is listed in the European and Mediterranean Plant Protection Organization (EPPO) A1 list (EPPO, online).
Oak wilt caused by B. fagacearum has only been reported from the US. The disease is present in 26 states in southern, central and eastern USA, ranging from Texas to New York State. Distribution data are available by county. The most recent distribution map is from June 2020, with the new occurrences from 2017 and 2018 (Figure 1). Counties where oak wilt is no longer active after 5 years’ monitoring without new findings are removed from the list (EFSA, online). The number of oak wilt‐free counties varies by state and goes from one or two counties (e.g. Virginia) to the large majority (e.g. New York State). There are no national official surveillance protocols, but state natural resource agencies are in charge of detecting and monitoring oak wilt and reporting the data to the USDA Forest Service FHAAST (Forest Health Assessment & Applied Sciences Team). In federal land, the USDA forest service itself, more precisely the Forest Health Protection staff, is responsible for official surveillance. Surveillance activity is particularly intensive along the edges of the known disease range. Examples of tools used for surveillance include fixed‐wing aircrafts, helicopters, UAV (unmanned aerial vehicles), followed up with ‘ground‐truthing’ and ground surveys (EFSA, online). Diagnostic laboratories perform both the molecular and the standard isolation test (provided by Yang and Juzwik, 2017) on samples, particularly those taken outside the known oak wilt range (EFSA, online).
Figure 1.
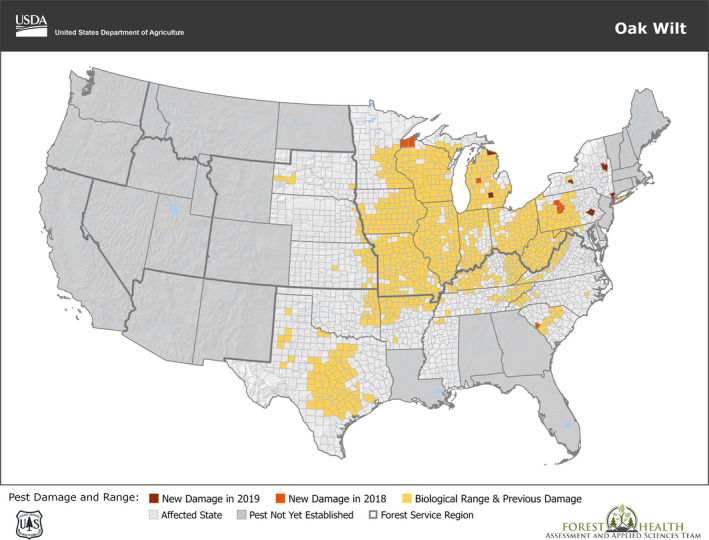
Current distribution of Bretziella fagacearum in the US. Map updated to 2019, provided by USDA
There are few data available on disease prevalence and incidence in individual forest stands. In the 110,000 ha large Anoka County (Minnesota) with an estimated 5.92 million oaks, 885 actively expanding disease centres occupying 547 ha were reported in 2010 (Juzwik et al., 2011). According to Harrington (2013), oak wilt is scattered across much of the eastern US but is still ‘filling in’ within that range and is also expanding at the edges. In West Virginia, the disease is present in 52 of 55 counties, but it is sporadic, and losses are generally not heavy (Juzwik, 2009). Heavy loss of timber value due to oak wilt has been reported in the Upper Midwest (Haugen et al., 2009), where the disease can be severe, particularly on sandy soils. In Missouri in the south, oak wilt is widespread, but the disease centres are relatively small and losses not great (Juzwik, 2009). Texas has been heavily affected by oak wilt since probably the 1930s, with thousands of aesthetically significant oak trees killed each year (Appel, 1995b) (note that Texas is not an exporter of oak logs to the EU).
3.2.1. Possibility of spread within a forest stand
Spread of oak wilt within forest stands mainly occurs through root grafts, which results in distinct disease foci (Juzwik et al., 2011). Local spread by vectors is also possible, but it is considered less relevant than transmission through root grafting (Harrington, 2013). For pathogen transmission, root grafts need to allow the movement of xylem content from one tree to another: this is more frequently observed in light textured soils (e.g. sands) than in heavier textured soils (e.g. silt loam) (Menges, 1978; Prey and Kuntz, 1995). In the Upper Midwest, several oak trees can share connected root systems, particularly on deep sandy soils. Expansion of disease foci on such soils has been estimated to reach from 7.6 m/year in Minnesota to 12 m/year in Michigan (Juzwik et al., 2011). No rates of disease foci expansion have been reported for the Appalachians. In this region, diverse hardwood stands with numerous species occur, which limit disease development because of infrequent occurrence of interspecific root grafts (Juzwik et al., 2011).
3.2.2. Possibility of spread to new forest stands
Spread of B. fagacearum to new forest stands can occur by insects carrying spores of the pathogen (Juzwik et al., 2011). Spread by nitidulid beetles is mostly limited to 1 km (Juzwik et al., 1985) and spread over longer distances is spotty (Menges and Loucks, 1984; Shelstad et al., 1991). Fresh wounds on oak trees, which attract the beetles, are needed for successful vector transmission.
Lastly, the pathogen may spread to new areas through the transport of contaminated firewood and logs (Harrington, 2013), logs being also the most likely pathway for spread to new continents (Chalkley, 2016). No evidence is available on the role of nursery stock or seed in spreading B. fagacearum (Juzwik et al., 2011; EFSA PLH Panel, 2018a).
3.3. Vectors and potential vectors of Bretziella fagacearum
3.3.1. North American vectors
The Dossier does not contain information about vector species associated with oak logs with bark in the US but are generically referred to as ‘beetles’.
However, from the literature, there is evidence for a group of nitidulid beetles (Coleoptera Nitidulidae, a group of beetles ubiquitous in forest habitats all over the world), including at least six species that are associated with Bretziella mats and could potentially act as vectors (Appel et al., 1990; Jagemann et al., 2018). The number of nitidulid beetle species provided during the hearing was 14 or 15 (EFSA, online). Different species of sap beetles are associated with young and mature mats but only two were shown to carry spores (Colopterus truncatus Randall and Carpophilus sayi Parsons). C. truncatus has an earlier emergence peak than C. sayi in the US. All sites with oak wilt centres in Wisconsin yielded beetles with viable fungal propagules, with the frequency of association ranging from 1% to 50%. Sites asymptomatic for oak wilt contained both beetle species, but no vector‐borne viable pathogen (Jagemann et al., 2018). In Texas, the level of contamination of nitidulid beetles caught in both deciduous and live oak outbreak areas varied between 0.3% and 2%. Trapping devices include funnel traps baited with fermenting dough and a commercial pheromone were developed (Jagemann et al., 2018) but not used in regular surveillance (EFSA, online).
No interception of Colopterus truncatus and Carpophilus sayi was found in EUROPHYT (online).
Other beetle species associated with the fungus in North America are the bark beetles Pseudopityophthorus minutissimus (Zimmermann) and P. pruinosus Eichhoff (Coleoptera Curculionidae Scolytinae) and the brentid beetle Arrhenodes minutus (Drury) (EFSA PLH Panel, 2018a). These three species are currently regulated in the Commission Implementing Regulation (EU) 2019/2072.
P. minutissimus and P. pruinosus are poorly known species of bark beetles in North America, with scattered distribution (P. minutissimus more northern, P. pruinosus more southern) and few (tens) collection reports (https://scan-bugs.org/portal/collections/list.php). They are small (less than 2 mm long) and mainly associated with weakened or dead branches but can also attack the stems. Adults have maturation feeding in twigs, leaf petioles and young acorn stems. They can be a vector of B. fagacearum. EFSA has recently published a pest categorisation (EFSA PLH Panel, 2019b) in which it is shown that they have a large potential to establish in the EU. No interception on these two species was found in EUROPHYT (online).
A. minutus is commonly known as the ‘oak timberworm’ in North America where it is a widespread and common species in oak forest, as documented by updated maps (https://bugguide.net/node/view/49667/data) and at least 597 collection reports (https://scan-bugs.org/portal/collections/list.php). It is associated with timber, also large diameters and it colonises trees through oviposition in fresh wounds. Larvae develop in 2–4 years. Adults are 7–25 mm long and feed on sap oozing from bark. EFSA has recently published a pest categorisation of A. minutus (EFSA PLH Panel, 2019c). It can be a vector of B. fagacearum and it has a large potential to establish in the EU. One interception of A. minutus on white oak timber is present in EUROPHYT (online).
3.3.2. Potential vectors in the EU
The association between an exotic pathogen and a native vector is always difficult to assess and predictions about the outcomes have large uncertainty.
The European bark beetle species Scolytus intricatus (Ratzeburg) (Coleoptera: Curculionidae) is briefly mentioned in the Dossier (pp. 6 and 23) as a potential vector of the fungus, being a target of the measures included in the proposed systems approach. This bark beetle is common all over the EU and it is characterised by a breeding phase in the phloem of the tree, just below the bark and a facultative maturation feeding in twigs. Because of the similarity with the species of the genus Scolytus associated with elm (Ulmus spp.), it has been considered a potential vector of the fungus. However, the probability that the beetles can become in contact with the fungal propagules is low as they do not get deep into the sapwood or frequent the mats (Doganlar and Schopf, 1984; Yates, 1984). The association between the elm bark beetles and the elm fungus (Ophiostoma novo‐ulmi) is very strict because the fungus propagules are formed in the breeding galleries of the beetles and maturation feeding on healthy trees may easily spread the fungus.
It has been questioned whether B. fagacearum could become established in an ecosystem without suitable nitidulid vectors (Harrington, 2013). In the EU, there are several species of nitidulid beetles that occur in oak forests. Some of them are the same as those occurring in North America, as Carpophilus hemipterus (L.), Glischrochilus fasciatus (Olivier) and G. quadrisignatus (Say), that were occasionally associated with B. fagacearum in the US (Juzwik et al., 2004). Species of the genus Cryptarcha Shukard are also associated with B. fagacearum in North America and the genus includes other species widespread in the EU (Pasqual et al., 2013).
Therefore, the possibility that nitidulid beetles present in the EU are associated with the mats of B. fagacearum if the fungus is introduced cannot be excluded, given the very similar traits as North American species.
3.3.3. Role of vectors and potential vectors in pathogen establishment in the EU
In the Dossier, only the European oak bark beetle S. intricatus is addressed, while there are other potential vectors that need to be considered. S. intricatus does not fully match the requirements to be a suitable vector as its life cycle marginally crosses that of B. fagacearum, being the beetle not fully associated with outer sapwood nor with fungal mats. The acquisition of viable propagules of B. fagacearum by S. intricatus would be even less likely for white oaks than for red oaks, because fungal mats are usually absent or rare on white oaks. In addition, the depth of the fungus in logs is expected to be greater in the former than in the latter (EFSA, online), and this might link to the probability of mat formation.
None of the vectors of the oak wilt pathogen in North America, either regulated or not regulated in the EU, have been reported to occur in the EU so far (EFSA PLH Panel, 2019b,c). However, there is at least one case of interception of a vector regulated in the EU (A. minutus) and there are other cases of interceptions of beetles in association with the import of non‐fumigated oak logs with bark from the US, in line with Art. 8 of the Commission Decision 2005/359/EC. Several consignments of oak logs with bark from US were intercepted because the consignments were not fumigated or not properly fumigated, suggesting that entry of the oak wilt pathogen and its insect vectors from US may happen. These beetles may leave the logs when opening or unloading the containers and can establish the fungus in potential hosts in the EU. In addition to bark beetles, also nitidulids, either native or introduced, are reported as vectors or potential vectors of B. fagacearum in the EU. Should vectors enter within logs infected by the pathogen, this may lead to establishment of the disease. Establishment may also occur if EU potential vectors come into contact with fertile mats present on the imported logs.
4. The commodity
4.1. Description
The commodity to be imported is logs with bark belonging to white and red oak species (Quercus spp.).
As shown in Table 3, most oak wood exported from the US to the EU in the period 2017–2019 was lumber (1.35 million cubic board metres (CBM)). During this time period, 28,148 CBM of white and red oak logs with or without bark were exported to the EU. More white oak logs with or without bark (26,014.95 CBM) compared with red oak logs (2,133.05 CBM) were exported from the US to the EU during this time period.
Table 3.
White and red oak lumber and logs, with or without bark exported from the US to the EU from 2017 to 2019 (EFSA, online)
| Year | Cubic board metres | ||||
|---|---|---|---|---|---|
| Logs with and without bark | Lumber | ||||
| White oak | Red oak | Oak (species not supplied) | White oak | Red oak | |
| 2017 | 7,184.97 | 235.28 | 10,690.00 | 293,769.12 | 69,371.78 |
| 2018 | 6,880.58 | 918.56 | 223.97 | 546,870.92 | 26,909.37 |
| 2019 | 11,949.40 | 979.21 | 220.69 | 247,258.74 | 155,528.94 |
| Total: | 26,014.95 | 2,133.05 | 11,134.66 | 1,087,898.78 | 251,810.09 |
| Total: | Logs: 28,148 | Lumber: 1.35 million | |||
The large prevalence of imports of white oak, compared to red oak, is also supported by recent data reported in Hardwood Review eGlobal (Europe) (2020) (Table 4).
Table 4.
US hardwood log exports to EU (Hardwood Review eGlobal, Europe, August 2020)
| Species | Volume (m3) May 2020 | Volume (m3) January–May 2019 | Volume (m3) January–May 2020 | Change |
|---|---|---|---|---|
| White oak | 1,378 | 6,244 | 6,806 | +9.0% |
| Red oak | 23 | 531 | 498 | –6.2% |
The main use of the US exported oak logs with bark is veneer production (EFSA, online), although EU import data show that a small proportion is devoted to other uses (e.g. wine barrels), that can be submitted to different processing steps and eventually to differences in applicable RROs (e.g. ‘cooking’, step 10, RRO18, in Table 8).
The main EU importing countries are Austria, Germany, Portugal and Spain: from May 2019 to April 2020, these countries reported a total of at least 350 consignments.
Data on volumes of export on a monthly basis are not available (EFSA, online), although data on the number of mills (from those surveyed) receiving oak logs from US are higher from October to April and lower in the remaining months of the year (the Dossier).
The size of logs is variable: diameters can range from 35.5 to 91.5 cm, with a minimal length of 2.6 m (EFSA, online). This size meets the requirements for the production of veneers (Roberto Zanuttini, personal communication).
4.2. Production areas
Based on a survey reported in the Dossier, oak logs intended for export come from 10 US states: Missouri, Ohio, Pennsylvania, Tennessee, Indiana, Virginia, New York, Illinois, Wisconsin and North Carolina (the Dossier).
4.3. Production and handling processes
4.3.1. Source of logs for export and growing conditions
The oak logs for export come from natural mixed forests. Logs from plantations of hardwoods in the US are not used for oak log export (EFSA, online). The hardwood forests from which oak is harvested extend from New York to North Carolina; therefore, the tree species composition of these mixed oak stands varies with latitude: e.g. maple and basswood in the northern areas to oak‐hickory mixed stands in the southern areas (EFSA, online).
There is no standard density for oaks grown in mixed forests. Data expressed as number of stems per unit of surface are not readily available. Basal area is the more common metric used in the US. Estimated basal area per acre (equal to the sum of all basal areas for each oak tree) for stands with high‐quality oaks that yield Grade 1 logs range from 80 to 100 ft2 (7.4–9.3 m2) in the southern tier of states (e.g. Indiana) and from 90 to 110 ft2 (8.4–10.2 m2) in the northern tier (e.g. Pennsylvania) (EFSA, online).
Oak stands selected for harvest of high‐quality logs are on sites with heavier textured soils, in particular for northern red oak, Quercus rubra, the soils are loam to silt loam; for white oak, Quercus alba, the sites have well‐drained loam soils, e.g. silt loam or silty‐clay loam (EFSA, online).
4.3.2. Production cycle
Forests stands are naturally regenerated. Oak logs with bark for export to the EU come from private land and only about 2% of oak logs harvested in the US are suitable for export to EU mills to produce veneer (EFSA, online). Oak logs intended for export are harvested from private land, which is mainly certified according to official guidelines on the quality of forest management and production, but it cannot be excluded that oak logs also come from uncertified forests (EFSA, online).
Harvesting of the logs is achieved by selective cutting. Private landowners generally contract a certified forester, qualified at the national or state level for providing professional advice (the Dossier), to handle their timber sale.
Buyers, who include loggers, lumber or veneer mills, and log exporters, inspect the trees on the forest track before bidding (the Dossier).
The trees are marked for harvesting in the growing season (mostly in the summer and early autumn and less in the spring) and all checked systematically. The oak wilt symptoms are not visible during the dormant stage, underlying the importance of marking the trees during summer–early autumn. Industry practices do permit marking in late spring.
The assessment based on visual inspection of the crown and bole with the support of published information (diagnostic guides such as O'Brien et al., 2011) is the standard diagnostic protocol applied by certified foresters: trees showing more than 10% of dieback are not marked for harvesting, nor reported to phytosanitary services. A certified forester observing oak wilt symptoms typically reports it to the landowner (EFSA, online).
A trained forester can identify oak wilt with confidence in northern red oak, where symptoms development is very rapid, with the crown completely wilted within 6–8 weeks after infection, or with initial wilting in the summer and complete wilting the next year, immediately after the spring leafing starts. In the white oak, instead, oak wilt identification can be more difficult: in this species, the fungus can remain undetected for many years (e.g. 20‐year infection observed in a Q. alba tree). In addition, as previously reported, fungal mats are usually absent or rare on white oak, and this may hamper a prompt detection of the disease at stand level. The white oak shows symptoms of decline with gradual crown dieback of about 10% dieback each year. Certified foresters observing a white oak with at least 10% of crown dieback do not select it, although this observation is not always easy.
If one oak tree is found to be symptomatic by the certified forester selecting the trees in a stand for harvest of high‐quality oak logs, the forester would: (1) not select that tree for selective harvest, and (2) notify the land manager or landowner of the finding. The decision of when and how to manage the diseased tree is made by the land manager or the landowner with input from a forest health specialist. When the forest health specialist is involved, samples are taken to confirm the presence of B. fagacearum (EFSA, online). The forest health specialists provide advice to the landowners under request, but it is not clear whether they routinely check the situation of diseased trees and have any possibility to verify whether symptomatic trees are marked (EFSA, online).
Non‐infected oak trees can be reliably harvested from stands where oak wilt has been detected. This harvesting depends on the size of the stand in relation to the location, extent and species of oak tree(s) infected with oak wilt (EFSA, online). However, asymptomatic yet infected trees could go undetected and hence could be harvested.
An array of informational and decision‐making tools to design stand or site‐level treatment prescriptions are available for use by land managers (e.g. see EFSA, online). There are a series of operations regulated and mandatory, particularly if the operations are part of a larger forest certification programme or landowners or land managers are receiving state and/or federal assistance funds to treat oak wilt on their lands. For example, the private landowners enrolled in the managed forest land programme administered by the Wisconsin Department of Natural Resources (DNR) are required to treat oak wilt according to the Wisconsin Council on Forestry and Wisconsin DNR Oak‐Harvesting Guidelines (2020). For public and private sector lands where ‘cost‐share’ oak wilt suppression funds are provided by the US Forest Service, adherence to the programme participant guidelines mentioned previously is mandatory and includes required data reporting to the agency (EFSA, online).
Seasonality of harvesting is considered as a means for reducing the risk of spread of oak wilt in the US. Harvesting between late spring and early summer (from June to mid‐July, depending upon latitude) is not recommended due to the potential: (a) to create unintended xylem‐penetrating wounds on residual trees while harvesting target trees: these wounds would attract nitidulid beetles, potentially vectoring B. fagacearum; and (b) to move logs from oak wilt‐affected trees, which have produced sporulation mats, to non‐affected areas (EFSA, online). In case of an outbreak, all the trees behind the root graft barrier line can be cut and used for firewood or brought to a local mill for domestic use, if they are properly handled to not spread the fungal mats (EFSA, online).
4.3.3. Export procedure
Logs are sorted by species and graded at the harvest site landing. Buyers, including US log exporters and end users from the EU and their delegated agents inspect logs at the landing site, and tag logs they have purchased. A unique identification (ID) tag number is placed on each purchased oak log and is used to track the log by species, grade and dimensions in the purchase tally (the Dossier).
Oak logs are transported to an export log yard (the Dossier). There is no specific distance between log yards and forests and no rules for time, although logs have to be shipped as soon as possible (1–2 weeks) to limit their degradation (which is continuous and faster during summer) (EFSA, online). The process of collection and transport of logs from the forest can be subjected to some delay in the case of rain. Because oak logs lose quality and value every day they remain at the harvest site landing (sun, water and heat increase the rate of degradation even in winter months), the supply chain is incentivised to quickly move oak logs into containers for transit to wood processors, including EU mills. Oak logs may also be transported to a transload facility for loading into shipping containers (the Dossier). Special equipment is required for loading oak logs into shipping containers that is not available at the harvest site landing. At export log yards and transload facilities, logs receive additional inspections. Each log is inspected by a quality control employee for species, grade and dimensions, matching this information with the ID tag number and the purchase tally prepared at the landing to confirm the correct log is received. The circumference of each log is visually divided into four equal sized quadrants called ‘sides’ (EFSA, online). Each side is visually inspected along the entire length of the log and at each end of the log for defects. The ends of the logs are trimmed one day before the inspection to select for export to EU mills. If a side has no visible defect, it is rated as ‘clear.’ Logs must have three or four sides rated as clear with no defects, C3S (clear three sides) or C4S (clear four sides), respectively. Logs are also inspected by buyers (the Dossier). At export log yards, a packing list of logs sold to the buyer is prepared with the tag numbers listed. At export log yards and transload facilities, logs are inspected by USDA authorised inspectors within 14 days before loading into shipping containers to issue the required phytosanitary certificate (the Dossier).
Oak logs with bark requiring fumigation are then transported to the port to be fumigated in shipping containers before export. Details on the fumigation process are provided below in Section 5.2. Shipment to the EU takes about 2 weeks (the Dossier).
4.4. Overview of interceptions
The EU MSs that have reported to the European Commission on the import of US oak logs with bark, as required by Commission Decision 2005/359/EC (Art. 10), between 2016 and 2020 are Austria, Germany, Portugal and Spain.
As correctly mentioned in the Dossier, consignments of oak logs with bark were never rejected due to finding B. fagacearum, but other reasons for interception are still relevant to the assessment of the risk.
Table 5.
List of interceptions found in EUROPHYT (online) on oak logs with bark from the US on only non‐compliance with special requirements (fumigation) and/or presence of harmful organisms (the correctness of this table has been verified by NPPOs)
| Year | Oak species | Shipment starting after 15 October and finishing before 30 April | Interception country | Destination country | Reason for interception |
|---|---|---|---|---|---|
| 2005 | Q. alba | France | France | One adult beetle identified as Arrhenodes sp. and later attributed to Arrhenodes minutus as it is the only species of the genus present in North America (EFSA PLH Panel, 2019c) | |
| 2000 | Q. alba | Germany | Germany | Not submitted to fumigation treatment | |
| 2009 | Q. rubra | Germany | Germany | Not submitted to fumigation treatment | |
| 2010 | Q. alba | Germany | Germany | Living ants in the wood | |
| 2012 | Q. alba | Germany | Czechia | Non‐compliance with treatment specifications | |
| 2017 | Q. alba | X | Germany | Germany | Presence of Melittomma sericeum |
| 2015 | Q. rubra | Spain | Spain | Non‐compliance with technical arrangements | |
| 2015 | Q. alba | Spain | Spain |
Not submitted to fumigation treatment Signs of generalised infection Presence of Bostrichidae beetles |
|
| 2016 | Q. alba | Spain | Spain | Inadequate fumigation | |
| 2017 | Q. alba | Spain | Spain | Inadequate fumigation | |
| 2018 | Q. alba | X | Portugal | Portugal | Not submitted to fumigation treatment |
On a few occasions, the logs were not or non‐adequately, fumigated. In other cases, the presence of arthropods was observed on the commodity, proving that, also in those cases, the fumigation treatment had failed, unless not applied due to the winter period (i.e. interception of M. sericeum in Germany).
5. The systems approach
5.1. Description of the systems approach (ISPM 14)
The management of pests of round wood can require the application of a series of phytosanitary measures that can be applied in a systems approach (FAO, 2017a).
A systems approach is described in ISPM 14 (FAO, 2019) as a combination of at least two phytosanitary measures that are applied independently of each other to meet phytosanitary import inspections. These measures may include actions taken in the preharvest stage and post‐harvest stage. A combination of less severe measures could attain a similar level of risk compared to a single (more restrictive) measure, such as import ban.
5.2. The USDA APHIS systems approach
The systems approach proposed by the USDA APHIS in the Dossier (Figure 2) is very similar to the current import option 1 (fumigated white and red oaks with bark), but it differs in the following phytosanitary treatments:
Instead of fumigation with MB, SF is used.
Containers are opened only at the mill. Note that this is different from the current practice in Spain where phytosanitary checks are made at the port of entry, but this corresponds to the current practice in other countries (Germany, Austria and Portugal) (NPPOs replies to EFSA questionnaire).
Logs are put in continuous wet storage. The current import option prescribes wet storage starting at the time of flushing of oak trees in the surrounding of the mill at latest.
Figure 2.
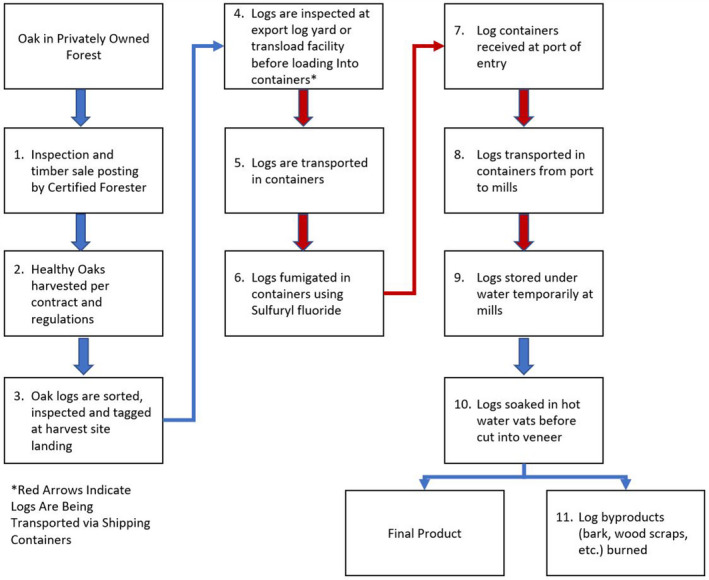
Flow chart of the proposed systems approach using sulfuryl fluoride to mitigate the introduction of oak wilt fungus (Bretziella fagacearum) in oak logs with bark (Quercus spp.) exported from the US to the EU (from the Dossier by USDA)
The first measure takes place in the US and the latter two in the EU. Of the three changes suggested in the systems approach (compared with the current derogation 2005/359/EC), the most important is the difference in the fumigant. Therefore, the comparison between the SF and MB fumigation treatment was handled after a systematic literature search, data extraction (Appendix A) and EKE (Appendix B).
5.2.1. Fumigation with sulfuryl fluoride
The proposed SF fumigation schedule for control of oak wilt in oak logs is carried out under the following conditions: oaks logs are placed in containers. In the container, a fumigation hose is placed along with a fan to ensure air circulation. SF is brought in the containers at a dosage of 0.24 kg/m3. SF is added to raise the concentration to 280 g/m3 at regular intervals, i.e. at 0.5, 2, 24, 48 and 72 h. Between these time points, the concentration drops leading to an average concentration of 240 kg/m3 for the first 72 h levelling off to 200 g/m3 at 96 h. Temperature is kept at 15.6°C. The accumulated dosage is at least 22,500 g‐h/m3.
6. Evaluation of risk reduction options proposed in a systems approach
In the current situation, the import of oak logs with bark from US involves actions taken at the country of origin and at destination. The derogation only regulates some aspects related to the trade of the commodity, while part of the actions taken in the US before shipment and part of the actions taken in the EU after arrival are common practice, but not explicitly regulated.
In this section, a description of the different actions, regulated and non‐regulated, is provided, assessing effectiveness and uncertainty specific to each RRO.
6.1. Quantitative assessment of the pest freedom of oak logs with bark from the US at the point of entry in the EU
6.1.1. Assessment of the proposed risk reduction options in the US
In Table 6, all the RROs proposed by USDA APHIS to be undertaken in US are summarised and an indication of their effectiveness on B. fagacearum is provided. The outcomes of this analysis, together with the information received from USDA (the Dossier and EFSA, online) and the additional evidence collected with a systematic literature search (Appendix A), have been used as a base to perform the EKE (Appendix B) whose results are provided in Section 6.1.2.
6.1.2. Outcome of Expert Knowledge Elicitation
Table 7 and Figure 3 show the outcome of the EKE on pest freedom for B. fagacearum at the point of entry in the EU of containers loaded in US with logs with bark of Quercus spp. taking into account the RROs under the current system (regulated under Commission Decision 2005/359/EC) (i.e. 1, oak logs with bark fumigated with MB, and 2, white oak logs with bark non‐fumigated under the conditions listed in Art. 8 of the same derogation), and under the alternative system proposed by USDA APHIS for oak logs with bark fumigated with SF.
Table 7.
Conclusion on the likelihood of pest freedom after evaluation of the risk reduction options for oak logs with bark coming from US and designated for export to the EU under the current system (regulated under Commission Decision 2005/359/EC) (i.e. oak logs with bark fumigated with methyl bromide or white oak logs with bark non‐fumigated under the conditions listed in Art. 8 of the same derogation) and under the alternative system proposed by USDA APHIS for oak logs with bark fumigated with sulfuryl fluoride. The median value is indicated by ‘M’ and the range is indicated from ‘L’ to ‘U’. For more information on pest freedom categories, see the legends under the table
| Number | Systemsapproach | Sometimespest} free | More} {often thannot} pest free | Frequentlypest} {free | Very} {frequentlypest} {free | Extremelyfrequentlypest free | Pest} free withsome} {exceptionalcases | Pest} free with fewexceptional} cases | Almost alwayspest free |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Fumigation with MB | L; M | U | ||||||
| 2 | Fumigation with SF | L | M | U | |||||
| 3 | Without fumigation (only white oak) | L | M | U |
| Pest freedom category | Pest free containers out of 10,000 | Legend of pest freedom categories | ||
|---|---|---|---|---|
| Sometimes pest free | ≤ 5,000 | L | Pest freedom category includes the elicited lower bound of the 90% uncertainty range | |
| More often than not pest free | 5,000 to ≤ 9,000 | M | Pest freedom category includes the elicited median | |
| Frequently pest free | 9,000 to ≤ 9,500 | U | Pest freedom category includes the elicited upper bound of the 90% uncertainty range | |
| Very frequently pest free | 9,500 to ≤ 9,900 | |||
| Extremely frequently pest free | 9,900 to ≤ 9,950 | |||
| Pest free with some exceptional cases | 9,950 to ≤ 9,990 | |||
| Pest free with few exceptional cases | 9,990 to ≤ 9,995 | |||
| Almost always pest free | 9,995 to ≤ 10,000 | |||
Figure 3.
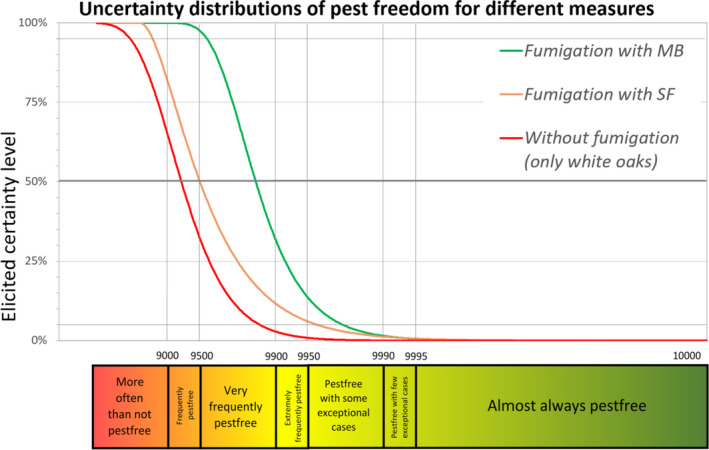
Comparison of the likelihood of pest freedom after the evaluation of the risk reduction options for logs with bark of Quercus spp. designated for export to the EU from the US for Bretziella fagacearum visualised as descending distribution function
- Likelihood of pest freedom under the current system (regulated under Commission Decision 2005/359/EC) (i.e. oak logs with bark fumigated with methyl bromide or white oak logs with bark non‐fumigated under the conditions listed in Art. 8 of the same derogation) and under the alternative system proposed by USDA APHIS for oak logs with bark fumigated with sulfuryl fluoride are compared.
The difference between the scenario with MB and that proposed in the Dossier with SF is essentially the type of fumigant. Therefore, the Panel performed a literature review (Appendix A) to identify potential comparative studies where both fumigants were used, with conditions similar to those applied to the commodity, and specific to the effectiveness of SF against B. fagacearum. A very limited number of studies met these criteria. The evidence summarised in the extraction table (Appendix A) together with a series of notions obtained while reviewing the collected literature, was used to support the EKE. The main observations on the use and efficacy of SF can be summarised as follows:
The first studies on the use SF as fumigant refer to its efficacy for the control of structural and commodity insect pests (Kenaga, 1957; Stewart, 1957), due to its toxicity to insects, stability at a wide temperature range, rapid penetration into substrates, non‐reactivity to many different materials (Armstrong et al., 2014).
The commercial name of SF is Vikane (the trade name ‘ProFume’ is also used for PT18 under Directive 98/8/EC and for applications under Directive 91/414/EEC;), a structural fumigant targeted to drywood termites.
In the EU, SF is an approved active substance, listed in the EU Pesticides Database,7 whose ‘use supported by available data is against insect pests in stored product, such as emptied cereal mills or empty grain storage areas’ (Appendix II of the European Commission review report, 2016).
Penetration is higher for SF than MB in dry wood but lower in hydrated wood, most likely due to the greater water solubility of MB (Scheffrahn and Thoms, 1993; Ren et al., 2011; Bonifácio et al., 2014). The commodity under assessment is freshly cut wood which has higher moisture content than dried wood.
The efficacy of SF is greater on insects than on B. fagacearum, as evidenced by the lower doses needed to kill insects (e.g. Barak et al., 2006, 2010) compared to the doses needed to kill the pathogen (e.g. Juzwik et al., 2017; Uzunovic et al., 2017; Yang et al., 2019). As mentioned in the Dossier: the doses proposed in the systems approach should be sufficient to eliminate all life stages of timber‐infesting insects in the fumigated oak logs, including potential vectors of the oak wilt fungus. However, uncertainty should be raised in case of wood boring insect species, given the absence of studies on infested commercial sized logs, where the penetration of the fumigant could not be able to reach the deepest layers of the log within the exposure time and therefore the insect.
In respect to insects, concerns exist on the efficacy of SF on some life stages of insects, in particular on the observed high tolerance of eggs (Mizobuchi et al., 1996; Soma et al., 1996; Zhang, 2006), to the point at which SF fumigation of US logs was rejected by China in 2013 (Armstrong et al., 2014).
In terms of number of sapwood locations positive to the pathogen after treatments, the efficacy of SF against B. fagacearum is lower than that of MB (Yang et al., 2019). SF was also less efficient than ethanedinitrile (EDN) against other tree pathogens (Seabright et al., 2020).
Further details on the EKE are provided in Appendix B.
Figure 4 provides an explanation of the descending distribution function describing the likelihood of pest freedom after the evaluation of the proposed RROs under the alternative system for oak logs with bark fumigated with SF.
Figure 4.
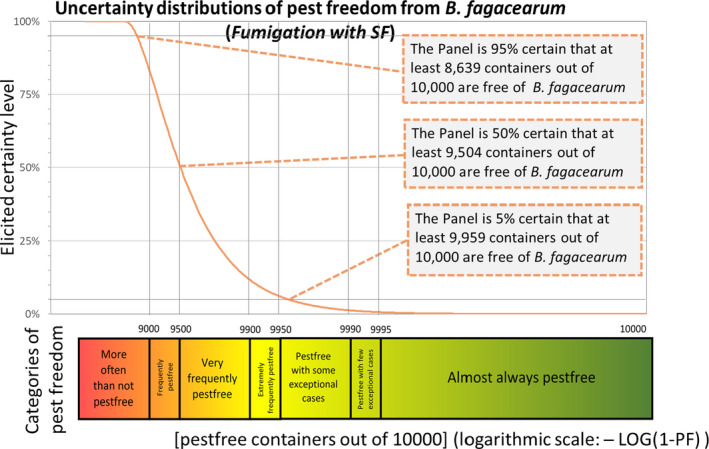
Explanation of the descending distribution function describing the likelihood of pest freedom for B. fagacearum at the entry point in the EU of containers loaded in US with logs with bark of Quercus spp. after evaluation of the proposed risk reduction options
- The example in the figure above represents the current derogation of imported oak logs with bark treated with sulfuryl fluoride.
6.2. Non‐quantitative assessment of the proposed risk reduction options in the EU
In Table 8, all the RROs proposed by USDA APHIS to be undertaken in the EU are summarised and an indication of their effectiveness on B. fagacearum is provided. Additional information and clarifications were received from NPPOs, contacted via email.
7. Conclusions
The risk associated with the importation into the EU of oak logs with bark from US in relation to the presence of B. fagacearum under the systems approach proposed by USDA APHIS with logs fumigated with SF was assessed and compared with the current system (regulated under Commission Decision 2005/359/EC), which includes either fumigation of logs with MB or, for white oaks exported in certain periods of the year and to certain regions of the EU, non‐fumigation. The risk was assessed in a quantitative manner, using expert judgement, until the point of entry into the EU whereas a non‐quantitative assessment was conducted for all the subsequent steps until the mills in the EU.
The quantitative assessment until the point of entry in the EU and the supporting evidence suggest that none of the systems is fully effective in preventing the introduction of B. fagacearum:
The likelihood of pest freedom of containers at the point of entry in the EU under the systems approach proposed by USDA APHIS with logs fumigated with SF was estimated as ‘very frequently pest free’ with the 90% uncertainty range spanning from ‘more often than not pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 8,639 and 10,000 containers per 10,000 would be free from B. fagacearum.
The likelihood of pest freedom at the point of entry in the EU under the current system with fumigation of logs with MB was estimated as ‘very frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,573 and 10,000 containers per 10,000 would be free from B. fagacearum.
The likelihood of pest freedom at the point of entry in the EU under the current system without fumigation of white oak logs was estimated as ‘frequently pest free’ with the 90% uncertainty range spanning from ‘more often than not pest free’ to ‘very frequently pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 7,803 and 10,000 containers per 10,000 would be free from B. fagacearum.
The results of the quantitative assessment until the point of entry in the EU indicate that the most effective import option was the current import option using MB, followed by the systems approach proposed by USDA APHIS with oak logs fumigated with SF. The least effective import option was the systems approach based on delivering white oak logs in certain periods of the year to certain regions of the EU without fumigation.
The risk of entry of B. fagacearum into the EU does not necessarily correspond to the likelihood of establishment of the pest in the EU, due to several reasons:
After entry, a series of RROs is regulated in the EU under current derogation and contributes to reduce the risk of transfer of the pathogen to a suitable host. This holds for all the import options assessed. Notably, the import option on white oak without fumigation, compared with the two other import options assessed in the opinion, is subject to more stringent measures in the EU (listed under Art. 8 of Commission Decision 2005/359/EC), which will make the risk associated with this import option closer to import options with fumigation.
B. fagacearum needs suitable environmental conditions and the presence of suitable hosts for establishment to occur in the EU.
Efficient insect vectors, either native to the EU territory or imported from North America, are also essential for establishment. Several consignments of oak logs with bark from US were intercepted because either infested by insects or not properly fumigated suggesting that an entry of infested logs with insect vectors from US may happen.
In conclusion, the weakest point in the systems approach proposed in the Dossier up to the point of entry in the EU is the performance of SF on oak logs with bark compared to MB.
Other critical aspects common to the three system approaches assessed in the opinion have been identified:
Most of RROs conducted in the US and that could reduce the risk of importing infested logs to EU are performed on a voluntary basis or they are regulated only in some states and in some circumstances; in particular: certification of forest stands, periods of marking and cutting of trees, communication of the presence of oak wilt symptoms to the phytosanitary services, removal of diseased trees.
Current detection procedures hinging on isolations of the fungus from small branches (EPPO, 2001) are not applicable for inspections of logs before export and import.
EU inspections of US oak logs do not require sampling and laboratory testing in the absence of symptoms.
The transport in closed containers from US to the mill is not compulsory.
Cooking of logs before processing does not apply to products other than veneer.
The way residues are destroyed is not specified in Commission Decision 2005/359/EC.
An increase in the likelihood of pest freedom for B. fagacearum of oak logs with bark from US is expected, should the indicated measures in the US be regulated in all states. RROs proposed to be undertaken in the EU are expected to further reduce the risk of establishment of B. fagacearum, should these RROs be regulated, correctly implemented and checked by the NPPOs.
8. Recommendations
Oak logs with bark are a commodity that currently involves a limited number of MSs and 28 thousand CBM, which represents a small proportion of the total volume of oak wood traded from the US (2% of the total volume imported during the last 3 years). B. fagacearum has been reported as viable in sawn lumber up to 24 weeks after sawing (Tainter et al., 1984). Given these reasons, the assessment of the potential risk of entry of B. fagacearum with oak lumber from US is worth being investigated.
Given the results of the quantitative assessment, the import of non‐fumigated white oak logs with the restrictions listed under Art 8. seems to pose a high risk. That conclusion would require further quantitative assessment on the risk of establishment and efficacy of RROs in the EU. This pathway also raises concerns about potential introduction of other pests associated with this commodity.
This opinion allowed the identification of a series of actions, in the US and in the EU that are effective in reducing the level of risk but are currently not regulated (Section 6.1.1 Table 6, Section 6.2 Table 8). A pathway analysis may help to identify the most effective ones, provided a thorough data collection from MSs and private sector (EU sawmills) is implemented. For instance, the transport of logs in closed shipping containers up to the arrival at the mill is crucial in limiting the spread of any pest from the commodity and is currently commonly applied but not regulated. Alternatively, containers could be opened for inspections in the port entry far away from suitable hosts.
Conversely, critical steps could involve actions already regulated and their identification would better target disease control. For instance, if the wet storage of logs under a sprinkler system fails or is not correctly applied, the likelihood of establishment of the pest may even increase as a result of fungal mat development and uptake of inoculum of the pathogen by potential native insect vectors.
Therefore, should the derogation be renewed by incorporating the proposals of USDA APHIS, the Panel would like to stress:
the importance of developing technical solutions for a fumigation reaction test for SF;
a study on the effectiveness of SF against B. fagacearum under commercial conditions (e.g. logs with bark up to 90 cm diameter) to reduce the uncertainty on its efficacy;
the need for standardised inspection, sampling and detection procedures in line with ISPM 31 (FAO, 2016), including LAMP assays or other molecular tools already available (Yang and Juzwik, 2017), to be used during inspections for export and import even in the absence of symptoms;
the opportunity to revisit whether some of the measures listed in Art. 8 of the Derogation (i.e. seasonality and latitude as from conditions (c) and (e)) are still relevant under the current climate change scenario and the fact that other potential vectors in the EU can play a role in the establishment of B. fagacearum (e.g. nitidulid beetles).
Finally, the literature review (Appendix A) and the hearing with USDA APHIS (EFSA, online) allowed the identification of alternative treatments (e.g. vacuum steam treatment and EDN), which could represent promising alternatives able to further reduce the level of risk related to the import of oak logs with bark even compared with MB. However, technical hurdles need to be taken before these alternative treatments can be implemented (EFSA, online).
9. Documentation as provided to EFSA
A systems approach to mitigate the introduction of oak wilt fungus (Bretziella fagacearum) in oak logs (Quercus species) with Bark Exported from the United States to the EU. Dossier by USDA APHIS submitted to EFSA by DG SANTE on July 2020.
Abbreviations
- APS
American Phytopathological Society
- CBM
Cubic board metres CBM
- EDN
Ethanedinitrile
- EKE
Expert knowledge elicitation
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organisation
- FHAAST
Forest Health Assessment & Applied Sciences Team of USDA
- ISPM
International Standards for Phytosanitary Measures
- MB
Methyl bromide
- MS
Member State
- NPPO
National Plant Protection Organisation
- PCR
Polymerase chain reaction
- PLH
Plant Health
- RRO
Risk Reduction Option
- SF
Sulfuryl fluoride
- USDA APHIS
Animal and Plant Health Inspection Service of United States Department of Agriculture
- VS
Vacuum steam treatment
Appendix A – Literature review on Fumigants
1.
The literature search was performed in Web of Science on all the databases using the following string:
TOPIC: (((Bretziella OR Ceratocystis OR Endoconidiophora) AND fagacearum) OR (oak wilt) OR ((Chalara OR Thielaviopsis) AND quercina)) AND TOPIC:
AND
TOPIC: ((methyl bromide) OR (sulfuryl fluoride))
No language, date or document type restrictions were applied to the search strategy.
Results after the last run of the search string (25 October 2020): 38 documents published between 1955 and 2020. Titles and abstracts were screened, and 26 papers were considered relevant for inclusion in the table below.
Further references cited in the screened articles or belonging to the grey literature were included in a second phase.
The evidence table, which includes 46 papers, summarises all the main evidences extracted from these references and assesses their specific relevance in support to this opinion.
References
Armstrong JW, Brash D and Waddell BC, 2014. Comprehensive literature review of fumigants and disinfestation strategies, methods and techniques pertinent to potential use as quarantine treatments for New Zealand export logs. Plant & Food Research SPTS No. 10678, Plant & Food Research, Palmerston North, New Zealand.
Barak AV, Wang Y, Xu L, Rong Z, Hang X and Zhan G, 2005. Methyl bromide as a quarantine treatment for Anoplophora glabripennis (Coleoptera: Cerambycidae) in regulated wood packing material. Journal of Economic Entomology, 98, 1911–1916.
Barak AV, Wang Y, Zhan G, Wu Y, Xu L, and Huang Q, 2006. Sulfuryl fluoride as a quarantine treatment for Anoplophora glabripennis (Coleoptera: Cerambycidae) in regulated wood packing material. Journal of Economic Entomology, 99, 1628–1635.
Barak AV, Messenger M, Neese P, Thoms E and Fraser I, 2010. Sulfuryl fluoride treatment as a quarantine treatment for emerald ash borer (Coleoptera: Buprestidae) in ash logs. Journal of Economic Entomology, 103, 603–611.
Bonifácio LF, Sousa E, Naves P, Inácio ML, Henriques J, Mota M et al., 2014. Efficacy of sulfuryl fluoride against the pinewood nematode, Bursaphelenchus xylophilus (Nematoda: Aphelenchidae), in Pinus pinaster boards. Pest Management Science 70, 6–13.
Cartwright JB, Edwards DW and McMullen MJ, 1953. Sterilization of timber with methyl bromide. Nature, 172, 552–553.
Dwinell LD, Thoms E and Prabhakaran S, 2003. Effect of sulfuryl fluoride on the pinewood nematode in pine wood. In: Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions, November 3–6, San Diego, California, pp. 1–4.
Hall M, Najar‐Rodriguez A, Adlam A, Hall A and Brash D, 2017. Sorption and desorption characteristics of methyl bromide during and after fumigation of pine (Pinus radiata D. Don) logs. Pest Management Science, 73, 874–879.
Kenaga EE, 1957. Some biological, chemical and physical properties of sulfuryl fluoride as an insecticidal fumigant. Journal of Economic Entomology, 50, 1–6.
Juzwik J, Yang A, Chen Z, White MS, Shugrue S and Mack R, 2019. Vacuum steam treatment eradicates viable Bretziella fagacearum from logs cut from wilted Quercus rubra. Plant Dis. 103, 276–283. https://doi.org/10.1094/pdis-07-18-1252-re
Juzwik J, Yang A, Myers S, Furtado M and Taylor A, 2017. Survival of Ceratocystis fagacearum following red oak log fumigation with sulfuryl fluoride Proceedings of the Annual Meeting the American Phytopathological Society (APS). Phytopathology, 107, 45 pp.
Liese W and Ruetze M, 1985. Development of a fumigation treatment of oak logs against Ceratocystis fagacearum. EPPO Bulletin, 15, 29–36.
Liese W, Knigge H and Ruetze M, 1981. Fumigation experiments with methyl‐bromide on oak wood. Material und Organismen, 16, 265–280.
Macdonald WL, Schmidt EL and Harner EJ, 1985. Methyl‐bromide eradication of the oak wilt fungus from red and white oak logs. Forest Products Journal, 35, 11‐1611‐1.
Mizobuchi M, Matsuoka I, Soma Y, Kishino H, Yabuta S, Imamura M, Mizuno T, Hirose Y and Kawakami F, 1996. Susceptibility of forest insect pests to sulfuryl fluoride. 2. Ambrosia beetles. Research Bulletin of the Plant Protection Service Japan, 32, 77–82.
Najar‐Rodriguez AJ, Hall MKD, Adlam AR, Hall AJ, Burgess SB, Somerfield KG, Page BBC and Brash DW, 2015. Developing new fumigation schedules for the phytosanitary treatment of New Zealand export logs: comparative toxicity of two fumigants to the burnt pine longhorn beetle, Arhopalus ferus. New Zealand Plant Protection, 68, 19–25.
Osbrink WLA, Scheffrahn RH, Su N‐Y and Rust MK, 1987. Laboratory comparisons of sulfuryl fluoride toxicity and mean time of mortality among ten termite species (Isoptera: Hodotermitidae, Kalotermitidae, Rhinotermitidae). Journal of Economic Entomology, 80, 1044–1047.
Parameswaran N and Ruetze M, 1984. Effect of methyl bromide fumigation on the ultrastructure of Ceratocystis fagacearum (Bretz) Hunt. Material und Organismen, 19, 133–140.
Ren YL, Lee BH and Padovan B, 2011. Penetration of methyl bromide, sulfuryl fluoride, ethanedinitrile and phosphine into timber blocks and the sorption rate of the fumigants. Journal of Stored Products Research, 47, 63–68.
Ruetze M and Liese W, 1985. A postfumigation test (TTC) for oak logs. Holzforschung, 39, 327–330. https://doi.org/10.1515/hfsg.1985.39.6.327
Scheffrahn RH and Thoms EM, 1993. Penetration of sulfuryl fluoride and methyl bromide through selected substrates during fumigation. Down to Earth, 48, 15–19.
Schmidt EL, 1982. Methyl bromide eradication of the oak wilt fungus from lumber. International Journal of Wood Preservation, 2, 123–126.
Schmidt EL, 1983. Minimum temperature for methyl‐bromide eradication of Ceratocystis‐fagacearum in red oak log pieces. Plant Disease, 67, 1338–1339.
Schmidt EL, 1985. Control of mold and stain on methyl‐bromide fumigated red oak sapwood. Forest Products Journal, 35, 61–62.
Schmidt EL and Christopherson ER, 1997. Effects of fumigants on parenchyma viability in red oak log sections. Forest Products Journal, 47, 61–63.
Schmidt EL and Kreber B, 1998. Effects of two fumigants and a fungicide formulation on the development of kiln brown stain in radiata pine lumber. Holz als Roh ‐ und Werkstoff, 56, 416‐420.
Schmidt EL, Macdonald WL, Rutze MM et al., 1982. Methyl‐bromide eradication of the oak wilt fungus in logs and lumber. Phytopathology, 72, 979.
Schmidt EL, Ruetze MM and French DW, 1982. Methyl bromide treatment of oak wilt infected logs: Laboratory and preliminary field fumigations. Forest Products Journal, 32, 46‐49.
Schmidt E, Juzwik J and Schneider B, 1997. Sulfuryl fluoride fumigation of red oak logs eradicates the oak wilt fungus. Holz Als Roh‐Und Werkstoff, 55, 315—318.
Schmidt EL, Cassens DL and Jordan BA, 2001a. Control of graystain in yellow‐poplar lumber by log fumigation with sulfuryl fluoride. Forest Products Journal, 51, 50–52.
Schmidt EL, Kreber B and Boon S, 2001b. Fumigation of red beech logs for reducing gray stain in lumber. Forest Products Journal, 51, 89–91.
Schortemeyer M, Thomas K, Haack RA, Uzunovic A, Hoover K, Simpson JA and Grgurinovic CA, 2011. Appropriateness of probit‐9 in the development of quarantine treatments for timber and timber commodities. Journal of Economic Entomology, 104, 717–731.
Seabright KW, Davila‐Flores A, Myers SW and Taylor A, 2020. Efficacy of methyl bromide and alternative fumigants against pinewood nematode in pine wood samples. Journal of Plant Diseases and Protection, 127, 393–400.
Simpson WT, 2001. Chapter 10 – Log and lumber storage. USDA Agricultural Handbook AH‐188: Dry Kiln Operator's Manual, 220–238.
Soma Y, Yabuta S, Mizoguti M, Kishino H, Matsuoka I, Goto M, Akagawa T, Ikeda T and Kawakami F, 1996. Susceptibility of forest insect pests to sulfuryl fluoride. 1. Wood borers and bark beetles. Research Bulletin of the Plant Protection Service Japan, 32, 69–76.
Soma Y, Mizobuchi M, Oogita T, Misumi T, Kishono H, Akagawa T and Kawakami F, 1997. Susceptibility of forest insect pests to sulfuryl fluoride. 3. Susceptibility to sulfuryl fluoride at 25°C. Research Bulletin of the Plant Protection Service Japan, 33, 25–30.
Soma Y, Naito H, Misumi T, Mizobuchi M, Tsuchiya Y, Matsuoka I, Kawakami F, Hirata K and Komatsu H, 2001. Effects of some fumigants on pine wood nematode, Bursaphelenchus xylophilus, infecting wooden packages. Susceptibility of pine wood nematode to methyl bromide, sulfuryl fluoride and methyl isothiocyanate. Research Bulletin of the Plant Protection Service Japan, 37, 19–26.
Sprenkel RJ and Williams LH, 1988. Ovicidal activity of Vikane gas fumigant on anobiid and lyctid beetle eggs. Down to Earth, 44, 15–19.
Stewart D, 1957. Sulfuryl fluoride‐a new fumigant for control of the drywood termite Kalotermes minor (Hagen). Journal of Economic Entomology, 50, 7–11.
Su N‐Y and Scheffrahn RH, 1986. Field comparison of sulfuryl fluoride susceptibility among three termite species (Isoptera: Kalotermitidae, Rhinotermitidae) during structural fumigation. Journal of Economic Entomology, 79, 903–908.
Su N‐Y and Scheffrahn RH, 1990. Efficacy of sulfuryl fluoride against four beetle pests of museums (Coleoptera: Dermestidae, Anobiidae). Journal of Economic Entomology, 83, 879–882.
Su N‐Y, Osbrink WLA and Scheffrahn RH, 1989. Concentration‐time relationship for fumigant efficacy of sulfuryl fluoride against the Formosan subterranean termite (Isoptera: Rhinotermitidae). Journal of Economic Entomology, 82, 156–158.
Thoms EM and Scheffrahn RH, 1994. Control of pests by fumigation with Vikane gas fumigant (sulfuryl fluoride). Down to Earth, 49, 23–30.
Tubajika KM and Barak AV, 2006. Efficacy of sulfuryl fluoride and methyl bromide against wood‐inhabiting fungi. Proceedings of the Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions, Orlando, Florida, USA, 6–9 November 2006, 147 pp.
Tubajika KM and Barak AV, 2007. Methyl iodide and sulfuryl fluoride as quarantine treatments for solid wood packing material. Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions: [proceedings] 2007, 131 pp.
Tubajika KM and Barak AV, 2011. Fungitoxicity of methyl iodide, sulfuryl fluoride, and methyl bromide to Ceratocystis fagacearum in red oak, maple, poplar, birch and pine wood. American Journal of Plant Sciences, 2, 268–275.
University of Kentucky, 2016. Example Fumigants. In: Fumigation Training Manual. UK Pesticide Safety Education Program. University of Kentucky, College of Agriculture, Departments of Entomology. Cooperative Extension Service. Available online: http://www.uky.edu/Ag/Entomology/PSEP/fumexamples.html
Uzunovic A, Mukherjee A, Elder P and Myers SW, 2017. Rapid screening of sulfuryl fluoride as a potential phytosanitary treatment for a broad selection of fungi relevant to forestry. Forest Products Journal, 67, 4–12.
Williams LH and Sprenkel RJ, 1990. Ovicidal activity of sulfuryl fluoride to anobiid and lyctid beetle eggs of various ages. Journal of Entomological Science, 25, 366–375.
Woodward RP and Schmidt EL, 1995. Fungitoxicity of sulfuryl fluoride to Ceratocystis‐fagacearum in‐vitro and in wilted red oak log sections. Plant Disease, 79, 1237–1239.
Yang A, Juzwik J, White M, Chen Z, Shugrue S and Mack R, 2017. Vacuum steam as a promising alternative to methyl bromide for killing Ceratocystis fagacearum in Quercus rubra logs for global export. Proceedings of the Annual Meeting the American Phytopathological Society (APS). Phytopathology, 107, 47 pp.
Yang A, Seabright K, Juzwik J, Myers SW and Taylor A, 2019. Survival of the oak wilt fungus in logs fumigated with sulfuryl fluoride and methyl bromide. Forest Products Journal, 69, 87–95.
Yu SY, 2014. The Toxicology and Biochemistry of Insecticides. Publisher CRC Press, 2nd Edition, 348 pp.
Yu D, Barak AV, Jiao Y, Chen Z, Zhang G, Chen Z, Kang L and Yang W, 2010. Sulfuryl fluoride as a quarantine treatment for Chlorophorus annularis (Coleoptera: Cerambycidae) in Chinese bamboo poles. Journal of Economic Entomology, 103, 277–283.
Zhang Z, 2006. Use of sulfuryl fluoride as an alternative fumigant to methyl bromide in export log fumigation. New Zealand Plant Protection, 59, 223–227.
Appendix B – Elicited values for pest freedom
1.
This appendix provides the rating based on expert judgement on the likelihood of pest freedom for the oak logs with bark imported to EU from the US under three different scenarios:
Fumigation with methyl bromide (MB).
Fumigation with sulfuryl fluoride (SF).
Only white oak logs from October to April, without fumigation.
B.1. Overall likelihood of pest freedom of consignments treated with methyl bromide
B.1.1. Reasoning for a scenario which would lead to a reasonably low number of infested consignments (lower limit)
A series of conditions common to the three EKE (on MB, SF and white oak without fumigation) illustrates this scenario:
The disease incidence is low, apart from some disease pockets: even when widespread, in the stand it has a patchy distribution and is not expected to be associated with more than 25% of trees.
The logs originate from certified forests.
The forest management is conducted in an effective manner (e.g. diseased trees including root systems are removed, trench applied to stop oak wilt spread) and achieves a reduction of the infection rate.
The logs originate from mixed forests with low frequency of oak trees, characterised by low stand density, where conditions are unfavourable to root grafting and therefore to underground spread of the disease.
Most of the logs come from areas which are still disease free (e.g. most counties in New York State) and with low vectors density.
In case of a new outbreak, the landowner/manager takes immediate action.
The operator marks only asymptomatic trees, most probably excluding also those that, despite asymptomatic, are surrounded by wilting ones.
Marking of trees is carried out when diseased oaks are easier to recognise (i.e. during the vegetative season).
Marked trees are felled in winter, during reduced vectors activity.
Harvested logs are stored for a very short time, the wood moisture content remains high and unfavourable for fungal mat formation, avoiding an increase in the fungus load.
The inspections before shipment are very effective in detecting the pathogen presence on the logs.
In addition to the general conditions:
Most of the logs are coming from stands mainly composed of red oak expressing symptoms in a few weeks.
MB penetrates fresh logs quite well and effectively minimises the pathogen presence during fumigation.
The fumigation is implemented correctly, and the fumigant reaches the target pest at an effective concentration.
B.1.2. Reasoning for a scenario which would lead to a reasonably high number of infested consignments (upper limit)
A series of conditions common to the three EKE (on MB, SF and white oak without fumigation) illustrate this scenario:
The disease incidence is high.
The logs originate from uncertified forests.
The forest management is not effective in reducing the infestation rate (e.g. trees are felled from stands where oak wilt is known to be present).
The logs originate from mixed stands characterised by high frequency of oak species and high trees density, supporting disease spread via root grafting.
Most of the logs come from infested areas where B. fagacearum is already widespread (e.g. Wisconsin, Michigan) and therefore where strict surveillance is less likely to occur. In the worst‐case scenario, this could result in a high risk of the presence of one infested log in the container.
The logs are harvested from stands with high vectors density.
In the event of a new outbreak, no actions are immediately taken by the landowner/manager.
The operator marks asymptomatic trees, but is not aware of the distribution of the diseased trees during the previous season(s). In addition, the selected trees are usually of big size, therefore very old, with a higher probability of having been infected, at some point.
Marking of trees is carried out close to harvest, outside the vegetative season, when diseased trees are more difficult to recognise.
Most of the marked trees are felled in periods of intense vectors activity.
Harvested logs are submitted to a long storage, which causes a decrease in moisture content and increase in the fungus load.
The visual inspections before shipment are not sufficient to identify the infested logs.
In addition to the general conditions:
Most of the logs come from stands mainly composed of white oak, which is asymptomatic or poorly symptomatic (less than 10% of crown showing symptoms) and develops symptoms much slower than red oak.
MB is not fully effective, particularly with larger logs, which can reach 90 cm in diameter, and on white oaks, where the fungus can be present deeper in the sapwood, it is expected that on larger logs, the penetration potential of MB is reduced.
Fumigation is not always carried out correctly or accidentally left out (as confirmed by the interception data).
B.1.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested consignments (median)
There is a good amount of literature on the efficacy of MB as a fungicide: even when most of the evidence does not come from trials on logs, there is still empirical evidence from its use for decades.
Proportion of asymptomatic trees.
Interceptions due to ineffective fumigation.
Most of oak logs for export come from certified forests, where they are mostly handled in the correct manner.
Overall, the incidence of the disease is not very high (10%).
B.1.4. Reasoning for the precision of the judgement describing the remaining uncertainties (first and third quartile/interquartile range)
The main uncertainty reflects the absence of information about the disease incidence in the stand.
The elicited and fitted values for B. fagacearum in containers fumigated with methyl bromide agreed by the Panel are shown in Tables B.1 and B.2 and in Figure B.1.
Table B.1.
Elicited and fitted values of the uncertainty distribution of pest infestation by B. fagacearum in containers fumigated with methyl bromide per 10,000 containers
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 5.0 | 80.0 | 150.0 | 250.0 | 500.0 | ||||||||||
| Fitted | 7.4 | 14.3 | 23.6 | 39.3 | 58.2 | 80.5 | 102.8 | 150.5 | 209.0 | 246.6 | 296.1 | 354.0 | 427.0 | 495.2 | 580.0 |
The EKE results are the Weibull distribution (1.4040, 195.45) fitted with @Risk version 7.5.
Table B.2.
The uncertainty distribution of containers free of B. fagacearum when fumigated with methyl bromide per 10,000 containers calculated from Table B.1
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EKE | 9,500 | 9,750 | 9,850 | 9,920 | 9,995 | ||||||||||
| Fitted | 9,420 | 9,505 | 9,573 | 9,646 | 9,704 | 9,753 | 9,791 | 9,849 | 9,897 | 9,920 | 9,942 | 9,961 | 9,976 | 9,986 | 9,993 |
The EKE results are the fitted values.
Figure B.1.
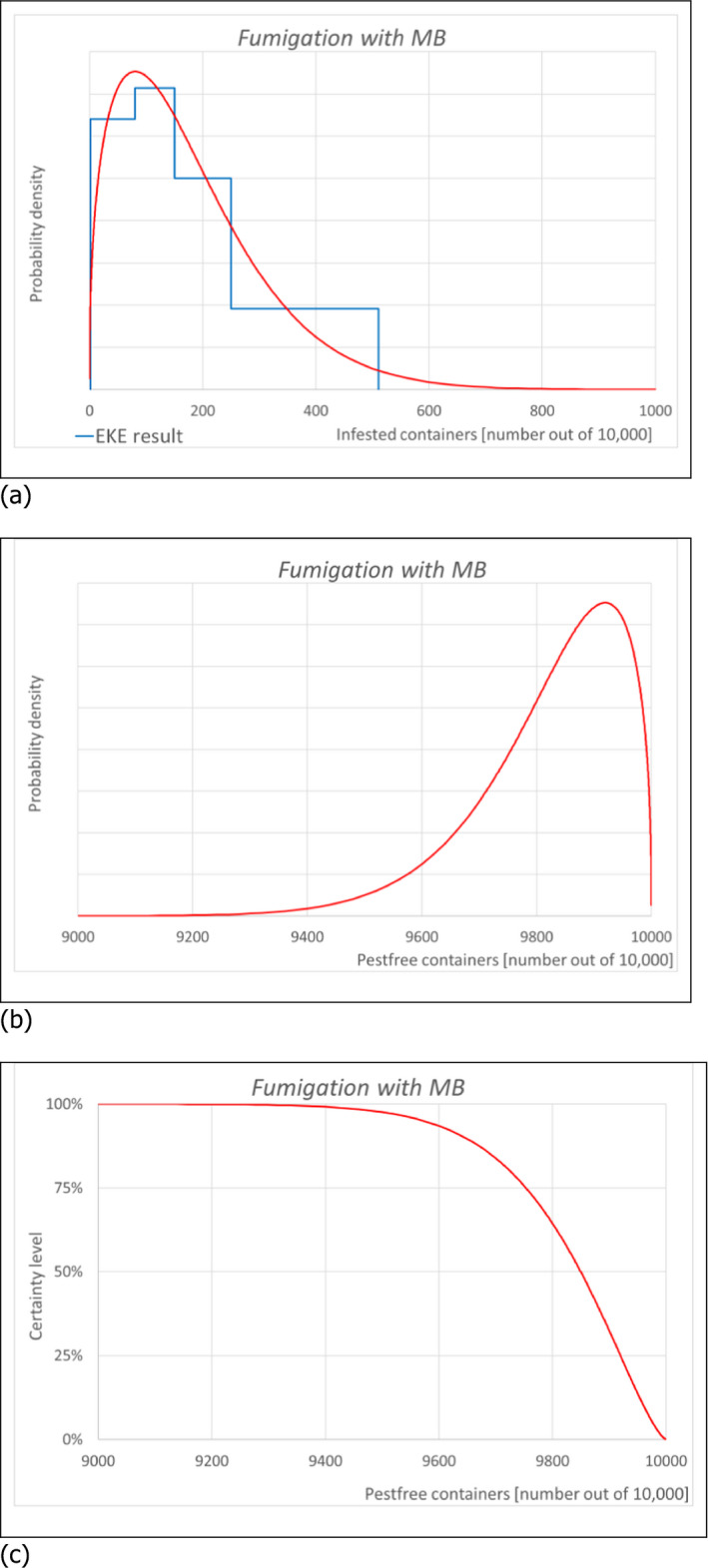
(a) Elicited uncertainty of pest infestation per 10,000 containers (histogram in blue–vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free containers per 10,000 (i.e. =1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 containers
Based on the numbers of estimated infested containers, the pest freedom was calculated (i.e. = 10,000 – the number of infested containers per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table B.2.
B.2. Overall likelihood of pest freedom of consignments treated with sulfuryl fluoride
B.2.1. Reasoning for a scenario which would lead to a reasonably low number of infested consignments (lower limit)
The conditions common to the three EKE (on MB, SF and white oak without fumigation) are provided under the reasoning of MB (Section B.1.1 of this Appendix).
In addition to the general conditions:
Most of the logs are coming from stands mainly composed of red oak, which will show symptoms in a few weeks, if the disease is present.
Studies in which SF performs better than MB on full, fresh logs are not available. Yang et al. (2019) observed a similar level of pathogen reduction between logs treated with MB or SF. This would support a best‐case scenario in which the effectiveness of SF is the same as for MB.
B.2.2. Reasoning for a scenario which would lead to a reasonably high number of infested consignments (upper limit)
The conditions common to the three EKE (on MB, SF and white oak without fumigation) are provided under the reasoning of MB (Section B.1.2 of this Appendix).
In addition to the general conditions:
Most of the logs come from stands mainly composed of white oak that is asymptomatic or poorly symptomatic (less than 10% of crown showing symptoms) and develops symptoms much slower than red oak.
Yang et al. (2019) results indicated that in the ‘worst case’ the number of positive sapwood samples (i.e. oak wilt pathogen still alive) was higher after SF than after MB treatments. Furthermore, the limited penetration capacity of SF should be summed up. Seabright et al. (2020) showed in fact how SF is much less effective than MB on fresh wood: its effectiveness is lower on logs, as penetration capacity is a function of distance, and is contrasted by high wood moisture content and by bark presence.
This scenario is expected to reflect the conditions due to latent infections.
B.2.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested consignments (median)
The value of the median is estimated based on the fact that fumigation with SF has an effect, even when lower than MB. Given that the proportion of its effectiveness is already reflected by the upper limit, the threefold increase from MB to SF provided at the upper bound is kept for the median.
There is confidence that the probability of having less consignments infested is higher than that of having very high number of containers infected.
B.2.4. Reasoning for the precision of the judgement describing the remaining uncertainties (first and third quartile/interquartile range)
The main uncertainty is related to the limited number of studies on SF efficacy of killing the pathogen in fresh logs: Yang et al. (2019) describes a trial carried out on a limited number of logs, smaller than those commonly traded to the EU, and provides an unexplained result of more positive samples on logs treated with higher dosage than on logs treated with lower dosage.
The elicited and fitted values for B. fagacearum in containers fumigated with sulfuryl fluoride agreed by the Panel are shown in Tables B.3 and B.4 and in Figure B.2.
Table B.3.
Elicited and fitted values of the uncertainty distribution of pest infestation by B. fagacearum in containers fumigated with sulfuryl fluoride per 10,000 containers
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 5.0 | 250.0 | 450.0 | 900.0 | 1,500.0 | ||||||||||
| Fitted | 7.8 | 20.0 | 40.8 | 83.9 | 143.8 | 223.0 | 307.5 | 496.0 | 722.9 | 859.8 | 1,023.9 | 1,190.7 | 1,361.4 | 1,484.4 | 1,595.7 |
The EKE results are the BetaGeneral distribution (0.98193, 2.1041, 0, 1800) fitted with @Risk version 7.5.
Table B.4.
The uncertainty distribution of containers free of B. fagacearum when fumigated with sulfuryl fluoride per 10,000 containers calculated in Table B.3
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EKE | 8,500 | 9,100 | 9,550 | 9,750 | 9,995 | ||||||||||
| Fitted | 8,404 | 8,516 | 8,639 | 8,809 | 8,976 | 9,140 | 9,277 | 9,504 | 9,693 | 9,777 | 9,856 | 9,916 | 9,959 | 9,980 | 9,992 |
The EKE results are the fitted values.
Figure B.2.
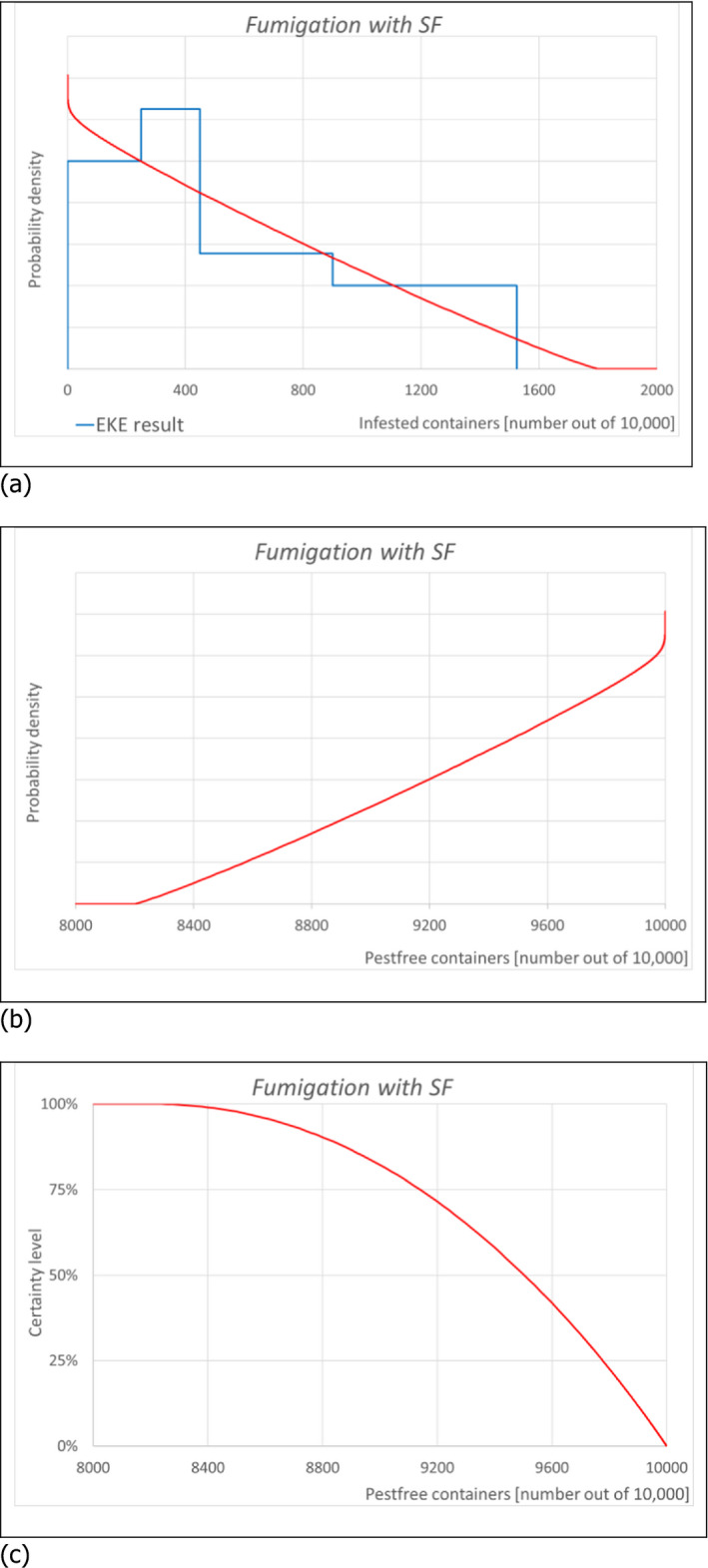
(a) Elicited uncertainty of pest infestation per 10,000 containers (histogram in blue, vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free containers per 10,000 (i.e. = 1 − pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 containers
Based on the numbers of estimated infested containers, the pest freedom was calculated (i.e. = 10,000 – the number of infested containers per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table B.4.
B.3. Overall likelihood of pest freedom of consignments of white oak without fumigation
B.3.1. Reasoning for a scenario which would lead to a reasonably low number of infested consignments (lower limit)
The conditions common to the three EKE (on MB, SF and white oak without fumigation) are provided under the reasoning of MB (Section B.1.1 of this Appendix).
The white oak is less likely to be infected and the pathogen generally does not form mats on this oak species. Furthermore, the winter harvest should reduce the process of mats formation after felling the trees. These two aspects would lower the risk, but they are counterbalanced by the potential absence of symptoms on infected white oak and the omission of fumigation.
B.3.2. Reasoning for a scenario which would lead to a reasonably high number of infested consignments (upper limit)
The conditions common to the three EKE (on MB, SF and white oak without fumigation) are provided under the reasoning of MB (Section B.1.2 of this Appendix).
The worst case would be given by 10% of infested trees, with no or only minor disease symptoms (less than 10% crown dieback) and a distribution of infested logs coming from the same tree in different containers.
B.3.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested consignments (median)
The value of the median is estimated based on the omission of fumigation treatment: this value cannot be lower than the values estimated for logs treated with the different fumigants.
B.3.4. Reasoning for the precision of the judgement describing the remaining uncertainties (first and third quartile/interquartile range)
Uncertainty is mainly related to the contradicting effect of symptoms absence: lower susceptibility, but longer asymptomatic period. Furthermore, connected with the symptom's absence, there is also the limited knowledge on the proportion of infested white oak in the stands. For these reasons, the maximum uncertainty is located on the lower part of the curve.
The elicited and fitted values for B. fagacearum in containers of white oak without fumigation agreed by the Panel are shown in Tables B.5 and B.6 and in Figure B.3.
Table B.5.
Elicited and fitted values of the uncertainty distribution of pest infestation by B. fagacearum in containers of white oak without fumigation per 10,000 containers
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 20.0 | 400.0 | 750.0 | 1,200.0 | 3,000.0 | ||||||||||
| Fitted | 53.5 | 91.3 | 138.7 | 214.8 | 302.9 | 406.5 | 510.2 | 736.7 | 1,023.3 | 1,214.2 | 1,472.5 | 1,785.8 | 2,196.9 | 2,597.0 | 3,114.4 |
The EKE results are the Gamma distribution (1.8020, 497.13) fitted with @Risk version 7.5.
Table A.1.
Evidence table summarising the study results on methyl bromide (MB) and sulfuryl fluoride (SF) efficacy
| Fumigant | Plant | Scope/pest | Type of samples | Concentration | Duration temperature | Results/efficacy | Additional information | Reference | Limitations/uncertainties |
|---|---|---|---|---|---|---|---|---|---|
| MB | Pinus virginiana and P. strobus | Bursaphelenchus xylophilus |
Fresh (average moisture contents above 133%) chips, blocks and logs with bark inoculated with fungi and PWN MB SF EDN PH3 |
Blocks: 120 mg L−1 on SD 140% 240 mg L−1 on SD 162% |
24 and 48 h 20.0 ± 0.5°C |
MB effective in preventing recovery of PWN from 48 h treatments |
Log samples from white pine from Sandwich, MA EDN resulted the best option for providing quarantine level control of PWN on logs with bark |
Seabright et al., 2020 | Artificially inoculated wood |
| SF |
Chips: 50—90 mg L−1 on SD 162% Blocks: 80—180 mg L−1 on SD 121% |
SF effective at all concentrations on chips SF ineffective at all concentrations on blocks PH3 and SF penetrate dry wood quickly, but do not penetrate wet wood well |
|||||||
| MB | Quercus rubra | Bretziella fagacearum | Natural infection and artificial inoculation 2 Vacuum steam (VS) treatments:
|
Vacuum steam treatment is very effective alternative to MB: no viable pathogen or its DNA detected in post‐treatment logs | Juzwik et al., 2019 | VS treatment | |||
| MB | Quercus ellipsoidalis | Bretziella fagacearum | naturally infected logs: 14 artificially inoculated logs: 20 (year 1) and 48 (year 2) | 240 g/m3 | 72 h | 2017: 4(12) positive logs, with 4 outer and 3 inner positive locations | Rates of fungus isolation for artificially inoculated logs > for naturally infected logs | Yang et al., 2019 |
Limited number of samples which hampered a statistically supported comparison between performances of SF and MB Logs in the experiment are smaller than those traded from US to EU Not clear why SF treatments at higher concentrations (2016) or for longer times (2017) were less effective than those applying milder conditions |
| SF |
128 g/m3 240 g/m3 280 g/m3 320 g/m3 |
72 h 96 h |
2016: 2(6) positive artificial inoculated logs at 280 g/m3; 1(5) positive artificial inoculated logs at 320 g/m3 and 1(4) naturally infected logs at 320 g/m3 2017: 5(12) positive logs at 128 g/m3 with 4 outer and 3 inner positive locations, 5(12) at 240 g/m3 with 9 outer and 5 inner positive locations Rates of fungus isolation were slightly higher for SF than for MB SF diffusion in relation with distance and time shows an extreme variability, which could limit the possibility to determine the time and dose required for SF to accumulate in the oak sapwood region where the fungus resides Full reliance on the toxicity of SF might not be sufficient to eradicate B. fagacearum in the logs cut from diseased trees |
||||||
| MB | Pinus radiata | Quantification of sorption and desorption characteristics of MB by recently harvested pine logs | Logs stored at 4 ± 1°C for up to 4 weeks, then cut ≈270 mm long by 250 mm diameter; barked, partially (50%) or fully debarked; end‐grain sealed or unsealed | 342 or 854 ml of pure MB gas delivered to chambers to dose 48 or 120 g m−3, respectively |
16 h: concentration of MB was measured at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 14 and 16 h after fumigation 10 or 20 ± 2°C |
After 16 h, an average of 70.7 ± 2.5% of the initial concentration remained in the treated space when end‐grains were sealed surface area of a log is the most important factor influencing MB (de)sorption desorption rates: greater surface à greater (de)sorption |
Logs from a commercial stand of 18‐year‐old trees, near Palmerston North, New Zealand | Hall et al., 2017 | Pine timber can differ substantially from oak timber and even when the trial is conducted on fresh logs, they were stored for 4 weeks before fumigating and also cut in smaller parts |
| SF | Quercus ellipsoidalis and B. rubra | Bretziella fagacearum | Natural infection and artificial inoculation 1 control 3 fumigation rates |
240 g/m3 280 g/m3 320 g/m3 |
Pathogen not isolated at any location after treatment at 240 g/m3 only | Juzwik et al., 2017 |
Conference proceeding: limited details Not clear why SF treatments at higher concentrations were less effective than those applying milder conditions |
||
| SF | 23 fungal species, among which Bretziella fagacearum | Each fungal isolate represented by 3 colonised pieces of grain in one borosilicate tube for each treatment combination |
40 mg/L 80 mg/L 120 mg/L 160 mg/L 200 mg/L 240 mg/L |
24 h 48 h 72 h 15°C and 20°C |
Maximum efficacy observed at 72 h The two isolates of B. fagacearum survived at any SF dosage |
Uzunovic et al., 2017 | Lab test on fungal colonies on agar: penetration of SF into wood products was not part of this study | ||
| MB | Quercus rubra | Bretziella fagacearum | 5 replicates, each with 1 artificially inoculated and 2 with natural infected logs 2 VS treatments:
|
After treatment, pathogen not isolated from any locations for either schedule | minimal quality defects could be associated with VS | Yang et al., 2017 |
Conference proceeding: limited details Not MB but VS treatment |
||
| SF | Fumigation Training Manual | Used to fumigate closed structures and their contents for drywood and Formosan termites, wood‐infesting beetles, bed bugs, carpet beetles, clothes moths, cockroaches and rodents | University of Kentucky, 2016 | Manual | |||||
| MB |
Arhopalus ferus Fumigation schedules for EDN |
MB: 4 h EDN: 3 h 10°C 20°C |
Most tolerant life stage to MB: larvae most susceptible life stage to MB: adults toxicity EDN > MB | Najar‐Rodriguez et al., 2015 | Exposed insects | ||||
| MB | Review on fumigants for New Zealand export logs | Fumigation of logs for export |
80 g/m3 at ≥ 15°C 120 g/m3 at ≤ 15°C |
16 h | Penetrability
|
SF was developed to control drywood termites (warm climates), now used as structural fumigant, on stored products and against museum pests Pine logs from the US fumigated with SF cannot be exported to China |
Armstrong et al., 2014 | ||
| SF |
Toxic to insects under all temperature and exposure conditions, non‐flammable, non‐explosive, easily dispersed, non‐reactive with a wide range of materials, non‐sorptive in commodities, rapid penetration, no impact on the atmospheric ozone layer Very low effectiveness against insect eggs, requires greater concentrations to obtain adequate level of control ‘The only possible second choice after EDN for further study as a MB alternative’ |
||||||||
| SF | Pinus pinaster | Bursaphelenchus xylophilus | Boards treated than checked for PWN presence at 24 h, 72 h and 21 days after treatment |
1 dosage range for each temperature: 3169–4407 1901–4051 1385–2141 gh m−3 |
24 h 15°C 20°C 30°C |
SF effective in trials at 15 and 30°C PWN survival to 20°C treatment (mortality 94.06—100%) without a dose–response relationship is difficult to explain |
Insect eggs less susceptible to SF Water saturated pinewood limits SF penetration. |
Bonifácio et al., 2014 | Other type of pathogen, penetrating deeper in the wood |
| MB | In the soil, it is used for preplant soil fumigation with chloropicrin to control nematodes, insects and fungi. Used also on commodities | Boiling point 4.5°C | MB has shown to be difficult to replace due to its low cost and effectiveness against a wide variety of pests | Yu, 2014 | Generic review about insecticides, no original data | ||||
| SF | For termite control | Boiling point = –55.2°C | |||||||
| MB | Pseudotsuga menziesii | Comparing timber penetration of 4 fumigants: MB, SF, C2N2, PH3 | timber blocks were conditioned for 2 months at 25 ± 2 °C and 60% relative humidity 3 sample ports for each block (at 5, 10 and 15 cm), in 2 blocks in the same chamber, for 4 duplicate treatments |
MB: 48 mg/L SF: 48 mg/L C2N2: 48 mg/L PH3: 1 mg/L |
48 h 23—25°C |
Rate and extent of penetration was for C2N2 and PH3 > SF During fumigation, 70% MB > 63% C2N2 > 35% SF > 25% PH3 were absorbed by the timber block Ct (Concentration x time) products of C2N2 after 48 h achieve elimination of nematodes and wood pathogens, while Ct products of SF and PH3 cannot eradicate all insect stages |
C2N2 presented the best performances, being a fumigant with the potential to replace MB for control of insects, nematodes and wood pathogens | Ren et al., 2011 | The penetration or sorption rates for the fumigants with wet wood blocks were not tested |
| SF | |||||||||
| Use of probit‐9 mortality as standard for assessing the efficacy of quarantine treatments of insects | Review | Schortemeyer et al., 2011 | Probit‐9 works on treatments for insect pests, not for pathogens. (USDA, US Federal Register, 2007) | ||||||
| MB | Betula,Pinus resinosa,Acer,Populus | Bretziella fagacearum | Artificially inoculated wood blocks of birch, maple, poplar and red pine treated with MB, MI or SF |
160 g/m3 240 g/m3 |
0.5 h 2 h 4 h 24 h 48 h 72 h 20 ± 2°C |
0 pathogen recovery in the 4 plant species: MB and MI:
SF: 240 g/m3 for 72 h MB and SF at 240 g/m3 are not effective or adequate to control all wood fungi tested. Their ineffectiveness to kill B. fagacearum in red oak and poplar samples may possibly due to their deep penetration into wood, to exposure time used in this study, wood characteristics, sorption or to other unknown factors |
Same results presented by Tubajika and Barak, 2007 (results are the same although not all details match, e.g. temperature of trials): it was decided to refer to the most recent version and to exclude from the table the other | Tubajika and Barak, 2011 |
Wood blocks Artificially inoculated Dry wood (for WPM) |
| SF | |||||||||
| SF | Fraxinus | Agrilus planipennis |
Logs and large branches cut to 70—72 cm Cargo container commercial fumigation |
104 g/m3 112 g/m3 136 g/m3 144 g/m3 |
24 h 48 h 10.0°C 15.6°C |
Dose for larvae > eggs probably due to reduced metabolism induced by storage conditions Ash is a wood of density > pine: this can reduce penetration up to 92% for SF and up to 98% for MB |
Barak et al., 2010 | Target is an insect pest | |
| SF | Bambusa | Chlorophorus annularis |
Trial 1: fixed temperature Trial 2: different combinations of temperatures and doses Trial 3: in a commercial container |
64 g/m3 80 g/m3 96 g/m3 112 g/m3 |
24 h 15.9°C 21.5°C 23.0°C 26.0°C |
SF was fully effective at all combinations of doses and temperatures Recommended schedules
|
Yu et al., 2010 | Target is an insect pest | |
| SF | Populus spp. |
Anoplophora glabripennis Fumigation schedule |
20—112 g/m3 |
0.5, 2, 4, 12, 24 h 4.4°C 10.0°C 15.6°C 21.1°C |
Efficacy affected by lower temperatures and higher moisture wood | Barak et al., 2006 |
Target is an insect pest Trial on debarked wood for WPM |
||
| MB | Populus,Quercus rubra | 14 fungal species, among which Bretziella fagacearum |
Artificially inoculated blocks of red oak and poplar sapwood Soil block tests |
16 g/m3 32 g/m3 48 g/m3 64 g/m3 80 g/m3, 96 g/m3, 112 g/m3 |
0.5, 1, 2, 4, 24 h 21 ± 2°C |
MB and SF fumigation were not effective in soil block tests against C. fagacearum (and other pathogen species): all tested fungi were recovered at all concentrations for both the fumigants The dose of 80 g/m3 (both for MB and SF) is not effective in killing all wood‐inhabiting fungi |
Tubajika and Barak, 2006 |
Artificially inoculated blocks WPM |
|
| SF | |||||||||
| SF | 2 insect + 8 fungi species |
Arhopalus tristis: 4 replicates/treatment, with 20 adults and at least 50 eggs per replicate Hylastes ater: 4 replicates/treatment, with 20 adults and 10 larvae per replicate Fungi: 5 replicates/treatment, 1 Petri dish per replicate |
5 treatments: 0 g/m3 15 g/m3 30 g/m3 60 g/m3 120 g/m3 |
24 h 15°C |
Mortality: A. tristis adults, H. ater adults and larvae: 100% at ≥ 15 g/m3 Mortality A. tristis eggs: 100% at 120 g/m3 Mortality fungi: 100% for all 8 species at ≥ 30 g/m3 |
fungi species: Cladosporium herbarum,Phlebiopsis gigantean,Schizophyllum commun,Armillaria novae‐zelandiae,Botryodiplodia theobromae,Ophiostoma novo‐ulmi,Phytophthora cinnamomi and Sphaeropsis sapinea | Zhang, 2006 | Exposed insects and eggs, fungi in Petri dishes | |
| MB | Populus spp. |
Anoplophora glabripennis Fumigation schedule |
48 g/m3 (= 3 lb/1,000 cu ft) 56 g/m3 64 g/m3 80 g/m3 (= 5 lb/1,000 cu ft) |
0.5, 2, 4, 12, 24 h 4.4°C 10.0°C 15.6°C 21.1°C |
Drier wood is more easily penetrated by MB: this means that the required Ct obtained with high moisture wood is satisfactory for wood of any moisture content | Barak et al., 2005 |
Target is an insect pest Trial on debarked wood for WPM |
||
| SF | Pinus echinata |
Bursaphelenchus xylophilus |
Naturally infested pine sticks and logs Trial 1: 2 replicates of 20 sticks/treatment Trial 2: 25 sticks/temperature Trial 3: field experiment with slabs, cants and lumber in 9 fumigation chambers |
30 g/m3 60 g/m3 |
24 h 20°C 25°C 30°C |
Trial 1: at 60 g/m3 and 20°C 10% positive Trial 2: at 60 g/m3 and ≥ 25°C, 0 positive Trial 3: at 997–1751 g‐h/m3 and 35.3°C on average (max 40.9°C), 0 positive |
Dwinell et al., 2003 |
Other type of pathogen, on lumber and smaller pieces of dried wood Few replicates |
|
| SF | Liriodendron tulipifera | SF fumigation of logs to prevent non‐fungal graystain when logs are processed according to a commercial mill operation | 2 trees: each log cut into 3 bolts, one for each treatment:
|
|
72 h | SF successful in prevention of graystain. But killing of parenchyma also promotes fungal blue stain during storage and air‐drying | In this trial SF, which was not tested for its fungicide capacity, caused an increase in fungal activity | Schmidt et al., 2001a | Very limited number of samples |
| SF | Nothofagus fusca | SF fumigation of logs to prevent non‐fungal graystain after normal commercial lumber processing | 8 trees cut 4—11 weeks before fumigation. Each log cut into 3 bolts, one for each treatment:
|
|
72 h | SF more effective in preventing graystain if logs are fumigated sooner than 4 weeks | red beech logs harvested at the West Coast of the South Island, New Zealand | Schmidt et al., 2001b |
Limited number of samples Trial on kiln‐dried lumber |
| MB | Pinus |
Bursaphelenchus xylophilus |
conifer wooden board and lumber treated with MB, SF or MITC |
20 g/m3 30 g/m3 40 g/m3 60 g/m3 80 g/m3 |
24 h 48 h 15°C |
Complete mortality at
|
Soma et al., 2001 | Other type of pathogen on board and lumber | |
| SF | Some survivors at 60 g/m3 for 48 h | ||||||||
| MB | Pinus radiata | Fumigation against kiln brown stain | A) Fumigation of commercial lumber7 sets of 20 replicates: • Treatments within 24 h at 1. Control, 2. MB, 3. SF low dose, 4. SF higher dose • Treatments after 10 days in a wet condition à 5. Control, 6. MB, 7. SF higher doseB) Fumigation of freshly cut log sections3 sets coming from 3 log sections of 3 trees:8. Control,9. MB,10. SF higher dose | 240 g of MB per cubic metre | 72 h5—21°C | Severe intensity of stain 48 h after fumigation 1. Control: 19/20 2.MB: 7/20 3. SF low dose: 7/20 4.SF higher dose: 8/20Severe intensity of stain 48 h after fumigation5. Control: 17/20 6. MB: 13/20 7. SF higher dose: 8/20Severe intensity of stain on lumber cut from fumigated log sections8. Control: 32/34 samples with stain 9. MB: 25/32 samples with stain10. SF higher dose: 9/30 samples with stainPenetration SF > MB on freshly felled log sections | 20 pieces of lumber from sapwood portions of logs in a sawmill of Rotorua, New Zealand.Overall stain still unacceptable (20–30% lumber free of stain) after both treatments. | Schmidt and Kreber, 1998 | Trial on kiln‐dried lumber. |
| SF | • SF low dose: 240 g of SF per cubic metre• SF higher dose: 360 g of SF per cubic metre | ||||||||
| SF | Quercus rubra | Bretziella fagacearum | Logs with bark coming from 5 naturally infected trees.2 logs from each tree: distributed in 2 piles.Discs sampled at 5 locations on each log.80 isolation attempts for each disc. | 27,400 g h/m335,010 g h/m3 | 72 h | After SF fumigation, no B. fagacearum was isolated, at any dose, while other microbial species were not eradicated.The mean detection rate of live oak wilt fungus in oak pre‐fumigation (14.4%), was very low, if for example compared to Woodward and Schmidt (1995) (53%). It was even lower in the deeper sapwood layers (3%). | Natural infections via root grafting: trees with 60–100% of foliage wilted in late July, from north‐central Minnesota. | Schmidt et al., 1997 | Low rate of natural infection before treatment |
| SF | 14 species of forest insect pests | 24 h25°C | the least susceptible in:Cerambycidae: S. japonicus egg (DL95: 57.4 g/m3 and complete mortality: 100 g/m3)Scolytidae: X. pferii egg and larva not killed at 100 g/m3Curculionidae: P. nitidus egg not killed at 50 g/m3All life stages of other species killed at low doses (10–40 g/m3).Some hatched larvae of ambrosia beetles developed to adults. | Cerambycidae (Semanotus japonica,Callidiellium rufipenne,Monochamus alternatus), Scolytidae (Cryphalus fulvus,Ips cembrae,Phloeosinus perlatus,Xyleborus pferii,X. validus,Xylosandrus gemanus,X. brevis,Scolytoplatypus tycon), Platypodidae (Platypus calamus,P. quercivorus), Curculionidae (Pissodes nitidus) | Soma et al., 1997 | Target are insect pestsTrials on exposed insects | |||
| SF | 5 species of ambrosia beetles | 5–11 doses | 24 h48 h15°C | 15 g/m3 for 24 h killed 100% X. pfeili,X. validus and X. germanus adults, and P. calamus and P. quercivorus larvae and adults.40 g/m3 for 24 h killed 11.1% X. validus and X. germanus larvaeFor X. pfeili• Eggs (the most resistant stage):80 g/m3 for 24 h killed 19.0%40 g/m3 for 48 h killed 11.1%50 g/m3 for 48 h killed 23.1%• Larvae50 g/m3 for 48 h killed 98.8%• Adults:20 g/m3 for 48 h killed 100% | Xyleborus pfeili,Xyleborus validus,Xylosandrus germanus,Platypus calamus and Platypus quercivorusVery difficult for the eggs to estimate applied dose for attaining 100% mortality | Mizobuchi et al., 1996 | Target are insect pestsTrials on exposed insects | ||
| SF | 17 species of forest insect pests | 5–7 doses | 24 h for larval, pupal and adult stages48 h for egg stages25°C | Resistance eggs > larvae, pupae and adultsThe most resistantEggs: • C. fulvus• Larvae: Sirahoshizo sp. (the most resistant stage of all larval, pupal and adult stages).130 g/m3: practical dose of SF for attaining 100% mortality of C. fulvus egg (which was the most resistant stage). | cryptomeria bark borer, Semanotus japonicus, small cedar longicorn beetle, Callidiellum rufipenne, Japanese pine sawyer, Monochamus alternatus, small pine bark beetle, Cryphalus fulvus, larch ips, Ips cembrae, thuja bark beetle, Phloeosinus perlatus,Sirahoshizo sp. | Soma et al., 1996 | Target are insect pestsTrials on exposed insects | ||
| SF | Quercus rubra | Bretziella fagacearum | 2 isolates from naturally infected red oak trees.2 cultures for each isolate.Logs from naturally infected trees. | Fumigation of cultures.16 g/m3,40 g/m3,60 g/m3,80 g/m3,100 g/m3,120 g/m3Fumigation of logs.160 g/m3,220 g/m3,280 g/m3 | 24 h48 h21—23°C | SF effective on cultures at 80 g/m3 for 48 h and 120 g/m2 for 24 hSF effective on logs at 280 g/m3 for 72 h% of fungal isolation on infected oak logs pre‐fumigation (53%) was much higher than that observed by Schmidt et al. (1997) | No discoloration of fumigated woodFirst report on SF fungitoxicity | Woodward and Schmidt, 1995 | The way the fumigation treatment was performed (EFSA, online) |
| SF | 42 arthropod species | LAD99 comparative table | Eggs require 4‐ to 54‐fold the dosage of SF needed to kill adults of the same speciesLimited ovicidal activity due to poor penetration through eggshell and embryonic membranes | Control of the eggs unnecessary for social insects | Thoms and Scheffrahn, 1994 | Target are insect pestsNot all the cited studies are published | |||
| MB | Quercus rubra, Swietenia sp., Pinus echinata, Pseudotsuga menziesii, Tsuga heterophylla | Penetration analyses through wood: disks of 5.8 cm in diameter of 2 hardwood heartwoods (1.9 cm thick) and 3 coniferous sapwoods (2.5 cm thick).After tests at ambient moisture content (6.8–12.1%), further tests after brush application of 2 or 3 coats of acrylic latex gloss enamel or following hydration above fibre saturation (31.5 to 41.7%) | 22.2°C | Penetration• Through pine end grain: extremely rapid• Through parallel grain surface: markedly slower• Q. rubra and P. menziesii: the lowest among tested species• Reduced by painting and hydration• SF > MB in painted wood• SF > MB in dry wood, MB > SF in hydrated wood | Pine tracheids facilitate rapid gas penetration due to their orientation, creating channels.Rapid penetration of fumigants occurs through direct gas passages created by insect galleries rather than through wood penetration. | Scheffrahn and Thoms, 1993 | Means of 4 replicates. | ||
| SF | |||||||||
| SF | Anthrenus flavipes,Attagenus megatoma,Lasioderma serricorne | Insects in metal cages | 12 SF concentrations | 22 h26.5 h | Susceptibility adults > larvae > eggs (7–30 times more SF required)156 mg h/L to kill 99% A. flavipes larvae | Su and Scheffrahn, 1990 | Target are beetle pests of museumsTrials on exposed insects | ||
| SF | Euvrilletta peltata and Lyctus brunneus | Fumigations lyctid:• 3.2 (= 289 mg‐h/L) → 11.6% survival for eggs of all ages (70.2% for 2‐day‐old eggs)• 5.2 (= 470 mg‐h/L) → 3.9% survival for eggs of all ages (24.7% for 2‐day‐old eggs)times the drywood termite dosageFumigations anobiid:No differences between 3.2‐ and 5.2‐fold rates on eggs; being the least susceptible 2–4‐day‐old eggs. | 90 mg‐h/L for 22→2°C: drywood termite dosage | Williams et al., 1990 | Target are beetle pests of museumsTrials on exposed insects | ||||
| SF | Captotermes formosanus | Termites in petri dishes | 6 accumulated concentrations | 12 exposure times | Exposure time is equally important to concentration | Su et al., 1989 | Target are insect pestsTrials on exposed insects | ||
| SF | Euvrilletta peltata,Lyctus brunneus | Eggs survival during tent fumigations of a houseEggs from 1 to 7 day‐old | • 289 mg‐h/L (= 3.2 times drywood termite dosage) • 470 mg‐h/L (= 5.2 times drywood termite dosage) | 72 h25°C | 289 mg‐h/L →11.6% survival for all ages of eggs; 70.2% survival 2‐day‐old eggs 470 → mg‐h/L survival for all ages of eggs; 24.7% survival 2‐day‐old eggsNo difference between the two dosages for E. peltatathe least susceptible: aged 2—4‐day‐old eggs | Sprenkel and Williams, 1988 | Target are insect pests | ||
| SF | 10 termite species belonging to Hodotermitidae, Kalotermitidae, Rhinotermitidae | 30 termites/group | 22 h27°C | Species sensitivity• Max: R. flavipes and R. tibialis• Min: I. minorpost‐fumigation grand mean time of mortality• Max: R. tibialis• Min: I. snyderi | Zootermopsis angusticollis,Cryptotermes cavifrons,Incisitermes minor,I. snyderi,Neotermes jouteli,Kalotermes approximatus,Coptotermes formosanus,Reticulitermes tibialis,R. flavipes,Prorhinotermes simplex | Osbrink et al., 1987 | Target are insect pestsTrials on exposed insects | ||
| SF | Pinus elliottii | Incisitermes schwarzi,Cryptotermes cavifrons and Coptotermes formosanus | Termites in petri dishes and wooden enclosures removed from each structure at 2‐h intervals for 20 h. | 3 mg/L,6 mg/L,12 mg/L | I. schwarzi and C. cavifrons: 100% mortality from accumulated dosages of 28–49 mg h/L after 72 h.C. formosanus: 100% mortality in wood enclosures at higher dosages of ~95 mg h/L. | Su and Scheffrahn, 1986 | Target are insect pestsTrials on exposed insects | ||
| MB | Quercus alba,Q. rubra | Bretziella fagacearum | Fumigation of cultures, oak logs with bark, logs from artificially inoculated living trees fumigated in practical conditions (under tarpaulin) | 240 g/m3 | 0—6 °C | MB effective on cultures at 32 g/m3 for 72 h or 20 g/m3 for 96 hMB effective on logs at 240 g/m3 for 72 h, also in field conditions | 240 g/m3 is the maximum dose that can be safely handled under practical conditions.No wood discoloration caused by MB. | Liese and Ruetze, 1985 | |
| MB | Quercus robur | Colour reaction test | Tetrazolium chloride (TTC) reaction test | 180 g/m3 240 g/m3 | 72 h | TTC‐indicator can be an effective post‐fumigation test for oak logs as it reliably verifies MB treatment efficacyMB doses insufficient in killing B. fagacearum do not eliminate parenchyma cells either | TTC‐test gives positive in case of living cells containing an active dehydrogenase; therefore, it indicates viability of parenchyma cells but also of wood colonising microorganisms | Ruetze and Liese, 1985 | TTC‐test |
| MB | Quercus rubra | Bretziella fagacearum | 4 naturally infected boles.Log sections with bark | 240 g/m3 | 5°C0°C−5°C−10°C | Treatments at 0°C and higher were all effective.Storage at 0°C after treatment could support efficacy at −5°C. Treatments at −10°C were ineffective | The infected trees had 75% foliage wilted | Schmidt, 1983 | |
| MB | Quercus alba,Q. rubra | Bretziella fagacearum | Short log sections in lab chamber fumigations2.4 m logs with bark in outdoor trials | 240 g/m3 | 72 h5°C | Pathogen eradication in lab conditionsPathogen frequency very limited to a small % of controls in outdoor trials.48 h fumigation not effective | Schmidt et al., 1982 | Abstract only | |
| MB | Attagenus piceus,Tribolium confusum | Insecticide toxicityRelative penetration properties in various commodities | 8 mg/L | 4°C,15°C,26°C | Under similar conditions, and at all concentrations, temperatures andexposure periods, toxicity SF > MBIncreased SF concentrations,exposure times and/or fumigation temperatures required to kill eggs when compared to conditions required to kill larvae, pupae or adults. | Same range of insect toxicity as MB, egg stage being significantly more tolerant tofumigation | Kenaga, 1957 | Target are insect pestsTrials on exposed insects | |
| SF | 14 insect species | ||||||||
| MB | Kalotermes minor | 2 replicates of 25 termitesMortality counts 5–14 days after exposure | 1—50 mg/L | 0—24 h6—38°C | MB and SF equally toxic, but SF more sensitive to temperature change. | Toxicology: SF is 1/3 toxic than MB | Stewart, 1957 | Target are insect pestsTrials on exposed insects | |
| SF | |||||||||
| MB | Picea abies | Sterilisation of fungal infested timber | Artificially inoculated blocks, followed by isolation and incubation of fungal populations |
6 lb./1,000 ft 12 lb./1,000 ft 20 lb./1,000 ft |
24 h 72 h 96 h |
Most effective: 12 lb. per 1,000 cu. ft. for 96 h Moderately effective: 20 lb. per 1,000 cu. ft. for 24 h |
Cartwright et al., 1953 | Artificially inoculated blocks |
Figure B.3.
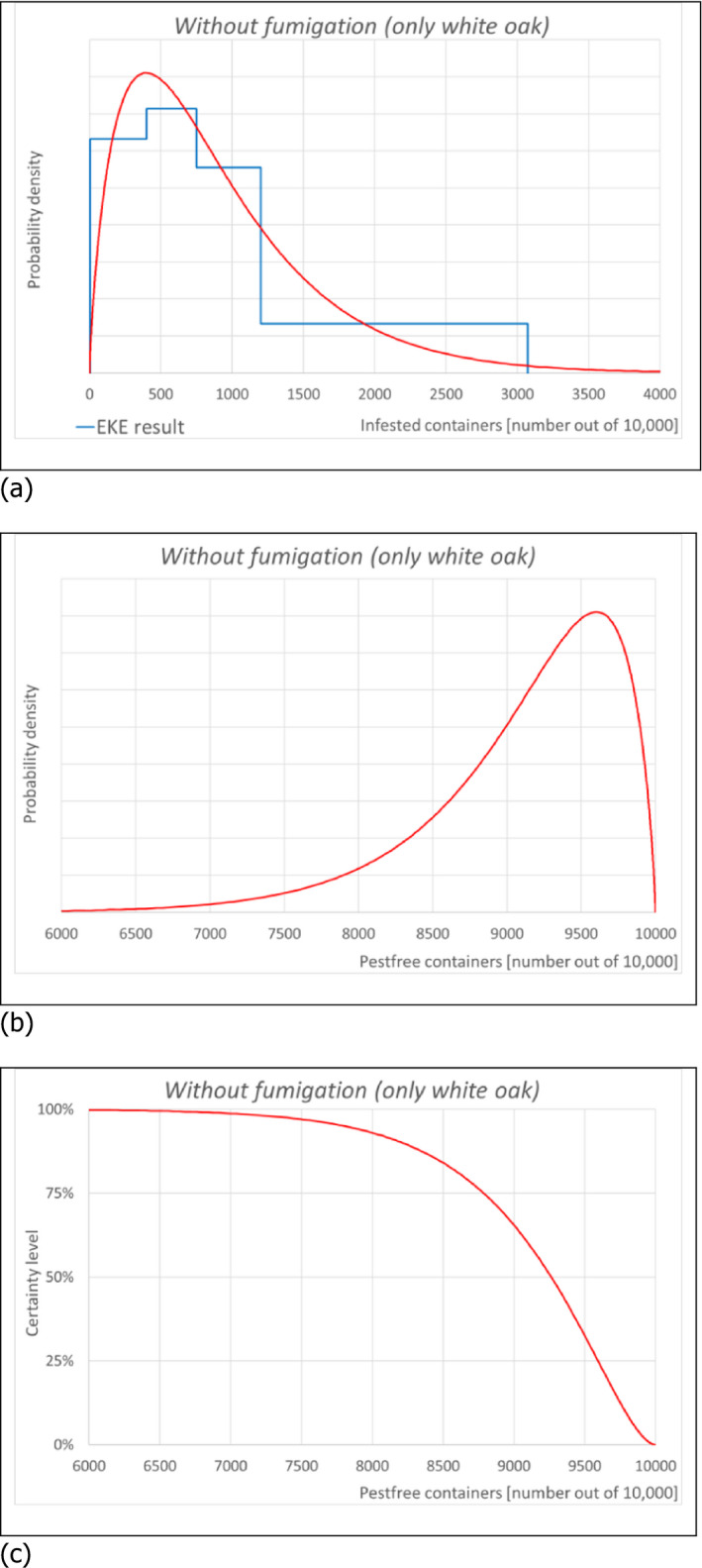
(a) Elicited uncertainty of pest infestation per 10,000 containers (histogram in blue, vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free containers per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 containers
Based on the numbers of estimated infested containers, the pest freedom was calculated (i.e. = 10,000 – the number of infested containers per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table B.6.
Table B.6.
The uncertainty distribution of containers of white oak without fumigation free of B. fagacearum per 10,000 containers calculated in Table B.5
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EKE | 7,000 | 8,800 | 9,250 | 9,600 | 9,980 | ||||||||||
| Fitted | 6,886 | 7,403 | 7,803 | 8,214 | 8,528 | 8,786 | 8,977 | 9,263 | 9,490 | 9,594 | 9,697 | 9,785 | 9,861 | 9,909 | 9,947 |
The EKE results are the fitted values.
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Dehnen‐Schmutz K, Di Serio F, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Douma JC, Rigling D, Mosbach‐Schulz O, Stancanelli G, Tramontini S and Gonthier P, 2020. Scientific Opinion on the commodity risk assessment of oak logs with bark from the US for the oak wilt pathogen Bretziella fagacearum under an integrated systems approach. EFSA Journal 2020;18(12):6352, 67 pp. 10.2903/j.efsa.2020.6352
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00547
Panel members: Claude Bragard, Katharina Dehnen‐Schmutz, Francesco Di Serio, Paolo Gonthier, Marie‐Agnès Jacques, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L Reignault, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent, Jonathan Yuen and Lucia Zappalà.
Acknowledgements: EFSA wishes to acknowledge the contributions of Prof. Roberto Zanuttini, who provided information on the processing of oak wood and veneer production in the EU, of USDA APHIS (the Animal and Plant Health Inspection Service of United States Department of Agriculture) representatives during the hearing process, and of EU NPPOs (National Plant Protection Organisations) for the replies to the questionnaire.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figures 1 and 2: © USDA
Adopted: 26 November 2020
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, pp. 1–112.
Commission Implementing Regulation (EU) 2019/2072 of 28 November 2019 establishing uniform conditions for the implementation of Regulation (EU) 2016/2031 of the European Parliament and the Council, as regards protective measures against pests of plants, and repealing Commission Regulation (EC) No 690/2008 and amending Commission Implementing Regulation (EU) 2018/2019. OJ L 319, 10.12.2019, pp. 1–279.
Commission Decision 2005/359/EC of 29 April 2005 providing for a derogation from certain provisions of Council Directive 2000/29/EC as regards oak (Quercus L.) logs with bark attached, originating in the United States of America. OJ L 114, 4.5.2005, pp. 14–19. Current consolidated version: 27.11.2010.
Commission Decision 2006/750/EC of 31 October 2006 amending Decision 2005/359/EC as regards the ports of unloading of oak (Quercus L.) logs with bark attached, originating in the United States. OJ L 302, 1.11.2006, pp. 49–50.
Commission Decision 2010/723/EU of 26 November 2010 extending the period of validity of Decision 2005/359/EC providing for a derogation from certain provisions of Council Directive 2000/29/EC as regards oak (Quercus L.) logs with bark attached, originating in the United States of America. OJ L 312, 27.11.2010, pp. 30–30.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, pp. 1–24.
The EU Pesticides database is the reference database for member states about the pesticide residues, active substances and products and their values. Accessible from: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database
References
- Appel DN, 1995a. Epidemiology of oak wilt in Texas In Appel DN, Billings RF. (eds.). Oak Wilt Perspectives: The Proceedings of the National Oak Wilt Symposium, June 22–25, 1992, Austin, Texas. Information Development, Inc., Houston, Texas, pp. 21–28. [Google Scholar]
- Appel DN, 1995b. The oak wilt enigma: perspectives from the Texas epidemic. Annual Review of Phytopathology, 33, 103–118. [DOI] [PubMed] [Google Scholar]
- Appel DN, Kurdyla T and Lewis R, 1990. Nitidulids as vectors of the oak wilt fungus and other Ceratocystis spp. in Texas. European Journal of Forest Pathology, 20, 412–417. [Google Scholar]
- Armstrong JW, Brash D and Waddell BC, 2014. Comprehensive literature review of fumigants and disinfestation strategies, methods and techniques pertinent to potential use as quarantine treatments for New Zealand export logs. Plant & Food Research SPTS No. 10678, Plant & Food Research, Palmerston North, New Zealand.
- Barak AV, Wang Y, Zhan G, Wu Y, Xu L and Huang Q, 2006. Sulfuryl fluoride as a quarantine treatment for Anoplophora glabripennis (Coleoptera: Cerambycidae) in regulated wood packing material. Journal of Economic Entomology, 99, 1628–1635. [DOI] [PubMed] [Google Scholar]
- Barak AV, Messenger M, Neese P, Thoms E and Fraser I, 2010. Sulfuryl fluoride treatment as a quarantine treatment for emerald ash borer (Coleoptera: Buprestidae) in ash logs. Journal of Economic Entomology, 103, 603–611. [DOI] [PubMed] [Google Scholar]
- Bonifácio LF, Sousa E, Naves P, Inacio ML, Henriques J, Mota M, Barbosa P, Drinkall MJ and Buckley S, 2014. Efficacy of sulfuryl fluoride against the pinewood nematode, Bursaphelenchus xylophilus (Nematoda: Aphelenchidae), in Pinus pinaster boards. Pest Management Science, 70, 6–13. [DOI] [PubMed] [Google Scholar]
- Bruhn JN, Pickens JB and Stanfield DB, 1991. Probit analysis of oak wilt transmission through root grafts in red oak stands. Forest Science, 37, 28–44. [Google Scholar]
- Chalkley D, 2016. Invasive fungi fact sheets – Oak wilt – Ceratocystis fagacearum. Systematic Mycology and Microbiology Laboratory. ARS, USDA. Available online: https://nt.ars-grin.gov/taxadescriptions/factsheets/index.cfm?thisapp=Ceratocystisfagacearum [Accessed: 10 November 2020]
- Cones WL, 1967. Oak wilt mats on white oak in West Virginia. Plant Disease Reporter, 51, 430–431. [Google Scholar]
- Doganlar M and Schopf R, 1984. Some biological aspects of the European oak bark beetle, Scolytus intricatus (Ratz.) (Col., Scolytidae) in the northern parts of Germany (BRD). Zeitschrift für Angewandte Entomologie, 97, 153–162. [Google Scholar]
- EFSA , online. Minutes of the Working Group on US oak logs with systems approach for oak wilt. Available online: http://www.efsa.europa.eu/sites/default/files/wgs/plant-health/wg-us-oak-logs-system-approach-oak-wilt.pdf [Accessed: 20 October 2020].
- EFSA PLH Panel (EFSA Panel on Plant Health), 2018a. Scientific Opinion on the pest categorisation of Bretziella fagacearum . EFSA Journal 2018;16(2):5185, 30 pp. 10.2903/j.efsa.2018.5185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2018b. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2019a. Guidance on commodity risk assessment for the evaluation of high risk plants dossiers. EFSA Journal 2019; 17(4):5668, 20 pp. 10.2903/j.efsa.2019.5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2019b. Scientific Opinion on the pest categorisation of Pseudopityophthorus minutissimus and P. pruinosus . EFSA Journal 2019;17(1):5513, 27 pp. 10.2903/j.efsa.2019.5513 [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2019c. Scientific opinion on the pest categorisation of Arrhenodes minutus . EFSA Journal 2019;17(2):5617, 26 pp. 10.2903/j.efsa.2019.5617 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2018. Scientific Opinion on the principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122,235 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhard AW, 1955. Occurrence of oak wilt fungus mats and pads on members of the red and white oak groups in Iowa. Plant Disease Reporter, 39, 254–255. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), 2001. Diagnostic protocols for regulated pests: ceratocystis fagacearum. EPPO Bulletin, 31, 41–44. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), online. EPPO Global Database. Available online: https://www.eppo.int/ [Accessed: 26 October 2020]
- EUROPHYT , online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT. Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 14 October 2020].
- FAO (Food and Agriculture Organization of the United Nations), 2016. ISPM (International standards for phytosanitary measures) No. 31. Methodologies for sampling of consignments. FAO, Rome. Available online: https://www.ippc.int/en/publications/588/
- FAO (Food and Agriculture Organization of the United Nations), 2017a. ISPM (International standards for phytosanitary measures) No. 39. International movement of wood. FAO, Rome. Available online: https://www.ippc.int/en/publications/84341/
- FAO (Food and Agriculture Organization of the United Nations), 2017b. ISPM (International standards for phytosanitary measures) No. 5. Glossary of phytosanitary terms. FAO, Rome. Available online: https://www.ippc.int/en/publications/622/
- FAO (Food and Agriculture Organization of the United Nations), 2019. ISPM (International standards for phytosanitary measures) No. 14. The use of integrated measures in a systems approach for pest risk management. FAO, Rome. Available online: https://www.ippc.int/en/publications/607/
- Gibbs JN and French DW, 1980. The transmission of oak wilt. Research Paper NC‐185, US Department of Agriculture, Forest Service, North Central Research Station. St Paul, Minnesota. [Google Scholar]
- Gutierrez Garzon AR, Bettinger P, Siry J, Abrams J, Cieszewski C, Boston K, Mei B, Zengin H and Yeşil A, 2020. A comparative analysis of five forest certification programs. Forests, 11, 863. [Google Scholar]
- Hardwood Review eGlobal, Europe , 2020. August 2020, 9, 16 pp. [Google Scholar]
- Harrington TC, 2013. Ceratocystis Diseases In: Gonthier P. and Nicolotti G. (eds.). Infectious Forest Diseases. CAB International, Wallingford, UK: pp. 230–255. [Google Scholar]
- Haugen L, O'Brien J, Pokorny J, Mielke M and Juzwik J, 2009. Oak wilt in the north central region. Proceedings of the National Oak Wilt Symposium, June 4–7, 2007. RF Billings and DN Appel, eds. Texas Forest Service Publication, 149–157.
- Jagemann SM, Juzwik J, Tobin PC and Raffa KF, 2018. Seasonal and regional distributions, degree‐day models, and phoresy rates of the major sap beetle (Coleoptera: Nitidulidae) vectors of the oak wilt fungus, Bretziella fagacearum, in Wisconsin. Environmental Entomology, 47, 1152–1164. [DOI] [PubMed] [Google Scholar]
- Jones TW, 1973. Killing the Oak wilt fungus in logs. Forest Products Journal, 23, 52–54. [Google Scholar]
- Juzwik J, 2009. Epidemiology and occurrence of oak wilt in Midwestern, Middle and South Atlantic states., Austin, Texas: pp. 49–59. [Google Scholar]
- Juzwik J, French DW and Jeresek J, 1985. Overland spread of the oak wilt fungus in Minnesota. Journal of Arboriculture, 11, 323–327. [Google Scholar]
- Juzwik J, Skalbeck TC and Neuman MF, 2004. Sap beetle species (Coleoptera: Nitidulidae) visiting fresh wounds on healthy oaks during spring in Minnesota. Forest Science, 50, 757–764. [Google Scholar]
- Juzwik J, Appel DA, MacDonald WL and Burks S, 2011. Challenges and successes in managing oak wilt in the United States. Plant Disease, 95, 888–900. [DOI] [PubMed] [Google Scholar]
- Juzwik J, Yang A, Myers S, Furtado M and Taylor A, 2017. Survival of Ceratocystis fagacearum following red oak log fumigation with sulfuryl fluoride Proceedings of the Annual Meeting the American Phytopathological Society (APS). Phytopathology, 107, 45. [Google Scholar]
- Juzwik J, Yang A, Chen Z, White MS, Shugrue S and Mack R, 2019. Vacuum steam treatment eradicates viable Bretziella fagacearum from logs cut from wilted Quercus rubra . Plant Disease, 103, 276–283. 10.1094/PDIS-07-18-1252-RE [DOI] [PubMed] [Google Scholar]
- Kenaga EE, 1957. Some biological, chemical and physical properties of sulfuryl fluoride as an insecticidal fumigant. Journal of Economic Entomology, 50, 1–6. [Google Scholar]
- Kuntz JE and Drake CR, 1957. Tree wounds and long distance spread of oak wilt. Phytopathology, 47, 22. [Google Scholar]
- Menges ES, 1978. Patterns of oak mortality in Midwestern oak forests. Proceedings of the Central Hardwood Forestry Conference, 2, 508–528. [Google Scholar]
- Menges ES and Loucks OR, 1984. Modeling a disease‐caused patch disturbance: Oak wilt in the Midwestern United States. Ecology, 65, 487–498. [Google Scholar]
- Mizobuchi M, Matsuoka I, Soma Y, Kishino H, Yabuta S, Imamura M, Mizuno T, Hirose Y and Kawakami F, 1996. Susceptibility of forest insect pests to sulfuryl fluoride. 2. Ambrosia beetles. Research Bulletin of the Plant Protection Service Japan, 32, 77–82. [Google Scholar]
- O'Brien JG, Mielke ME, Starkey D and Juzwik J, 2011. How to identify, prevent and control oak wilt. NA‐FR-01‐11. Northeastern Area S&PF, U.S. Dept. of Agric., For. Serv., Newtown Square, PA. Available online: https://www.fs.usda.gov/naspf/publications/how-identify-prevent-and-control-oak-wilt
- Pasqual C, Kovar I, van Vondel BJ and Audisio P, 2013. Fauna Europaea: Nitidulidae, Cryptarchinae. Cryptarcha spp. Fauna Europaea version, 2017, 06 Available online: https://fauna-eu.org [Google Scholar]
- Prey A and Kuntz JE, 1995. Epidemiology of oak wilt outside of Texas In Appel DN, Billings RF. (eds.). Oak Wilt Perspectives: The Proceedings of the National Oak Wilt Symposium. June 22–25, 1992, Austin, TX. Information Development Inc., Houston, TX, pp. 15‐20. [Google Scholar]
- Ren YL, Lee BH and Padovan B, 2011. Penetration of methyl bromide, sulfuryl fluoride, ethanedinitrile and phosphine into timber blocks and the sorption rate of the fumigants. Journal of Stored Products Research, 47, 63–68. [Google Scholar]
- Robinet CJ, Douma C, Piou D and van der Werf W, 2016. Application of a wood pathway model to assess the effectiveness of options for reducing risk of entry of oak wilt into Europe. Forestry, 89, 456–472. [Google Scholar]
- Scheffrahn RH and Thoms EM, 1993. Penetration of sulfuryl fluoride and methyl bromide through selected substrates during fumigation. Down to Earth, 48, 15–19. [Google Scholar]
- Seabright KW, Davila‐Flores A, Myers SW and Taylor A, 2020. Efficacy of methyl bromide and alternative fumigants against pinewood nematode in pine wood samples. Journal of Plant Diseases and Protection, 127, 393–400. [Google Scholar]
- Shelstad D, Queen L, French D and Fitzpatrick D, 1991. Describing the spread of oak wilt using a geographic information system. Journal of Arboriculture, 17, 192–199. [Google Scholar]
- Soma Y, Yabuta S, Mizoguti M, Kishino H, Matsuoka I, Goto M, Akagawa T, Ikeda T and Kawakami F, 1996. Susceptibility of forest insect pests to sulfuryl fluoride. 1. Wood borers and bark beetles. Research Bulletin of the Plant Protection Service Japan, 32, 69–76. [Google Scholar]
- Stewart D, 1957. Sulfuryl fluoride‐a new fumigant for control of the drywood termite Kalotermes minor (Hagen). Journal of Economic Entomology, 50, 7–11. [Google Scholar]
- Tainter FH, MacDonald WL and Harner EJ, 1984. Survival of the oak wilt fungus in air‐dried lumber. European Journal of Forest Pathology, 14, 9–16. [Google Scholar]
- USDA (United States Department of Agriculture), online. US National Fungus Collections Databases. Available online: https://nt.ars-grin.gov/sbmlweb/fungi/databases.cfm
- Uzunovic A, Mukherjee A, Elder P and Myers SW, 2017. Rapid screening of sulfuryl fluoride as a potential phytosanitary treatment for a broad selection of fungi relevant to forestry. Forest Products Journal, 67, 4–12. [Google Scholar]
- Yang A and Juzwik J, 2017. Use of nested and real‐time PCR for the detection of Ceratocystis fagacearum in the sapwood of diseased oak species in Minnesota. Plant Disease, 101, 480–486. [DOI] [PubMed] [Google Scholar]
- Yang A, Seabright K, Juzwik J, Myers SW and Taylor A, 2019. Survival of the oak wilt fungus in logs fumigated with sulfuryl fluoride and methyl bromide. Forest Products Journal, 69, 87–95. [Google Scholar]
- Yates MG, 1984. The biology of the oak bark beetle, Scolytus intricatus (Ratzeburg) (Coleoptera: Scolytidae), in southern England. Bulletin of Entomological Research, 74, 569–579. [Google Scholar]
- Zhang Z, 2006. Use of sulfuryl fluoride as an alternative fumigant to methyl bromide in export log fumigation. New Zealand Plant Protection, 59, 223–227. [Google Scholar]


