Abstract
Objective
Although it is reported that patients with coronavirus disease 2019 (COVID-19) disease who have comorbidities are at higher risk to suffer adverse clinical outcomes, there are inadequate evidence to clarify the association between COVID-19 and asthma. On this ground, this study aims to systematically analyze the clinical characteristics of COVID-19 patients with asthma.
Methods
In this single-center, retrospective and observational cohort study, 21 COVID-19 patients with asthma and 100 non-asthma COVID-19 patients were statistically matched by propensity score based on age, sex and comorbidities. Meanwhile, a collection and comparison concerning demographic indicators, clinical and laboratory examinations, treatments and outcomes were conducted between two groups to specify their differences.
Results
Statistically, the COVID-19 patients with asthma had a higher proportion of ICU admission (14.3% [3/21] vs. 2.1% [2/96] p = 0.040) than those who do not have. On top this, a higher level of inflammatory responses, such as interleukin 6, interleukin 8, procalcitonin, leukocytes, neutrophils and CD4+ T cells was presented in asthma patients. Moreover, the increase of organ damage indices like D-dimer, lactate dehydrogenase and high-sensitivity cardiac troponin I, were more pronounced in COVID-19 patients with asthma.
Conclusions
Exacerbated inflammatory responses and multiple organ damages were triggered in COVID-19 patients with asthma, which highlights more intensive surveillance and supportive treatment.
Keywords: COVID-19, asthma, D-dimer, procalcitonin
Introduction
Coronavirus disease 2019 (COVID-19) has become a global pandemic hitting more than 200 countries. As of June 3, 2020, there have been 6,242,974 confirmed cases of COVID-19, including 378,485 deaths (1,2). It has caused a serious threat to human health. What’s worse, the number of confirmed cases is still mounting. Under such circumstances, greater protective measures are imperative among high-risk populations, especially the elderly and people with other comorbidities.
Notably, COVID-19 patients with underlying diseases would have adverse clinical outcomes (3). As reported, diabetes, hypertension, and other comorbidities were predictors of severity in that these diseases lead to an increased risk of death for COVID-19 patients (2,4,5). Asthma is a global health problem that affects around 300 million individuals, and approximately 250,000 people die prematurely each year (6). According to statistics, the prevalence of the disease is 6.4% and 4.2% in the adult population of Wuhan and China, respectively (7,8). However, asthmatics who received therapy with inhaled corticosteroids accounted for only 5.6%, still much lower than that of some European countries, such as 17% in Italy and 49% in the United Kingdom (9). As a chronic respiratory disorder, asthma is characterized by inherently chronic airway inflammation involved with a variety of cells such as eosinophils and mast cells, and this inflammation is often accompanied by increased airway reactivity (10). Due to the limited study illustrating the association between COVID-19 and asthma, however, it is urgent to analyze and summarize the clinical characteristics and changes in specific indicators of COVID-19 patients with asthma. Thus, we made a hypothesis that patients both suffering COVID-19 and asthma were more likely to present severe clinical characteristics and worse outcomes than those without asthma.
Herein, a single-center, retrospective and observational cohort study was performed for the purpose to systematically profile the clinical characteristics of COVID-19 patients with asthma by comparing biological indicators between 21 COVID-19 patients and 100 matched non-asthma COVID-19 patients.
Materials and methods
Study design and participants
All COVID-19 patients (1864) admitted to Tongji Hospital, Tongji College of Huazhong University of Science and Technology, Wuhan between January 26 and March 2, 2020, were retrospectively recruited as a cohort. The primary outcome of the cohort study is death and the secondary outcomes include ICU admission and hospital length of stay. The detailed electronic medical records were collected according to the corresponding time. Those COVID-19 patients who were diagnosed with asthma (N = 21) in the cohort before our study were enrolled and 100 patients without asthma were matched by propensity score at an approximate ratio of 1:5 based on age and sex and comorbidities including hypertension, diabetes, coronary heart disease et al (Table 1). This study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology and granted a waiver of informed consent from study participants.
Table 1.
Demographic, clinical, and treatment of COVID-19 patients with asthma and non-asthma.
| Indicators | Total | Asthma |
Non-asthma |
p Value | ||
|---|---|---|---|---|---|---|
| N = 121 | N = 21 | N = 100 | ||||
| Characteristics# | N = 121 | N = 21 | N = 100 | |||
| Age(years) | 57.52 ± 14.71 | 55.10 ± 16.70 | 58.03 ± 14.30 | 0.408a | ||
| Sex (N = 121) | 0.654c | |||||
| Male | 41 (33.88%) | 8 (38.10%) | 33 (33.00%) | |||
| Female | 80 (66.12%) | 13 (61.90%) | 67 (67.00%) | |||
| Comorbidities# | N = 121 | N = 21 | N = 100 | |||
| Hypertension | 32 (26.5%) | 6 (28.6%) | 26 (26.0%) | 0.808c | ||
| Diabetes | 16 (13.2%) | 2 (9.5%) | 14 (14.0%) | 0.844d | ||
| Coronary heart disease | 10 (8.3%) | 3 (14.3%) | 7 (7.0%) | 0.505d | ||
| Chronic pulmonary disease | 5 (4.1%) | 2 (9.5%) | 3 (3.0%) | 0.207e | ||
| Cerebral infarction | 3 (2.5%) | 1 (4.8%) | 2 (2.0%) | 0.439e | ||
| Cirrhosis or hepatitis | 1 (0.8%) | 0 (0.0%) | 1 (1.0%) | 1.000e | ||
| Pulmonary tuberculosis | 1 (0.8%) | 0 (0.0%) | 1 (1.0%) | 1.000e | ||
| Chronic bronchitis | 1 (0.8%) | 0 (0.0%) | 1 (1.0%) | 1.000e | ||
| Tumor | 2 (1.7%) | 0 (0.0%) | 2 (2.0%) | 1.000e | ||
| Chronic kidney disease | 1 (0.8%) | 0 (0.0%) | 1 (1.0%) | 1.000e | ||
| others | 22 (18.2%) | 6 (28.6%) | 16 (16.0%) | 0.295d | ||
| Initial symptoms | N = 109 | N = 21 | N = 88 | |||
| Fever | 78 (71.6%) | 15 (71.4%) | 63 (71.6%) | 0.988c | ||
| Cough | 69 (63.3%) | 11 (52.4%) | 58 (65.9%) | 0.248c | ||
| Dyspnea | 29 (26.6%) | 3 (14.3%) | 26 (29.6%) | 0.155c | ||
| Chest tightness | 13 (11.9%) | 3 (14.3%) | 10 (11.4%) | 1.000d | ||
| Expectoration | 37 (33.9%) | 1 (4.8%) | 36 (40.9%) | 0.002*,c | ||
| Diarrhea | 16 (14.7%) | 4 (19.1%) | 12 (13.6%) | 0.775d | ||
| Myalgia | 14 (12.8%) | 2 (9.5%) | 12 (13.6%) | 0.886d | ||
| Fatigue | 14 (12.8%) | 2 (9.5%) | 12 (13.6%) | 0.886d | ||
| Chills | 13 (11.9%) | 0 (0.0%) | 13 (14.8%) | 0.133d | ||
| Anorexia | 8 (7.3%) | 0 (0.0%) | 8 (9.1%) | 0.332d | ||
| Treatment | ||||||
| ICU admission (N = 117) | 5 (4.3%) | N = 21 | 3 (14.3%) | N = 96 | 2 (2.1%) | 0.040*,e |
| Hospital length of stay (N = 121)d | 14.00 (8.00–22.00) | N = 21 | 17.00 (9.00–29.00) | N = 100 | 14.00 (8.00–21.75) | 0.233b |
| Invasive ventilation (N = 121) | 5 (4.1%) | N = 21 | 2 (9.5%) | N = 100 | 3 (3.0%) | 0.207e |
| Non-invasive ventilation (N = 121) | 4 (3.3%) | N = 21 | 0 (0.0%) | N = 100 | 4 (4.0%) | 1.000e |
| High-flow oxygen therapy (N = 121) | 87 (71.9%) | N = 21 | 17 (82.00%) | N = 100 | 70 (70.0%) | 0.310c |
| Days of High-flow oxygen therapy (N = 87)d | 12.00 (5.00–22.00) | N = 17 | 14.00 (8.00–28.00) | N = 70 | 11.00 (5.00–22.00) | 0.251b |
| Glucocorticoid therapy (N = 121) | 23 (19.0%) | N = 21 | 5 (23.8%) | N = 100 | 18 (18.0%) | 0.756d |
| Days of glucocorticoid therapy (N = 23)d | 7.00 (4.25–11.75) | N = 5 | 11.00 (5.00–12.00) | N = 18 | 7.00 (4.00–9.00) | 0.730b |
| CT findings | N = 113 | N = 20 | N = 93 | |||
| Ground-glass opacity | 67 (59.3%) | 9 (45.0%) | 58 (62.4%) | 0.152c | ||
| Patchy shadows | 84 (74.3%) | 18 (90.0%) | 66 (71.0%) | 0.077c | ||
| Consolidation | 13 (11.5%) | 4 (20.0%) | 9 (9.7%) | 0.354d | ||
| Reticulation | 3 (2.7%) | 2 (10.0%) | 1 (1.1%) | 0.080e | ||
| Interlobular septal thickening | 1 (0.9%) | 1 (5.0%) | 0 (0.0%) | 0.177e | ||
| Pleural thickening | 10 (8.9%) | 3 (15.0%) | 7 (7.5%) | 0.526d | ||
| Hydropericardium | 1 (0.9%) | 0 (0.0%) | 1 (1.1%) | 1.000e | ||
| Lymphadenia | 23 (20.4%) | 2 (10.0%) | 21 (22.6%) | 0.336d | ||
| Bilateral pulmonary | 102 (90.3%) | 20 (100.0%) | 82 (88.2%) | 0.229d | ||
| Left lung | 6 (5.3%) | 0 (0.0%) | 6 (6.5%) | 0.537d | ||
| Right lung | 4 (3.5%) | 0 (0.0%) | 4 (4.3%) | 1.000e | ||
| Admission severity | N = 121 | N = 21 | N = 100 | 0.589c | ||
| Mild | 81 (66.9%) | 13 (61.9%) | 68 (68.0%) | |||
| Severe | 40 (33.1%) | 8 (38.1%) | 32 (32.0%) | |||
| Outcome | N = 121 | N = 21 | N = 100 | 0.439e | ||
| Survivor | 118 (97.5%) | 20 (95.2%) | 98 (98.0%) | |||
| Non-survivor | 3 (2.5%) | 1 (4.8%) | 2 (2.0%) | |||
Abbreviation: COVID-19, Coronavirus disease 2019; CT, Computerized; tomography; ICU, Intensive Care Unit; IQR, Interquartile range; SD, standard deviation.
Continuous variables were described as median (IQR) or mean (SD). p values were calculated by Mann–Whitney U non-parameter test for skewed distributed data and Student’s t-test was used for normal distributed data. Categorical variables were expressed as number (%). p values were calculated by Pearson χ2 test or Fisher's exact test.
aStudent’s t-test.
bMann–Whitney U non-parameter test.
cPearson χ2 test.
dPearson’s χ² test with Yates' continuity correction.
eFisher's exact test. *p < 0.05.
#Factors adjusted in the propensity score matching.
Data collection
We extracted demographics, clinical, CT image examination, laboratory examination, treatment, and outcome data of all study objects from the customized uniform electronic medical records system of Tongji Hospital, Tongji Medical College, Huazhong University of Sciences and Technology, Wuhan. The detailed and standardized information of demographic data, underlying comorbidities, initial symptoms, vital signs, and chest computed tomography (CT) were recorded at hospital admission. The admission and in-hospital data of these patients were collected, reviewed, and verified by a trained team of physicians. Two trained investigators independently viewed the data to verify the accuracy and all charts were checked by the principal investigators. The extracted indicators were based on items of WHO/International Severe Acute Respiratory and Emerging Infection Consortium case record form for severe acute respiratory infections with consideration of reflecting conditions regarding COVID-19 as much as possible (11). Laboratory examinations including routine bloods, immune cell subsets, inflammatory cytokines and biomarkers, and tests about cardiac function, renal function, liver function, pancreatic function, coagulation, and metabolism were detected on the first diagnosed date. All comorbidities and uncertain records were collected and clarified through communication with involved patients and their families. Up to now, all patients have discharged or died.
Definition
The COVID-19 patients were confirmed on RNA detection of SARS-CoV-2 in throat-swab specimens by real-time reverse transcription PCR or radiologic evidence of pneumonia and infiltrate on chest CT scan according to WHO interim guidance (12). The included COVID-19 patients were over 18 years old. The asthma patients had been ascertained through clinical symptoms, allergen test, lung function examination and exclusion of other interfering diseases according to the National Heart Lung and Blood Institute’s Guidelines for the Diagnosis and Management of Asthma and GINA guidelines before our study (13). Fever was defined as over 37.3 °C for axillary temperature.(5) All patients were clinically typed into two categories (mild and severe) upon admission, according to the WHO guidelines and the Seventh Revised Trial Version of the COVID-19 Diagnosis and Treatment Guidance (2020) of China (14,15). The criteria for discharge included (i) absence of fever for at least 3 days; (ii) substantial improvement in both lungs in chest CT and clinical respiratory symptoms, and (iii) throat-swab samples negative for SARS-CoV-2 RNA obtained at least 24 h apart (5).
Statistical analysis
Continuous variables were described as mean (SD) if they are normal distributed or median (IQR) if not. Categorical variables were presented as number (%). To eliminate the possible confounding effects derived from sex, age and comorbidities, propensity score matching with a 1:5 ratio was performed to identify a group of patients with similar baseline characteristics. Sex and comorbidities were encoded as binary variables with a caliper width set as 0.4 of the standard deviation of the logit of the propensity score. After matching, we compared the differences with corresponding statistic methods to guarantee the homogeneousness in age, sex and comorbidities between two groups. The absence of statistical differences between two groups was considered favorable matching. For continuous variables, Student’s t-test was used for normal distributed data and Mann–Whitney U non-parameter test for non-normal distributed data. The Pearson’s χ2 test, Pearson’s χ2 test with Yates' continuity correction or Fisher’s exact test were employed for categorical variables. All statistical analyses were performed by the SPSS 23.0 software and R (3.6.2). p < 0.05 was considered statistically significant.
Results
Clinical characteristics of COVID-19 patients with asthma
As shown in Table 1, the most commonly observed symptoms on admission were fever (15/21, [71.4%]), cough (11/21, [52.4%]), diarrhea (4/21, [19.1%]), chest tightness (3/21, [14.3%]) and dyspnea (3/21, [14.3%]) for COVID-19 patients with asthma. However, in non-asthma COVID-19 patients, fever (63/88, [71.6%]) was followed by cough (58/88, [65.9%]), expectoration (36/88, [40.9%]), dyspnea (26/88, [29.6%]) and chills (13/88, [14.8%]). Particularly, lower prevalence of expectoration existed in COVID-19 patients with asthma (1/21, [4.8% vs. 36/88, [40.9%]; p = 0.002).
We observed substantial differences in laboratory findings between COVID-19 patients with or without asthma, especially in liver, myocardial impairment and inflammation-related indicators (Table 2). Higher level of lactate dehydrogenas (LDH, 247.00[194.00–270.00] vs. 201.00[174.00–237.00] U/l, p = 0.032) was presented in COVID-19 patients with asthma, as well as high-sensitivity cardiac troponin I (hs-cTnI, 5.20[1.30–12.40] vs. 1.20[0.60–2.58] pg/mL, p = 0.002) and D-dimer (0.73[0.30–1.87] vs. 0.32[0.22–0.63] µg/ml, p = 0.026).
Table 2.
Laboratory examination of COVID-19 patients with asthma and non-asthma.
| Indicators | Total | Asthma | Non-asthma | p Value | ||
|---|---|---|---|---|---|---|
| N = 121 | N = 21 | N = 100 | ||||
| Laboratory examination | ||||||
| Inflammatory cytokines and biomarkers | ||||||
| IL-6 (N = 117), pg/ml | 1.57 (1.50–2.45) | N = 17 | 2.35 (1.50–3.61) | N = 100 | 1.53 (1.50–2.21) | 0.041*,b |
| IL-8 (N = 116), pg/ml | 7.55 (5.00–12.43) | N = 16 | 11.60 (7.05–19.58) | N = 100 | 7.50 (5.00–11.33) | 0.044*,b |
| IL-10 (N = 116), pg/ml | 5.00 (5.00–5.00) | N = 16 | 5.00 (5.00–5.15) | N = 100 | 5.00 (5.00–5.00) | 0.134b |
| TNF-α (N = 116), pg/ml | 6.45 (4.80–8.00) | N = 16 | 7.35 (5.63–11.15) | N = 100 | 6.40 (4.80–7.90) | 0.172b |
| IL-1β (N = 116), pg/ml | 5.00 (5.00–5.00) | N = 16 | 5.00 (5.00–5.03) | N = 100 | 5.00 (5.00–5.00) | 0.319b |
| IL-2R (N = 116), U/ml | 311.00 (238.75–485.50) | N = 16 | 350.00 (280.00–493.75) | N = 100 | 305.50 (237.75–483.00) | 0.359b |
| hs-CRP (N = 102), mg/l | 2.20 (1.90–5.08) | N = 18 | 2.75 (1.90–6.08) | N = 84 | 2.20 (1.90–4.78) | 0.629b |
| Procalcitonin (N = 111), ng/ml | 0.03 (0.02–0.05) | N = 17 | 0.05 (0.03–0.10) | N = 94 | 0.03 (0.02–0.05) | 0.036*,b |
| Immune cell subsets | ||||||
| Lymphocytes, # (N = 121), ×109/l | 1.43 (1.04–1.81) | N = 21 | 1.20 (0.91–2.05) | N = 100 | 1.44 (1.05–1.80) | 0.398b |
| Lymphocytes, % (N = 121) | 26.95 ± 11.41 | N = 21 | 21.69 ± 12.69 | N = 100 | 28.06 ± 10.88 | 0.019*,a |
| CD3 + CD4+ T cells, # (N = 26), /μl | 388.00 (70.29–652.00) | N = 7 | 670.00 (460.00–905.50) | N = 19 | 76.43 (69.13–424.50) | 0.007*,b |
| CD3-CD19+ B cells, # (N = 26), /μl | 161.00 (133.00–210.00) | N = 7 | 158.00 (150.50–215.50) | N = 19 | 164.00 (127.00–207.00) | 0.644b |
| CD3 + CD19- T cells, # (N = 26), /μl | 1108.50 ± 484.01 | N = 7 | 1254.43 ± 512.55 | N = 19 | 1054.74 ± 475.89 | 0.390a |
| CD3 + CD8+ T cells, # (N = 26), /μl | 382.88 ± 217.49 | N = 7 | 481.00 ± 252.92 | N = 19 | 346.74 ± 198.12 | 0.237a |
| CD3-CD16 + CD56+ NK cells, # (N = 26), /μl | 180.50 (141.00–235.00) | N = 7 | 169.00 (157.50–196.50) | N = 19 | 211.00 (118.00–252.00) | 0.611b |
| T cells + B cells + NK cells, # (N = 26), /μl | 1507.15 ± 582.84 | N = 7 | 1656.29 ± 621.45 | N = 19 | 1452.21 ± 575.53 | 0.466a |
| Organ damage indexes | ||||||
| ALT (N = 121), U/L | 19.00 (13.00–32.00) | N = 21 | 26.00 (13.00–43.00) | N = 100 | 18.00 (13.00–30.25) | 0.384b |
| AST (N = 121), U/L | 24.66 ± 14.20 | N = 21 | 27.62 ± 20.15 | N = 100 | 24.04 ± 12.66 | 0.442a |
| ALB (N = 121), g/L | 40.20 (37.20–42.10) | N = 21 | 39.00 (35.20–41.20) | N = 100 | 40.25 (37.63–42.13) | 0.292b |
| GLO (N = 121), g/L | 29.56 ± 3.64 | N = 21 | 30.29 ± 4.51 | N = 100 | 29.41 ± 3.44 | 0.313a |
| ALB/GLO (N = 121) | 1.35 ± 0.23 | N = 21 | 1.29 ± 0.28 | N = 100 | 1.36 ± 0.22 | 0.209a |
| Urea (N = 121), mmol/L | 4.50 (3.90–5.50) | N = 21 | 3.90 (3.00–5.00) | N = 100 | 4.50 (4.08–5.60) | 0.062b |
| LDH (N = 114), U/L | 208.50 (176.25–247.75) | N = 21 | 247.00 (194.00–270.00) | N = 93 | 201.00 (174.00–237.00) | 0.032*,b |
| ALP (N = 114), U/L | 65.00 (53.00–76.00) | N = 21 | 67.00 (56.00–81.00) | N = 93 | 64.00 (52.00–75.00) | 0.161b |
| Uric Acid (N = 121), μmol/l | 281.10 (225.00–337.00) | N = 21 | 282.50 (238.00–363.00) | N = 100 | 281.05 (222.75–330.58) | 0.634b |
| Myohemoglobin (N = 80), ng/ml | 29.10 (22.98–46.28) | N = 16 | 29.10 (24.93–67.53) | N = 64 | 29.05 (22.88–43.00) | 0.431b |
| CK (N = 93), U/l | 60.00 (45.00–89.00) | N = 17 | 60.00 (45.00–110.00) | N = 76 | 60.00 (45.50–84.50) | 0.992b |
| CK-MB (N = 78), U/l | 0.70 (0.40–1.10) | N = 16 | 0.85 (0.58–1.10) | N = 62 | 0.65 (0.40–1.18) | 0.453b |
| hs-cTnI (N = 121), pg/ml | 1.30 (0.70–3.50) | N = 21 | 5.20 (1.30–12.40) | N = 100 | 1.20 (0.60–2.58) | 0.002*,b |
| Platelet, # (N = 121), ×10^9/l | 224.00 (175.00–258.00) | N = 21 | 225.00 (173.00–272.00) | N = 100 | 223.00 (176.50–256.25) | 0.880b |
| D-dimer (N = 119), ug/ml | 0.36 (0.22–0.80) | N = 21 | 0.73 (0.30–1.87) | N = 98 | 0.32 (0.22–0.63) | 0.026*,b |
| APTT (N = 117), s | 37.30 (34.70–40.20) | N = 21 | 34.10 (32.30–40.60) | N = 96 | 37.40 (35.60–40.05) | 0.088b |
| PT (N = 120), s | 13.40 (12.98–14.00) | N = 21 | 13.30 (13.10–14.10) | N = 99 | 13.40 (12.95–14.00) | 0.717b |
| TT (N = 54), s | 16.25 (15.50–17.20) | N = 21 | 15.90 (15.20–17.80) | N = 33 | 16.50 (15.80–17.10) | 0.384b |
| FDP (N = 89), g/l | 4.00 (4.00–4.00) | N = 19 | 4.00 (4.00–5.35) | N = 70 | 4.00 (4.00–4.00) | 0.158b |
| Fibrinogen (N = 116), g/l | 3.40 (2.82–3.88) | N = 21 | 3.72 (3.39–4.32) | N = 95 | 3.34 (2.79–3.76) | 0.069b |
| Glucose (N = 92), mmol/l | 5.41 (4.97–6.82) | N = 21 | 5.91 (4.84–7.11) | N = 71 | 5.40 (5.01–6.55) | 0.948b |
| Blood routine | ||||||
| Leukocytes, # (N = 121), ×10^9/l | 5.29 (4.52–7.41) | N = 21 | 6.66 (4.85–9.00) | N = 100 | 5.20 (4.50–7.05) | 0.036*,b |
| Erythrocytes, # (N = 121), ×10^12/l | 4.22 ± 0.471 | N = 21 | 4.29 ± 0.474 | N = 100 | 4.21 ± 0.472 | 0.500a |
| Neutrophils, # (N = 121), ×10^9/l | 3.25 (2.51–4.96) | N = 21 | 4.73 (3.22–6.85) | N = 100 | 3.16 (2.40–4.52) | 0.012*,b |
| Monocytes, # (N = 121), ×10^9/l | 0.47 (0.37–0.58) | N = 21 | 0.49 (0.29–0.71) | N = 100 | 0.47 (0.387–0.532) | 0.811b |
| Eosinophils, # (N = 121), ×10^9/l | 0.06 (0.03–0.13) | N = 21 | 0.04 (0.00–0.13) | N = 100 | 0.07 (0.03–0.133) | 0.173b |
| Basophils, # (N = 121), ×10^9/l | 0.02 (0.01–0.03) | N = 21 | 0.02 (0.01–0.03) | N = 100 | 0.02 (0.01–0.03) | 0.825b |
| Hemoglobin (N = 121), g/l | 129.36 ± 14.14 | N = 21 | 131.57 ± 13.81 | N = 100 | 128.89 ± 14.24 | 0.427a |
Abbreviation: COVID-19, Coronavirus disease 2019;IL-6, Interleukin 6; IL-8, Interleukin 8; IL-10, Interleukin 10; TNF-α, Tumor necrosis factor α;IL-1β, Interleukin 1β; IL-2R, Interleukin 2 receptor; hs-CRP, High-sensitivity C-reactive protein; CD, Cluster of differentiation; NK cells, Natural killer cells; ALT, Alanine transaminase; AST, Aspartate transaminase; ALB, Albumin; GLO, Globulin; LDH, Lactic dehydrogenase; ALP, Alkaline phosphatase; CK, Creatine; CK-MB, Creatine kinase-MB; hs-cTnI, High-sensitivity cardiac troponin I; APTT, activated partial thromboplastin time; PT, Prothrombin time; TT, Thrombin time; FDP, Fibrinogen degradation product; IQR, Interquartile range; SD, standard deviation.
Continuous variables were described as median (IQR) or mean (SD). p values were calculated by Mann–Whitney U non-parameter test for skewed distributed data and Student’s t-test was used for normal distributed data.
aStudent’s t-test.
bMann–Whitney U non-parameter test
*p < 0.05.
We also observed that the serum levels of inflammatory cytokines including interleukin 6(IL-6, 2.35 [1.50–3.61] vs. 1.53 [1.50–2.21] pg/ml, p = 0.041), interleukin 8 (IL-8, 11.60 [7.05–19.58] vs. 7.50 [5.00–11.33] pg/ml, p = 0.044) and procalcitonin (PCT, 0.05 [0.03–0.10] vs. 0.03 [0.02–0.05] ng/ml, p = 0.036) were increased in COVID-19 patients with asthma. Additionally, elevated levels of leukocytes (6.66[4.85–9.00] vs. 5.20[4.50–7.05] × 109/l, p = 0.036), neutrophils (4.73[3.22–6.85] vs. 3.16[2.40–4.52] × 109/l, p = 0.012) and percentage of neutrophils (69.38 vs. 60.96, p = 0.007; Supplementary Table 1) were observed in COVID-19 patients with asthma. As to immune cell subsets, the percentages of monocytes and lymphocytes were significantly decreased in COVID-19 patients with asthma (6.96 vs. 8.83, p = 0.004; 21.69 vs. 28.06, p = 0.019; respectively; Supplementary Table 1), as well as CD3-CD19+ B cells (13.05 vs. 23.98, p = 0.045; Supplementary Table 1). However, CD3+CD4+ T cells (670.00[460.00–905.50] vs. 76.43[69.13–424.50]/μl, p = 0.007) was presented a dramatically elevated level in COVID-19 patients with asthma. Other indicators were presented in the Supplementary Table 1.
Outcomes of COVID-19 patients with asthma
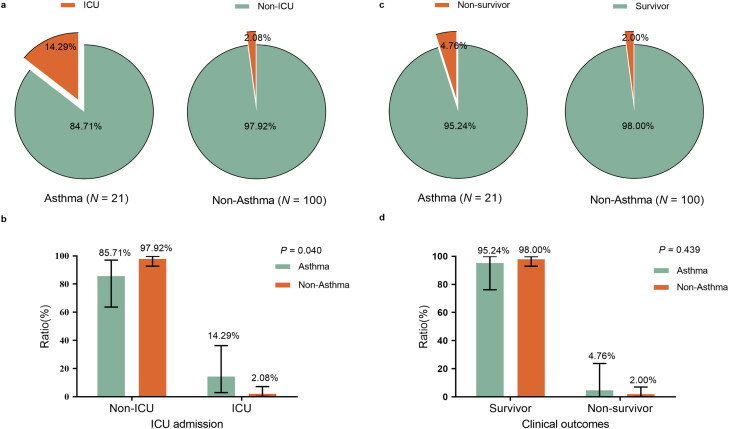
For the primary outcome during the observation period, the proportion of in-hospital death was 4.8% [1/21] in asthma patients and 2.0% [2/100] in non-asthma patients (p = 0.439; Table 1; Figure 1). In the terms of secondary outcomes, admission to ICU of the asthma patients is statistically significantly higher than that of the non-asthma patients (14.3% [3/21] vs. 2.1% [2/96]; p = 0.040) (Table 1; Figure 1). 61.9% [13/21] and 38.1% [8/21] of COVID-19 patients with asthma were classified into the mild and severe group on admission while 68(68.00%) and 32(32.00%) in non-asthma COVID-19 patients (p = 0. 589), respectively.
Figure 1.
Clinical outcomes and ICU admission of COVID-19 patients with asthma and without asthma. (a) Pie chart of ICU admission of COVID-19 patients with or without asthma. (b) Comparison of ICU admission of COVID-19 patients with or without asthma analyzed by Fisher’s exact test, p < 0.05 was considered statistically significant. (c) Pie chart of clinical outcomes of COVID-19 patients with or without asthma. (d) Comparison of clinical outcomes of COVID-19 patients with or without asthma analyzed by Fisher’s exact test, p < 0.05 was considered statistically significant.
Discussion
In this retrospective cohort study, we found that COVID-19 patients with asthma had a higher proportion of ICU admission than those without asthma. Additionally, fewer occurrence of expectoration and higher levels of IL-6, IL-8, PCT, leukocytes, neutrophils, CD4+T cells, D-dimer, LDH and hs-cTnI were observed, which suggested more aggravated inflammation storm and severer multiorgan injuries.
Expectoration is a common manifestation in COVID-19 patients (12), but not for COVID-19 patients with asthma in our study. It was observed amid the study that serum from rabbits with bronchial asthma resulted in ciliary dyskinesia (16). Together, the inflammation responses induced by asthma can result in more respiratory excreta and thicken the sputum, which might further decrease expectoration.
The aggravated inflammatory storm appears to be a prominent feature of COVID-19 patients with asthma. Supported by abundant evidence, T cells play an important role in asthma, particularly the Th2 cells in atopic allergic asthma (17). Compared to the non-asthma/A(H1N1)pdm09 group, the mouse model of asthma/A(H1N1)pdm09 infection showed excessive inflammation and higher virus replication in the bronchoalveolar lavage fluid (18,19). The assumption that COVID-19 patients with asthma presenting increased blood cytokines and inflammatory biomarkers might share similar mechanisms with it needs further exploration.
D-dimer deriving from the formation and lysis of cross-linked fibrin might be the indicator for the activation of fibrinolysis (5). SARS-Cov-2 infection can cause systemic pro-inflammatory cytokine responses, inducing the dysfunction of endothelial cells and then resulting in excessive generation of thrombin which can activate platelets and stimulate fibrinolysis (20). Likewise, the aggravated inflammatory storm in COVID-19 patients with asthma might intensify the fibrinolysis with elevated D-dimer.
Notably, it is reported that viral infection could deteriorate the illness of asthma patients (21,22). Up to one-third of adults with asthma who died or were hospitalized with pH1N1 suffered adverse clinical outcomes among pandemic influenza A in California (21). Furthermore, a recent study showed that asthma prolongs the incubation in COVID-19 patients (22), suggesting that individuals with asthma may require extra monitoring and care to prevent exacerbation. The increased IL-6, PCT, D-dimer, LDH, hs-cTnI, leukocytes, neutrophilia, and decreased lymphocytes studied in our study were all reported to be associated with adverse outcomes suffered by COVID-19 patients (5,23–28), among which the elevations of LDH and hs-cTnL indicate more severe liver and myocardial injuries, respectively. Besides, a higher prevalence of ICU admission and more pronounced inflammatory storm were exhibited among COVID-19 patients with asthma. These collectively provided evidence that the comorbidity of asthma might exasperate COVID-19, which highlights more intensive surveillance and protective treatment for cardiac and liver function.
The prevalence of asthma is heterogeneous, which is higher in industrialized countries than in developing countries generally (29). Additionally, lacking early concerns and treatment of asthma could account for a much lower prevalence of asthma in China than in other countries. Several studies indicated asthma was not associated with an increased risk of hospitalization and worse outcomes, which possibly resulted from the use of inhaled corticosteroids and a lower expression of ACE2 in asthmatics (30–33). While existing studies have suggested that corticosteroids may impair antiviral innate immune responses (34) and that ICS use leads to delayed virus clearance (35), which could exacerbate COVID-19 infection leading to poor prognosis. In addition, the merely observational analysis or non-matched comparison might cause several biases and obscure the effects of asthma on COVID-19. However, the detailed comparative analysis of laboratory characteristics with adjustments of potential confounding factors in our study might better illustrate this issue.
However, our study also has several limitations. First, the sample size is so small that statistical power is not enough for subtle differences and might have a certain false-negative rate. The samples with such a small size stop us from analyzing the risk factors of COVID-19 patients with asthma through COX proportional hazards model or logistic regression. Second, specific biological markers regarding asthma cannot be acquired and the missing data cannot be imputed on account of the nature of the retrospective study. Third, not all asthma patients were included in our study because of the absence of electronic medical records, which may cause selective bias and reduce the representativeness of samples. Fourth, the transmissibility of COVID-19 patients with asthma is still unclear. Moreover, the other asthma medications which are not compared may affect clinical results while they were rarely used. Fifth, though many comparisons were analyzed, we adopted an unadjusted α = 0.05 to avoid missing the differences between two groups to provide a more prompt message to clinicians, which statistically increased the possibility of Type I error. Future studies will be necessary to resolve these issues.
Conclusions
To our knowledge, this study systematically profiled the most detailed clinical characteristics of COVID-19 patients with asthma, suggesting that they had more aggravated inflammatory responses and multiple organ damages. In this case, more intensive surveillance and supportive treatment are required.
Supplementary Material
Acknowledgements
We acknowledge all patients and their families involved in the study and all health-care workers who are fighting against COVID-19 pneumonia.
Glossary
Abbreviations
- COVID-19
coronavirus disease 2019
- WHO
world health organization
- IQR
interquartile range
- SD
standard deviation
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- LDH
lactate dehydrogenase
- PCT
procalcitonin
- TC
total cholesterol
- LDL-C
low density lipoprotein
- hs-cTnI
high-sensitivity cardiac troponin I
- IL-6
interleukin 6
- IL-8
interleukin 8
- CD
Cluster of differentiation
- CT
computed tomographic
- ICU
intensive care unit
- IL-10
interleukin 10
- IL-13
interleukin 13
- TNF-α
tumor necrosis factor α
- TGF-β1
transforming growth factor-β1
- SARS
severe acute respiratory syndrome
- MERS
middle-east respiratory syndrome
Funding Statement
This work was supported by SARS-CoV-2 Pneumonia Emergency Technology Public Relations Project of Tongji Medical College, Huazhong University of Science and Technology under Grant 2020kfyXGYJ043 and National Key Research and Development Program of China under Grant 2016YFC1304403.
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request (Huilan Zhang, Email: huilanz_76@163.com).
Declaration of interests
The authors declare no conflicts of interest.
References
- 1.World Health Organization. Rolling updates on coronavirus disease (COVID-19). Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [last accessed 3 June 2020].
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, China Medical Treatment Expert Group for Covid-19, et al.. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian J, Miao X. Challenges and recommendations for cancer care in the COVID-19 pandemic. doi: 10.20892/j.issn.2095-3941.2020.0300 [DOI] [PMC free article] [PubMed]
- 4.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K, et al. . Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev 2020;36(7):e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bousquet J, Mantzouranis E, Cruz AA, Ait-Khaled N, Baena-Cagnani CE, Bleecker ER, Brightling CE, Burney P, Bush A, Busse WW, et al. . Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol 2010;126(5):926–938. doi: 10.1016/j.jaci.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, et al. . Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang K, Yang T, Xu J, Yang L, Zhao J, Zhang X, Bai C, Kang J, Ran P, Shen H, et al. . Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet 2019;394(10196):407–418. doi: 10.1016/S0140-6736(19)31147-X. [DOI] [PubMed] [Google Scholar]
- 9.Janson C, Chinn S, Jarvis D, Burney P.. Physician-diagnosed asthma and drug utilization in the European Community Respiratory Health Survey. Eur Respiratory J 1997;10(8):1795–1802. doi: 10.1183/09031936.97.10081795. [DOI] [PubMed] [Google Scholar]
- 10.Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD.. Asthma. Nat Rev Dis Primers 2015;1:15025 doi: 10.1038/nrdp.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO/International Severe Acute Respiratory and Emerging Infection. Consortium case record form for severe acute respiratory infections. Available from: https://media.tghn.org/medialibrary/2016/06/ISARIC-WHO-SARI_Case_Record_Form_7JAN16.pdf [last accessed 3 June 2020].
- 12.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Asthma Education and Prevention Program . Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Available from: http://www.nhlbi.nih.gov/ [last accessed 3 June 2020].
- 14.World Health Organization . Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. Available from: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected [last accessed 3 June 2020].
- 15.National Health Commission of the People’s Republic of China. 2020. Chinese management guideline for COVID-19 (version 7.0, in Chinese) [last accessed 3 March 2020].
- 16.Wilson GB, Fudenberg HH. Ciliary dyskinesia factors in cystic fibrosis and asthma . Nature 1977;266(5601):463–464. doi: 10.1038/266463a0. [DOI] [PubMed] [Google Scholar]
- 17.Amin K. The role of the T lymphocytes and remodeling in asthma. Inflammation 2016;39(4):1475–1482. doi: 10.1007/s10753-016-0380-9. [DOI] [PubMed] [Google Scholar]
- 18.Okada S, Hasegawa S, Hasegawa H, Ainai A, Atsuta R, Ikemoto K, Sasaki K, Toda S, Shirabe K, Takahara M, et al. . Analysis of bronchoalveolar lavage fluid in a mouse model of bronchial asthma and H1N1 2009 infection. Cytokine 2013;63(2):194–200. doi: 10.1016/j.cyto.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto Y, Hasegawa S, Matsushige T, Wakiguchi H, Nakamura T, Hasegawa H, Nakajima N, Ainai A, Oga A, Itoh H, et al. . Pulmonary inflammation and cytokine dynamics of bronchoalveolar lavage fluid from a mouse model of bronchial asthma during A(H1N1)pdm09 influenza infection. Sci Rep 2017;7(1):9128. doi: 10.1038/s41598-017-08030-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connors JM, Levy JH.. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mortensen E, Louie J, Pertowski C, Cadwell BL, Weiss E, Acosta M, Matyas BT.. Epidemiology and outcomes of adults with asthma who were hospitalized or died with 2009 pandemic influenza A (H1N1) – California, 2009. Influenza Other Respi Viruses 2013;7(6):1343–1349. doi: 10.1111/irv.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahdavinia M, Foster KJ, Jauregui E, Moore D, Adnan D, Andy-Nweye AAB, Khan S, Bishehsari F.. Asthma prolongs intubation in COVID-19. J Allergy Clin Immunol 2020;8:2388–2391. doi: 10.1016/j.jaip.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Zhou H, Zhou Y, Wu X, Zhao Y, Lu Y, Tan W, Yuan M, Ding X, Zou J, et al. . Risk factors associated with disease severity and length of hospital stay in COVID-19 patients. J Infect 2020;81(1):e95–e97. doi: 10.1016/j.jinf.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou W, Zhang W, Jin R, Liang L, Xu B, Hu Z.. Risk factors for disease progression in hospitalized patients with COVID-19: a retrospective cohort study. Infect Dis (London, England) 2020:1–8. doi: 10.1080/23744235.2020.1759817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang JJY, Lee KS, Ang LW, Leo YS, Young BE.. Risk factors of severe disease and efficacy of treatment in patients infected with COVID-19: a systematic review, meta-analysis and meta-regression analysis. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu L, Chen S, Fu Y, Gao Z, Long H, Wang J-M, Ren H-W, Zuo Y, Li H, Wang J, et al. . Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin Infect Dis 2020;52(7):498–505. doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Li Q, Jiang C, Zhou Y, Liu S, et al. . Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Y-Y, Wang R-R, Zhang D-W, Tu Y-H, Chen C-S, Ji S, Li C-X, Li X-Y, Zhou M-X, Cao W-S, et al. . Risk factors for severe COVID-19: evidence from 167 hospitalized patients in Anhui, China. J Infect 2020;81(1):e89–e92. doi: 10.1016/j.jinf.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015 . The Lancet. Respirat Med 2017;5:691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Foer D, Bates DW, Boyce JA, Zhou L.. Risk factors for hospitalization, intensive care, and mortality among patients with asthma and COVID-19. J Allergy Clin Immunol 2020;146:808–812. doi: 10.1016/j.jaci.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heffler E, Detoraki A, Contoli M, Papi A, Paoletti G, Malipiero G, Brussino L, Crimi C, Morrone D, Padovani M, et al. . COVID-19 in Severe Asthma Network in Italy (SANI) patients: clinical features, impact of comorbidities and treatments. Allergy 2020. doi: 10.1111/all.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grandbastien M, Piotin A, Godet J, Abessolo-Amougou I, Ederlé C, Enache I, Fraisse P, Tu Hoang TC, Kassegne L, Labani A, et al. . SARS-CoV-2 pneumonia in hospitalized asthmatic patients did not induce severe exacerbation. J Allergy Clin Immunol Pract 2020;8(8):2600–2607. doi: 10.1016/j.jaip.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beurnier A, Jutant EM, Jevnikar M, Boucly A, Pichon J, Preda M, Frank M, Laurent J, Richard C, Monnet X, et al. . Characteristics and outcomes of asthmatic patients with COVID-19 pneumonia who require hospitalisation. Eur Respir J 2020;56(5):2001875. doi: 10.1183/13993003.01875-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson JL, Carroll M, Yang IA, Reynolds PN, Hodge S, James AL, Gibson PG, Upham JW.. Reduced antiviral interferon production in poorly controlled asthma is associated with neutrophilic inflammation and high-dose inhaled corticosteroids. Chest 2016;149(3):704–713. doi: 10.1016/j.chest.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Southworth T, Pattwell C, Khan N, Mowbray SF, Strieter RM, Erpenbeck VJ, Singh D.. Increased type 2 inflammation post rhinovirus infection in patients with moderate asthma. Cytokine 2020;125:154857 doi: 10.1016/j.cyto.2019.154857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request (Huilan Zhang, Email: huilanz_76@163.com).