Abstract
Aims
Patients with heart failure (HF) have impaired quality of life (QoL). The randomized controlled trial PHARM‐CHF investigated whether an interdisciplinary intervention consisting of regular contacts with the community pharmacy and weekly dosing aids improves medication adherence in patients with HF. It is unknown how an intervention involving frequent structured pharmacy visits affects QoL. Our aim was to explore adherence to the intervention and effects on QoL.
Methods and results
Among 237 patients, n = 110 were randomized to pharmacy care and n = 127 to usual care. The pharmacy care group received a medication review followed by (bi‐)weekly dose dispensing and counselling. The median follow‐up was 2.0 years [inter‐quartile range (IQR) 1.2–2.7]. Median interval between pharmacy visits was 8.4 days (IQR 8.0–10.3) and the visits lasted in median 14 min (IQR 10–15). Median adherence to the intervention was 96% (IQR 84–100). QoL at 365 days was predefined as a main secondary and at 730 days as another secondary endpoint in PHARM‐CHF. QoL was measured by the Minnesota Living with Heart Failure Questionnaire; and for 111 patients (n = 47 in the pharmacy care group and n = 64 in the usual care group), data were available at baseline, and after 365 and 730 days (mean age 74 years; 41% female). Improvement in QoL was numerically higher in the pharmacy care group after 365 days and was significantly better after 730 days (difference in total scores −7.7 points [−14.5 to −1.0]; P = 0.026) compared to the usual care group. In all subgroups examined, this treatment effect was preserved. Improvements in the physical and emotional dimensions were numerically higher in the pharmacy care group after 365 days and were significantly better after 730 days: −4.0 points [−6.9 to −1.2]; P = 0.006, and −1.9 points [−3.7 to −0.1]; P = 0.039, respectively.
Conclusions
A pharmacy‐based interdisciplinary intervention was well received by the patients and suggests clinically important improvements in QoL.
Keywords: Chronic heart failure, Health‐related quality of life, Community pharmacy services, Interdisciplinary care, Randomized controlled trial
Introduction
Heart failure (HF) is an increasingly prevalent condition, limiting functional capacity associated with impaired quality of life (QoL) and mortality imposing a high burden on health care systems. 1 The QoL of HF patients is independent of the left ventricular ejection fraction (LVEF) 2 and was similar in patients with preserved (HFpEF) and reduced (HFrEF) LVEF in contemporary randomized clinical trials (RCTs) after accounting for variation in demographics, functional status, and symptom burden. 3 Poor QoL, measured by the Minnesota Living with Heart Failure Questionnaire (MLHFQ), is associated with increased risk of all‐cause death as well as the combined endpoint of cardiac death or hospitalization for worsening HF. 4 QoL of HF patients is more impaired than in age‐matched patients without chronic diseases and those with other co‐morbidities. 5 , 6
Apart from morbidity (hospital readmissions) and mortality, QoL is, therefore, a key target in the management of patients with HF. 7 , 8 Regulatory agencies are also increasingly recognizing the importance of QoL outcomes in HF, and patient‐reported outcomes are increasingly being used as endpoint in clinical trials. Moreover, health technology assessments include QoL as important patient‐relevant outcome. 3 , 9 , 10
In the outpatient setting, HF patients have to take care of their own daily therapy, usually without continuous support or surveillance of health care professionals. Pharmacists may provide successful interventions that improve patient outcomes. 11 , 12 , 13 Specifically, pharmacy‐based interventions may help HF patients in their medication management. 14 , 15
However, randomized evidence on improving medication adherence and QoL of HF patients in the outpatient setting is scarce. The PHARM‐CHF RCT found that an interdisciplinary pharmacist/physician intervention (pharmacy care) in comparison with usual care improves adherence to HF medication. 16 The intervention of PHARM‐CHF involved very frequent and structured visits to the community pharmacy. It is unknown whether this strategy itself and its effects on medication adherence have positive or negative effects on the QoL. QoL at 365 days was predefined as a main secondary and at 730 days as another secondary endpoint in PHARM‐CHF. 17 Our aim was, therefore, to explore adherence to the intervention and effects on QoL after 365 and 730 days.
Methods
Study design
A full description of the study design has been previously published. 16 , 17 In brief, PHARM‐CHF was an investigator‐initiated, prospective multicentre RCT with blinded adjudication of hospitalization events. Patients aged 60 years and older with chronic HF (CHF) defined by HF symptoms, currently treated with a diuretic and hospitalized for HF within the last 12 months or increased BNP (≥350 pg/mL) or N‐terminal pro‐BNP concentrations (NT‐proBNP; ≥1400 pg/mL), were recruited by study physicians. Patients were randomized via a secure web interface tool (www.pharm-chf.de) in a 1:1 ratio to the intervention (pharmacy care) or control group (usual care).
The intervention consisted of the following components: first, patients visited their attending physician for primary assessments (including QoL) and received a current medication list. A comprehensive medication review was performed by pharmacists in the community pharmacy. They compared the physician‐documented regimen with the current drug regimen reported by the patient in a brown bag interview. The pharmacist consulted with the attending physician on identified discrepancies and other drug‐related problems to consolidate the medication plan.
Pharmacy care continued by (bi‐)weekly visits to the community pharmacy including receiving a filled weekly dosing aid, measurement of blood pressure and pulse rate, and counselling. The type of the dosing aid (dosette, pill box) was at the discretion of the pharmacist and in agreement with the patient. The interaction in the community pharmacy included questions on the general health and HF symptoms such as shortness of breath, in addition to asking about potential problems with the pharmacotherapy. The intervention has been described in detail in the previously published design paper. 17
Patients in the usual care group continued to visit pharmacies of their choice to fill prescriptions without further intervention. Usual care mainly consisted of dispensing prescribed medication, including counselling by the pharmacist or pharmacy technician on the safe and appropriate use of the drugs. In Germany, medication review or providing medication in a weekly dosing aid is neither part of usual care nor reimbursed. 16 , 17
The PHARM‐CHF trial (ClinicalTrials.gov identifier: NCT01692119) was conducted according to the principles stated in the current version of the Declaration of Helsinki, International Conference on Harmonization Good Clinical Practice, and to local and national regulations. Documented approvals from independent ethics committees were obtained for all participating centres and written informed consent from all patients. 16 , 17
Outcome measures
Health‐related QoL was measured by the MLHFQ. The MLHFQ is one of the best characterized instruments to assess QoL 18 and has been highly rated in systematic reviews. 19 , 20 , 21 The MLHFQ total score ranges from 0 to 105 (0 = best and 105 = worst QoL; minimal clinically important difference 5 points), assessed by the patients at the day of their appointment with their physician at baseline, and after 365 and 730 days. A total score of <24 signifies a good QoL, a score between 24 and 45 signifies a moderate QoL, and a score of >45 signifies a poor QoL. The MLHFQ provides as well scores for the physical (eight items, range 0–40) and emotional (five items, range 0–25) dimensions. 18 , 19 , 22 , 23 , 24 According to the Statistical Analysis Plan as of 10 February 2019, change in MLHFQ overall score between baseline and 365 days was specified as a main secondary outcome and between baseline and 730 days as another secondary outcome.
Statistical analyses
Baseline characteristics are summarized as number of patients (%) for categorical variables and as mean (±SD) or median [inter‐quartile range (IQR)] for continuous variables. Changes in MLHFQ scores after 365 and after 730 days to baseline in both study groups were compared by analysis of covariance models adjusted for the baseline value. We analysed patients with data available at baseline, and after 365 and 730 days. We calculated the Pearson correlation coefficients to screen for correlations between variables and the MLHFQ total score. Variation in change of MLHFQ total scores from baseline to 730 days among patients is depicted graphically with a waterfall plot. A P‐value < 0.05 was considered statistically significant. All statistical analyses were performed using SAS® version 9.4 (SAS Institute, Cary, NC, USA).
Results
The study was performed at 31 sites including general practitioners, internal medicine specialists, and both office‐based and hospital‐based cardiologists, and 69 community pharmacies in nine different states of Germany. Among 237 patients, n = 110 were randomized to pharmacy care and n = 127 to usual care. For 111 patients (n = 47 in the pharmacy care group and n = 64 in the usual care group), MLHFQ data were available at baseline, and after 365 and 730 days. The remaining patients did not attend the final study visit at 730 days (owing to death, relocation, withdrawal of consent, or other reasons for dropout), or data on QoL were missing at baseline, at 365 days or 730 days' follow‐up. At baseline, the mean age of the 111 patients was 74 years (range 60–86), and 41% were female. At baseline, 21% had a LVEF < 40%, 37% a LVEF between 40% and 49%, and 42% a LVEF ≥ 50%. At baseline, 49% were in New York Heart Association (NYHA) functional class III and 5% in class IV. Both groups had similar baseline characteristics (Table 1 ). On average, patients suffered from seven co‐morbidities, received eight different drugs, and took 10 doses at three different time points per day; 17% of the patients were suspected to currently have depression [nine‐item Patient Health Questionnaire (PHQ‐9) score ≥ 10]. 16 Changes in PHQ‐9 scores and NYHA functional class were not statistically different between groups at 365 days and 730 days compared to baseline.
TABLE 1.
Baseline characteristics of the quality of life cohort, according to treatment group
| Characteristic | Pharmacy care (n = 47) | Usual care (n = 64) |
|---|---|---|
| Age, mean ± SD, years | 73.3 ± 6.3 | 74.5 ± 6.5 |
| Median (IQR) | 73.0 (69–78) | 75.0 (70–79) |
| ≥75 years, n (%) | 28 (60) | 33 (52) |
| Female sex, n (%) | 17 (36) | 28 (44) |
| BMI, a kg/m2, mean ± SD | 28.8 ± 4.5 | 29.4 ± 4.8 |
| LVEF, b mean ± SD, % | 50.4 ± 14.0 | 47.1 ± 14.6 |
| LVEF < 40%, n (%) | 7 (15) | 16 (25) |
| LVEF 40–49%, n (%) | 18 (38) | 23 (36) |
| LVEF ≥ 50%, n (%) | 22 (47) | 25 (39) |
| NYHA class, % | ||
| I/II | 49 | 44 |
| III/IV | 51 | 56 |
| Time since last hospitalization for HF, mean ± SD, years | 0.36 ± 0.67 | 0.26 ± 0.28 |
| Within the past 3 months, n (%) | 19 (42) | 25 (41) |
| Attending DMP CHD, module HF, yes, n (%) | 15 (32) | 20 (31) |
| Different co‐morbidities, mean ± SD | 7.5 ± 2.6 | 6.8 ± 2.2 |
| Medication, n (%) | ||
| No. drug packages, mean ± SD | 8.3 ± 2.8 | 8.2 ± 3.0 |
| No. single doses/day, mean ± SD | 9.6 ± 3.5 | 10.3 ± 4.0 |
| No. drug intakes/day, median (IQR) | 3.0 (2–3) | 3.0 (2–3) |
| HF‐medication, c n (%) | ||
| ACEi/ARB | 39 (83) | 54 (84) |
| Beta‐blocker | 45 (96) | 62 (97) |
| MRA | 14 (30) | 27 (42) |
| MLHFQ d total score, mean ± SD | 36.4 ± 20.5 | 37.4 ± 21.5 |
| Good (<24), n (%) | 13 (28) | 21 (33) |
| Moderate (24–45), n (%) | 19 (40) | 17 (27) |
| Poor (>45), n (%) | 15 (32) | 26 (41) |
| Physical dimension, mean ± SD | 18.6 ± 9.2 | 18.5 ± 9.9 |
| Emotional dimension, mean ± SD | 6.2 ± 5.8 | 6.5 ± 5.3 |
| Depression (PHQ‐9 e ), mean ± SD | 5.5 ± 4.8 | 6.2 ± 4.3 |
| PHQ‐9 score ≥ 10, % | 15 | 19 |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CHD, coronary heart disease; CV, cardiovascular; DMP, disease management programme; HF, heart failure; IQR, inter‐quartile range; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association (functional class); SD, standard deviation.
The body mass index (BMI) is the weight in kilogrammes divided by the square of the height in metres.
According to available chart data for n = 40 in the pharmacy care and n = 53 in the usual care group.
All patients received a diuretic.
Minnesota Living with Heart Failure Questionnaire (MLHFQ); total score 0–105 (0 = best QoL, 105 = worst QoL); a score < 24 signifies a good, a score between 24 and 45 a moderate, and a score > 45 a poor heart failure‐related quality of life.
Nine‐item Patient Health Questionnaire (score 0–27); patients with a score ≥ 10 are suspected to currently have depression.
Adherence to the intervention
The median follow‐up for the 237 patients randomized was 2.0 years (IQR 1.2–2.7). Median adherence to the pharmacy‐based intervention (n = 110) was 96% (IQR 84–100), and the median interval between pharmacy visits was 8.4 days (IQR 8.0–10.3) 16 ; 81% of the patients in the pharmacy care group opted for weekly visits and the remaining 19% for biweekly visits. The median (bi‐)weekly visit to the pharmacy lasted 14 min (IQR 10–15).
Health‐related quality of life
With a median MLHFQ total score of 37 (IQR 19–52), HF‐related QoL of the patients at baseline was moderate (Tables 1 and 2 ). Improvement in QoL was numerically higher in the pharmacy care group, compared with the usual care group, after 365 days and was significantly better after 730 days (difference in MLHFQ total scores −7.7 points [−14.5 to −1.0]; P = 0.026) (Table 2 ), 16 with no significant difference between the intention‐to‐treat and per‐protocol populations (data not shown). Interindividual variation in change of MLHFQ total score 730 days to baseline is shown as waterfall plot in Figure 1 . An improvement in the MLHFQ total score between baseline and 730 days of at least −5 points was observed in 47% of the patients in the pharmacy care group and in 38% of the usual care group [odds ratio (OR) 0.68, 95% confidence interval (CI) [0.32 to 1.46], P = 0.33]. A deterioration of the QoL (≥5 points) was observed in 34% and 45% of the patients (OR 1.61, 95% CI [0.74 to 3.50], P = 0.23).
TABLE 2.
Heart failure‐related quality of life (Minnesota Living with Heart Failure Questionnaire)
| MLHFQ | Days post randomization | Pharmacy care Mean ± SD (n = 47) | Usual care Mean ± SD (n = 64) | Intervention effecta (95% CI) | P‐value |
|---|---|---|---|---|---|
| Total score (0–105) | 0 | 36.4 ± 20.5 | 37.4 ± 21.5 | — | 0.789 |
| 365 | 31.4 ± 20.0 | 37.5 ± 22.1 | −5.5 (−12.2 to 1.1) | 0.102 | |
| 730 | 32.5 ± 19.3 | 40.8 ± 19.8 | −7.7 (−14.5 to −1.0) | 0.026 | |
| Physical dimension (0–40) | 0 | 18.6 ± 9.2 | 18.5 ± 9.9 | — | 0.977 |
| 365 | 16.6 ± 9.6 | 19.0 ± 10.0 | −2.4 (−5.4 to 0.5) | 0.107 | |
| 730 | 15.9 ± 7.7 | 19.9 ± 8.9 | −4.0 (−6.9 to −1.2) | 0.006 | |
| Emotional dimension (0–25) | 0 | 6.2 ± 5.8 | 6.5 ± 5.3 | — | 0.799 |
| 365 | 5.3 ± 5.0 | 6.4 ± 5.9 | −1.0 (−2.9 to 0.9) | 0.319 | |
| 730 | 5.6 ± 5.2 | 7.6 ± 4.9 | −1.9 (−3.7 to −0.1) | 0.039 |
CI, confidence interval; MLHFQ, Minnesota Living with Heart Failure Questionnaire.
Analysis of covariance of change to baseline (adjusted for baseline quality of life).
FIGURE 1.
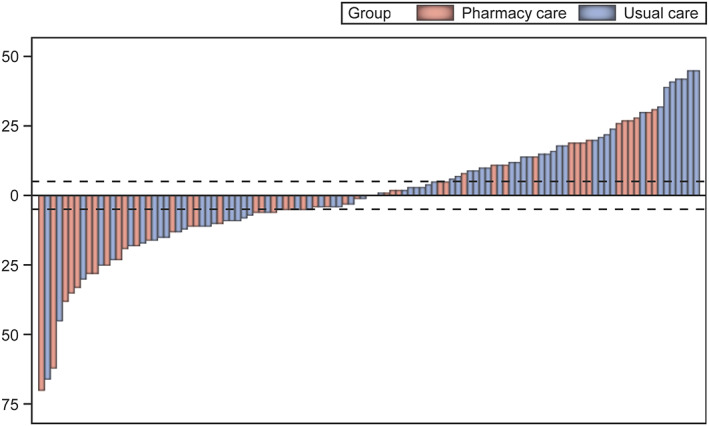
Waterfall plot change of the Minnesota Living with Heart Failure Questionnaire (MLHFQ) total score from baseline to 730 days for each patient of the intervention (pharmacy care) and control (usual care) groups. The dotted lines represent the minimal clinically important difference for improvement (−5 points) and worsening (+5 points) of heart failure‐related quality of life.
Improvement in the MLHFQ physical dimension score was numerically higher in the pharmacy care group, compared with the usual care group, after 365 days and was significantly better after 730 days (−4.0 points [−6.9 to −1.2]; P = 0.006). The MLHFQ emotional dimension score after 365 days improved only in the pharmacy care group, and the difference to the usual care group became significant after 730 days: −1.9 points [−3.7 to −0.1]; P = 0.039 (Table 2).
Sensitivity analyses
In all subgroups pre‐specified for the medication adherence endpoints, 17 plus the groups of patients classified as adherent [proportion of days covered (PDC) at least 80% during 365 or 730 days post randomization], the treatment effect for the MLHFQ total score was preserved. A consistent improvement in QoL after 2 years in patients receiving pharmacy care when compared with usual care was demonstrated. For all subgroups, there was no significant interaction (Figure 2 ).
FIGURE 2.
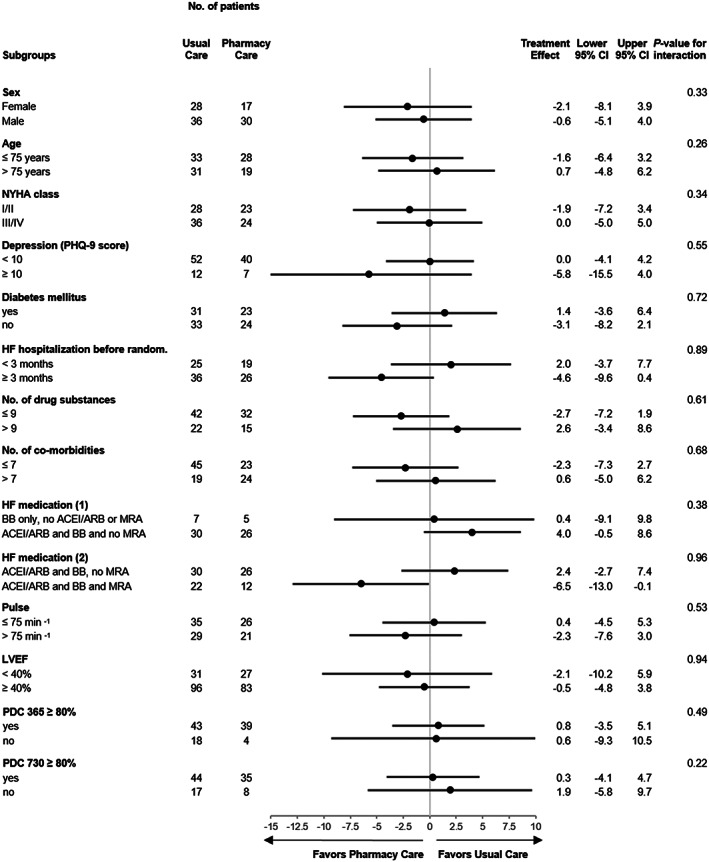
Forest plot of sensitivity analyses for the MLHFQ total score. Shown are data of baseline‐adjusted changes after 730 days, using analyses of covariance with each subgroup as a covariate, and treatment and the interaction between treatment and subgroup as covariates. The point estimate and the 95% confidence intervals (CIs) are stated for each subgroup. The P‐values of the interaction term (treatment and subgroups) are presented. ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta‐blocker; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; PDC, proportion of days covered; PHQ‐9, nine‐item Patient Health Questionnaire; random., randomization.
Change in MLHFQ total score at 730 days to baseline in patients with signs of depression (PHQ‐9 score ≥ 10, n = 19, mean change −15.5, 95% CI [−27.1 to −4.0]) and patients without signs of depression (PHQ‐9 score < 10, n = 92, mean change 3.6, 95% CI [−0.5 to 7.7]) is shown as boxplots in Figure 3 . With the use of the Pearson correlation coefficient, there was a weak correlation between signs of depression and change of MLHFQ total score at 730 days to baseline (r = −0.269, P < 0.002). Correlation coefficients for all other variables were <0.2.
FIGURE 3.
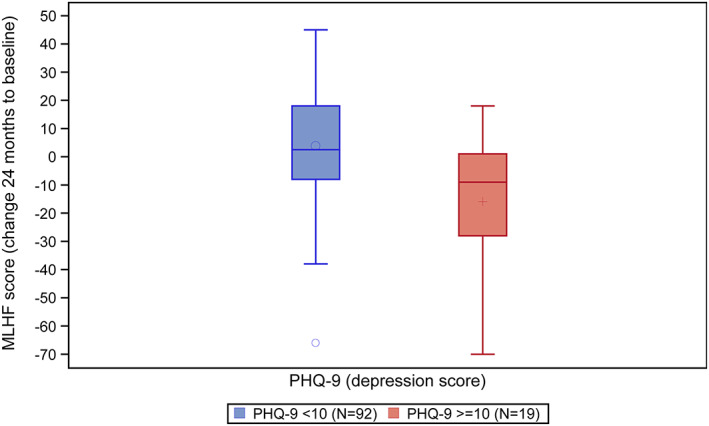
Boxplots of the changes of the Minnesota Living with Heart Failure (MLHF) Questionnaire scores 730 days to baseline in the subgroup of patients without [nine‐item Patient Health Questionnaire (PHQ‐9) score < 10, n = 92] and with (PHQ‐9 score ≥ 10, n = 19) signs of depression.
Discussion
The main finding of this analysis suggests that the interdisciplinary intervention involving (bi‐)weekly structured visits to the community pharmacy led to long‐term and quantitatively important improvements in QoL in elderly patients with CHF. HF patients are often symptomatic and have a poor QoL. 5 , 25 Improving QoL is acknowledged as a fundamental goal of HF management in the guidelines. 26 , 27
Heart failure‐specific QoL in our study at baseline was moderate (median score 37) and independent of LVEF, which is, for example, comparable with findings in the CHARM programme with a mean MLHFQ summary score of 41. 2
A recent systematic review and meta‐analysis included 18 RCTs comparing pharmacist‐involved multidisciplinary interventions with usual care. Among the five RCTs measuring QoL, only four studies reported significant improvement and difference. None reported QoL data beyond 12 months. 13 For example, Korajkic et al. explored the impact of a 3 months' pharmacist intervention on patient‐guided diuretic dose adjustment in ambulatory patients with HF. Seventy‐five patients were recruited and 1:1 randomized. QoL measured by the MLHFQ (total score) was significantly lower in the intervention group (38 ± 20) compared with the control group (48 ± 19; P = 0.03). In a comparable pharmaceutical care RCT, n = 104 patients in each group completed the 12 month trial. 15 QoL measured by the MLHFQ improved significantly in the intervention when compared with the usual care group.
Overall, QoL outcomes beyond 12 months in chronic diseases are very rarely available. 6 , 14 , 21 , 28 , 29 , 30 , 31 Therefore, it remains unclear whether other interventions without significant between‐group differences at 6 months or 1 year may have led to improvements in the long run. In general, however, it seems unlikely that shorter interventions would result in an improved QoL in the long run.
Above all and compared with the generally modest, if significant, improvement of QoL by other HF interventions, 6 , 21 , 32 , 33 including device therapy or telemonitoring, 30 , 31 the 7.7 points' change in the MLHFQ score in favour of pharmacy care is of significant clinical importance. Of note, patients' QoL improved independent of sex, age, EF, disease severity, burden of illnesses, or pill burden. The data suggest a more pronounced effect of the intervention on the physical compared with the emotional dimension of the MLHFQ. Although in agreement with findings of an RCT exploring the impact of a 3 months' pharmacist intervention on QoL of ambulatory patients with HF in Australia and measured by the MLHFQ, 14 we do not have a conclusive explanation for a significantly different impact on physical compared with emotional components of HF‐related QoL.
Depression is common in HF and associated with adverse clinical outcomes. 34 , 35 The PHQ‐9 is a commonly used instrument facilitating not only diagnosis but also estimation of severity of depressive symptoms. 36 Depression as assessed by the PHQ‐9 was shown to independently predict health care use and mortality in patients with HF. 35 The 21‐item MLHFQ focuses on the burden of HF in individuals' well‐being. 37 , 38 A signal for an improved QoL from baseline to 730 days in patients with compared with patients without signs of depression was observed. However, this subgroup of patients was rather small. Although there was no significant difference in the primary medication adherence outcomes for patients with and without suspected depression in PHARM‐CHF, 16 the potential impact on QoL of patients with HF and depression should be explored in a specifically designed trial.
Whether the MLHFQ is sensitive to detect differences in QoL on the basis of the change in level of adherence to medication remains unclear. Recently, Chambela et al. suggested a relationship of an improvement in QoL, measured by the MLHFQ with an increase in medication adherence. This RCT compared pharmaceutical care with standard care in 81 patients with Chagas disease and HF. 39 Uchmanowicz and colleagues concluded that with an increasing QoL, the level of adherence to therapeutic recommendations among elderly hypertensive patients increases. 40 Further findings support an association between medication adherence and QoL among patients with hypertension (and/or diabetes). 41 Whether this association holds true for CHF patients remains unclear. Our data do not suggest a significantly different effect of the intervention on HF‐related QoL between patients with high (PDC ≥ 80%) compared with low adherence to HF medications (PDC < 80%), both during 365 and 730 days post randomization.
Our study design and the intervention applied are difficult to compare with those of the literature. Previous RCTs applied different, interdisciplinary or multidisciplinary interventions for usually <1 year, in different settings. 14 , 15 , 42 , 43 , 44 , 45 , 46 In the majority of studies, QoL did not differ between groups. 42 , 43 , 44 , 45 , 46 For example, the HeartMed RCT utilized community pharmacists to provide two home visits 2 to 8 weeks after discharge including a drug review and self‐management and lifestyle advice to 149 intervention patients for 6 months. Eligible patients were adults, admitted as an emergency in which ‘heart failure was an important ongoing clinical condition’. The investigators found no difference in hospitalizations or self‐reported adherence. MLHFQ was completed by 78 intervention patients and 80 control patients at 6 months (66% of surviving intervention patients and 67% of surviving controls). Whereas intervention patients' scores increased (worsened) slightly, those for control patients decreased (improved) slightly. 46
A recent systematic review on disease management interventions for HF found that QoL was generally poorly reported (median follow‐up was 6 months), with high attrition. Low‐quality evidence indicated that clinic‐based interventions may result in little or no difference in QoL. 21
Nine of 11 structured telephone support studies, and five of 11 telemonitoring studies reported significant improvements in QoL. 47 For example, Ferrante et al. found a 4.4‐point difference in the MLHFQ total score by a telephone intervention vs. usual care. 48 The recent TIM‐HF II telemonitoring RCT found no significant differences in the changes in the MLHFQ total score between baseline and 12 months. 31
In general, previous HFpEF and HFrEF RCTs have observed only a modest improvement of QoL by pharmacotherapy. 3 , 6 , 29 A recent systematic review and meta‐analysis identified nine trials reporting drug treatment effects on QoL measured by the MLHFQ. 49 Overall estimate showed that pharmacotherapy resulted in better, although modestly improved, QoL scores (−1.63 points, 95% CI [−2.94 to −0.31], P = 0.001). For example, in the TOPCAT trial studying symptomatic HFpEF patients, use of spironolactone was associated with a modest improvement in QoL. Adjusted mean changes measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ), for the spironolactone group, were significantly better than those for the placebo group at 4‐month (1.54; P = 0.002), 12‐month (1.35; P = 0.02), and 36‐month (1.86; P = 0.02) visits. 29 As for the MLHFQ, the minimal clinically important difference in the KCCQ score is 5 points. 50
In the recent PARAGON‐HF trial studying symptomatic HFpEF patients, between baseline and month 8, there was a mean decrease (hence, worse QoL) in the KCCQ clinical summary score of 1.6 points in the sacubitril–valsartan group and 2.6 points in the valsartan group (between‐group difference, 1.0 point; 95% CI [0.0 to 2.1]). 33
Limitations
The findings of this study should be interpreted in consideration of the following potential limitations. First, patients were not blinded to the intervention, and this may have biased their reports of their health status. The significant differences in the changes in QoL scores to baseline between 365 and 730 days do not suggest a relevant bias, however. Second, the statistical analysis plan did neither pre‐specify the subgroups with regard to QoL nor report any sensitivity analysis to impute scores for patients who died or with missing values. Third, QoL information was not available for all patients at all three time points. However, the rates of missing MLHFQ values were similar for both groups, and the number of deaths was not significantly different. 16 Fourth, the number of patients in the intervention and the control groups is relatively small. Hence, especially the subgroup analyses should be interpreted with caution. Finally and given that HF‐specific QoL after 365 days was a main outcome and after 730 days another secondary outcome in PHARM‐CHF, our findings have to be considered exploratory, warranting future randomized studies.
Conclusions
A pharmacy‐based interdisciplinary intervention, when compared with usual care, not only improved adherence to HF medication 16 but also suggested long‐term clinically important improvements in HF‐related QoL. Patients' adherence to the intervention was high.
Conflict of interest
None to declare.
Funding
This work was supported by ABDA – Federal Union of German Associations of Pharmacists, Berlin; Apotheker‐Foundation Westphalia‐Lippe, Muenster; Chamber of Pharmacists North Rhine, Dusseldorf; Lesmueller Foundation, Munich; and Foundation Pharmaceutical Care Berlin, Germany. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgements
We are grateful to all physicians, pharmacists, study nurses, and staff at the investigative sites and especially to all patients involved.
Schulz, M. , Griese‐Mammen, N. , Schumacher, P. M. , Anker, S. D. , Koehler, F. , Ruckes, C. , Rettig‐Ewen, V. , Wachter, R. , Trenk, D. , Böhm, M. , and Laufs, U. (2020) The impact of pharmacist/physician care on quality of life in elderly heart failure patients: results of the PHARM‐CHF randomized controlled trial. ESC Heart Failure, 7: 3310–3319. 10.1002/ehf2.12904.
References
- 1. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol 2016; 13: 368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lewis EF, Lamas GA, O'Meara E, Granger CB, Dunlap ME, McKelvie RS, Probstfield JL, Young JB, Michelson EL, Halling K, Carlsson J, Olofsson B, McMurray JJV, Yusuf S, Swedberg K, Pfeffer MA. Characterization of health‐related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail 2007; 9: 83–91. [DOI] [PubMed] [Google Scholar]
- 3. Chandra A, Vaduganathan M, Lewis EF, Claggett BL, Rizkala AR, Wang W, Lefkowitz MP, Shi VC, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, McMurray JJV, Solomon SD. Health‐related quality of life in heart failure with preserved ejection fraction: the PARAGON‐HF Trial. JACC Heart Fail; 7: 862–874. [DOI] [PubMed] [Google Scholar]
- 4. Kato N, Kinugawa K, Seki S, Shiga T, Hatano M, Yao A, Hirata Y, Kazuma K, Nagai R. Quality of life as an independent predictor for cardiac events and death in patients with heart failure. Circ J 2011; 75: 1661–1669. [DOI] [PubMed] [Google Scholar]
- 5. Vaishnava P, Lewis EF. Assessment of quality of life in severe heart failure. Curr Heart Fail Rep 2007; 4: 170–177. [DOI] [PubMed] [Google Scholar]
- 6. Lewis EF, Claggett BL, McMurray JJV, Packer M, Lefkowitz MP, Rouleau JL, Liu J, Shi VC, Zile MR, Desai AS, Solomon SD, Swedberg K. Health‐related quality of life outcomes in PARADIGM‐HF. Circ Heart Fail 2017; 10: pii: e003430. [DOI] [PubMed] [Google Scholar]
- 7. Lewis EF. Assessing the impact of heart failure therapeutics on quality of life and functional capacity. Curr Treat Options Cardiovasc Med 2013; 15: 425–436. [DOI] [PubMed] [Google Scholar]
- 8. Rumsfeld JS, Alexander KP, Goff DC, Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, Spertus JA, Sullivan MD, Treat‐Jacobson D, Zerwic JJ. Cardiovascular health: The importance of measuring patient‐reported health status: a scientific statement from the American Heart Association. Circulation 2013; 127: 2233–2249. [DOI] [PubMed] [Google Scholar]
- 9. U.S. Department of Health and Human Services Food and Drug Administration . Treatment for heart failure: endpoints for drug development: guidance for industry. Draft Guidance 06/18/19. Rockville, MD, USA; 2019.
- 10. Institute for Quality and Efficiency in Health Care. General methods. Version 5.0 of 10 July 2017. https://www.iqwig.de/en/methods/methods-paper.3020.html (13 February 2020). [PubMed]
- 11. McKay C, Park C, Chang J, Brackbill M, Choi J‐Y, Lee JH, Kim SH. Systematic review and meta‐analysis of pharmacist‐led transitions of care services on the 30‐day all‐cause readmission rate of patients with congestive heart failure. Clin Drug Investig 2019; 39: 703–712. [DOI] [PubMed] [Google Scholar]
- 12. West LM, Williams JB, Faulkenberg KD. The impact of pharmacist‐based services across the spectrum of outpatient heart failure therapy. Curr Treat Options Cardiovasc Med 2019; 21: 59. [DOI] [PubMed] [Google Scholar]
- 13. Parajuli DR, Kourbelis C, Franzon J, Newman P, McKinnon RA, Shakib S, Whitehead D, Clark RA. Effectiveness of the pharmacist‐involved multidisciplinary management of heart failure to improve hospitalizations and mortality rates in 4630 patients: a systematic review and meta‐analysis of randomized controlled trials. J Card Fail 2019; 25: 774–756. [DOI] [PubMed] [Google Scholar]
- 14. Korajkic A, Poole SG, MacFarlane LM, Bergin PJ, Dooley MJ. Impact of a pharmacist intervention on ambulatory patients with heart failure: a randomised controlled study. J Pharm Pract Research (Journal of Pharmacy Practice and Research) 2011; 41: 126–131. [Google Scholar]
- 15. Sadik A, Yousif M, McElnay JC. Pharmaceutical care of patients with heart failure. Br J Clin Pharmacol 2005; 60: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schulz M, Griese‐Mammen N, Anker SD, Koehler F, Ihle P, Ruckes C, Schumacher PM, Trenk D, Böhm M, Laufs U. Pharmacy‐based interdisciplinary intervention for patients with chronic heart failure: results of the PHARM‐CHF randomized controlled trial. Eur J Heart Fail 2019; 21: 1012–1021. [DOI] [PubMed] [Google Scholar]
- 17. Laufs U, Griese‐Mammen N, Krueger K, Wachter A, Anker SD, Koehler F, Rettig‐Ewen V, Botermann L, Strauch D, Trenk D, Böhm M, Schulz M. PHARMacy‐based interdisciplinary program for patients with Chronic Heart Failure (PHARM‐CHF): rationale and design of a randomized controlled trial, and results of the pilot study. Eur J Heart Fail 2018; 20: 1350–1359. [DOI] [PubMed] [Google Scholar]
- 18. Bilbao A, Escobar A, García‐Perez L, Navarro G, Quirós R. The Minnesota Living with Heart Failure Questionnaire: comparison of different factor structures. Health Qual Life Outcomes 2016; 14: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garin O, Herdman M, Vilagut G, Ferrer M, Ribera A, Rajmil L, Valderas JM, Guillemin F, Revicki D, Alonso J. Assessing health‐related quality of life in patients with heart failure: a systematic, standardized comparison of available measures. Heart Fail Rev 2014; 19: 359–367. [DOI] [PubMed] [Google Scholar]
- 20. Kelkar AA, Spertus J, Pang P, Pierson RF, Cody RJ, Pina IL, Hernandez A, Butler J. Utility of patient‐reported outcome instruments in heart failure. JACC Heart Fail 2016; 4: 165–175. [DOI] [PubMed] [Google Scholar]
- 21. Takeda A, Martin N, Taylor RS, Taylor SJ. Disease management interventions for heart failure. Cochrane Database Syst Rev 2019; 1: CD002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aaronson NK, Acquadro C, Alonso J, Apolone G, Bucquet D, Bullinger M, Bungay K, Fukuhara S, Gandek B, Keller S. International Quality of Life Assessment (IQOLA) Project. Qual Life Res 1992; 1: 349–351. [DOI] [PubMed] [Google Scholar]
- 23. Munyombwe T, Höfer S, Fitzsimons D, Thompson DR, Lane D, Smith K, Astin F. An evaluation of the Minnesota Living with Heart Failure Questionnaire using Rasch analysis. Qual Life Res 2014; 23: 1753–1765. [DOI] [PubMed] [Google Scholar]
- 24. Behlouli H, Feldman DE, Ducharme A, Frenette M, Giannetti N, Grondin F, Michel C, Sheppard R, Pilote L. Identifying relative cut‐off scores with neural networks for interpretation of the Minnesota Living with Heart Failure questionnaire. Conf Proc IEEE Eng Med Biol Soc 2009; 2009: 6242–6246. [DOI] [PubMed] [Google Scholar]
- 25. Gallagher AM, Lucas R, Cowie MR. Assessing health‐related quality of life in heart failure patients attending an outpatient clinic: a pragmatic approach. ESC Heart Fail 2019; 6: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Heart Failure Association (HFA) of the ESC: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 2016: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 27. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 28. Mohammed MA, Moles RJ, Chen TF. Impact of pharmaceutical care interventions on health‐related quality‐of‐life outcomes: a systematic review and meta‐analysis. Ann Pharmacother 2016; 50: 862–881. [DOI] [PubMed] [Google Scholar]
- 29. Lewis EF, Kim H‐Y, Claggett B, Spertus J, Heitner JF, Assmann SF, Kenwood CT, Solomon SD, Desai AS, Fang JC, McKinlay SA, Pitt BA, Pfeffer MA. Impact of spironolactone on longitudinal changes in health‐related quality of life in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail 2016; 9: e001937. [DOI] [PubMed] [Google Scholar]
- 30. Jayaram NM, Khariton Y, Krumholz HM, Chaudhry SI, Mattera J, Tang F, Herrin J, Hodshon B, Spertus JA. Impact of telemonitoring on health status. Circ Cardiovasc Qual Outcomes 2017; 10: pii: e004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan B‐A, Winkler S, Vettorazzi E, Bruch L, Oeff M, Zugck C, Doerr G, Naegele H, Störk S, Butter C, Sechtem U, Angermann C, Gola G, Prondzinsky R, Edelmann F, Spethmann S, Schellong SM, Schulze PC, Bauersachs J, Wellge B, Schoebel C, Tajsic M, Dreger H, Anker SD, Stangl K. Efficacy of telemedical interventional management in patients with heart failure (TIM‐HF2): a randomised, controlled, parallel‐group, unmasked trial. The Lancet 2018; 392: 1047–1057. [DOI] [PubMed] [Google Scholar]
- 32. Ferreira JP, Zannad F. Patient‐reported and morbidity–mortality endpoints: can one have the best of both worlds? Eur J Heart Fail 2019; 21: 71–73. [DOI] [PubMed] [Google Scholar]
- 33. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin‐Colet J, Cleland J, Düngen H‐D, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019; 391: 1609–1620. [DOI] [PubMed] [Google Scholar]
- 34. Piepenburg SM, Faller H, Gelbrich G, Störk S, Warrings B, Ertl G, Angermann CE. Comparative potential of the 2‐item versus the 9‐item patient health questionnaire to predict death or rehospitalization in heart failure. Circ Heart Fail 2015; 8: 464–472. [DOI] [PubMed] [Google Scholar]
- 35. Moraska AR, Chamberlain AM, Shah ND, Vickers KS, Rummans TA, Dunlay SM, Spertus JA, Weston SA, McNallan SM, Redfield MM, Roger VL. Depression, healthcare utilization, and death in heart failure: a community study. Circ Heart Fail 2013; 6: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rector TS, Kubo SH, Cohn JN. Patients' self‐assessment of their congestive heart failure: part 2: content, reliability and validity of a new measure, The Minnesota Living with Heart Failure Questionnaire. Heart Failure 1987; 3: 198–209. [Google Scholar]
- 38. Mohammed MA, Moles RJ, Chen TF. Pharmaceutical care and health related quality of life outcomes over the past 25 years: have we measured dimensions that really matter? Int J Clin Pharm 2018; 40: 3–14. [DOI] [PubMed] [Google Scholar]
- 39. Chambela MC, Mediano MFF, Carneiro FM, Ferreira RR, Waghabi MC, Mendes VG, Oliveira LS, de Holanda MT, de Sousa AS, da Costa AR, Xavier SS, da Silva GMS, Saraiva RM. Impact of pharmaceutical care on the quality of life of patients with heart failure due to chronic Chagas disease: randomized clinical trial. Br J Clin Pharmacol; 86: 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Uchmanowicz B, Chudiak A, Mazur G. The influence of quality of life on the level of adherence to therapeutic recommendations among elderly hypertensive patients. Patient Prefer Adherence 2018; 12: 2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khayyat SM, Mohamed MMA, Khayyat SMS, Hyat Alhazmi RS, Korani MF, Allugmani EB, Saleh SF, Mansouri DA, Lamfon QA, Beshiri OM, Abdul HM. Association between medication adherence and quality of life of patients with diabetes and hypertension attending primary care clinics: a cross‐sectional survey. Qual Life Res 2019; 28: 1053–1061. [DOI] [PubMed] [Google Scholar]
- 42. Bouvy ML, Heerdink ER, Urquhart J, Grobbee DE, Hoes AW, Leufkens HGM, Hoe AW. Effect of a pharmacist‐led intervention on diuretic compliance in heart failure patients: a randomized controlled study. J Card Fail 2003; 9: 404–411. [DOI] [PubMed] [Google Scholar]
- 43. Murray MD, Young J, Hoke S, Tu W, Weiner M, Morrow D, Stroupe KT, Wu J, Clark D, Smith F, Gradus‐Pizlo I, Weinberger M, Brater DC. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med 2007; 146: 714–725. [DOI] [PubMed] [Google Scholar]
- 44. Azad N, Molnar F, Byszewski A. Lessons learned from a multidisciplinary heart failure clinic for older women: a randomised controlled trial. Age Ageing 2008; 37: 282–287. [DOI] [PubMed] [Google Scholar]
- 45. Hogg W, Lemelin J, Dahrouge S, Liddy C, Armstrong CD, Legault F, Dalziel B, Zhang W. Randomized controlled trial of anticipatory and preventive multidisciplinary team care: for complex patients in a community‐based primary care setting. Can Fam Physician 2009; 55: e76–e85. [PMC free article] [PubMed] [Google Scholar]
- 46. Holland R, Brooksby I, Lenaghan E, Ashton K, Hay L, Smith R, Shepstone L, Lipp A, Daly C, Howe A, Hall R, Harvey I. Effectiveness of visits from community pharmacists for patients with heart failure: HeartMed randomised controlled trial. BMJ 2007; 334: 1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Inglis SC, Clark RA, Dierckx R, Prieto‐Merino D, Cleland JGF. Structured telephone support or non‐invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev 2015: CD007228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferrante D, Varini S, Macchia A, Soifer S, Badra R, Nul D, Grancelli H, Doval H. Long‐term results after a telephone intervention in chronic heart failure: DIAL (Randomized Trial of Phone Intervention in Chronic Heart Failure) follow‐up. J Am Coll Cardiol 2010; 56: 372–378. [DOI] [PubMed] [Google Scholar]
- 49. Zheng SL, Chan FT, Nabeebaccus AA, Shah AM, McDonagh T, Okonko DO, Ayis S. Drug treatment effects on outcomes in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. Heart 2018; 104: 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005; 150: 707–715. [DOI] [PubMed] [Google Scholar]


