Abstract
Aims
The aim of this study is to study the introduction of sacubitril/valsartan (sac/val) in Sweden with regards to regional differences, clinical characteristics, titration patterns, and determinants of use and discontinuation.
Methods and results
A national cohort of heart failure was defined from the Swedish Prescribed Drug Register and National Patient Register. A subcohort with additional data from the Swedish Heart Failure Registry (SwedeHF) was also studied. Cohorts were subdivided as per sac/val prescription and registration in SwedeHF. Median sac/val prescription rate was 20 per 100 000 inhabitants. Between April 2016 and December 2017, we identified 2037 patients with ≥1 sac/val prescription, of which 1144 (56%) were registered in SwedeHF. Overall, patients prescribed with sac/val were younger, more frequently male, and had less prior cardiovascular disease than non‐sac/val patients. In SwedeHF subcohort, patients prescribed with sac/val had lower ejection fraction. Overall, younger age [hazard ratio 2.81 (95% confidence interval 2.45–3.22)], registration in SwedeHF [1.97 (1.83–2.12)], male gender [1.50 (1.37–1.64)], ischaemic heart disease [1.50 (1.39–1.62)], lower left ventricular ejection fraction [3.06 (2.18–4.31)], and New York Heart Association IV [1.50 (1.22–1.84)] were predictors for sac/val use. As initiation dose in the sac/val cohort, 38% received 24/26 mg, 54% 49/51 mg, and 9% 97/103 mg. Up‐titration to the target dose was achieved in 57% of the overall cohort over a median follow‐up of 6 months. The estimated treatment persistence for any dose at 360 days was 82%.
Conclusions
Implementation of sac/val in Sweden was slow and varied five‐fold across different regions; younger age, male, SwedeHF registration, and ischaemic heart disease were among the independent predictors of receiving sac/val. Overall, treatment persistence and tolerability was high.
Keywords: Sacubitril/valsartan, Sweden, Real‐world, Registry
Introduction
Sacubitril/valsartan (sac/val) demonstrated a 20% relative risk reduction vs. enalapril for the primary composite outcome of cardiovascular death and heart failure (HF) hospitalization in patients with HF with reduced ejection fraction (HFrEF) in the PARADIGM‐HF trial. 1 Following PARADIGM‐HF, sac/val was approved by the Food and Drug Administration and European Medicines Agency and received a Class I recommendation in the European and US HF guidelines. 2 , 3
Sacubitril/valsartan is reimbursed in Sweden since April 2016. Sweden has a government‐funded healthcare system with a decentralized healthcare budget in 21 regions. To avoid unequal regional implementation of new drugs, the Swedish Association of Local Authorities and Regions (www.skr.se) has a structured national introduction programme; sac/val was part of this introduction programme. The proportion of HFrEF patients eligible for sac/val depend on the PARADIGM‐HF criteria and whether an equivalent of 10 or 20 mg daily of enalapril is considered required, on guidelines, and on label criteria. Three available studies reported eligibility ranging from 21% to 60% in Hull, UK, 4 34% to 76% in the Swedish Heart Failure Registry (SwedeHF), 5 and 12% to 84% in the ESC‐EORP‐HFA HF‐LT registry. 6 Actual implementation has been described elsewhere only in the USA 7 ; however, the factors determining the choice to initiate sac/val, as well as dose up‐titration and treatment persistence, are unknown. Therefore, we assessed (i) implementation of sac/val across Sweden, (ii) predictors of initiation/discontinuation, (iii) dose up‐titration, and (iv) treatment persistence.
Methods
Study design
We carried out a non‐interventional cohort study of patients aged ≥18 years with HF utilizing retrospective, real‐world, patient‐level data from Swedish registers, as described in the succeeding text, all of which are linked based on a unique personal identification number. HF patients with guideline‐directed medical therapy and patients with ≥1 sac/val prescription starting from April 2016 were included in the study and followed until December 2017.
Swedish National Patient Register (NPR)
The Swedish National Patient Register (NPR) is updated annually and contains ICD‐10 code administrative data from all completed inpatient admissions and specialized outpatient care, and provided the overall national cohort together with the Swedish Prescribed Drug Register (SPDR). It also provided extensive co‐morbidities for multivariable adjustment.
Swedish Prescribed Drug Register (SPDR)
The SPDR contains data on the medication prescribed and actually dispensed to the individual patient.
Swedish Heart Failure Register (SwedeHF)
Swedish Heart Failure Register (SwedeHF) is a national disease register including important HF‐related clinical, laboratory, and EF data of patients with HF. Hospitals and outpatient clinics report data into the register voluntarily at the time of hospital discharge or outpatient visit.
Cause of Death Register
The statistics on causes of death comprise all deaths, covering all Swedish residents, whether the person in question was a Swedish citizen or not and irrespective of whether the deaths occurred in Sweden or not. This was not used for causes of death but for death date for defining the end of follow‐up date for patients who died during the study period.
Longitudinal integration database for health insurance and labour market studies (LISA by Swedish acronym)
This database from statistics Sweden includes all individuals ≥16 years of age that were registered in Sweden as of 31 December for each year. It includes information on employment, sector of employment, income, the highest level of education, family status including the number of children, and marital status. These factors may affect prescription and filling of prescriptions and were used for multivariable adjustment.
For the included cohorts, socio‐demographics were taken from LISA (pre‐index date), co‐morbidities from NPR (pre‐index), clinical characteristics from SwedeHF (index), and prescription data from SPDR (pre‐index).
Cohorts
The overall national cohort included patients with either (i) a prescription of angiotensin‐converting enzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB) and beta‐blocker (BB) within 6 months, where the prescription date of the latter (index date) is between 1 April 2016 and 31 December 2017 and a hospitalization visit or visit to a specialist before index date with an ICD‐10 code of HF as main diagnosis, or (ii) a dispatched (filled) prescription of sac/val between 1 April 2016 and 31 December 2017. Furthermore, a subcohort of the overall cohort was identified by linkage to SwedeHF. Sac/val titration patterns were analysed in patients with minimum two filled sac/val prescriptions and 6 months of follow‐up. Key parameters included maximum individual dose reached, time to reach maximum dose, and down‐titration and discontinuation.
Statistics
During follow‐up, an individual can start and discontinue the sac/val treatment. Data were descriptively summarized for the subgroup receiving sac/val at the time of starting the treatment. For the subgroup of individuals never treated with sac/val during the follow‐up, the data were summarized at the study entry (see also Figure 2 legend). Continuous variables are presented as mean or median and categorical variables as number and percentage. For extraction, the last non‐missing value was used in SwedeHF.
FIGURE 2.
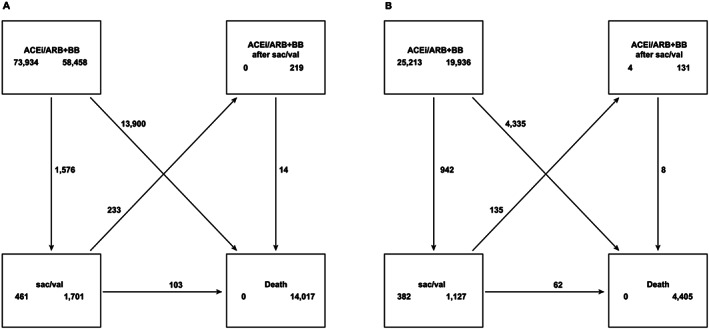
Transition pattern in a multi‐state model in the overall national cohort (Swedish National Patient Register) (A) and the Swedish Heart Failure Registry subcohort (B). (A) Patients transitioning treatment [all heart failure patients on angiotensin‐converting enzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB) + beta‐blocker (BB) to sacubitril/valsartan (sac/val)] and patients transitioning from sac/val treatment to ACEi/ARB + BB (sac/val non‐persistent patients) in overall national cohort (Swedish National Patient Register). (B) Patients transitioning treatment (all HF patients on ACEi/ARB + BB to sac/val) and patients transitioning from sac/val treatment to ACEi/ARB + BB (sac/val non‐persistent patients) in the Swedish Heart Failure Registry subcohort.
Note: Illustration of the transitions between the predefined states within the study period. The numbers in the boxes below the state names are the number of individuals starting in the state (left) and ending in the state (right). Numbers along the arrows are the number of individuals moving between the states. Baseline characteristics for the individuals when entering the sac/val state and for the individuals never entering the sac/val state at the time when entering the ACEi/ARB + BB state are summarized in Table 1.
Multi‐state models (Figures 3 and 4) assessed independent predictors for sac/val use for discontinuation. Two multivariable Cox models, corresponding to transitions from standard treatment to sac/val and from sac/val to standard treatment, were fitted. All continuous variables were modelled using restricted cubic splines with three degrees of freedom. Each variable's contribution to the model was assessed using the individual χ 2 – d.f. values, where d.f. denotes the degrees of freedom. The proportional hazards assumption was assessed by plotting the scaled Schoenfeld residuals vs. time for each variable. The number of individuals with follow‐up >540 days was small; therefore, persistence was assessed to a maximum follow‐up of 540 days. Persistence was defined by the days of supply dispensed, and treatment was defined to stop if no prescription was dispatched during a predefined grace period of 90 days.
FIGURE 3.
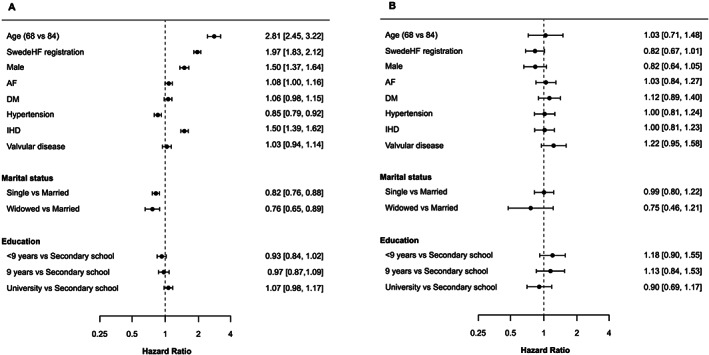
Hazard ratios of determinants for the transition to treatment with sacubitril/valsartan (sac/val) (A) and discontinuation from sac/val (B) in a multi‐state model in patients from overall national cohort.
Note: Hazard ratios for transitions to treatment with sac/val (A) and discontinuation from sac/val (B) using a grace period of 90 days. A hazard ratio >1 in (A) means a higher likelihood of getting sac/val, while a hazard ratio >1 in (B) means a higher likelihood of discontinuation of sac/val. AF, atrial fibrillation; DM, diabetes mellitus; IHD, ischaemic heart disease.
FIGURE 4.
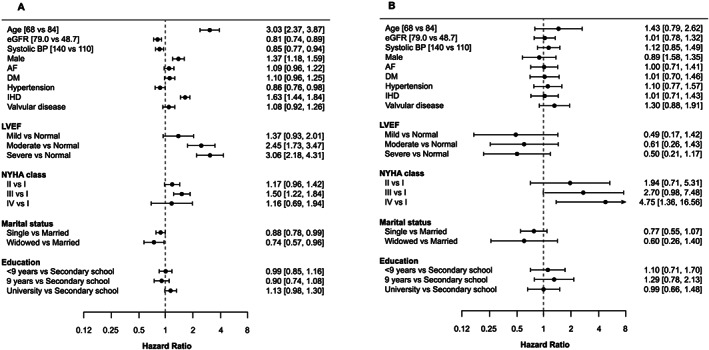
Hazard ratios of determinants for transition to treatment with sacubitril/valsartan (sac/val) (A) and discontinuation from sac/val (B) in a multi‐state model in patients from the Swedish Heart Failure Registry subcohort.
Note: Hazard ratios for transitions to treatment with sac/val (A) and discontinuation from sac/val (B) using a grace period of 90 days in patients with registration in the Swedish Heart Failure Registry. A hazard ratio >1 in (A) means a higher likelihood of getting sac/val, while a hazard ratio >1 in (B) means a higher likelihood of discontinuation of sac/val. AF, atrial fibrillation; BP, blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; IHD, ischaemic heart disease; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Ethics
This study was carried out in accordance with the Guidelines for Good Pharmacoepidemiology Practices and with the ethical principles laid down in the Declaration of Helsinki. This study also fulfils the criteria of a ‘European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) study’ and follows the ‘ENCePP Code of Conduct’. The study had ethics approval. No identifiable personal information of the participants in this retrospective observational study will be provided to any outside entity. All registers except SwedeHF are administrative and do not require patient consent. SwedeHF is a quality register and does not require consent, but patients are informed of entry into national quality registers and allowed to opt‐out.
Results
Implementation across Sweden
The median sac/val prescription rate ranged from 8 to 39 per 100 000 inhabitants across regions in Sweden (Figure 1 ), the median rate across Sweden was 20 per 100 000 inhabitants.
FIGURE 1.
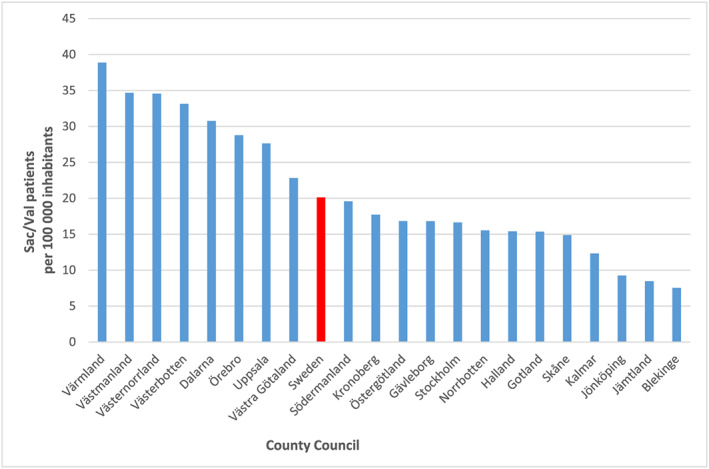
Total number of patients with dispatched sacubitril/valsartan (sac/val) prescription per region during the 21 month introduction period.
Baseline characteristics
Between April 2016 and December 2017, we identified 2037 patients with ≥1 sac/val prescription in the drug register, of which 1144 (coverage 56.1%) were registered in SwedeHF. During the corresponding period, the general HF population that filled the standard HF treatment prescription (other than sac/val; i.e. ACEI, ARB, and BB) was 72 358, of which 21 202 (coverage 29.3%) were registered in SwedeHF. In Table 1 , baseline characteristics were summarized at the time of treatment start for the sac/val subgroup and at the time of study entry for the non‐sac/val subgroup (the non sac/val users during the study period) in the overall national cohort and in the corresponding subcohorts with SwedeHF registrations. In the sac/val subgroup, patients were predominantly male (80% in overall national cohort and 80% in SwedeHF subcohort), with a mean age of 67.9 and 67.4 years. Left ventricular ejection fraction (LVEF) is not recorded in the patient register but in the SwedeHF subcohort (which contains EF); EF was <40% in 91%, but 40–49% in 7.2% and even ≥50% in 2.2%. More patients receiving sac/val had diabetes mellitus and ischaemic heart disease, while there was a lesser prevalence of atrial fibrillation. Sac/val patients had similar N‐terminalpro–B‐type natriuretic peptide but received considerably more HF treatments than non‐sac/val patients, and sac/val patients with index in 2016 had more frequently recent HF hospitalizations than non‐sac/val patients.
TABLE 1.
Baseline and clinical characteristics of overall national cohort (NPR and SPDR) and SwedeHF subcohort with or without sac/val
| Characteristics | Overall national cohort (NPR and SPDR) | SwedeHF subcohort | ||
|---|---|---|---|---|
| +sac/val | −sac/val | +sac/val | −sac/val | |
| N = 2037 | N = 72 358 | N = 1144 | N = 21 202 | |
| Socio‐demographics and socio‐economics | ||||
| Age (years), mean ±SD | 67.9 ± 11.3 | 75.2 ± 12.1 | 67.4 ± 11.2 | 74.0 ± 12.1 |
| ≥75 years, n (%) | 628 (30.8) | 41 248 (57.0) | 327 (28.6) | 11 238 (53.0) |
| Male, n (%) | 1633 (80.2) | 43 172 (59.7) | 915 (80) | 13 743 (64.8) |
| Married, n (%) | 1135 (56.1) | 32 162 (44.5) | 632 (55.3) | 9726 (45.9) |
| Education, n (%) | ||||
| Secondary school | 910 (45.5) | 28 715 (40.4) | 520 (46.1) | 8592 (41.3) |
| University | 452 (22.6) | 12 909 (18.2) | 246 (21.8) | 3743 (18.0) |
| Cardiovascular diseases a , n (%) | ||||
| Hypertension | 1101 (54.1) | 47 961 (66.3) | 616 (53.8) | 13 321 (62.8) |
| Ischaemic heart diseases | 1077 (52.9) | 33 801 (46.7) | 631 (55.2) | 10 073 (47.5) |
| Atrial fibrillation | 902 (44.3) | 37 570 (51.9) | 532 (46.5) | 11 632 (54.9) |
| Non‐cardiovascular diseases a , n (%) | ||||
| Diabetes mellitus | 563 (27.6) | 19 036 (26.3) | 332 (29.0) | 5628 (26.5) |
| Pulmonary diseases | 707 (34.7) | 30 091 (41.6) | 445 (38.9) | 9035 (42.6) |
| Anaemia | 85 (4.2) | 4560 (6.3) | 57 (5.0) | 1377 (6.5) |
| Stroke or TIA | 242 (11.9) | 11 879 (16.4) | 153 (13.4) | 3252 (15.3) |
| Psychiatric disorder | 7 (0.3) | 397 (0.5) | 3 (0.3) | 109 (0.5) |
| Malignant cancer | 210 (10.3) | 10 127 (14.0) | 128 (11.2) | 2947 (13.9) |
| Clinical status, n (%) | ||||
| NYHA I | — | — | 82 (8.6) | 2207 (13.7) |
| NYHA II | — | — | 456 (47.6) | 8595 (53.2) |
| NYHA III | — | — | 406 (42.4) | 5069 (31.4) |
| NYHA IV | — | — | 14 (1.5) | 280 (1.7) |
| LVEF, n (%) | ||||
| ≥50% | 25 (2.2) | 3479 (17.3) | ||
| 40–49% | 81 (7.2) | 4067 (20.3) | ||
| 30–39% | — | — | 402 (35.5) | 5855 (29.2) |
| <30% | — | — | 623 (55.1) | 6659 (33.2) |
| Treatments, n (%) | ||||
| ACEi/ARB | 1999 (98.1) | 72 358 (100.0) | 1136 (99.3) | 21 202 (100.0) |
| BB | 2015 (98.9) | 72 358 (100.0) | 1141 (99.7) | 21 202 (100.0) |
| MRA | 1852 (90.9) | 37 858 (52.3) | 1080 (94.4) | 13 372 (63.1) |
| ACEi/ARB + BB | 1990 (97.7) | 72 358 (100.0) | 1135 (99.2) | 21 202 (100.0) |
| ACEi/ARB + BB + MRA | 1830 (89.8) | 37 858 (52.3) | 1076 (94.1) | 13 372 (63.1) |
| CRT | — | — | 222 (19.5) | 1120 (5.4) |
| ICD | — | — | 342 (30) | 1411 (6.8) |
| Ivabradine | 59 (2.9) | 246 (0.3) | 40 (3.5) | 117 (0.6) |
| Anti‐diabetics | 640 (31.4) | 19 534 (27.0) | 366 (32.0) | 5710 (26.9) |
| SGLT‐2 inhibitors | 58 (2.8) | 437 (0.6) | 30 (2.6) | 112 (0.5) |
| Laboratory values | ||||
| Haemoglobin (g/dL), median (IQR) | — | — | 138.0 (128.0–149.0) | 136.0 (124.0–147.0) |
| Potassium (mmol/L), median (IQR) | — | — | 4.3 (4.0–4.6) | 4.2 (3.9–4.5) |
| NT‐proBNP (pg/mL), median (IQR) | — | — | 2090.0 (1056.8–4001.0) | 2060.0 (860.0–4482.2) |
| Pre‐index HF hospitalizations/outpatient visits, n (%) | ||||
| ≥1 (12 month pre‐index HF hospitalization) b | 188 (38.8) | 16 985 (24.5) | 119 (41.3) | 5032 (24.5) |
| ≥1 (30 day pre‐index HF hospitalization) b | 76 (15.7) | 5430 (7.8) | 49 (17.0) | 1327 (6.5) |
| ≥1 (12 month pre‐index HF outpatient visit) | 345 (71.3) | 24 294 (35.0) | 223 (77.4) | 7798 (38.0) |
| ≥1 (30 day pre‐index HF outpatient visit) b | 231 (47.7) | 6108 (8.8) | 147 (51.0) | 1577 (7.7) |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta‐blocker; CRT, cardiac resynchronization therapy; HF, heart failure; ICD, implantable cardioverter defibrillator; IQR, inter‐quartile range; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NPR, Swedish National Patient Register; NT‐proBNP, N‐terminal pro–B‐type natriuretic peptide; NYHA, New York Heart Association; sac/val, sacubitril/valsartan; SD, standard deviation; SGLT‐2, sodium–glucose cotransporter 2; SPDR, Swedish Prescribed Drug Register; SwedeHF, Swedish Heart Failure Registry; TIA, transient ischaemic attack.
The baseline characteristics are summarized for individuals when entering the sac/val state and for the individuals never entering the sac/val state at the time when entering the ACEi/ARB + BB state as illustrated in Figure 2 . Percentages for the subgroup are excluding missing data.
Only data from 2016.
Only data from 2016. Limited number of sac/val patients with index 2016, hence caution in interpretation as patients retrieving a sac/val treatment in 2016 can differ from patients retrieving it in 2017.
Predictors of use and non‐use of sacubitril/valsartan
Patients transitioning treatment from ACEi/ARB + BB (in the non sac/val group) to sac/val and patients transitioning from sac/val treatment to ACEi/ARB + BB (sac/val non‐persistent patients) for the overall national cohort and the SwedeHF subcohort are presented in Figure 2 A and 2 B , respectively. Among the individuals treated with sac/val (n = 2037), 1576 (77%) switched from ACEi/ARB + BB to sac/val during the study, and 461 (23%) entered the study directly with a sac/val prescription; 233 patients (11.4%) moved back to ACEi/ARB + BB from sac/val group during the study (Figure 2 A ). Similar patient flows were observed for patients registered in SwedeHF subcohort (Figure 2 B ).
Parameters associated with the baseline use and non‐use of sac/val for both the overall national cohorts and SwedeHF subcohort in order of apparent importance are shown in Figures 3 and 4 , respectively. Age was the most important predictor for sac/val use with younger patients more likely to have sac/val prescribed [hazard ratio (HR) 2.81 (95% confidence interval 2.45–3.22), for age 68 vs. 84]. Registration in SwedeHF was also associated with a higher likelihood of having sac/val prescribed with an HR of 1.97 (1.83–2.12). In addition, men were more likely to have sac/val prescribed than women [HR 1.50 (1.37–1.64)], as well as patients with a previous diagnosis of ischaemic heart disease were more likely [HR 1.50 (1.39–1.62)] to receive sac/val. Patients with a previous hypertension diagnosis and being single or widowed were less likely to have sac/val prescribed. Similar to the total sac/val overall cohort, in the SwedeHF subcohort, age was the most important predictor for sac/val use with younger patients three times more likely to have sac/val prescribed [HR 3.03 (2.37–3.87), for age 84 vs. 68]. Higher estimated glomerular filtration rate, higher systolic blood pressure, female gender, and to a lesser extent a previous hypertension diagnosis decreased the likelihood of having sac/val prescribed. A previous diagnosis of ischaemic heart disease, the severity of HF, and a higher New York Heart Association (NYHA) Class (III vs. I) were markedly associated with sac/val prescriptions.
Only modest associations (range: 0.75–1.22) were seen between the selected predictors and discontinuation of sac/val use. Previous valvular disease and lower grade of education seemed to increase the likelihood of discontinuation. In addition, the remaining symptomatic (NYHA IV vs. I) despite the use of sac/val was the most important predictor for discontinuation.
Titration pattern and dose tolerability
The baseline characteristics of sac/val patients stratified by sac/val initiation dose are presented in Table 2 . At initiation, 37.8% received the lowest dose (24/26 mg), 53.5% the recommended start dose (49/51 mg), and 8.7% the target dose (97/103 mg). Patients prescribed with 24/26 vs. 97/103 mg were older, more often female, and had lower LVEF (<30%), higher N‐terminal pro–B‐type natriuretic peptide, and lower haemoglobin (Table 2 ). Almost one‐third of the patients initiated on 24/26 and 49/51 mg were not up‐titrated and remained at the same dose during the 6 month follow‐up (Figure A1 ). Up‐titration to the target dose (97/103 mg) was achieved in 57% of the total population and more common in patients initiated on 49/51 vs. 24/26 mg (66.7% vs. 31.0%), and few patients initiated on 49/51 and 97/103 mg were permanently down‐titrated throughout the 6 month follow‐up (1.5% and 1.9%, respectively). At the end of 1 year, the estimated treatment persistence for any dose was 82% with a grace period of 90 days (Figure A2 ).
TABLE 2.
Baseline characteristics of patients stratified by initiation dose of sac/val
| 24/26 mg | 49/51 mg | 97/103 mg | |
|---|---|---|---|
| N = 771 | N = 1089 | N = 177 | |
| Age (years), mean ±SD | 69.3 ± 10.8 | 67 ± 11.1 | 67.5 ± 13.3 |
| ≥75 years, n (%) | 264 (34.2) | 303 (27.9) | 61 (34.5) |
| Male, n (%) | 587 (76.1) | 895 (82.2) | 151 (85.3) |
| Cardiovascular diseases, n (%) | |||
| Hypertension | 417 (54.1) | 579 (53.2) | 105 (59.3) |
| Diabetes mellitus | 211 (27.4) | 301 (27.6) | 51 (28.8) |
| Ischaemic heart diseases | 437 (56.7) | 553 (50.8) | 87 (49.2) |
| Atrial fibrillation | 354 (45.9) | 465 (42.7) | 83 (46.9) |
| Non‐cardiovascular diseases, n (%) | |||
| Pulmonary diseases | 332 (43.1) | 325 (29.8) | 50 (28.2) |
| Anaemia | 34 (4.4) | 44 (4.0) | 7 (4.0) |
| Stroke or TIA | 83 (10.8) | 137 (12.6) | 22 (12.4) |
| Psychiatric disorder | 4 (0.5) | 3 (0.3) | 0 (0.0) |
| Cancer | 86 (11.2) | 103 (9.5) | 21 (11.9) |
| Clinical status a , n (%) | |||
| NYHA I | 25 (6.8) | 52 (10.1) | 5 (6.8) |
| NYHA II | 173 (46.9) | 250 (48.4) | 33 (45.2) |
| NYHA III | 164 (44.4) | 209 (40.5) | 33 (45.2) |
| NYHA IV | 7 (1.9) | 5 (1.0) | 2 (2.7) |
| LVEF, n (%) | |||
| 30–39% | 145 (33.7) | 204 (34.4) | 53 (49.1) |
| <30% | 250 (58.1) | 328 (55.3) | 45 (41.7) |
| Treatments, n (%) | |||
| ACEi/ARB | 750 (97.3) | 1078 (99.0) | 171 (96.6) |
| BB | 757 (98.2) | 1084 (99.5) | 174 (98.3) |
| MRA | 681 (88.3) | 1002 (92.0) | 169 (95.5) |
| ACEi/ARB + BB | 743 (96.4) | 1076 (98.8) | 171 (96.6) |
| ACEi/ARB + BB + MRA | 668 (86.6) | 996 (91.5) | 166 (93.8) |
| Laboratory values a | |||
| Haemoglobin (g/dL), mean (SD) | 135.0 (123.0, 145.0) | 140.0 (129.8, 151.0) | 140.0 (133.0, 150.0) |
| Potassium (mmol/L), mean (SD) | 4.3 (4.0, 4.6) | 4.3 (4.0, 4.5) | 4.4 (4.1, 4.6) |
| NT‐proBNP (pg/mL), median (Q1, Q3) | 2524.0 (1349.0, 5190.0) | 1960.0 (880.0, 3770.0) | 1680.0 (871.0, 3128.2) |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta‐blocker; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro–B‐type natriuretic peptide; NYHA, New York Heart Association; sac/val, sacubitril/valsartan; SD, standard deviation; TIA, transient ischaemic attack.
Co‐morbidities extracted from the Swedish National Patient Register were only available until December 2016.
Clinical and laboratory data for the subgroup of the individuals also registered in Swedish Heart Failure Registry.
Discussion
The current study is to our knowledge the first to describe a nationwide implementation of sac/val with a focus on key patient characteristics, dose patterns, and determinants of use and discontinuation of sac/val in Europe. This study leveraged the unique data infrastructure in Sweden by linking nationwide health registers with data on pharmacy dispensation, resource utilization, mortality, and socio‐economic information with clinically rich disease‐specific registers.
The nationwide study showed that the overall implementation of sac/val was slow and the prescription rates varied almost five‐fold across county regions, ranging from 8 to 39 per 100 000 inhabitants. This suggests that even though a structured national introduction programme was in place with a recommendation for use, local clinical practice significantly influenced the prescription patterns. The prevalence of HF in Sweden is ~2–3%; of these, ~50% have HFrEF, which results in 1000–1500 HFrEF patients per 100 000 inhabitants. However, in our study, the median prescriptions in Sweden during the first 2 year introduction period were only 20 per 100 000 inhabitants, indicating that <1 in 50 (2%) HFrEF patients received sac/val. This number is very low given that in the ESC‐EORP‐HFA HF‐LT Registry study, almost 84% of HF patients met the sac/val label criteria, and 12% and 28% met the PARADIGM‐HF and guideline criteria for sac/val if requiring ≥20 and ≥10 mg enalapril, respectively. 8 A study by Norberg et al. reported 24% eligibility for sac/val among patients with HFrEF across Umeå, Sweden, as per the PARADIGM‐HF criteria. Furthermore, the study by Simpson et al. 5 using data from SwedeHF reported 34% and 76% eligibility for sac/val in symptomatic patients with HFrEF, depending on the required ACEi/ARB dose, respectively. Therefore, even if we take the more stringent inclusion criteria, the prescription rate of <2% in the current study is disconcertingly low, despite an increasing trend in the prescription of sac/val over the study period.
There could be many reasons behind the slow and poor implementation. When compared with the PARADIGM‐HF population, patients prescribed with sac/val in the overall cohort in the current study were older (and those not prescribed with sac/val even older) and had more co‐morbidities; thus, the eligibility may have been lower than in published studies. Cost‐effectiveness studies 8 , 9 suggest a cost per quality‐adjusted life year of much less than the generally accepted €500 000 in Sweden, but given the high prevalence of HF, there may have been concerns over aggregate costs to the healthcare system and displacement of other needs. Another important reason behind non‐prescription of sac/val is hypotension, 10 but in the current study, mean blood pressure was in the normal range. Overall, the slow uptake is more likely a reflection of poor implementation of HFrEF therapy in general 11 , 12 , 13 , 14 rather than patient characteristics influencing the eligibility. DeVore et al. 7 in the USA in the CHAMP‐HF study reported similar findings of improper implementation. The implementation of sac/val was only 15% in eligible patients across 121 outpatient centres. Our study highlights clinical inertia among healthcare professionals to start sac/val as per guideline recommendations and despite a national structured treatment recommendation. It is plausible that local practice and physician preference have affected the implementation patterns. Theoretically, comfort with the use of ACEi/ARBs, therapeutic inertia towards symptomatic patients with NYHA II, questions about safety and tolerability, and lack of experience may have acted as potential barriers to sac/val implementation.
As in the CHAMP‐HF study, 7 compared with non‐sac/val patients, sac/val patients in the current study were younger, more often male, and highly symptomatic (NYHA III/IV) with higher pre‐index HF hospitalization/outpatient visits and presence of ischaemic heart disease. Interestingly, patients with a previous hypertension diagnosis were less likely to have sac/val prescribed. Overall, being symptomatic is one of the important determinants for initiation of sac/val, but the remaining symptomatic despite treatment with sac/val was also the most important determinant that leads to discontinuation.
A majority (n = 6203, 79%) of the HF patients registered in SwedeHF with EF < 40% who were not prescribed with sac/val received an ACEi or an ARB; only 40% received ≥75% of the optimum dose (data not shown). Similar results concerning dose titration of ACEi/ARB are reported in other studies. 15 More than half (57%) of sac/val patients with median ≥6 month follow‐up received the targeted dose (97/103 mg b.i.d.). This suggests that the dose tolerability of sac/val in real‐world clinical practice is at least equivalent to ACEi/ARB. The remaining 43% patients who did not reach the target dose is probably a true reflection from real‐world clinical setting in which many of HF patients are generally a decade older with multiple co‐morbidities including renal failure, which is different from clinical trials where many severe co‐morbidities are excluded. This finding is in line with other HF therapies like ACEi/ARB or BB where various studies have highlighted that majority of the patients are not on guideline‐recommended target doses 16 , 17 and patients with higher age, lower body weight, and reduced kidney function are less likely to achieve target doses. 18 Furthermore, administration in twice daily may compromise compliance; however, it is difficult to believe that twice daily regimens alone are largely attributable to suboptimal up‐titration as many ACEi including enalapril and captopril are also administrated twice daily. In a recent study from CHAMP‐HF registry, only 10% of the patients received the recommended sac/val dose (49/51 mg) at the first prescription and less than half of all patients achieved the maximum target dose. Findings are comparable with the randomized TRANSITION study, which assessed the proportion of patients initiated on sac/val after an acute HF event and reaching the target dose (97/103 mg b.i.d.) by Week 10 (approximately half of the patients). 19 Overall, the estimated persistence to sac/val was high, and 82% of patients were on treatment after 1 year. Similar results of estimated sac/val treatment persistence at 71% were reported in patients from Germany with at least one sac/val prescription between January 2016 and June 2017 and 12 month pre‐index activity (defined by any treatment every 6 months). 20 In the current study, a small percentage of patients (2%) did not receive prior ACEi/ARB within 6 months of sac/val initiation. These patients could represent a divergence from HF guidelines, possibly due to clinicians prioritizing the early benefit of sac/val and potentially wishing to minimize extra visits involved in starting and up‐titrating ACEi/ARB before switching to sac/val. The recent European Society of Cardiology consensus report for the management of HF recommended initiation of sac/val rather than ACEi/ARB for patients hospitalized with new‐onset HF or decompensated chronic HF to reduce the short‐term risk of adverse events and to simplify management. 21
The study has limitations. The method of last value carried forward might introduce bias as some of the clinical measures were collected from historical records. Data from the patient register were only available until 2016 at the time of the linkage, and co‐morbidities, number of hospitalization, and outpatient visits are therefore restricted to individuals with an index date before 2017, which decreases the overall subjects in the overall cohort with this type of data. For the non‐sac/val cohort, we miss the individuals fulfilling inclusion criteria during 2017 because the data from NPR are available until 2016. As we go forward in time to define whether an individual is still on treatment when assessing persistence, we might introduce immortal time bias.
Inclusion of data on all individuals that filled at least one prescription of sac/val during the study period 2016–2017 is the biggest strength of the study. A complete data set on all eligible patients defined from national registers, including co‐morbidities, concomitant drugs, and socio‐economics through linkage of the different registers, adds further to the robustness of the results.
In conclusion, the implementation of sac/val in Sweden during the first 2 years after the introduction was lower than expected, and there was wide variation in prescription rates across regions despite a centralized structured implementation programme, highlighting the presence of clinical inertia and influence of local physician practice. Patients receiving sac/val during this first period were younger, more often male, and had lower LVEF and higher use of other HF therapies than those not prescribed with sac/val. This study confirmed that dose tolerability of sac/val in real‐world clinical practice is at least equivalent to ACEi/ARB and treatment persistence for any dose of sac/val at the end of 1 year was high.
Funding
Novartis supported the study.
Conflict of interest
M.U. has related to the present work grant to author's institution from Novartis and unrelated to the present work personal fees from GlaxoSmithKline, AstraZeneca, Novartis, Merck Sharp & Dohme, Boehringer Ingelheim, and Vifor‐Fresenius. L.H.L. has related to the present work grant to author's institution from Novartis and unrelated to the present work personal fees from Merck, Sanofi, Bayer, Pharmacosmos, Abbott, Medscape, and Myokardia, grants and personal fees from Vifor‐Fresenius, AstraZeneca, Relypsa, Novartis, Mundipharma, and Boehringer Ingelheim, and grants from Boston Scientific. U.D. has nothing related to the present work and unrelated to the present work grant to author's institution from AstraZeneca and Boehringer Ingelheim and personal fee from Amgen, Novartis, and AstraZeneca. B.S. has related to the present work grant to author's institution from Novartis and unrelated to the present work grant to author's institution from AstraZeneca and Abbott. E.L. has nothing related or unrelated to the present work. A.L. and M.C.‐S. are employees of Novartis. D.J. was an employee of Novartis during the conduct of the study.
Acknowledgement
Medical writing support was provided by Amit Garg of Novartis Healthcare Private Limited, Hyderabad, India.
Appendix A.
FIGURE A1.
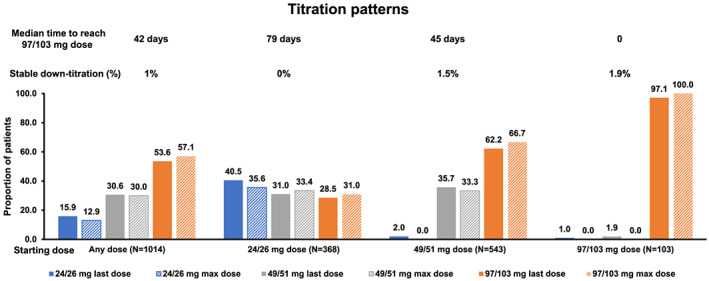
Proportion of patients on sacubitril/valsartan treatment reaching the specified doses during the study period (sacubitril/valsartan overall national cohort).
Note: X ‐axis shows groups of starting doses (any, 24/26, 49/51, and 97/103 mg). N, number of sac/val patients with minimum of two filled sac/val prescriptions and 6 months follow‐up. Last dose, last dose within 6 months; Max dose, max dose within 6 months
FIGURE A2.
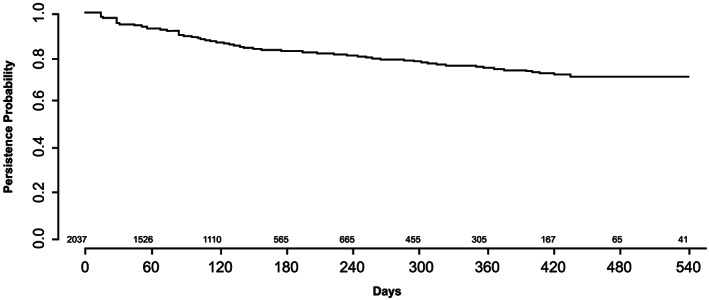
Persistence of sacubitril/valsartan (sac/val) treatment.
Note: Individuals were considered persistent if the next dispatched sac/val prescription was within the grace period from the end of current sac/val prescriptions supply. Numbers of individuals with follow‐up time >540 days were small; therefore, the analyses of persistence were restricted to a maximum follow‐up of 540 days.
Fu, M. , Vedin, O. , Svennblad, B. , Lampa, E. , Johansson, D. , Dahlström, U. , Lindmark, K. , Vasko, P. , Lundberg, A. , Costa‐Scharplatz, M. , and Lund, L. H. (2020) Implementation of sacubitril/valsartan in Sweden: clinical characteristics, titration patterns, and determinants. ESC Heart Failure, 7: 3633–3643. 10.1002/ehf2.12883.
References
- 1. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Investigators P‐H, Committees . Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M , Document R . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 3. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 4. Pellicori P, Urbinati A, Shah P, MacNamara A, Kazmi S, Dierckx R, Zhang J, Cleland JGF, Clark AL. What proportion of patients with chronic heart failure are eligible for sacubitril–valsartan? Eur J Heart Fail 2017; 19: 768–778. [DOI] [PubMed] [Google Scholar]
- 5. Simpson J, Benson L, Jhund PS, Dahlstrom U, McMurray JJV, Lund LH. “Real world” eligibility for sacubitril/valsartan in unselected heart failure patients: data from the Swedish Heart Failure Registry. Cardiovasc Drugs Ther. [journal article] 2019; 33: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kapelios CJ, Lainscak M, Savarese G, Laroche C, Seferovic P, Ruschitzka F, Coats A, Anker SD, Crespo‐Leiro MG, Filippatos G, Piepoli MF, Rosano G, Zanolla L, Aguiar C, Murin J, Leszek P, McDonagh T, Maggioni AP, Lund LH, Heart Failure Long‐Term Registry Investigators . Sacubitril/valsartan eligibility and outcomes in the ESC‐EORP‐HFA Heart Failure Long‐Term Registry: bridging between European Medicines Agency/Food and Drug Administration label, the PARADIGM‐HF trial, ESC guidelines, and real world. Eur J Heart Fail 2019; 21: 1383–1397. [DOI] [PubMed] [Google Scholar]
- 7. DeVore AD, Hill CL, Thomas L, Sharma PP, Albert NM, Butler J, Patterson JH, Spertus JA, Williams FB, Duffy CI, McCague K, Hernandez AF, Fonarow GC. Patient, provider, and practice characteristics associated with sacubitril/valsartan use in the United States. Circ Heart Fail 2018; 11: e005400. [DOI] [PubMed] [Google Scholar]
- 8. Albert NM, Swindle JP, Buysman EK, Chang C. Lower hospitalization and healthcare costs with sacubitril/valsartan versus angiotensin‐converting enzyme inhibitor or angiotensin‐receptor blocker in a retrospective analysis of patients with heart failure. J Am Heart Assoc [Article] 2019; 8: e011089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van der Pol S, Postma MJ. CV3—Cost‐effectiveness of sacubitril/valsartan in Germany: applying the efficiency threshold. Value Health [Conference Abstract] 2018; 21: S6. [Google Scholar]
- 10. Scardovi AB, Boccanelli A. Valsartan/sacubitril in heart failure and hypotension: how, when and why. Eur Heart J Suppl [Short Survey] 2019; 21: B88–B89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Savarese G, Sartipy U, Friberg L, Dahlstrom U, Lund LH. Reasons for and consequences of oral anticoagulant underuse in atrial fibrillation with heart failure. Heart (British Cardiac Society) 2018; 104: 1093–1100. [DOI] [PubMed] [Google Scholar]
- 12. Savarese G, Carrero JJ, Pitt B, Anker SD, Rosano GMC, Dahlstrom U, Lund LH. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: an analysis of 11 215 patients from the Swedish Heart Failure Registry. Eur J Heart Fail 2018; 20: 1326–1334. [DOI] [PubMed] [Google Scholar]
- 13. Norberg H, Bergdahl E, Lindmark K. Eligibility of sacubitril‐valsartan in a real‐world heart failure population: a community‐based single‐centre study. ESC heart failure 2018; 5: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thorvaldsen T, Benson L, Dahlstrom U, Edner M, Lund LH. Use of evidence‐based therapy and survival in heart failure in Sweden 2003–2012. Eur J Heart Fail 2016; 18: 503–511. [DOI] [PubMed] [Google Scholar]
- 15. Maggioni AP, Anker SD, Dahlstrom U, Filippatos G, Ponikowski P, Zannad F, Amir O, Chioncel O, Leiro MC, Drozdz J, Erglis A, Fazlibegovic E, Fonseca C, Fruhwald F, Gatzov P, Goncalvesova E, Hassanein M, Hradec J, Kavoliuniene A, Lainscak M, Logeart D, Merkely B, Metra M, Persson H, Seferovic P, Temizhan A, Tousoulis D, Tavazzi L, Heart Failure Association of the ESC . Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12 440 patients of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2013; 15: 1173–1184. [DOI] [PubMed] [Google Scholar]
- 16. Peri‐Okonny PA, Mi X, Khariton Y, Patel KK, Thomas L, Fonarow GC, Sharma PP, Duffy CI, Albert NM, Butler J, Hernandez AF, McCague K, Williams FB, DeVore AD, Patterson JH, Spertus JA. Target doses of heart failure medical therapy and blood pressure: insights from the CHAMP‐HF registry. JACC Heart Fail 2019; 7: 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greene SJ, Fonarow GC, DeVore AD, Sharma PP, Vaduganathan M, Albert NM, Duffy CI, Hill CL, McCague K, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Butler J. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol 2019; 73: 2365–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Norberg H, Pranic V, Bergdahl E, Lindmark K. Differences in medical treatment and clinical characteristics between men and women with heart failure—a single‐centre multivariable analysis. Eur J Clin Pharmacol 2020; 76: 539–546. [DOI] [PubMed] [Google Scholar]
- 19. Wachter R, Senni M, Belohlavek J, Straburzynska‐Migaj E, Witte KK, Kobalava Z, Fonseca C, Goncalvesova E, Cavusoglu Y, Fernandez A, Chaaban S, Bohmer E, Pouleur AC, Mueller C, Tribouilloy C, Lonn E, Buraiki JAL, Gniot J, Mozheiko M, Lelonek M, Noe A, Schwende H, Bao W, Butylin D, Pascual‐Figal D, Investigators T . Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail [Article in Press] 2019; 21: 998–1007. [DOI] [PubMed] [Google Scholar]
- 20. Bruce Wirta S, Wachter R, Balas B, Klebs S, Fonseca AF, Proenca CC, Dworak M, Schlienger R, Engelhard J, Maier T, Kostev K. Abstracts of the Heart Failure 2018 and the World Congress on Acute Heart Failure, 26–29 May 2018, Vienna, Austria. Eur J Heart Fail 2018; 20: 5–638.29878595 [Google Scholar]
- 21. Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, de Boer RA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 1169–1186. [DOI] [PubMed] [Google Scholar]


