Abstract
Aims
Heart failure (HF) and type 2 diabetes (T2D), common co‐morbidities, translate into worse patient prognoses and higher direct costs than for either condition alone. Empagliflozin has been shown to markedly reduce cardiovascular (CV) deaths and HF hospitalizations (HHF) in HF patients with T2D. This study evaluated the lifetime cost‐effectiveness of supplementing standard of care (SoC) with empagliflozin, relative to SoC alone, in HF patients with T2D from the UK payer perspective.
Methods and results
An existing discrete‐event simulation model was adapted for the economic evaluation. Risk equations developed from time‐dependent parametric survival analyses using patient‐level HF subpopulation data from the EMPA‐REG OUTCOME trial were employed to predict CV and renal events. Non‐CV death, utility weights, and costs were drawn from UK sources. Quality‐adjusted life years (QALYs) and costs were discounted at 3.5% per annum. Relative to SoC, empagliflozin with SoC yielded fewer first HHF, recurrent HHF, CV death, and non‐fatal myocardial infarction but more non‐fatal stroke events. Empagliflozin with SoC vs. SoC alone was associated with increased average life expectancy (10.80 vs. 9.59 LYs) and quality of life (6.27 vs. 5.62 QALYs), though at higher lifetime cost (£18 197 vs. £16 829) per person, resulting in an incremental cost‐effectiveness ratio of £2093 per QALY. The probability of empagliflozin being cost‐effective in the HF subpopulation at a £20 000 per QALY willingness‐to‐pay threshold was 91%.
Conclusions
This analysis suggests that adding empagliflozin to SoC in HF patients with T2D constitutes a cost‐effective use of UK healthcare resources and may provide long‐term health benefits to patients.
Keywords: Chronic heart failure, Cost‐effectiveness, Empagliflozin, Sodium–glucose cotransporter 2 inhibitor, Type 2 diabetes, UK
Introduction
Patients with co‐morbid heart failure (HF) and type 2 diabetes (T2D) have a worse prognosis than patients with either disease alone. 1 The frequent hospital admissions and increased medical management required for these patients contribute to the economic burden associated with concurrent HF and T2D. 2
Historically, glucose‐lowering medications have demonstrated neutral or even unfavourable effects on HF outcomes. 3 Clinical trials for sodium–glucose cotransporter 2 (SGLT2) inhibitors to investigate exercise capacity in HF have shown neutral or inconsistent results 4 or are still ongoing. 5 , 6 However, recent cardiovascular (CV) outcomes trials have shown that SGLT2 inhibitors reduce the risk of CV death and HF hospitalization in T2D patients across a spectrum of CV risk, as well as in patients with HF but without T2D. 7 The effects of empagliflozin (Jardiance®) in T2D patients with established CV disease (CVD) were demonstrated in the EMPA‐REG OUTCOME (EMPAgliflozin Removal of Excess of Glucose OUTCOME) trial, in which empagliflozin reduced CV death by 38% [hazard ratio (HR): 0.62; 95% confidence interval (CI): 0.49–0.77] and hospitalization for HF by 35% (HR: 0.65; 95% CI: 0.50–0.85) vs. standard of care (SoC). 8 The subgroup of patients with HF at baseline (10%) had reductions in these endpoints similar to those in the overall trial population. 9
In order to provide information for the optimal use of healthcare resources, this analysis was designed to estimate the cost‐effectiveness of empagliflozin with SoC vs. SoC alone in HF patients with T2D over a lifetime horizon from the UK payer perspective. A model that assessed empagliflozin with SoC compared with SoC alone in patients with T2D and established CVD in the UK was previously published. 10 The current model incorporates new risk equations based on a sub‐analysis of patients with HF at baseline in the EMPA‐REG OUTCOME trial to predict clinical event rates.
Methods
Overview of economic model
A discrete‐event simulation model developed by Kansal and colleagues was adapted to predict CV and renal events over a lifetime in HF patients with T2D in the EMPA‐REG OUTCOME trial. A detailed description of that model was previously published. 10 Briefly, the present economic model assigned baseline characteristics for individual patients. A series of parametric models based on analysis of EMPA‐REG OUTCOME trial HF subpopulation data were used to draw random times from event‐specific time‐to‐event distributions. Modelled clinical events were handled as competing risks. The events comprised first, and subsequent, hospitalization for worsening HF, CV death, non‐fatal myocardial infarction (MI), non‐fatal stroke, hospitalization for unstable angina, transient ischaemic attack, revascularization, macroalbuminuria, renal injury, and renal failure. These events were defined in the same way as those reported by Kansal et al., except that separate risk equations were fit for hospitalization for first and subsequent worsening HF events. Patients were also subject to non‐CV‐related death using UK age‐adjusted and sex‐adjusted all‐cause mortality data. 11 Adverse events of empagliflozin treatment (e.g. genital infection) were not modelled, as these events are generally short lived and typically do not require hospitalization or incur inpatient costs. Individual patient history was accrued as simulated patients experienced clinical events, and this history altered subsequent risks of modelled events. The model tracked cumulative incidence of clinical events, direct medical costs, life years (LYs), and quality‐adjusted life years (QALYs) over time for each patient. Costs and QALYs were discounted by 3.5% per annum based on published pharmacoeconomic guidelines for the UK. 12 Mean outcomes over all the patients in each treatment group were calculated and compared. A model diagram is shown in Figure 1 .
Figure 1.
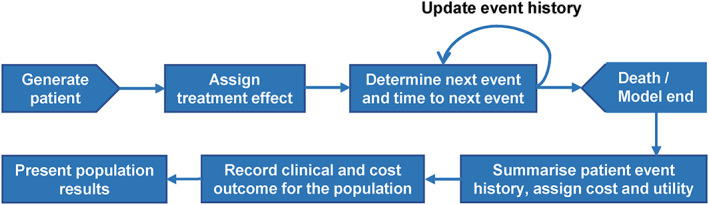
Simulation model process.
Patient population
The modelled population was the same as participants with HF at baseline enrolled in the EMPA‐REG OUTCOME trial. The population consisted of adults with T2D and atherosclerotic CVD who were not restricted by left ventricular ejection fraction or New York Heart Association class. Trial participants were largely (70%) male, with an average (standard deviation) age of 64.5 (8.8) years, haemoglobin A1c of 8.07 (0.86) percent, and an estimated glomerular filtration rate of 68.7 (20.4) mL/min/1.73 m2. The HF subpopulation had a slightly lower proportion of male patients than the population without HF and was slightly older; in addition, estimated glomerular filtration rate in the HF subset of patients was lower, although haemoglobin A1c was similar. Detailed trial inclusion criteria and baseline characteristics of this subpopulation have been documented elsewhere. 9
The simulated population was created by randomly sampling complete individual profiles with replacement from the observed subject‐level data describing characteristics of participants with chronic HF collected at baseline in the EMPA‐REG OUTCOME trial (N = 706), which considers the natural correlations among risk factors and medical histories. Each sampled patient record was duplicated, and identical patients were assigned to each treatment in the model: empagliflozin with SoC and SoC alone. A cohort size of 5000 patients was sufficient to obtain convergence for lifetime simulations.
Clinical inputs
Risk equations based on parametric proportional hazards regression analyses of patient‐level EMPA‐REG OUTCOME trial HF subpopulation data (462 patients randomized to empagliflozin and 244 patients randomized to placebo) were used to predict the time to the next clinical event for the simulated individual patients. When the analytic time horizon exceeded the trial duration, the model extrapolated the risk functions of CV and renal events. Event risks were adjusted for baseline characteristics, treatment allocation (empagliflozin with SoC or SoC), and time‐dependent variables. Non‐fatal CV events could recur (e.g. a patient could experience more than one non‐fatal MI); renal events were non‐recurring. A systematic statistical analysis approach was employed to develop individual patient‐level risk equations for the model, as previously described by Kansal and colleagues for analysis in the overall EMPA‐REG OUTCOME trial population. 10
In this study, first and subsequent hospitalization for worsening HF were modelled using separate risk equations. The time‐to‐event estimates of other clinical events were adjusted for history of hospitalization for worsening HF (whether it occurred), if history of HF hospitalization had a statistically significant effect or was considered an important prognostic factor for future risk. A total of 11 risk equations were developed. A comparison of the statistical fits is provided in Supporting Information, Table S1 . Graphs comparing the alternative curves over the trial duration and long term are shown in Supporting Information, Figures S1 – S11 . The risk equations included in the model appear in Table S2 .
The risk of non‐CV death was derived from UK life tables provided by the Office for National Statistics. 11 Gompertz distributions were fit to the probabilities separately for men and women to estimate the time to event. The non‐CV death risk equation variables are shown in Table S3 .
During validation, observed HRs for empagliflozin with SoC compared with SoC in the EMPA‐REG OUTCOME trial HF subpopulation were calculated. Risk equations for each clinical event applied in the model over the 3‐year mean trial duration reproduced the within‐trial outcomes (Table S4 ).
Health‐related quality of life
Model utility weights were obtained from Sullivan and Ghushchyan, 13 who estimated the health‐related quality of life (HRQoL) of diabetes‐related chronic conditions based on an analysis of 20 705 patients with diabetes and valid EQ‐5D scores who participated in the 2000–2011 Medical Expenditure Panel Survey. The utility weight corresponding to hospitalization for unstable angina was assumed the same as that for MI. The effect of revascularization on HRQoL was taken from an alternative source. 14 A summary of utilities is given in Table 1 .
TABLE 1.
Inputs for the cost‐effectiveness model
| Input parameter | Deterministic value | Probabilistic distribution | Source |
|---|---|---|---|
| Empagliflozin drug cost (monthly) | £39.75 | Not applicable | MIMS 16 |
| Cost per episode (2018 values) | Mean (SE) | Gamma (All costs) | |
| HF (first or subsequent) | £4633 (£463) a | α = 100; β = 46 | Alva, 2015 17 |
| CV death | £3413 (£341) a | α = 100; β = 34 | Alva, 2015 17 |
| Non‐fatal MI | £7523 (£752) a | α = 100; β = 75 | Alva, 2015 17 |
| Non‐fatal stroke | £11 044 (£1104) a | α = 100; β = 110 | Alva, 2015 17 |
| Unstable angina | £726 (£73) a | α = 100; β = 7 | NHS ref costs 2016–17 (EB13A–EB13D) 20 |
| Transient ischaemic attack | £2773 (£277) a | α = 100; β = 28 | Wardlaw, 2014 22 |
| Revascularization | £1691 (£169) a | α = 100; β = 17 | NHS ref costs 2016–17 (YQ50A–YQ50F) 20 |
| Macroalbuminuria | £8554 (£855) a | α = 100; β = 86 | Gordios, 2004 18 |
| Renal injury | £619 (£62) a | α = 100; β = 6 | Kent, 2015 19 |
| Renal failure | £38 160 (£3816) a | α = 100; β = 382 | NICE guideline NG28 Appendix F 21 |
| Utility values | Mean (95% CI) | Beta (All decrements) | |
| Baseline utility | 0.785 (0.707, 0.864) b | α = 178; β = 49 | Clarke, 2002 31 |
| HF disutility | −0.050 (−0.064, −0.036) | α = 47; β = 884 | Sullivan, 2016 13 |
| Non‐fatal MI disutility | −0.047 (−0.057, −0.036) | α = 73; β = 1486 | Sullivan, 2016 13 |
| Non‐fatal stroke disutility | −0.060 (−0.074, −0.046) | α = 66; β = 1038 | Sullivan, 2016 13 |
| Unstable angina disutility | −0.047 (−0.057, −0.036) | α = 73; β = 1486 | Sullivan, 2016 13 ; assumed same as MI |
| Transient ischaemic attack disutility | −0.070 (−0.131, −0.008) | α = 5; β = 61 | Sullivan, 2016 13 |
| Revascularization disutility | −0.030 (−0.036, −0.024) c | α = 93; β = 3011 | Lindgren, 2007 14 |
| Macroalbuminuria disutility | −0.038 (−0.059, −0.016) | α = 12; β = 291 | Sullivan, 2016 13 |
| Renal injury disutility | −0.038 (−0.059, −0.016) | α = 12; β = 291 | Sullivan, 2016 13 |
| Renal failure disutility | −0.038 (−0.059, −0.016) | α = 12; β = 291 | Sullivan, 2016 13 |
| Utility effect of multiple events (additive to utility) | Mean | ||
| 2 diabetes‐related complications | 0.017 | Not applicable | Sullivan, 2016 13 |
| 3 diabetes‐related complications | 0.042 | Not applicable | Sullivan, 2016 13 |
| 4 diabetes‐related complications | 0.070 | Not applicable | Sullivan, 2016 13 |
| ≥5 diabetes‐related complications | 0.087 | Not applicable | Sullivan, 2016 13 |
CI, confidence interval; CV, cardiovascular; HF, heart failure; MI, myocardial infarction; NHS, National Health Service; NICE, National Institute for Health and Care Excellence; SE, standard error.
All event costs are inflated according to Personal Social Services Research Unit Hospital and Community Health Services index for the UK.
The SE is calculated as 10% of the mean value.
The 95% CI is calculated as ±10% of the mean value.
The 95% CI is calculated as ±20% of the mean value.
Utility weights for clinical events were applied to a baseline tariff value for the UK. The model combined weights for multiple events by applying individual decrements additively, and an adjustment factor dependent on the number of events was applied to account for overlapping effects. For example, the utility score for a patient in the UK with non‐fatal MI and non‐fatal stroke history would be 0.695, which is equivalent to 0.785 (baseline utility) − 0.047 (disutility associated with non‐fatal MI) − 0.060 (disutility for stroke) + 0.017 (adjustment factor for individuals with two clinical events).
Costs and perspective
This analysis was undertaken from the perspective of the UK National Health Service and focused on accumulation of direct costs reported in British pound sterling (GBP). All costs were inflated to 2018 GBP, where necessary (Table 1 ). 15 These included the drug acquisition cost of empagliflozin (£36.59 per pack of 28 tablets), the value for which was extracted from MIMS UK drug database, 16 as well as healthcare expenditures associated with the management of acute clinical events, which were derived from UK databases and published literature. 17 , 18 , 19 , 20 , 21 , 22 Medication costs other than those involved in empagliflozin were not considered, because empagliflozin is modelled as an add‐on to SoC. Per‐episode event costs were applied in the model when a patient experienced a clinical event. The model excluded maintenance costs associated with clinical events on the grounds that these were indirectly accounted for via the increased risk of future events, as previously employed by Kansal et al. 10 Adverse events associated with empagliflozin (e.g. genital infection) were not included in the model, as these events generally lead to short episodes of care and low management costs. The analysis assumed that other management costs would be similar across both treatment arms, and these costs were therefore not modelled.
Model analysis
Clinical event rates, LYs, total costs, and total QALYs were calculated over a lifetime horizon. Incremental outcomes were reported. Cost‐effectiveness was measured using the incremental cost‐effectiveness ratio (ICER) for a willingness‐to‐pay (WTP) threshold of £20 000 per QALY, the lower threshold accepted by the National Institute for Health and Care Excellence in the UK. 12
Deterministic sensitivity analyses (DSA) were conducted by varying model inputs individually or in combinations to assess their influence on the base case. An analysis over 10 years was run to evaluate the influence of the time horizon on model outcomes. The effect of discount rates on costs and QALYs was investigated by symmetrically setting the rates to 0% and 5% per annum. Upper and lower confidence limits were used to vary utility weights. For cost inputs, where these data were not available, an assumed change of ±20% was applied. The influence of event rates derived from the EMPA‐REG OUTCOME trial HF subpopulation analysis was assessed by scaling the rate of each clinical event by a constant HR (0.9–1.1) that applies over the entire model time horizon.
A probabilistic sensitivity analysis (PSA) was carried out considering the uncertainty in all model inputs simultaneously over 1000 iterations, with incremental effectiveness and cost estimates displayed on the cost‐effectiveness plane. Through testing for convergence of the ICER, 1000 iterations were deemed sufficient to minimize the Monte Carlo error. Inputs for each iteration of the microsimulation process were sampled from gamma (costs) or beta (utility weights) distributions (Table 1 ). The PSA considered correlation among the risk equation coefficients using Cholesky decomposition of the covariance matrices.
Results
Base case analysis
The base case analysis found that empagliflozin added to SoC was associated with a reduction in the rates of all CV and renal events relative to SoC alone, except non‐fatal stroke and renal failure (Table 2 ). Although a higher event rate for non‐fatal stroke indirectly contributed to CV deaths, as implied by the positive covariate in the CV death risk equation (Table S2 ), the CV death rate with empagliflozin with SoC remained lower than with SoC (6.07 vs. 7.37 events per 100 patient‐years).
TABLE 2.
Base case cost‐effectiveness analysis over a lifetime horizon
| Event rates per 100 patient‐years | |||
|---|---|---|---|
| Empagliflozin with SoC | SoC | Hazard ratio | |
| First hospitalization for worsening HF | 3.64 | 4.61 | 0.79 |
| Subsequent hospitalization for worsening HF | 2.78 | 4.92 | 0.56 |
| CV death | 6.07 | 7.37 | 0.82 |
| Non‐fatal MI | 2.67 | 3.31 | 0.81 |
| Non‐fatal stroke | 2.57 | 1.54 | 1.67 |
| Hospitalization for unstable angina | 2.43 | 2.81 | 0.87 |
| Transient ischaemic attack | 0.18 | 0.50 | 0.36 |
| Revascularization | 2.83 | 3.12 | 0.91 |
| Macroalbuminuria | 6.46 | 11.02 | 0.59 |
| Renal injury | 1.17 | 1.70 | 0.69 |
| Renal failure | 0.28 | 0.14 | 1.94 |
| Non‐CV death | 3.19 | 3.07 | NA |
| Empagliflozin with SoC | SoC | Incremental | |
|---|---|---|---|
| Undiscounted life expectancy (years) | 10.80 | 9.59 | 1.21 |
| Discounted QALY | 6.27 | 5.62 | 0.65 |
| Discounted costs over patients' lifetime | |||
| Drug acquisition cost | £4009 | £0 | £4009 |
| Event management cost | £14 188 | £16 829 | −£2642 |
| Total cost | £18 197 | £16 829 | £1367 |
| ICER, £ per QALY | — | — | £2093 |
CV, cardiovascular; HF, heart failure; ICER, incremental cost‐effectiveness ratio; MI, myocardial infarction; NA, not applicable; QALY, quality‐adjusted life year; SoC, standard of care.
Empagliflozin with SoC compared with SoC showed greater life expectancy, translating to more QALYs per person, while also generating increased expenditures over a lifetime horizon. Lower total clinical event costs accrued in the empagliflozin with SoC vs. the SoC arm, with a difference of £2642 per patient that partially offset the acquisition cost associated with empagliflozin. Thus, despite the increase in drug expenditures attributable to the supplementation of SoC by empagliflozin, the improvement in average HRQoL produced an ICER of £2093 per QALY. This lies well below the £20 000 per QALY WTP threshold, 12 indicating that empagliflozin with SoC is highly cost‐effective relative to SoC alone.
Sensitivity analyses
Overall, the DSA results were consistent with the base case (Figure 2 ). In the analyses with a 10‐year time horizon and higher event management costs, empagliflozin with SoC was found to be a dominant strategy (more effective and less costly) when compared with SoC. Variations in the discount rate to costs, the price of empagliflozin, and the discount rate to QALYs were most influential on the ICER. All scenarios produced ICERs well below the WTP threshold of £20 000 per QALY.
Figure 2.
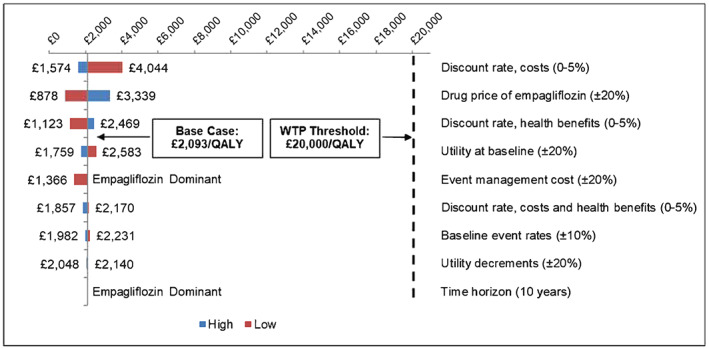
Deterministic sensitivity analyses results. QALY, quality‐adjusted life year; WTP, willingness‐to‐pay.
The PSA produced relatively broad 95% CIs around mean event rates in both treatment arms (Figure 3 ), given that the modelled time‐to‐event estimates were based upon only a subpopulation of the EMPA‐REG OUTCOME trial. The overall clinical effect translated to a median ICER of £1955 per QALY (interquartile range of £168 to £4851 per QALY). Empagliflozin with SoC provided a QALY benefit over, and cost less than (i.e. dominated), SoC in 18% of iterations and overall was cost‐effective at a WTP threshold of £20 000 per QALY in 91% of iterations (Figure 4 ).
Figure 3.
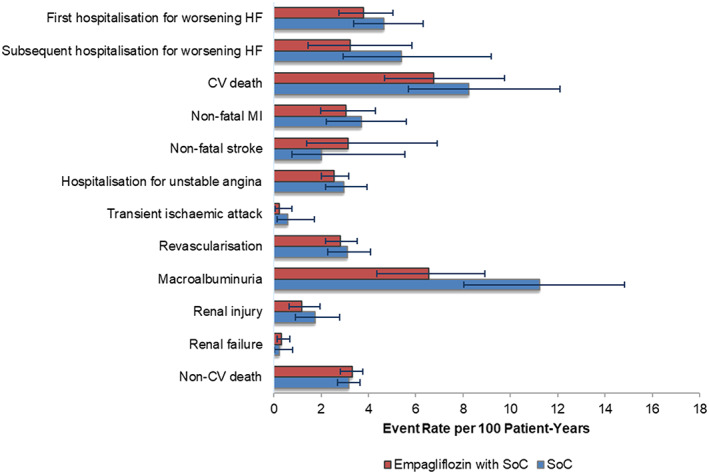
Probabilistic sensitivity analyses event rates. CV, cardiovascular; HF, heart failure; MI, myocardial infarction; SoC, standard of care.
Figure 4.
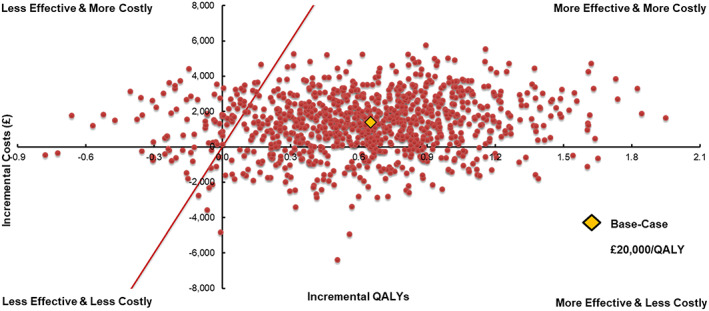
Probabilistic sensitivity analyses scatterplot. QALYs, quality‐adjusted life years.
Discussion
This study compared health benefits and cost outcomes of empagliflozin with SoC vs. SoC alone based on the HF subpopulation in the EMPA‐REG OUTCOME trial. The analysis showed modestly increased total lifetime expenditures (£1367) with greater average life expectancy (1.21 years) and more QALYs (0.65) for HF patients with T2D using empagliflozin with SoC. The ICER was £2093 per QALY in the base case analysis, falling well below the £20 000 per QALY WTP threshold employed in the UK. Accounting for parameter uncertainty did not impact these conclusions. Notably, because the relative benefit of empagliflozin was realized relatively quickly, it was found in the DSA to dominate SoC in treating HF patients with T2D when considering a relatively short time horizon (i.e. 10 years vs. lifetime) and increased event management costs (20% higher).
The model framework draws upon a previous cost‐effectiveness model in the UK setting based on the EMPA‐REG OUTCOME trial overall population. 10 Localizations of the cost‐effectiveness model to other healthcare settings (USA, Canada, Greece, and Italy) 23 , 24 , 25 , 26 and a sub‐analysis in Asian patients (Japanese setting) 27 and HF patients (US setting) 28 of the EMPA‐REG OUTCOME trial have been reported. This analysis focused on the trial HF subpopulation considering the healthcare system in the UK, and the cost‐effectiveness results were broadly consistent with other studies, despite differences in the population and financial structures of health care across markets.
Several clinical trials have shown benefit of SGLT2 inhibitors in the prevention of HF. 7 , 8 , 29 , 30 The populations of these trials were heterogeneous, and further research is needed to better understand the specific effect of individual agents within the class. Data from two major ongoing clinical trials evaluating empagliflozin vs. placebo in addition to SoC in patients with HF (with or without T2D) with reduced ejection fraction (EMPEROR‐Reduced) or preserved ejection fraction (EMPEROR‐Preserved) will generate future evidence beyond the scope of the EMPA‐REG OUTCOME trial. Evidence from these trials will provide further insights on effectiveness outcomes with empagliflozin in HF patients and the potential to generate resource and cost savings to healthcare systems globally. Future research may consider the cost‐effectiveness of empagliflozin to other SGLT2 inhibitors or sacubitril/valsartan in the same population and setting.
The main limitations of this economic evaluation primarily stem from the trial subpopulation data. The EMPA‐REG OUTCOME trial was powered to detect treatment effects in the overall enrolled population with T2D and established CVD. Thus, the HF subpopulation (N = 706) is subject to broader CIs around mean event rates than the overall trial results, and most events showed non‐significant differences, including those with large point estimates. Nonetheless, a vast majority (91%) of PSA iterations generating ICERs that fell below the £20 000 UK WTP threshold suggests a very strong likelihood that empagliflozin with SoC is cost‐effective (i.e. that the true ICER falls beneath the threshold). Also, the characterization of the HF subpopulation may be subject to misclassification. Investigator‐reported HF at baseline was based on the narrow Standardized MedDRA Query ‘cardiac failure’ rather than measures of cardiac function or biomarkers such as N‐terminal pro‐B‐type natriuretic peptide. As such, there is a risk that some patients without a diagnosis of HF at baseline might have had HF or some degree of left ventricular dysfunction that was not fully evident. Data from the EMPEROR‐Reduced and EMPEROR‐Preserved trials are expected to provide further insights on the benefit of empagliflozin in patients with HF with and without T2D at baseline.
Another limitation is that the model does not account for the costs or HRQoL impacts resulting from adverse events commonly associated with SGLT2 inhibitors (e.g. genital infections). These events are typically mild and self‐treated and thus should not meaningfully affect modelled overall direct healthcare costs or QALYs. Furthermore, the model was not equipped to capture intensification of treatment beyond the trial duration, which, as noted, necessitated adoption of conservative treatment assumptions that may result in underestimating the cost‐effectiveness of empagliflozin with SoC, as compared with SoC alone. As noted elsewhere, results from the EMPA‐REG OUTCOME trial indicate that intensification of treatment‐lowering therapy was more common in the placebo arm relative to the empagliflozin arms. 10
This analysis suggests that empagliflozin is cost‐effective for patients with HF and T2D in the UK context and probably also in similar healthcare economies. This should inform decisions on funding and changes to current clinical practice.
Conflict of interest
J.T.G., M.B., S.K., A.U., S.L., and N.H. are employees of Boehringer Ingelheim. J.F. was an employee of Boehringer Ingelheim during the conduct of this work. O.S.R., S.B.B., and M.S. are, and during the conduct of this work, A.R.K. and J.L. were, employees of Evidera, a healthcare research firm that provides consulting and other research services to the biopharmaceutical and medical device industry. In these salaried positions, they work(ed) with a variety of companies and are(were) explicitly precluded from accepting any payment or honoraria directly from those companies for services rendered. Evidera received payment from Boehringer Ingelheim for collaboration on this project and article.
Funding
This work was supported by Boehringer Ingelheim International GmbH.
Supporting information
Table S1. Comparison of fitted models using AIC and BIC.
Table S2. Parameters of risk equations for CV and renal events.
Table S3. Parameters of risk equations for non‐CV death.
Table S4. Validation of three‐year HRs for empagliflozin plus SoC vs. SoC alone.
Figure S1. Comparison of statistical fits vs. observed data‐hospitalisation for worsening heart failure, first.
Figure S2. Comparison of statistical fits vs. observed data–hospitalisation for worsening heart failure, subsequent.
Figure S3. Comparison of statistical fits vs. observed data–cardiovascular death.
Figure S4. Comparison of statistical fits vs. observed data–non‐fatal myocardial infarction.
Figure S5. Comparison of statistical fits vs. observed data–non‐fatal stroke.
Figure S6. Comparison of statistical fits vs. observed data–hospitalisation for unstable angina.
Figure S7. Comparison of statistical fits vs. observed data–transient ischaemic attack.
Figure S8. Comparison of statistical fits vs. observed data–revascularisation.
Figure S9. Comparison of statistical fits vs. observed data–macroalbuminuria.
Figure S10. Comparison of statistical fits vs. observed data–renal injury.
Figure S11. Comparison of statistical fits vs. observed data–renal failure.
Acknowledgements
The authors gratefully acknowledge Janet Dooley of the Evidera Editorial and Design team for her editorial assistance.
Reifsnider, O. S. , Kansal, A. R. , Franke, J. , Lee, J. , George, J. T. , Brueckmann, M. , Kaspers, S. , Brand, S. B. , Ustyugova, A. , Linden, S. , Stargardter, M. , and Hau, N. (2020) Cost‐effectiveness of empagliflozin in the UK in an EMPA‐REG OUTCOME subgroup with type 2 diabetes and heart failure. ESC Heart Failure, 7: 3910–3918. 10.1002/ehf2.12985.
References
- 1. Seferovic PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, Paulus WJ, Komajda M, Cosentino F, de Boer RA, Farmakis D, Doehner W, Lambrinou E, Lopatin Y, Piepoli MF, Theodorakis MJ, Wiggers H, Lekakis J, Mebazaa A, Mamas MA, Tschope C, Hoes AW, Seferovic JP, Logue J, McDonagh T, Riley JP, Milinkovic I, Polovina M, van Veldhuisen DJ, Lainscak M, Maggioni AP, Ruschitzka F, McMurray JJV. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 853–872. [DOI] [PubMed] [Google Scholar]
- 2. Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med 2012; 29: 855–862. [DOI] [PubMed] [Google Scholar]
- 3. Nassif M, Kosiborod M. Effect of glucose‐lowering therapies on heart failure. Nat Rev Cardiol 2018; 15: 282–291. [DOI] [PubMed] [Google Scholar]
- 4. Healio.com/Cardiology. EMPERIAL top‐line results: empagliflozin for HFpEF, HFrEF fails to improve exercise ability December 13, 2019. https://www.healio.com/news/cardiology/20191213/emperial-topline-results-empagliflozin-for-hfpef-hfref-fails-to-improve-exercise-ability (12 June 2020).
- 5. ClinicalTrials.gov. DETERMINE‐reduced—dapagliflozin effect on exercise capacity using a 6‐minute walk test in patients with heart failure with reduced ejection fraction. ClinicalTrials.gov Identifier: NCT03877237 March 15, 2019. updated March 23, 2020. https://clinicaltrials.gov/ct2/show/NCT03877237 (12 June 2020).
- 6. ClinicalTrials.gov. DETERMINE‐preserved—dapagliflozin effect on exercise capacity using a 6‐minute walk test in patients with heart failure with preserved ejection fraction. ClinicalTrials.gov Identifier: NCT03877224 March 15, 2019. updated April 3, 2020. https://clinicaltrials.gov/ct2/show/NCT03877224 (June 12 2020).
- 7. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, De Mets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM, DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 8. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, EMPA‐REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 9. Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, EMPA‐REG OUTCOME Trial Investigators . Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA‐REG OUTCOME® trial. Eur Heart J 2016; 37: 1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kansal A, Reifsnider OS, Proskorovsky I, Zheng Y, Pfarr E, George JT, Kandaswamy P, Ruffolo A. Cost‐effectiveness analysis of empagliflozin treatment in people with Type 2 diabetes and established cardiovascular disease in the EMPA‐REG OUTCOME trial. Diabet Med 2019; 36: 1494–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Office for National Statistics (ONS) . National life tables: UK. 2012–2014 24 September 2019. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables (8 January 2020).
- 12. National Institute for Health and Care Excellence (NICE) . The guidelines manual. Process and methods [PMG6]: 7. Assessing cost effectiveness November 2012. https://www.nice.org.uk/process/pmg6/chapter/assessing-cost-effectiveness (8 January 2020).
- 13. Sullivan PW, Ghushchyan VH. EQ‐5D scores for diabetes‐related comorbidities. Value Health 2016; 19: 1002–1008. [DOI] [PubMed] [Google Scholar]
- 14. Lindgren P, Graff J, Olsson AG, Pedersen TJ, Jonsson B, IDEAL Trial Investigators . Cost‐effectiveness of high‐dose atorvastatin compared with regular dose simvastatin. Eur Heart J 2007; 28: 1448–1453. [DOI] [PubMed] [Google Scholar]
- 15. Curtis L, Burns A. Unit Costs of Health and Social Care 2018. University of Kent, Canterbury: Personal Social Services Research Unit (PSSRU); 2018. https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2018/ (8 January 2020). [Google Scholar]
- 16. MIMS.co.UK. MIMS UK drug database. 2018: Haymarket Media Group, Ltd.; 2018. https://www.mims.co.uk/ (8 January 2020).
- 17. Alva ML, Gray A, Mihaylova B, Leal J, Holman RR. The impact of diabetes‐related complications on healthcare costs: new results from the UKPDS (UKPDS 84). Diabet Med 2015; 32: 459–466. [DOI] [PubMed] [Google Scholar]
- 18. Gordois A, Scuffham P, Shearer A, Oglesby A. The health care costs of diabetic nephropathy in the United States and the United Kingdom. J Diabetes Complications 2004; 18: 18–26. [DOI] [PubMed] [Google Scholar]
- 19. Kent S, Schlackow I, Lozano‐Kuhne J, Reith C, Emberson J, Haynes R, Gray A, Cass A, Baigent C, Landray MJ, Herrington W, Mihaylova B, SHARP Collaborative Group . What is the impact of chronic kidney disease stage and cardiovascular disease on the annual cost of hospital care in moderate‐to‐severe kidney disease? BMC Nephrol 2015; 16: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Health Service (NHS) . NHS reference costs, 2016–17 (Schedule EB13A‐EB13D and YQ50A‐YQ50F).
- 21. National Institute for Health and Care Excellence (NICE) . Type 2 diabetes in adults: management. NICE guideline [NG28]. Type 2 diabetes: appendix F December 2015 Last updated: August 2019 https://www.nice.org.uk/guidance/NG28/documents/type-2-diabetes-appendix-f (8 January 2020).
- 22. Wardlaw J, Brazzelli M, Miranda H, Chappell F, McNamee P, Scotland G, Quayyum Z, Martin D, Shuler K, Sandercock P, Dennis M. An assessment of the cost‐effectiveness of magnetic resonance, including diffusion‐weighted imaging, in patients with transient ischaemic attack and minor stroke: a systematic review, meta‐analysis and economic evaluation. Health Technol Assess 2014; 18: 1–368 v‐vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gourzoulidis G, Tzanetakos C, Ioannidis I, Tsapas A, Kourlaba G, Papageorgiou G, Maniadakis N. Cost‐effectiveness of empagliflozin for the treatment of patients with type 2 diabetes mellitus at increased cardiovascular risk in Greece. Clin Drug Investig 2018; 38: 417–426. [DOI] [PubMed] [Google Scholar]
- 24. Iannazzo S, Mannucci E, Reifsnider O, Maggioni AP. Cost‐effectiveness analysis of empagliflozin in the treatment of patients with type 2 diabetes and established cardiovascular disease in Italy, based on the results of the EMPA‐REG OUTCOME study. Farmeconomia Health Econ Ther Pathways 2017; 18: 43–53. [Google Scholar]
- 25. Kansal A, Zheng Y, Proskorovsky I, Krotneva M, Kandaswamy P, Ruffolo A. Modeling cardiovascular (CV) outcomes of treatment with empagliflozin in type 2 diabetes based on hard outcomes data. Abstract PDB37, at ISPOR 21st Annual International Meeting, 21–25 May 2016, Washington, DC, USA. Value Health 2016; 19: A203. [Google Scholar]
- 26. Mettam SR, Bajaj H, Kansal AR, Kandaswamy P. Abstract PDB52 at ISPOR 19th Annual European Congress, 29 October–2 November 2016, Vienna AustriaCost Effectiveness of Empagliflozin in Patients with T2DM and High CV Risk in Canada. Value Health 2016; 19: A674. [Google Scholar]
- 27. Kaku K, Haneda M, Sakamaki H, Yasui A, Murata T, Ustyugova A, Chin R, Hirase T, Shibahara T, Hayashi N, Kansal A, Kaspers S, Okamura T. Cost‐effectiveness analysis of empagliflozin in Japan based on results from the Asian subpopulation in the EMPA‐REG OUTCOME trial. Clin Ther 2019; 41: 2021–2040 e11. [DOI] [PubMed] [Google Scholar]
- 28. Reifsnider O, Kansal A, Franke J, George JT, Brueckmann M, Kaspers S, Brand S, Ustyugova A, Linden S, Hau N. Cost‐effectiveness of empagliflozin treatment in patients with type 2 diabetes and chronic heart failure based on a subgroup of EMPA‐REG OUTCOME in the United States. American Heart Association Scientific Sessions; Nov 10–14; Chicago, IL, USA. 2018.
- 29. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 30. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 31. Clarke PM, Gray AM, Briggs A, Farmer AJ, Fenn P, Stevens RJ, Matthews DR, Stratton IM, Holman RR, UK Prospective Diabetes Study Group . A model to estimate the lifetime health outcomes of patients with Type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 2004; 47: 1747–1759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of fitted models using AIC and BIC.
Table S2. Parameters of risk equations for CV and renal events.
Table S3. Parameters of risk equations for non‐CV death.
Table S4. Validation of three‐year HRs for empagliflozin plus SoC vs. SoC alone.
Figure S1. Comparison of statistical fits vs. observed data‐hospitalisation for worsening heart failure, first.
Figure S2. Comparison of statistical fits vs. observed data–hospitalisation for worsening heart failure, subsequent.
Figure S3. Comparison of statistical fits vs. observed data–cardiovascular death.
Figure S4. Comparison of statistical fits vs. observed data–non‐fatal myocardial infarction.
Figure S5. Comparison of statistical fits vs. observed data–non‐fatal stroke.
Figure S6. Comparison of statistical fits vs. observed data–hospitalisation for unstable angina.
Figure S7. Comparison of statistical fits vs. observed data–transient ischaemic attack.
Figure S8. Comparison of statistical fits vs. observed data–revascularisation.
Figure S9. Comparison of statistical fits vs. observed data–macroalbuminuria.
Figure S10. Comparison of statistical fits vs. observed data–renal injury.
Figure S11. Comparison of statistical fits vs. observed data–renal failure.


