Abstract
Ruffini's corpuscles are present as long fusiform encapsulated sensory structures in different tissues including the skin. Although physiological analyses strongly suggest their existence in glabrous digital skin, such localisation remains unconfirmed. Here, we have investigated the occurrence of typical Ruffini's corpuscles in 372 sections of human digital skin obtained from 186 subjects of both sexes and different ages (19–92 years). S100 protein, neuron‐specific enolase and neurofilament proteins were detected, and the basic immunohistochemical profile of these corpuscles was analysed. Fewer than 0.3 Ruffini's corpuscles/mm2 were detected, with density distribution across the fingers being F4 > F3 > F2 > F1 > F5 and absolute values being F2 > F1 > F3 > F4 > F5. Axons displayed neuron‐specific enolase immunoreactivity, glial cells forming the core contained S100 protein, and the capsule was positive for CD34 but not Glut1, demonstrating an endoneurial origin. Present results demonstrate the existence of Ruffini's corpuscles in human glabrous digital skin at very low densities. Moreover, the identified Ruffini's corpuscles share the basic immunohistochemical characteristics of other dermal sensory corpuscles.
Keywords: cutaneous sensory corpuscles, glabrous skin, immunohistochemistry, Ruffini's corpuscles
Typical Ruffini's corpuscles in human glabrous digital skin. Ax: axon; c: capsule; gc: glial core.
The dermis contains sensory corpuscles that mediate tactile stimuli.
Ruffini's corpuscles are fusiform type II slowly adapting low‐threshold sensors.
This study confirms the existence of Ruffini's corpuscles in human digital skin.
Human glabrous digital skin contains fewer than 0.3 Ruffini's corpuscles per mm2.
Ruffini's corpuscles share the same immunohistochemical profile as other sensors.
![]()
1. INTRODUCTION
Mechanosensory afferents can be distinguished morphologically based on their sensory terminals in the skin (i.e. sensory corpuscles) and the speed with which they conduct action potentials; most of them respond to tactile stimuli with specific firing patterns (Rice and Albrecht, 2008; Gardner and Johnson, 2013). The dermis in vertebrate skin contains a variety of sensory corpuscles that mediate different types of sensibility, especially touch (McGlone and Reilly, 2010; McGlone et al., 2014). These structures, known as low‐threshold mechanoreceptors (LTMRs), encode non‐painful mechanical stimuli and relay the information to the peripheral processes of primary sensory neurons (Abraira and Ginty, 2013; Fleming and Luo, 2013; Zimmerman et al., 2014).
Four types of LTMRs have been identified in mammalian glabrous skin: type I (forming Merkel cell‐neurite complexes) and type II (Ruffini's corpuscles) slowly adapting (SA) LTMRs, as well as type I (Meissner's corpuscles) and type II (Pacinian corpuscles) rapidly adapting (RA) LTMRs (Rice and Albrecht, 2008; Fleming and Luo, 2013; Zimmerman et al., 2014). Type I SA LTMRs detect fine touch, type II SA LTMRs mediate stretching information, and RA LTMRs tune to vibration and motion across the skin (Johnson, 2001; Jones and Smith, 2014; Owens and Lumpkin, 2014; Olson et al., 2016).
The morphologies (Munger and Ide, 1988; Zelená, 1994; Rice and Albrecht, 2008), development (Feito et al., 2018), ageing (García‐Piqueras et al., 2019) and immunohistochemical profiles (Vega et al., 2009; Feito et al., 2016; García‐Piqueras et al., 2020) of Meissner's and Pacinian corpuscles in human glabrous skin have been well characterised. Conversely, little information is available about other dermal sensory corpuscles, especially Ruffini's corpuscle (Munger and Ide, 1988; Rice and Albrecht, 2008). Type II SA LTMRs have been extensively characterised physiologically (Johansson and Vallbo, 1979; Wellnitz et al., 2010) but not morphologically. In many cases, type II SA LTMR responses were recorded in nerve fibres innervating a tissue (Johansson and Vallbo, 1979; Rasmusson and Turnbull, 1986), but the presence of Ruffini's corpuscles in such tissue has not been confirmed (Rice and Rasmusson, 2000; Paré et al., 2002). In humans, a single Ruffini‐like corpuscle was reported in the skin of the index finger (Paré et al., 2003), which is much less than what would be expected based on physiological recordings.
Cutaneous Ruffini's corpuscles form elongated structures with tapered ends localised in the dermis. They consist of a single axon with numerous terminal branches embedded in a core of Schwann‐related cells and collagen, all surrounded by a multilayered capsule of perineurial origin (Halata, 1977; Munger and Ide, 1988; Rice and Albrecht, 2008; Fleming and Luo, 2013). Sensory corpuscles morphologically similar to Ruffini's corpuscles have been identified in the skin of monkeys and raccoons (Rice and Rasmusson, 2000; Paré et al., 2002).
Given the scarcity of information on cutaneous Ruffini's corpuscles, the present study aimed to verify the existence of Ruffini's corpuscles in human digital glabrous skin, its morphology and immunohistochemical characteristics.
2. METHODS
2.1. Quantitative study
The presence of typical Ruffini's corpuscles was investigated in 372 sections of human digital skin processed for detection of S100 protein (S100P; see below for details of immunohistochemical staining and the antibody used) belonging to the histological collection of the SINPOS research group at the University of Oviedo (Registro Nacional de Biobancos, Sección colecciones, Ref. C‐0001627, responsible OG‐S). The antigenicity of the stored sections was tested in 10 randomly selected slides, and no evident changes were observed with respect to the newly processed ones. Samples were from the distal phalanx of all fingers of the hand, with predominance of fingers 1 and 2, and were obtained from 186 subjects (two sections per subject taken at least 100 µm apart) free of peripheral neurological disease, who suffered incidental amputation of the fingertip. This material was collected and processed at our laboratory from 1998 until 2019, corresponded to subjects with an age range of 19 to 92 years, and stored as 10 µm thick sections in consecutively numbered slides. The collection of the skin samples was performed within 6‐8 h after amputation, cleaned in cold physiological solution and fixed in 4% buffered formaldehyde, and then processed for routinely embedding in paraffin. Basic data on case materials are presented in Table 1.
Table 1.
Data on case materials and density of Ruffini's corpuscles in human glabrous digital skin
| Gender |
| Males: 157 (77%); Females: 47 (23%) |
| Age: 19–92 years |
| 10–20: 2 (1%); 21–40: 65 (32%); 41–60: 101 (50%); 61–80: 33 (16%); 80–100: 3 (1%) |
| Number of samples: 186 |
| F1: 70 (37.5%); F2: 85 (45.5%); F3: 21 (11.5%); F4: 3 (2.5%); F5: 7 (3.5%) |
| Number of fields: 1860 (372 sections)* |
| F1: 350 (1400 mm2); F2: 425 (1700 mm2); F3: 105 (420 mm2); F4: 15 (60 mm2); F5: 35 (140 mm2) |
| Number of Ruffini's corpuscles: 461 |
| F1: 113 (24.5%); F2: 256 (55.5%); F3: 74 (16%); F4: 16 (3.5%); F5: 2 (0.5%) |
| Number of Ruffini's corpuscles per mm2: |
| F1: 0.08; F2: 0.15; F3: 0.17; F4: 0.26; F5: 0.01 |
F, finger.
Each section was scanned with a SCN400F scanner (Leica Biosystems™, Newcastle, UK) and annotated using the SlidePath Gateway LAN software (Leica Biosystems™). Then, on 4x500 µm enlarged images, a grid of 4 mm2 was applied randomly in five non‐overlapping fields of dermis (20 mm2 per section; 40 mm2 per subject), and Ruffini's corpuscles within the grid were counted by two independent observers. Results are expressed as absolute numbers of Ruffini's corpuscles on each finger and as density of Ruffini's corpuscles per mm2 (see Table 1 and Figure S1).
2.2. Immunohistochemical characterisation of Ruffini's corpuscles
Selected skin sections (n = 50) obtained from five skin samples (10 subjects, age range 19–73 years) were subjected to single and dual immunohistochemical staining to characterise Ruffini's corpuscles. These subjects were independent of those included in Table 1. This experiment was approved by the Ethical Committee for Biomedical Research of the Principality of Asturias, Spain (Cod. CElm, PAst: Proyecto 266/18). All materials were obtained in compliance with Spanish law (RD 1301/2006; Ley 14/2007; DR 1716/2011; Orden ECC 1414/2013).
The skin samples were routinely processed for paraffin embedding, cut on 10 µm thick sections perpendicular to the skin surface, and mounted on gelatine‐coated microscope slides. Thereafter, deparaffinised and rehydrated sections were processed for indirect immunohistochemistry using the Leica Bond™ Polymer Refine Detection Kit (Leica Biosystems™) following the manufacturer's instructions. Owing to the continuity between the axon and periaxonic cells of sensory corpuscles and the cells of nerve trunks (Vega et al., 2009), we examined the expression of axonal (neuron‐specific enolase: NSE; clone BBS/NC/VI‐H14 used diluted 1:1000; from Dako), Schwann‐related cells (S100 protein: S100P; raised in rabbit, used diluted 1:1000; from Dako), endoneurial (CD34 antigen; clone QB‐END/10, purchased prediluted; from Master Diagnostica) or perineurial (glucose transporter 1: Glut1; raised in rabbit, used diluted 0.5 µg/L; from Rocklin) markers in deep cutaneous dermal corpuscles and particularly those whose morphology was reminiscent of Ruffini's corpuscles.
Dual immunofluorescence was carried out to investigate the co‐localisation of S100P with NSE and S100P with CD34 or Glut1. Non‐specific binding in deparaffinised and rehydrated sections was reduced by incubation in 5% bovine serum albumin in Tris‐buffered saline, pH 7.4, for 30 min. Sections were then incubated overnight at 4°C in a humidified chamber with a 1:1 (v/v) mixture of anti‐S100P and anti‐NSE, anti‐S100P and anti‐CD34 or anti‐S100P and anti‐Glut1 antibodies. After rinsing, the sections were incubated for 1 h with Alexa Fluor 488‐conjugated goat anti‐rabbit IgG (1:1000; Serotec™), rinsed again, and incubated for 1 h with a Cy3‐conjugated donkey anti‐mouse antibody (1:50; Jackson‐ImmunoResearch™; Baltimore). Both steps were performed at room temperature in a dark, humidified chamber. Thereafter, sections were washed, mounted with Fluoromount Gold (ThermoFisher), and finally counterstained with 4′,6‐diamidino‐2‐phenylindole (DAPI; 10 ng/mL) to label the nuclei. Triple‐stained sections were examined under a Leica DMR‐XA automatic fluorescence microscope coupled with Leica Confocal Software, version 2.5 (Leica Microsystems), and the captured images were processed using ImageJ version 1.43 g software at Master Biophotonics Facility, McMaster University (www.macbiophotonics.ca).
As controls, representative sections were processed in the same way as described above using non‐immune rabbit or mouse sera instead of primary antibodies or by omitting the primary antibodies during incubation.
3. RESULTS
For the purpose of this study, dermal nerve formations with the following morphologies were considered as typical Ruffini's corpuscles: (a) fusiform or spindle‐shaped in longitudinal sections, comprising a highly branched axon (sections processed for NSE or NFP) or a core of disorganised Schwann‐like cells (sections processed for S100P) surrounded by a capsule of varying thickness; (b) circular in cross‐sections, with the same immunohistochemical characteristics and capsulated. This characterisation was chosen because oblique sections of dermal nerves are frequently misidentified as Ruffini's corpuscles.
3.1. Occurrence and density of Ruffini's corpuscles
The number of Ruffini's corpuscles identified with certainty was very low. We evaluated randomly chosen areas of 20 mm2 from 372 sections of human digital skin obtained from 186 subjects. The occurrence of typical Ruffini's corpuscles was noted in all sections, with absolute values being F2 > F1 > F3 > F4 > F5, and density per mm2 being F4 > F3 > F2 > F1 > F5. In all cases, the density remained below 0.3 corpuscles/mm2 (Table 1).
3.2. Immunohistochemical characterisation of cutaneous Ruffini's corpuscles
Typical Ruffini's corpuscles were identified in the central and deep zones of the dermis. They exhibited fusiform or circular morphology and consisted of a branched axon embedded in a cluster of Schwann‐like cells surrounded by a capsule of variable thicknesses (Figures 1 and 2). A subcapsular space between the capsule and the core of glial cells, filled by flattened cells, was also noted (Figure 2c).
Figure 1.
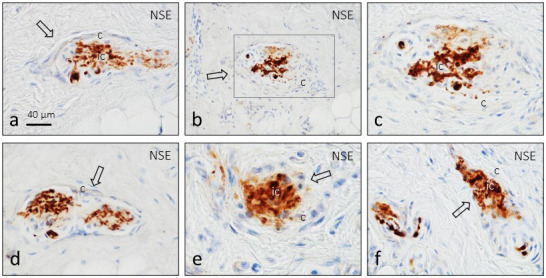
Longitudinal (a–d and f) and transversal (e) sections of human digital Ruffini's corpuscles (arrows) subjected to immunolabelling of axon branches (NSE: neuron‐specific enolase). (c) is an enlargement of (b). c, capsule
Figure 2.
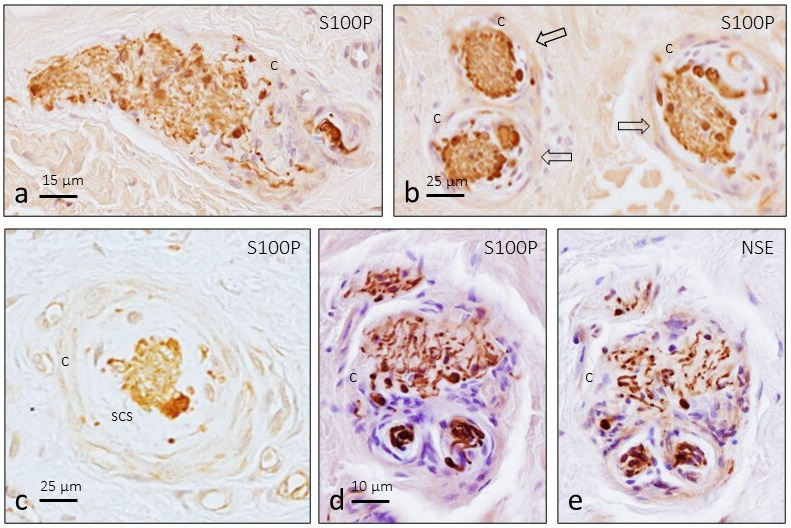
Longitudinal (a) and transversal (b and c) sections of human digital Ruffini's corpuscles (arrows) subjected to immunolabelling of glial cells (S100P: S100 protein). Serial sections (d and e) immunolabelled for S100P and NSE showing a transversal section of a single Ruffini's corpuscle associated with two smaller unidentified sensory corpuscles (black arrows). c, capsule; scs, subcapsular space
We rarely observed any association between structures that could be regarded as Ruffini's corpuscles and other smaller corpuscles surrounded by a common capsule (Figure 2d and 2e).
The composition of Ruffini's corpuscles was also studied by fluorescence and confocal microscopy. NSE and S100P showed complementary distribution patterns by immunohistochemistry and highlighted the intense axonal arborisation and irregular arrangement of Schwann‐like cells (Figure 3). All structures identified as Ruffini's corpuscles possessed a complete capsule that displayed intense immunoreactivity against CD34 (an endoneurial marker) (Figure 4) but not Glut1 (a perineurial marker; data not shown). Interestingly, CD34‐positive septa partially segmented the inner nucleus (Figure 3e and 3f).
Figure 3.
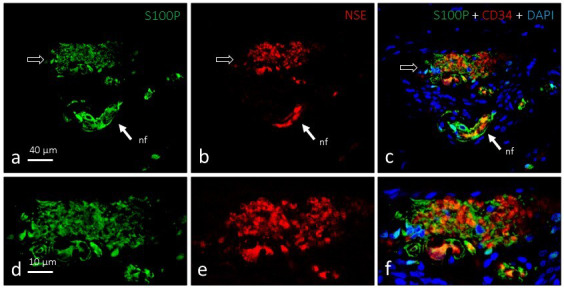
Dual immunofluorescence for S100 protein (a and d; green fluorescence) and neuron‐specific enolase (b and e; red fluorescence) in a single Ruffini's corpuscle (arrows). The axonal branch and glial cells exhibit complementary localisation (c and f), are intimately linked, and occupy most of the corpuscle. nf, nerve fibre supplying the corpuscle. Objective: 63×/1.40 oil; pinhole: 1.37; XY resolution: 139.4 nm; and Z resolution: 235.8 nm
Figure 4.
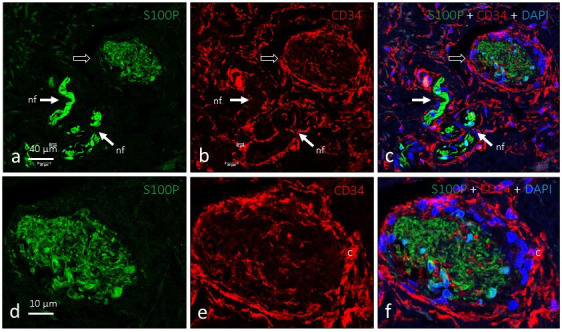
Dual immunofluorescence for S100 protein (a and d; green fluorescence) and CD34 (b and e; red fluorescence) in a single Ruffini's corpuscle (arrows). Glial cells occupy the core of the corpuscle and the capsule is of endoneurial origin (c and f). nf: nerve fibre. Objective: 63X/1.40 oil; pinhole: 1.37; XY resolution: 139.4 nm; and Z resolution: 235.8 nm
4. DISCUSSION
Ruffini's corpuscles were described for the first time in the dermis under a light microscope by Angelo Ruffini (1894) and at ultrastructural level by Chambers et al. (1972). They are long fusiform encapsulated structures (0.2–2 mm in length and 50–200 µm in diameter) containing a core formed by a repeatedly branched axon located within collagen bundles (Chambers et al., 1972). As in other types of cutaneous sensory corpuscles (Cobo et al., 2020), the core of Ruffini's corpuscles also contains Schwann‐related cells. Furthermore, beneath the capsule is a fluid‐filled subcapsular space. Functionally, Ruffini's corpuscles represent type II SA LTMRs that detect stretching and tangential forces generated in the skin, as well as the direction of motion of an object (Johnson, 2001; Jones and Smith, 2014; Owens and Lumpkin, 2014; Olson et al., 2016).
The present study was designed to identify and immunohistochemically characterise Ruffini's corpuscles in human digital skin. This sensory corpuscle is mentioned in books of neuroanatomy and histology and, whereas some authors affirm that ‘they are broadly expressed in the dermis’ (Roudaut et al., 2012), their existence in glabrous skin has not been confirmed. They have, however, been documented in hairy skin (Chambers et al., 1972; Biemesderfer et al., 1978; Halata, 1988; Munger and Ide, 1988; Zimmerman et al., 2014, as well as in non‐skin tissues, such as the joints’ ligaments and capsule (Halata, 1988; Hogervorst and Brand, 1998).
Early reports of Ruffini's corpuscles in glabrous skin (see Halata, 1988) have not been corroborated by more recent investigations in different species including humans (Rice and Rasmusson, 2000; Paré et al., 2002, 2003; Wellnitz et al., 2010). In humans, Paré et al. (2003) observed a single presumptive Ruffini‐like corpuscle in the index finger and typical Ruffini corpuscles were only found at the base of finger nails of Macaca fascicularis (Paré et al., 2002). Nevertheless, type II SA LTMRs have been recorded in human microneurography studies, although their proportions appeared to differ greatly among the populations of assessed fibres (Johansson and Vallbo, 1979; Macefield et al., 1990; Phillips et al., 1992).
Here, we observed typical Ruffini's corpuscles in all fingers, albeit at a very low density of fewer than 0.3 corpuscles per mm2 (Table 1). The discrepancy in Ruffini's corpuscle density between our findings and those of Paré et al. (2003) may be explained by the number of sections examined, as well as by the markers used to identify them. Paré et al. (2003) used exclusively axonal markers (200‐kDa phosphorylated subunit of NFP), whereas we employed also markers for glial cells, which are known to form the core of the corpuscles. The approach chosen in the present study allows for more accurate identification of sensory corpuscles, because they are better preserved and can be examined for longer times in fixed samples.
One question remains nevertheless unanswered. Electrophysiological studies of glabrous skin demonstrate high activity compatible with type II SA LTMR; however, our study shows that Ruffini's corpuscles are scarce or non‐existent in this tissue. Hence, it remains unclear which sensory corpuscles are the source of such activity. One possibility is that other sensors, morphologically dissimilar to Ruffini's corpuscles and distributed in the dermis, may exert the same function as that of Ruffini's corpuscles. Paré et al. (2002) and ourselves (Cobo et al., unpublished) have observed numerous sensory nerve formations characteristic of glabrous skin, such as Meissner's and Pacinian corpuscles, in human digital dermis. Furthermore, Rice and Albrecht (2008) affirmed that type II SA fibres may actually be innervating diffuse patterns of Merkel's cells.
Our immunohistochemical profile of Ruffini's corpuscles coincides with that expected for this type of sensor and is similar to those observed for other skin mechanoreceptors (Vega et al., 2009). The axon that forms Ruffini's corpuscles were positive for NSE and NFP, Schwann‐like glial cells displayed an intense immunoreaction against S100P, and the capsule was related to endoneurium (García‐Piqueras et al., 2020) rather than perineurium origin as claimed by other authors (Halata, 1977; Munger and Ide, 1988).
Taken together, the present study demonstrates that Ruffini's corpuscles exist in human glabrous digital skin at very low densities, and that they share the basic immunohistochemical characteristics of other dermal sensory corpuscles.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest regarding the publication of this paper.
AUTHOR CONTRIBUTIONS
RC, YG‐M and LC performed the experiments. JF and OG‐S collected the material in compliance with ethical guidelines and performed part of the experiments. RC, JM‐C, JC, OG‐S and JG‐P quantified the data. JG‐P and JAV designed the study, analysed the data and wrote the manuscript.
Supporting information
Fig S1
ACKNOWLEDGEMENTS
This study was supported in part by a grant from Gerencia Regional de Salud de Castilla y León to JF and JAV (GRS 1882/A/18). YG‐M was supported by a ‘Severo Ochoa’ grant from the Government of the Principality of Asturias (Ref. BP17‐044). The authors thank Dr. Marta Guervos (Servicios Comunes de Investigación, Microscopia Confocal, Universidad de Oviedo) and Marta Sánchez‐Pitiot (Grupo de Histopatología Molecular, Instituto Universitario de Oncología del Principado de Asturias) for technical assistance.
Cobo R, García‐Mesa Y, Cárcaba L et al. Verification and characterisation of human digital Ruffini’s sensory corpuscles. J. Anat. 2020;238:13–19. 10.1111/joa.13301
Ramón Cobo and Yolanda García‐Mesa contributed equally to this paper.
DATA AVAILABILITY STATEMENT
Data supporting the findings of this study will be available from the corresponding author upon reasonable request.
REFERENCES
- Abraira, V.E. & Ginty, D.D. (2013) The sensory neurons of touch. Neuron, 79, 618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemesderfer, D. , Munger, B.L. , Binck, J. , & Dubner, R. (1978) The pilo‐Ruffini complex: A non‐sinus hair and associated slowly‐adapting mechanoreceptor in primate facial skin. Brain Research, 142, 197–222. [DOI] [PubMed] [Google Scholar]
- Chambers, M.R. , Andres, K.H. , von Duering, M. & Iggo, A. (1972) The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Quarterly Journal of Experimental Physiology and Cognate Medical Sciences, 57, 417–445. [DOI] [PubMed] [Google Scholar]
- Cobo, R. , García‐Mesa, Y. , García‐Piqueras, J. , Feito, J. , Martín‐Cruces, J. , García‐Suárez, O. and José A. Vega (2020) The glial cell of human cutaneous sensory corpuscles: Origin, characterization, and putative roles, In: Suzuki T. (Ed.), Somatosensory and motor research (pp. 19–33). London, UK: IntechOpen; 10.5772/intechopen.91815 [DOI] [Google Scholar]
- Feito, J. , Ramos‐García, J.L. , Gago, Á. Cobo, J.L. , García‐Suárez, O. , Junquera, L.M. and Vega, J.A. (2016) Pacinian corpuscles in a cervical chondrocutaneous remnant: A case report and update of Pacinian corpuscles. American Journal of Dermatopathology, 38, 231–235. [DOI] [PubMed] [Google Scholar]
- Feito, J. , García‐Suárez, O. , García‐Piqueras, J. García‐Mesa, Y. , Pérez‐Sánchez, A. , Suazo, I. et al. (2018) The development of human digital Meissner's and Pacinian corpuscles. Annals of Anatomy ‐ Anatomischer Anzeiger, 219, 8–24. [DOI] [PubMed] [Google Scholar]
- Fleming, M.S. and Luo, W. (2013) The anatomy, function, and development of mammalian Aβ low‐threshold mechanoreceptors. Frontiers in Biology (Beijing), 8, 408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Piqueras, J. , García‐Mesa, Y. , Cárcaba, L. , Feito, J. , Torres‐Parejo, I. , Martín‐Biedma, B. et al (2019) Ageing of the somatosensory system at the periphery: age‐related changes in cutaneous mechanoreceptors. Journal of Anatomy, 234, 839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Piqueras, J. , Cobo, R. , Cárcaba, L. , García‐Mesa, Y. , Feito, J. , Cobo, J. et al (2020) The capsule of human Meissner corpuscles: Immunohistochemical evidence. Journal of Anatomy, 236, 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner, E.P. and Johnson, K.O. (2013) Touch In: Kandel E.R., Schwartz J.H., Jessell T.M., Siegelbaum S.A. & Hudspeth A.J. (Eds.) Principles of neural science. New York: McGraw‐Hill, pp. 498–529. [Google Scholar]
- Halata, Z. (1977) The ultrastructure of the sensory nerve endings in the articular capsule of the knee joint of the domestic cat (Ruffini corpuscles and Pacinian corpuscles). Journal of Anatomy, 124, 717–729. [PMC free article] [PubMed] [Google Scholar]
- Halata, Z. (1988) Ruffini corpuscle – a stretch receptor in the connective tissue of the skin and locomotion apparatus. Progress in Brain Research, 74, 221–229. [DOI] [PubMed] [Google Scholar]
- Hogervorst, T. and Brand, R.A. (1998) Mechanoreceptors in joint function. Journal of Bone and Joint Surgery. American Volume, 80, 1365–1378. [DOI] [PubMed] [Google Scholar]
- Johansson, R.S. and Vallbo, A.B. (1979) Tactile sensibility in the human hand: Relative and absolute densities of four types of mechanoreceptive units in glabrous skin. Journal of Physiology, 286, 283–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, K.O. (2001) The roles and functions of cutaneous mechanoreceptors. Current Opinion in Neurobiology, 11, 455–461. [DOI] [PubMed] [Google Scholar]
- Jones, L.A. and Smith, A.M. (2014) Tactile sensory system: encoding from the periphery to the cortex. Wiley Interdisciplinary Reviews: Systems Biology and Medicine, 6, 279–287. [DOI] [PubMed] [Google Scholar]
- McGlone, F. , Wessberg, J. & Olausson, H. (2014) Discriminative and affective touch: Sensing and feeling. Neuron, 82, 737–755. [DOI] [PubMed] [Google Scholar]
- McGlone, F. & Reilly, D. (2010) The cutaneous sensory system. Neuroscience and Biobehavioral Reviews, 34, 148–159. [DOI] [PubMed] [Google Scholar]
- Macefield, G. , Gandevia, S.C. & Burke, D. (1990) Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. Journal of Physiology, 429, 113–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger, B.L. and Ide, C. (1988) The structure and function of cutaneous sensory receptors. Archives of Histology and Cytology, 51, 1–34. [DOI] [PubMed] [Google Scholar]
- Olson, W. , Dong, P. , Fleming, M. , and Luo, W. (2016) The specification and wiring of mammalian cutaneous low‐threshold mechanoreceptors. Wiley Interdisciplinary Reviews: Developmental Biology, 5, 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens, D.M. & Lumpkin, E.A. (2014) Diversification and specialization of touch receptors in skin. Cold Spring Harbor Perspectives in Medicine, 4, a013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré, M. , Smith, A.M. & Rice, F.L. (2002) Distribution and terminal arborizations of cutaneous mechanoreceptors in the glabrous finger pads of the monkey. The Journal of Comparative Neurology, 445, 347–359. [DOI] [PubMed] [Google Scholar]
- Paré, M. , Behets, C. & Cornu, O. (2003) Paucity of presumptive Ruffini corpuscles in the index finger pad of humans. The Journal of Comparative Neurology, 456, 260–266. [DOI] [PubMed] [Google Scholar]
- Phillips, J.R. , Johansson, R.S. & Johnson, K.O. (1992) Responses of human mechanoreceptive afferents to embossed dot arrays scanned across fingerpad skin. Journal of Neuroscience, 12, 827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson, D.D. and Turnbull, B.G. (1986) Sensory innervation of the raccoon forepaw: 2. Response properties and classification of slowly adapting fibers. Somatosensory Research, 4, 63–75. [DOI] [PubMed] [Google Scholar]
- Rice, F.L. and Albrecht, P.J. (2008) Cutaneous mechanisms of tactile perception: Morphological and chemical organization of the innervation to the skin In: Smith D., Firestein S. and Beauchamp G. (Eds.) The Senses: A Comprehensive reference. San Diego: Academic Press, pp. 1–32. [Google Scholar]
- Rice, F.L. & Rasmusson, D.D. (2000) Innervation of the digit on the forepaw of the raccoon. The Journal of Comparative Neurology, 417, 467–490. [DOI] [PubMed] [Google Scholar]
- Roudaut, Y. , Lonigro, A. , Coste, B. , Hao, J. , Delmas, P. & Crest, M. (2012) Touch sense: Functional organization and molecular determinants of mechanosensitive receptors. Channels (Austin), 6, 234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffini, A. (1894) Sur un nouvel organe nerveux terminal et sur la présence des corpuscles Golgi‐Mazzoni dans le cojonctifsous‐cutané de la pulpe des doigts de l´homme. Archives Italiennes de Biologie, 21, 249–265. [Google Scholar]
- Vega, J.A. , García‐Suárez, O. , Montaño, J.A. , Pardo, B. and Cobo, J.M. (2009) The Meissner and Pacinian sensory corpuscles revisited new data from the last decade. Microscopy Research and Technique, 72, 299–309. [DOI] [PubMed] [Google Scholar]
- Wellnitz, S.A. , Lesniak, D.R. , Gerling, G.J. , and Lumpkin, E.A. (2010) The regularity of sustained firing reveals two populations of slowly adapting touch receptors in mouse hairy skin. Journal of Neurophysiology, 103, 3378–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelená, J. (1994) Nerves and Mechanoreceptors: The Role of Innervation in the Development and Maintenance of Mammalian Mechanoreceptors. New York: Chapman & Hall. [Google Scholar]
- Zimmerman, A. , Bai, L. & Ginty, D.D. (2014) The gentle touch receptors of mammalian skin. Science, 346, 950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Data Availability Statement
Data supporting the findings of this study will be available from the corresponding author upon reasonable request.


