Abstract
Aims
Previous studies have demonstrated that moderate/severe tricuspid regurgitation (TR) is associated with adverse outcome in patients with heart failure (HF) with reduced ejection fraction. Little is known about the prevalence and prognostic value of TR in patients of stage B HF and those with stage C HF with preserved ejection fraction (HFpEF). We aimed to investigate the prevalence and prognosis of TR in patients with HFpEF.
Methods and results
From 2013 to 2017, 2014 patients with stage B (n = 1341) or C (n = 673) HFpEF were enrolled in the study. Detailed transthoracic echocardiogram was performed, and the severity of TR was graded as no, mild, moderate, and severe. The mean age of the study population was 66.7 ± 14.1 years old, and 46% were men. Mean left ventricular ejection fraction was 62.2 ± 5.5%. The prevalence of moderate/severe TR increased from stage B to C HF (8% to 16%, respectively, P < 0.01). Older age, hyperlipidaemia, atrial fibrillation, left ventricular mass, and right ventricular systolic pressure were independently associated with moderate/severe TR (P < 0.05 for all). With a median follow‐up of 3.8 (2.9–4.7) years, 346 patients died and 234 developed HF requiring hospitalization. Kaplan–Meier curve revealed that the presence of moderate/severe TR was associated with all‐cause mortality, HF requiring hospitalization and cardiovascular death (log‐rank test P < 0.01). Multivariable analysis demonstrated that moderate (hazard ratio = 1.5; 95% confidence interval: 1.1–2.2; P < 0.05) and severe TR (hazard ratio = 2.1; 95% confidence interval: 1.3–3.3; P < 0.01) were independently associated with mortality, HF requiring hospitalization and cardiovascular death.
Conclusions
The presence of moderate/severe TR is not uncommon in patients with stage B HF and stage C HFpEF. Importantly, moderate/severe TR was independently associated with mortality and HF requiring hospitalization.
Keywords: Heart failure with preserved ejection fraction, Tricuspid regurgitation, Predictors, Prognosis
Introduction
The presence of trivial and mild tricuspid regurgitation (TR) is often detected during transthoracic echocardiography assessment and has long been thought to be clinically benign. 1 Likewise, an incidental finding of moderate and severe TR can be found in 7% in community setting and 12% in hospitalized patients with heart failure (HF). 2 , 3 , 4 The clinical relevance of TR has been further demonstrated in studies showing that significant TR is strongly associated with adverse outcome in various populations. 2 , 3 , 4 Consequently, an increasing number of studies are focusing on evaluation of the natural progression and outcome of TR.
In 2001, the ACC/AHA classified HF as stage A (those who are at risk for developing HF but have no structural disorder of the heart), B (patients with a structural disorder of the heart but who have never developed symptoms of HF), C (patients with past or current symptoms of HF associated with underlying structural heart disease), or D (refractory/end‐stage HF) to help clinicians provide tailored treatment and develop a surveillance strategy for individual patients. 5 Recent studies have demonstrated that significant TR is associated with all‐cause mortality and HF requiring hospitalization in patients with stage B and C HF with reduced ejection fraction (HFrEF). 2 Nonetheless, the prevalence as well as the prognostic implication of significant TR in patients at risk of stage B HF and those with stage C HF with preserved ejection fraction (HFpEF) is uncertain. The aim of the present study was to investigate the prevalence and severity of TR in asymptomatic stage B and symptomatic stage C HFpEF. Further, the predictive value of moderate and severe TR for mortality and HF requiring hospitalization was evaluated.
Methods
Study population
Consecutive patients who underwent detailed outpatient echocardiography were recruited retrospectively from 2013 to 2017. Patients were included if they were (i) diagnosed with stage B or C HF based on 2013 ACCF/AHA Guideline for the Management of Heart Failure 6 ; (ii) had left ventricular (LV) ejection fraction (LVEF) ≥ 50% on echocardiography; and (iii) had integrated clinical and echocardiographic characterization at baseline. Among the 4942 patients retrieved, 2928 were excluded for the following reasons: LVEF < 50% (n = 1794), incomplete echocardiographic parameter measurement (n = 106), no risk factors for HF (n = 95), and presence of stage A HF (868) or stage D HF (n = 37). We further excluded patients with congenital or infiltrative heart disease (sarcoidosis or amyloidosis), previous valve surgery, pacemaker or defibrillator implantation, end‐stage renal failure, severe liver dysfunction, or cancer (n = 28). The final analysis comprised 2014 patients with stage B HF and stage C HFpEF (Figure 1 ). This was a single‐centre study conducted in Queen Mary Hospital, and 11 clinicians participated in performing echocardiography.
Figure 1.
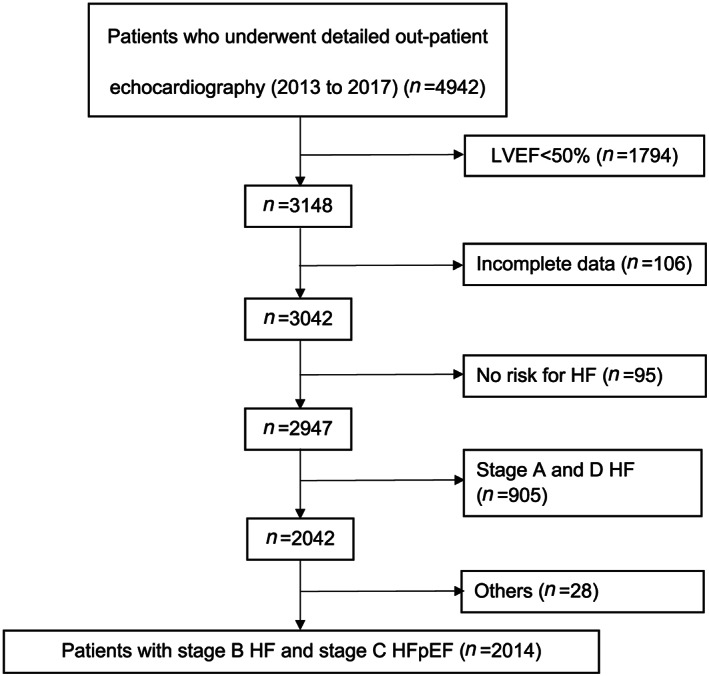
Flow chart of the cohort. HF, heart failure; HFpEF, heart failure with preserved ejection fraction; LVEF, left ventricular ejection fraction.
Follow‐up
The primary endpoints were the composite of all‐cause mortality and HF requiring hospitalization. The exact cause of mortality was retrieved and subsequently classified as cardiovascular or non‐cardiovascular. Major adverse cardiovascular events (MACEs) were defined as all‐cause mortality and HF requiring hospitalization. The study was approved by the ethics committee of the West Cluster Hospital Authority, and all patients had signed written consent. Clinical outcome of the patients was retrieved by the electronic clinical medical record system of the Hospital Authority of Hong Kong, China, where detailed records of all hospitalization and cause of mortality for each individual were available.
Echocardiography
All echocardiography was performed using a commercially available ultrasound system (Vingmed Vivid 7 or E9, General Electric Vingmed Ultrasound, Milwaukee, WI, USA). A 3.5 MHz probe was used to obtained images that were stored in cine‐loop format (three cardiac cycles). Two‐dimensional M‐mode echocardiography was used to measure interventricular septum, LV dimension, and posterior wall thickness at end‐diastole in the parasternal long axis view according to the guideline. 7 LV mass was calculated according to the Devereux formula. 8 LVEF, LV end‐diastolic volume, and end‐systolic volume were derived from the standard apical four‐chamber and two‐chamber views using the modified biplane Simpson method. Diastolic dysfunction grade was classified according to recommendations of the ASE/EACVI that were based on the measurement of E, A, E/A, E/e` ratio, and TR peak velocity. 9 The degree of TR was visually assessed using an integrative, semiquantitative approach and graded as none, mild, moderate, or severe. The TR jet was interrogated using continuous‐wave (CW) Doppler in multiple views including right ventricular (RV) inflow, parasternal short axis at the aortic valve level, and apical four‐chamber view. Hepatic venous flow was sampled using pulsed‐wave Doppler to look for systolic flow reversal in severe TR. Pulmonary artery systolic pressure was estimated by right ventricular systolic pressure (RVSP) that was calculated from peak TR velocity by continuous‐wave Doppler using simplified Bernoulli equation and combining this value with the estimated right atrial pressure: RVSP = 4(V)2 + right atrial pressure. The severity of a left‐sided valvular lesion including mitral regurgitation or mitral stenosis, aortic regurgitation, or aortic stenosis was classified according to the current recommendations. 10
Statistical analysis
Continuous variables are expressed as mean ± standard deviation and analysis of variance with Bonferroni post hoc testing used for comparison of intergroup differences. Categorical variables are expressed as frequency or proportion and compared using Mann–Whitney U test. Univariable logistic regression was used to evaluate the association between cardiac risk factors and the severity of TR that was divided into two groups (no/mild vs. moderate/severe). Variables with P < 0.1 were further tested in multivariable logistic regression. Kaplan–Meier curve was used to estimate the impact of TR on all‐cause mortality, hospitalization, and both by dividing TR at baseline into quartiles and log‐rank used to compare survival rates. Cox proportional hazards regression models adjusted for age, gender, LVEF, atrial fibrillation (AF), and HF stage were conducted to estimate the influence of TR on adverse events. All statistical analyses were performed using SPSS 25.0 (Chicago, Illinois, USA). A two‐sided P value <0.05 for all tests was considered statistically significant.
Results
The mean age of the cohort was 67 years, and 46% were men. Their clinical demographics are shown in Table 1 . Over half of the cohort had hypertension, one third had diabetes mellitus, and 40% had hyperlipidaemia. Around 20% of the study population had underlying AF and ischaemic heart disease.
Table 1.
Clinical characteristics of the entire cohort and by TR groups
| Clinical characteristics | Overall (N = 2014) | No TR (N = 955) | Mild TR (N = 837) | Moderate TR (N = 168) | Severe TR (N = 54) | P value |
|---|---|---|---|---|---|---|
| Age, years | 66.7 ± 14.1 | 64.0 ± 13.8 | 67.9 ± 14.3 | 73.0 ± 11.5 | 75.1 ± 12.1 | <0.01 |
| Male, n (%) | 934 (46) | 497 (52) | 343 (41) | 76 (45) | 18 (33) | <0.01 |
| Hb, g/dL | 12.6 ± 2.1 | 12.6 ± 2.1 | 12.7 ± 2.1 | 12.4 ± 2.3 | 12.4 ± 2.2 | 0.50 |
| Cr, umol/L | 91.5 ± 41.8 | 91.0 ± 42.3 | 91.6 ± 42.2 | 90.0 ± 35.3 | 102.6 ± 45.3 | 0.31 |
| Clinical history | ||||||
| HT, n (%) | 1142 (57) | 575 (60) | 454 (54) | 86 (51) | 27 (50) | <0.01 |
| DM, n (%) | 665 (33) | 370 (39) | 243 (29) | 38 (23) | 14 (26) | <0.01 |
| HL, n (%) | 851 (42) | 426 (45) | 364 (44) | 46 (27) | 15 (28) | <0.01 |
| AF, n (%) | 446 (22) | 119 (13) | 197 (24) | 90 (54) | 40 (74) | <0.01 |
| IHD, n (%) | 482 (24) | 237 (25) | 195 (23) | 39 (23) | 11 (20) | 0.38 |
| HF stage | ||||||
| HF stage B, n (%) | 1341 (67) | 690 (72) | 537 (64) | 98 (58) | 16 (30) | <0.01 |
| HF stage C, n (%) | 673 (33) | 265 (28) | 300 (36) | 70 (42) | 38 (70) | <0.01 |
AF, atrial fibrillation; Cr, creatinine; DM, diabetes mellitus; Hb, haemoglobin; HF, heart failure; HL, hyperlipidaemia; HT, hypertension; IHD, ischaemic heart disease; TR, tricuspid regurgitation.
Values are expressed as mean ± standard deviation or n (%).
Those with P value < 0.05 were in bold which means the variable is significant.
Patients were stratified as having no TR (n = 955, 47%), mild TR (n = 837, 42%), moderate TR (n = 168, 8%), or severe TR (n = 54, 3%) (Figure 2 ). A higher degree of TR was associated with older age and female gender and was less common in patients with diabetes mellitus and hyperlipidaemia but more common in patients with AF (Table 1 ). The prevalence of mild, moderate, and severe TR increased from stage B to C. In particular, the prevalence of moderate TR (7% vs. 10%, P < 0.01) and severe TR (1% vs. 6%, P < 0.01) increased from HF stage B and C, respectively (Figure 2 ).
Figure 2.
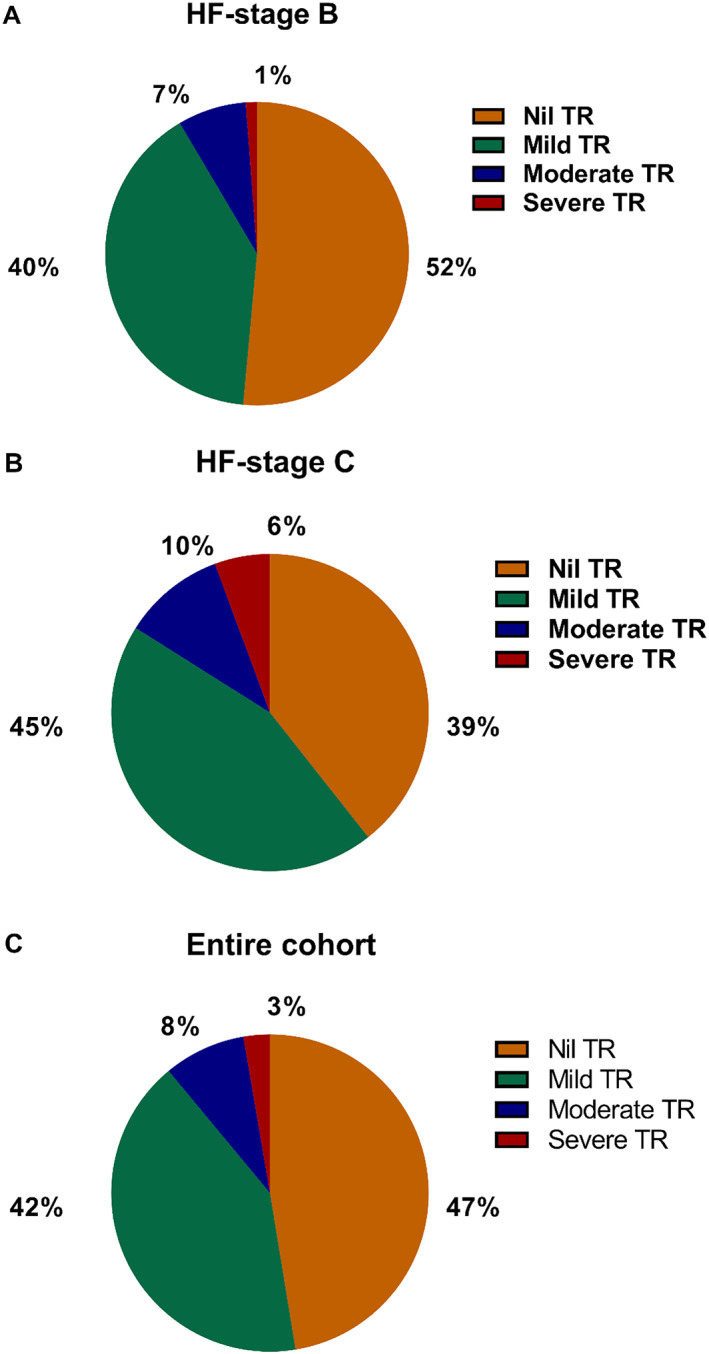
The prevalence of TR in (A) HF stage B, (B) HF stage C, and (C) entire cohort. HF, heart failure; TR, tricuspid regurgitation.
Detailed echocardiography parameters are shown in Table 2 . In the entire cohort, the mean LVEF was 62% and 33% of patients had diastolic dysfunction. Patients with a higher degree of TR were more likely to have a greater LV dimension, lower LVEF, higher RVSP, and higher prevalence of significant mitral regurgitation and aortic regurgitation (Table 2 ). Nonetheless, diastolic dysfunction was similar in patients with different grades of TR. Univariate analysis demonstrated that age, diabetes mellitus, hyperlipidaemia, AF, LV mass, LVEF, RVSP, and HF stage were associated with severity of TR. Upon multivariable adjustment with univariate significant variables, only older age, lower LV mass, higher RVSP, and presence of AF remained significantly associated with higher degree of TR (Table 3 ).
Table 2.
Echocardiographic characteristics of the entire cohort and by TR groups
| Echocardiographic characteristics | Overall (N = 2014) | No TR (N = 955) | Mild TR (N = 837) | Moderate TR (N = 168) | Severe TR (N = 54) | P value |
|---|---|---|---|---|---|---|
| LVDd, cm | 4.5 ± 0.6 | 4.5 ± 0.6 | 4.5 ± 0.6 | 4.5 ± 0.7 | 4.6 ± 0.8 | 0.50 |
| LVDs, cm | 2.9 ± 0.5 | 2.9 ± 0.5 | 3.0 ± 0.5 | 3.0 ± 0.5 | 3.1 ± 0.6 | <0.01 |
| LVEDV, mL | 94.5 ± 30.0 | 93.6 ± 28.6 | 94.5 ± 29.1 | 97.2 ± 34.6 | 100.8 ± 44.2 | 0.26 |
| LVM, g/m2 | 194.4 ± 59.3 | 199.6 ± 59.4 | 192.7 ± 56.2 | 178.9 ± 62.1 | 187.9 ± 87.9 | <0.01 |
| LVEF | 62.2 ± 5.5 | 62.7 ± 5.6 | 62.0 ± 5.3 | 61.4 ± 5.0 | 58.6 ± 5.3 | <0.01 |
| RVSP, mmHg | 34.5 ± 9.5 | 32.6 ± 9.6 | 35.3 ± 9.2 | 38.4 ± 8.8 | 39.3 ± 8.9 | <0.01 |
| DD, n (%) | 671 (33) | 305 (32) | 317 (38) | 41 (24) | 8 (15) | 0.33 |
| MR ≥ 2, n (%) | 197 (10) | 66 (7) | 103 (12) | 21 (13) | 7 (13) | <0.01 |
| MS ≥ 2, n (%) | 50 (2) | 17 (2) | 28 (3) | 3 (2) | 2 (4) | 0.11 |
| AR ≥ 2, n (%) | 109 (5) | 40 (4) | 53 (6) | 13 (8) | 3 (6) | 0.02 |
| AS ≥ 2, n (%) | 106 (5) | 54 (6) | 41 (5) | 8 (5) | 3 (6) | 0.62 |
AR, aortic regurgitation; AS, aortic stenosis; DD, diastolic dysfunction; LVDd, left ventricular dimension diastole; LVDs, left ventricular dimension systole; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; MR, mitral regurgitation; MS, mitral stenosis; RVSP, right ventricular systolic pressure; TR, tricuspid regurgitation.
Those with P value < 0.05 were in bold which means the variable is significant.
Table 3.
Univariate and multivariate logistic regression models for the association with the severity of tricuspid regurgitation
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | CI | P | OR | CI | P | |
| Age | 1.05 | 1.03–1.06 | <0.01 | 1.05 | 1.03–1.07 | <0.01 |
| Male | 0.83 | 0.63–1.10 | 0.20 | |||
| HT | 0.77 | 0.58–1.02 | 0.07 | 0.82 | 0.48–1.40 | 0.47 |
| DM | 0.59 | 0.43–0.82 | <0.01 | 0.65 | 0.37–1.16 | 0.14 |
| HL | 0.48 | 0.36–0.66 | <0.01 | 0.55 | 0.33–0.93 | 0.02 |
| AF | 6.57 | 4.90–8.80 | <0.01 | 4.71 | 3.89–7.67 | <0.01 |
| LVEDV | 1.00 | 1.00–1.01 | 0.07 | 1.02 | 1.00–1.03 | 0.02 |
| LV mass | 1.00 | 0.99–1.00 | <0.01 | 0.99 | 0.99–1.00 | 0.01 |
| LVEF | 0.94 | 0.92–0.97 | <0.01 | 0.96 | 0.92–1.00 | 0.07 |
| RVSP | 1.05 | 1.03–1.06 | <0.01 | 1.04 | 1.02–1.06 | <0.01 |
| MR ≥ 2 | 1.41 | 0.92–2.17 | 0.11 | |||
| AR ≥ 2 | 1.49 | 0.86–2.58 | 0.16 | |||
| HF stage | 2.06 | 1.55–2.73 | <0.01 | 1.24 | 0.76–2.05 | 0.39 |
AF, atrial fibrillation; AR, aortic regurgitation; CI, confidence interval; DM, diabetes mellitus; HF, heart failure; HL, hyperlipidaemia; HT, hypertension; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; OR, odds ratio; RVSP, right ventricular systolic pressure.
Those with P value < 0.05 were in bold which means the variable is significant.
Survival
During a median follow‐up of 3.8 (2.9–4.7) years, 346 patients died (112 cardiovascular and 234 non‐cardiovascular causes) and 234 developed HF requiring hospitalization. Kaplan–Meier curves for MACE, all‐cause mortality, cardiovascular mortality, non‐cardiovascular mortality, and HF requiring hospitalization are shown in Figure 3 . Patients with moderate/severe TR were more likely to experience individual adverse outcome compared with those with mild TR and no TR.
Figure 3.
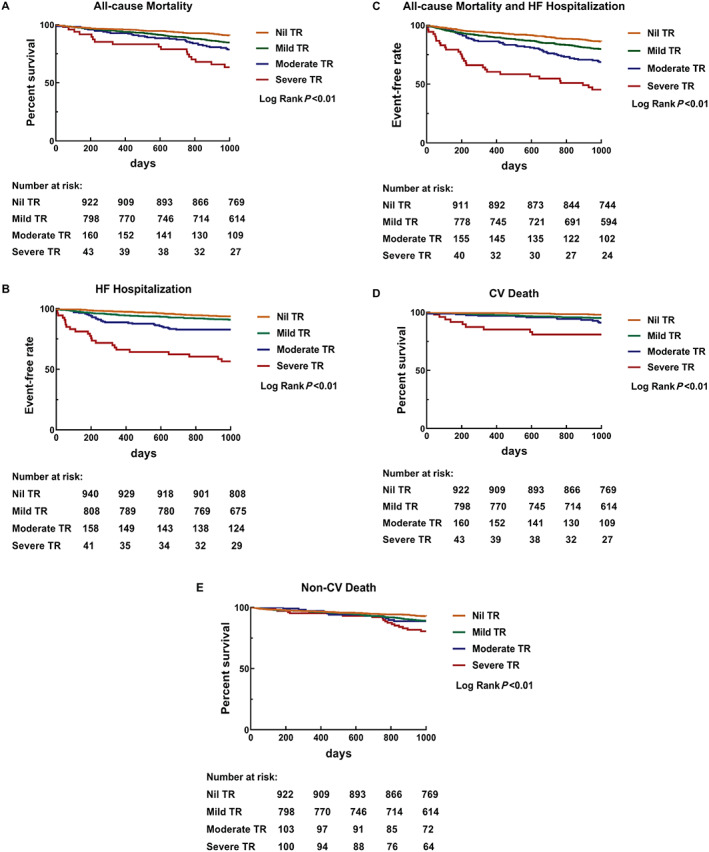
Kaplan–Meier curve for the impact of TR grades on (A) all‐cause mortality, (B) HF hospitalization, (C) all‐cause mortality and HF hospitalization, (D) cardiovascular death (CV‐death), and (E) non‐cardiovascular death (non‐CV death). In all subgroups, increasing TR grade is significantly associated with long‐term outcomes. HF, heart failure; TR, tricuspid regurgitation.
Cox regression for adverse outcome is shown in Table 4 . When compared with those with no TR, moderate/severe TR was independently associated with MACE, all‐cause mortality, and HF requiring hospitalization. Interestingly, moderate and severe TR was associated with cardiovascular mortality, while no such association was noted for non‐cardiovascular mortality.
Table 4.
TR impact on adverse outcomes
| TR grade | Unadjusted model | Adjusted for age, sex, EF, AF, RVSP, and HF stage | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| All‐cause mortality and HF hospitalization | ||||
| No TR | Reference | Reference | ||
| Mild TR | 1.5 (1.2–1.8) | <0.01 | 1.2 (0.9–1.5) | 0.21 |
| Moderate TR | 2.4 (1.8–3.2) | <0.01 | 1.5 (1.1–2.2) | 0.03 |
| Severe TR | 5.7 (3.9–8.2) | <0.01 | 2.1 (1.3–3.3) | <0.01 |
| All‐cause mortality | ||||
| No TR | Reference | Reference | ||
| Mild TR | 1.6 (1.3–2.1) | <0.01 | 1.3 (1.0–1.8) | 0.08 |
| Moderate TR | 2.4 (1.7–3.4) | <0.01 | 1.6 (1.0–2.5) | 0.05 |
| Severe TR | 4.3 (2.7–7.0) | <0.01 | 1.8 (1.0–3.2) | 0.01 |
| HF hospitalization | ||||
| No TR | Reference | Reference | ||
| Mild TR | 1.5 (1.1–2.0) | <0.01 | 1.1 (0.8–1.6) | 0.60 |
| Moderate TR | 2.9 (2.0–4.4) | <0.01 | 1.7 (1.0–2.8) | 0.04 |
| Severe TR | 8.9 (5.7–13.9) | <0.01 | 3.2 (1.8–5.6) | <0.01 |
| CV death | ||||
| No TR | Reference | Reference | ||
| Mild TR | 2.8 (1.8–4.5) | <0.01 | 1.8 (1.0–3.3) | 0.06 |
| Moderate TR | 4.9 (2.7–8.9) | <0.01 | 1.8 (0.8–4.1) | 0.05 |
| Severe TR | 11.8 (5.8–24.0) | <0.01 | 3.0 (1.2–7.4) | 0.02 |
| Non‐CV death | ||||
| No TR | Reference | Reference | ||
| Mild TR | 1.3 (1.0–1.8) | 0.04 | 1.2 (0.8–1.7) | 0.37 |
| Moderate TR | 1.8 (1.2–2.8) | 0.01 | 1.6 (0.9–2.7) | 0.12 |
| Severe TR | 2.4 (1.2–4.8) | 0.01 | 1.2 (0.5–2.8) | 0.74 |
AF, atrial fibrillation; CI, confidence interval; CV, cardiovascular death; EF, ejection fraction; HF, heart failure; HR, hazard ratio; RVSP, right ventricular systolic pressure; TR, tricuspid regurgitation.
Discussion
This study evaluating patients with stage B and C HFpEF demonstrated that the presence of TR is associated with old age, AF, and higher HF stage. Importantly, moderate/severe TR was significantly associated with mortality and HF requiring hospitalization independent to LVEF, AF, and other clinical risk factors.
Prevalence
The presence of TR is usually secondary to left‐sided heart disease and is a common finding on routine echocardiography, in any degree, in as high as 65% to 85% of cases. 1 In a community screening study, isolated significant TR, defined as that without significant comorbidities, structural left‐sided valve disease, pulmonary hypertension, or overt cardiac disease, was found in 8.1% of residents. 3 The frequency of TR as well as valvular pathology was evaluated in a study of 5223 adults who underwent echocardiography at three Veterans Affairs medical centres: moderate/severe TR was present in 15.7%. 11 As expected, patients with HF symptoms are more likely to have significant TR compared with those who are asymptomatic. This was evidenced in a study of 576 patients with congestive HF in whom moderate/severe TR was present in 19% (56% of patients with LVEF < 35%). 12 In another larger cohort that evaluated 13 026 patients with HFrEF, TR was detected in up to 88%, with 23% having moderate/severe TR. 2 Building on this, our results demonstrate that in asymptomatic patients at risk of HF (stage B) and those with stage C HFpEF, the prevalence of moderate/severe TR was 11.0%. In patients with chronic HF, the presence of TR correlated with LVEF and may explain the lower prevalence of significant TR in our study compared with studies that involved patients with HFrEF. 12 Interestingly, the association of TR with LV systolic function persists even in patients with preserved LVEF, as evidenced by the present study. This finding highlights the strong influence of LV systolic function on severity of TR, even before the development of overt systolic dysfunction. Future studies are warranted to evaluate the relation of subclinical systolic dysfunction, as measured by sensitive markers such as myocardial strain, with severity of TR. A prior study has further demonstrated that diastolic dysfunction plays a key role in the development of HF symptoms in patients with hypertensive heart disease and normal ejection fraction. 13
Another finding of the present study demonstrated that patients with stage C HFpEF had a higher incidence of moderate/severe TR than stage B HF (16% vs. 8%), which can be partly explained by the clustering of risk factors such as renal dysfunction, aging, and AF. Significant TR can occur in up to 34% of patients with acute symptomatic HF, 14 and the degree of TR has further been correlated with specific symptoms such as dyspnoea, ankle oedema, and jugular vein dilatation in patients with HFrEF. 2 Consequently, clinicians should consider the presence of significant TR once patients have developed HF symptoms since the prevalence of moderate/severe TR increases.
Age is another key factor that determines the prevalence of TR. In a study that evaluated an elderly population aged >65 years with no known history of valvular heart disease, moderate/severe TR was present in 2.7% and was more prevalent than left‐sided valve lesion. 15 In the Framingham Heart Study, the prevalence of moderate and severe TR was up to 1.5% and 5.6% in men and women aged >70 years, respectively. 16 Similarly, our previous study 17 and others 2 , 3 , 18 , 19 have consistently demonstrated that age is independently associated with severity of TR. The estimated prevalence of moderate/severe TR is 1.6 million in the USA and is perceived to steadily increase as a result of our rapidly aging global population. 20 In addition to age, AF has been shown to be closely associated with TR. 19 In particular, TR can occur in up to 25% of patients with >10 year history of AF. 21 The mechanism is likely to be multifactorial and include factors such as bilateral atrial enlargement, 22 tricuspid annulus dilatation, 23 tricuspid valve deformation, and right heart remodelling. 24 Our finding concurs with a previous study in patients with LVEF > 50% that demonstrated a similarly high prevalence of AF (74%) in patients with isolated TR, 25 while only approximately half the patients with HFrEF had concomitant AF (48%). 2 The discrepancy can be partly explained by patients with HFrEF being more often accompanied by an enlarged right ventricular chamber, which results in tricuspid annulus dilatation. The dilated right heart may directly cause TR that exceeds the role of AF in the progress of TR in HFrEF. In contrast, HFpEF is less accompanied by right heart dilatation compared with HFrEF, and thus, the role of AF contributing to the development of TR may become more significant. Future studies would nonetheless be required to evaluate the different impacts of AF contributing to the development of TR between patients with HFpEF and HFrEF. Whether aggressive rhythm control in patients with AF such as by catheter ablation may reduce the progression of TR would also require future elucidation. 26
Prognosis
The tricuspid valve has often been considered a forgotten valve, in part due to its secondary nature in left‐sided heart disease and a protracted latent asymptomatic period. Studies have consistently documented that TR is not a benign entity and the presence of moderate/severe TR is associated with adverse outcome. In a large retrospective analysis that included a population with heterogeneous characteristics, moderate/severe TR was associated with all‐cause mortality. 11 In another observational study that included 576 patients with systolic HF, moderate/severe TR was associated with all‐cause mortality in mild to moderate congestive HF but not in those with advanced disease. 12 In patients with acute HF, moderate/severe TR was associated with HF hospitalization or mortality, particularly in those with concomitant pulmonary hypertension. 14 Recently, a large cohort that involved stage B and C HFrEF patients further confirmed that higher TR severity is associated with all‐cause mortality, independent of pulmonary hypertension and other clinical risk factors. 2 A prior study has further demonstrated that TR during exercise was significantly associated with mortality and HF hospitalization in patients with history of HFpEF and warranted future evaluation in patients with HFpEF. 27 Extending these findings, our present study demonstrated that moderate/severe TR is independently associated with mortality and HF hospitalization in asymptomatic patients at risk of HF (stage B) and stage C HFpEF. In particular, patients with moderate TR had a 1.5‐fold increased risk of mortality and HF requiring hospitalization that increased to 2.1‐fold in those with severe TR. One of the merits of the present study is the detailed follow‐up where the exact cause of mortality was acknowledged unlike other studies. 2 , 3 , 4 Our results highlight that moderate/severe TR is associated with cardiovascular mortality but is neutral for non‐cardiovascular mortality. Also confirming that the mechanism of TR‐related mortality is driven by cardiovascular complications, the present study further recommends that clinicians should focus on prevention of cardiovascular outcome when significant TR is detected in those at risk of HF or with HFpEF. Due to the small population of patients with significant TR among those at risk of HF, future studies with a larger study population should be performed to evaluate the prognostic role of moderate/severe TR in this group of patients.
Clinical implications
Our current findings demonstrate that in asymptomatic patients at risk of HF (stage B) and those with stage C HFpEF patients, TR is common and is associated with adverse outcome. This observation emphasizes that preemptive detection of TR evaluated by Doppler echocardiography is warranted. Further, HFpEF and AF are inextricably linked and increasingly encountered due to our aging population. Intriguingly, it has been shown that the development of AF may impose a greater adverse outcome in patients with HFpEF than in those with HFrEF. 28 This notion is further confirmed by a recent substudy of the TOPCAT trial that demonstrated that in patients with HFpEF, concomitant AF was associated with a more than twofold increased risk of cardiovascular events compared with non‐AF patients. 29 Nonetheless, the underlying reason why AF resulted in a worse outcome in patients with HFpEF is uncertain. It has been firmly established that AF contributes to the development of TR, and our present study further confirms that significant TR contributes to adverse outcome in patients at risk of HF and those with HFpEF. The current finding may explain why AF causes a higher prevalence of significant TR that contributes to an elevated risk for adverse events in patients with HFpEF. In the same context, although there is no definitive treatment to improve survival in patients with HFpEF, preventing the development of TR may be a potential therapeutic option to reduce adverse outcome. Studies such as those that use a novel transcatheter tricuspid valve procedure 30 to reduce significant TR should be performed to investigate the potential survival benefit in patients with HFpEF and significant TR.
Limitations
Patients were retrospectively identified from our echocardiography database, but all clinical data were prospectively measured at clinical diagnosis. Nonetheless, electronic formats were not available for all echocardiography examinations, meaning that operator variability for severity of TR could not be assessed. Although reporting of TR severity was based on the same criteria, echocardiography examinations were performed by different operators and potential interobserver and intraobserver variability could exist. Further, the grading of TR was semiquantitative. Assessment using a quantitative method such as regurgitant volume and effective regurgitant orifice may provide a more consistent evaluation. 31 TR is highly load dependent and it is difficult to accurately differentiate patients who are volume overloaded from those with persistent TR. Future prospective studies with comprehensive assessment of mechanism and quantitative evaluation of TR severity are warranted. Prior studies have indicated that pulmonary artery pressure and right ventricular function could influence TR in patients with HFpEF and increased the risk of cardiovascular events. 32 , 33 , 34 However, the present study did not systematically evaluate patients' right ventricular function systemically and would require validation by future prospective studies. Echocardiographic parameters such as left atrial volume index, global longitudinal strain, atrial strain, and serum NT‐proBNP were not evaluated in the present study and warranted evaluation by future prospective study. In the present study, prospective echocardiography to assess longitudinal changes of LV remodelling or function was not systemically performed, which requires elucidation by future studies.
Conclusions
The presence of significant TR is common in patients with stage B HF and stage C HFpEF. Comorbidities such as hypertension, diabetes mellitus, and atrial arrhythmia may drive the development for patients at risk of HF (stage A or B) to HFpEF. Clinicians should provide treatment to patients at risk of HF according to the guidelines in order to prevent the progression of symptomatic HF. The prognostic implication of TR suggests that the presence of TR may play an integral part and represent a potential therapeutic target for patients at risk of HF and those with HFpEF.
Conflict of interest
None declared.
Funding
The study was funded by the Shenzhen Key Medical Discipline Construction Fund and the Sanming Project of Cardiology, the university of Hong Kong Shenzhen hospital, Sanming grant from the Ministry of Health, Shenzhen, China.
Acknowledgements
We thank the medical and nursing staff of the Division of Cardiology, The University of HongKong‐Shenzhen hospital and Queen May Hospital.
Ren, Q. , Li, X. , Fang, J. , Chen, Y. , Wu, M.‐Z. , Yu, Y.‐J. , Liao, S. , Tse, H.‐F. , and Yiu, K.‐H. (2020) The prevalence, predictors, and prognosis of tricuspid regurgitation in stage B and C heart failure with preserved ejection fraction. ESC Heart Failure, 7: 4051–4060. 10.1002/ehf2.13014.
References
- 1. Antunes MJ, Rodríguez‐Palomares J, Prendergast B, de Bonis M, Rosenhek R, al‐Attar N, Barili F, Casselman F, Folliguet T, Iung B, Lancellotti P, Muneretto C, Obadia JF, Pierard L, Suwalski P, Zamorano P. on behalf of the ESC Working Groups of Cardiovascular Surgery and Valvular Heart DiseaseManagement of tricuspid valve regurgitation: position statement of the European Society of Cardiology Working Groups of Cardiovascular Surgery and Valvular Heart Disease. Eur J Cardiothorac Surg 2017; 52: 1022–1030. [DOI] [PubMed] [Google Scholar]
- 2. Benfari G, Antoine C, Miller WL, Thapa P, Topilsky Y, Rossi A, Michelena HI, Pislaru S, Enriquez‐Sarano M. Excess mortality associated with functional tricuspid regurgitation complicating heart failure with reduced ejection fraction. Circulation 2019; 140: 196–206. [DOI] [PubMed] [Google Scholar]
- 3. Topilsky Y, Maltais S, Medina Inojosa J, Oguz D, Michelena H, Maalouf J, Mahoney DW, Enriquez‐Sarano M. Burden of tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc Imaging 2019; 12: 433–442. [DOI] [PubMed] [Google Scholar]
- 4. Chorin E, Rozenbaum Z, Topilsky Y, Konigstein M, Ziv‐Baran T, Richert E, Keren G, Banai S. Tricuspid regurgitation and long‐term clinical outcomes. Eur Heart J Cardiovasc Imaging 2020; 21: 157–165. [DOI] [PubMed] [Google Scholar]
- 5. Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, Noble RJ, Packer M, Silver MA, Stevenson LW, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Jacobs AK, Hiratzka LF, Russell RO, Smith SC Jr, American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure)., International Society for Heart and Lung Transplantation., Heart Failure Society of America. ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure) . Developed in Collaboration With the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation 2001; 104: 2996–3007. [DOI] [PubMed] [Google Scholar]
- 6. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey de Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride P, McMurray J, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation . American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 7. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 8. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977; 55: 613–618. [DOI] [PubMed] [Google Scholar]
- 9. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD, Houston, Texas; Oslo, Norway; Phoenix, Arizona; Nashville, Tennessee; Hamilton, Ontario, Canada; Uppsala, Sweden; Ghent and Liège, Belgium; Cleveland, Ohio; Novara, Italy; Rochester, Minnesota; Bucharest, Romania; and St. Louis, Missouri . Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 1321–1360. [DOI] [PubMed] [Google Scholar]
- 10. Zoghbi WA, Adams D, Bonow RO, Enriquez‐Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017; 30: 303–371. [DOI] [PubMed] [Google Scholar]
- 11. Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long‐term survival. J Am Coll Cardiol 2004; 43: 405–409. [DOI] [PubMed] [Google Scholar]
- 12. Neuhold S, Huelsmann M, Pernicka E, Graf A, Bonderman D, Adlbrecht C, Binder T, Maurer G, Pacher R, Mascherbauer J. Impact of tricuspid regurgitation on survival in patients with chronic heart failure: unexpected findings of a long‐term observational study. Eur Heart J 2013; 34: 844–852. [DOI] [PubMed] [Google Scholar]
- 13. Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Ommen SR, Redfield MM, Bursi F, Kass DA. Cardiac structure and ventricular‐vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation 2007; 115: 1982–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mutlak D, Lessick J, Khalil S, Yalonetsky S, Agmon Y, Aronson D. Tricuspid regurgitation in acute heart failure: is there any incremental risk? Eur Heart J Cardiovasc Imaging 2018; 19: 993–1001. [DOI] [PubMed] [Google Scholar]
- 15. d'Arcy JL, Coffey S, Loudon MA, Kennedy A, Pearson‐Stuttard J, Birks J, Frangou E, Farmer AJ, Mant D, Wilson J, Myerson SG, Prendergast BD. Large‐scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J 2016; 37: 3515–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol 1999; 83: 897–902. [DOI] [PubMed] [Google Scholar]
- 17. Yiu KH, Chen Y, Liu JH, Lin Q, Liu M, Wu M, Wang R, Zhen Z, Zou Y, Lam YM, Ng MY. Burden and contributing factors associated with tricuspid regurgitation. a hospital‐based study Hosp Pract (1995) 2017; 55: 504–512. [DOI] [PubMed] [Google Scholar]
- 18. Itzhaki Ben Zadok O, Sagie A, Vaturi M, Shapira Y, Schwartzenberg S, Kuznitz I, Shochat T, Bental T, Yedidya I, Aravot D, Kornowski R, Sharony R. Long‐term outcomes after mitral valve replacement and tricuspid annuloplasty in rheumatic patients. Ann Thorac Surg 2019; 107: 539–545. [DOI] [PubMed] [Google Scholar]
- 19. Zhao SX, Soltanzad N, Swaminathan A, Ogden WD, Schiller NB. Frequency and associated clinical features of functional tricuspid regurgitation in patients with chronic atrial fibrillation. Am J Cardiol 2017; 119: 1371–1377. [DOI] [PubMed] [Google Scholar]
- 20. Stuge O, Liddicoat J. Emerging opportunities for cardiac surgeons within structural heart disease. J Thorac Cardiovasc Surg 2006; 132: 1258–1261. [DOI] [PubMed] [Google Scholar]
- 21. Abe Y, Akamatsu K, Ito K, Matsumura Y, Shimeno K, Naruko T, Takahashi Y, Shibata T, Yoshiyama M. Prevalence and prognostic significance of functional mitral and tricuspid regurgitation despite preserved left ventricular ejection fraction in atrial fibrillation patients. Circ J 2018; 82: 1451–1458. [DOI] [PubMed] [Google Scholar]
- 22. Najib MQ, Vinales KL, Vittala SS, Challa S, Lee HR, Chaliki HP. Predictors for the development of severe tricuspid regurgitation with anatomically normal valve in patients with atrial fibrillation. Echocardiography 2012; 29: 140–146. [DOI] [PubMed] [Google Scholar]
- 23. Kim HK, Kim YJ, Park JS, Kim KH, Kim KB, Ahn H, Sohn DW, Oh BH, Park YB, Choi YS. Determinants of the severity of functional tricuspid regurgitation. Am J Cardiol 2006; 98: 236–242. [DOI] [PubMed] [Google Scholar]
- 24. Utsunomiya H, Itabashi Y, Mihara H, Berdejo J, Kobayashi S, Siegel RJ, Shiota T. Functional tricuspid regurgitation caused by chronic atrial fibrillation: a real‐time 3‐dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging 2017; 10: e004897. [DOI] [PubMed] [Google Scholar]
- 25. Fender EA, Petrescu I, Ionescu F, Zack CJ, Pislaru SV, Nkomo VT, Cochuyt JJ, Hodge DO, Nishimura RA. Prognostic importance and predictors of survival in isolated tricuspid regurgitation: a growing problem. Mayo Clin Proc 2019; 94: 2032–2039. [DOI] [PubMed] [Google Scholar]
- 26. Black‐Maier E, Ren X, Steinberg BA, Green CL, Barnett AS, Rosa NS, al‐Khatib SM, Atwater BD, Daubert JP, Frazier‐Mills C, Grant AO, Hegland DD, Jackson KP, Jackson LR, Koontz JI, Lewis RK, Sun AY, Thomas KL, Bahnson TD, Piccini JP. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm 2018; 15: 651–657. [DOI] [PubMed] [Google Scholar]
- 27. Donal E, Lund LH, Oger E, Reynaud A, Schnell F, Persson H, Drouet E, Linde C, Daubert C. Value of exercise echocardiography in heart failure with preserved ejection fraction: a substudy from the KaRen study. Eur Heart J Cardiovasc Imaging 2016; 17: 106–113. [DOI] [PubMed] [Google Scholar]
- 28. Linssen GC, Rienstra M, Jaarsma T, Voors AA, van Gelder IC, Hillege HL, van Veldhuisen DJ. Clinical and prognostic effects of atrial fibrillation in heart failure patients with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail 2011; 13: 1111–1120. [DOI] [PubMed] [Google Scholar]
- 29. Cikes M, Claggett B, Shah AM, Desai AS, Lewis EF, Shah SJ, Anand IS, O'Meara E, Rouleau JL, Sweitzer NK, Fang JC, Saksena S, Pitt B, Pfeffer MA, Solomon SD. Atrial fibrillation in heart failure with preserved ejection fraction: the TOPCAT trial. JACC Heart Fail 2018; 6: 689–697. [DOI] [PubMed] [Google Scholar]
- 30. Asmarats L, Puri R, Latib A, Navia JL, Rodés‐Cabau J. Transcatheter tricuspid valve interventions: landscape, challenges, and future directions. J Am Coll Cardiol 2018; 71: 2935–2956. [DOI] [PubMed] [Google Scholar]
- 31. Tribouilloy CM, Enriquez‐Sarano M, Capps MA, Bailey KR, Tajik AJ. Contrasting effect of similar effective regurgitant orifice area in mitral and tricuspid regurgitation: a quantitative Doppler echocardiographic study. J Am Soc Echocardiogr; 15: 958–965. [DOI] [PubMed] [Google Scholar]
- 32. Gorter TM, Hoendermis ES, van Veldhuisen DJ, Voors AA, Lam CSP, Geelhoed B, Willems TP, van Melle JP. Right ventricular dysfunction in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. Eur J Heart Fail 2016; 18: 1472–1487. [DOI] [PubMed] [Google Scholar]
- 33. Aschauer S, Kammerlander AA, Zotter‐Tufaro C, Ristl R, Pfaffenberger S, Bachmann A, Duca F, Marzluf BA, Bonderman D, Mascherbauer J. The right heart in heart failure with preserved ejection fraction: insights from cardiac magnetic resonance imaging and invasive haemodynamics. Eur J Heart Fail 2016; 18: 71–80. [DOI] [PubMed] [Google Scholar]
- 34. Medvedofsky D, Aronson D, Gomberg‐Maitland M, Thomeas V, Rich S, Spencer K, Mor‐Avi V, Addetia K, Lang RM, Shiran A. Tricuspid regurgitation progression and regression in pulmonary arterial hypertension: implications for right ventricular and tricuspid valve apparatus geometry and patients outcome. Eur Heart J Cardiovasc Imaging 2017; 18: 86–94. [DOI] [PubMed] [Google Scholar]


