Abstract
Aims
Mild or moderate aortic regurgitation (AR) has only little effect on cardiovascular outcome in people with normal left ventricular ejection fraction (EF); therefore, it is not perceived as a major clinical problem. This study investigates whether mild or moderate AR is associated with increased short‐term mortality in patients hospitalized for treatment of acute heart failure (AHF) and whether mild or moderate AR impacts differently on short‐term mortality in AHF patients with reduced EF (AHFrEF), mid‐range EF (AHFmrEF), or preserved EF (AHFpEF).
Methods and results
This mono‐centric study included 505 consecutive adult patients hospitalized for de novo or worsening chronic HF not related to acute ischaemia or severe valvular pathology in the echocardiogram at index hospitalization. Cox regression analysis studied the impact of AR on all‐cause mortality (ACM) over the 150 days' study period. Mild or moderate AR was associated with increased ACM (HR 1.75 [95% CI: 1.1–2.7]; P = 0.009). The prevalence of mild or moderate AR in the study population was 42% and not significantly different between AHFpEF (n = 227), AHFmrEF (n = 86), and AHFrEF (n = 192) study participants (37.9% vs. 50.0% vs. 42.7%; P = 0.144). In AHFpEF patients, the age‐adjusted hazard for ACM was increased in patients with AR compared with patients without AR (HR 2.17 [95% CI: 1.1–4.2]; P = 0.002). The age‐adjusted hazard for ACM was increased by a trend in AHFmrEF with AR (HR 7.11, [95% CI: 0.9–57.8]; P = 0.067) and not different between the AHFrEF groups (HR 0.95 [95% CI: 0.5–1.8]; P = 0.875).
Conclusions
Mild or moderate AR increased ACM only in AHFpEF patients, highlighting a distinct clinical relevance.
Keywords: Acute heart failure, Aortic regurgitation, All‐cause mortality
Introduction
Epidemiological studies show that the low prevalence of aortic valve insufficiency at younger age increases to a 13–29% level in the elderly. 1 , 2 , 3 A similar increase has been shown for the prevalence of symptomatic heart failure (HF) in the general population, which is low at younger age but affects >40% of people at the age of 70 years and above. 4 , 5 This suggests that coincidence of aortic regurgitation (AR) and HF should occur in particular in the aged.
For patients with symptomatic and asymptomatic severe AR with or without reduced left ventricular ejection fraction (LVEF), the therapeutic strategy is set out. 6 However, the clinical importance of mild or moderate AR remains unclear in particular in HF, although about 20% of hospitalized patients with non‐severe AR have also HF as shown in a more recent epidemiological hospital‐based survey. 7
We therefore studied the impact of mild or moderate AR on all‐cause mortality (ACM) in patients with hospitalization for treatment of acute HF (AHF). This study population was chosen because acute decompensation represents a fair argument for the presence of HF, which can be difficult to diagnose in particular when LVEF is in the mid‐range or preserved. 8 , 9 In fact, this approach has already been successfully adopted in an earlier study investigating characteristics of the HF patients with preserved LVEF. 10 Another argument in favour of application of the research question to these patients is their high burden of adverse cardiovascular outcome. In theory, the latter should permit detection of interaction even in smaller‐size groups with short‐term follow‐up.
Methods
Study population
This study combines two local prospective registries (years 2005–2009, n = 402; years 2015–2018, n = 221). 11 , 12 Both registries had prospectively recruited consecutive adult AHF patients with presentation to the emergency wards followed by hospitalization at the Lausanne University Hospital. Screening excluded patients with exacerbation of obstructive pulmonary disease, acute pulmonary embolism or stress‐related cardiomyopathy, acute myocardial ischaemia, or acute mechanical cause from acute coronary syndrome; patients after recent cardiac surgery; or patients with echocardiography performed short term before hospitalization. 9 Inclusion criteria were (i) age ≥ 18 years; (ii) hospitalization for AHF treatment at the CHUV; (iii) transthoracic echocardiographic exam during index hospitalization; and (iv) written consent. Additional exclusion criteria were (i) pregnancy; (ii) comorbidity with survival time considered to be <1 year on the basis of the patient's medical history including primary pulmonary artery hypertension; (iii) severe aortic, mitral, and tricuspid regurgitation or stenosis on index echocardiography; (iv) AHF caused by acute metabolic, toxic or infectious disorders; (v) AHF with accompanying cerebrovascular insult; and (ii) prior aortic valve replacement. The study protocol was approved by the local ethics committee (CER Vaud 2019–01158).
Acquisition of anthropometric, biological, and clinical data
Anthropometric, biological, clinical admission data and medical history were collected from the individual patients' electronic health report at the Lausanne University Hospital (T. A., G. T., and N. S.). Data accuracy was tested by revisiting 30% of randomly selected patients' data revealing 99.7% correctness (T. A. and N. S.). Standard transthoracic echocardiographic images and parameters were acquired by board‐certified cardiologists. Echocardiographic analysis was performed offline using EchoPac software, version 4.0.4 (GE Medical Systems) (G. T., N. B., J. R., and P. M.). LVEF was assessed using the Simpson method; the severity of valvular regurgitations was graded using a multiparametric assessment as recommended by the European Association of Cardiovascular Imaging. 13 , 14
Types of heart failure
Participants were classified to suffer from chronic HF (CHF) when presenting clinical signs of CHF before index admission as documented in the patients' history. Patients were classified to have AHF with preserved EF (AHFpEF) when LVEF was ≥50%, AHF with mid‐range EF (AHFmrEF) when LVEF was ≥40–49%, and AHF with reduced EF (AHFrEF) when LVEF was <40% in analogy to the European Society of Cardiology guidelines for the diagnosis and treatment of AHF and CHF. 8
Study outcome: all‐cause mortality over the study period of 150 days after hospital admission for treatment of decompensated heart failure
ACM was seized over a 150 days' period starting on the day of hospital admission; 99.8% of study patients had a length of hospital stay < 30 days.
Statistical analysis
Analyses were performed with Stata® 13.1 (StataCorp, College Station, TX, USA). As normal distribution could not be ensured for most of the variables, all continuous variables are shown as medians with inter‐quartile ranges. Categorical variables were shown as per cent (absolute number).
Associations with AR were tested using the Mann–Whitney U‐test for continuous variables and the χ 2 test for categorical variables. The Kruskal–Wallis test with the Mann–Whitney U‐test and the χ 2 as a post‐hoc test were performed to compare interval variables with categorical variables between the three different types of HF.
Cox regression analysis was used to identify parameters that were associated with ACM over the study period. The hazard ratio (HR) accompanied by its 95% CI was used as a measure of strength for Cox regression analysis results.
Variables that showed at least very weak evidence (P < 0.2) for an association with type of HF (Tables S1 and S2), mild or moderate AR, and ACM were included in the multivariable Cox regression analysis to study the impact of mild or moderate AR on ACM according to the type of HF. Thus, an interaction term between type of HF and AR was forced in the multivariable model. To get to the final model, covariables with a non‐significant P‐value (P > 0.05) were removed in a stepwise backward approach. The test of proportional hazards assumption was used for the final model to test the prerequisite to use a Cox regression model.
Results
Screening and inclusion into the study population
Screening excluded myocardial ischaemia (n = 129) and echocardiography at short term before index hospitalization (n = 118). Thirty patients were excluded after initial inclusion because of severe valvular pathology at index echocardiography; 34 patients were excluded because of severe non‐cardiac pathology suspected to impact on short‐term mortality. Five hundred five study participants were retained for the final analysis (cohort 2005–2009 and 2015–2018, n = 311 and n = 194, respectively).
Demographic, clinical, medical, and biological characteristics of acute heart failure patients without or with mild or moderate aortic regurgitation
The prevalence of mild or moderate AR was 42% (211/505) in this study population; 43% (216/505) were female; 52% (261/505) had a history of previous myocardial infarction.
AR patients were older (80.5 [74.2–85.7] vs. 77.8 [68.7–84.3] years; P = 0.002), the body mass index (BMI) was lower (25.8 [23.5–31.1] vs. 26.9 [24–31.6]; P = 0.014), and diabetes and smoking were less prevalent (62 [29.4%] vs. 133 [45.2%], P < 0.001; 103 [48.8%] vs. 173 [59%], P = 0.023). All other cardiovascular risk factors were not significantly different between groups (Table 1). Patients with AR were less frequently implanted with an internal cardiac defibrillator (8 [3.8%] vs. 25 [8.5%], P = 0.035), treated with statins (77 [36.7%] vs. 134 [45.7%], P = 0.042), oral antidiabetics (26 [12.4%] vs. 59 [20.1%], P = 0.023), or insulin (27 [12.9%] vs. 64 [21.8%], P = 0.01) (Table 1). The serum glucose level was lower at admission in patients without AR (6.8 [5.9–8.4] vs. 7.4 [6.0–10.1] mmol/L, P = 0.041). Electrolytes, renal function, and haematological parameters were not significantly different between patients with or without AR (Table 2).
Table 1.
Demographic and clinical characteristics of acute heart failure patients without or with mild or moderate aortic regurgitation
| n | All (n = 505) | Patients with AR (n = 211) | Patients without AR (n = 294) | P‐value | ||||
|---|---|---|---|---|---|---|---|---|
| Demographic and clinical parameters | ||||||||
| Age (years) | 505 | 79.3 | [71–85] | 80.5 | [74.2–85.7] | 77.8 | [68.7–84.3] | 0.002 |
| Female gender (%) | 505 | 216 | (42.8) | 96 | (45.5) | 120 | (40.8) | 0.29 |
| BMI (kg/m2) | 504 | 26.4 | [23.5–31.1] | 25.8 | [22.8–30.1] | 26.9 | [24–31.6] | 0.014 |
| SBP (mmHg) admission | 505 | 136 | [120–156] | 137 | [120–160] | 136 | [120–156] | 0.711 |
| SBP (mmHg) discharge | 500 | 124 | [109–138] | 126 | [109–135] | 124 | [109–140] | 0.573 |
| DBP (mmHg) admission | 505 | 80 | [68–90] | 80 | [68–92] | 79 | [68–90] | 0.281 |
| DBP (mmHg) discharge | 499 | 66 | [58–75] | 66 | [57–74] | 67 | [59–76] | 0.101 |
| HR (b.p.m.) admission | 505 | 89 | [75–107] | 88 | [71–107] | 90 | [75–107] | 0.751 |
| HR (b.p.m.) discharge | 499 | 75 | [65–85] | 74 | [65–84] | 75 | [66–85] | 0.21 |
| Co‐morbidity | ||||||||
| COPD (%) | 505 | 84 | (16.6) | 28 | (13.3) | 56 | (19.0) | 0.086 |
| Smoking status (%) | 504 | 276 | (54.8) | 103 | (48.8) | 173 | (59.0) | 0.023 |
| Hx of Afib/flutter (%) | 505 | 284 | (56.2) | 129 | (61.1) | 155 | (52.7) | 0.06 |
| Hx of MI (%) | 505 | 261 | (51.7) | 105 | (49.8) | 156 | (53.1) | 0.464 |
| Dyslipidaemia (%) | 502 | 293 | (58.4) | 118 | (56.5) | 175 | (59.7) | 0.464 |
| Hypertension (%) | 505 | 422 | (83.6) | 177 | (83.9) | 245 | (83.3) | 0.869 |
| Diabetes mellitus (%) | 505 | 195 | (38.6) | 62 | (29.4) | 133 | (45.2) | <0.001 |
| QRS duration (ms) | 505 | 98.0 | [80–120] | 100.0 | [80–120] | 92.0 | [80–120] | 0.304 |
| Medical therapy | ||||||||
| ICD (%) | 505 | 33 | (6.5) | 8 | (3.8) | 25 | (8.5) | 0.035 |
| Pacemaker (%) | 505 | 57 | (11.3) | 25 | (11.8) | 32 | (10.9) | 0.736 |
| Statin (%) | 503 | 211 | (41.9) | 77 | (36.7) | 134 | (45.7) | 0.042 |
| Beta‐blocker (%) | 505 | 247 | (48.9) | 100 | (47.4) | 147 | (50.0) | 0.563 |
| ACEI (%) | 505 | 183 | (36.2) | 80 | (37.9) | 103 | (35.0) | 0.507 |
| ARB (%) | 505 | 145 | (28.7) | 56 | (26.5) | 89 | (30.3) | 0.361 |
| ARNI (%) | 505 | 1 | (0.2) | 0 | (0.0) | 1 | (0.3) | 0.396 |
| MRA (%) | 505 | 78 | (15.4) | 36 | (17.1) | 42 | (14.3) | 0.395 |
| Loop diuretic (%) | 505 | 292 | (57.8) | 123 | (58.3) | 169 | (57.5) | 0.856 |
| Antidiabetic drug (%) | 504 | 85 | (16.9) | 26 | (12.4) | 59 | (20.1) | 0.023 |
| Insulin (%) | 504 | 91 | (18.1) | 27 | (12.9) | 64 | (21.8) | 0.01 |
ACEI, angiotensin‐converting enzyme inhibitor; AR, aortic regurgitation of mild or moderate severity; ARB, angiotensin receptor blocker; ARNI, angiotensin II receptor blocker neprilysin inhibitor; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; Hx of Afib/flutter, history of persistent/paroxysmal/permanent atrial fibrillation or flutter; Hx of HF, history of heart failure; Hx of MI, history of myocardial infarction; ICD, implantable cardioverter defibrillator; LADi, left atrium diameter index; LVEDDi, left ventricular end‐diastolic diameter index; LVEF, left ventricular ejection fraction; LVMi, left ventricular mass index; MRA, mineralocorticoid receptor antagonist; SBP, systolic blood pressure.
Table 2.
Biological characteristics of acute heart failure patients without or with mild or moderate aortic regurgitation
| n | All (n = 505) | Patients with AR (n = 211) | Patients without AR (n = 294) | P‐value | ||||
|---|---|---|---|---|---|---|---|---|
| Haemoglobin (g/L) | 505 | 123 | [110–140] | 123.0 | [110–139] | 123.0 | [109–140] | 0.923 |
| Haematocrit (%) | 503 | 37 | [34–42] | 38 | [34–42] | 37 | [33–42] | 0.845 |
| RDW (%) | 505 | 14.9 | [13.9–16.2] | 14.9 | [13.9–16.3] | 15.0 | [13.9–16.2] | 0.958 |
| Leucocytes (G/L) | 505 | 8.5 | [6.8–10.7] | 8.7 | [7.0–11.1] | 8.4 | [6.7–10.3] | 0.344 |
| Glucose (mmol/L) | 500 | 7.0 | [6.0–9.2] | 7.4 | [6.0–10.1] | 6.8 | [5.9–8.4] | 0.041 |
| Creatinine (μmol/L) | 504 | 108 | [84–147] | 106 | [82–145] | 110 | [87–150] | 0.130 |
| Sodium (mmol/L) | 504 | 139 | [136–142] | 139 | [136–142] | 140 | [137–142] | 0.715 |
| Potassium (mmol/L) | 504 | 4.3 | [3.9–4.7] | 4.3 | [3.9–4.8] | 4.3 | [3.9–4.7] | 0.301 |
| Cholesterol (mmol/L) | 391 | 3.9 | [3.2–4.6] | 3.8 | [3.1–4.6] | 4.0 | [3.2–4.7] | 0.123 |
AR, aortic regurgitation; RDW, red cell distribution width.
Echocardiographic characteristics of acute heart failure patients without or with mild or moderate aortic regurgitation
Patients with AR had a larger indexed LV end‐diastolic diameter (LVEDD) (30 [27–34] vs. 28.2 [25–32] mm/m2, P = 0.004); LV mass index was higher (120 [93–144] vs. 109 [86–132] g/m2, P = 0.004). The prevalence of mild or moderate mitral regurgitation (MR) (91.4% [n = 192] vs. 76.5% [n = 225], P < 0.001) and tricuspid regurgitation (85.2% [n = 178] vs. 73.8% [n = 217], P = 0.002) was higher in patients with AR. Mild or moderate aortic stenosis with or without AR was not significantly different between both groups (14.7% [n = 31] vs. 15.0% [n = 44], P = 0.932) (Table 3).
Table 3.
Echocardiographic characteristics of acute heart failure in patients with or without moderate aortic regurgitation
| n | All (n = 505) | Patients with AR (n = 211) | Patients without AR (n = 294) | P‐value | ||||
|---|---|---|---|---|---|---|---|---|
| LVEDDi (mm/m2) | 425 | 29.0 | [26–33] | 30.0 | [27–34] | 28.2 | [25‐32] | 0.004 |
| LVMi (g/m2) | 405 | 114 | [90–136] | 120 | [93–144] | 109 | [86–132] | 0.004 |
| LADi (mm/m2) | 414 | 25.0 | [22‐28] | 25.0 | [23‐28] | 25.0 | [22‐28] | 0.369 |
| LVEF (%) | 505 | 45.0 | [30–60] | 41.0 | [30–60] | 46.0 | [30–60] | 0.502 |
| Mitral regurgitation (%) | 504 | 417 | (82.7) | 192 | (91.4) | 225 | (76.5) | <0.001 |
| Mitral stenosis (%) | 505 | 10 | (2.0) | 4 | (1.9) | 6 | (2.0) | 0.908 |
| Aortic regurgitation (%) | 505 | 211 | (41.8) | 211 | (100.0) | 0 | (0.0) | <0.001 |
| Aortic stenosis (%) | 505 | 75 | (14.9) | 31 | (14.7) | 44 | (15.0) | 0.932 |
| Tricuspid regurgitation (%) | 503 | 395 | (78.5) | 178 | (85.2) | 217 | (73.8) | 0.002 |
LADi, indexed left atrial diameter; LVEDDi, left ventricular end‐diastolic diameter indexed to body surface; LVEF, left ventricular ejection fraction; LVMi, left ventricular mass index.
Parameters associated with all‐cause mortality in univariable Cox regression analysis
Parameters that were associated ACM over the study period (P < 0.05) were dyslipidaemia (HR 0.44 [0.29–0.67], P < 0.001), BMI per kg/m2 (HR 0.92 [0.89–0.96], P < 0.001), systolic blood pressure at admission per mmHg (HR 0.99 [0.98–0.99], P = 0.004), age per year (HR 1.04 [1.01–1.06], P = 0.002), mild or moderate AR (HR 1.75 [1.15–2.66], P = 0.009), HFmrEF (HR 0.38 [0.18–0.82], P = 0.013 compared with HFrEF), pacemaker (HR 1.26 [1.08–3.2], P = 0.025), red cell distribution width per % (HR 1.08 [1.01–1.15], P = 0.029), mineralocorticoid receptor antagonist treatment (HR 1.71 [1.04–2.81], P = 0.035), LVMi per g/m2 (HR 1.01 [1.00–1.01], P = 0.037), statin treatment (HR 0.62 [0.40–0.98], P = 0.039), and LVEDDi per mm/m2 (HR 1.04 [1. 00–1.08], P = 0.040) (Table 4).
Table 4.
Univariable analysis showing associations of parameters with 150 days' all‐cause mortality in all acute heart failure patients
| Hazard ratio [95% CI] | P‐value | ||
|---|---|---|---|
| Type of heart failure | |||
| AHFrEF | 1.00 | Baseline | |
| AHFmrEF | 0.38 | [0.18–0.82] | 0.013 |
| AHFpEF | 0.70 | [0.45–1.08] | 0.109 |
| Demographic and clinical parameters | |||
| Age, per year | 1.04 | [1.01–1.06] | 0.002 |
| Male gender | 0.99 | [0.65–1.5] | 0.950 |
| BMI, per kg/m2 | 0.92 | [0.89–0.96] | <0.001 |
| SBP admission, per mmHg | 0.99 | [0.98–0.99] | <0.001 |
| SBP discharge, per mmHg | 0.98 | [0.97–1.00] | 0.004 |
| DBP admission, per mmHg | 0.99 | [0.98–1.00] | 0.053 |
| DBP discharge, per mmHg | 0.99 | [0.98–1.01] | 0.483 |
| HR admission, per b.p.m. | 1.00 | [0.99–1.01] | 0.982 |
| HR discharge, per mmHg | 1.00 | [0.99–1.02] | 0.685 |
| Co‐morbidity | |||
| COPD | 0.61 | [0.32–1.18] | 0.143 |
| Smoking status | 0.91 | [0.60–1.38] | 0.646 |
| Hx of Afib/flutter | 1.26 | [0.82–1.93] | 0.300 |
| Hx of MI | 0.91 | [0.60–1.39] | 0.667 |
| Dyslipidaemia | 0.44 | [0.29–0.67] | <0.001 |
| Hypertension | 0.64 | [0.39–1.05] | 0.075 |
| Diabetes mellitus | 0.76 | [0.49–1.18] | 0.223 |
| QRS duration, per ms | 1.00 | [1.00–1.01] | 0.122 |
| Echocardiography | |||
| LVEDDi, per mm/m2 | 1.04 | [1. 00–1.08] | 0.040 |
| LVMi, per g/m2 | 1.01 | [1.00–1.01] | 0.037 |
| LADi, per mm/m2 | 1.03 | [0.99–1.08] | 0.188 |
| LVEF, per % | 0.99 | [0.98–1.00] | 0.212 |
| Mitral regurgitation | 1.33 | [0.72–2.45] | 0.358 |
| Mitral stenosis | 1.22 | [0.30–4.94] | 0.785 |
| Aortic regurgitation | 1.75 | [1.15–2.66] | 0.009 |
| Aortic stenosis | 1.32 | [0.77–2.26] | 0.320 |
| Tricuspid regurgitation | 1.14 | [0.67–1.94] | 0.618 |
| Medical therapy | |||
| ICD | 1.26 | [0.58–2.72] | 0.563 |
| Pacemaker | 1.86 | [1.08–3.2] | 0.025 |
| Statin | 0.62 | [0.40–0.98] | 0.039 |
| Beta‐blocker | 0.69 | [0.45–1.06] | 0.088 |
| ACEI | 0.95 | [0.61–1.47] | 0.818 |
| ARB | 0.71 | [0.43–1.17] | 0.176 |
| ARNI | — | — | — |
| MRA | 1.71 | [1.04–2.81] | 0.035 |
| Loop diuretic | 0.99 | [0.65–1.51] | 0.953 |
| Antidiabetic drug | 0.54 | [0.27–1.08] | 0.083 |
| Insulin | 0.93 | [0.53–1.62] | 0.799 |
| Laboratory values | |||
| Haemoglobin, per g/L | 0.99 | [0.99–1. 00] | 0.32 |
| Haematocrit, per % | 0.99 | [0.96–1.03] | 0.598 |
| RDW, per % | 1.08 | [1.01–1.15] | 0.029 |
| Leucocytes, per G/L | 1.01 | [0.97–1.04] | 0.694 |
| Glucose, per mmol/L | 0.97 | [0.91–1.04] | 0.437 |
| Creatinine, per μmol/L | 1.00 | [1.00–1.00] | 0.133 |
| Sodium, per mmol/L | 1.00 | [0.97–1.03] | 0.844 |
| Potassium, per mmol/L | 0.98 | [0.85–1.14] | 0.824 |
| Cholesterol, per mmol/L | 0.95 | [0.79–1.14] | 0.571 |
BMI, body mass index; DBP, diastolic blood pressure; LVEDDi, left ventricular end‐diastolic diameter index; LVMI, left ventricular mass index; MRA, mineralocorticoid receptor antagonist; RDW, red cell distribution width; SBP, systolic blood pressure.
Kaplan–Meier estimates of survival in accordance to the type of heart failure
Figure S1 demonstrates that Kaplan–Meier estimates of survival differ significantly between the different types of HF with the best survival for patients with HFmrEF and lower survival for HFpEF and HFrEF patients (P = 0.024). Figure S2 shows similar results when multivariable analysis and Cox Hazard proportional analysis were applied to picture survival.
Interaction of mild or moderate aortic regurgitation and type of heart failure on all‐cause mortality over the study period using multivariable Cox regression analysis
ACM was higher in the group of AHF patients with AR (HR 1.75, [95% CI: 1.1–2.7], P = 0.009) despite the overall more favourable cardiovascular risk profile of this group. The final model of multivariable Cox regression analysis with mild or moderate AR as dependent variable included all variables of the univariable Cox regression that showed at least very weak evidence (P < 0.2) for an association with first, mild, or moderate AR, second, 150 days' ACM, and, third, type of HF (Table 4). There was no evidence for a violation of the proportional hazard assumption (P = 0.369). Figure 1 illustrates the interaction of mild or moderate AR and the type of HF on ACM. The hazard of HFpEF patients without AR was used as reference. Within the HFpEF group, the presence of AR increased the HR significantly when compared with the absence of AR (HR: 2.17 [95% CI: 1.13–4.15], P = 0.02). Compared with the reference, we observed a more than twofold increased hazard in HFrEF without AR (HR: 2.55 [95% CI: 1.33–4.87], P = 0.005) and in HFrEF with AR (HR: 2.43 [95% CI 1.26–4.69], P = 0.008). The HR of HFmrEF patients without AR was not different to respective HFpEF patients (HR: 0.2 [95% CI: 0.03–1.52], P = 0.874), and presence of AR in HFmrEF patients showed a trend towards increased ACM in the HFmrEF group (HR: 7.11 [95% CI: 0.87–57.79], P = 0.067) (Figure 1).
Figure 1.
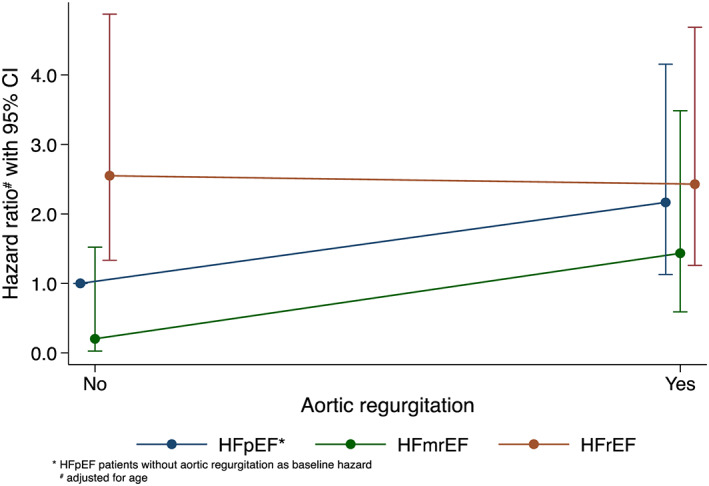
Illustration of the interaction of mild or moderate AR and type of heart failure on 150 days' ACM using multivariable Cox regression. The hazards in the different groups are compared with those of HFpEF without AR as baseline hazard. ACM, all‐cause mortality; AR, aortic regurgitation; HFpEF, heart failure with preserved ejection fraction.
Tables S2 and S3 demonstrate demographics and clinical parameters in HFpEF patients with or without mild to moderate AR. HFpEF patients with mild to moderate AR had a higher prevalence of female gender (57 [66.3%] vs. 70 patients [49.6%], P = 0.014), while smoking (32 [37.2%] vs. 77 [55%] patients, P = 0.009) and diabetes mellitus (21 [24.4%] vs. 53 [37.6%] patients, P = 0.04) were less prevalent. Furthermore, diabetes was less often treated with insulin (9 [10.5%] vs. 31 [22%], P = 0.027). Biological parameters were not significantly different between groups.
Discussion
This study adds to the current knowledge in AHF that AR of mild or moderate severity is a determinant of short‐term ACM in AHF patients with preserved LVEF. AR is highly prevalent in patients with preserved LVEF hospitalized for treatment of AHF, suggesting that efforts to reduce the clinical impact of AR have the potential to improve outcome in these patients.
Prevalence of aortic regurgitation and chronic heart failure in the general population and in heart failure populations
Mild to moderate AR is in general not regarded as a major clinical problem in patients with normal LVEF 15 , 16 despite its high prevalence in the age groups > 70 years. 2 , 3 HF is also highly prevalent in the elder age groups, 4 making coincidental occurrence of HF with AR likely. In fact, mild or moderate AR was observed in 8.3% of ambulatory patients with HF symptoms in a community study involving 79.043 echocardiographic studies. 17 Further evidence supporting a role of AR in HF derives from a retrospective population‐level epidemiological study in Scotland, which showed that 25% of hospitalized patients with a diagnosis of AR had HF. The fact that only 3.3% of all these hospitalized AR cases had aortic valve replacement surgery suggests that the majority of the HF cases had mild or moderate AR. 7 Although not providing definite proof of a pathophysiological role of mild to moderate AR, the increase of the prevalence of AR in parallel with the clinical severity of HF was nonetheless intriguing and the reason for the present study. In fact, 42% of study participants in this study had mild or moderate AR, which not only exceeds considerably the respective prevalence reported elsewhere 2 , 3 , 7 , 17 but may suggest likewise that the prevalence of mild or moderate AR increases in parallel with the severity of HF.
Characteristics of the study population
The present study population included only 505 AHF patients; we, therefore, discuss first whether the basic characteristics of the study population such as portion of three HF types, gender, age, and mortality permit broader application of the study results.
A prominent characteristic of the present cohort is the 61% prevalence of participants with mid‐range or preserved LVEF. In fact, the prevalence of AHF patients with LVEF ≥ 40% was lower in many previous AHF registries, in particular when these studies had recruited participants during the first years of the millennium. 18 However, the more recent prospective, multicentre Kyoto Congestive Heart Failure (KCHF) registry, which had recruited AHF patients in the years 2014–2016, reported a similar proportion of AHF patients with LVEF ≥ 40% (61%). 19 In addition, a similar proportion of AHF patients with LVEF ≥ 40% (62%) was reported from the Get With The Guidelines registry, which had recruited AHF patients in the years 2005–2010. 20 Of note, the number of AHF patients with LVEF ≥ 40% had progressively increased between 2005 and 2010 from 48% to 53% in the latter registry 21 ; in addition, a similar trend had been reported for AHF admissions in the Olmsted county where the percentage of patients with preserved LVEF increased from 38% to 54% in the years 1986 to 2002. 22
With respect to gender distribution, the present study population is comparable with that of other AHF registries, 18 and the 56% of female patients in the HFpEF group correspond to the female proportion reported in many HFpEF studies. 23 Mean age was high in the present study population but not different to reports from the KCHF or the Get With The Guidelines registry 19 , 20 , 21 although higher when compared with AHF registries in the past. 18 However, patients with LVEF < 40% were younger than patients with LVEF ≥ 40%, similar to other reports. 20 Of note, 30 and 90 days' mortality of the present study population correspond to results reported from Canadian Enhanced Feedback for Effective Cardiac Treatment (EFFECT) and OPTIMIZE‐HF registry, respectively. 24 Last but not the least, the survival was best in HFmrEF patients but lower in HFpEF and HFrEF patients, corresponding to results reported from the Get With The Guidelines registry. 20 Altogether, this remarkable similarity of the characteristics of the present study population with other more contemporary AHF study cohorts suggests broad applicability of the results of the present study.
The role of aortic regurgitation for all‐cause mortality in acute heart failure patients
AR was in our study population associated with an increased risk for ACM despite the short period of follow‐up. This association was observed against the background of a more favourable cardiovascular risk profile of the AHF patients with AR who were less often smokers or diabetics. However, we did not observe the negative correlation between AR and diabetes that had been reported from the Strong Heart study. 25
AR furthermore remained independently related with ACM in AHF patients with preserved LVEF, raising the question of why AR is of prognostic relevance in AHF with preserved LVEF but not in AHF with either mid‐range or reduced LVEF. HFpEF is characterized by disorder of muscular and hemodynamic processes affecting propagation of blood flow during early diastole of the left ventricle. In the study participants with preserved LVEF and AR, we observed a numerically higher left ventricular mass index and a numerically increased frequency of mild or moderate tricuspid regurgitation. This combination is compatible with an increased left ventricular end‐diastolic pressure (LVEDP), suggesting that the small regurgitant volume of mild or moderate aortic valve insufficiency resulted in a disproportionate increase of the LVEDP in the small and stiff left ventricles of these patients. This effect may be even worsen left ventricular dysfunction volume when patients already suffer from increased volume charge resulting from mild or moderate MR. This pathophysiolgical concept can furthermore explain why the small regurgitant volume is of prognostic relevance in HFpEF patients but not in HFmrEF or HFrEF patients. In fact, their LV end‐diastolic volume usually is larger, which should dampen the rise of the LVEDP with a small aortic regurgitant volume.
However, it is also important to note that even a small aortic regurgitant volume will result in a coronary steal with a subsequently reduced intracoronary blood flow. The coronary steal maybe of little relevance at rest but may nonetheless induce subendocardial hypoxia with exertion when the LVEDP increases disproportionally and the coronary steal reduces the filling of the pre‐existing pathological microcirculation of the HFpEF heart at the same time. 26
AR may furthermore disturb the diastolic vortex, which is a swirling structure responsible for entering a significant fraction of LV filling volume at no energetic or pressure cost. In the normal heart, the vortex is responsible for entering about 13% the filling volume, while this portion is reduced to 5% in hypertrophic cardiomyopathy. 27 Impaired generation of this swirling structure has also been shown in HFpEF, 28 and perturbation of the vortex function has been associated with increased cardiovascular mortality and rehospitalization. 29 Biomechanical models of the left ventricle have visualized that the regurgitant jet of mild or moderate AR impedes vortex formation as a function of its severity, 30 suggesting that already a small regurgitant volume should worsen the pre‐existing pathologic vortex formation in patients with preserved LVEF. Support of this hypothesis derives from the observation that mild AR after transcatheter aortic valve replacement increased mortality in patients with a baseline LVEF of 50 ± 13% as reported from a series of patients operated at the Cleveland Clinic. 31 Certainly, these patients do not represent the classical stable HF patient with preserved LVEF patient but nevertheless provide evidence that new mild or moderate AR significantly impacts on mortality within a time period of not even 3 years.
Therapeutic implications
Improvement of AHF patients with preserved LVEF and AR was associated with a decrease of systolic and diastolic blood pressure and reduction of heart rate in addition. More recently, independent association has been found between diastolic blood pressure or resting heart rate with mortality in chronic moderate to severe AR. 32 This investigation showed that diastolic blood pressure < 70 mmHg and resting heart rate > 60 b.p.m. increased ACM. Corresponding studies are missing in AHF patients with mild to moderate AR but should be useful for guidance of therapeutic management of these patients. Careful monitoring of these parameters in the ambulatory setting may therefore prevent decompensation, in particular in AHFpEF patients with AR. Whether implantation of a hemodynamic monitoring device in the pulmonary circulation is an option if traditional surveillance fails warrants investigation.
Limitations
The relatively small study population and the mono‐centric study design represent a methodological limitation with respect to the applicability of the study results. Nevertheless, study participants were recruited from consecutive patients presenting to the emergency department, and key characteristics of the study participants are comparable with those of other AHF cohorts. Furthermore, the post‐hoc study design did not allow for retrospective distinction of the cause of mortality in particular in those study participants followed up by the 2005–2009 cohort. However, the short‐term follow‐up and the careful exclusion of AHF patients with adverse prognosis related to co‐morbidity suggests that case fatality was likely due to cardiovascular cause. In the acknowledgement of these limitations, the study results of this study remain therefore hypothesis generating.
Conclusions
The results of this study suggest that AR of mild or moderate severity is of prognostic relevance in patients with preserved LVEF and hospitalization for treatment of AHF. This observation merits attention because coincidence of AR and HF is frequent in the elderly. Therefore, better understanding of the interaction between AR and LV dysfunction in HFpEF patients is necessary and will help to reduce the negative impact of mild or moderate AR in these patients.
Conflict of interest
None declared.
Funding
This study was supported by funds from the Centre hospitalier universitaire vaudois (CHUV), enabling prospective data collection for the cohort 2005–2009. The data collection for the study cohort 2015–2018 was made possible by funds from the Schweizerische Herzstiftung attributed to Pierre Monney, MD, and Roger Hullin, MD. Tamila Abdurashidova received financial support from the Swiss Excellence scholarship, a programme launched by the Swiss government (Direktion für Entwicklung und Zusammenarbeit).
Supporting information
Table S1. Demographical and clinical data of all AHF patients separated by type of heart failure.
Table S2. Demographic and clinical characteristics of AHF patients with and without AR in patients with preserved LVEF (n = 227).
Table S3. Biological characteristics of AHF patients with and without AR in patients with preserved ejection fraction (n = 227).
Figure S1. Kaplan–Meier Estimates of survival in accordance to the type of heart failure.
Figure S2. Multivariable analysis and Cox Hazard Proportional Analysis of Survival with acute heart failure with reduced, mid‐range, and preserved ejection fraction.
Abdurashidova, T. , Monney, P. , Tzimas, G. , Soborun, N. , Regamey, J. , Daux, A. , Barras, N. , Kirsch, M. , Müller, M. , and Hullin, R. (2020) Non‐severe aortic regurgitation increases short‐term mortality in acute heart failure with preserved ejection fraction. ESC Heart Failure, 7: 3901–3909. 10.1002/ehf2.12983.
Martin Müller and Roger Hullin shared last authorship.
References
- 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet 2006; 368: 1005–1011. [DOI] [PubMed] [Google Scholar]
- 2. Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993; 21: 1220–1225. [DOI] [PubMed] [Google Scholar]
- 3. Singh JP, Evans JC, Levy D, Marson MG, Freed LA, Fuller DL, Lehmann B, Benjamin EJ. Prevalence and clinical determinants of, mitral, tricuspid, and aortic regurgitation (The Framingham Heart Study). Am J Cardiol 1999; 83: 897–902. [DOI] [PubMed] [Google Scholar]
- 4. Shah AM, Clagett B, Loehr LR, Chang PP, Matsushita K, Kitzman D, Konety S, Kuchawska‐Newton A, Sueta CA, Mosley TH, Wright JD, Coresh J, Heiss G, Folsom AR, Solomon SD. Heart failure stages among the older adults in the community. The Atherosclerosis Risk in Communities Study. Circulation 2017; 135: 224–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cowie MR, Wood DA, Coats AJS, Thompson G, Poole‐Wilson GA, Suresh V, Sutton GC. Incidence and etiology of heart failure. A population‐based study. Eur Heart J 1999; 20: 421–428. [DOI] [PubMed] [Google Scholar]
- 6. Baumgartner H, Falk V, Bax JJ, DeBonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Munoz DR, Rosenhek R, Djögren J, Mas PT, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017; 38: 2739–2791. [DOI] [PubMed] [Google Scholar]
- 7. Berry C, Lloyd SM, Wang Y, MacDonal A, Ford I. The changing course of aortic valve disease in Scotland: temporal trends in hospitalizations and mortality and prognostic importance of aortic stenosis. Eur Heart J 2013; 34: 1538–1547. [DOI] [PubMed] [Google Scholar]
- 8. Ponikowski P, Voors A, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JP, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016; 37: 2129–2200.27206819 [Google Scholar]
- 9. Ho JE, Zern EK, Wooster L, Bailey CS, Cunningham T, Eisman AS, Hardin KM, Zampierello GA, Jarolim P, Pappagianopoulos PP, Malhotra R, Nayor M, Lewis GD. Differential clinical profiles, exercise responses, and outcomes associated with existing HFpEF definitions. Circulation 2019; 140: 353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang C‐C, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015; 131: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sotiropoulos K, Yerly P, Monney P, Garnier A, Regamey J, Hugli O, Martin D, Metrich M, Antonietti J‐P, Hullin R. Red cell distribution width and mortality in acute heart failure patients with preserved and reduced ejection fraction. ESC Heart Fail 2016; 2: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Monney P, Barras N, Tzimas G, Abdurashidova T, Regamey J, Vinet E, Crisinel V, Lauriers N, Garnier A, Yerly P, Hugli O, Hullin R. Discharge echocardiographic parameters of RV and LV function but not of changes in cardiac unloading are related to 12‐months prognosis during hospitalization for acute decompensated heart failure. Eur Heart J 2019; 40: 778. [Google Scholar]
- 13. Lancelotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European association of cardiovascular imaging. Eur Heart J 2013; 14: 611–644. [DOI] [PubMed] [Google Scholar]
- 14. Zoghbi WA, Enriquez‐Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weismann NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003; 16: 777–802. [DOI] [PubMed] [Google Scholar]
- 15. Badiani S, van Zalen J, Sacheeka S, Hart L, Topham A, Patel N, Sturridge L, Marshall A, Sulke N, Furniss S, Lloyd G. Clinical events and echocardiographic lesion progression rate in subjects with mild or moderate aortic regurgitation. Echo Res Pract 2017; 4: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Detaint D, Maalouf J, Tribouilloy C, Mahoney DW, Schaff HV, Tajik TA, Enriquez‐Sarano M. Congestive heart failure complicating aortic regurgitation with medical and surgical management: a prospective study of traditional and quantitative echocardiographic markers. J Cardiothor Cardiovasc Surg 2008; 136: 1549–1557. [DOI] [PubMed] [Google Scholar]
- 17. Marciniak A, Glover K, Sharma R. Cohort profile: prevalence of valvular heart disease in community patients with suspected heart failure in UK. BMJ Open 2017; 7: e012240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 19. Yaku H, Ozasa N, Morimoto T, Inuzuka Y, Tamaki Y, Yamamoto E, Yoshikawa Y, Kitai T, Taniguchi R, Iguchi M, Kato M, Takahashi M, Jinnai T, Ikeda T, Nagao K, Kawai T, Komasa A, Nishikawa R, Kawase Y, Morinaga T, Su K, Kawato M, Sasaki K, Toyofuku M, Furukawa Y, Nakagawa Y, Ando K, Kadota K, Shizuta S, Ono K, Sato Y, Kuwahara K, Kato T, Kimura T, KCHF Study Investigators . Demographics, management, and in‐hospital outcome of hospitalized acute heart failure patients in contemporary real clinical practice in Japan. Circ J 2018; 82: 2811–2819. [DOI] [PubMed] [Google Scholar]
- 20. Cheng RK, Cox M, Neely ML, Heidenreich PA, Bhatt DL, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J 2014; 168: 721–730. [DOI] [PubMed] [Google Scholar]
- 21. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC. Trends in patients hospitalized with heart failure and preserved ejection fraction: prevalence, therapies, and outcomes. Circulation 2012; 126: 65–75. [DOI] [PubMed] [Google Scholar]
- 22. Owan TE, Hodge DO, Herges RM, Jacobsen RS, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 23. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017; 14: 591–602. [DOI] [PubMed] [Google Scholar]
- 24. Fonarow GC, Gattis Stough W, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB for the OPTIMIZE‐HF . Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure. J Am Coll Cardiol 2007; 50: 768–777. [DOI] [PubMed] [Google Scholar]
- 25. Lebowitz NE, Bella JN, Roman MJ, Liu JE, Fishman DP, Paranicas M, Lee ET, Fabsitz RR, Welty TK, Howard BV, Devereux DB. Prevalence and correlates of aortic regurgitation in American Indians: the Strong Heart study. J Am Coll Cardiol 2000; 36: 461–467. [DOI] [PubMed] [Google Scholar]
- 26. Srivaratharajah K, Coutinho T, de Kemp R, Liu P, Hadda H, Stadnick E, Davies RA, Chih S, Dwivedi G, Guo A, Wells GA, Bernick J, Beanlands R, Mielniczuk LM. Reduced myocardial flow in heart failure patients with preserved ejection raction. Circ Heart Fail 2016; 9: e002562. [DOI] [PubMed] [Google Scholar]
- 27. Martinez‐Legazpi P, Bermejo J, Benito Y, Yotti R, Perez de Villar C, Gonzalez‐Mansilla C, Barrio A, Villacorta E, Sanchez PL, Fernandez‐Aviles F, del Alamo JC. Contribution of the diastolic vortex ring to left ventricular filling. J Am Coll Cardiol 2014; 64: 1711–1721. [DOI] [PubMed] [Google Scholar]
- 28. Spinarova M, Meluzin J, Podrouzkova H, Stepanova R, Spinorova L. New echocardiographic parameters in the diagnosis of heart failure with preserved ejection fraction. Int J Cardiovasc Imaging 2018; 34: 229–235. [DOI] [PubMed] [Google Scholar]
- 29. Poh KK, Lee LC, Shen L, Chong E, Tan YL, Chai P, Yeo TC, Wood MJ. Left ventricular fluid dynamics in heart failure: echocardiographic measurement and utilities of vortex formation time. Eur Heart J 2012; 13: 385–393. [DOI] [PubMed] [Google Scholar]
- 30. Okafor I, Raghav V, Condado JF, Midha PA, Kumar G, Yoganathan AG. Aortic regurgitation generates a kinematic obstruction which hinders left ventricular filling. Ann Biomed Eng 2017; 45: 1305–1314. [DOI] [PubMed] [Google Scholar]
- 31. Jones BM, Tuzcu EM, Krishnaswamy A, Popovic Z, Mick S, Roselli EE, Gul S, Devgun J, Mistry S, Jaber WA, Svensson LG, Kapadia SR. Prognostic significance of mild aortic regurgitation in predicting mortality after transcatheter aortic valve replacement. J Cardiothorac Surg 2016; 152: 783–790. [DOI] [PubMed] [Google Scholar]
- 32. Yang L‐T, PellIkka PA, Enriquez‐Sarano M, Scott CG, Padang R, Mankad SV, Schaff HV, Michelena HI. Diastolic blood pressure and heart rate are independently associated with mortality in chronic aortic regurgitation. J Am Coll Cardiol 2020; 75: 29–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographical and clinical data of all AHF patients separated by type of heart failure.
Table S2. Demographic and clinical characteristics of AHF patients with and without AR in patients with preserved LVEF (n = 227).
Table S3. Biological characteristics of AHF patients with and without AR in patients with preserved ejection fraction (n = 227).
Figure S1. Kaplan–Meier Estimates of survival in accordance to the type of heart failure.
Figure S2. Multivariable analysis and Cox Hazard Proportional Analysis of Survival with acute heart failure with reduced, mid‐range, and preserved ejection fraction.


