Abstract
Aims
Systemic metabolic impairment is the key pathophysiology of heart failure (HF) with preserved ejection fraction (HFpEF). Fatty acid‐binding protein 4 (FABP4) is highly expressed in adipocytes and secreted in response to lipolytic signals. We hypothesized that circulating FABP4 levels would be elevated in patients with HFpEF, would correlate with cardiac structural and functional abnormalities, and could predict clinical outcomes.
Methods and results
Serum FABP4 measurements and echocardiography were performed in patients with HFpEF (n = 92) and those with coronary artery disease free of HF (n = 20). Patients were prospectively followed‐up for a composite endpoint of all‐cause mortality or HF hospitalization. Compared with patients with coronary artery disease, those with HFpEF had higher FABP4 levels [12.5 (9.1–21.0) vs. 43.5 (24.6–77.4) ng/mL, P < 0.0001]. FABP4 levels were associated with cardiac remodelling (left ventricular mass index: r = 0.29, P = 0.002; left atrial volume index: r = 0.40, P < 0.0001), left ventricular systolic and diastolic dysfunction (global longitudinal strain: r = −0.24, P = 0.01; E/e′ ratio: r = 0.29, P = 0.002; and N‐terminal pro‐B‐type natriuretic peptide: r = 0.62, P < 0.0001), and right ventricular dysfunction (tricuspid annular plane systolic excursion: r = −0.43, P < 0.0001). During a median follow‐up of 9.1 months, there were 28 primary endpoints in the HFpEF cohort. Event‐free survival was significantly decreased in patients with FABP4 levels ≥43.5 ng/mL than in those with FABP4 levels <43.5 ng/mL (P = 0.003).
Conclusions
Serum FABP4 levels were increased in HFpEF and were associated with cardiac remodelling and dysfunction, and poor outcomes. Thus, FABP4 could be a potential biomarker in the complex pathophysiology of HFpEF.
Keywords: Adipose tissue, Fatty acid‐binding protein, Heart failure, HFpEF
Introduction
Approximately one‐half of the patients with heart failure (HF) have a preserved left ventricular (LV) ejection fraction (HFpEF), and the prevalence of this syndrome is increasing at an alarming pace in developed countries. 1 Recent studies have clarified that HFpEF is a complex and heterogeneous syndrome. Patients with HFpEF have multiple cardiac and extracardiac abnormalities and several factors that drive these abnormalities, such as metabolic co‐morbidities, cellular senescence, endothelial dysfunction, systemic inflammation and fibrosis, and alterations in nitric oxide–cyclic guanosine monophosphate–protein kinase G signalling pathways. 2 However, the underlying mechanisms remain unclear.
Adipose tissue is an endocrine organ that actively secretes cytokines and adipokines, which contribute to obesity‐related metabolic and cardiovascular diseases. 3 Fatty acid‐binding protein 4 (FABP4), which is also known as adipocyte fatty acid‐binding protein and adipocyte protein 2, is one of the most abundant proteins in adipocytes and primarily acts as an intracellular lipid chaperone. 4 During the last decade, numerous human studies have shown that higher serum FABP4 levels are observed in various immunometabolic diseases, including type 2 diabetes, metabolic syndrome, non‐alcoholic fatty liver disease, atherosclerosis, acute myocardial infarction, stroke, and cancer. 5 , 6 , 7 Although the associations are robust, the role of FABP4 in disease pathogenesis remains enigmatic.
Studies using FABP4 loss‐of‐function or gain‐of‐function models in mice showed that FABP4 induces inflammatory cytokines, reduces myocardial contraction, induces lipid accumulation in cardiomyocytes, and inhibits endothelial nitric oxide synthase. 8 , 9 , 10 , 11 Despite the growing number of studies suggesting the extracellular function of FABP4 in the cardiovascular system, whether secreted FABP4 plays a hormonal role remains unclear.
Fatty acid‐binding protein 4 is secreted from adipocytes in response to increased lipolytic signals that are elicited by catecholamine and natriuretic peptides. 12 Circulating FABP4 levels are increased during a bout of exercise in humans, and an induction of FABP4 is highly correlated with that of norepinephrine and work load, as assessed by cardiopulmonary exercise test. 13 Adrenergic hyperactivation is the hallmark of HF, and adipose tissue lipolysis may be profoundly activated in HF. 14 However, whether FABP4 has a potential as a biomarker for HFpEF remains unknown.
Hence, we sought to examine whether circulating FABP4 levels would be elevated in patients with HFpEF and to determine whether the magnitude of elevation would correlate with cardiac structural and functional abnormalities and clinical outcomes in patients with HFpEF.
Methods
Study population
Patients with HFpEF [ejection fraction (EF) ≥45%] (HFpEF group) who were admitted to Gunma University Hospital between 2015 and 2018 were enrolled in this prospective study. Given the known difficulties in diagnosing HFpEF and to ensure the unequivocal presence of HFpEF, we ensured that all patients in this study had been recently hospitalized for HF that was treated with intravenous diuretics. Patients with reduced EF (EF < 45%), valvular heart disease (greater than moderate left‐sided regurgitation and greater than mild stenosis), unstable coronary disease or recent revascularization, constrictive pericarditis, high‐output HF, or cardiomyopathy were excluded. All patients who were in a compensated state, that is, whose recent worsening HF had been resolved with diuretics, underwent clinical history taking, blood sampling, and resting echocardiography.
Patients free of HF who were referred for coronary angiography for angina pectoris evaluation (n = 9) or follow‐up after percutaneous coronary intervention (n = 11) were included as a comparator group [coronary artery disease (CAD) group, n = 20]. The CAD group had no evidence of elevated LV filling pressures based on echo‐Doppler findings or natriuretic peptide levels. Written informed consent was obtained from all patients before study participation. This study was approved by the Gunma University Hospital Clinical Research Review Board. The authors had full access to the data and take responsibility for data integrity. The investigation conforms to the principles outlined in the Declaration of Helsinki.
Biomarker measurements
Venous blood samples were obtained from the patients after an overnight fast. Serum haemoglobin, creatinine, glucose, lipid profiles, and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) levels were measured using routine automated laboratory procedures. Serum FABP4 levels were measured using a commercially available enzyme‐linked immunosorbent assay kit (BioVendor R&D, Modřice, Czech Republic). As specified by the manufacturer, the lower limit of detection of serum FABP4 was 0.05 ng/mL. The accuracy and reproducibility of the kit have been described previously. The intra‐assay and inter‐assay coefficients of variation were 3.7–6.4% and 2.6–5.3%, respectively. 5 Serum levels of interleukin‐6 (IL‐6) and tumour necrosis factor (TNF)‐α were also measured using sandwich enzyme‐linked immunoassays (R&D Systems, Minneapolis, MN, USA). The reported intra‐assay and inter‐assay coefficients of variation were 1.6–4.2% and 3.3–6.4% for IL‐6 and 2.2–3.0% and 7.3–8.4% for TNF‐α, respectively.
Assessment of cardiac structure and function
Two‐dimensional, M‐mode, Doppler and tissue Doppler echocardiography were performed within 2 days of blood sampling. Echocardiographic measurements were performed according to the American Society of Echocardiography guidelines. 15 LV systolic function was evaluated using systolic mitral annular tissue Doppler velocity (s′) and global longitudinal strain (GLS). Speckle tracking analyses were performed offline with commercially available software (EchoPAC PC, GE, Milwaukee, WI, USA). We reported the absolute GLS values to avoid unnecessary confusion. Moreover, LV diastolic function was assessed using transmitral velocities [early and late diastolic inflow velocities (E and A) and deceleration time of the E (DecT)], early diastolic septal mitral annular tissue velocity (e′), and E/e′ ratio. Maximum left atrial (LA) volume was calculated using the method of discs. LA volume and LV mass were indexed to the body surface area. The ratio of E/e′ and LV end‐diastolic volume was determined as non‐invasive estimates of LV stiffness. 16 Right ventricular (RV) diameters were measured at end‐diastole using RV‐focused views. RV systolic function was assessed by tricuspid annular plane systolic excursion (TAPSE).
Outcome assessment
Patient follow‐up was initiated on the day of blood sampling. The primary composite endpoint of this study was the first occurrence of all‐cause mortality or HF hospitalization. HF hospitalization was defined as dyspnoea and pulmonary oedema on chest radiographs, requiring intravenous diuretic treatment. Investigators obtained follow‐up data from the patient medical records, telephone interviews, and notices of death from other hospitals.
Statistical analysis
Data are reported as mean (standard deviation), median (inter‐quartile range), or number (%), unless otherwise specified. Between‐group differences were compared using an unpaired t‐test, Wilcoxon rank‐sum test, or χ 2 test, as appropriate. Pearson's or Spearman's correlation coefficient was used to assess relationships between two variables of interest, as appropriate. Event‐free rates were assessed using Kaplan–Meier curve analysis, and the prognostic value was determined using Cox proportional hazards models, in which non‐normally distributed data were log transformed. Given the limited number of events, the independence and robustness of FABP4 were assessed using progressive models. All tests were two sided, with P values <0.05 considered statistically significant. All analyses were performed using JMP 14.0.0 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
Age was similar between the HFpEF and CAD groups (Table 1 ). Compared with patients in the CAD group, those in the HFpEF group were predominantly female and anaemic and had a higher prevalence of atrial fibrillation (AF). The prevalence of dyslipidaemia and CAD was lower in the HFpEF group. In striking contrast to Western HFpEF populations, overweight/obesity was rare (23%) in the HFpEF population in our study, which is consistent with the findings from a recent Asian HFpEF registry. 17 Compared with patients with CAD, those with HFpEF were more frequently treated with loop diuretics and aldosterone blockers. Low‐density lipoprotein cholesterol and estimated glomerular filtration rate were lower, and IL‐6, TNF‐α, and NT‐proBNP levels were higher in the HFpEF group than in the CAD group.
Table 1.
Baseline characteristics
| CAD (n = 20) | HFpEF (n = 92) | P value | |
|---|---|---|---|
| Age (years) | 70.3 ± 7.8 | 73.0 ± 12.8 | 0.4 |
| Female, n (%) | 6 (30) | 54 (59) | 0.02 |
| Body surface area (m2) | 1.65 ± 0.20 | 1.50 ± 0.20 | 0.003 |
| Body mass index (kg/m2) | 22.7 ± 3.5 | 22.3 ± 3.6 | 0.7 |
| Overweight/obesity, n (%) | 4 (20)/1 (5) | 19 (21)/2 (2) | 0.8 |
| NYHA Class I/II/III, n (%) | — | 48 (52)/36 (39)/8 (9) | — |
| Co‐morbidities | |||
| Diabetes mellitus, n (%) | 7 (35) | 25 (27) | 0.5 |
| Hypertension, n (%) | 14 (70) | 66 (72) | 0.9 |
| Dyslipidaemia, n (%) | 13 (65) | 32 (36) | 0.02 |
| Atrial fibrillation, n (%) | 2 (10) | 42 (47) | 0.003 |
| Coronary artery disease, n (%) | 19 (95) | 30 (34) | <0.0001 |
| Medications | |||
| ACEI or ARB, n (%) | 9 (45) | 47 (52) | 0.6 |
| Beta‐blocker, n (%) | 7 (35) | 43 (47) | 0.3 |
| Loop diuretic, n (%) | 0 (0) | 68 (77) | <0.0001 |
| Aldosterone blocker, n (%) | 0 (0) | 45 (51) | <0.0001 |
| Any diuretic, n (%) | 0 (0) | 74 (80) | <0.0001 |
| Laboratories | |||
| Haemoglobin (g/dL) | 13.2 ± 1.7 | 11.5 ± 2.0 | 0.0006 |
| Glucose (mg/dL) | 106.0 (87.5–137.8) | 112.5 (96.0–164.8) | 0.5 |
| eGFR (mL/min/1.73 m2) | 68.9 ± 15.1 | 53.4 ± 31.5 | 0.04 |
| LDL cholesterol (mg/dL) | 109.6 ± 31.8 | 91.8 ± 31.2 | 0.03 |
| HDL cholesterol (mg/dL) | 48.6 ± 12.7 | 46.7 ± 15.8 | 0.6 |
| Triglyceride (mg/dL) | 103.0 (82.5–140.0) | 92.0 (66.0–132.0) | 0.3 |
| Interleukin‐6 (pg/mL) | 2.8 (2.2–4.3) | 17.4 (9.3–27.6) | <0.0001 |
| TNF‐α (pg/mL) | 6.3 (4.9–8.0) | 14.2 (10.3–27.5) | <0.0001 |
| NT‐proBNP (pg/mL) | 193.5 (103.0–303.3) | 1395.0 (756.3–3640.0) | <0.0001 |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; HFpEF, heart failure with preserved ejection fraction; LDL, low‐density lipoprotein; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; TNF, tumour necrosis factor.
Data are mean ± standard deviation, median (inter‐quartile range), or n (%).
Cardiac structure and function
Systolic, diastolic, and mean blood pressures and heart rate were similar between the two groups (Table 2 ). The HFpEF group had larger LV end‐diastolic volume and mass indices than the CAD group. The LVEF, s′, and GLS were significantly lower in the HFpEF group, suggesting impaired LV contractile function. Moreover, compared with the patients with CAD, those with HFpEF displayed an impaired LV diastolic function, with higher mitral E‐wave and E/e′ ratio and larger LA volume index. RV mid‐diameter was larger, and RV systolic function based on TAPSE was lower in patients with HFpEF than in those with CAD.
Table 2.
Cardiac structure and function
| CAD (n = 20) | HFpEF (n = 92) | P value | |
|---|---|---|---|
| Haemodynamics | |||
| Systolic BP (mmHg) | 120.8 ± 15.7 | 126.1 ± 22.0 | 0.3 |
| Diastolic BP (mmHg) | 67.2 ± 8.1 | 67.1 ± 12.9 | 1.0 |
| Mean BP (mmHg) | 85.0 ± 8.5 | 86.8 ± 14.2 | 0.6 |
| Heart rate (b.p.m.) | 70.5 ± 12.9 | 74.0 ± 14.8 | 0.3 |
| LV structure and function | |||
| LV end‐diastolic volume index (mL/m2) | 43.1 ± 15.0 | 61.4 ± 20.3 | 0.004 |
| LV mass index (g/m2) | 89.1 ± 16.4 | 108.9 ± 30.4 | 0.006 |
| LV ejection fraction (%) | 65.6 ± 6.9 | 57.8 ± 9.4 | 0.0007 |
| LV global longitudinal strain (%) | 20.5 ± 4.4 | 13.9 ± 4.4 | <0.0001 |
| Diastolic function | |||
| Mitral E wave (cm/s) | 62.6 ± 16.0 | 87.5 ± 29.0 | 0.0003 |
| Mitral A wave (cm/s) | 82.5 ± 20.3 | 83.0 ± 26.2 | 0.9 |
| Deceleration time (ms) | 259.1 ± 65.1 | 207.0 ± 68.9 | 0.003 |
| Mitral annular e′ (cm/s) | 5.2 ± 1.1 | 5.2 ± 1.7 | 0.9 |
| s′ velocity (cm/s) | 6.7 ± 1.3 | 5.4 ± 1.6 | 0.002 |
| E/e′ ratio | 11.4 (9.7–14.3) | 17.6 (12.9–22.7) | <0.0001 |
| LA volume index (mL/m2) | 24.9 ± 9.7 | 54.6 ± 26.7 | <0.0001 |
| TR velocity (m/s) | 2.1 ± 0.4 | 2.6 ± 0.5 | 0.0008 |
| RV structure and function | |||
| RV basal diameter (mm) | 33.0 ± 6.3 | 35.8 ± 6.9 | 0.1 |
| RV mid‐diameter (mm) | 24.0 ± 4.5 | 27.9 ± 6.1 | 0.007 |
| RV long diameter (mm) | 63.4 ± 5.9 | 62.2 ± 8.3 | 0.5 |
| TAPSE (mm) | 22.2 ± 3.2 | 16.4 ± 4.9 | 0.0005 |
A wave, late diastolic mitral inflow velocity; BP, blood pressure; CAD, coronary artery disease; E wave, early diastolic mitral inflow velocity; e′, early diastolic mitral annular tissue velocity; HFpEF, heart failure with preserved ejection fraction; LA, left atrial; LV, left ventricular; RV, right ventricular; s′, systolic mitral annular tissue velocity; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Data are mean ± standard deviation or median (inter‐quartile range).
Fatty acid‐binding protein 4 levels in heart failure with preserved ejection fraction and their correlations with clinical and echocardiographic markers
Overall, FABP4 levels tended to be higher in women than in men [40.4 (23.4–75.3) vs. 27.3 (13.5–70.3) ng/mL, P = 0.07] and were significantly higher in patients with AF than in those without [62.5 (30.3–85.7) vs. 26.2 (14.4–46.8) ng/mL, P < 0.0001]. Compared with patients with CAD, those with HFpEF showed significantly higher FABP4 levels [12.5 (9.1–21.0) vs. 43.5 (24.6–77.4) ng/mL, P < 0.0001; Figure 1 ]. FAPB4 levels remained significantly higher in HFpEF than in CAD after adjusting for sex, AF, diuretics use, LVEF, haemoglobin levels, and renal function (estimated glomerular filtration rate) (P = 0.0009).
Figure 1.
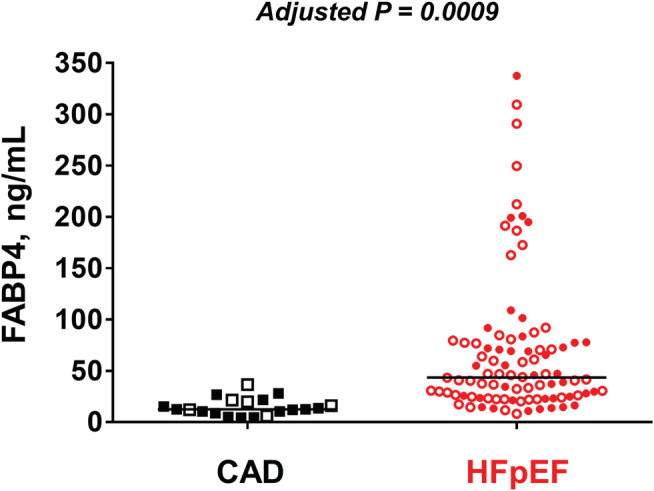
Comparison of fatty acid‐binding protein 4 (FABP4) levels between heart failure with preserved ejection fraction (HFpEF) and coronary artery disease (CAD) groups. Compared with patients with CAD, those with HFpEF had higher FABP4 levels. Even after adjustment for sex, atrial fibrillation, diuretics use, left ventricular ejection fraction, haemoglobin levels, and renal function, the association remained significant (P = 0.0009). Red closed circle indicates men with HFpEF; red open circle, women with HFpEF; black closed square, men with CAD; and black open square, women with CAD.
Fatty acid‐binding protein 4 levels were highly correlated with IL‐6 (r = 0.79, P < 0.0001) and TNF‐α levels (r = 0.67, P < 0.0001; Figure 2 ). Serum FABP4 levels were also correlated with greater LV mass index (r = 0.29, P = 0.002), higher tricuspid regurgitation velocity (r = 0.33, P = 0.002) and E/e′ ratio (r = 0.29, P = 0.002), and larger LA volume index (r = 0.40, P < 0.0001; Figure 3A ). Moreover, higher FABP4 levels were correlated with NT‐proBNP levels (r = 0.62, P < 0.0001; Figure 3B ) and increased LV stiffness (r = 0.30, P = 0.006). These results consistently suggest an association between FABP4 and LV diastolic dysfunction. By contrast, FABP4 levels were unrelated to LV systolic function as assessed by EF (P > 0.1), although they were related to s′ (r = −0.19, P = 0.04) and GLS (r = −0.24, P = 0.01). The association between FABP4 levels and LV diastolic function also remained significant in patients with HFpEF only (LV mass index, r = 0.21; LA volume index, r = 0.21; LV stiffness, r = 0.30; and NT‐proBNP, r = 0.50; all P < 0.05). Patients with HFpEF with FABP4 > 150 ng/mL (n = 12) exhibited more severe abnormalities in diastolic function, with a trend towards larger LV mass index (124.4 ± 42.9 vs. 106.6 ± 27.7 g/m2, P = 0.06) and LA volume index (68.1 ± 38.3 vs. 52.6 ± 24.2 mL/m2, P = 0.06), and significantly higher NT‐proBNP levels [7050.0 (1540.0–15375.0) vs. 1260.0 (647.5–3332.5) pg/mL, P = 0.002]. Higher FABP4 levels were also correlated with lower TAPSE (r = −0.43, P < 0.0001).
Figure 2.
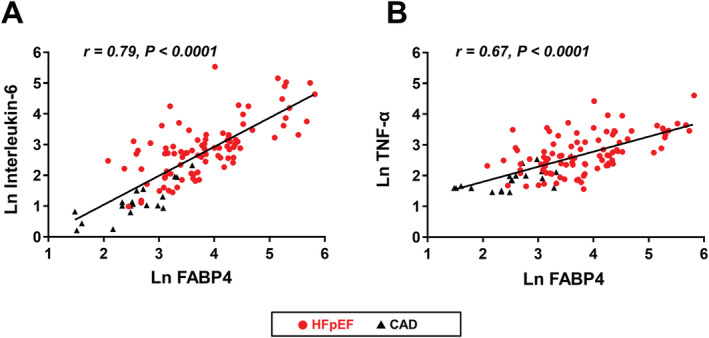
Correlation of fatty acid‐binding protein 4 (FABP4) levels with (A) interleukin‐6 (IL‐6) and (B) tumour necrosis factor‐α (TNF‐α). FABP4 levels were correlated with IL‐6 and TNF‐α.
Figure 3.
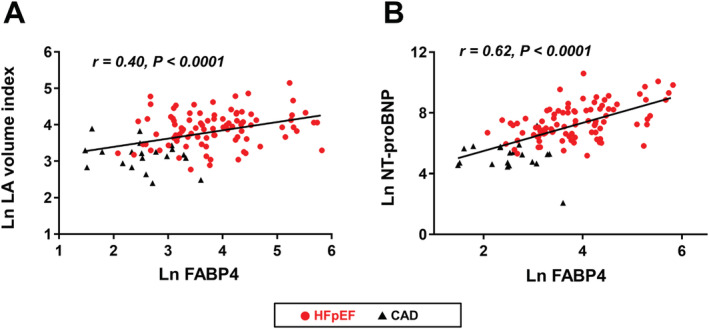
Fatty acid‐binding protein 4 (FABP4) levels, left atrial (LA) size, and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) levels. Increases in FABP4 levels were correlated with (A) larger LA volume index and (B) higher NT‐proBNP levels. Other abbreviations as in Figure 1 .
Prognostic impact of fatty acid‐binding protein 4 in heart failure with preserved ejection fraction
The status of four patients who declined to participate during the follow‐up (n = 1) or were lost to follow‐up (n = 3) was unknown. In the remaining 88 patients with HFpEF, there were 28 primary endpoints (6 all‐cause mortality and 22 HF hospitalizations) over a median follow‐up of 9.1 months (inter‐quartile range 1.3–26.0). Compared with the patients with HFpEF with FABP4 levels below the median value (<43.5 ng/mL), those with FABP4 levels above the median value (≥43.5 ng/mL) had a higher prevalence of AF and renal dysfunction, and higher NT‐proBNP, IL‐6, and TNF‐α levels, with a tendency towards larger LA volume index (Supporting Information, Table S1 ); nevertheless, age, sex, body mass index, other co‐morbidities, and the use of neurohormonal blockers were similar. Moreover, Kaplan–Meier analyses showed that event‐free survival was significantly decreased in patients with FABP4 levels ≥43.5 ng/mL than in those with FABP4 levels <43.5 ng/mL (log‐rank P = 0.003; Figure 4 ). In an unadjusted Cox hazard model, elevated FABP4 levels were associated with a two‐fold increased risk of worsened outcomes (hazard ratio per 1 unit = 2.16, 95% confidence interval, 1.32–3.51; P = 0.002). FABP4 remained an independent predictor in each multivariable Cox model (Table 3 ).
Figure 4.
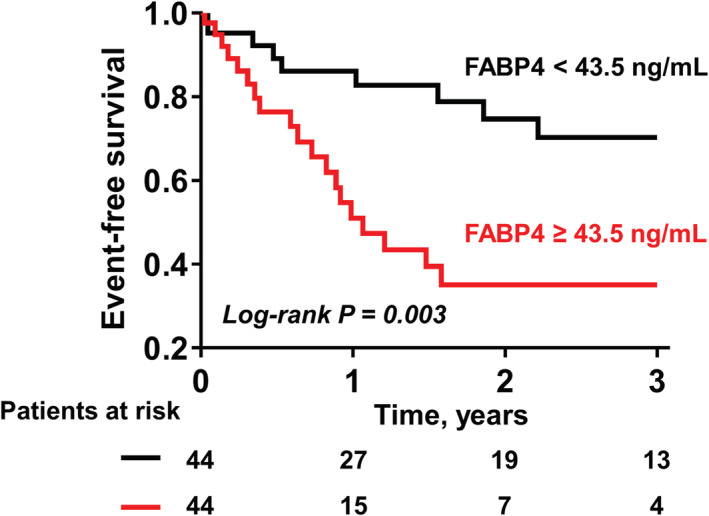
Kaplan–Meier curve analysis for predicting a composite outcome. Compared with patients with HFpEF with FABP4 levels below the median value (<43.5 ng/mL), those with higher FABP4 levels (≥43.5 ng/mL) had an increased risk of a composite endpoint of all‐cause mortality or hospitalization due to heart failure. Abbreviations as in Figure 1 .
Table 3.
Multivariable Cox regression analyses for predicting the primary outcome
| HR (95% CI) a | P value | |
|---|---|---|
| Age + AF adjusted | 2.16 (1.26–3.71) | 0.005 |
| Age + haemoglobin adjusted | 2.05 (1.21–3.46) | 0.007 |
| Age + Ln eGFR adjusted | 1.82 (1.07–3.07) | 0.03 |
| Age + Ln LA volume index adjusted | 2.14 (1.30–3.51) | 0.003 |
| Age + Ln NT‐proBNP adjusted | 1.89 (1.11–3.25) | 0.02 |
| Haemoglobin + Ln eGFR adjusted | 1.83 (1.05–3.16) | 0.03 |
| Haemoglobin + Ln NT‐proBNP adjusted | 1.91 (1.05–3.53) | 0.04 |
| Ln eGFR + Ln NT‐proBNP adjusted | 1.80 (1.04–3.16) | 0.04 |
AF, atrial fibrillation; eGFR, estimated glomerular filtration rate; LA, left atrial; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Hazard ratios (HRs) and 95% confidential intervals (CIs) for Ln FABP4 per 1 unit increase.
Sensitivity analysis
We performed a sensitivity analysis excluding patients with HFpEF with EF of 45–49% (n = 11). FABP4 levels remained higher in patients with HFpEF with EF ≥ 50% than in those with CAD [46.7 (25.8–78.6) vs. 12.5 (9.1–21.0) ng/mL, P < 0.0001]. Consistent with the findings from the primary analyses, FABP4 levels were correlated with LV mass index (r = 0.30, P = 0.002), LA volume index (r = 0.35, P = 0.0004), E/e′ ratio (r = 0.27, P = 0.006), NT‐proBNP (r = 0.65, P < 0.0001), GLS (r = −0.31, P = 0.002), and TAPSE (r = −0.43, P < 0.0001). In addition, event‐free survival was significantly lower in patients with FABP4 levels above the median value (≥46.7 ng/mL) than in those with FABP4 levels below the median value (<46.7 ng/mL) (log‐rank P = 0.009; Supporting Information, Figure S1 ).
Discussion
This study provides evidence of the associations of higher serum FABP4 levels with cardiac remodelling and dysfunction and with adverse clinical outcomes in patients with HFpEF. Given that FABP4 secretion from adipocytes is profoundly stimulated through lipolysis pathways, the results of our study indicate that serum FABP4 may be a potential biomarker for excessive lipolysis, which may be linked to the specific pathophysiology of HFpEF (Figure 5 ).
Figure 5.
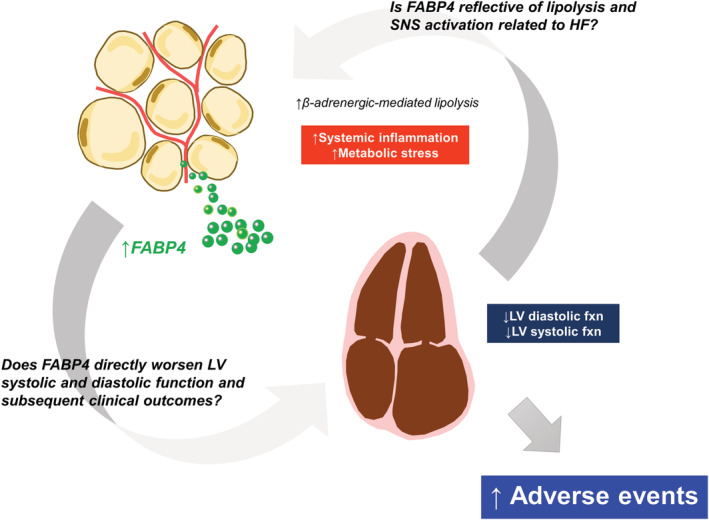
Conceptual model linking lipolysis, left ventricular (LV) systolic and diastolic dysfunctions, and poor outcome in HFpEF. Systemic inflammation and metabolic stress may enhance β‐adrenergic‐mediated lipolysis in HFpEF, which in turn leads to an elevation in FABP4 levels. The increase in FABP4 may directly worsen LV diastolic and systolic functions, thereby contributing to poor clinical outcomes. Conversely, heart failure‐related sympathetic nervous system (SNS) activation may lead to catecholamine‐induced lipolysis, or possibly both exacerbate each other. fxn, function; and other abbreviations as in Figure 1 .
Elevation in fatty acid‐binding protein 4 levels in heart failure with preserved ejection fraction
Fatty acid‐binding protein 4 is highly expressed in adipose tissue and plays an important role as an intracellular lipid chaperone that mediates lipid metabolism. 4 Increasing evidence indicates that FABP4 is actively released from adipose tissue in response to several metabolic stresses. 18 Numerous studies have shown that FABP4 levels are independently associated with increased risk of cardiovascular diseases and poor prognosis following stroke. 5 , 6 In addition, Liu et al. reported elevated FABP4 levels in a mixed cohort of patients with HFpEF and HF with reduced EF (HFrEF) compared with patients without HF. 19 In our study, despite the similar prevalence of metabolic co‐morbidities, such as hypertension, diabetes, and obesity between the HFpEF and CAD groups and lower CAD prevalence in the former, patients with HFpEF showed higher FABP4 levels than those with CAD. Even after adjusting for sex, AF, and renal function, which are known to influence serum FABP4 levels, 20 , 21 as well as other confounders, FABP4 levels remained higher in HFpEF than in CAD.
We have recently found that FABP4 levels are rapidly increased at the onset of acute myocardial infarction and during an acute bout of exercise on a cycle ergometer. 7 , 13 Our in vitro data also showed that FABP4 was released from adipocytes through a β3‐adrenergic receptor‐mediated mechanism. 7 These findings suggest that FABP4 secretion from adipocytes is attributed to β‐adrenergic‐mediated lipolysis. Moreover, a previous study reported that patients with HFpEF have a sympathetic–parasympathetic imbalance with reduced heart rate variability compared with HF‐free patients 22 and that increased activation of sympathetic nerve traffic and β‐adrenergic system plays a role in the pathophysiology of HFpEF. 23 Although the mechanisms of FABP4 elevation in HFpEF remain speculative, these observations suggest that the increased lipolysis through excessive sympathetic nerve activation induces FABP4 secretion from adipocytes. In addition, our data showing a highly significant association between FABP4 levels and inflammation markers (IL‐6 and TNF‐α) also suggest that systemic inflammation may contribute to FABP4 elevation.
The obesity prevalence in our HFpEF population was much lower than that in Western HFpEF populations, which is consistent with the findings from a recent Asian HFpEF registry. 17 FABP4 secretion may be determined by adiposity and sympathetic nervous activation. 13 Our data indicate that sympathetic nervous activation could increase FABP4 secretion possibly by lipolytic signal even in non‐obese patients. Nonetheless, further study is needed to determine the exact mechanisms of the increase in FABP4 level, and our results should be confirmed in Western populations.
Correlation of fatty acid‐binding protein 4 levels with echocardiographic parameters
Heart failure with preserved ejection fraction is characterized as a heterogeneous syndrome with multiple cardiac and extracardiac abnormalities, and LV diastolic dysfunction plays a fundamental, overarching role in the pathophysiology of HFpEF. 24 Previous studies of patients with obesity showed a correlation between FABP4 levels and diastolic function indices. 25 , 26 In our study, FABP4 levels were consistently associated with diastolic dysfunction markers, including LA volume index, E/e′ ratio, LV mass index, and LV diastolic stiffness. We further demonstrated that elevations in FABP4 level are associated with higher NT‐proBNP levels.
Although the LVEF is preserved in HFpEF (by definition), patients with HFpEF characteristically display depressed LV systolic function based on strain or tissue Doppler imaging. 27 Impaired LV systolic function in HFpEF is associated with an increased risk of adverse outcomes. 28 Similar to the studies in the general population and in patients with HFrEF, 25 , 29 FABP4 levels were unrelated to LVEF in our study. Nevertheless, we demonstrated a significant inverse correlation between FABP4 and s′ as well as GLS. Moreover, an experimental study reported that FABP4 directly suppresses cardiomyocyte contraction. 9 FABP4 has also been shown to induce inflammatory cytokines, promote myocardial triglyceride accumulation, and impair endothelial nitric oxide synthase activity. 8 , 10 , 11 Our data and those in previous studies indicate that FABP4 could directly worsen LV systolic and diastolic functions in patients with HFpEF. In the current study, RV function was depressed in patients with HFpEF compared with controls, and FABP4 levels were associated with the severity of RV dysfunction (i.e. TAPSE). This also suggests that FABP4 could play a potential role in developing RV dysfunction in HFpEF.
However, a previous report from the Cardiovascular Health Study showed that circulating FABP4 levels are not associated with incident HFpEF (EF ≥ 55%). 30 We could not determine whether FABP4 acts as an adipokine mediating the worsening of systolic and diastolic functions or whether FABP4 is a surrogate marker of lipolysis and sympathetic nervous system activation related to systolic and diastolic dysfunctions (Figure 5 ). Thus, further research is warranted to explore the underlying mechanisms.
Prognostic impact of fatty acid‐binding protein 4 in heart failure with preserved ejection fraction
In this study, higher FABP4 levels were associated with an increased risk of adverse outcomes (all‐cause mortality or HF hospitalization) in HFpEF and remained independently associated with the outcomes in each multivariable model. These findings are consistent with those of recent two reports investigating multiple plasma markers for the risk stratification of HFpEF, in which FABP4 was a highly significant predictor of poor outcome. 31 , 32 In addition, our findings also support the conclusion drawn from a network analysis enriched by knowledge‐based interaction, which identified the inflammatory process as the pathway specifically related to HFpEF. 33 Nevertheless, further studies are required to determine whether FABP4 drives poor outcomes possibly through alterations in LV systolic and diastolic functions or is reflective of the severity of lipolysis induced by adrenergic hyperactivation in patients with HFpEF.
Clinical implications
No proven treatment to improve the outcomes in patients with HFpEF has been established. Phenotypic heterogeneity has been recognized as a primary cause of clinical trial failures in HFpEF 34 ; there is an unmet need to categorize different phenotypes within the broader spectrum of HFpEF into pathophysiologically homogenous groups. 35 Based on the data in our study, we propose that FABP4 could be a biomarker that represents an HFpEF phenotype of increased adipose tissue lipolysis. Further study is needed to examine whether circulating FABP4‐guided therapy could reduce adipose tissue lipolysis and thus improve the outcome in patients with HFpEF.
Limitations
This was a single‐centre study from a tertiary referral centre; thus, inherent flaws related to selection and referral bias exist. The sample size was small, which could bias the results. All participants were Japanese, and the obesity prevalence was much lower in our study population than in the Western population. Thus, our results should be confirmed in Western populations before further extrapolation. The control group was not normal as they were referred for coronary angiography and, thus, had a higher prevalence of CAD as well as systemic hypertension, which could bias the results. However, the fact that the control population was more diseased than a truly normal healthy control population only biases our data towards the null. In addition, CAD and hypertension are established risk factors for developing HFpEF syndrome; thus, our control population may be considered as Stage B HFpEF. We believe that the inclusion of CAD in the correlation analyses added insights into the continuous relationships between FABP4 and cardiac structure and function across the spectrum of the HFpEF syndrome. Moreover, we did not measure the markers of sympathetic nervous activation, such as circulating or urinary catecholamines. The limited number of events did not allow us to perform a single multivariable Cox hazard model, including multiple variables. Although we evaluated the robustness of FABP4 using multiple Cox proportional hazard models to avoid overfitting, the independent predictive value may be overestimated in such models. Thus, our results should be confirmed in a larger external cohort. Finally, a comparison of the relationship of FABP4 and LV structure and function between HFpEF and HFrEF would be interesting and may provide insights into the pathophysiological difference between the two HF phenotypes; however, our study focused on the pathophysiological significance of FABP4 in patients with HFpEF.
Conclusions
Patients with HFpEF exhibited elevated circulating FABP4 levels, and the magnitude of the increase was associated with cardiac remodelling, LV systolic and diastolic dysfunctions, and RV dysfunction. Higher FABP4 levels were also associated with poor clinical outcomes independent of established prognostic markers. Hence, FABP4 may serve as a potential biomarker in the complex pathophysiology of HFpEF.
Conflict of interest
M.O. is supported by research grants from the Fukuda Foundation for Medical Technology, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Nippon Shinyaku, and the Japanese Circulation Society.
Funding
This work was supported by the MSD Life Science Foundation, Public Interest Incorporated Foundation, and Japan Heart Foundation Research Grant (to H.S.).
Supporting information
Table S1. Comparisons in Demographics and Echocardiographic Indices between HFpEF Patients with Lower and Higher FABP4 Levels.
Figure S1. Supporting Information.
Acknowledgement
The authors thank Reiko Oda for the data collection.
Harada, T. , Sunaga, H. , Sorimachi, H. , Yoshida, K. , Kato, T. , Kurosawa, K. , Nagasaka, T. , Koitabashi, N. , Iso, T. , Kurabayashi, M. , and Obokata, M. (2020) Pathophysiological role of fatty acid‐binding protein 4 in Asian patients with heart failure and preserved ejection fraction. ESC Heart Failure, 7: 4256–4266. 10.1002/ehf2.13071.
References
- 1. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017; 14: 591–602. [DOI] [PubMed] [Google Scholar]
- 2. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 3. Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 2006; 55: 1537–1545. [DOI] [PubMed] [Google Scholar]
- 4. Hotamisligil GS, Bernlohr DA. Metabolic functions of FABPs—mechanisms and therapeutic implications. Nat Rev Endocrinol 2015; 11: 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu A, Wang Y, Xu JY, Stejskal D, Tam S, Zhang J, Wat NMS, Wong WK, Lam KSL. Adipocyte fatty acid‐binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem 2006; 52: 405–413. [DOI] [PubMed] [Google Scholar]
- 6. Tso AWK, Xu A, Sham PC, Wat NMS, Wang Y, Fong CHY, Cheung BMY, Janus ED, Lam KSL. Serum adipocyte fatty acid binding protein as a new biomarker predicting the development of type 2 diabetes: a 10‐year prospective study in a Chinese cohort. Diabetes Care 2007; 30: 2667–2672. [DOI] [PubMed] [Google Scholar]
- 7. Obokata M, Iso T, Ohyama Y, Sunaga H, Kawaguchi T, Matsui H, Iizuka T, Fukuda N, Takamatsu H, Koitabashi N, Funada R, Takama N, Kasama S, Kaneko Y, Yokoyama T, Murakami M, Kurabayashi M. Early increase in serum fatty acid binding protein 4 levels in patients with acute myocardial infarction. Eur Heart J Acute Cardiovasc Care 2018; 7: 561–569. [DOI] [PubMed] [Google Scholar]
- 8. Furuhashi M, Fuseya T, Murata M, Hoshina K, Ishimura S, Mita T, Watanabe Y, Omori A, Matsumoto M, Sugaya T, Oikawa T, Nishida J, Kokubu N, Tanaka M, Moniwa N, Yoshida H, Sawada N, Shimamoto K, Miura T. Local production of fatty acid‐binding protein 4 in epicardial/perivascular fat and macrophages is linked to coronary atherosclerosis. Arterioscler Thromb Vasc Biol 2016; 36: 825–834. [DOI] [PubMed] [Google Scholar]
- 9. Lamounier‐Zepter V, Look C, Alvarez J, Christ T, Ravens U, Schunck W‐H, Ehrhart‐Bornstein M, Bornstein SR, Morano I. Adipocyte fatty acid‐binding protein suppresses cardiomyocyte contraction: a new link between obesity and heart disease. Circ Res 2009; 105: 326–334. [DOI] [PubMed] [Google Scholar]
- 10. Rodríguez‐Calvo R, Girona J, Rodríguez M, Samino S, Barroso E, de Gonzalo‐Calvo D, Guaita‐Esteruelas S, Heras M, van der Meer RW, Lamb HJ, Yanes O, Correig X, Llorente‐Cortés V, Vázquez‐Carrera M, Masana L. Fatty acid binding protein 4 (FABP4) as a potential biomarker reflecting myocardial lipid storage in type 2 diabetes. Metabolism 2019; 96: 12–21. [DOI] [PubMed] [Google Scholar]
- 11. Aragonès G, Saavedra P, Heras M, Cabré A, Girona J, Masana L. Fatty acid‐binding protein 4 impairs the insulin‐dependent nitric oxide pathway in vascular endothelial cells. Cardiovasc Diabetol 2012; 11: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doehner W, Frenneaux M, Anker SD. Metabolic impairment in heart failure: the myocardial and systemic perspective. J Am Coll Cardiol 2014; 64: 1388–1400. [DOI] [PubMed] [Google Scholar]
- 13. Iso T, Sunaga H, Matsui H, Kasama S, Oshima N, Haruyama H, Furukawa N, Nakajima K, Machida T, Murakami M, Yokoyama T, Kurabayashi M. Serum levels of fatty acid binding protein 4 and fat metabolic markers in relation to catecholamines following exercise. Clin Biochem 2017; 50: 896–902. [DOI] [PubMed] [Google Scholar]
- 14. Szabó T, Postrach E, Mähler A, Kung T, Turhan G, von Haehling S, Anker SD, Boschmann M, Doehner W. Increased catabolic activity in adipose tissue of patients with chronic heart failure. Eur J Heart Fail 2013; 15: 1131–1137. [DOI] [PubMed] [Google Scholar]
- 15. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–271. [DOI] [PubMed] [Google Scholar]
- 16. Kasner M, Sinning D, Burkhoff D, Tschöpe C. Diastolic pressure–volume quotient (DPVQ) as a novel echocardiographic index for estimation of LV stiffness in HFpEF. Clin Res Cardiol 2015; 104: 955–963. [DOI] [PubMed] [Google Scholar]
- 17. Tromp J, Teng T‐H, Tay WT, Hung CL, Narasimhan C, Shimizu W, Park SW, Liew HB, Ngarmukos T, Reyes EB, Siswanto BB, Yu C‐M, Zhang S, Yap J, MacDonald M, Ling LH, Leineweber K, Richards AM, Zile MR, Anand IS, Lam CSP, ASIAN‐HF Investigators . Heart failure with preserved ejection fraction in Asia. Eur J Heart Fail 2019; 21: 23–36. [DOI] [PubMed] [Google Scholar]
- 18. Cao H, Sekiya M, Ertunc ME, Burak MF, Mayers JR, White A, Inouye K, Rickey LM, Ercal BC, Furuhashi M, Tuncman G, Hotamisligil GS. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab 2013; 17: 768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu M, Zhou M, Bao Y, Xu Z, Li H, Zhang H, Zhu W, Zhang J, Xu A, Wei M, Jia W. Circulating adipocyte fatty acid‐binding protein levels are independently associated with heart failure. Clin Sci (Lond) 2013; 124: 115–122. [DOI] [PubMed] [Google Scholar]
- 20. Lind L, Sundström J, Stenemo M, Hagström E, Ärnlöv J. Discovery of new biomarkers for atrial fibrillation using a custom‐made proteomics chip. Heart 2017; 103: 377–382. [DOI] [PubMed] [Google Scholar]
- 21. Shrestha S, Sunaga H, Hanaoka H, Yamaguchi A, Kuwahara S, Umbarawan Y, Nakajima K, Machida T, Murakami M, Saito A, Tsushima Y, Kurabayashi M, Iso T. Circulating FABP4 is eliminated by the kidney via glomerular filtration followed by megalin‐mediated reabsorption. Sci Rep 2018; 8: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arora R, Krummerman A, Vijayaraman P, Rosengarten M, Suryadevara V, Lejemtel T, Ferrick KJ. Heart rate variability and diastolic heart failure. Pacing Clin Electrophysiol 2004; 27: 299–303. [DOI] [PubMed] [Google Scholar]
- 23. Packer M. Derangements in adrenergic–adipokine signalling establish a neurohormonal basis for obesity‐related heart failure with a preserved ejection fraction. Eur J Heart Fail 2018; 20: 873–878. [DOI] [PubMed] [Google Scholar]
- 24. Obokata M, Reddy YNV, Borlaug BA. Diastolic dysfunction and heart failure with preserved ejection fraction: understanding mechanisms by using noninvasive methods. JACC Cardiovasc Imaging 2020; 13: 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fuseya T, Furuhashi M, Yuda S, Muranaka A, Kawamukai M, Mita T, Ishimura S, Watanabe Y, Hoshina K, Tanaka M, Ohno K, Akasaka H, Ohnishi H, Yoshida H, Saitoh S, Shimamoto K, Miura T. Elevation of circulating fatty acid‐binding protein 4 is independently associated with left ventricular diastolic dysfunction in a general population. Cardiovasc Diabetol 2014; 13: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baessler A, Lamounier‐Zepter V, Fenk S, Strack C, Lahmann C, Loew T, Schmitz G, Blüher M, Bornstein SR, Fischer M. Adipocyte fatty acid‐binding protein levels are associated with left ventricular diastolic dysfunction in morbidly obese subjects. Nutr Diabetes 2014; 4: e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kraigher‐Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, McMurray JJV, Solomon SD. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014; 63: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation 2015; 132: 402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cabré A, Valdovinos P, Lázaro I, Bonet G, Bardají A, Masana L. Parallel evolution of circulating FABP4 and NT‐proBNP in heart failure patients. Cardiovasc Diabetol 2013; 12: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Djoussé L, Bartz TM, Ix JH, Kochar J, Kizer JR, Gottdiener JS, Tracy RP, Mozaffarian D, Siscovick DS, Mukamal KJ, Zieman SJ. Fatty acid‐binding protein 4 and incident heart failure: the Cardiovascular Health Study. Eur J Heart Fail 2013; 15: 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hage C, Michaëlsson E, Linde C, Donal E, Daubert J‐C, Gan L‐M, Lund LH. Inflammatory biomarkers predict heart failure severity and prognosis in patients with heart failure with preserved ejection fraction: a holistic proteomic approach. Circ Cardiovasc Genet 2017; 10: e001633. [DOI] [PubMed] [Google Scholar]
- 32. Chirinos JA, Orlenko A, Zhao L, Basso MD, Cvijic ME, Li Z, Spires TE, Yarde M, Wang Z, Seiffert DA, Prenner S, Zamani P, Bhattacharya P, Kumar A, Margulies KB, Car BD, Gordon DA, Moore JH, Cappola TP. Multiple plasma biomarkers for risk stratification in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2020; 75: 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tromp J, Westenbrink BD, Ouwerkerk W, van Veldhuisen DJ, Samani NJ, Ponikowski P, Metra M, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Lang CC, Ng LL, Zannad F, Zwinderman AH, van der Hillege HL, Meer P, Voors AA. Identifying pathophysiological mechanisms in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2018; 72: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 34. Shah SJ, Kitzman DW, Borlaug BA, Van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype‐specific treatment of heart failure with preserved ejection fraction. Circulation 2016; 134: 73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017; 136: 6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparisons in Demographics and Echocardiographic Indices between HFpEF Patients with Lower and Higher FABP4 Levels.
Figure S1. Supporting Information.


