Abstract
Aims
The EMPA‐REG OUTCOME trial demonstrated reductions in cardiovascular (CV) death and heart failure (HF) outcomes with empagliflozin, a sodium–glucose co‐transporter 2 inhibitor, in patients with type 2 diabetes and established CV disease over a study period of 3 years. We aimed to investigate the early benefit–risk profile of empagliflozin in patients enrolled in the EMPA‐REG OUTCOME trial according to HF status at baseline.
Methods and results
The effects of treatments on glycated haemoglobin, systolic blood pressure and body weight, and on the HF endpoints of hospitalization for HF (HHF), HHF or CV death, and HHF or all‐cause mortality were evaluated at 12 weeks, 6 months, and 1 year after randomization. Occurrence of adverse events (AEs) during these time points was also evaluated. Compared with placebo, empagliflozin lowered glycated haemoglobin, systolic blood pressure, and body weight and rates of all the HF endpoints, as early as at 12 weeks, regardless of HF status at baseline. Favourable clinical and metabolic effects were maintained over time. AEs were generally higher in those with HF than without HF; however, compared with placebo, empagliflozin did not increase risk of developing AEs over the first year of treatment.
Conclusions
In the EMPA‐REG OUTCOME trial, the use of empagliflozin led to early and beneficial effects on clinical, metabolic, and HF outcomes in patients with type 2 diabetes with or without HF at baseline, which were already apparent within 12 weeks from initiation of treatment. Over the first year of treatment, no safety concern was detected with the use of empagliflozin.
Keywords: Empagliflozin, EMPA‐REG OUTCOME, Trial, Heart failure, Diabetes
Background
Prior to advent of sodium–glucose co‐transporter 2 inhibitors (SGLT2is), many interventions that improve metabolic markers of dysglycaemia did not convincingly reduce the risk of common complications of type 2 diabetes (T2D) such as macrovascular events or an early death, over several years of treatment. 1 , 2 Some, such as glitazones, might have actually caused harm, increasing risk of fluid retention and worsening heart failure (HF). 3 The EMPA‐REG OUTCOME trial showed that an SGLT2i, empagliflozin, reduced the risk of major cardiovascular (CV) events by 14%, CV mortality by 38%, and hospitalization for HF (HHF) by 35% in patients with T2D and established CV disease. 4 These effects were evident early after treatment initiation and were consistent in those with and without HF. 5 Subsequent trials suggested that other SGLT2is, such as canagliflozin and dapagliflozin, also reduce HHF 6 , 7 in patients with T2D with established CV disease or with multiple CV risk factors. It is unclear if the rapid and beneficial effects observed with the use of empagliflozin are counterbalanced by an increased risk of adverse events, particularly in the more vulnerable HF population.
Aims
We investigated the early benefits on clinical, metabolic, and HF outcomes, as well as safety, associated with the use of empagliflozin in patients with and without HF at baseline enrolled in the EMPA‐REG OUTCOME trial.
Methods
This is a post hoc analysis of the EMPA‐REG OUTCOME trial. Briefly, the trial enrolled 7020 participants with T2D and established CV disease, of whom 706 (10%) had an investigator‐reported history of HF at baseline based on the narrow standardized Medical Dictionary for Regulatory Activities query (SMQ) ‘cardiac failure’ (as defined in Table 1 ). Patients were randomized in a 1:1:1 ratio to once‐daily empagliflozin (at a dose of either 10 or 25 mg) or placebo and followed for a median of 3.1 years. Detailed inclusion and exclusion criteria and results of primary and secondary outcomes can be found elsewhere. 4 In the current study, we evaluated the effects of treatments (pooled empagliflozin arms vs. placebo) on a broad range of outcomes of interest, including time to first HF outcomes (HHF, HHF or CV death, and HHF or all‐cause mortality), metabolic [glycated haemoglobin (HbA1c)] or clinical outcomes [systolic blood pressure (SBP) and body weight], and the occurrence of adverse events, at 12 weeks, 6 months, and 1 year after randomization in people with or without HF at baseline. HF outcome data were explored descriptively at 12 weeks and assessed by Cox regression models at 6 months and 1 year, whereas safety data were explored descriptively. The Cox model included the interaction of presence of HF at baseline by treatment to evaluate the treatment effect in patients with and without HF at baseline separately. The model further included covariate terms for age, gender, body mass index, HbA1c, estimated glomerular filtration rate, and geographical region. The effects on the clinical and metabolic outcomes (HbA1c, SBP, and body weight) were evaluated using a mixed effect model repeat measurement model, which included the baseline of the endpoint (for SBP or body weight) and baseline HbA1c as linear covariates and their interaction with visit, estimated glomerular filtration rate category, geographical region, baseline body mass index category, and the last week the patient could have had a measurement of the endpoint and treatment by HF at baseline by visit interaction as fixed effects. All P‐values constitute exploratory analyses and are reported without adjustment for multiplicity. Statistical analyses were performed using SAS® Version 9.4.
Table 1.
Summary of key baseline characteristics
| Heart failure at baseline a (N = 706) | No heart failure at baseline (N = 6314) | P‐value | |
|---|---|---|---|
| Age, mean (SD), years | 64.5 (8.8) | 63.0 (8.6) | <0.01 |
| Male, n (%) | 495 (70.1) | 4521 (71.6) | 0.41 |
| Weight, mean (SD), kg | 91.3 (19.4) | 85.8 (18.8) | <0.01 |
| BMI, mean (SD), kg/m2 | 32.1 (5.5) | 30.5 (5.2) | <0.01 |
| HbA1c, mean (SD), % | 8.07 (0.86) | 8.07 (0.85) | 0.96 |
| SBP, mean (SD), mmHg | 134 (18) | 136 (17) | 0.02 |
| DBP, mean (SD), mmHg | 77 (10) | 77 (10) | 0.61 |
| eGFR, mean (SD), mL/min/1.73 m2 | 68.7 (20.4) | 74.6 (21.4) | <0.01 |
| Therapy | |||
| Metformin | 446 (63.2) | 4747 (75.2) | <0.01 |
| Insulin | 394 (55.8) | 2993 (47.4) | <0.01 |
| Sulphonylurea | 266 (37.7) | 2740 (43.4) | <0.01 |
| Dipeptidyl peptidase‐4 inhibitor | 68 (9.6) | 728 (11.5) | 0.13 |
| Thiazolidinedione | 14 (2.0) | 285 (4.5) | <0.01 |
| Glucagon‐like peptide‐1 agonist | 23 (3.3) | 173 (2.7) | 0.43 |
| ACE‐I/ARB | 612 (86.7) | 5054 (80.0) | <0.01 |
| Beta‐blockers | 559 (79.2) | 3995 (63.3) | <0.01 |
| MRA | 169 (23.9) | 272 (4.3) | <0.01 |
| Diuretics | 506 (71.7) | 2529 (40.1) | <0.01 |
| Loop diuretics | 334 (47.3) | 755 (12.0) | <0.01 |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; MRA, mineralocorticoid receptor antagonist; SBP, systolic blood pressure; SD, standard deviation.
Based on narrow standardized Medical Dictionary for Regulatory Activities query (SMQ) ‘cardiac failure’, which comprised these preferred terms: acute pulmonary oedema; cardiac failure; cardiac failure, acute; cardiac failure, chronic; cardiac failure, congestive; cardiogenic shock; cardiopulmonary failure; left ventricular failure; pulmonary oedema; and right ventricular failure.
Results
Baseline characteristics of the study population have been published previously, 4 , 5 and are summarized in Table 1 . Compared with patients without HF, those with HF were older [mean age (standard deviation): 64.5 (8.8) vs. 63.0 (8.6) years, P < 0.01], had greater body weight [91.3 (19.4) vs. 85.8 (18.8) kg, P < 0.01] and body mass index [32.1 (5.5) vs. 30.5 (5.2) kg/m2, P < 0.01], and had lower systolic blood pressure [(134 (18) vs 136 (17), P = 0.02), but similar HbA1c [8.07 (0.86) vs. 8.07 (0.85) %, P = 0.96].
In patients with HF at baseline, the adjusted mean differences (95% confidence interval) in HbA1c change from baseline between those randomized to empagliflozin or placebo at 12 weeks, 6 months, and 1 year after randomization were −0.55 (−0.67, −0.44), −0.54 (−0.68, −0.40), and −0.53 (−0.68, −0.38) %, respectively (P < 0.0001 for all), with similar results in those without HF [−0.57 (−0.60, −0.53), −0.53 (−0.58, −0.49), and −0.48 (−0.53, −0.43) %, respectively (P < 0.0001 for all); P for interaction for HF vs. no HF 0.82, 0.94, and 0.54 at 12 weeks, 6 months, and 1 year, respectively]. Compared with placebo, empagliflozin lowered SBP [adjusted mean differences in the change in SBP from baseline at 12 weeks, 6 months, and 1 year were −2.43 (−4.53, −0.33) (P = 0.023), −2.32 (−4.50, −0.14) (P = 0.037), and −2.51 (−4.76, −0.26) (P = 0.029) mmHg, respectively, in those with HF and −4.03 (−4.74, −3.32), −4.58 (−5.31, −3.84), and −3.48 (−4.23, −2.72) mmHg, respectively (P < 0.0001 for all), in those without HF; P for interaction 0.16, 0.05, and 0.42 at 12 weeks, 6 months, and 1 year, respectively] and body weight at 12 weeks, 6 months, and 1 year, regardless of HF status [adjusted mean differences in changes in body weight: −1.01 (−1.41, −0.60), −1.67 (−2.22, −1.12), and −1.96 (−2.61, −1.30) kg, respectively (P < 0.0001 for all), in those with HF and −1.39 (−1.53, −1.25), −1.93 (−2.12, −1.75), and −1.94 (−2.16, −1.72) kg, respectively (P < 0.0001 for all), in those without HF; P for interaction for HF vs. no HF 0.08, 0.37, and 0.96 at 12 weeks, 6 months, and 1 year, respectively] (Figure 1 ).
Figure 1.

Effects on glycated haemoglobin (HbA1c; top panel), systolic blood pressure (SBP; middle panel), and body weight (lower panel) with empagliflozin vs. placebo during first year of treatment in patients with (on the left) or without (on the right) heart failure. Error bars represent 95% confidence intervals.
As early as 12 weeks after randomization, and compared with those taking placebo, patients treated with empagliflozin had a lower rate of HHF [0 (0%) vs. 7 (2.9%) and 5 (0.1%) vs. 3 (0.1%) amongst those with and without HF, respectively], HHF or CV death [1 (0.2%) vs. 10 (4.1%) and 9 (0.2%) vs. 8 (0.4%) in patients with and without HF, respectively], and HHF or all‐cause mortality [1 (0.2%) vs. 10 (4.1%) and 13 (0.3%) vs. 12 (0.6%) amongst those with and without HF]. Beneficial effects on HF outcomes were also observed in the modelled analyses after 6 months and 1 year in patients with and without HF (Figure 2 ).
Figure 2.
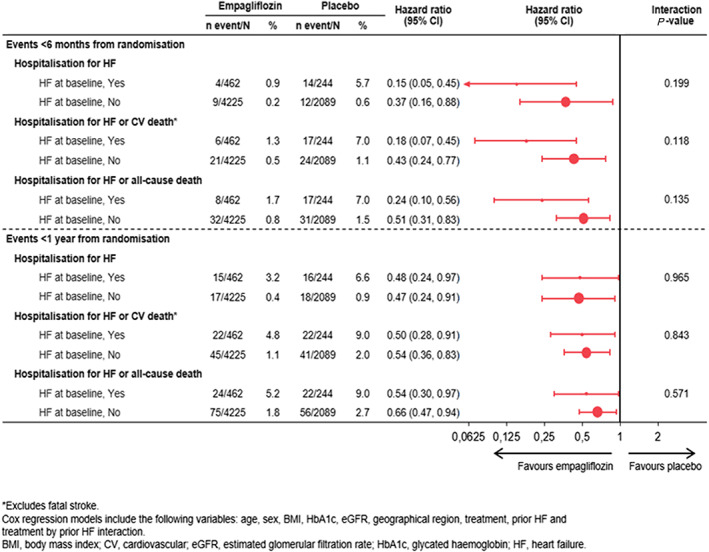
Effects on heart failure (HF) outcomes with empagliflozin vs. placebo at 6 months and 1 year by HF status at baseline. CI, confidence interval; CV, cardiovascular.
During both 6 month and 1 year time periods, the overall rates of adverse events and serious adverse events were numerically higher in those with HF than without HF, particularly in the placebo arm (Table 2 ). At 1 year, there were no differences in hyperkalaemia, volume depletion, or hypotension between the empagliflozin and placebo treatment arms for patients with and without HF (Table 2 ).
Table 2.
AEs of special interest occurring within 6 months and 1 year after randomization
| <6 months | <1 year | |||||||
|---|---|---|---|---|---|---|---|---|
| Patients with heart failure at baseline | Patients without heart failure at baseline | Patients with heart failure at baseline | Patients without heart failure at baseline | |||||
| Placebo (N = 244) | Empagliflozin (N = 462) | Placebo (N = 2089) | Empagliflozin (N = 4225) | Placebo (N = 244) | Empagliflozin (N = 462) | Placebo (N = 2089) | Empagliflozin (N = 4225) | |
| Any AE, n (%) | 176 (72.1) | 296 (64.1) | 1400 (67.0) | 2700 (63.9) | 206 (84.4) | 363 (78.6) | 1694 (81.1) | 3246 (76.8) |
| Any serious AE, n (%) | 51 (20.9) | 53 (11.5) | 268 (12.8) | 427 (10.1) | 79 (32.4) | 105 (22.7) | 447 (21.4) | 764 (18.1) |
| Hypoglycaemia a , n (%) | 33 (13.5) | 60 (13.0) | 301 (14.4) | 725 (17.2) | 50 (20.5) | 76 (16.5) | 388 (18.6) | 915 (21.7) |
| Hypoglycaemia requiring assistance (%) | 0 (0) | 1 (0.2) | 8 (0.4) | 17 (0.4) | 0 (0) | 3 (0.6) | 13 (0.6) | 27 (0.6) |
| Acute kidney injury b , n (%) | 1 (0.4) | 1 (0.2) | 5 (0.2) | 6 (0.1) | 1 (0.4) | 2 (0.4) | 7 (0.3) | 12 (0.3) |
| Hyperkalaemia c , n (%) | 1 (0.4) | 3 (0.6) | 11 (0.5) | 13 (0.3) | 3 (1.2) | 4 (0.9) | 23 (1.1) | 23 (0.5) |
| Volume depletion d , n (%) | 3 (1.2) | 11 (2.4) | 32 (1.5) | 73 (1.7) | 10 (4.1) | 20 (4.3) | 47 (2.2) | 111 (2.6) |
| Hypotension e , n (%) | 3 (1.2) | 8 (1.7) | 28 (1.3) | 51 (1.2) | 7 (2.9) | 14 (3.0) | 37 (1.8) | 77 (1.8) |
AEs, adverse events.
The Medical Dictionary for Regulatory Activities Version 18.0 was used to classify AEs by preferred terms.
Hypoglycaemia defined as any hypoglycaemic event that had a glucose value ≤70 mg/dL or where assistance was required.
Based on reported AEs of the preferred term in the Medical Dictionary for Regulatory Activities ‘acute kidney injury’.
Based on the Medical Dictionary for Regulatory Activities preferred terms ‘hyperkalaemia’ and ‘blood potassium increased’.
Based on eight Medical Dictionary for Regulatory Activities preferred terms ‘blood pressure (BP) ambulatory decreased’, ‘BP decreased’, ‘BP systolic decreased’, ‘dehydration’, ‘hypotension’, ‘hypovolaemia’, ‘orthostatic hypotension’, and ‘syncope’.
Based on 10 Medical Dictionary for Regulatory Activities preferred terms ‘blood pressure (BP) ambulatory decreased’, ‘BP decreased’, ‘BP systolic decreased’, ‘BP diastolic decreased’, ‘BP orthostatic decreased’, ‘diastolic hypotension’, ‘hypotension’, ‘mean arterial pressure decreased’, ‘orthostatic hypotension’, and ‘orthostatic intolerance’.
Conclusions
In the EMPA‐REG OUTCOME trial, treatment with empagliflozin led to favourable clinical and metabolic effects and decreased rates of HF events, in patients with T2D with or without HF at baseline. These beneficial effects occurred as early as 12 weeks after initiation of treatment. The use of empagliflozin was not associated with an increased risk of adverse events compared with placebo during first year of treatment.
The mechanisms of the CV benefits associated with empagliflozin are likely to be multifactorial and largely independent from an improved glucose control. Previous post hoc analyses of the EMPA‐REG OUTCOME trial suggested that CV benefits of empagliflozin were consistent in all patients, regardless of baseline HbA1c or the magnitude of its change after 12 weeks of treatment 8 ; conversely, markers of plasma volume, such as haematocrit and haemoglobin, were the most important mediators of the reduction in the risk of CV death. 9 Therefore, reduction in cardiac preload and afterload, caused by an osmotic diuresis, may be one of the key mechanisms underlying the rapid reduction in SBP, body weight, and subsequent risk of HF outcomes. As these effects were also observed in patients without HF, it is possible that many individuals with T2D and established CV disease have asymptomatic, or undiagnosed, cardiac dysfunction and/or preclinical congestion, which might lead to a greater CV risk. 10 However, a reduction in left ventricular mass, 11 or an increase in erythropoietin levels and erythropoiesis, 12 might also explain favourable clinical effects associated with empagliflozin.
Other hypotheses have been postulated to explain through which mechanisms empagliflozin exerts its effects and include a more efficient cardiac metabolism 13 and prevention of cardiac fibrosis. 14 In animal models, empagliflozin also prevented cardiac cytosolic calcium and sodium accumulation via inhibition of the sodium–hydrogen exchanger, processes that could contribute to T2D and development of HF, and improved mitochondrial function. 15
Recently, McMurray and colleagues reported that an SGLT2i, dapagliflozin, was superior to placebo in reducing risk of CV death and worsening HF in patients with HF and reduced left ventricular ejection fraction (HFrEF), regardless of T2D, 16 without increasing the frequency of adverse events related to volume depletion, renal dysfunction, and hypoglycaemia. This further supports the hypothesis that control of glycaemia is not the key mechanism by which SGLT2is exert their beneficial effects in people with T2D at high CV risk.
Patients with HF are more vulnerable to developing adverse events, and it is therefore reassuring that the overall frequency of adverse events and serious adverse events early after treatment initiation was numerically lower in the empagliflozin arm. We observed no increase in acute kidney injury in those with HF as compared with those without HF treated in the empagliflozin arm. This is supported by another recent sub‐analysis of EMPA‐REG OUTCOME focusing on renal outcomes, which showed that empagliflozin reduced the risk of incident or worsening nephropathy in those with and without HF during the entire length of the study. 17
The current analysis has some limitations. Firstly, this analysis was developed post hoc. Secondly, levels of circulating natriuretic peptides or left ventricular ejection fraction were not captured at baseline, and we could not differentiate amongst different HF phenotypes. Furthermore, as expected for the population enrolled in EMPA‐REG OUTCOME trial, rates of HF events were low during the first year of follow‐up. Lastly, as in most clinical trials, adverse events were not adjudicated but coded via Medical Dictionary for Regulatory Activities terms.
Large ongoing trials of empagliflozin [the EMPEROR Program (NCT03057951 and NCT03057977) and the EMPERIAL Trials (NCT03448406 and NCT03448419)], 18 , 19 along with more detailed analyses from DAPA‐HF trial, 16 will provide additional evidence for the effect and safety of SGLT2is in patients with HF and either reduced or preserved ejection fraction, regardless of T2D, and clarify further their complex mechanisms of action.
Conflict of interest
P.P. has received travel support from Boehringer Ingelheim. D.F. has received honoraria from Sanofi, Merck & Co., Amgen, AstraZeneca, Eli Lilly and Company, and Boehringer Ingelheim. C.W. has received honoraria for consultancy and lecturing from Abbvie, Actelion, Amgen, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Janssen, Eli Lilly and Company, Protalix, Sanofi Genzyme, and Shire. B.Z. has received research grants awarded to his institution from Boehringer Ingelheim and Novo Nordisk and honoraria from Janssen, Sanofi, Eli Lilly and Company, Boehringer Ingelheim, Novo Nordisk, and Merck Sharp & Dohme. J.L. has received consultancy fees from Abbott, AstraZeneca, Boehringer Ingelheim, CVRx, Novartis, Relypsa, Impulse Dynamics, V‐Wave Medical, and Edwards Lifesciences and grants from AstraZeneca, Sensible Medical, Volumetrix, and the National Institutes of Health. A.P.O., C.Z., J.G., and M.B. are employees of Boehringer Ingelheim.
Pellicori, P. , Ofstad, A. P. , Fitchett, D. , Zeller, C. , Wanner, C. , George, J. , Zinman, B. , Brueckmann, M. , and Lindenfeld, J. (2020) Early benefits of empagliflozin in patients with or without heart failure: findings from EMPA‐REG OUTCOME. ESC Heart Failure, 7: 3401–3407. 10.1002/ehf2.12891.
References
- 1. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 2. Zoungas S, Chalmers J, Neal B, Billot L, Li Q, Hirakawa Y, Arima H, Monaghan H, Joshi R, Colagiuri S, Cooper ME, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Lisheng L, Mancia G, Marre M, Matthews DR, Mogensen CE, Perkovic V, Poulter N, Rodgers A, Williams B, MacMahon S, Patel A, Woodward M, the ADVANCE‐ON Collaborative Group . Follow‐up of blood‐pressure lowering and glucose control in type 2 diabetes. N Engl J Med 2014; 371: 1392–1406. [DOI] [PubMed] [Google Scholar]
- 3. Hernandez AV, Usmani A, Rajamanickam A, Moheet A. Thiazolidinediones and risk of heart failure in patients with or at high risk of type 2 diabetes mellitus: a meta‐analysis and meta‐regression analysis of placebo‐controlled randomized clinical trials. Am J Cardiovasc Drugs 2011; 11: 115–128. [DOI] [PubMed] [Google Scholar]
- 4. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, EMPA‐REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 5. Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, EMPA‐REG OUTCOME® trial investigators . Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA‐REG OUTCOME® trial. Eur Heart J 2016; 37: 1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rådholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, de Zeeuw D, Fulcher G, Barrett TD, Shaw W, Desai M, Matthews DR, Neal B. Canagliflozin and heart failure in type 2 diabetes mellitus. Circulation 2018; 138: 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Bonaca MP, Ruff CT, Desai AS, Goto S, Johansson PA, Gause‐Nilsson I, Johanson P, Langkilde AM, Raz I, Sabatine MS, Wiviott SD. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 2019; 139: 2528–2536. [DOI] [PubMed] [Google Scholar]
- 8. Inzucchi SE, Kosiborod M, Fitchett D, Wanner C, Hehnke U, Kaspers S, George JT, Zinman B. Improvement in cardiovascular outcomes with empagliflozin is independent of glycemic control. Circulation 2018; 138: 1904–1907. [DOI] [PubMed] [Google Scholar]
- 9. Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, Schmoor C, Ohneberg K, Johansen OE, George JT, Hantel S, Bluhmki E, Lachin JM. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA‐REG OUTCOME trial. Diabetes Care 2018; 41: 356–363. [DOI] [PubMed] [Google Scholar]
- 10. Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez‐Eronini AA, Pollack PS, Hirshberg B, Frederich R, Lewis BS, McGuire DK, Davidson J, Steg PG, Bhatt DL, for the SAVOR‐TIMI 53 Steering Committee and Investigators . Heart failure, saxagliptin and diabetes mellitus: observations from the SAVOR‐TIMI 53 randomized trial. Circulation 2014; 130: 1579–1588. [DOI] [PubMed] [Google Scholar]
- 11. Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, Zuo F, Quan A, Farkouh ME, Fitchett DH, Goodman SG, Goldenberg RM, Al‐Omran M, Gilbert RE, Bhatt DL, Leiter LA, Jüni P, Zinman B, Connelly KA. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA‐HEART CardioLink‐6 randomized clinical trial. Circulation 2019; 140: 1693–1702. [DOI] [PubMed] [Google Scholar]
- 12. Mazer CD, Hare GMT, Connelly PW, Gilbert RE, Shehata N, Quan A, Teoh H, Leiter LA, Zinman B, Jüni P, Zuo F, Mistry N, Thorpe KE, Goldenberg RM, Yan AT, Connelly KA, Verma S. Effect of empagliflozin on erythropoietin levels, iron stores and red blood cell morphology in patients with type 2 diabetes and coronary artery disease. Circulation 2019; 141: 704–707. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13. Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA‐REG OUTCOME study? A unifying hypothesis. Diabetes Care 2016; 39: 1115–1122. [DOI] [PubMed] [Google Scholar]
- 14. Lee HC, Shiou YL, Jhuo SJ, Chang CY, Liu PL, Jhuang WJ, Dai ZK, Chen WY, Chen YF, Lee AS. The sodium–glucose co‐transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats. Cardiovasc Diabetol 2019; 18: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, Jancev M, Hollmann MW, Weber NC, Coronel R, Zuurbier CJ. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 2018; 61: 722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM, DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 17. Butler J, Zannad F, Fitchett D, Zinman B, Koitka‐Weber A, von Eynatten M, Zwiener I, George J, Brueckmann M, Cheung AK, Wanner C. Empagliflozin improves kidney outcomes in patients with or without heart failure. Circ Heart Fail 2019; 12: e005875. [DOI] [PubMed] [Google Scholar]
- 18. Abraham WT, Ponikowski P, Brueckmann M, Zeller C, Macesic H, Peil B, Brun M, Ustyugova A, Jamal W, Salsali A, Lindenfeld J, Anker SD, EMPERIAL Investigators and National Coordinators . Rationale and design of the EMPERIAL‐Preserved and EMPERIAL‐Reduced trials of empagliflozin in patients with chronic heart failure. Eur J Heart Fail 2019; 21: 932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Packer M, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Anker SD, Zannad F, EMPEROR‐Reduced Trial Committees and Investigators . Evaluation of the effect of sodium–glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR‐Reduced trial. Eur J Heart Fail 2019; 21: 1270–1278. [DOI] [PubMed] [Google Scholar]


