Abstract
Aims
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is associated with a high risk of sudden cardiac death. Three different prediction models for the indication of implanted cardioverter defibrillator (ICD) are now available: the 5 year ARVC risk score, the International Task Force Consensus (ITFC) criteria, and the Heart Rhythm Society (HRS) criteria. We compared these three prediction models in a validation cohort of patients with definite ARVC.
Methods and results
In a cohort of 140 patients with definite ARVC, the 5 year ARVC risk score and the ITFC and HRS criteria were compared for the prediction of a major combined endpoint of sudden cardiac death, appropriate ICD intervention, resuscitated cardiac arrest, and sustained ventricular tachycardia. During the follow‐up, 65 major events occurred. The 5 year ARVC risk score with a threshold >10%, derived from the maximally selected rank statistic, predicted 62 (95%) events [odds ratio (OR) 9.1, 95% confidence interval (CI) 2.6–32, P = 0.0006], the ITFC criteria 53 (81%, OR 4.8, 95% CI 2.2–10.3, P = 0.0001), and the HRS criteria 29 (45%, OR 4.2, 95% CI 1.9–9.3, P = 0.0003). At the analysis of decision curve for ICD implantation, a 5 year ARVC risk score >10% showed a greater net benefit than the ITFC and HRS criteria over a wide range of threshold probability of events. Finally, at multivariate analysis, the 5 year ARVC risk score >10% was the only independent predictor of major events.
Conclusions
The 5 year score with a threshold of >10% was more effective for predicting events than the ITFC and HRS criteria.
Keywords: Arrhythmogenic cardiomyopathy, International Task Force Consensus, 5 year ARVC risk score, Heart Rhythm Society criteria, Prognosis
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is characterized by a broad spectrum of clinical presentation ranging from an asymptomatic presentation to heart failure, 1 , 2 but the most important risk associated with this cardiomyopathy is sudden cardiac death (SCD), which is sometimes the first clinical presentation. Early diagnosis and prognostic stratification are great challenges in ARVC. 3 Three different prediction models of SCD or malignant ventricular arrhythmias are now available. In 2015, the International Task Force Consensus proposed a simple algorithm (ITFC criteria) to stratify the risk of SCD in patients with definite ARVC. 4
In 2019, a novel 5 year ARVC risk score model for the estimation of 5 year risk of arrhythmic events in ARVC was recently proposed by Cadrin‐Tourigny et al. 5 from a registry of 18 institutions of Europe and the USA.
More recently, a consensus document of the Heart Rhythm Society (HRS) and collaborating organizations produced new criteria (HRS criteria) for the indication of implanted cardioverter defibrillator (ICD) in a broad spectrum of arrhythmogenic cardiomyopathy including ARVC. 6
The aim of the present study was to evaluate the effectiveness of these three prediction models (ITFC criteria, 5 year ARVC risk score, and the HRS criteria) for primary prevention of the major combined endpoint of SCD and its surrogates by using an external validation cohort of consecutive patients with definite diagnosis of ARVC.
Methods
The three risk models were retrospectively evaluated considering baseline characteristics of the population of the PROAC prospective registry. 7 In this multicentre registry, 150 consecutive patients with definite ARVC were prospectively enrolled in six different institutions to evaluate the prognostic role of cardiac magnetic resonance (CMR) presentation in patients with definite ARVC. The diagnosis of definite ARVC was based on revised Task Force criteria 8 : patients had a ‘definite’ diagnosis when fulfilling two or one major and two or four minor criteria from different categories. We excluded all patients with contraindications to CMR: glomerular filtration rate <30 mL/min, CMR incompatible devices, and claustrophobia. The study was approved by the institutional review board of each institution participating in the registry.
Genetic analysis was performed using next‐generation sequencing or direct sequencing of candidate genes recognized as associated with arrhythmogenic cardiomyopathy, depending on availability in the single centre. In all cases, mutations of desmosomal genes [plakophilin‐2 (PKP2); desmoplakin (DSP); plakoglobin (JUP); desmoglein‐2 (DSG2); and desmocollin‐2 (DSC2)] were systematically assessed according to current guidelines, 9 and pathogenic and/or likely pathogenic variants are called ‘mutations’ throughout the manuscript.
Cardiac magnetic resonance was performed in all the patients as previously reported. 7 , 10 The following parameters of right ventricular (RV) involvement were considered: (i) regional wall motion abnormalities (akinesia or dyskinesia), (ii) RV end‐diastolic volume to body surface area ≥110 mL/m2 in men or ≥100 mL/m2 in women, or (iii) RV ejection fraction ≤45%.
International Task Force Consensus risk evaluation
The risk of SCD was also evaluated as suggested by the ITFC statement algorithm 4 in which ICD implantation for primary prevention is indicated by the presence of ≥1 of the following criteria: (i) non‐sustained ventricular tachycardia (NSVT), (ii) cardiogenic syncope, and (iii) moderate‐to‐severe ventricular dysfunction of the right ventricle and/or left ventricle.
Arrhythmogenic right ventricular cardiomyopathy risk score assessment
The ARVC risk score was recently proposed to estimate the risk of malignant ventricular arrhythmias in patients with definite ARVC. 5 Data from the PROAC study population were retrospectively used to calculate the 5 year risk score. The prediction model was calculated using the following formula:
where S 0(t) is the baseline survival probability at time t, which is 0.801 when t = 5 years (the 5 year risk = 1 − 0.802exp(A)), where A was calculated according the equation:
Dichotomic parameters, such as sex, syncope (defined as transient loss of consciousness and postural tone with spontaneous recovery with a likely arrhythmic mechanism, excluding vasovagal aetiology), and NSVT (defined as haemodynamically stable VT at ≥100 b.p.m., for ≥3 beats <30 s) were considered as 1 = positive and 0 = negative (Table 1 ).
Table 1.
Prediction models for predicting malignant ventricular arrhythmias
| Prediction model/criteria | First author, reference | Model/criteria for primary prevention |
|---|---|---|
| International Task Force Consensus (ITFC) criteria | Corrado 4 | ICD indication for the presence of ≥1 of the following criteria: NSVT, cardiogenic syncope, and moderate‐to‐severe ventricular dysfunction (of RV and/or LV) |
| 5 year ARVC risk score | Cadrin‐Tourigny 5 | 5 year risk is calculated as 1 − 0.802exp(A), where A = Male sex × 0.49 + age × −0.022 + recent syncope × 0.66 + NSVT × 0.81 + Ln(24 h PVC count) × 0.17 + number of leads with inverted T wave at basal ECG × 0.11 + RVEF × −0.025 |
| Heart Rhythm Society (HRS) criteria a | Towbin 6 | ICD indication for the presence of ≥1 of the following criteria: (i) haemodynamically tolerated sustained VT or syncope suspected due to ventricular arrhythmia; (ii) LVEF < 35%; and (iii) the presence of two or one major and two or four minor of the following criteria: major criteria were NSVT, inducibility to VT at EPS, and LVEF ≤ 49%; minor criteria were male sex, >1000 PVCs per 24 h, right ventricular dysfunction (as for major criteria of the 2010 Task Force Criteria), proband status, and two or more desmosomal variants. If both NSVT and PVC criteria are present, then only NSVT can be used |
HRS algorithm was adapted for ARVC (genetic assessment for phospholamban, FLNC, or lamin A/C mutation was not performed).
LV, left ventricle; LVEF, left ventricular ejection fraction; NSVT, non‐sustained ventricular tachycardia; PVC, premature ventricular contraction; RV, right ventricle; RVEF, right ventricular ejection fraction; VT, ventricular tachycardia. ARVC, arrhythmogenic right ventricular cardiomyopathy; ECG, electrocardiogram; EF, ejection fraction; EPS, electrophysiological study;
Heart Rhythm Society criteria
The HRS criteria for indication of ICD implantation in primary prevention were checked in our population with the exclusion of genetic criteria (phospholamban, FLNC mutation, or lamin A/C) that were absent in our cohort of definite ARVC. 6 Briefly, HRS criteria were satisfied in the presence of at least one of these criteria: (i) haemodynamically tolerated sustained VT (sVT) or syncope suspected due to ventricular arrhythmia; (ii) left ventricular (LV) ejection fraction <35%; and (iii) the presence of two or one major and two or four minor of the following criteria: major criteria were (a) NSVT, (b) inducibility to VT during electrophysiological study, and (c) LV ejection fraction ≤49%; minor criteria were (a) male sex, (b) >1000 premature ventricular contractions per 24 h, (c) RV dysfunction (as major criteria of the 2010 Task Force Criteria), (d) proband status, and (e) two or more desmosomal variants. If both NSVT and premature ventricular contraction criteria are present, only NSVT was used.
Clinical follow‐up
Follow‐up was performed in all patients for a median of 5 years (25th–75th range, 2–8 years) after the CMR examination. A physician compiled a clinical questionnaire during periodic ambulatory visits or via telephone contact. The clinical questionnaire included the definition of major combined endpoint of SCD and its surrogates [resuscitated cardiac arrest, appropriate implantable cardioverter defibrillator (ICD) intervention, and episodes of sVT lasting ≥30 s at ≥100 b.p.m.]. ICD interventions were designated as appropriate if triggered by life‐threatening arrhythmias: VT above the programmed ICD cut‐off (12 intervals at >180 b.p.m.) or ventricular fibrillation. A complete interrogation of the ICD was performed by the referring physician to confirm the appropriateness of the intervention.
Statistical analysis
Values are presented as mean ± standard deviation or as median and 25th–75th for variables with normal and non‐normal distribution, respectively. A P value <0.05 was considered significant. Values with non‐normal distribution via the Kolmogorov–Smirnov test were logarithmically transformed into parametric analysis. Categorical variables were compared by the χ 2 test or Fisher's exact test when appropriate. Continuous variables were compared by Student's independent t‐test and ANOVA or Wilcoxon non‐parametric test when appropriate. The Kaplan–Meier analysis was used to compare survival between groups. Time‐dependent area under the curve (AUC) for the 5 year ARVC risk score and the ITFC and HRS criteria was obtained using the timeROC package of R software.
A maximally selected rank statistic analysis was performed to define the optimal cut‐off of the 5 year ARVC risk score for survival analysis using the maxstat package of R software. Decision curve analysis was generated to evaluate the net benefit of the 5 year ARVC score compared with the ITFC criteria. 11
Univariate and multivariate logistic regression analyses were used to explore the impact of each significant variable in the analysis to predict the occurrence of a combined endpoint (cardiac death, appropriate ICD intervention, and resuscitated cardiac arrest). For multivariate analysis, we included all variables with a significant P value in the univariate. The risk of multicollinearity among the covariates was evaluated by the variance inflation factor. Variance inflation factor values <10 indicated a low risk of multicollinearity.
The number needed to treat (NNT) of each risk model was calculated as NNT = 1/ARR, in which ARR is the absolute risk reduction, measured as the difference between the event rate found in the population and the estimated event rate using the risk model for the ICD indication.
Results
The final population included 140 patients (mean age 42 ± 17 years, 97 male). Patient characteristics are reported in Table 2 . The ITFC criteria for ICD implantation for primary prevention were satisfied in 89 patients (63%), whereas the HRS criteria in 41 patients (29%). The ARVC risk score was calculated for all the patients, using the parameters of Table 2 . The median 5 year ARVC risk score was 23% (12–33). Twelve patients (9%) had a 5 year risk of ≤5%, >5%, and ≤15% in 33 patients (24%), >15% and ≤25% in 28 patients (20%), >25% and ≤50% in 50 patients (36%), and finally, >50% in 17 patients. Overall, 128 patients (91.4%) had an estimated 5 year risk >5%.
Table 2.
Basal characteristics of the whole population
| Parameters | Value |
|---|---|
| n | 140 |
| Age (years) | 42 ± 17 |
| Male, n (%) | 97 (69) |
| Weight (kg) | 71 ± 14 |
| Height (cm) | 172 ± 10 |
| Systemic hypertension, n (%) | 30 (21) |
| Diabetes, n (%) | 7 (5) |
| Dyslipidaemia, n (%) | 29 (21) |
| Family history of CAD, n (%) | 9 (6) |
| TF major criteria | |
| Family history of ARVC/D, n (%) | 32 (23) |
| Positive genetic analysis (overall), n (%) | 54 (39) |
| PKP2, n (%) | 27 (20) |
| DSP, n (%) | 14 (10) |
| JUP, n (%) | 5 (4) |
| DSG2, n (%) | 8 (5) |
| DSC2, n (%) | 0 |
| Negative genetic analysis, n (%) | 41 (29) |
| Unknown gene type, n (%) | 45 (32) |
| ECG major repolarization criterion, n (%) | 41 (29) |
| ECG major depolarization criterion, n (%) | 7 (5) |
| Arrhythmias major criterion, n (%) | 37 (26) |
| Echocardiographic TF major criterion, n (%) | 56 (40) |
| CMR major criterion, n (%) | 40 (29) |
| EMB major criterion, n (%) | 14 (10) |
| TF minor criteria | |
| Family history minor criterion, n (%) | 32 (23) |
| ECG minor repolarization criterion, n (%) | 38 (28) |
| ECG minor depolarization criterion, n (%) | 19 (14) |
| Arrhythmias minor criterion, n (%) | 88 (63) |
| Echocardiographic TF minor criterion, n (%) | 8 (5) |
| CMR minor criterion, n (%) | 15 (11) |
| EMB minor criterion, n (%) | 6 (4) |
| ARVC/D diagnosis | |
| ≥2 major criteria, n (%) | 95 (68) |
| 1 major and ≥2 minor criteria, n (%) | 43 (31) |
| ≥4 minor criteria, n (%) | 2 (1) |
| Therapy | |
| Beta‐blockers, n (%) | 125 (90) |
| ACE inhibitors, n (%) | 26 (19) |
| Antiarrhythmic drug, n (%) | 42 (30) |
| Diuretic, n (%) | 14 (10) |
| ARVC score parameters | |
| Age (years) | 42 ± 17 |
| Male, n (%) | 97 (69) |
| Cardiac syncope, n (%) | 19 (14) |
| NSVT, n (%) | 79 (56) |
| 24 h PVC count, median (25th–75th) | 3110 (265–7450) |
| Leads with inverted T wave, median (25th–75th) | 1 (0–3) |
| RV ejection fraction (%), n (%) | 53 (13) |
ACE, angiotensin‐converting enzyme; ARVC, arrhythmogenic right ventricular cardiomyopathy; CAD, coronary artery disease; CMR, cardiac magnetic resonance; ECG, electrocardiogram; EMB, endomyocardial biopsy; NSVT, non‐sustained ventricular tachycardia; PVC; premature ventricular complex; RV, right ventricular; TF, Task Force.
The median 5 year ARVC risk was of 29% (20–42) in patients with ITFC indication for ICD and 11% 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 in those without (P < 0.001). Patients with HRS criteria had a median 5 year ARVC risk of 31% (21–54), while those not satisfying these criteria had a median of 19% (10–28%), P < 0.001.
Follow‐up
During the whole follow‐up duration (median 5 years, 25th–75th, 2–8 years), the major combined endpoint was documented in 65 patients (46%). The events were 3 SCD, appropriate ICD intervention in 33 patients, 12 patients with aborted cardiac arrest, and 17 patients with episode of sVT. During the follow‐up, 51 patients underwent ICD implantation.
Using the ITFC criteria, 89 patients (63%) should have been implanted with ICD. Among the patients with major combined endpoint, 53 (81%) would have been predicted by the ITFC criteria [odds ratio (OR) 4.8, 95% confidence interval (CI) 2.2–10.3, P = 0.0001]. The ITFC criteria had a sensitivity of 82% (95% CI 70–90), a specificity of 52% (95% CI 40–64), positive predictive value of 60% (95% CI 53–65%), and negative predictive value of 76% (95% CI 65–84) to predict major events. The NNT of the ITFC criteria was 2.6.
Heart Rhythm Society criteria would have prevented 29 (45%) events (OR 4.2, 95% CI 1.9–9.3, P = 0.0003) with indication for ICD in 41 (29%) patients. HRS criteria had a sensitivity of 43% (95% CI 31–55), a specificity of 84% (95% CI 74–91), positive predictive value of 70% (95% CI 57–81%), and negative predictive value of 62% (95% CI 56–67) to predict major events. The NNT of the HRS criteria was 4.8.
As evident in Figure 1 , at maximally selected rank statistic, the optimal cut‐off of 5 year ARVC risk score was >10% for the analysis of the survival free from major combined events. Using this threshold of >10% of the 5 year ARVC risk score, 114 (81%) patients should have been implanted, and 62 out of 65 major events (95%) would have been prevented. The 5 year risk score >10% had 95% sensitivity (95% CI 87–99) and 31% specificity (95% CI 20–42), positive predictive value of 54% (95% CI 50–58), and negative predictive value of 88% (95% CI 71–96) to predict major combined endpoint (OR 9.1, 95% CI 2.6–32, P = 0.0006). The NNT for the 5 year ARVC risk score >10% was 2.3.
Figure 1.
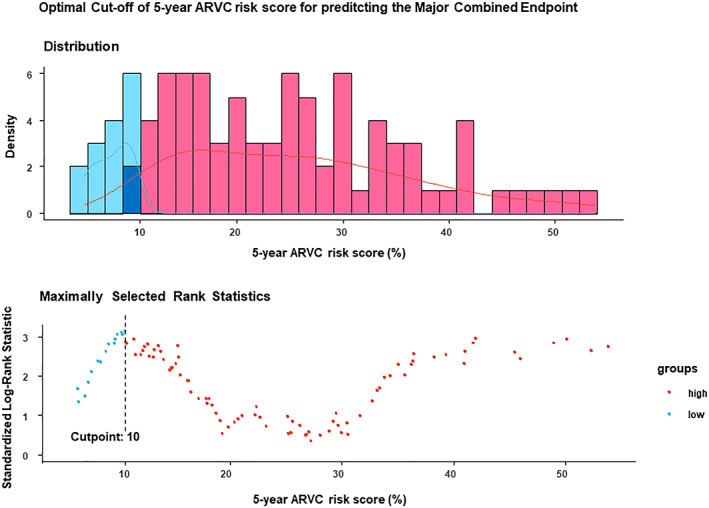
Maximally selected rank statistic of 5 year arrhythmogenic right ventricular cardiomyopathy (ARVC) risk score to predict major combined events: the optimal threshold of 5 year ARVC score was >10%.
Compared with the ITFC criteria, a 5 year ARVC score >10% would have prevented 14% more events (P = 0.01) but with 25.8% more ICD implantations (P = 0.005). Compared with the HRS criteria, the 5 year ARVC score >10% would have been capable of preventing 50% more events (P < 0.0001) but with almost three times more ICD implantations.
Kaplan–Meier curves evaluating the survival free from major combined endpoint of the 5 year ARVC score and the ITFC and HRS criteria are shown in Figure 2 .
Figure 2.
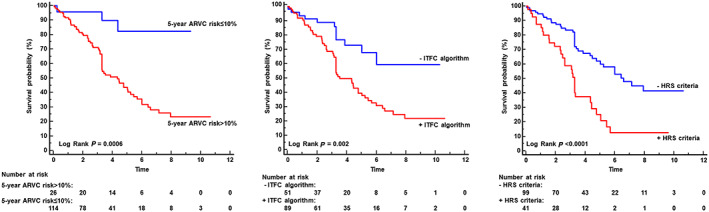
Kaplan–Meier survival free from event curves: in the left panel, Kaplan–Meier curves showed that patients with 5 year ARVC risk score >10% had worse prognosis than those with lower values of risk score. As showed in the middle panel, patients satisfying the International Task Force Consensus (ITFC) criteria had worse prognosis than those without. Finally, Kaplan–Meier curve of right panel demonstrated the prognostic role of Heart Rhythm Society (HRS) criteria.
Time‐dependent receiver operating characteristic curves of 5 year ARVC risk score and the ITFC and HRS criteria predict major cardiac combined endpoint, as shown in Figure 3 . The median time‐dependent AUC of the HRS criteria was significantly higher than that of 5 year ARVC (0.65 vs. 0.60, P = 0.002) and of the ITFC criteria (0.58, P < 0.0001).
Figure 3.
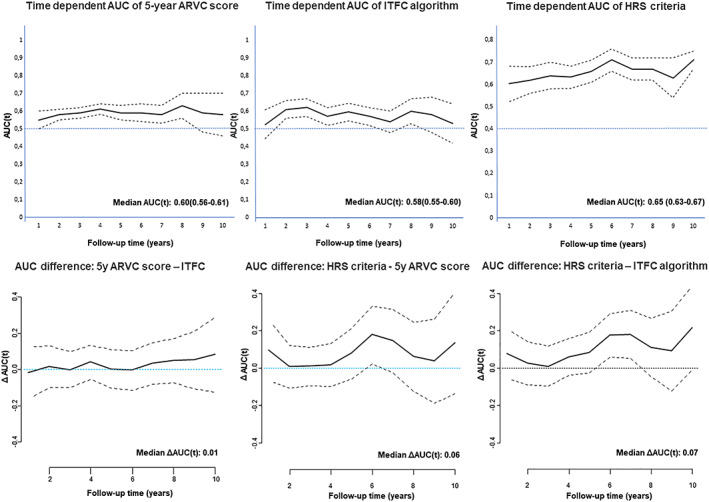
Time‐dependent area under the curve (AUC) for predicting major combined endpoint: the time‐dependent AUC curves of the 5 year ARVC score (continuous variable), of the International Task Force Consensus (ITFC) algorithm, and of the Heart Rhythm Society (HRS) criteria are showed in the upper panels, respectively, from left to right. In the lower panels, the time‐dependent AUC difference between 5 year ARVC score and the ITFC, ARVC, and HRS criteria and between HRS and ITFC algorithm are showed.
The decision analysis curves for ICD implantation are shown in Figure 4 . As evident, the 5 year risk score >10% had a greater net benefit compared with other thresholds of the risk score. Compared with the ITFC and HRS criteria, the 5 year ARVC risk >10% had a higher net benefit in the range of a probability threshold 0.12–0.37.
Figure 4.
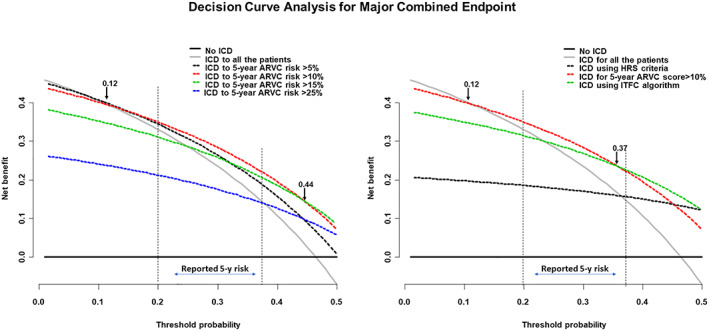
Decision curve analysis of implanted cardioverter defibrillator (ICD) implantation for preventing the major combined endpoint. In the left panel, the net benefit curves of different thresholds of 5 year arrhythmogenic right ventricular cardiomyopathy (ARVC) risk score are compared, demonstrating that the >10% threshold had a higher net benefit compared with the other thresholds and to the ‘ICD to all the patients’ approach for a wide range of threshold probability, including the range of probability corresponding to the reported 5 year risk of sudden death in ARVC. In the right panels, the net benefit curves of the 5 year ARVC score >10%, of the International Task Force Consensus (ITFC) algorithm, and of the Heart Rhythm Society (HRS) criteria were compared. The 5 year ARVC score >10% had a greater net benefit than other models in a wide range of probability including the range of reported 5 year risk of sudden death of ARVC.
Univariate and multivariable logistic regression analyses for prediction of the major combined endpoint are reported in Table 3 . At univariate analysis, the 5 year ARVC risk score (continuous), a 5 year ARVC risk score >10% (dichotomous), the ITFC criteria, the HRS criteria, NSVT, and syncope were significantly associated with the major events. These parameters were evaluated in two multivariate logistic regression models. In the first model, in which the 5 year ARVC risk score was included as continuous variable, no parameter was a significant independent predictor of events. In the second model, the 5 year ARVC risk score >10% (dichotomous) was included. In this model, the 5 year ARVC risk score >10% (OR 5.4, 95% CI 1.5–26, P = 0.02) was the only independent predictor of major events.
Table 3.
Univariate and multivariate logistic regression analyses for predicting the original ARVC risk study combined endpoint
| Univariate | |||
|---|---|---|---|
| OR | 95% CI | P value | |
| Age | 1.02 | 0.98–1.04 | 0.08 |
| Sex = male | 1.14 | 0.72–2.79 | 0.70 |
| 24 h PVC count | 0.99 | 0.99–1.01 | 0.88 |
| RVEF | 1.01 | 0.98–1.04 | 0.55 |
| RVEF < 40% | 0.73 | 0.27–2.03 | 0.54 |
| LVEF < 35% | 0.52 | 0.19–1.9 | 0.55 |
| No. of inverted T wave | 2.04 | 0.77–5.8 | 0.16 |
| Syncope | 1.88 | 1.34–2.78 | <0.001 |
| NSVT | 5.8 | 2.8–12.5 | <0.001 |
| ITFC algorithm | 4.8 | 2.3–10.7 | <0.001 |
| HRS criteria | 4..2 | 2–9.6 | <0.001 |
| 5 year ARVC risk score | 1.03 | 1.01–1.05 | 0.009 |
| 5 year ARVC risk >10% | 7.7 | 2.8–27 | 0.004 |
| Multivariate model with 5 year ARVC risk score (continuous) | ||||
|---|---|---|---|---|
| OR | 95% CI | P value | VIF | |
| NSVT | 4.4 | 0.82–34 | 0.10 | 5.5 |
| Syncope | 1.1 | 0.3–4.4 | 0.88 | 1.5 |
| ITFC algorithm | 0.8 | 0.1–4.7 | 0.80 | 5.5 |
| HRS criteria | 1.9 | 0.7–5.3 | 0.21 | 1.5 |
| 5 year ARVC risk score | 1.01 | 0.98–1.03 | 0.81 | 1.6 |
| McFadden R 2 | 0.12 | |||
| Multivariate model with 5 year ARVC risk score >10% (dichotomic) | ||||
|---|---|---|---|---|
| OR | 95% CI | P value | VIF | |
| NSVT | 4.5 | 0.85–35 | 0.10 | 5.1 |
| Syncope | 1.1 | 0.3–4.2 | 0.90 | 1.4 |
| ITFC algorithm | 0.5 | 0.1–2.6 | 0.39 | 5.2 |
| HRS criteria | 1.9 | 0.7–5.4 | 0.19 | 1.5 |
| 5 year ARVC risk score >10% | 5.4 | 1.5–26 | 0.02 | 1.2 |
| McFadden R 2 | 0.21 | |||
ARVC, arrhythmogenic right ventricular cardiomyopathy; CAD, coronary artery disease; CI, confidence interval; HRS, Heart Rhythm Society; ITFC, international task force algorithm; LVEF, left ventricular ejection fraction; NSVT, non‐sustained ventricular tachycardia; OR, odds ratio; PVC; premature ventricular complex; RVEF, right ventricular ejection fraction; VIF, variance inflation factor.
Discussion
In the present study, we sought to compare and validate three different risk models for ARVC, namely, the ARVC risk score, the ITFC criteria, and the HRS criteria by using a validation cohort of patients with definite ARVC. We found that the 5 year ARVC risk score with the optimal threshold of >10% was the most sensitive model for predicting events and had a higher net benefit than the other models.
Chronologically, the ITFC algorithm was the first proposed model for the indication for ICD implantation in patients with definite ARVC. 4 Based on this algorithm, ICD implantation should be indicated in patients with severe RV and/or LV dysfunction or in those with at least one major risk factor, such as NSVT, syncope, or moderate RV and/or LV dysfunction. In our population, the ITFC criteria were satisfied in 63% of patients and would have prevented 81.4% of cardiac events. However, this was the model with the lowest time‐dependent AUC for predicting events.
The HRS criteria were proposed by Towbin et al. in 2019. 6 These criteria were not generated specifically for ARVC, but they were aimed to provide ICD indication for a broad spectrum of arrhythmogenic cardiomyopathies. For some aspects, these criteria were similar to the ITFC criteria, but in HRS criteria, the relative ‘weight’ of LV dysfunction was higher than that of RV dysfunction, which was considered just a minor criterion. Moreover, in the Task Force Consensus document by Corrado et al., 12 inducibility to VT at electrophysiological study was considered a minor risk factor because of the reported low predictive accuracy. In contrast, in the HRS criteria, inducibility to VT in electrophysiological study was a major criterion. However, the reported class of evidence referred to a study by Orgeron et al., 13 in which inducibility to VT was an independent predictor of ventricular arrhythmias but not of SCD.
In our study, the HRS criteria were satisfied in only 41 patients and missed more than half of the events and had the lowest sensitivity (43%) and highest specificity (84%) among the prediction models. Despite this finding, the time‐dependent AUC of HRS criteria was higher than that of the other models.
The 5 year ARVC risk score model for estimation of the 5 year risk of arrhythmic events in ARVC was proposed by Cadrin‐Tourigny et al. in 2019 after analysing data from a registry of 18 institutions. 5 This score included dichotomic variables, such as gender, syncope, and episode of VT at 24 h Holter electrocardiogram monitoring, and quantitative parameters, such as the number or inverted T waves at basal 12‐lead electrocardiogram, the number of premature ventricular complexes at Holter monitoring, and the RVEF. The ARVC risk score was generated using a combined endpoint of SCD, cardiac arrest, and appropriate ICD therapy but also including episodes of sVT lasting ≥30 s at ≥100 b.p.m. or with haemodynamic compromise requiring cardioversion. This score was then generated using episodes of slow sVT without haemodynamic compromise, which were not surrogates of SCD.
Furthermore, in Cadrin‐Tourigny's study, among the 340 patients with mutations, 258 (76%) patients had a PKP2 mutation, while DSP was involved in 7% and DSG2 in 5% of patients. Because of this asymmetrical distribution of mutated genes, the effectiveness of this risk score to predict events in patients with DSP or DSG2 mutation was not guaranteed. Indeed, DSP and DSG2 mutations were more frequently associated with LV involvement and with a higher risk of sudden death than the PKP2 mutation. 7 , 14 Recently, two multicentre studies demonstrated that LV involvement is associated with worse prognosis than lone RV involvement. Our population had a greater prevalence of DSP and DSG2 mutations and LV involvement than that of Cadrin‐Tourigny's study, which may explain the higher percentage of events of our study.
Finally, in the study by Cadrin‐Tourigny et al., 5 a threshold of 5 year ARVC risk score to indicate ICD implantation was not explicitly proposed. We tried to overcome this limitation, and by using the maximally selected rank statistic, we found that a 5 year risk score >10% was the best cut point for predicting the major combined endpoint as it was the threshold with the greatest net benefit for the indication of ICD implantation, when compared with other thresholds. With this threshold, the 5 year ARVC score was capable of predicting 95% of events, which was14% and 50% more than the ITFC and HRS criteria, respectively, but at the price of more ICD implantations.
The 5 year ARVC risk score >10% had a greater net benefit compared with the ITFC and HRS criteria, particularly in the threshold probability range corresponding to the 5 year reported risk of SCD of ARVC (range 20–35%). Finally, in the multivariate analysis, the 5 year ARVC risk score >10% was the only independent predictor of major cardiac events confirming its superiority to the other prediction models.
These results are not surprising because the 5 year ARVC score was the only prediction model to have been directly generated from ARVC patient data, whereas the other two models had been based on class of evidence or on expert consensus and were not even tested in patients until the present study.
Interestingly, in the multivariate analysis, the 5 year ARVC risk score, as a continuous variable, was not a significant predictor of events, probably because of the great dispersion of 5 year score values found in this population, ranging from 2.8% to 73% in the whole cohort and from 7% to 73% among patients with cardiac events.
The 5 year ARVC risk score had greater accuracy than other models to predict events. Using this score with >10% criterion, 95% of events would have been prevented but at cost of implantation of ICD in 81% of the patients. Thus, even this score system is far from being an optimal prediction model. Actually, patients with ARVC are intrinsically at higher risk of cardiac arrhythmic events, and to prevent such events, ICD should be implanted in a great percentage of patients.
In the present study, we excluded CMR‐derived parameters, such as tissue abnormalities (fat infiltration and late gadolinium enhancement) of RV and also LV involvement. Recently, the prognostic role of LV involvement was demonstrated in patients with definite diagnosis of ARVC and in patients with borderline or possible ARVC. 7 , 15 In this validation study, we preferred not to consider CMR parameters that were not included for the generation of ARVC risk score or for the ITFC criteria. However, LV involvement in ARVC is relevant because it is found in approximately 50% of patients 16 and is associated with a different prevalence of pathogenic mutation and possibly to a different clinical presentation. 17 , 18 , 19
Some study limitations should be mentioned. First, the study population was smaller than that in the original Cadrin‐Tourigny's study. However, major events were recorded in 46% of patients in this validation cohort, and this rate was sufficient to test the ARVC risk score.
Second, the characteristics of population were different from the original ARVC risk score study, thus resulting in a higher prevalence of cardiac events. However, this difference was an advantage in our study, because ARVC is a complex condition with many different presentations, and the ARVC risk score should be tested in different presentations.
In our validation cohort, only two patients had LV ejection fraction <35%, and only a minority of patients underwent an electrophysiological study because the prediction models were retrospectively calculated. Thus, the indication for an electrophysiological study was given by the referral cardiologist based on clinical evaluation.
We enrolled patients and performed the clinical follow‐up prospectively; however, the evaluation of the three risk models was performed retrospectively using patient baseline characteristics.
Finally, the definition of the appropriate ICD intervention (for episode >180 b.p.m. for 12 intervals) was not up to date. However, all ICD interventions occurred for ventricular arrhythmias at heart rates >200 b.p.m., and during the follow‐up, no episode of slow VT was recorded.
Conclusions
The 5 year ARVC risk score with a threshold of >10% was more accurate for predicting the occurrence of major cardiac events than the ITFC and HRS criteria and allowed us to predict 95% of cardiac events in this cohort of patients.
Conflict of interest
None declared.
Aquaro, G. D. , De Luca, A. , Cappelletto, C. , Raimondi, F. , Bianco, F. , Botto, N. , Barison, A. , Romani, S. , Lesizza, P. , Fabris, E. , Todiere, G. , Grigoratos, C. , Pingitore, A. , Stolfo, D. , Dal Ferro, M. , Merlo, M. , Di Bella, G. , and Sinagra, G. (2020) Comparison of different prediction models for the indication of implanted cardioverter defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy. ESC Heart Failure, 7: 4080–4088. 10.1002/ehf2.13019.
References
- 1. Sen‐Chowdhry S, Syrris P, Prasad SK, Hughes SE, Merrifield R, Ward D, Pennell DJ, McKenna WJ. Left‐dominant arrhythmogenic cardiomyopathy: an under‐recognized clinical entity. J Am Coll Cardiol 2008; 52: 2175–2187. [DOI] [PubMed] [Google Scholar]
- 2. Bennett RG, Haqqani HM, Berruezo A, Della Bella P, Marchlinski FE, Hsu CJ, Kumar S. Arrhythmogenic cardiomyopathy in 2018–2019: ARVC/ALVC or both? Heart Lung Circ 2019; 28: 164–177. [DOI] [PubMed] [Google Scholar]
- 3. Calkins H, Corrado D, Marcus F. Risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation 2017; 136: 2068–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corrado D, Wichter T, Link MS, Hauer RN, Marchlinski FE, Anastasakis A, Bauce B, Basso C, Brunckhorst C, Tsatsopoulou A, Tandri H, Paul M, Schmied C, Pelliccia A, Duru F, Protonotarios N, Estes NM 3rd, McKenna WJ, Thiene G, Marcus FI, Calkins H. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Circulation 2015; 132: 441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cadrin‐Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale A, Bourfiss M, Fortier A, Lie ØH, Saguner AM, Svensson A, Andorin A, Tichnell C, Murray B, Zeppenfeld K, van den Berg MP, Asselbergs FW, Wilde AAM, Krahn AD, Talajic M, Rivard L, Chelko S, Zimmerman SL, Kamel IR, Crosson JE, Judge DP, Yap SC, van der Heijden JF, Tandri H, Jongbloed JDH, Guertin MC, van Tintelen JP, Platonov PG, Duru F, Haugaa KH, Khairy P, Hauer RNW, Calkins H, Te Riele ASJM, James CA. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2019; 40: 1850–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, Daubert JP, de Chillou C, DePasquale EC, Desai MY, Estes NAM 3rd, Hua W, Indik JH, Ingles J, James CA, John RM, Judge DP, Keegan R, Krahn AD, Link MS, Marcus FI, McLeod CJ, Mestroni L, Priori SG, Saffitz JE, Sanatani S, Shimizu W, van Tintelen JP, Wilde AAM, Zareba W. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm 2019; 16: e301–e372. [DOI] [PubMed] [Google Scholar]
- 7. Aquaro GD, De Luca A, Cappelletto C, Raimondi F, Bianco F, Botto N, Lesizza P, Grigoratos C, Minati M, Dell’Omodarme M, Pingitore A, Stolfo D, Ferro MD, Merlo M, Di Bella G, Sinagra G. Prognostic Value of Magnetic Resonance Phenotype in Patients With Arrhythmogenic Right Ventricular Cardiomyopathy. Journal of the American College of Cardiology. 2020;75: 22:2753–2765. 10.1016/j.jacc.2020.04.023 [DOI] [PubMed] [Google Scholar]
- 8. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force criteria. Circulation 2010; 121: 1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier‐Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aquaro GD, Barison A, Todiere G, Grigoratos C, Ait Ali L, Di Bella G, Emdin M, Festa P. Usefulness of combined functional assessment by cardiac magnetic resonance and tissue characterization versus task force criteria for diagnosis of arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol 2016; 118: 1730–1736. [DOI] [PubMed] [Google Scholar]
- 11. Vickers AJ, Van Calster B, Steyerberg EW. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ 2016; 352: i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Corrado D, Leoni L, Link MS, Della Bella P, Gaita F, Curnis A, Salerno JU, Igidbashian D, Raviele A, Disertori M, Zanotto G, Verlato R, Vergara G, Delise P, Turrini P, Basso C, Naccarella F, Maddalena F, Estes NA 3rd, Buja G, Thiene G. Implantable cardioverter‐defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2003; 108: 3084–3091. [DOI] [PubMed] [Google Scholar]
- 13. Orgeron GM, James CA, Te Riele A, Tichnell C, Murray B, Bhonsale A, Kamel IR, Zimmerman SL, Judge DP, Crosson J, Tandri H, Calkins H. Implantable cardioverter‐defibrillator therapy in arrhythmogenic right ventricular dysplasia/cardiomyopathy: predictors of appropriate therapy, outcomes, and complications. J Am Heart Assoc 2017; 6: e006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith ED, Lakdawala NK, Papoutsidakis N, Aubert G, Mazzanti A, McCanta AC, Agarwal PP, Arscott P, Dellefave‐Castillo LM, Vorovich EE, Nutakki K, Wilsbacher LD, Priori SG, Jacoby DL, McNally EM, Helms AS. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation 2020; 141: 1872–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aquaro GD, Pingitore A, Di Bella G, Piaggi P, Gaeta R, Grigoratos C, Altinier A, Pantano A, Strata E, De Caterina R, Sinagra G, Emdin M. Prognostic role of cardiac magnetic resonance in arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol 2018; 122: 1745–1753. [DOI] [PubMed] [Google Scholar]
- 16. Te Riele AS, James CA, Philips B, Rastegar N, Bhonsale A, Groeneweg JA, Murray B, Tichnell C, Judge DP, Van Der Heijden JF, Cramer MJ, Velthuis BK, Bluemke DA, Zimmerman SL, Kamel IR, Hauer RN, Calkins H, Tandri H. Mutation‐positive arrhythmogenic right ventricular dysplasia/cardiomyopathy: the triangle of dysplasia displaced. J Cardiovasc Electrophysiol 2013; 24: 1311–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Syrris P, Ward D, Asimaki A, Evans A, Sen‐Chowdhry S, Hughes SE, McKenna WJ. Desmoglein‐2 mutations in arrhythmogenic right ventricular cardiomyopathy: a genotype‐phenotype characterization of familial disease. Eur Heart J 2007; 28: 581–588. [DOI] [PubMed] [Google Scholar]
- 18. Bauce B, Basso C, Rampazzo A, Beffagna G, Daliento L, Frigo G, Malacrida S, Settimo L, Danieli G, Thiene G, Nava A. Clinical profile of four families with arrhythmogenic right ventricular cardiomyopathy caused by dominant desmoplakin mutations. Eur Heart J 2005; 26: 1666–1675. [DOI] [PubMed] [Google Scholar]
- 19. Jacob KA, Noorman M, Cox MG, Groeneweg JA, Hauer RN, van der Heyden MA. Geographical distribution of plakophilin‐2 mutation prevalence in patients with arrhythmogenic cardiomyopathy. Neth Hear J 2012; 20: 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]


