Abstract
Precise descriptions of coronavirus disease 2019 (COVID‐19)‐related cardiac damage as well as underlying mechanisms are scarce. We describe clinical presentation and diagnostic workup of acute myocarditis in a patient who had developed COVID‐19 syndrome 1 month earlier. A healthy 40‐year‐old man suffered from typical COVID‐19 symptoms. Four weeks later, he was admitted because of fever and tonsillitis. Blood tests showed major inflammation. Thoracic computed tomography was normal, and RT–PCR for SARS‐CoV‐2 on nasopharyngeal swab was negative. Because of haemodynamic worsening with both an increase in cardiac troponin and B‐type natriuretic peptide levels and normal electrocardiogram, acute myocarditis was suspected. Cardiac echographic examination showed left ventricular ejection fraction at 45%. Exhaustive diagnostic workup included RT–PCR and serologies for infectious agents and autoimmune blood tests as well as cardiac magnetic resonance imaging and endomyocardial biopsies. Cardiac magnetic resonance with T2 mapping sequences showed evidence of myocardial inflammation and focal lateral subepicardial late gadolinium enhancement. Pathological analysis exhibited interstitial oedema, small foci of necrosis, and infiltrates composed of plasmocytes, T‐lymphocytes, and mainly CD163+ macrophages. These findings led to the diagnosis of acute lympho‐plasmo‐histiocytic myocarditis. There was no evidence of viral RNA within myocardium. The only positive viral serology was for SARS‐CoV‐2. The patient and his cardiac function recovered in the next few days without use of anti‐inflammatory or antiviral drugs. This case highlights that systemic inflammation associated with acute myocarditis can be delayed up to 1 month after initial SARS‐CoV‐2 infection and can be resolved spontaneously.
Keywords: Myocarditis, COVID‐19, Pathological analysis
Introduction
Since the start of the coronavirus disease 2019 (COVID‐19) outbreak, it has been considered that SARS‐CoV‐2‐related heart damage was relatively frequent according to the rate of increase in cardiac troponin levels and because of the presence of angiotensin‐converting enzyme 2 receptors—required to infect cells—within myocardium. In addition, the increase in cardiac biomarkers is associated with worse outcome in infected patients. 1 , 2 However, descriptions of such cardiac involvement and underlying mechanisms are scarce and incomplete, and the existence and mechanisms of acute myocarditis related to SARS‐CoV‐2 infection remain questioned. 3 , 4
The present report describes a case of biopsy‐proven myocarditis in the setting of SARS‐CoV‐2 infection and helps to discuss mechanisms of cardiac involvement. The patient provided written informed consent, and the diagnostic procedures were conducted in accordance with institutional guidelines about the protection of human subjects.
Case report
A 40‐year‐old man, without past medical history or co‐morbidities except an obesity (body mass index = 34.8 kg/m2), described symptoms of COVID‐19 including fever, intense fatigue, myalgia, and anosmia. He isolated himself at home and took paracetamol without any diagnostic test. After a few days, most symptoms resolved. Four weeks later, he presented at the emergency unit of this hospital with fever, odynophagia, and left neck pain. The initial examination revealed a body temperature at 39.9°C, blood pressure at 110/60 mmHg, heart rate at 123 b.p.m., and tonsillitis with cervical adenopathy. On biological test, leukocytes blood count was 25 × 109/L (neutrophils 22 × 109/L, eosinophils 0.04 × 109/L, and lymphocytes 0.90 × 109/L). All markers of serum inflammation were highly increased, that is, C‐reactive protein 604 mg/L, fibrinogen 12.5 g/L, procalcitonin 14 μg/L, and D‐dimer 5700 ng/L. Because of the outbreak of COVID‐19, a nasopharyngeal swab was performed on admission and the RT–PCR assay returned negative for SARS‐CoV‐2. Blood cultures were negative. Intravenous antibiotic therapy—ceftriaxone and metronidazole—was started in the event of cellulitis. There was no purulent collection and dental granuloma nor tonsil phlegmon requiring surgery.
The patient was referred at Day 2 of hospitalization in the intensive care unit because of respiratory and haemodynamic worsening although ear–nose–throat infection symptoms improved. The electrocardiogram showed sinus tachycardia (110 b.p.m., PR duration = 150 ms, QRS duration = 80 ms, and no repolarization abnormality). Blood levels of cardiac troponin (high‐sensitivity troponin I) were increased at 485 ng/L (N < 34) and B‐type natriuretic peptide (BNP) at 2960 ng/L. Blood level of interleukin‐6 (Roche Diagnostics) was highly increased at 75.6 μg/L (N < 7). A thoracic contrast‐enhanced computed tomography displayed only a moderate bilateral pleural effusion without any sign of SARS‐CoV‐2 pneumonia or pulmonary embolism. Cardiac echography revealed a decrease in left ventricular ejection fraction at 45%, low cardiac output (3 L/min), and both subtle hypertrophy and akinesia of posterolateral left ventricular wall with small pericardial effusion opposite. At Day 3, a coronary angiogram overruled the hypothesis of obstructive coronary disease. Cardiac magnetic resonance (CMR) at 1.5 T (Figure 1 ) showed normal left ventricular size (left ventricular end‐diastolic volume index: 75 mL/m2) and mild systolic dysfunction (left ventricular ejection fraction: 45%) with global hypokinesia. The presence of myocardial inflammation was confirmed by T2 mapping (global T2 relaxation times: 62 ms; centre‐specific cut‐off value for acute myocarditis: ≥55 ms). Late gadolinium enhancement imaging (inversion time by using the Look‐Locker technique: 280 ms) showed focal lateral subepicardial enhancement with prolonged T1 relaxation times (global T1 relaxation times: 1160 ms; centre‐specific cut‐off value for acute myocarditis: ≥1000 ms). Cardiac magnetic resonance imaging also showed small pericardial effusion. Overall, revised 2018 Lake Louise criteria for diagnosis of acute myocarditis were fulfilled. Because of the clinical severity of the acute heart failure with left ventricular dysfunction and pulmonary oedema requiring intensive oxygenotherapy and high doses of diuretics, endomyocardial biopsies of left ventricle (posterolateral wall) were performed on Day 4. Pathological analysis found an interstitial oedema, small foci of necrosis, interstitial and perivascular infiltrates composed of CD138+ CD79a+ CD20− plasmocytes, CD3+ CD8+ T‐lymphocytes (7 cells/mm2), few neutrophils, and a dense and diffuse infiltration by CD163+ macrophages (Figure 2 ). Exhaustive research of viruses was performed by RT–PCR on frozen myocardial fragments and was negative for the following: adenovirus, B19 parvovirus, parainfluenza 1, 2, 3, and 4, influenza A and B, rhinovirus, enterovirus, coronavirus 229E, HKU1, NL63, OC43 and MERS, human herpes virus 6 and 8, cytomegalovirus, Epstein–Barr virus, varicella‐zoster virus, HIV, and SARS‐CoV‐2. RT–PCR for herpes simplex virus 1 and 2, varicella‐zoster virus, adenovirus, enterovirus, human herpes virus 6A and B, and parvovirus B19, as well as Elisa tests for HIV, CMV, and EBV, were also performed on blood and were negative. Only SARS‐CoV‐2 serology returned positive: IgG blood level was increased with signal cut‐off ratio at 3.9 (positive threshold at 1.4, Abbott Diagnostics) whereas there was no IgM. The following measurements were also performed to rule out autoimmune disease: the blood research of antinuclear, anti‐DNA, anti‐extractable nuclear antigen, and anti‐neutrophil cytoplasm antibodies were negative, and the measurement of complement components C3 and C4 was normal.
Figure 1.
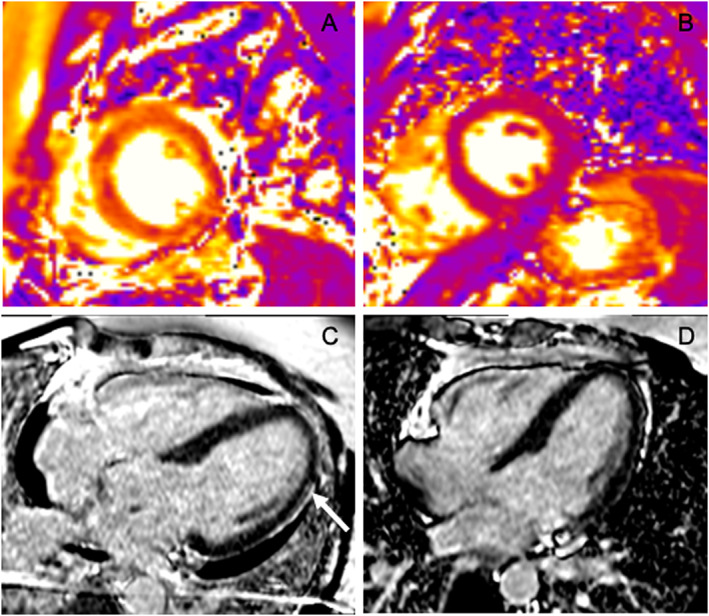
(A) Initial T2 mapping sequence in short axis view showing intense interstitial myocardial oedema (native T2 = 62 ms). (B) One month later, T2 mapping sequence in short axis view showing normalization of native T2 = 44 ms, suggesting disappearing of myocardial oedema. (C) Initial phase‐sensitive inversion recovery sequences in four‐chamber view showing pericardial effusion and focal lateral subepicardial late gadolinium enhancement (white narrow). (D) One month later, phase‐sensitive inversion recovery sequences in four‐chamber view showing no pericardial effusion and no subepicardial late gadolinium enhancement.
Figure 2.
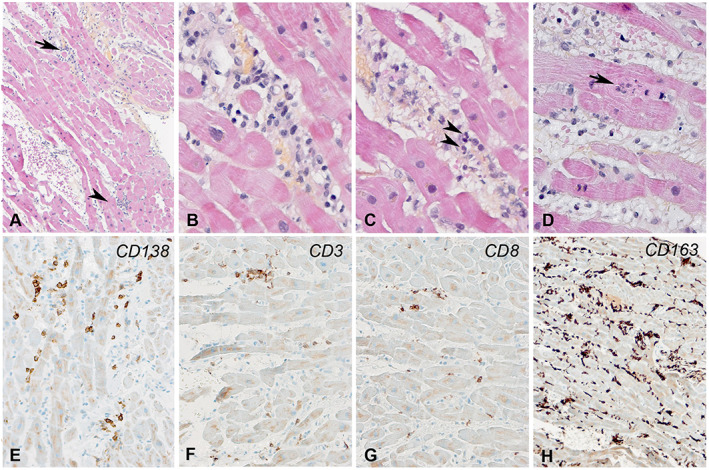
(A–D) Endomyocardial biopsy showing multiple foci of lymphocytes (arrow and arrowhead) in a diffuse inflammatory and oedematous background. (B) A higher magnification of the interstitial and perivascular inflammatory foci shown by an arrow in (A). (C) A few neutrophils are shown by arrowheads. (D) Myocyte necrosis, infiltrated by inflammatory cells (arrow). (E–H) The inflammatory cells were composed of numerous CD138+ plasmocytes, CD3+ CD8+ T cells, and numerous CD163+ macrophages.
Medical treatment of heart failure included angiotensin‐converting enzyme inhibitors and beta‐blockers, no anti‐inflammatory drug because of ear–nose–throat infection requiring antibiotic treatment, and as transient atrial fibrillation occurred at Day 6, anticoagulant and anti‐arrhythmic therapy were added. The patient rapidly recovered and was discharged at Day 9 while C‐reactive protein was at 44 mg/L, cardiac troponin I 71 ng/L, and BNP 197 ng/L, and echocardiographic examination was normal. One month after hospital discharge, clinical examination was completely normal (no fever and no signs of heart failure). CMR and transthoracic echocardiogram showed disappearance of myocardial oedema (global T2 relaxation time = 44 ms, global T1 relaxation time = 1006 ms), recovering of left ventricular ejection fraction (60%), absence of pericardial effusion, and myocardial late gadolinium enhancement. The hypersensitive troponin I and BNP levels were <14 and 21 ng/L, respectively. Biological markers of systemic inflammation returned to normal values, that is, C‐reactive protein <4 mg/L, procalcitonin <0.02 mg/L, and D‐dimers <270 ng/L.
Discussion
A few case reports of acute heart failure and/or left ventricular dysfunction with abnormalities in CMR suggesting myocarditis have been published in the setting of acute SARS‐CoV‐2 infection (Table 1 ), 4 , 5 , 6 , 7 , 13 but diagnosis of active myocarditis was proven by endomyocardial biopsy in only one case. 8 Our case report highlights the importance of exhaustive and careful workup to specify diagnosis and causes of acute myocarditis using both blood tests, echo, CMR analysis, and also endomyocardial biopsies if necessary. 9 , 10 After carefully ruling out usual aetiologies of myocarditis such as common viruses, bacteria, autoimmune disease, giant cells, and hypereosinophilic syndrome, we hypothesized that SARS‐CoV‐2‐mediated systemic inflammation could be the cause of this acute cardiac inflammatory response. The nearly 1‐month delay between acute heart failure and initial COVID‐related symptoms of our patient is unusual. All previously published cases occurred within the first week after initial symptoms of COVID. Our hypothesis is that prolonged systemic inflammation (‘cytokines storm’) led to both tonsillitis and dense myocardial inflammation. Sala et al. reported recently the first evidence of acute myocarditis by endomyocardial biopsy in a patient with COVID‐19. 8 As in our patient, they did find evidence of SASR‐CoV‐2 within myocardium using RT–PCR. Similarities with Tako‐Tsubo syndrome were also underlined in this case.
Table 1.
Publishe`d case reports of patients with heart failure and suspicion of acute myocarditis related to SARS‐CoV‐2 infection
| Authors | Time from onset of COVID symptoms | Diagnostic test for COVID | Clinical manifestations | Blood tests | CMR | Cardiac biopsy | Treatments | Outcome |
|---|---|---|---|---|---|---|---|---|
| Doyen et al. 5 | Day 7 | PCR on nasal swab | 65 years, cough, fever, dyspnoea | TnI 9000 ng/L | Normal LVEF | No | Hydrocortisone | |
| Subepicardial inferolateral LGE | ||||||||
| Luetkens et al. 6 | Day 2 | PCR on nasal swab | 53 years, fever, dry cough | CRP 13 mg/L | No | No | Dobutamine | |
| TnT 0.24 ng/mL | Hydroxychloroquine, lopinavir/ritonavir, methylprednisolone | |||||||
| NT‐pro‐BNP 5647 pg/mL | ||||||||
| Kim et al. 7 | Day 1 | PCR on nasal swab | 21 years | TnI 1.26 ng/mL | LVEF 40% | No | ||
| NT‐pro‐BNP 929 pg/mL | T1 = 1431 ms, transmural LGE | |||||||
| Sala et al. 8 | Day 3 | PCR on nasal swab | 37 years, dyspnoea, chest pain, diarrhoea, cardiogenic shock | TnT >10 000 ng/L | No | No | Corticosteroids | Full recovery within 1 week |
| NT‐pro‐BNP >21 000 ng/L | Noradrenalin | |||||||
| Immunoglobulins | ||||||||
| Diuretics | ||||||||
| Milrinone | ||||||||
| Tazocilline | ||||||||
| Caforio et al. 9 | Day 7 | PCR on nasal swab | 53 years, cough, fever, fatigue | Increase in TnT and BNP | LVEF 35% | No | Dobutamine, lopinavir/ritonavir, steroids, chloroquine | Progressive stabilization |
| Diffuse LGE, pericardial effusion | ||||||||
| Ferreira et al. 10 | Day 3 | PCR on nasal swab | 37 years, chest pain, dyspnoea, diarrhoea | TnT >10 000 ng/mL | No | No | Diuretic, milrinone, norepinephrine, methylprednisolone, immunoglobulin, piperacillin, sulbactam | Full recovery within 3 weeks |
| Creatine Kinase Myocardite Band (CPKMB) 112.9 ng/mL | ||||||||
| NT‐pro‐BNP 21 025 ng/L | ||||||||
| Zhou 3 | Day 3 | PCR on nasal swab | 43 years, dyspnoea, chest pain | CRP 18 mg/L | LVEF 43% | Diffuse CD3+ T‐lymphocytic inflammatory infiltrates | Lopinavir/ritonavir, hydroxychloroquine |
Full recovery of LVEF at Day 7 Persistence of a mild hypokinesia at basal and mid‐left ventricular segments; at the same sites |
| TnT 135 ng/L | Diffuse LGE | |||||||
| NT‐pro‐BNP 521 ng/L | ||||||||
| Tavazzi et al. 11 | Day 4 | PCR on nasal swab | 69 years, respiratory distress and cardiogenic shock | CRP 52 mg/dL | No | Low‐grade macrophagic interstitial and endocardial cardiac inflammation | Noradrenaline, IABP, ECMO | |
| TnI 4332 ng/L | ||||||||
| Bonnet et al. 12 | Day 30 | PCR on nasal swab | 19 years, respiratory distress and cardiogenic shock, LVEF 20% | PCT 155 μg/L | No | No necrosis | Diuretics, antibiotics, inotropic and vasopressive drugs | |
| TnI 4200 ng/L | Inflammatory infiltrates (T‐lymphocytes and neutrophils) | |||||||
| NT‐pro‐BNP 17 377 pg/mL |
CMR, cardiac magnetic resonance; CRP, C‐reactive protein; ECMO, extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pump; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; NT‐pro‐BNP, N‐terminal pro‐brain natriuretic peptide; PCR, polymerase chain reaction; PCT, procalcitonin; Tn, cardiac troponin levels.
In another patient with severe pulmonary inflammation and cardiogenic shock requiring extracorporeal membrane oxygenation, endomyocardial biopsy showed a low‐grade macrophagic interstitial and endocardial cardiac inflammation, without evidence of acute myocarditis. 11 The severe left ventricular dysfunction recovered spontaneously. Interestingly, the authors observed viral particles within macrophages but not within myocytes, suggesting the role of SARS‐CoV‐2‐mediated cardiac inflammation by either transient viraemia or infected macrophage migration. In our patient, myocardial infiltrates also consisted of macrophages as shown in Figure 2 but without evidence of virus according to PCR analysis. Some similarities can be also found with a study 14 that reported Kawasaki‐like disease in children in the setting of COVID‐19 pandemic. These young patients suffered from multisystem inflammatory syndrome and acute heart failure with complete recovery in a few days, suggesting that myocardial involvement could be due to myocardial stunning/oedema more than to myocardial necrotic damage. Most of them had negative nasopharyngeal RT–PCR, but SARS‐CoV‐2 serology testing was positive in 89% of patients. This study shows also that 60% of patients had cervical lymphadenopathy. Very recently, Bonnet et al. reported a short clinical case of biopsy‐proven severe myocarditis related to COVID‐19 in a young adult male. 12 As in our case, symptoms of fulminant myocarditis were delayed by 1 month after the initial SARS‐CoV‐2 infection, and endomyocardial biopsy failed to demonstrate the presence of virus within myocardium.
Conclusions
We report an exhaustive description of acute lympho‐plasmo‐histiocytic myocarditis related to severe systemic inflammation. Our case is unique by its delay after the initial COVID‐19 infection. Myocarditis is a difficult diagnosis with various underlying mechanisms and therapeutic issues. This report underlines the importance of careful workup to conclude such diagnosis. Our patient recovered without use of anti‐inflammatory drugs or antiviral drugs. Finally, it is important to remind that SARS‐CoV‐2 infection can lead to systemic inflammatory response with organ involvement up to several weeks after initial infection that highlights the importance of clinical surveillance even after resolving of initial symptoms.
Conflict of interest
None declared.
Nicol, M. , Cacoub, L. , Baudet, M. , Nahmani, Y. , Cacoub, P. , Cohen‐Solal, A. , Henry, P. , Adle‐Biassette, H. , and Logeart, D. (2020) Delayed acute myocarditis and COVID‐19‐related multisystem inflammatory syndrome. ESC Heart Failure, 7: 4371–4376. 10.1002/ehf2.13047.
References
- 1. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020; 27: e201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol 2020; 25: e200950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou R. Does SARS‐CoV‐2 cause viral myocarditis in COVID‐19 patients? Eur Heart J 2020; 41: ehaa392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peretto G, Sala S, Caforio AL. Acute myocardial injury, MINOCA, or myocarditis? Improving characterization of coronavirus‐associated myocardial involvement. Eur Heart J 2020; 41: ehaa396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doyen D, Moceri P, Ducreux D, Dellamonica J. Myocarditis in a patient with COVID‐19: a cause of raised troponin and ECG changes. Lancet, 2020; 395:1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luetkens JA, Isaak A, Zimmer S, Nattermann J, Sprinkart AM, Boesecke C, Rieke GJ, Zachoval C, Heine A, Velten M, Duerr GD. Diffuse myocardial inflammation in COVID‐19 associated myocarditis detected by multiparametric cardiac magnetic resonance imaging. Circ Cardiovasc Imaging 2020; 13: e010897. [DOI] [PubMed] [Google Scholar]
- 7. Kim IC, Kim JY, Kim HA, Han S. COVID‐19‐related myocarditis in a 21‐year‐old female patient. Eur Heart J 2020; 41: 1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, de Cobelli F, Tresoldi M, Cappelletti AM, Basso C, Godino C, Esposito A. Acute myocarditis presenting as a reverse Tako‐Tsubo syndrome in a patient with SARS‐CoV‐2 respiratory infection. Eur Heart J 2020; 41: 1861–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, European Society of Cardiology Working Group on Myocardial and Pericardial Diseases . Current stats of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013. Sep; 34: 2636–2648, 2648a‐2648d. [DOI] [PubMed] [Google Scholar]
- 10. Ferreira VM, Schulz‐Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: Expert recommendations. J Am Coll Cardiol 2018; 72: 3158–3176. [DOI] [PubMed] [Google Scholar]
- 11. Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID‐19 cardiogenic shock. Eur J Heart Fail 2020; 22: 911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonnet M, Chamapagnac A, Lantelme P, Harbaoui B. Endomyocardial biopsy findings in Kawasaki‐like disease associated with SARS‐CoV‐2. Eur Heart J 2020; ehaa588 10.1093/eurheartj/ehaa588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doyen D, Moceri P, Ducreux D, Dellamonica J. Myocarditis in a patient with COVID‐19: a cause of raised troponin and ECG changes. Lancet 2020; 395: 1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Belhadjer B, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, Auriau J, Grimaud M, Oualha M, Beghetti M, Wacker J. Acute heart failure in multisystem inflammatory syndrome in children (MIS‐C) in the context of global SARS‐CoV‐2 pandemic. Circulation 2020; 142: 429–436. [DOI] [PubMed] [Google Scholar]


