Abstract
Aims
The prevalence of heart failure (HF) after acute myocardial infarction (AMI) is common. Contemporary data are lacking on the prognostic utility of the measurement of biomarker for patients with AMI complicated by HF according to preserved (HFpEF) and reduced ejection fraction (HFrEF). We aim to assess the association between D‐dimer levels and all‐cause mortality in patients with AMI complicated by different HF subtypes during hospitalization in the context of other risk factors.
Methods and results
We enrolled 4495 patients with AMI with complete clinical and laboratory variable assessments in this cohort. D‐dimer levels were measured on admission immediately at baseline. We used Cox proportional hazards analysis to assess this association accounting for 18 relevant clinical variables. During the index hospitalization, 589 patients with AMI developed HFpEF, 513 patients with AMI developed HFrEF, and 3393 patients with AMI did not develop HF. The patients were divided into HFpEF, HFrEF, and non‐HF groups accordingly. The median length of follow‐up was 1 year (range: 1 to 24 months). During the whole follow‐up, 58 (15.5%), 107 (27.9%), and 96 (4.2%) of the patients experienced death event in HFpEF, HFrEF, and non‐HF groups, respectively. In each group, the patients were divided into high or low D‐dimer levels according to D‐dimer concentration (145 ng/mL). In the fully adjusted model, the risk of all‐cause mortality of those patients with high D‐dimer levels was 2.09 [95% confidence intervals (CI): 1.08 to 4.02, P = 0.02] times as high as the risk of patients with low D‐dimer levels in HFpEF group. When analysing D‐dimer as a continuous variable, this associations still existed. But there was no significant association between D‐dimer concentration and all‐cause mortality in HFrEF [hazard ratio (HR): 1.25, CI: 0.76 to 2.04, P = 0.37] or non‐HF (HR: 1.56, CI: 0.98 to 2.47, P = 0.06), respectively, after fully adjustment for other key clinical variables.
Conclusions
High D‐dimer levels on admission were found to be strongly associated with the subsequent cumulative incidence of all‐cause mortality in patients with AMI complicated by HFpEF.
Keywords: D‐dimer, Acute myocardial infarction, Heart failure with preserved ejection fraction, Mortality, Survival
Introduction
Heart failure (HF) is common after acute myocardial infarction (AMI) 1 , 2 which is considered to be one of its major precursors (contributing to more than 50% of HF in patients). 3 , 4 The occurrence of acute HF in patients with AMI is recognized as a significant predictor of increased morbidity and mortality. 5 , 6 However, important changes in the epidemiology of HF after AMI have taken place over the last few decades, characterized by a decline in its incidence 1 and a change in the case mix according to left ventricular dysfunction, characterized by an increasing proportion of HF cases presenting with preserved ejection fraction, 1 for which treatment benefits are less established 7 and its mortality rate is comparable to that of patients with reduced EF. 8 Heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF) are two distinct HF phenotypes with different etiologic factors and pathophysiologic mechanism 9 and need different treatments as well. 7 Contemporary data are lacking on the prognostic utility of the measurement of biomarker for AMI patients complicated by HF according to preserved/reduced ejection fraction. Thus, a measurement of biomarker that identifies high‐risk patients could potentially be applied to identify those patients who may benefit from greater clinical monitoring and alterations in available therapies.
D‐dimer, a degradation product of cross‐linked fibrin, have been found to be associated with a risk of subsequent thrombotic events, all‐cause mortality, and risk of cardiovascular disease, particularly in patients with vascular disease or coronary heart disease. 10 , 11 , 12 , 13 Our previous study also showed that D‐dimer was associated with the incidence of HF and all‐cause mortality in patients with AMI. 14 In this study, we aim to focus on the prognostic utility of D‐dimer assessment in patients with AMI complicated by different HF subtypes during the index hospitalization.
Methods
Study design and data collection
With the support of National Key R&D Program of China, this prospective, hospital‐based AMI cohort study has been established since 2017 in the 2nd affiliated hospital of Harbin Medical University. 14 A total of 5041 consecutive patients with diagnosed AMI and written consents on admission, including ST‐segment elevation (STEMI) and non‐ST‐segment elevation myocardial infarction (NSTEMI), admitted to the cardiac care unit of the 2nd affiliated hospital of Harbin Medical University were recruited between February 2017 and April 2019. 14 As 195 patients who had prior HF as well as heart valve disease and pericardial constriction, liver disease, infection, uraemia, malignancy, deep venous thrombosis or other thrombotic diseases, or had received thrombolysis therapy before admission, and a further 28 patients who had not D‐dimer assessment during hospitalization were excluded, then a total of 4818 potentially eligible patients were enrolled.
As a further 314 patients had missing data and 9 patients developed HF with unknown type on admission, in the final count, there were 4495 patients with complete clinical and laboratory variable assessments enrolled in this complete data cohort for all‐cause mortality analysis.
Follow up
A total of 4423 patients alive at discharge consented to an additional 1 to 24 months follow‐up. All participants were followed up for the occurrence of death through their complete inpatient and outpatient community medical records from the admission to the occurrence of death or date of the last follow‐up or 28 May 2020. The regular follow‐up visits were at 1, 3, 6, 12, 18, and 24 months. To ensure data quality, the diagnosis of all events was further validated through reviewing medical records by two cardiac doctors. The reconciliation between the two doctors was made if they had a disagreement in the diagnosis. This study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Harbin Medical University.
Definitions
Acute myocardial infarction was diagnosed if a patient had a rise and/or fall of cardiac troponin I (cTNI) with at least one value exceeding the 99th percentile of a normal reference population with one of the following criteria: chest pain lasting 20 min or longer, diagnostic serial electrocardiographic changes consisting of new pathologic Q waves, or ST‐segment and T‐wave changes, or new left bundle branch block. 15 , 16
The definition of HF was made mainly based on the Framingham study 17 and 2016 ESC guideline. 18 Patients with HF post‐AMI were defined as symptoms of dyspnoea with one or more of the following but without non‐cardiac causes of breathlessness: pulmonary rales, oedema of lower limbs, radiographic evidence of pulmonary congestion, third heart sound together with persistent sinus tachycardia, and treatment with diuretic or intravenous vasodilator therapy for HF. Each first incident HF event was categorized as HFpEF (LVEF ≥50%), HFrEF (LVEF <50%), or unclassified HF (no LV function assessment available and would be excluded from this study). 19 , 20 HFpEF diagnosis was established in AMI patients with the above typical symptoms or signs of HF, NT‐proBNP > 300 pg/mL, 21 LVEF ≥ 50%, or other evidences of diastolic dysfunction as evaluated using echocardiography. The key functional alterations are left atrial volume index (LAVI) > 34 mL/m2 or increased left ventricular mass index (LVMI) ≥ 95 g/m2 in women and ≥115 g/m2 in men, plus an E/e′ ≥ 13 and a mean e′septal and lateral wall <9 cm/s. 18
Endpoint
The endpoint was all‐cause mortality in patients with AMI complicated by HFpEF, HFrEF, or non‐HF, respectively.
Common clinical and laboratory assessments
The blood samples were collected on initial presentation to hospital and prior to administration of anticoagulant or antiplatelet use and PCI and were sent to the laboratory for testing immediately. Baseline population characteristics were collected from medical records, prior medication, and self‐reports. Routine blood test, myocardial injury assessment, coagulation function test, liver and nephric function tests, and echocardiography were performed on admission and reviewed when needed. Multivessel disease was defined as at least two major vessels (≥2 mm diameter) with >70% stenosis of the diameter measured by quantitative coronary angiography. 22 Uraemia was defined as a glomerular filtration rate (GFR) of less than 15 mL/min/1.73 m2, or the need for treatment with dialysis or transplantation. 23 The estimated GFR (eGFR) was calculated using baseline serum creatinine concentrations. 24 The management of all data and quality control was performed with an electronic data capture system (Thinvent Technology Ltd, Nanchang, China).
Statistical analysis
Continuous variables were presented as median (25th and 75th percentile) and compared using Mann–Whitney U test. Categorical variables were presented as number (percentages) compared using χ 2 test or Fisher exact test. Normality of continuous variables was assessed by the Kolmogorov–Smirnov test.
The patients with AMI were divided into HFpEF, HFrEF, and non‐HF groups. In each group, the patients were divided into high or low D‐dimer levels according to D‐dimer concentration (145 ng/mL). The cumulative incidence of death of different groups was estimated using Kaplan–Meier method. Univariable and multivariable Cox proportional hazards analyses were constructed to examine the association between D‐dimer levels and all‐cause mortality in each group. Multivariate Cox regression model adjusted for risk factors that are known to influence the endpoint including sex, age, body mass index (BMI), smoking status, AMI‐types and the histories of hypertension, diabetes and myocardial infarction, amino‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and high sensitivity C reactive protein (hs‐CRP), cTNI, eGFR, total cholesterol (TC), percutaneous coronary intervention (PCI), and medical treatment. The regression results are reported by per SD increase in D‐dimer (as a continuous variable) and reported according to D‐dimer binary levels (as a categorical variable) using the low level as the referent to aid in interpretation. A two‐tailed P value of <0.05 was considered statistically significant in this study. All the analyses were performed with EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA).
Results
Patient population
A total of 4495 patients with AMI with complete data were enrolled in this study. During the index hospitalization, 589 patients developed HFpEF, 513 patients developed HFrEF, and 3393 patients did not develop HF. Characteristics of the study population were shown in Table 1 . The median length of follow‐up was 1 year (range: 1 to 24 months). Patients complicated by HFrEF seemed to have higher level of D‐dimer, NT‐proBNP, and hs‐CRP or lower level of eGFR than patients complicated by HFpEF and followed by patients without HF. Patients complicated by HFpEF also had a higher prevalence of hypertension than patients with HFrEF and followed by patients without HF.
Table 1.
Patients with complete data on all baseline variables
| Overall (n = 4495) | HFpEF (n = 589) | HFrEF (n = 513) | Non‐HF (n = 3393) | P value | |
|---|---|---|---|---|---|
| Age (years) | 62 (53–68) | 67 (61–74) | 65 (58–72) | 60 (51–67) | <0.001 |
| Male | 3100 (68.97) | 308 (52.29) | 334 (65.11) | 2458 (72.44) | <0.001 |
| BMI (kg/m2) | 24.69 (22.49–27.34) | 24 (21.85–26.63) | 24.80 (22.12–27.07) | 24.93 (22.82–27.46) | <0.001 |
| Smoking (current + ex) | 2913 (64.81) | 341 (57.89) | 301 (58.67) | 2271 (66.93) | <0.001 |
| Previous history | |||||
| Hypertension | 2361 (52.53) | 353 (59.93) | 284 (55.36) | 1724 (50.81) | <0.001 |
| Diabetes | 1074 (23.89) | 154 (26.15) | 164 (31.97) | 756 (22.28) | 0.942 |
| MI | 440 (9.79) | 63 (10.70) | 94 (18.32) | 283 (8.34) | 0.176 |
| AMI types | |||||
| STEMI | 3062 (68.12) | 392 (66.55) | 369 (71.93) | 2301 (67.82) | 0.018 |
| CAG | 3280 (96.67) | 579 (98.30) | 486 (94.74) | 3280 (96.67) | <0.001 |
| Multivessel disease | 2002 (44.54) | 316 (53.65) | 309 (60.23) | 1377 (40.58) | <0.001 |
| PCI | 3732 (83.03) | 505 (85.74) | 426 (83.04) | 2801 (82.55) | 0.003 |
| LVEDd (mm) | 46 (42.90–49) | 45.50 (42.30–48.30) | 52.60 (48–57) | 45.60 (42.60–48.30) | 0.06 |
| EF (%) | 61 (54–62) | 59 (55–62) | 40.80 (36–45) | 61 (57–63) | <0.001 |
| Laboratory covariates | |||||
| eGFR (mL/min/1.73 m2) | 82.11 (66.14–97.06) | 69.07 (49.66–84.28) | 67.93 (51.51–86.64) | 85.97 (71.16–99.55) | <0.001 |
| cTNI (ng/L) | 32.22 (8.95–93.37) | 41.39 (12.34–122.51) | 61.98 (13.88–149.91) | 28.64 (7.93–80.91) | <0.001 |
| NT‐proBNP (pmol/L) | 996 (342.50–2599.50) | 4535 (1775–6835) | 5653 (2811–10 694) | 669 (243–1461) | <0.001 |
| Hs‐CRP (mg/L) | 5.75 (2.34–12.02) | 10.27 (3.51–13.63) | 12.06 (6.46–14.12) | 4.70 (2.02–10.92) | <0.001 |
| D‐dimer (ng/mL) | 102 (59–201) | 145 (87–289) | 185 (101–433) | 88 (52–166) | <0.001 |
| TG (mmol/L) | 1.38 (0.97–1.97) | 1.32 (0.93–1.89) | 1.29 (0.96–1.78) | 1.41 (0.98–2.02) | <0.001 |
| TC (mmol/L) | 4.54 (3.90–5.27) | 4.41 (3.76–5.24) | 4.30 (3.67–5.13) | 4.58 (3.95–5.31) | 0.008 |
| Medical treatment | |||||
| Aspirin | 4363 (97.06) | 566 (96.10) | 469 (91.42) | 3328 (98.08) | <0.001 |
| Clopidogrel or Ticagrelor | 4685 (97.2) | 571 (96.94) | 470 (91.62) | 3341 (98.47) | <0.001 |
| Statins | 4324 (96.20) | 563 (95.59) | 463 (90.25) | 3298 (97.20) | 0.185 |
| ACEI/ARB | 3312 (73.68) | 447 (75.89) | 376 (73.29) | 2489 (73.36) | <0.001 |
ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; CAG, coronary angiography; cTnI, cardiac troponin I; EF, ejection fraction; eGFR, estimated glomerular filtration rate; Hs‐CRP, high sensitivity C reactive protein; LVEDd, left ventricular end‐diastolic dimension; NT‐proBNP, amino‐terminal pro‐B‐type natriuretic peptide; PCI, percutanous coronary intervention; STEMI, ST‐segment elevation myocardial infarction; TC, total cholesterol; TG, triglyceride.
Values are median (25th and 75th percentile) or number (percentages).
In each group, the patients were divided into high or low D‐dimer levels according to D‐dimer concentration (145 ng/mL). Patients with high D‐dimer levels in each group were more likely to be older and have a lower eGFR, NT‐proBNP, and hs‐CRP (Table 2 ).
Table 2.
Patient characteristics at baseline in different groups divided by HF types and D‐dimer levels
| HFpEF (n = 589) | HFrEF (n = 513) | Non‐HF (n = 3393) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <145 ng/mL | ≥145 ng/mL | <145 ng/mL | ≥145 ng/mL | <145 ng/mL | ≥145 ng/mL | ||||
| n = 294 | n = 295 | P value | n = 200 | n = 313 | P value | n = 2420 | n = 973 | P value | |
| Age (years) | 66 (59–71) | 70 (63–76) | <0.001 | 62.50 (52–69) | 66 (60–75) | <0.001 | 57 (50–65) | 65 (57–71) | <0.001 |
| Male | 154 (52.38) | 154 (52.20) | 0.966 | 142 (71) | 192 (61.34) | 0.025 | 1864 (77.02) | 594 (61.05) | <0.001 |
| BMI (kg/m2) | 24.25 (21.90–26.89) | 23.59 (21.83–26.09) | 0.06 | 24.80 (22.12–27.07) | 23.67 (21.48–25.95) | 0.004 | 25.13 (23.04–27.68) | 24.44 (22.22–26.89) | <0.001 |
| Smoking (current + ex) | 170 (57.82) | 171 (57.97) | 0.972 | 123 (61.50) | 178 (56.87) | 0.299 | 1643 (67.89) | 628 (64.54) | <0.001 |
| Previous history | |||||||||
| Hypertension | 175 (59.52) | 178 (60.34) | 0.84 | 103 (51.50) | 181 (57.83) | 0.16 | 1184 (48.93) | 540 (55.50) | <0.001 |
| Diabetes | 81 (27.55) | 73 (24.75) | 0.439 | 69 (34.50) | 95 (30.35) | 0.326 | 540 (22.31) | 216 (22.20) | 0.942 |
| MI | 38 (12.93) | 25 (8.47) | 0.081 | 48 (24) | 46 (14.70) | 0.008 | 192 (7.93) | 91 (9.35) | 0.176 |
| AMI types | |||||||||
| STEMI | 202 (68.71) | 190 (64.41) | 0.269 | 144 (72) | 225 (71.88) | 0.977 | 1612 (66.61) | 689 (70.81) | 0.018 |
| CAG | 291 (98.98) | 288 (97.63) | 0.685 | 188 (94) | 298 (95.21) | 0.55 | 2356 (97.36) | 924 (94.96) | <0.001 |
| Multivessel disease | 154 (52.38) | 162 (54.92) | 0.537 | 114 (57) | 195 (62.30) | 0.232 | 936 (38.68) | 441 (45.32) | <0.001 |
| PCI | 259 (88.10) | 246 (83.39) | 0.102 | 167 (83.50) | 259 (82.75) | 0.825 | 2027 (83.76) | 774 (79.55) | 0.003 |
| LVEDd (mm) | 45.60 (42.65–48.10) | 45.20 (42–48.65) | 0.674 | 52.85 (48.48–57.60) | 52.50 (47–57) | 0.133 | 45.60 (42.80–48.40) | 45.30 (42.20–48.10) | 0.06 |
| EF (%) | 59 (55–62) | 59 (55–62) | 0.196 | 40.85 (37–45) | 40.70 (35–45) | 0.454 | 62 (58–63) | 61 (56–62) | <0.001 |
| Laboratory covariates | |||||||||
| eGFR (mL/min/1.73 m2) | 76.88 (62.45–92.03) | 58.87 (42.70–76.20) | <0.001 | 81.40 (66.46–93.59) | 60.63 (43.38–76.38) | <0.001 | 88.48 (74.67–101.75) | 77.96 (63.49–92.96) | <0.001 |
| cTNI (ng/L) | 48.77 (14.17–120.47) | 37.81 (11.04–126.67) | 0.54 | 74.51 (17.16–146.01) | 54.19 (12.32–163.93) | 0.417 | 26.59 (7.46–77.44) | 34.36 (9.88–93.26) | <0.001 |
| NT‐proBNP (pmol/L) | 3823 (1115–5298.50) | 5407 (3241–8668.50) | <0.001 | 4045.50 (1799–7365.25) | 7060 (3873–14 274) | <0.001 | 559.50 (202–1211) | 1069 (425–2033) | <0.001 |
| Hs‐CRP (mg/L) | 6.36 (2.44–12.62) | 11.74 (5.41–14.11) | <0.001 | 11.16 (4.33–13.95) | 12.83 (8.27–14.26) | <0.001 | 3.96 (1.73–9.57) | 7.17 (2.97–12.40) | <0.001 |
| TG (mmol/L) | 1.36 (0.97–2.02) | 1.27 (0.91–1.81) | 0.091 | 1.29 (0.98–1.79) | 1.28 (0.94–1.77) | 0.742 | 1.44 (0.99–2.11) | 1.32 (0.96–1.86) | <0.001 |
| TC (mmol/L) | 4.50 (3.79–5.25) | 4.38 (3.73–5.21) | 0.303 | 4.37 (3.78–5.22) | 4.30 (3.60–5.08) | 0.196 | 4.62 (3.98–5.33) | 4.51 (3.89–5.25) | 0.008 |
| Medical treatment | |||||||||
| Aspirin | 289 (98.30) | 277 (93.90) | 0.006 | 190 (95) | 279 (89.14) | 0.021 | 2387 (98.64) | 941 (96.71) | <0.001 |
| Clopidogrel or ticagrelor | 291 (98.98) | 280 (94.92) | 0.004 | 192 (96) | 278 (88.82) | 0.004 | 2394 (98.93) | 947 (97.33) | <0.001 |
| Statins | 288 (97.96) | 275 (93.22) | 0.005 | 191 (95.50) | 272 (86.90) | 0.001 | 2358 (97.44) | 940 (96.61) | 0.185 |
| ACEI/ARB | 231 (78.57) | 216 (73.22) | 0.129 | 164 (82) | 212 (67.73) | <0.001 | 1821 (75.25) | 668 (68.65) | <0.001 |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CAG, coronary angiography; cTnI, cardiac troponin I; EF, ejection fraction; eGFR, estimated glomerular filtration rate; Hs‐CRP, high sensitivity C reactive protein; LVEDd, left ventricular end‐diastolic dimension; NT‐proBNP, amino‐terminal pro‐B‐type natriuretic peptide; PCI, percutaneous coronary intervention; STEMI, ST‐segment elevation myocardial infarction; TC, total cholesterol; TG, triglyceride.
Values are median (25th and 75th percentile) or number (percentages).
All‐cause mortality
During the whole follow‐up, 107 (27.9%), 58 (15.5%), and 96 (4.2%) of the patients experienced death event in HFrEF, HFpEF, and non‐HF groups, respectively (Figure 1 ). In each group, the incidence of death was higher in patients with high D‐dimer levels than patients with low D‐dimer levels (Supplemental Table).
Figure 1.
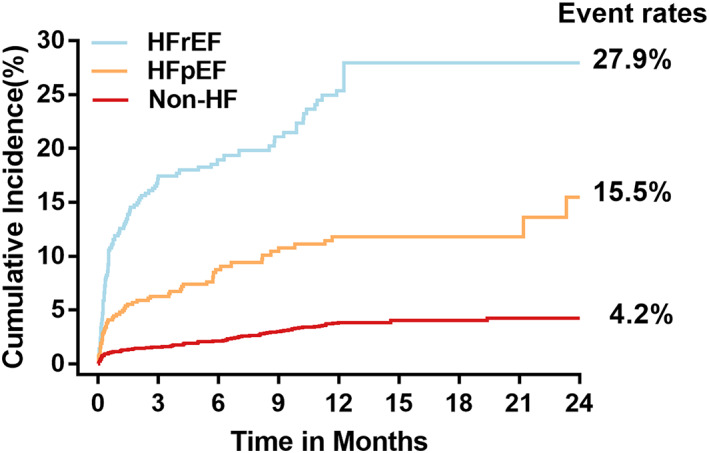
Cumulative incidence of mortality among patients with AMI complicated by HFrEF, HFpEF, or non‐HF, respectively.
Cox regression analysis showed that elevated D‐dimer was strongly associated with mortality in the unadjusted model in the total AMI population and the patients in different groups (Table 3 ). We also observed that the risk of all‐cause mortality among patients with high D‐dimer levels were 1.61 [95% confidence intervals (CI): 1.20 to 2.17, P = 0.002] times as high as the risk among patients with low D‐dimer levels for the total AMI population in the fully adjusted model. When analysing D‐dimer as a continuous variable, this association remained with a 20% increased risk of all‐cause mortality (95% CI: 1.04 to 1.37) for each SD increase in D‐dimer.
Table 3.
Hazard ratios (95% confidence intervals) associated with D‐dimer for outcomes in patients with AMI in different HF groups
| D‐dimer <145 ng/mL | D‐dimer ≥145 ng/mL | P value | 1‐SD increase in D‐dimer | P value | |
|---|---|---|---|---|---|
| All patients | |||||
| Unadjusted | 1 | 3.12 (2.38, 4.10) | <0.001 | 1.60 (1.44, 1.78) | <0.001 |
| Adjusted model | 1 | 1.61 (1.20, 2.17) | 0.002 | 1.20 (1.04, 1.37) | 0.01 |
| HFpEF | |||||
| Unadjusted | 1 | 3.46 (1.89, 6.31) | <0.001 | 1.79 (1.41, 2.26) | <0.001 |
| Adjusted model | 1 | 2.09 (1.08, 4.02) | 0.02 | 1.43 (1.05, 1.94) | 0.024 |
| HFrEF | |||||
| Unadjusted | 1 | 2.27 (1.46, 3.53) | <0.001 | 1.34 (1.14, 1.58) | <0.001 |
| Adjusted model | 1 | 1.25 (0.76, 2.04) | 0.37 | 0.97 (0.78, 1.21) | 0.81 |
| Non‐HF | |||||
| Unadjusted | 1 | 3.77 (2.51, 5.66) | <0.001 | 1.92 (1.62, 2.28) | <0.001 |
| Adjusted model | 1 | 1.56 (0.98, 2.47) | 0.06 | 1.40 (1.10, 1.77) | 0.001 |
Unadjusted model adjusted for: none. Adjusted model adjusted for: sex, age, BMI, smoking status, AMI‐types and the histories of hypertension, diabetes, and myocardial infarction, NT‐proBNP, cTNI, hs‐CRP, eGFR, TC, PCI, and medical treatments including aspirin, clopidogrel or ticagrelor, and statins.
When divided the patients with AMI into HFpEF, HFrEF, or non‐HF groups, we observed that the risk of all‐cause mortality among patients with D‐dimer values in high levels was 2.09 (95% CI: 1.08 to 4.02, P = 0.02) times as high as the risk among patients with D‐dimer values in low levels in HFpEF group after fully adjustment for other variables. When analysing D‐dimer as a continuous variable, this association remained with a 43% increased risk of all‐cause mortality (95% CI: 1.05 to 1.94, P = 0.024) for each SD increase in D‐dimer. But there was no significant association between D‐dimer concentration and all‐cause mortality in HFrEF [hazard ratio (HR): 1.25, CI: 0.76 to 2.04, P = 0.37] or non‐HF (HR: 1.56, CI: 0.98 to 2.47, P = 0.06), respectively, after fully adjusting for other key clinical variables.
Discussion
Summary
In the present study, we demonstrated an association between plasma concentrations of D‐dimer assessed on admission and the subsequent cumulative incidence of all‐cause mortality in patients with AMI complicated by HFpEF, which was independent of traditional clinical factors and usual biomarkers. In addition, we observed a non‐significant association between D‐dimer levels and the incidence of all‐cause mortality in patients with AMI complicated by HFrEF or non‐HF.
Comparison with existing literature
After AMI, HF strongly increases the risk of mortality independently of key confounders, including MI severity, co‐morbidity, and acute treatment. 5 Patients with HFrEF and HFpEF after AMI shared a similar prognosis. 5 , 25 The prevalence of HFpEF relative to HFrEF was rising at an alarming rate of 1% per year, yet survival improved over time for those with reduced EF but not for those with preserved EF. 25 Hence, there is a strong need to identify patients with HFpEF post‐AMI who are at high risk of death and improve prevention strategies in the management. What is more, contemporary data are lacking on the prognostic utility of the measurement of biomarker for AMI patients complicated by HF according to preserved/reduced ejection fraction. To the best of our knowledge, our study is the first to evaluate the prognostic utility of D‐dimer assessment in AMI patients complicated by different HF subtypes, and we also assess the association among AMI patients complicated by non‐HF in addition.
In line with our results, some previous studies conducted in patients with coronary heart disease or acute coronary syndrome also found that D‐dimer was predictive for the occurrence of mortality. 26 , 27 , 28 , 29 With respect to the HF population, a study showed that a plasma concentration of D‐dimer >250 ng/mL increased almost threefold risk for all‐cause mortality during a follow‐up period of 6 years in elderly patients with HF. 30 What was different to us is that D‐dimer was found to be associated with cardiovascular mortality in hospitalized patients with HFrEF in another study. 31 A recent research also revealed that higher D‐dimer levels were associated with an increased risk of all‐cause death irrespective of the subtypes of HF including HFrEF and HFpEF. 32 The difference among these results may be that our study was conducted in the AMI population accompanied by HFpEF, HFrEF, or non‐HF during the index admission, but the other studies were only conducted in the pure HF population or coronary heart disease population.
HFpEF and HFrEF are two distinct HF phenotypes with different etiologic factors and pathophysiologic mechanism. 9 Myocardial remodelling of HFpEF differs from HFrEF, where remodelling is driven by loss of cardiomyocyte. The size of myocardial infarct, myocyte loss, and myocardial necrosis are the principal causes of HFrEF development after AMI. 33 However, the development of HFpEF in patients with AMI is complex: Ischaemic and necrotic myocardium promotes the process of cardiac systolic and diastolic dysfunction, and the stunned myocardium in surrounding and the surviving cardiomyocytes can present transient impairment of contraction and relaxation, 34 especially affecting the diastolic function which requires consuming oxygen and glucose. 35 The extent of ventricular enlargement after infarction is related to the magnitude of the initial damage to the myocardium, and although an increase in cavity size tends to restore stroke volume despite a persistently reduced ejection fraction, ventricular dilation has been associated with a reduction in survival. 33 Thus, the mortality of HFrEF group was more than the HFpEF group just as our study showed.
D‐dimer, acting as a marker of a thrombotic burden and reflecting the turnover of fibrin secondary to plaque rupture at any vascular site and plasmin‐mediated fibrin degradation, also a sensitive marker of ongoing thrombosis, may be directly linked to an inflammatory vascular state. 13 , 36 There are two points that may explain the association between D‐dimer concentration and all‐cause mortality in patients with AMI complicated by HFpEF. At one hand, hospitalized HF is often accompanied with vascular abnormalities, increased coagulability, and impaired blood flow. 37 It is reasonable to conceive that early elevation of D‐dimer may reflect the development or the severity of several chronic diseases, including cardiovascular disease, cancer, or infectious diseases which lead to augmented mortality. On the other hand, the mechanism behind the association between D‐dimer and death may be related to a long‐term risk of thrombotic events in association with various pathogenic pathways, including atherosclerosis, inflammatory, and infectious disease processes. 29 Hence, the patients with high D‐dimer levels may be associated with a cumulative incidence of death just as our study suggested.
However, the association between D‐dimer and mortality in patients with HFrEF was not statistically significant in our study, possibly due to the significant increasing levels and remarkable prognostic value of NT‐proBNP and cTNI that attenuated the effect of D‐dimer. What is more, the association between D‐dimer and mortality in patients with non‐HF was not significant, possibly due to the overall low levels of D‐dimer in this population.
Limitations
Some limitations should be acknowledged in interpreting these data. First, these results emanated from a single‐centre study and was performed in a Northeast Chinese population, and thus, they may not be applicable to other populations. Second, we did not conduct serial measurements for D‐dimer levels at other time points, particularly the peak levels after the occurrence of AMI; however, the D‐dimer levels on admission also gave us strong information for the prognosis of patients with AMI complicated by HFpEF during the index hospitalization. Third, this was an observational cohort study, and the observed differences in clinical outcomes and D‐dimer levels may be subject to possible confounders which we were unable to control for. However, our findings were strongly statistically significant and clinically meaningful, and relevant results were shown by viewing the numerical data.
Conclusions
In patients with AMI complicated by HFpEF, a high D‐dimer level is an important independent and sustained risk factor for all‐cause mortality. It predicts risk independently and in addition to known traditional risk factors and biomarkers, with the potential to guide management decisions.
Conflict of interest
None declared.
Funding
This work was supported by National Key R&D Program of China (no. 2016YFC1301100, no. 2016YFC1301101) and Major Instrument Development Project of National Natural Science Foundation of China (no. 81827806).
Supporting information
Data S1. Supporting Information
Acknowledgements
The authors acknowledge Jonathan Stock for correcting English and Duolao Wang for guiding the statistical analysis of the manuscript selflessly.
Zhang, X. , Wang, S. , Sun, L. , Fang, S. , and Yu, B. (2020) Prognostic value of D‐dimer in acute myocardial infarction complicated by heart failure with preserved ejection fraction. ESC Heart Failure, 7: 4118–4125. 10.1002/ehf2.13027.
Contributor Information
Shaohong Fang, Email: fangshaohong7802@163.com.
Bo Yu, Email: dryu_hmu@163.com.
References
- 1. Gerber Y, Weston SA, Berardi C, McNallan SM, Jiang R, Redfield MM, Roger VL. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am J Epidemiol 2013; 178: 1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D'Agostino RB, Levy D, Kannel WB, Vasan RS. Long‐term trends in the incidence of heart failure after myocardial infarction. Circulation 2008; 118: 2057–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Minicucci MF, Azevedo PS, Polegato BF, Paiva SA, Zornoff LA. Heart failure after myocardial infarction: clinical implications and treatment. Clin Cardiol 2011; 34: 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lippi G, Cervellin G. Risk assessment of post‐infarction heart failure. Systematic review on the role of emerging biomarkers. Crit Rev Clin Lab Sci 2014; 51: 13–29. [DOI] [PubMed] [Google Scholar]
- 5. Gerber Y, Weston SA, Enriquez‐Sarano M, Berardi C, Chamberlain AM, Manemann SM, Jiang R, Dunlay SM, Roger VL. Mortality associated with heart failure after myocardial infarction: a contemporary community perspective. Circ Heart Fail 2016; 9: e002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Emanuelsson H, Karlson BW, Herlitz J. Characteristics and prognosis of patients with acute myocardial infarction in relation to occurrence of congestive heart failure. Eur Heart J 1994; 15: 761–768. [DOI] [PubMed] [Google Scholar]
- 7. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J 2011; 32: 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA 2006; 296: 2209–2216. [DOI] [PubMed] [Google Scholar]
- 9. Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation 2011; 123: 2006–2013 discussion 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. di Castelnuovo A, de Curtis A, Costanzo S, Persichillo M, Olivieri M, Zito F, Donati MB, de Gaetano G, Iacoviello L. Association of D‐dimer levels with all‐cause mortality in a healthy adult population: findings from the MOLI‐SANI study. Haematologica 2013; 98: 1476–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ridker PM, Hennekens CH, Cerskus A, Stampfer MJ. Plasma concentration of cross‐linked fibrin degradation product (D‐dimer) and the risk of future myocardial infarction among apparently healthy men. Circulation 1994; 90: 2236–2240. [DOI] [PubMed] [Google Scholar]
- 12. Lowe GD, Rumley A. Use of fibrinogen and fibrin D‐dimer in prediction of arterial thrombotic events. Thromb Haemost 1999; 82: 667–672. [PubMed] [Google Scholar]
- 13. Lee LV, Ewald GA, McKenzie CR, Eisenberg PR. The relationship of soluble fibrin and cross‐linked fibrin degradation products to the clinical course of myocardial infarction. Arterioscler Thromb Vasc Biol 1997; 17: 628–633. [DOI] [PubMed] [Google Scholar]
- 14. Zhang X, Wang S, Liu J, Wang Y, Cai H, Wang D, Fang S, Yu B. D‐dimer and the incidence of heart failure and mortality after acute myocardial infarction. Heart 2020. [DOI] [PubMed] [Google Scholar]
- 15. Thygesen K, Alpert JS, White HD. Joint ESCAAHAWHFTFftRoMI. Universal definition of myocardial infarction. J Am Coll Cardiol 2007; 50: 2173–2195. [DOI] [PubMed] [Google Scholar]
- 16. Santoro GM, Carrabba N, Migliorini A, Parodi G, Valenti R. Acute heart failure in patients with acute myocardial infarction treated with primary percutaneous coronary intervention. Eur J Heart Fail 2008; 10: 780–785. [DOI] [PubMed] [Google Scholar]
- 17. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 18. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 19. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed) 2016; 69: 1167. [DOI] [PubMed] [Google Scholar]
- 20. Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, Bartz TM, Santhanakrishnan R, Lee DS, Chan C, Liu K, Blaha MJ, Hillege HL, van der Harst P, van Gilst WH, Kop WJ, Gansevoort RT, Vasan RS, Gardin JM, Levy D, Gottdiener JS, de Boer RA, Larson MG. Predicting heart failure with preserved and reduced ejection fraction: the international collaboration on heart failure subtypes. Circ Heart Fail 2016; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Kardiol Pol 2016; 74: 1037–1147. [DOI] [PubMed] [Google Scholar]
- 22. Thiele H, Akin I, Sandri M, de Waha‐Thiele S, Meyer‐Saraei R, Fuernau G, Eitel I, Nordbeck P, Geisler T, Landmesser U, Skurk C, Fach A, Jobs A, Lapp H, Piek JJ, Noc M, Goslar T, Felix SB, Maier LS, Stepinska J, Oldroyd K, Serpytis P, Montalescot G, Barthelemy O, Huber K, Windecker S, Hunziker L, Savonitto S, Torremante P, Vrints C, Schneider S, Zeymer U, Desch S, CULPRIT‐SHOCK Investigators . One‐year outcomes after PCI strategies in cardiogenic shock. N Engl J Med 2018; 379: 1699–1710. [DOI] [PubMed] [Google Scholar]
- 23. Levey AS, Coresh J. Chronic kidney disease. Lancet 2012; 379: 165–180. [DOI] [PubMed] [Google Scholar]
- 24. Levey AS, Stevens LA, Schmid CH, Zhang Y(L), Castro AF III, Feldman HI, Kusek JW, Eggers P, van Lente F, Greene T, Coresh J, for the CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 26. Oldgren J, Linder R, Grip L, Siegbahn A, Wallentin L. Coagulation activity and clinical outcome in unstable coronary artery disease. Arterioscler Thromb Vasc Biol 2001; 21: 1059–1064. [DOI] [PubMed] [Google Scholar]
- 27. Moss AJ, Goldstein RE, Marder VJ, Sparks CE, Oakes D, Greenberg H, Weiss HJ, Zareba W, Brown MW, Liang CS, Lichstein E, Little WC, Gillespie JA, van Voorhees L, Krone RJ, Bodenheimer MM, Hochman J, Dwyer EM Jr, Arora R, Marcus FI, Watelet LFM, Case RB. Thrombogenic factors and recurrent coronary events. Circulation 1999; 99: 2517–2522. [DOI] [PubMed] [Google Scholar]
- 28. Mjelva OR, Ponitz V, Brugger‐Andersen T, Grundt H, Staines H, Nilsen DW. Long‐term prognostic utility of pentraxin 3 and D‐dimer as compared to high‐sensitivity C‐reactive protein and B‐type natriuretic peptide in suspected acute coronary syndrome. Eur J Prev Cardiol 2016; 23: 1130–1140. [DOI] [PubMed] [Google Scholar]
- 29. Simes J, Robledo KP, White HD, Espinoza D, Stewart RA, Sullivan DR, Zeller T, Hague W, Nestel PJ, Glasziou PP, Keech AC, Elliott J, Blankenberg S, Tonkin AM, For the LIPID Study Investigators . D‐dimer predicts long‐term cause‐specific mortality, cardiovascular events, and cancer in patients with stable coronary heart disease. Circulation 2018; 138: 712–723. [DOI] [PubMed] [Google Scholar]
- 30. Alehagen U, Dahlstrom U, Lindahl TL. Elevated D‐dimer level is an independent risk factor for cardiovascular death in out‐patients with symptoms compatible with heart failure. Thromb Haemost 2004; 92: 1250–1258. [DOI] [PubMed] [Google Scholar]
- 31. Zorlu A, Yilmaz MB, Yucel H, Bektasoglu G, Refiker Ege M, Tandogan I. Increased D‐dimer levels predict cardiovascular mortality in patients with systolic heart failure. J Thromb Thrombolysis 2012; 33: 322–328. [DOI] [PubMed] [Google Scholar]
- 32. Yan W, Liu J, Liu H, Lu J, Chen J, Rong R, Song L, Tang H, Li J, He K. Elevated D‐dimer levels predict adverse outcomes in hospitalised elderly patients with chronic heart failure. Intern Med J 2019; 49: 1299–1306. [DOI] [PubMed] [Google Scholar]
- 33. Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990; 81: 1161–1172. [DOI] [PubMed] [Google Scholar]
- 34. Braunwald E, Kloner RA. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation 1982; 66: 1146–1149. [DOI] [PubMed] [Google Scholar]
- 35. Solomon SD, Glynn RJ, Greaves S, Ajani U, Rouleau JL, Menapace F, Arnold JMO, Hennekens C, Pfeffer MA. Recovery of ventricular function after myocardial infarction in the reperfusion era: the healing and early afterload reducing therapy study. Ann Intern Med 2001; 134: 451–458. [DOI] [PubMed] [Google Scholar]
- 36. Weitz JI, Fredenburgh JC, Eikelboom JW. A test in context: D‐dimer. J Am Coll Cardiol 2017; 70: 2411–2420. [DOI] [PubMed] [Google Scholar]
- 37. Haas S. Venous thromboembolism in medical patients—the scope of the problem. Semin Thromb Hemost 2003; 29: 17–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information


