Abstract
Background
Centers for Disease Control and Prevention (CDC) guidelines recommend 240 mg gentamicin plus 2 g azithromycin for the treatment of gonorrhea in cephalosporin-allergic patients. The efficacy of gentamicin alone in the treatment of pharyngeal gonorrhea is uncertain.
Methods
Between September 2018 and March 2019, we enrolled men who have sex with men with nucleic acid amplification test–diagnosed pharyngeal gonorrhea in a single-arm, unblinded clinical trial. Men received a single 360-mg intramuscular dose of gentamicin and underwent test of cure by culture 4–7 days later. The study measured creatinine at enrollment and test of cure, serum gentamicin concentration postdose to establish peak concentration (Cmax), and standard antimicrobial minimum inhibitory concentrations (MICs) by agar dilution. The trial was designed to establish a point estimate for gentamicin’s efficacy for pharyngeal gonorrhea. We planned to enroll 50 evaluable participants; assuming gentamicin was 80% efficacious, the trial would establish a 95% confidence interval (CI) of 66%–90%. We planned interim analyses at n = 10 and n = 25.
Results
The study was stopped early due to poor efficacy. Of 13 enrolled men, 10 were evaluable, and only 2 (20% [95% CI, 2.5%–55.6%]) were cured. Efficacy was not associated with gentamicin Cmax or MIC. No participants experienced renal insufficiency. The mean creatinine percentage change was +5.2% (range, −6.7% to 21.3%). Six (46%) participants experienced headache, all deemed unrelated to treatment.
Conclusions
Gentamicin alone failed to eradicate Neisseria gonorrhoeae from the pharynx. Clinicians should use caution when treating gonorrhea with the CDC’s current alternative regimen (gentamicin 240 mg plus azithromycin 2 g) given increases in azithromycin resistance and gentamicin’s poor efficacy at the pharynx.
Clinical Trials Registration
Keywords: sexually transmitted infections, gonorrhea, pharynx, clinical trial, Neisseria gonorrhoeae
Gentamicin, currently the backbone antibiotic of the Centers for Disease Control and Prevention’s alternative treatment regimen for gonorrhea, failed to eradicate the gonococcus from the pharynx in 80% of cases in this nonblinded clinical trial.
As the second most common bacterial sexually transmitted infection in the United States (US), Neisseria gonorrhoeae (NG) infects mucosal surfaces and leads to major morbidities such as pelvic inflammatory disease, ectopic pregnancies, and infertility in women. NG also increases the risk of human immunodeficiency virus (HIV) transmission and acquisition [1]. One of the complicating characteristics of the gonococcus is its ability to rapidly develop and acquire lasting antimicrobial resistance, as evidenced by its serial development of resistance to sulfonamides, penicillins, tetracyclines, and fluoroquinolones over the past 75 years [2]. Current gonococcal treatment consists of an injectable third-generation cephalosporin (ceftriaxone) in combination with a macrolide (azithromycin). However, the rapid rise of azithromycin resistance, which by 2017 was 4.4% of tested urethral NG isolates in the US [3], threatens this regimen.
With no new drugs currently available, recycling older drugs—therapies that have never or rarely been used routinely for NG in the United States—might be a viable option to maintain an antimicrobial armamentarium while awaiting the arrival of novel agents. Gentamicin has been used as single-dose therapy for urogenital gonorrhea in Malawi for years [4, 5], and a meta-analysis suggested that, as a single agent, the drug is 91% effective for urogenital infection [6]. Although this level of efficacy does not meet the World Health Organization’s (WHO) criteria for an effective gonococcal agent (≥95%) [6], it suggests that gentamicin has significant efficacy and could be a good therapy, particularly if used in combination with another drug. In vitro data suggest that the addition of gentamicin to cefixime or ceftriaxone was not synergistic but may improve pharmacodynamics [7], and a 2012 noncomparative clinical trial found that a single 240-mg dose of gentamicin administered intramuscularly (IM) plus 2 g of azithromycin administered orally (PO) was 100% effective against genital and extragenital NG infections; however, extragenital infections were rare (1 rectal and 10 pharyngeal) [8]. This trial led to gentamicin 240 mg IM plus azithromycin 2 g PO becoming a Centers for Disease Control and Prevention (CDC)–recommended alternative regimen in 2015 [1]. However, the contribution of gentamicin to the efficacy observed in the trial is uncertain as azithromycin 2 g alone was highly effective against macrolide-susceptible NG, and only 0.5% of isolates tested in this study had an azithromycin minimum inhibitory concentration (MIC) ≥2 μg/mL [8].
Although many clinicians do not routinely test patients for pharyngeal gonorrhea, the infection is common [9], yet pharyngeal gonorrhea is more difficult to eradicate than infections occurring at other anatomic sites [10]. Although ceftriaxone is >96% effective [1] in eradicating the gonococcus from the throat, persons reporting penicillin or β-lactam hypersensitivity cannot be treated with ceftriaxone. An older aminoglycoside, spectinomycin, which is no longer available in the US, demonstrated poor efficacy at the throat, but the reasons for poor response are unclear and it is unknown whether gentamicin would yield a similar response [10]. Thus, we evaluated the efficacy of a single 360-mg IM dose of gentamicin alone for the treatment of pharyngeal gonorrhea.
METHODS
Study Design and Study Population
This study was designed as a single-arm, nonblinded, demonstration trial. We planned to recruit 60 men who have sex with men (MSM) who screened positive for pharyngeal gonorrhea, had not yet received treatment, and presented to the Public Health–Seattle & King County (PHSKC) sexually transmitted diseases (STD) clinic for treatment. At the PHSKC STD clinic, all MSM patients who report performing fellatio in the last 60 days are screened for pharyngeal gonorrhea as part of routine clinical care using a nucleic acid amplification test (NAAT; Aptima Combo 2, Hologic, Marlborough, Massachusetts). We excluded potential study participants with any of the following: age <16 years; known allergy to any aminoglycoside; history of renal disease (eg, diagnosis of solitary kidney, chronic renal insufficiency, renal cell carcinoma); concurrent nephrotoxic drugs or muscle relaxants; history of diabetes, hearing loss or tinnitus; diagnosis with concurrent syphilis or chlamydial infection necessitating an additional antibiotic at the clinic visit; or inability to return for a follow-up visit. We did not exclude potential study participants based on HIV status or concomitant untreated NG infection at the urethra and/or rectum. This study was approved by the University of Washington’s Human Subjects Division and was registered at ClinicalTrials.gov (NCT03632109).
Study Procedures
Following informed consent, the study clinician obtained swabs for gonococcal culture at the pharynx and all other NAAT NG-positive anatomic sites. Specimens for culture were obtained with a polyester swab, plated directly onto selective Thayer-Martin media, and placed in a candle (carbon dioxide) jar in a 37°C incubator within 15 minutes of collection. Plates were transported to the Neisseria Reference Laboratory daily.
After specimen collection, participants received 360 mg gentamicin IM. We elected to use 360 mg of gentamicin rather than the 240-mg dose currently recommended by the CDC based on established pharmacokinetic/pharmacodynamic (PK/PD) data. Gentamicin exhibits concentration-dependent bactericidal activity, meaning that the ratio of the peak serum concentration (Cmax) relative to the MIC is the strongest predictor of efficacy [11]. Although the ideal Cmax/MIC ratio for NG has not been determined, for other gram-negative bacteria peak gentamicin levels between 2 and 10 times the MIC have been considered optimal [12–14]. The Gonococcal Isolate Surveillance Project, the antimicrobial resistance surveillance program in the US, began testing gonococcal isolates for gentamicin susceptibility in 2015; the current US gentamicin MIC90 is 8 µg/mL [3]. For persons weighing 50–90 kg, a 360-mg dose of gentamicin is expected to achieve serum levels between 16 µg/mL and 27 µg/mL, corresponding to Cmax/MIC ratios of 2–7 for the majority of isolates in the US. However, serum concentrations and PK/PD targets developed in patients with sepsis or pneumonia may not translate well to pharyngeal infection compartment and NG [12–14]. Thus, an additional consideration in developing a single-dose treatment for pharyngeal gonorrhea was the maximum tolerable IM injection (~5 mL). Gentamicin is formulated as 40 mg/mL, meaning that a 360-mg dose requires a 9-mL volume of solution. For this study, the 360-mg dose was divided into two 4.5-mL injections, one in each gluteus maximus.
The study clinician collected blood specimens 30, 45, and 60 minutes following drug administration to define peak drug levels (Cmax); this blood specimen was also tested for baseline serum creatinine. Blood specimens were centrifuged immediately following collection; serum was aliquoted and frozen at −80°C until tested by the University of Washington Laboratory Medicine. Participants were counseled to abstain from all sex, especially oral sex and kissing, between treatment and follow-up.
Participants returned to the PHSKC STD clinic 4–7 (±1) days following treatment for test of cure (TOC). TOC was determined by culture at all infected anatomic sites using the same procedures described above. At the TOC visit, the study clinician queried participants about intervening sexual activity and side effects, and obtained another blood specimen to evaluate renal function (creatinine). Participants who experienced treatment failure were called to return to the clinic for treatment with ceftriaxone 250 mg plus 1 g azithromycin (standard of care). Participants were compensated for their time.
Outcomes
Our primary study outcome was clearance of NG at the pharynx, as demonstrated by a negative culture at TOC. We defined treatment failure as a positive culture 3–8 days after treatment. Clearance of NG at the rectum was a secondary outcome. We evaluated tolerability of the regimen using a standardized symptom questionnaire conducted at the TOC visit that specifically queried participants about intervening headaches, vision changes, hearing changes, tinnitus, urinary symptoms, nausea/vomiting, diarrhea, constipation, and rash and allowed for free response of otherwise uncaptured symptoms. Renal safety was evaluated by calculating the percentage change in serum creatinine between baseline and TOC.
Additional study aims included documenting the mean peak gentamicin levels following 360 mg IM of gentamicin stratified by weight, and correlating the achieved Cmax/MIC ratio with the primary outcome. Adjusted body weight was used to calculate the weight-based dose in obese patients [15]. Among treatment failures, we planned exploratory analyses comparing pre- and posttreatment MIC for evidence of resistance emergence.
Statistical Analysis
As this study was designed as a demonstration trial to determine the potential for a larger randomized controlled trial, we sought to establish a point estimate and 95% confidence interval (CI) of the efficacy of 360 mg gentamicin IM for pharyngeal gonorrhea. In determining the sample size, we reasoned that if gentamicin 360 mg alone were at least 80% effective at the pharynx, it could be combined with another drug as an effective regimen. Thus, assuming an 80% cure rate with 360 mg of gentamicin, we determined that a sample size of 50 would give a reasonable 95% CI (66%–90%) to suggest that the drug could potentially be used as part of a 2-drug treatment regimen. To account for loss to follow-up and negative NG culture at enrollment, we increased our sample size by 20% (N = 60).
We planned interim analyses after completing data collection on 10 and 25 evaluable participants, with α levels of .0001 and .003, respectively. Predetermined stopping rules were (1) an efficacy upper bound of a 100 × (1 – α)% 2-sided CI <80%; or (2) renal toxicity, as defined as a >40% increase in serum creatinine at TOC from baseline creatinine in >20% of participants. We chose 20% of participants because if 20% of participants experienced renal toxicity it was unlikely to be due to chance (95% CI, 7%–40%). An independent medical monitor reviewed interim analyses data.
The primary endpoint, the pharyngeal cure rate, was calculated as the number of cured participants divided by the total number of participants who received treatment and returned for follow-up testing in a modified intention-to-treat analysis. Although not a primary objective, we reported on the cure rate for rectal gonorrhea among those with concomitant infection. Participant tolerability of the treatment regimen was reported as the number and proportion of participants who reported side effects as descriptive statistics.
To estimate the ideal pharmacodynamic criterion, we log-transformed the Cmax/MIC ratios to calculate the geometric mean. As an exploratory analysis, we planned to compare pre- and posttreatment gentamicin MICs of treatment failures to estimate whether single-dose gentamicin therapy can select for resistance. We considered an increase in the MIC by 2 doubling dilutions (eg, from MIC of 2 µg/mL to 8 µg/mL) to represent a meaningful change in susceptibility [16].
RESULTS
Enrolled Participants
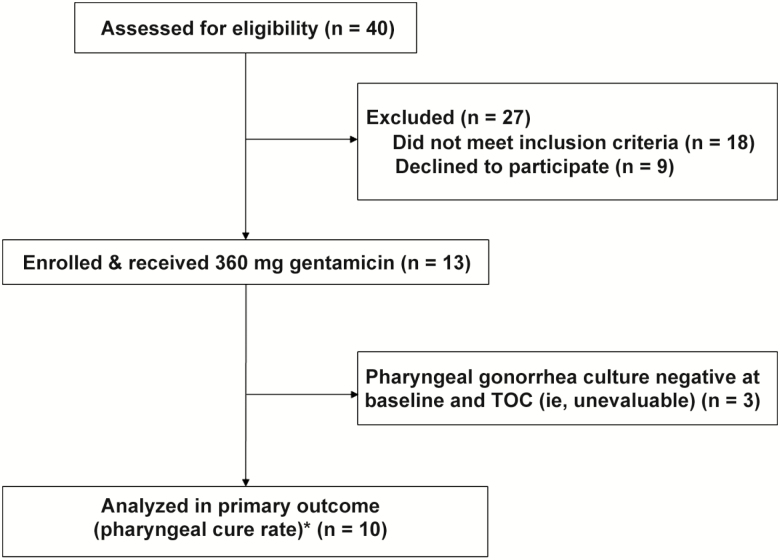
Between September 2018 and March 2019, we enrolled 13 MSM with pharyngeal gonorrhea in the Gent Study (Figure 1). All men were HIV negative, and >60% were taking tenofovir disoproxil fumarate/emtricitabine (Truvada) for HIV pre-exposure prophylaxis. The mean age of participants was 29.5 years. Table 1 describes the study population.
Figure 1.
Study flow diagram. *All 13 enrolled participants returned for test of cure. Abbreviation: TOC, test of cure.
Table 1.
Demographics and Baseline Characteristics of All Enrolled Men (N = 13)
| Characteristic | No. (%) or Means (Range) |
|---|---|
| Age, y, mean (range) | 29.3 (21–44) |
| Race | |
| White | 3 (23) |
| Black/African American | 1 (7.7) |
| Asian/Pacific Islander | 3 (23) |
| Other | 6 (46.2) |
| Weight, kg, mean (range) | 86.4 (53–133) |
| Height, in, mean (range) | 70.9 (68–76) |
| BMI, kg/m2, mean (range) | 26.6 (17.9–43.4) |
| Baseline creatinine, mg/dL, mean (range) | 0.86 (0.74–1.05) |
| HIV positive | 0 |
| On PrEP (Truvada) | 8 (61.5) |
| Gender of sex partners | |
| Male | 13 (100) |
| Female | 2 (15.4) |
| Transgender male | 3 (23) |
| No. of sex partners participant gave oral sex within ≤2 mo, mean (range) | 4.2 (0–10) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; PrEP, pre-exposure prophylaxis.
Efficacy and PK/PD
Of 10 evaluable participants with pharyngeal gonorrhea, only 2 (20% [95% CI, 2.5%–55.6%]) were cured. The upper bound of the 99.99% CI for efficacy after 10 evaluable participants was 81%. Although this did not precisely meet the early stopping criteria of <80% efficacy, a decision was made to stop the study for poor efficacy.
One participant reported sexual activity between treatment and TOC. However, he was deemed unevaluable due to negative cultures at both enrollment and TOC. Two men had rectal gonococcal infections, both of whom had negative rectal cultures at TOC.
The gentamicin Cmax ranged from 15 µg/mL to >30 µg/mL (upper limit of quantification) (Table 2), and Cmax was not associated with time of blood draw following gentamicin dose. In this young, healthy population, the equivalent weight-based dosing of the 360-mg dose ranged from 3.64 mg/kg to 6.84 mg/kg. The arithmetic mean Cmax/MIC ratio was 3.61 (range, 2.04 – 7.05). Cure was not associated with gentamicin Cmax or MIC (Table 3). Cure was also not associated with weight-based dosing nor the log-transformed Cmax/MIC ratio. However, the small number of cures does not allow robust correlation of PK/PD with outcomes. Among treatment failures (n = 8), none had a TOC gentamicin MIC >1 doubling dilution greater than the enrollment MIC. All cultured isolates (enrollment and TOC) had MIC ≤8 mg/L.
Table 2.
Clinical Outcomes of Pharyngeal and Rectal Infections Treated With 360 mg Intramuscular Gentamicin, Gentamicin Minimum Inhibitory Concentration (MIC), Peak Serum Concentration (Cmax), Weight-Based Dosing, and Cmax/MIC Ratio
| Study ID | Pharyngeal Treatment Outcome | Rectal Treatment Outcome | Gentamicin MIC, µg/mL | Cmax, µg/mL | Weight-based Dosing, mg/kg | Cmax/MIC Ratio |
|---|---|---|---|---|---|---|
| 1 | Failure | NA | 8 | 20.7 | 5.12 | 2.59 |
| 2 | Failure | NA | 8 | 24.7 | 4.36 | 3.09 |
| 3 | Failure | Cure | 8a | >30 | 6.73 | >3.75 |
| 4 | Unevaluable | NA | NA | >30 | 4.9 | NA |
| 5 | Cure | NA | 8 | 20.9 | 3.97 | 2.61 |
| 6 | Failure | NA | 8 | 16.3 | 3.89 | 2.04 |
| 7 | Cure | NA | 8 | 16.8 | 4.56 | 2.1 |
| 8 | Failure | NA | 4 | 28.2 | 6.84 | 7.05 |
| 9 | Failure | NA | 8 | Missing | 3.64b | NA |
| 10 | Unevaluable | Cure | 4c | 15 | 4.59 | 3.75c |
| 11 | Failure | NA | 4 | 15 | 3.75b | 3.75 |
| 12 | Unevaluable | NA | NA | 15.7 | 4.22b | NA |
| 13 | Failure | NA | 8 | Missing | 4.07 | NA |
Abbreviations: Cmax, peak serum concentration; ID, identifier; MIC, minimum inhibitory concentration; NA, not applicable.
aPharyngeal and rectal isolate with same gentamicin MIC.
bAdjusted body weight used to determined weight-based dosing for obese participants (body mass index >30 kg/m2).
cMIC and Cmax/MIC ratio are reported for rectal isolate.
Table 3.
Analysis of Pharmacokinetic/Pharmacodynamic Predictors of Cure of Pharyngeal Gonorrhea With 360 mg Intramuscular Gentamicin
| Predictor | Cure (n = 2) | Failure (n = 8) | P Value |
|---|---|---|---|
| Gentamicin MIC, μg/mL, median | 8 | 8 | .486 |
| Gentamicin Cmax, μg/mL, mean | 18.9 | 22.5 | .470 |
| Gentamicin weight-based dosing, mg/kg, mean | 4.26 | 4.80 | .596 |
| Cmax/MIC ratio, mean | 2.36 | 3.71 | .345 |
| Cmax/MIC ratio, geometric mean | 0.851 | 1.23 | .280 |
Abbreviations: Cmax, peak serum concentration; MIC, minimum inhibitory concentration.
Safety and Tolerability
Seven of the 13 (53.8%) participants reported an adverse event. All adverse events were considered mild to moderate and transient (Table 4). Six of the 7 (86%) participants who reported adverse events reported headaches. Most were mild to moderate (mean pain scale reported as 5 out of 10) and resolved prior to TOC. Renal safety was measured as the percentage change of creatinine between treatment and TOC. The mean creatinine percentage change was +5.2% (95% CI, .20%–10.1%), and ranged from −6.7% to 21.3%. All creatinine changes were considered not clinically significant.
Table 4.
Renal Safety and Adverse Events
| Event | No. (%) | Comment |
|---|---|---|
| Creatinine % change, mean | 5.2% (95% CI, .20%–10.1%); range, (−6.7% to 21.3%) | … |
| Any AE | 7 (53.8) | All mild to moderate |
| Serious AE | 0 (0) | … |
| Headache | 6 (46.2) | Pain (mean self-reported score, 5 out of 10; n = 4); Duration (n = 4): mean, 37 h (range, 1–72 h) |
| Hearing changes | 1 (7.7) | Intermittent muffled hearing |
| Urine changes | 1 (7.7) | Darker urine |
| Vomiting | 1 (7.7) | One episode |
| Fatigue | 2 (15.4) | Tired × 1 day |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: AE, adverse event; CI, confidence interval.
Following dosing, participants were asked to rate injection pain and compare it to other commonly used injections in STD clinics. Using a scale of zero to 10, mean injection pain was 2 (range, 1–7). Among participants who had previously received penicillin G benzathine (Bicillin L-A) by IM injection (n = 7), 86% believed IM gentamicin to be less painful than IM Bicillin L-A. Among participants who had received ceftriaxone (n = 8) by IM injection, 75% believed the gentamicin injection was more painful than ceftriaxone.
Discussion
Gentamicin alone, even at an elevated dose of 360 mg, was insufficient to eradicate NG from the pharynx. To our knowledge, this is the first study to document gentamicin’s efficacy for the treatment of pharyngeal gonorrhea in the absence of a second agent. These results are consistent with reports of spectinomycin’s inability to cure gonorrhea from the pharynx, as well as a recently published trial of gentamicin for gonorrhea.
Ross et al recently published a noninferiority, randomized controlled trial [17] comparing 240 mg gentamicin plus 1 g azithromycin to 500 mg ceftriaxone plus 1 g azithromycin for the treatment of gonorrhea. In contrast to the 2012 CDC trial, which used 240 mg of gentamicin with 2 g of azithromycin and achieved 100% cure at all anatomic sites [8], the gentamicin-containing regimen in the Ross et al study had a composite efficacy of 91% (95% CI, 88%–94%) and was deemed inferior to ceftriaxone (risk difference, −6.4% [95% CI, −10.4% to −2.4%]). At the pharynx, the gentamicin-containing regimen in the Ross et al study was only 80% (95% CI, 72%–88%) efficacious compared to 96% efficacy among patients treated with ceftriaxone (95% CI, 92%–99%). Considering these 3 gentamicin trials together, we believe that the high efficacy of gentamicin observed in the 2012 CDC trial likely reflects the efficacy of 2 g of azithromycin in treating pharyngeal gonorrhea caused by susceptible isolates, not the efficacy of gentamicin, which appears to be ineffective in treating pharyngeal gonorrhea.
Identifying gentamicin as yet another drug that fails to cure gonorrhea from the pharynx highlights the need to better understand the pathobiology of pharyngeal gonorrhea and the PK/PD of the antibiotics used to treat NG in the oropharynx. Although the advent of NAAT has aided our understanding of the epidemiology of this infection [18], we still do not know exactly where—at the microscopic level—in this anatomic site the bacteria reside, nor the host immunology that fosters persistence. This lack of understanding of the infection’s basic biology prevents investigators from determining the appropriate compartment from which to measure the local pharmacokinetics of drugs used to treat the infection, the most important predictor of treatment efficacy. Studies measuring drug concentrations in pharyngeal fluid have shown none to negligible cefixime levels despite the fact that the drug appears to have at least some activity in treating pharyngeal infections [19]. Tonsillar concentrations of ceftriaxone, which is >96% effective at the pharynx, have also been lower than anticipated [20]. Since it is unclear whether pharyngeal fluid or tonsillar concentrations are representative of the infection compartment, understanding the pathobiology of pharyngeal gonorrhea will be crucial to developing treatment regimens that are effective at the pharynx.
Although few in number, both of the 2 rectal infections were cured with gentamicin 360 mg alone. Ross and colleagues found the combination of gentamicin plus azithromycin to be 90% efficacious for rectal infections [17]. It remains unclear if the higher gentamicin dose enhanced the efficacy at nonpharyngeal sites. Notably, the gentamicin dose used in this study, 360 mg, was deemed safe and tolerable, which is an important finding as gentamicin does have good efficacy at urogenital sites [6, 17].
Our study has both strengths and limitations. Unlike prior studies, we evaluated the efficacy of gentamicin in the absence of a second drug, which we believe provided us with a clearer perspective on the drug’s efficacy. Also, our approach, evaluating gentamicin without a control group, proved to be an economical initial step in evaluating the drug, allowing us to rapidly determine that gentamicin does not merit further investigation as a treatment for pharyngeal gonorrhea. We believe that the single-arm unblinded demonstration study approach can be used more often as a means to rapidly identify potential existing antibiotics that can be used to treat infection where the outcome is bacterial eradication [21]. Unfortunately, we did not elucidate gentamicin’s PK/PD target for the eradication of NG from the pharynx. While the lack of statistical association is likely due to small numbers of evaluable participants, it is also possible that PK/PD parameters predictive for sepsis are not the same as those for pharyngeal infection. Last, although we do not anticipate outcome differences of pharyngeal NG by gender or HIV status, our study population did not include women or people living with HIV.
Very few currently available antimicrobials are reliably effective against the gonococcus. While ceftriaxone remains very effective at all anatomic sites, persons with verified β-lactam allergies should not receive ceftriaxone. Moreover, the requirement for intramuscular administration makes ceftriaxone’s use impractical in some settings. Additionally, dependence on a single agent is highly risky as ceftriaxone resistance has been reported [22]. At the same time, resistance to azithromycin, which CDC and European guidelines currently recommend as part of dual therapy, is rapidly growing, now occurring in >10% of surveillance isolates in Seattle–King County, Washington, where this study was conducted, a level that exceeds the threshold set by the WHO and CDC for removal of a drug from treatment guidelines (>5%) [1, 2]. Gentamicin fulfills a unique and necessary niche as one of a small number of drugs available to treat gonorrhea in patients at high risk for allergic drug reactions to cephalosporins. Unfortunately, gentamicin as monotherapy is not reliable for the treatment of pharyngeal gonorrhea. We recommend that clinicians test all patients for pharyngeal gonorrhea if they plan to treat for gonorrhea using a drug other than ceftriaxone, and that they routinely perform tests of cure in patients with pharyngeal gonorrhea treated with regimens that do not include ceftriaxone.
Notes
Acknowledgments. The authors thank Ann Collier, who volunteered as the independent medical monitor, and all study participants.
Financial support. This work was supported by the University of Washington Royalty Research Fund (grant number A127193 to L. A. B.) and the National Institutes of Health (NIH) (grant number K23 AI113185A to L. A. B.). B. J. W. is supported in part by the National Institute of Allergy and Infectious Diseases of the NIH (grant numbers 1R21AI132994-01A1 and 1R01AI136979-01).
Potential conflicts of interest. L. A. B. and M. R. G. have received research support, unrelated to this work, from Hologic. M. R. G. has received grants from GlaxoSmithKline, unrelated to this work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: International Society of Sexually Transmitted Diseases Research World Congress, Vancouver, Canada, 15 July 2019. Abstract number O06.1.
References
- 1. Centers for Disease Control and Prevention. STD treatment guidelines. Atlanta, GA: CDC, 2015. [Google Scholar]
- 2. Unemo M, Golparian D, Eyre DW. Antimicrobial resistance in neisseria gonorrhoeae and treatment of gonorrhea. Methods Mol Biol 2019; 1997:37–58. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. STD surveillance report, 2017. Atlanta, GA: CDC, 2018. [Google Scholar]
- 4. Brown LB, Krysiak R, Kamanga G, et al. Neisseria gonorrhoeae antimicrobial susceptibility in Lilongwe, Malawi, 2007. Sex Transm Dis 2010; 37:169–72. [DOI] [PubMed] [Google Scholar]
- 5. Ross JD, Lewis DA. Cephalosporin resistant Neisseria gonorrhoeae: time to consider gentamicin? Sex Transm Infect 2012; 88:6–8. [DOI] [PubMed] [Google Scholar]
- 6. Dowell D, Kirkcaldy RD. Effectiveness of gentamicin for gonorrhoea treatment: systematic review and meta-analysis. Sex Transm Infect 2012; 88:589–94. [DOI] [PubMed] [Google Scholar]
- 7. Barbee LA, Soge OO, Holmes KK, Golden MR. In vitro synergy testing of novel antimicrobial combination therapies against Neisseria gonorrhoeae. J Antimicrob Chemother 2014; 69:1572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis 2014; 59:1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan PA, Robinette A, Montgomery M, et al. Extragenital infections caused by Chlamydia trachomatis and Neisseria gonorrhoeae: a review of the literature. Infect Dis Obstet Gynecol 2016; 2016:5758387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moran JS. Treating uncomplicated Neisseria gonorrhoeae infections: is the anatomic site of infection important? Sex Transm Dis 1995; 22:39–47. [DOI] [PubMed] [Google Scholar]
- 11. Gentamicin. In: Kucers A, Crowe S, Grayson M, Foy J, eds. The use of antibiotics: a clinical review of antibacterial, antifungal and antiviral drugs. 5th ed. Oxford: Butterworth-Heinemann, 1997. [Google Scholar]
- 12. Ristuccia AM, Cunha BA. The aminoglycosides. Med Clin North Am 1982; 66:303–12. [DOI] [PubMed] [Google Scholar]
- 13. Fischer JH, Hedrick PJ, Riff LJ. Pharmacokinetics and antibacterial activity of two gentamicin products given intramuscularly. Clin Pharm 1984; 3:411–6. [PubMed] [Google Scholar]
- 14. Lacy MK, Nicolau DP, Nightingale CH, Quintiliani R. The pharmacodynamics of aminoglycosides. Clin Infect Dis 1998; 27:23–7. [DOI] [PubMed] [Google Scholar]
- 15. Pai MP, Bearden DT. Antimicrobial dosing considerations in obese adult patients. Pharmacotherapy 2007; 27:1081–91. [DOI] [PubMed] [Google Scholar]
- 16. Drago L, De Vecchi E, Nicola L, Colombo A, Gismondo MR. Selection of resistance of telithromycin against Haemophilus influenzae, Moraxella catarrhalis and streptococci in comparison with macrolides. J Antimicrob Chemother 2004; 54:542–5. [DOI] [PubMed] [Google Scholar]
- 17. Ross JDC, Brittain C, Cole M, et al. G-ToG Trial Team Gentamicin compared with ceftriaxone for the treatment of gonorrhoea (G-ToG): a randomised non-inferiority trial. Lancet 2019; 393:2511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barbee LA, Dombrowski JC, Kerani R, Golden MR. Effect of nucleic acid amplification testing on detection of extragenital gonorrhea and chlamydial infections in men who have sex with men sexually transmitted disease clinic patients. Sex Transm Dis 2014; 41:168–72. [DOI] [PubMed] [Google Scholar]
- 19. Barbee LA, Nayak SU, Blumer JL, et al. A phase 1 pharmacokinetic and safety study of extended-duration, high-dose cefixime for cephalosporin-resistant neisseria gonorrhoeae in the pharynx. Sex Transm Dis 2018; 45:677–83. [DOI] [PubMed] [Google Scholar]
- 20. Blumer JL, Reed MD, Kaplan EL, Drusano GL. Explaining the poor bacteriologic eradication rate of single-dose ceftriaxone in group a streptococcal tonsillopharyngitis: a reverse engineering solution using pharmacodynamic modeling. Pediatrics 2005; 116:927–32. [DOI] [PubMed] [Google Scholar]
- 21. Handsfield HH, Zenilman JM. Standards for treatment and control regimens in therapeutic trials for gonorrhea: lessons from a “failed” trial. Sex Transm Dis 2019; 46:287–9. [DOI] [PubMed] [Google Scholar]
- 22. Eyre DW, Town K, Street T, et al. Detection in the United Kingdom of the Neisseria gonorrhoeae FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, October to December 2018. Euro Surveill 2019; 24. [DOI] [PMC free article] [PubMed] [Google Scholar]