Abstract
Aims
Endothelin‐1 (ET‐1) is a potent vasoconstrictor, which regulates renal and vascular function. We aimed to relate plasma levels of ET‐1 with the clinical picture and outcomes in acute heart failure (AHF).
Methods and results
We studied 113 patients with AHF [mean age 65 ± 13 (years), median (upper and lower quartiles) N‐terminal pro‐B‐type natriuretic peptide, 5422 (2689; 8582) (pg/mL)], in whom plasma levels of ET‐1 were serially measured at admission (10.8 ± 5.2), Day 1 (9.5 ± 3.4), and Day 2 (8.9 ± 3.8) (pg/mL). The population was divided into tertiles across baseline ET‐1 levels. Patients in the highest ET‐1 tertile had predominant clinical signs of peripheral congestion; however, no difference was observed in pulmonary congestion and severity of dyspnoea. They also presented lower spot urine sodium at admission (75 ± 35 vs. 99 ± 43 vs. 108 ± 30), 6 h (84 ± 34 vs. 106 ± 43 vs. 106 ± 35), and Day 1 (75 ± 38 vs. 96 ± 36 vs. 100 ± 35) (mmol/L), when compared with the second and first tertile, respectively (all P < 0.05); furthermore, they received higher doses of intravenous furosemide from Day 2 and had longer intravenous diuretics, as median switch to oral furosemide was 4 (3; 4) vs. 3 (2; 4) vs. 2 (2; 3) (days), respectively, P < 0.05. There was no difference in serum creatinine, urea, and renal injury biomarkers (kidney injury molecule‐1, serum cystatin C, and urine neutrophil gelatinase‐associated lipocalin) between the ET‐1 tertiles. Higher values of ET‐1 measured at each time point were related with a higher risk of 1 year mortality.
Conclusions
Elevation of ET‐1 is related to clinical signs of peripheral congestion, low urine sodium excretion, and poor outcome in AHF.
Keywords: Natriuresis, Renal function, Spot urine sodium, Congestion, Endothelin‐1
Introduction
Endothelin‐1 (ET‐1) is a potent endogenous vasoconstrictor impacting the vascular tone of pulmonary and peripheral circulation. 1 , 2 , 3 The endothelin system has already been shown to play an important role in the pathophysiology of numerous pathologies including heart failure, where elevated blood levels of ET‐1 were linked to the severity of the disease and the outcomes. 4 , 5 , 6 , 7 However, in the settings of acute heart failure (AHF), the role of the endothelin system remains largely unknown. 8
Importantly, ET‐1 regulates renal function, and its role goes far beyond being a control of afferent and efferent glomerular artery tone. It directly affects the renal tubular cells, thus impacting urine production, including regulation of ion/water homeostasis. 9 As impaired water/sodium handling with extracellular water expansion and resultant congestion are all the hallmarks of AHF, we decided to examine the role of ET‐1 in this population. Thus, we aimed to (i) correlate serially measured ET‐1 levels with the clinical picture of AHF patients; (ii) find clinical and laboratory correlates of ET‐1 levels in the settings of AHF; and (iii) examine the associations of ET‐1 with renal function, markers of renal injury, and water/sodium handling abilities.
Methods
Study population
This is a single‐centre observational study. The population consists of AHF patients who were included into a registry that was run at our institution between 2010 and 2012. To enter the registry, patients needed to fulfil the following criteria: ≥18 years old, AHF as the primary cause of hospitalization [according to the European Society of Cardiology (ESC) guidelines criteria 10 , 11 ], presence of clinical signs/symptoms of fluid overload on admission with need for intravenous loop diuretics, and the patient's written agreement to participate. The following criteria excluded patients from the registry: cardiogenic shock, a clinical diagnosis of concurrent acute coronary syndrome/severe infection, known severe liver disease or renal disease requiring or with planned renal replacement therapy, exposure to nephrotoxic drugs, and evidence of urinary infection or bacteraemia. Patients were treated in accordance with the attending physicians' recommendations of the ESC guidelines 10 , 11 rather than by a protocol, although it was requested by the protocol to collect detailed information on in‐hospital management. The research was approved by the local ethics committee, and each individual gave written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Study design
In patients who entered the registry, a detailed questionnaire on demographics, clinical history, co‐morbidities, type of AHF (according to the 2008 ESC guidelines classification 10 ), previous therapies, and physical/clinical findings (at admission and during hospitalization) was filled in. In all patients, venous blood and urine samples were collected at pre‐specified time points (admission, 6 h, Day 1, Day 2, and discharge). Detailed information regarding urine sample collection has been described elsewhere. 12 Blood and urine samples were assessed in our local laboratory as part of our everyday clinical practice (please see succeeding text). Part of the collected material was centrifuged and immediately frozen at −70°C until further laboratory analysis.
Local laboratory measurements
The following laboratory parameters (among others) were assessed using standard methods and were immediately available for treating physicians:
haematology: haemoglobin, haematocrit, leukocytes (white blood cell), and platelets;
serum electrolytes: sodium (Na+) and potassium (K+);
renal and liver function tests: creatinine, estimated glomerular filtration rate (eGFR) by Modification of Diet in Renal Disease method was calculated, 13 blood urea nitrogen, aspartate aminotransferase (AST), alanine aminotransferase, bilirubin, and albumin; and
plasma N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) (method: immunoenzymatic, Siemens, Marburg, Germany) and troponin I (method: immunoenzymatic, single Dimension RxL Max, Siemens).
The assessment of spot urine Na+, urine creatinine, and urea levels was also completed in the local laboratory; however, these results were not available to treating physicians during hospitalization.
Additional laboratory measurements
The following biomarkers were assessed at the Singulex Laboratory form frozen blood and urine samples (taken at admission, Day 1, and Day 2): ET‐1, kidney injury molecule‐1 (KIM‐1), neutrophil gelatinase‐associated lipocalin (NGAL), cystatin C, and urine albumin. The analyses were performed on a diagnostic platform Singulex, Inc. (Alameda, CA, USA) with the Erenna immunoassay system, which uses a microparticle immunoassay and single‐molecule counting in a capillary flow system.
Clinical assessment
The clinical status of each patient was assessed (at admission, Day 1, and Day 2) based on signs and symptoms of heart failure, including dyspnoea (visual analogue scale: from 0 to 10 points, where 10 is the worst dyspnoea), oedema (0–4 point scale, where 4 is the worst oedema), pulmonary congestion (0–3 point scale, with 3 representing congestion reaching upper parts of lungs), and jugular venous pressure. We have also calculated the sodium retention score/congestion score as proposed by Cody, based on rales (0–3 points), peripheral oedema (0–4 points), weight change (−1 to 1 points), hepatomegaly (0 vs. 1 point), S3 gallop (0 vs. 1 point), and increased jugular venous pressure (0 vs. 1 point) (range of possible score: −1 to 11).
Clinical follow‐up
The status of every patient was followed for at least 12 months. Information was obtained directly from patients or their relatives (telephone contact), from the heart failure clinic database, or from the hospital system. No patient was lost to follow‐up. The primary endpoint of interest was all‐cause mortality at 1 year.
Statistical analysis
Continuous variables with a normal distribution were described using means ± standard deviation (SD), variables with skewed distribution were described by medians with (upper and lower quartiles), and categorized variables were given as numbers and percentages. The skewed distributed variables were log‐transformed where needed. The statistical significance of differences between the groups was assessed using t‐test, Mann–Whitney U‐test, χ 2 test, or Kruskal–Wallis one‐way analysis of variance by rank test, where appropriate. A multivariable regression (stepwise progressive) model was built among variables significantly correlated with ET‐1 in the univariate model. The Cox proportional hazards model was used to calculate the hazard ratio (HR) with corresponding 95% confidence interval (95% CI) for all‐cause mortality. The P < 0.05 was considered statistically significant. Statistical analyses were performed using STATISTICA 13 (StatSoft).
Results
The study population consists of 113 patients, 84 (74%) being male and with a mean (±SD) age of 65 ± 13 years, and mean (±SD) systolic blood pressure and heart rate at admission were 134 ± 35 (mmHg) and 90 ± 26 (b.p.m.), respectively. The median (upper and lower quartiles) NT‐proBNP was 5422 (2689–8582) (pg/mL). Detailed characteristics of the population are presented in Table 1 .
TABLE 1.
Baseline and in‐hospital characteristics of patients with acute heart failure
| Parameter | All (n = 113) | Endothelin‐1 at admission (pg/mL) | P | ||
|---|---|---|---|---|---|
|
1st tertile (n = 38) <7.9 |
2nd tertile (n = 37) 8.0–11.1 |
3rd tertile (n = 38) >11.2 |
|||
| Sex (male) | 84 (74%) | 23 (60%) | 27 (73%) | 34 (89%) | 0.01 |
| Age (years) | 65 ± 13 | 65 ± 13 | 65 ± 15 | 64 ± 14 | 0.87 |
| Heart rate (b.p.m.) | 90 ± 26 | 88 ± 25 | 87 ± 20 | 97 ± 30 | 0.21 |
| Systolic blood pressure at admission (mmHg) | 134 ± 35 | 142 ± 37 | 137 ± 38 | 124 ± 30 | 0.06 |
| Diastolic blood pressure at admission (mmHg) | 79 ± 18 | 81 ± 17 | 80 ± 19 | 76 ± 19 | 0.31 |
| Left ventricular ejection fraction (%) | 32 ± 13 | 35 ± 12 | 33 ± 13 | 29 ± 13 | 0.11 |
| Acute heart failure (de novo) | 28 (25%) | 10 (26%) | 9 (24%) | 9 (24%) | 0.96 |
| Heart failure aetiology | |||||
| Ischaemic | 60 (53%) | 22 (58%) | 19 (51%) | 19 (50%) | 0.76 |
| Hypertension | 9 (8%) | 3 (8%) | 4 (11%) | 2 (5%) | 0.81 |
| Admission | |||||
| Peripheral oedema (yes) | 86 (76%) | 24 (63%) | 29 (78%) | 31 (82%) | 0.15 |
| Pulmonary congestion (yes) | 105 (93%) | 35 (92%) | 35 (94%) | 35 (92%) | 0.88 |
| Dyspnoea (points) | 7.4 ± 2.3 | 7.2 ± 2.6 | 7.5 ± 2.7 | 7.4 ± 1.7 | 0.88 |
| Any dyspnoea (yes) | 103 (91%) | 34 (90%) | 34 (92%) | 35 (92%) | 0.92 |
| Ascites (yes) | 16 (14%) | 0 (0%) | 6 (16%) | 10 (26%) | <0.005 |
| Hepatomegaly (yes) | 34 (33%) | 4 (10%) | 11 (30%) | 19 (50%) | <0.005 |
| Blood count | |||||
| Haemoglobin (g/dL) | 13.1 ± 1.9 | 12.9 ± 1.7 | 12.7 ± 2.3 | 13.7 ± 1.6 | <0.05 |
| White blood count (G/L) | 9.0 ± 3.6 | 9.0 ± 4.0 | 9.4 ± 3.1 | 8.6 ± 3.7 | 0.65 |
| Platelets (G/L) | 215 ± 96 | 216 ± 83 | 211 ± 81 | 219 ± 121 | 0.92 |
| Bilirubin (mg/dL) | 1.1 (0.9; 1.7) | 1.0 (0.7; 1.4) | 0.9 (0.8; 1.5) | 1.6 (1.1; 2.1) | 0.001 |
| Aspartate transaminase (IU/L) | 26 (19; 36) | 22 (16; 36) | 26 (20; 36) | 28 (23; 38) | 0.24 |
| Alanine transaminase (IU/L) | 25 (17; 37) | 25 (17; 36) | 23 (17; 37) | 28 (18; 42) | 0.72 |
| Na (mmol/L) | 138 ± 5 | 139 ± 3.5 | 138 ± 6.1 | 137 ± 4.4 | 0.41 |
| Calculated eGFR (mL/min/1.73 m2) | 63 ± 28 | 67 ± 36 | 63 ± 24 | 58 ± 24 | 0.43 |
| Creatinine (mg/dL) | 1.29 ± 0.6 | 1.26 ± 0.8 | 1.24 ± 0.5 | 1.38 ± 0.48 | 0.56 |
| Urea (mg/dL) | 62 ± 40 | 56 ± 37 | 59 ± 37 | 71 ± 44 | 0.22 |
| NT‐proBNP (pg/mL) | 5422 (2689; 8582) | 4351 (2145; 6253) | 4800 (2674; 9111) | 7686 (4670; 12 276) | 0.002 |
| Troponin I (ng/mL) | 0.04 (0.02; 0.1) | 0.04 (0.2; 0.1) | 0.04 (0.02; 0.06) | 0.06 (0.02; 0.14) | 0.24 |
| Lactate (mmol/L) | 2.3 ± 1.1 | 2.0 ± 1.0 | 2.2 ± 0.9 | 2.5 ± 1.3 | 0.09 |
| Day 2 | |||||
| Peripheral oedema (yes) | 58 (51%) | 16 (42%) | 19 (51%) | 23 (60%) | 0.27 |
| Peripheral oedema (≥2 ++) | 22 (19%) | 3 (8%) | 7 (19%) | 12 (32%) | 0.02 |
| Pulmonary congestion (yes) | 18 (16%) | 3 (8%) | 7 (20%) | 8 (21%) | 0.19 |
| Dyspnoea (points) | 2.2 ± 2.5 | 1.9 ± 2.3 | 2.3 ± 2.5 | 2.4 ± 2.5 | 0.75 |
| Any dyspnoea (yes) | 31 (27%) | 7 (19%) | 10 (28%) | 14 (37%) | 0.21 |
| Chest pain at admission (yes) | 6 (5%) | 2 (5%) | 3 (8%) | 1 (3%) | 0.57 |
| Systolic blood pressure (mmHg) | 115 ± 19 | 119 ± 20 | 116 ± 19 | 110 ± 18 | 0.18 |
| Creatinine (mg/dL) | 1.22 ± 0.60 | 1.29 ± 0.85 | 1.15 ± 0.41 | 1.21 ± 0.41 | 0.61 |
| Urea (mg/dL) | 64 ± 37 | 63 ± 36 | 61 ± 34 | 68 ± 42 | 0.71 |
| Endothelin‐1 | |||||
| Endothelin‐1 at admission (pg/mL) | 10.8 ± 5.2 | 6.3 ± 1.5 | 9.6 ± 0.8 | 16.6 ± 4.7 | <0.001 |
| 9.7 (7.4; 12.8) | 6.6 (5.5; 7.4) | 9.7 (9.0; 10.0) | 15.2 (12.8; 18.9) | ||
| Endothelin‐1 at Day 1 (pg/mL) |
9.5 ± 3.4 |
6.9 ± 2.3 | 9.1 ± 2.2 | 12.1 ± 4.5 | <0.001 |
| 8.9 (6.9; 11.2) | 6.8 (5.6; 7.8) | 9.5 (7.4; 10.6) | 11.6 (8.9; 14.6) | ||
| Endothelin‐1 at Day 2 (pg/mL) | 8.9 ± 3.8 | 6.5 ± 1.9 | 8.9 ± 3.1 | 11.6 ± 4.3 | <0.001 |
| 8.4 (6.2; 10.8) | 6.4 (5.1; 7.6) | 9.2 (6.6; 10.1) | 11.4 (8.9; 13.6) | ||
eGFR, estimated glomerular filtration rate; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Data shown as n (%), mean ± standard deviation, or median (interquartile range).
The bold was used in variables with statistical significances.
The mean plasma ET‐1 at admission was 10.8 ± 5.2 (pg/mL) and decreased within subsequent days of hospitalization to 9.5 ± 3.4 at Day 1 and 8.9 ± 3.8 (pg/mL) at Day 2 (both P < 0.05 when compared with admission).
Plasma endothelin‐1 levels and patients' characteristics
The population was divided into tertiles across baseline plasma ET‐1 levels, and comparison of patients' characteristics by the tertiles of ET‐1 on admission is presented in Table 1 and supporting Table S3.
Patients in the highest ET‐1 tertile were more frequently male with the highest NT‐proBNP values and a trend towards the lowest systolic blood pressure on admission. They also had more often clinical signs of peripheral congestion on admission (which remained until Day 2) as evidenced by higher prevalence of ascites and hepatomegaly, but no difference in pulmonary congestion and severity of dyspnoea.
Patients were subsequently divided according to the 2008 ESC guidelines classification, 10 and the mean levels of plasma ET‐1 are presented in Table 2 . There was no difference in the ET‐1 levels between patients with AHF de novo and those with decompensated chronic heart failure; neither there was a difference between patients with preserved vs. reduced ejection fraction. Patients who presented on admission with pulmonary oedema or hypertensive AHF tended to have lower levels of ET‐1 at Day 2. Interestingly, those classified as having right heart failure on admission had higher ET‐1 levels at all three time points (vs. remaining patients) and had significantly higher values of ET‐1 at Days 1 and 2 when compared with the other types of AHF (both P < 0.05) (Figure 1 ). Analogically, patients with overt clinical signs of fluid overload (oedema, ascites, or hepatomegaly) had significantly higher levels of ET‐1 within the first days of hospitalization (Table 2 ).
TABLE 2.
Comparison of the endothelin‐1 plasma level (pg/mL) at admission, Day 1, and Day 2 in subgroups of patients with acute heart failure
| Variable | Time point | Mean endothelin‐1 (pg/mL) | P | |
|---|---|---|---|---|
| Yes | No | |||
| EF ≤ 40% | Admission | 11.2 ± 5.5 | 9.2 ± 3.7 | 0.08 |
| Day 1 | 9.6 ± 4.0 | 8.8 ± 3.0 | 0.40 | |
| Day 2 | 8.9 ± 4.0 | 8.7 ± 2.7 | 0.79 | |
| AHF de novo | Admission | 10.6 ± 4.9 | 10.9 ± 5.3 | 0.87 |
| Day 1 | 8.6 ± 3.0 | 9.7 ± 4.1 | 0.20 | |
| Day 2 | 8.5 ± 3.9 | 9.1 ± 3.8 | 0.50 | |
| Type of AHF according to ESC guidelines 10 | ||||
| Decompensated chronic heart failure | Admission | 9.9 ± 4.6 | 11.4 ± 5.5 | 0.11 |
| Day 1 | 9.2 ± 4.5 | 9.6 ± 3.4 | 0.52 | |
| Day 2 | 8.8 ± 3.8 | 8.9 ± 3.9 | 0.82 | |
| Pulmonary oedema | Admission | 9.9 ± 6.5 | 11.0 ± 4.9 | 0.39 |
| Day 1 | 8.3 ± 2.2 | 9.7 ± 4.1 | 0.14 | |
| Day 2 | 6.9 ± 2.2 | 9.4 ± 3.9 | 0.02 | |
| Hypertensive AHF | Admission | 10.7 ± 5.7 | 10.9 ± 5.0 | 0.87 |
| Day 1 | 8.3 ± 2.8 | 9.9 ± 4.1 | 0.06 | |
| Day 2 | 7.5 ± 2.6 | 9.5 ± 4.1 | 0.02 | |
| Right heart failure | Admission | 12.4 ± 5.1 | 10.1 ± 5.1 | 0.03 |
| Day 1 | 11.0 ± 3.2 | 8.7 ± 3.9 | 0.004 | |
| Day 2 | 10.8 ± 4.1 | 8.0 ± 3.4 | 0.002 | |
| Clinical signs of congestion | ||||
| Pulmonary congestion at admission | Admission | 10.8 ± 5.3 | 10.3 ± 4.4 | 0.75 |
| Day 1 | 9.4 ± 3.9 | 9.9 ± 3.9 | 0.72 | |
| Day 2 | 8.9 ± 3.9 | 9.4 ± 2.0 | 0.73 | |
| Hepatomegaly | Admission | 12.9 ± 4.6 | 9.9 ± 5.2 | 0.0005 |
| Day 1 | 11.3 ± 3.8 | 8.6 ± 3.6 | <0.0001 | |
| Day 2 | 10.6 ± 3.8 | 8.3 ± 3.6 | 0.01 | |
| Oedema at admission | Admission | 11.2 ± 5.4 | 9.5 ± 4.3 | 0.13 |
| Day 1 | 10.0 ± 4.2 | 7.8 ± 2.2 | 0.009 | |
| Day 2 | 9.5 ± 3.9 | 7.4 ± 2.9 | 0.01 | |
| Ascites at admission | Admission | 13.4 ± 4.4 | 10.4 ± 5.2 | 0.03 |
| Day 1 | 11.2 ± 2.4 | 9.1 ± 3.9 | 0.05 | |
| Day 2 | 12.6 ± 3.1 | 8.4 ± 3.6 | <0.0001 | |
AHF, acute heart failure; EF, ejection fraction; ESC, European Society of Cardiology.
The bold was used in variables with statistical significances.
FIGURE 1.
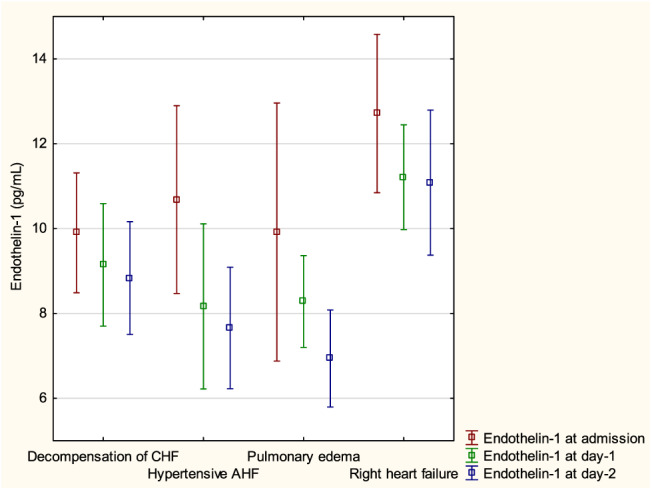
Mean endothelin‐1 by different types of acute heart failure (AHF). CHF, chronic heart failure.
Correlates of plasma levels of endothelin‐1 in acute heart failure
The ET‐1 level at admission significantly correlated with AST r = 0.48, alanine aminotransferase r = 0.39, total bilirubin r = 0.32, serum lactate r = 0.34, NT‐proBNP r = 0.37, spot urine Na+ r = −0.35, total dose of furosemide administrated within the first 48 h of hospitalization r = 0.20, heart rate r = 0.25, and systolic blood pressure at admission r = −0.22, all P < 0.05 (for more details, see Supporting Information, Table S1 ). The multivariable model revealed that heart rate, AST, and NT‐proBNP along with spot urine Na+ were independently related with ET‐1 level at admission.
Plasma levels of endothelin‐1 spot urine sodium, diuretic demand, and markers of renal injury
Patients in the highest tertile of ET‐1 at admission had significantly lower spot urine Na+ at admission, 6 h, and Day 1 when compared with the remaining tertiles (all P < 0.05), but the difference at Day 2 was not statistically significant (P = 0.28) (Figure 2). Sodium retention score at admission and Day 2 was significantly higher in those with the highest ET‐1. All the tertiles received the same doses of furosemide within the first day of hospitalization, but the third tertile group received higher doses from Day 2 and had longer intravenous diuretic treatment as median switch to oral furosemide was 4 (3; 4) vs. 3 (2; 4) vs. 2 (2; 3) (days) for tertiles from third to first, respectively, P < 0.05 (Table 3 ). There were no differences in serum KIM‐1, serum cystatin C, urine NGAL, and urine albumin between tertiles of ET‐1 (Table 3 ).
FIGURE 2.
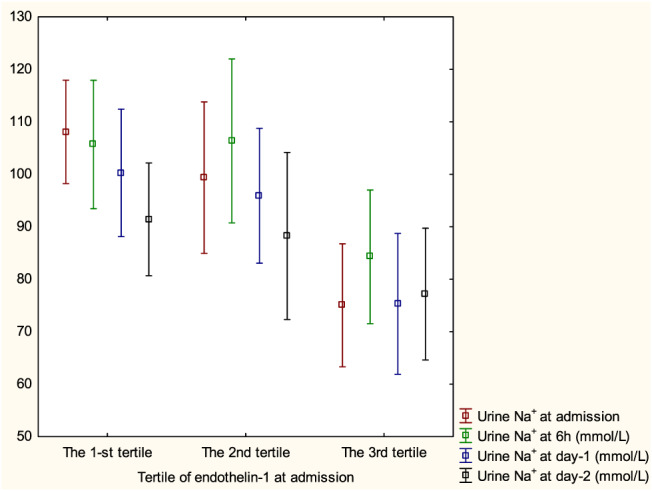
Pattern of longitudinal change of urine Na+ at admission, 6 h, 24 h, and 48 h by tertile of endothelin‐1 at admission.
TABLE 3.
Natriuresis, decongestion, and renal function by tertile of endothelin‐1 at admission
| Parameter | All | Tertile 1 | Tertile 2 | Tertile 3 | P |
|---|---|---|---|---|---|
| Urine Na+ at admission (mmol/L) | 95 ± 42 | 108 ± 30 | 99 ± 43 | 75 ± 35 | <0.001 |
| Urine Na+ at 6 h (mmol/L) | 99 ± 38 | 106 ± 35 | 106 ± 43 | 84 ± 34 | 0.03 |
| Urine Na+ at Day 1 (mmol/L) | 91 ± 38 | 100 ± 35 | 96 ± 36 | 75 ± 38 | 0.01 |
| Urine Na+ at Day 2 (mmol/L) | 86 ± 36 | 91 ± 31 | 88 ± 42 | 77 ± 34 | 0.28 |
| Weight reduction to Day 2 (kg) | −1.0 (−2.5; −0.5) | −1.0 (−2.0; −0.4) | −1.95 (−3.0; −1.0) | −1.0 (−2.4; 0) | 0.29 |
| Length of hospitalization (days) | 10 ± 8 | 7 ± 4 | 9 ± 8 | 14 ± 10 | <0.001 |
| NT‐proBNP at admission (pg/mL) | 5422 (2689; 8582) | 4351 (2145; 6253) | 4800 (2674; 9111) | 7686 (4670; 12 276) | 0.002 |
| NT‐proBNP at Day 2 (pg/mL) | 3169 (1781; 6528) | 2164 (1373; 4318) | 3760 (1639; 6086) | 5257 (3196; 8669) | 0.001 |
| NT‐proBNP at discharge (pg/mL) | 2648 (1434; 5493) | 1798 (1305; 4217) | 2382 (1329; 4787) | 3867 (2333; 7242) | 0.02 |
| Sodium retention score at admission | 4 (3; 5) | 4 (3; 5) | 4 (3; 5) | 5 (3; 5) | <0.05 |
| Sodium retention score at Day 2 | 0 (0;2) | 0 (−1; 1) | 0 (−1; 2) | 1 (0; 3) | <0.05 |
| Dose of furosemide (iv) 0–6 h (mg) | 40 (30; 60) | 40 (40; 80) | 60 (40; 60) | 50 (40; 60) | 0.79 |
| Dose of furosemide (iv) 6–24 h (mg) | 40 (20; 60) | 30 (0; 60) | 40 (20; 80) | 50 (24; 80) | 0.07 |
| Dose of furosemide (iv) 24–48 h (mg) | 40 (0; 80) | 0 (0; 60) | 60 (0; 100) | 60 (20; 100) | 0.01 |
| Median day of switch to oral furosemide (days) | 3 (2; 4) | 2 (2; 3) | 3 (2; 4) | 4 (3; 4) | <0.005 |
| Dose of furosemide oral at discharge (mg) | 80 (40; 120) | 40 (40; 80) | 40 (40; 120) | 100 (40; 120) | <0.005 |
| Use of inotrope (yes) | 12 (11%) | 2 (5%) | 2 (5%) | 8 (21%) | <0.05 |
| Serum KIM‐1 at admission (pg/mL) | 249 (153; 373) | 263 (139; 404) | 241 (161; 276) | 257 (168; 396) | 0.77 |
| Serum KIM‐1 at Day 1 (pg/mL) | 240 (162; 348) | 243 (131; 353) | 211 (168; 265) | 265 (177; 382) | 0.55 |
| Serum KIM‐1 at Day 2 (pg/mL) | 236 (155; 334) | 228 (123; 293) | 214 (153; 287) | 270 (183; 353) | 0.14 |
| Serum cystatin C at admission (μg/dL) | 164 (135; 226) | 173 (134; 212) | 148 (121; 212) | 172 (147; 277) | 0.18 |
| Serum cystatin C at Day 1 (μg/dL) | 163 (131; 252) | 172 (137; 232) | 143 (117; 235) | 167 (143; 298) | 0.25 |
| Serum cystatin C at Day 2 (μg/dL) | 164 (129; 234) | 164 (127; 215) | 159 (125; 228) | 175 (136; 258) | 0.79 |
| Urine NGAL at admission (ng/mL) | 15 (6; 32) | 21 (5; 68) | 12 (6; 27) | 15 (6; 23) | 0.15 |
| Urine NGAL at Day 1 (ng/mL) | 16 (8; 31) | 16 (9; 21) | 14 (5; 31) | 24 (9; 45) | 0.51 |
| Urine NGAL at Day 2 (ng/mL) | 18 (9; 38) | 20 (9; 38) | 17 (10; 41) | 22 (6; 32) | 0.76 |
| Urine albumin at admission (μg/mL) | 37 (12; 160) | 37 (12; 134) | 25 (11; 158) | 54 (12; 188) | 0.68 |
| Urine albumin at Day 1 (μg/mL) | 29 (6; 132) | 23 (6; 55) | 18 (3; 132) | 68 (12; 167) | 0.35 |
| Urine albumin at Day 2 (μg/mL) | 39 (6; 108) | 15 (7; 108) | 43 (5; 112) | 40 (8; 98) | 0.96 |
iv, intravenous; KIM‐1, kidney injury molecule‐1; NGAL, neutrophil gelatinase‐associated lipocalin; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Data shown as mean ± standard deviation or median (interquartile range).
The bold was used in variables with statistical significances.
Endothelin‐1 and in‐hospital course/post‐discharge outcomes
Patients in the highest tertile of ET‐1 had significantly longer total length of hospital stay, when compared with the second and first tertile: 14 ± 10 vs. 9 ± 8 and 7 ± 4 (days), respectively, both P < 0.05. Moreover, this group received the inotropes more frequently 8 (21%) than other tertiles 2 (5%) and 2 (5%), P < 0.05. During 1 year of follow‐up, 33 (29%) patients died. Higher values of ET‐1 measured at each time point were related with higher risk of 1 year mortality; HR (95% CI) for admission, Day 1, and Day 2 were 3.09 (1.34–7.12), 5.57 (2.07–14.98), and 3.74 (1.34–10.38), respectively, all P < 0.05. After correction for serum creatinine and systolic blood pressure, levels of ET‐1 remained significant prognosticator of 1 year mortality, HR (95% CI): 1.06 (1.00–1.12), P < 0.05 (Table S2 ). Kaplan–Meier survival curves by tertiles of ET‐1 at admission and Day 1 are shown in Figure 3 A and Figure 3 B .
FIGURE 3.
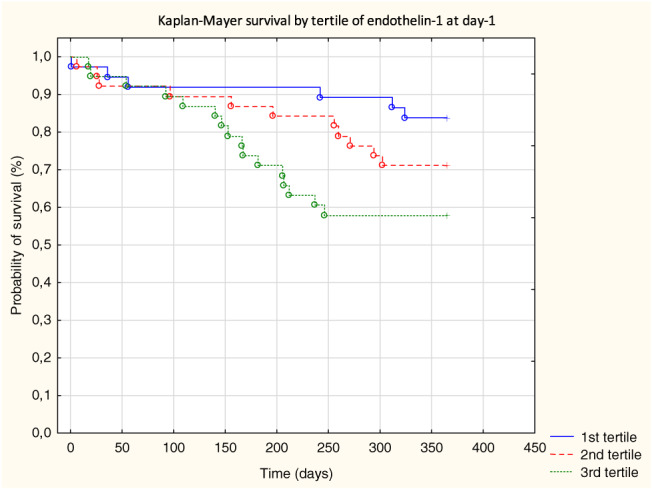
(A) Kaplan–Meier survival by tertile of endothelin‐1 at admission. (B) Kaplan–Meier survival by tertile of endothelin‐1 at Day 1.
Discussion
There are several new findings of our study. Firstly, we have linked ET‐1 concentrations with clinical characteristics of AHF patients. It might have been taken for granted that ET‐1 (as a potent vasoconstrictor) would be related with high blood pressure AHF. Surprisingly, we have shown a completely opposite pattern of endothelin concentration across the spectrum of AHF presentations. We found that high endothelin is related with clinical signs of peripheral congestion (fluid overload) and low urine sodium excretion rather than dyspnoea and pulmonary congestion. This observation may be of importance for a better understanding of AHF pathophysiology.
Secondly, we have presented the ET‐1 trajectory within the first 2 days of treatment in a broad spectrum of AHF patients. The marker decreased (along with decreasing congestion) within subsequent days of hospitalization. Our data agree with previous papers describing the marker in patients enrolled to ASCEND‐HF trial 8 and the VERITAS programme. 14 However, our population had a slightly higher first ET‐1 concentration (median 9.7 vs. 7.6 pg/mL) than was observed in ASCEND‐HF. There are several possible explanations for this fact. Firstly, the baseline sample in the trial was assessed with an approximately 18 h of delay from hospital presentation. Notably, the highest tertile of ET‐1 in ASCEND‐HF had a significantly shorter time of delay, further confirming this assumption. Secondly, we have examined unselected populations with half of them having systolic blood below 130 mmHg at admission and patients with clinical signs of right heart failure, which turned out to have the highest values of ET‐1. Furthermore, we have observed a decreasing pattern of ET‐1 change across all types of AHF, but the mean values of the marker were persistently, significantly higher in patients with clinical signs of right heart failure at all time points. The analogical pattern of interaction was shown during treatment of advanced chronic heart failure patients, where ET‐1 concentration was decreasing along with decreasing central venous pressure. 15
Thirdly, there are premises to believe that elevated ET‐1 may play an important role in congestion development in AHF. Patients with the highest values of ET‐1 had significantly lower urine sodium excretion, which is a strong driver of persistent congestion, poorer diuretic response, and worse outcome. 12 , 16 , 17 Secondly, it turned out that patients with clinical signs of peripheral congestion (viz. ascites, hepatomegaly, and peripheral oedema) had significantly higher ET‐1 within the first 2 days of hospitalization. On the other hand, patients with clinical signs of pulmonary congestion, dyspnoea, and elevated systolic blood pressure tended to have lower values of the marker. Moreover, the highest tertile group had significantly higher values of NT‐proBNP (from admission until discharge) and higher diuretic demand as they received higher doses of intravenous furosemide from Day 2 and they needed intravenous treatment for significantly longer period of time. Finally, the third ET‐1 tertile hospitalization had the longest hospital stay.
Our data show the relations between ET‐1 and renal function in AHF from a new perspective. Interestingly, there was no difference in ‘traditional’ markers of kidney function like creatinine, eGFR, and serum urea between ET‐1 tertiles. Neither were there differences in more sophisticated markers of kidney function/injury (like NGAL, KIM‐1, cystatin C, or urine albumin) across the ET‐1 levels. However, those patients with elevated ET‐1 had significantly lower urine sodium excretion, which only confirms the postulate for bi‐dimensional assessment of the kidney function in AHF, through the prism of natriuresis and eGFR, as two separate processes. 16 Moreover, our data suggest an interesting signal of interaction between congestion, liver dysfunction, elevation of serum lactates, and high ET‐1, all of which are signs of poor outcome in AHF. 18 , 19 , 20 , 21 , 22 The data from clinical trials that tested the endothelin receptor antagonists in heart failure also revealed an indirect connection between endothelin and water/sodium handling, as patients in active treatment arms of the studies tended to accumulate fluid. 23 Despite endothelin antagonists now being widely used to treat pulmonary hypertension, we believe its role in heart failure pathophysiology exceeds ‘simple’ pulmonary bed vasoconstriction.
Lastly, our findings may also have some practical importance. There were several attempts to use endothelin receptor antagonists in AHF, that is, by tezosentan (VERITAS programme) or by a serelaxin (RELAX‐HF study). The primary outcomes of both studies were not met. 24 , 25 However, our data suggest that the drugs might have been studied in a population with low ET‐1 concentration, as the clinical profile of patients from both studies (e.g. mean systolic blood pressure, median NT‐proBNP, and inclusion criteria) corresponded to the lowest ET‐1 tertile in our cohort. We may only speculate that those patients may have not benefited from the drug as the ET‐1 was not a major driver of their clinical deterioration. In our cohort, ET‐1 was related with laboratory signals of organ dysfunction (elevated liver function tests, NT‐proBNP, and signs of congestion). It therefore agrees with previous RELAX‐HF analyses, indicating that serelaxin might have organ protective abilities, especially in patients with hepatorenal dysfunction. 26 , 27 It would be intriguing to see a prospectively designed trial evaluating the clinical and renal effect of ET‐1 antagonist in AHF patients with high endothelin level. Moreover, the more personalized therapy should probably be adjusted to clinical response as well as to ET‐1 level during the active phase to reduce the risk of adverse events. At this stage, we may only speculate that modifications of ET‐1‐mediated pathways in heart failure may affect the renal water/sodium handling. However, this concept needs further research.
Although the ischaemic aetiology was the predominant (53%) cause of heart failure in our population, only approximately 5% of patients reported any chest pain at admission. This is surprisingly low, as some reports have shown that endothelin may be related with chest pain even in patients without over coronary artery disease. 28 , 29 , 30
Limitation
The study is based on the registry that was run between 2010 and 2012 in a single cardiology centre, which is an obvious limitation. The timeframe difference in samples collected in our population and ASCEND‐HF trial makes direct comparison of ET‐1 levels between those studies difficult for interpretation. Finally, due to the small number of patients and relatively small number of events, the prognostic significance of ET‐1 could not be tested extensively.
Conflict of interest
None declared.
Funding
This research was financially supported from the subsidy no. SUB.E190.19.052 for the Department of Heart Diseases, Wroclaw Medical University, Poland.
Supporting information
Table S1. Correlation between endothelin‐1 and clinical and laboratory variables.
Table S2. Predictive value of endothelin‐1 on one year mortality.
Table S3 The pharmacotherapy during hospitalization by endothelin‐1 tertiles.
Zymliński, R. , Sierpiński, R. , Metra, M. , Cotter, G. , Sokolski, M. , Siwołowski, P. , Garus, M. , Gajewski, P. , Tryba, J. , Samorek, M. , Jankowska, E. A. , Biegus, J. , and Ponikowski, P. (2020) Elevated plasma endothelin‐1 is related to low natriuresis, clinical signs of congestion, and poor outcome in acute heart failure. ESC Heart Failure, 7: 3536–3544. 10.1002/ehf2.13064.
References
- 1. Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988; 332: 411–415. [DOI] [PubMed] [Google Scholar]
- 2. Pacher R, Stanek B, Hülsmann M, Koller‐Strametz J, Berger R, Schuller M, Hartter E, Ogris E, Frey B, Heinz G, Maurer G. Prognostic impact of big endothelin‐1 plasma concentrations compared with invasive hemodynamic evaluation in severe heart failure. J Am Coll Cardiol 1996; 27: 633–641. [DOI] [PubMed] [Google Scholar]
- 3. Cody RJ, Haas GJ, Binkley PF, Capers Q, Kelley R. Plasma endothelin correlates with the extent of pulmonary hypertension in patients with chronic congestive heart failure. Circulation 1992; 85: 504–509. [DOI] [PubMed] [Google Scholar]
- 4. McMurray JJ, Ray SG, Abdullah I, Dargie HJ, Morton JJ. Plasma endothelin in chronic heart failure. Circulation 1992; 85: 1374–1379. [DOI] [PubMed] [Google Scholar]
- 5. Tsutamoto T, Hisanaga T, Fukai D, Wada A, Maeda Y, Maeda K, Kinoshita M. Prognostic value of plasma soluble intercellular adhesion molecule‐1 and endothelin‐1 concentration in patients with chronic congestive heart failure. Am J Cardiol 1995; 76: 803–808. [DOI] [PubMed] [Google Scholar]
- 6. Masson S, Latini R, Anand IS, Barlera S, Judd D, Salio M, Perticone F, Perini G, Tognoni G, Cohn JN. The prognostic value of big endothelin‐1 in more than 2,300 patients with heart failure enrolled in the Valsartan Heart Failure Trial (Val‐HeFT). J Card Fail 2006; 12: 375–380. [DOI] [PubMed] [Google Scholar]
- 7. Latini R, Masson S, Anand I, Salio M, Hester A, Judd D, Barlera S, Maggioni AP, Tognoni G, Cohn JN. The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val‐HeFT. Eur Heart J 2004; 25: 292–299. [DOI] [PubMed] [Google Scholar]
- 8. Perez AL, Grodin JL, Wu Y, Hernandez AF, Butler J, Metra M, Felker GM, Voors AA, McMurray JJ, Armstrong PW, Starling RC, O'Connor CM, Tang WHW. Increased mortality with elevated plasma endothelin‐1 in acute heart failure: an ASCEND‐HF biomarker substudy. Eur J Heart Fail 2016; 18: 290–297. [DOI] [PubMed] [Google Scholar]
- 9. Ramseyer VD, Cabral PD, Garvin JL. Role of endothelin in thick ascending limb sodium chloride transport. Endothelin in Renal Physio and Dis 2011; 5: 76–83. [DOI] [PubMed] [Google Scholar]
- 10. Dickstein K, Cohen‐Solal A, Filippatos G, McMurray JJV, Ponikowski P, Poole‐Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. ESC GUIDELINES ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J 2008; 29: 2388–2442. [DOI] [PubMed] [Google Scholar]
- 11. Mcmurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GYH, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur J Heart Fail 2012; 14: 803–869. [DOI] [PubMed] [Google Scholar]
- 12. Biegus J, Zymliński R, Sokolski M, Todd J, Cotter G, Metra M, Jankowska EA, Banasiak W, Ponikowski P. Serial assessment of spot urine sodium predicts effectiveness of decongestion and outcome in patients with acute heart failure. Eur J Heart Fail 2019; 21: 624–633. [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Annals of Inter Med 1999; 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 14. Milo‐Cotter O, Cotter‐Davison B, Lombardi C, Sun H, Bettari L, Bugatti S, Rund M, Metra M, Kaluski E, Kobrin I, Frey A, Rainisio M, McMurray JJV, Teerlink JR, Cotter‐Davison G. Neurohormonal activation in acute heart failure: results from VERITAS. Cardiology 2011; 119: 96–105. [DOI] [PubMed] [Google Scholar]
- 15. Johnson W, Omland T, Hall C, Lucas C, Myking OL, Collins C, Pfeffer M, Rouleau JL, Stevenson LW. Neurohormonal activation rapidly decreases after intravenous therapy with diuretics and vasodilators for class IV heart failure. J Am Coll Cardiol 2002; 39: 1623–1629. [DOI] [PubMed] [Google Scholar]
- 16. Mullens W, Damman K, Testani JM, Martens P, Mueller C, Lassus J, Tang WHHWW, Skouri H, Verbrugge FH, Orso F, Hill L, Dilek U, Lainscak M, Rossignol P, Metra M, Mebazaa A, Seferovic P, Ruschitzka F, Coats A. Evaluation of kidney function throughout the heart failure trajectory—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020; 22: 584–603. [DOI] [PubMed] [Google Scholar]
- 17. Testani JM, Hanberg JS, Cheng S, Rao V, Onyebeke C, Laur O, Kula A, Chen M, Wilson FP, Darlington A, Bellumkonda L, Jacoby D, Tang WHW, Parikh CR. Rapid and highly accurate prediction of poor loop diuretic natriuretic response in patients with heart failure. Circ Heart Fail 2016; 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Biegus J, Zymliński R, Sokolski M, Gajewski P, Banasiak W, Ponikowski P. Clinical, respiratory, haemodynamic, and metabolic determinants of lactate in heart failure. Pol Heart J 2018; 77: 47–52. [DOI] [PubMed] [Google Scholar]
- 19. Zymliński R, Biegus J, Sokolski M, Siwołowski P, Nawrocka‐Millward S, Todd J, Jankowska EA, Banasiak W, Cotter G, Cleland JG, Ponikowski P, Zymli R, Nawrocka‐Millward S, Todd J, Jankowska EA, Banasiak W, Cotter G, Cleland JG, Ponikowski P, Zymliński R, Biegus J, Sokolski M, Siwołowski P, Nawrocka‐Millward S, Todd J, Jankowska EA, Banasiak W, Cotter G, Cleland JG, Ponikowski P. Increased blood lactate is prevalent and identifies poor prognosis in patients with acute heart failure without overt peripheral hypoperfusion. Eur J Heart Fail 2018; 20: 1011–1018. [DOI] [PubMed] [Google Scholar]
- 20. Biegus J, Zymliński R, Sokolski M, Nawrocka S, Siwołowski P, Szachniewicz J, Jankowska EA, Banasiak W, Ponikowski P. Liver function tests in patients with acute heart failure. Pol Arch Internal Med 2012; 122: 471–479. [DOI] [PubMed] [Google Scholar]
- 21. Biegus J, Zymliński R, Sokolski M, Siwołowski P, Gajewski P, Nawrocka‐Millward S, Poniewierka E, Jankowska EAEA, Banasiak W, Ponikowski P. Impaired hepato‐renal function defined by the MELD XI score as prognosticator in acute heart failure. Eur J Heart Fail 2016; 18: 1518–1521. [DOI] [PubMed] [Google Scholar]
- 22. Biegus J, Zymliński R, Gajewski P, Sokolski M, Siwołowski P, Sokolska J, Swoboda K, Banasiak M, Banasiak W, Ponikowski P. Persistent hyperlactataemia is related to high rates of in‐hospital adverse events and poor outcome in acute heart failure. Pol Heart J 2019; 77: 355–362. [DOI] [PubMed] [Google Scholar]
- 23. Packer M, McMurray JJV, Krum H, Kiowski W, Massie BM, Caspi A, Pratt CM, Petrie MC, DeMets D, Kobrin I, Roux S, Swedberg K, Packer M, Caspi A, Kiowski W, Krum H, Pratt C, Swedberg K, Massie B, McMurray JM, Connally E, Petrie M, DeMets D, Anderson S, Barnet J, Cody R, Dargie H, Francis G, Greenberg B, Reichen J, Karrasch J, Krum H, Horowitz J, Amerena J, Sindone A, MacDonald P, Jeffrey I, Button I, DeAngelis E, Pacher R, Davies R, McAlister F, Tanser P, Sussex B, Baumann G, Fleck E, Olbrich HG, Werdan K, Klein H. Long‐term effect of endothelin receptor antagonism with bosentan on the morbidity and mortality of patients with severe chronic heart failure: primary results of the ENABLE trials. JACC: Heart Failure 2017; 5: 317–326. [DOI] [PubMed] [Google Scholar]
- 24. Metra M, Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Voors AA, Adams KF, Anker SD, Arias‐Mendoza A, Avendaño P, Bacal F, Böhm M, Bortman G, Cleland JGF, Cohen‐Solal A, Crespo‐Leiro MG, Dorobantu M, Echeverría LE, Ferrari R, Goland S, Goncalvesová E, Goudev A, Køber L, Lema‐Osores J, Levy PD, McDonald K, Manga P, Merkely B, Mueller C, Pieske B, Silva‐Cardoso J, Špinar J, Squire I, Stępińska J, van Mieghem W, von Lewinski D, Wikström G, Yilmaz MB, Hagner N, Holbro T, Hua TA, Sabarwal SV, Severin T, Szecsödy P, Gimpelewicz C, RELAX‐AHF‐2 Committees Investigators . Effects of serelaxin in patients with acute heart failure. N Engl J Med 2019; 381: 716–726.31433919 [Google Scholar]
- 25. McMurray JJV, Teerlink JR, Cotter G, Bourge RC, Cleland JGF, Jondeau G, Krum H, Metra M, O'Connor CM, Parker JD, Torre‐Amione G, van Veldhuisen DJ, Lewsey J, Frey A, Rainisio M, Kobrin I. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. JAMA 2007; 298: 2009–2019. [DOI] [PubMed] [Google Scholar]
- 26. Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Dorobantu MI, Grinfeld L, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Prescott MF, Edwards C, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin T, Teerlink JR. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX‐AHF) development program: correlation with outcomes. J Am Coll Cardiol 2013; 61: 196–206. [DOI] [PubMed] [Google Scholar]
- 27. Biegus J, Demissei B, Postmus D, Cotter G, Davison BA, Felker GM, Filippatos G, Gimpelewicz C, Greenberg B, Metra M, Severin T, Teerlink JR, Voors AA, Ponikowski P. Hepatorenal dysfunction identifies high‐risk patients with acute heart failure: insights from the RELAX‐AHF trial. ESC Heart Failure 2019; 6: 1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaski JC, Elliott PM, Salomone O, Dickinson K, Gordon D, Hann C, Holt DW. Concentration of circulating plasma endothelin in patients with angina and normal coronary angiograms. Heart 1995; 74: 620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cox ID, Bøtker HE, Bagger JP, Sonne HS, Kristensen BO, Kaski JC. Elevated endothelin concentrations are associated with reduced coronary vasomotor responses in patients with chest pain and normal coronary arteriograms. J Am Coll Cardiol 1999; 34: 455–460. [DOI] [PubMed] [Google Scholar]
- 30. Desideri G, Gaspardone A, Gentile M, Santucci A, Gioffrè PA, Ferri C. Endothelial activation in patients with cardiac syndrome X. Circulation 2000; 102: 2359–2364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Correlation between endothelin‐1 and clinical and laboratory variables.
Table S2. Predictive value of endothelin‐1 on one year mortality.
Table S3 The pharmacotherapy during hospitalization by endothelin‐1 tertiles.


