Abstract
Aims
Vitamin D supplementation is widely used in the clinical setting, but its effects on mortality and cardiovascular outcomes in patients with heart failure are unclear. This paper reports outcome data that were collected during follow‐up of 3 years after closure of the EVITA trial (a 3 year randomized, placebo‐controlled, intervention study with 4000 IU vitamin D daily in patients with advanced heart failure), to capture potential latency effects of vitamin D supplementation on clinical outcomes.
Methods and results
The prespecified primary endpoint was overall mortality. Secondary endpoints included hospitalization, mechanical circulatory support implantation, high urgent listing for heart transplantation, and heart transplantation. For group comparisons, we used Cox regression models with a time‐dependent categorical covariate. The calculated net difference in circulating 25‐hydroxyvitamin D between the vitamin D and placebo groups dropped from 60.9 nmol/L at the end of the active study period to 3.2 nmol/L at the end of the post‐intervention period. During the entire 6 year period, 73 patients (36.5%) died in the placebo group and 76 (38.8%) in the vitamin D group. Out of these 149 patients, 36 and 39 died during the first 3 years, and 37 and 37 during the second 3 years, respectively. The hazard ratio (HR) for mortality in the vitamin D versus the placebo group was 1.06 [95% confidence interval (CI): 0.68–1.66] for the first 3 years and 1.07 (95% CI: 0.68–1.70) for the 3 year post‐intervention follow‐up. Compared with the placebo group, the HRs for hospitalization and for mechanical circulatory support implant were significantly higher in the vitamin D group during vitamin D supplementation (HR = 1.31, 95% CI: 1.01–1.68 and HR = 2.01, 95% CI: 1.08–3.76, respectively) but not after vitamin D discontinuation (HR = 1.10, 95% CI: 0.62–1.94 and HR = 0.99, 95% CI: 0.38–2.56, respectively). There was no significant time‐dependent effect on the risk of high urgent listing for heart transplantation and heart transplantation.
Conclusions
No beneficial latency effects of vitamin D supplementation on overall mortality could be demonstrated. Instead, the disappearance of unfavourable findings in the vitamin D group (higher HRs for hospitalization and for mechanical circulatory support implant) after vitamin D discontinuation supports the assumption of adverse vitamin D effects on the cardiovascular system at doses of 4000 IU daily.
Keywords: Vitamin D, Heart failure, Mortality, Survival, Hospitalization, Mechanical circulatory support
Introduction
Observational data indicate that adequate vitamin D status may reduce all‐cause mortality risk, probably because of various beneficial vitamin D effects in different tissues, 1 but evidence from randomized controlled trials (RCTs) is inconsistent. 2 , 3 We recently demonstrated in patients with advanced heart failure (HF) and median circulating 25‐hydroxyvitamin D (25[OH]D) concentrations below 40 nmol/L that a daily vitamin D3 dose of 4000 IU for 3 years failed to significantly influence mortality risk. 4 An explanation for the null effect may be the relatively short study duration for this endpoint of only 3 years. Notably, a meta‐analysis of RCTs on vitamin D supplementation reported a significant decrease in all‐cause mortality in studies whose duration was longer than 3 years but not in studies of shorter duration. 5 In line with this meta‐analysis, a Mendelian randomization study 6 indicated that a genetically determined and thus lifelong 20 nmol/L lower 25(OH)D concentration is associated with a 30% increased mortality risk. Serum 25(OH)D is the generally accepted indicator of vitamin D status, and many guidelines recommend target serum 25(OH)D concentrations of ≥50 nmol/L. 7
Considerations for post‐intervention follow‐up analyses of vitamin D RCTs are supported by data on long‐term storage of vitamin D in the adipose tissue. 8 At the end of a 3 to 5 year intervention with a weekly dose of 20 000 IU vitamin D3 (equivalent to 2857 IU/day), 9 fat tissue vitamin D content was eight times higher in the vitamin D group than in the placebo group. Moreover, 1 year after vitamin D discontinuation circulating 25(OH)D concentrations were still substantially higher in the supplemented group than in the placebo group, and in the steady phase of decline, the half‐life of circulating 25(OH)D was calculated to be 255 days in the vitamin D group. 9 In another vitamin D supplementation study (5000 IU vitamin D3 daily for 1 year), 25(OH)D concentrations decreased over the 3 years of vitamin D discontinuation from 127 nmol/L at study end to 65 nmol/L after 1 year and 28 nmol/L after 3 years, 10 and thus to values which were present at study enrolment. 11
Because circulating 25(OH)D concentrations obviously remain elevated for a considerable time after vitamin D discontinuation, we performed a 3 year post‐intervention follow‐up of the EVITA trial to capture potential latency effects of vitamin D supplementation.
Methods
Participants
The EVITA (effect of vitamin D on mortality in HF) trial was a single‐centre study at the Heart and Diabetes Center North Rhine Westphalia, Bad Oeynhausen, Germany. Full details regarding study design, trial registration, and ethics committee approval can be found in the main trial report. 4 Participants were recruited from the HF unit of the Clinic for Thoracic and Cardiovascular Surgery at our heart centre. Inclusion criteria were an age between 18 and 79 years and New York Heart Association functional class II or higher. Key exclusion criteria were ‘high urgent’ listing for heart transplantation, hypercalcaemia, the daily intake of a vitamin D supplement of >800 IU, and baseline 25(OH)D levels ≥75 nmol/L.
Interventions
Participants were randomized into two groups to receive eight drops of an oily vitamin D preparation (Vigantol Oil, Merck, Darmstadt, Germany), resulting in a daily vitamin D3 intake of 4000 IU or eight drops of a matching vitamin D‐free oil daily (Migliol Oil; Merck) during a meal. Randomization was centralized, computer generated, and stratified by sex. All patients were in a long‐term HF programme, and trial medication was delivered during regular visits at the HF unit at our clinic.
Outcomes and follow‐up
Overall mortality was the primary prespecified endpoint in the main trial protocol. Secondary endpoints included hospitalization, mechanical circulatory support (MCS) implantation, high urgent listing for heart transplantation, and heart transplantation. This paper reports outcome data that had been observed during the trial and within 3 years of trial closure. This timing was selected because of the kinetics of serum 25(OH)D decline within 3 years of cessation of vitamin D supplementation. Mortality was assessed by using the following sources of information: a review of our medical records; an annual, standardized form (post‐discharge) completed by the patients themselves or by their family physician; and annual consultation of the respective registration office in case of missing post‐discharge forms. Secondary clinical endpoints were assessed by the same sources as used for the primary endpoint (with the exception of the registration office consultation).
Statistical analysis
Descriptive characteristics are summarized as median with 25th to 75th percentiles for continuous variables and counts and percentage of observations for categorical variables. The Mann–Whitney U‐test and the Wilcoxon signed ranks test were used for group comparisons, where appropriate. We used the equation by Martinaityte et al. 9 to estimate the difference in circulating 25(OH)D in our study groups at post‐intervention year 1, 2, and 3 as follows:
| (1) |
where 255 is the calculated half‐life in days of circulating 25(OH)D in the vitamin D group after vitamin D discontinuation, elapsed time is the time after vitamin D discontinuation, ln2 is the natural logarithm of 2 and is a constant of 0.693, Δ of 25(OH)D is the net difference in 25(OH)D at the end of the vitamin D supplementation period, and x is the calculated difference between the study groups in 25(OH)D at a given elapsed time after vitamin D discontinuation.
Event‐free survival time was modelled using Kaplan–Meier survival estimates and Cox regression models. Five outcome measures were explored, including one primary and four secondary endpoints: primary endpoint was time to death of any cause. Secondary endpoints were time to first hospitalization after study enrolment, MCS implantation, ‘high urgent’ listing for transplantation, or transplantation (censoring those who died during follow‐up). The explanatory variable in the models was the treatment group. Follow‐up was assessed using the Kaplan–Meier estimate of potential follow‐up. 12 The primary and secondary endpoints were all assessed by the intention‐to‐treat principle. In addition, the per‐protocol principle was used to assess overall mortality by excluding patients who dropped out prematurely (no adherence to the study treatment but still attending our HF unit) or were lost to follow‐up (no personal contact to the patient anymore) from data analyses. Because serum 25(OH)D concentrations decline after vitamin D discontinuation, we also used Cox regression models with a time‐dependent categorical covariate (first 3 year period and second 3 year period) to assess the potential effect of time on clinical outcomes. For each model, hazard ratios (HR) with 95% confidence intervals (CI) were calculated. Regarding the active study period of 3 years, the study had 80% power to detect a 62% reduction in the primary endpoint with vitamin D compared with placebo. This assumption was based on a two‐sided 5% significance level and 10% mortality per year. Regarding the 6 year follow‐up period, the study had 80% power to detect a 47% reduction in the primary endpoint with vitamin D compared with placebo. Analyses were performed using SPSS version 24.0 (IBM Corp, Armonk, NY, USA).
Results
Baseline characteristics
Baseline characteristics such as age and sex, anthropometry, clinical data, medication use, and vitamin D status are given in Table 1 . The majority of patients were male, were between 50 and 60 years of age, and had left ventricular ejection fraction values below 30%. The treatment‐resistant symptoms of HF in this study cohort are reflected by the high percentage of patients who were treated with angiotensin converting enzyme inhibitors/angiotensin II receptor blockers, beta‐blockers, and mineralocorticoid receptor antagonists. Notably, 20% of patients were already awaiting heart transplantation. None of the patients were treated with angiotensin receptor‐neprilysin inhibitors, neither during the active study period nor during the 3 year follow‐up period.
Table 1.
Baseline characteristics of the study groups
| Parameter | Placebo group (n = 201) | Vitamin D group (n = 199) |
|---|---|---|
| Age (years) | 54 (48–60) | 56 (48–62) |
| Gender (% male, n) | 82.6 (166) | 83.4 (166) |
| Body weight (kg) | 88 (75–98) | 88 (75–99) |
| Body height (cm) | 176 (170–183) | 176 (170–182) |
| Body mass index (kg/m2) | 27.8 (25.1–30.8) | 27.8 (24.5–31.6) |
| Diagnosis | ||
| DCM (%, n) | 49.8 (100) | 46.2 (92) |
| ICM (%, n) | 42.8 (86) | 46.8 (93) |
| Others (%, n) | 7.4 (15) | 7.0 (14) |
| Diabetes mellitus (%, n) | 22.9 (46) | 25.6 (51) |
| Anaemia (%, n) | 12.2 (24) | 18.2 (36) |
| Chronic obstructive pulmonary disease (%, n) | 8.0 (16) | 4.0 (8) |
| eGFR <60 mL/min/1.73 m2 | 30.3 (61) | 39.7 (79) |
| Left ventricular ejection fraction (%) | 27 (24–35) | 28 (23–34) |
| Medication | ||
| ACE‐inhibitors/ARBs (%, n) | 95.0 (191) | 97.0 (193) |
| Beta‐blockers (%, n) | 97.0 (195) | 94.0 (187) |
| Aldosterone‐antagonists (%, n) | 84.6 (170) | 79.9 (159) |
| Loop diuretics (%, n) | 82.6 (166) | 86.9 (173) |
| Thiazide‐diuretics (%, n) | 31.8 (64) | 35.7 (71) |
| Digoxin (%, n) | 42.3 (85) | 33.7 (67) |
| Lipid‐lowering drugs (%, n) | 52.2 (105) | 56.8 (113) |
| Vitamin D supplement use (%, n) | 0 (0) | 0 (0) |
| 25‐hydroxyvitamin D (nmol/L) | 35.2 (25.7–49.2) | 31.3 (21.5–44.8) |
| C‐reactive protein (mg/L) | 2.1 (0.9–3.9) | 2.4 (1.0–6.9) |
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers; DCM, dilated cardiomyopathy; eGFR, estimated glomerular filtration rate; ICM, ischaemic cardiomyopathy.
Median 25(OH)D concentrations were initially well below 50 nmol/L, and none of the study participants were taking vitamin D supplements (Table 1 ). At year 3 of the study, adherence rates in the vitamin D and placebo groups were 54.0% and 55.2%, respectively. At that time, median and 25th to 75th percentiles of circulating 25(OH)D in those patients who were alive and adherent were 92.8 nmol/L (62.5 to 128.3 nmol/L) and 40.8 nmol/L (31.3 to 58.0 nmol/L) in the vitamin D group (n = 84) and the placebo group (n = 87), respectively (P < 0.001). The changes in circulating 25(OH)D in the vitamin D and placebo groups between enrolment and study termination were 62.8 nmol/L (24.8 to 95.8 nmol/L; P < 0.001) and 1.9 nmol/L (−5.9 to 25.0 nmol/L; P = 0.019), respectively, indicating an average net change in circulating 25(OH)D by vitamin D supplementation of 60.9 nmol/L. At post‐intervention years 1, 2, and 3, the calculated differences in circulating 25(OH)D between the vitamin D and placebo groups were 23.0, 8.5, and 3.2 nmol/l, respectively.
Clinical outcomes
The study flow chart is given in Figure 1 . Completeness of study data for the primary endpoint in the vitamin D and placebo groups was 94.1% and 97.8%, respectively. During the 6 years of follow‐up, 73 patients (36.5%) died in the placebo group and 76 (38.8%) in the vitamin D group (Figure 2 ), with an HR for mortality in the vitamin D versus the placebo group of 1.07 (95% CI: 0.77–1.47; P = 0.70). In the vitamin D group, causes of death were cardiac‐related in 32 patients and non‐cardiac‐related, such as sepsis, pneumonia, hypoxic brain damage, pancreatitis, carcinoma, and intestinal ischaemia in 21, and unknown in 23. The corresponding data in the placebo group were 36 cardiac‐related, 11 non‐cardiac‐related, such as sepsis, liver failure, renal failure, carcinoma, and hypoxic brain damage, and 26 unknown. Mortality rates in the vitamin D and placebo group were 17.9% and 19.6% at the end of the active study period (P = 0.73), 23.4% and 25.7% at 1 year follow‐up (P = 0.65), 33.4% and 34.3% at 2 year follow‐up (P = 0.86), and 36.4% and 38.6% at 3 year follow‐up (P = 0.71), respectively. The time‐dependent Cox regression analysis in the vitamin D versus the placebo group revealed an HR for mortality of 1.06 (95% CI: 0.68–1.66; P = 0.80) for the first 3 year period and an HR of 1.07 (95% CI: 0.68–1.70; P = 0.76) for the second 3 year period. In the per‐protocol population, 6 year mortality in the placebo group and the vitamin D group was 40.2% and 40.4%, respectively, with an HR for the vitamin D versus the placebo group of 0.99 (95% CI: 0.66–1.49; P = 0.96; Supporting Information, Figure S1 ). Supporting Information, Figure S2 illustrates the cumulative incidence of mortality during follow‐up for those patients who survived the active study period.
Figure 1.
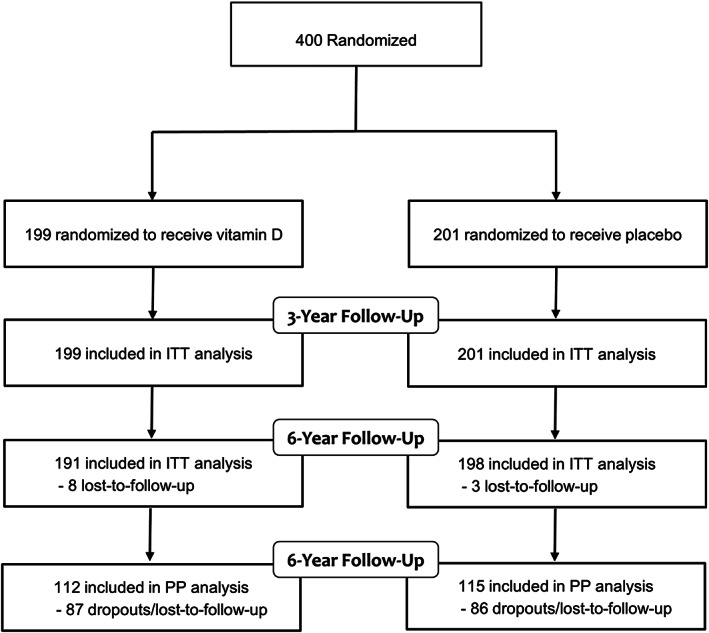
Study flow chart. ITT, intention‐to‐treat; PP, per‐protocol.
Figure 2.
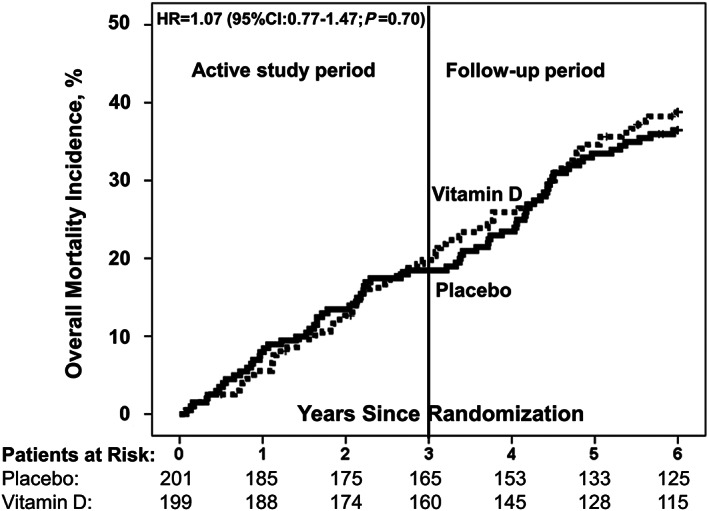
Cumulative incidence of all‐cause mortality by study group. HR, hazard ratio; CI, confidence interval vitamin D (dotted line); placebo (solid line); reference: placebo group.
Of the 400 study participants, 286 were hospitalized, 59 received MCS implants, 52 were ‘high urgent’ listed for heart transplantation, and 63 were transplanted during the 6 year follow‐up period. In Table 2 , results of the secondary endpoints are presented by study group. Overall, 6 year freedom from hospitalization was significantly lower in the vitamin D group than in the placebo group, with an HR for hospitalization in the vitamin D versus the placebo group of 1.27 (95% CI: 1.01–1.60; P = 0.045). Six years of risk of clinical events such as MCS implantation, ‘high urgent’ listing for heart transplantation, and heart transplantation did not differ significantly between study groups. However, we observed substantial time‐dependent differences between study groups with respect to MCS implant and also regarding hospitalization (Table 2 ): compared with the placebo group, the HRs for hospitalization and MCS implant were significantly higher in the vitamin D group only in the vitamin D supplementation period (P = 0.040 and P = 0.028, respectively) but not in the period after vitamin D discontinuation (P = 0.76 and P = 0.98, respectively). Figure 3 visually presents the cumulative event rates of the secondary endpoints over time.
Table 2.
Incidence and hazard ratio of secondary endpoints of the study participants
| Cox regression analysis hazard ratio (95% CI) | Time‐dependent Cox regression analysis hazard ratio (95% CI) | ||||
|---|---|---|---|---|---|
| Parameter | Placebo (n = 201) | Vitamin D (n = 199) | Year 0–6 | Year 0–3 | Year 4–6 |
| Hospitalization (%, n) | 68.5 (137) | 74.9 (149) | 1.27 (1.01–1.60) | 1.31 (1.01–1.68) | 1.10 (0.62–1.94) |
| MCS Implants (%, n) | 12.4 (25) | 17.1 (34) | 1.49 (0.89–2.52) | 2.01 (1.08–3.76) | 0.99 (0.38–2.56) |
| HU Listing for HTx (%, n) | 15.4 (26) | 15.5 (26) | 1.03 (0.60–1.77) | 1.01 (0.53–1.94) | 1.07 (0.40–2.84) |
| HTx (%, n) | 18.3 (30) | 20.4 (33) | 1.13 (0.69–1.85) | 1.06 (0.57–1.95) | 1.27 (0.55–2.94) |
CI, confidence interval; HTx, heart transplantation; HU, high urgent; MCS, mechanical circulatory support.
Figure 3.
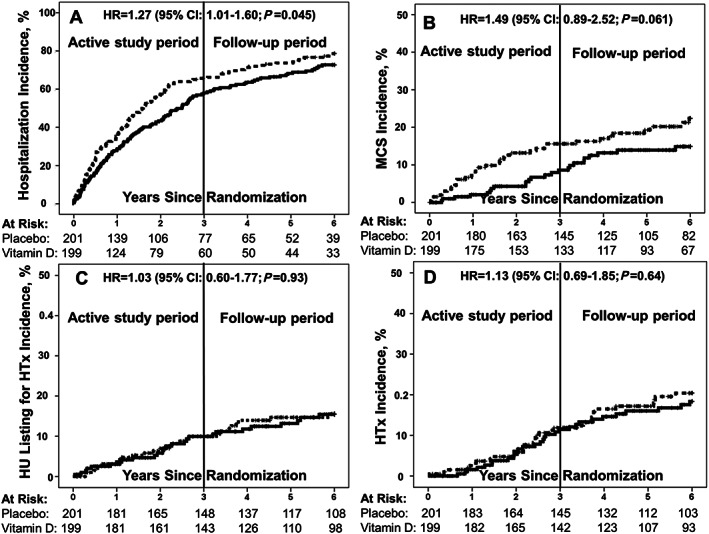
Cumulative incidence of hospitalization (A), mechanical circulatory support implant (B), high urgent listing for transplantation (C), and transplantation (D) by study group. Abbreviations: HR, hazard ratio; CI, confidence interval; MCS, mechanical circulatory support; HU, high urgent; HTx, heart transplantation vitamin D (dotted line); placebo (solid line); reference: placebo group.
Discussion
In this group of patients with treatment‐resistant symptoms of HF experiencing a high 6 year mortality risk of almost 40%, no significant latency effect of a 3 year supplementation with 4000 IU vitamin D daily could be demonstrated on overall mortality.
At the start of the 3 year post‐intervention follow‐up, the net difference in circulating 25(OH)D between the vitamin D and placebo group was on average 61 nmol/L. According to earlier data obtained following vitamin D discontinuation, 9 , 10 it can be safely assumed that in the vitamin D supplemented group, circulating 25(OH)D concentrations remain substantially elevated within the first 12 to 18 months post‐intervention but progressively decline to baseline 25(OH)D levels by the end of the 3 year extension period. Thus, our extended data analysis indicates that mid‐term to long‐term elevated circulating 25(OH)D concentrations may not result in reduced overall mortality in patients with advanced HF. Notably, in our per‐protocol population, the HR for mortality was also close to equality. The assumption of a null effect of vitamin D supplementation on overall mortality in patients with advanced HF is further strengthened by the similar mortality risk of the two study cohorts during the first and second 3 year periods of our trial. In our study, the majority of known causes of death were of cardiac origin. There is evidence that vitamin D supplementation may reduce cancer mortality but not cardiovascular mortality 2 and that higher circulating 25(OH)D concentrations may even tend to increase the latter. 6
Freedom from hospitalization was lower, and the need of MCS implants was higher in the vitamin D compared with the placebo group only in the first 3 year but not in the second 3 year period. The disappearance of these associations after vitamin D discontinuation indicates that vitamin D supplementation may have triggered adverse outcomes. We had already hypothesized that higher plasma calcium concentrations could be responsible for adverse vitamin D effects on the cardiovascular system and that especially patients with initial 25(OH)D concentrations >30 nmol/L and in‐study concentrations >100 nmol/L could be affected. 4 The assumed decline in circulating 25(OH)D after vitamin D discontinuation and the absence of higher HRs of hospitalization and MCS implant in the post‐intervention period is in line with this hypothesis. Moreover, in the era of donor heart shortage, MCS implant rather than heart transplantation is the last option to prevent death in patients with advanced HF in case of disease progression. This may explain why the risk of ‘high urgent’ listing for heart transplantation as well as heart transplantation did not differ significantly between our two study groups and did not change over time. A few studies in cohorts with a high prevalence of cardiovascular disease indicate that circulating 25(OH)D concentrations ≥100 nmol/L are associated with increased risk of major adverse cardiac and cerebrovascular events 13 and CVD mortality. 14 In an older population, 25(OH)D concentrations ≥50 nmol/L were positively associated with carotid intima media thickness, 15 the latter being predictive of cardiovascular events. 16
The daily vitamin D dose of 4000 IU was equivalent to the amount which the Institute of Medicine has set as the upper tolerable intake level for the general population. 17 However, the database for this threshold is limited, and our data indicate that this dose should not a priori be considered safe for patients with HF. Therefore, in the clinical setting, caution is necessary when using vitamin D doses beyond the nutritionally recommended daily intake of 600–800 IU 17 and when using cut‐off values of circulating 25(OH)D, which were created for the general population. Ideally, cut‐offs of circulating 25(OH)D concentrations and daily vitamin D doses should be established separately for each disease entity.
There are some limitations of our study that have to be addressed. First, the treatment options offered at our HF unit may have masked not only a beneficial but also an adverse vitamin D effect on overall mortality. Second, although we were able to calculate differences in circulating 25(OH)D between the vitamin D and placebo groups at post‐intervention years 1, 2, and 3, the post‐intervention follow‐up actually lacks 25(OH)D measurements. However, available pharmacokinetic data on circulating 25(OH)D after vitamin D discontinuation allowed us to estimate the decline in circulating 25(OH)D in the post‐intervention period with a great degree of confidence. Third, attrition rates were relatively high. Nevertheless, we were able to show important differences by study period with respect to critical secondary endpoints. Finally, we cannot definitively rule out that the differences between the study groups in secondary endpoints in the first 3 year period were chance findings and that the disappearance of these differences after vitamin D discontinuation was due to the phenomenon of regression to the mean. A main strength of our study is that no other vitamin D RCT in a patient population with a daily dose of at least 4000 IU reported on such long‐term outcome data. In light of a relatively wide use of vitamin D supplements, our findings provide important new data not only in terms of missing beneficial effects on hard clinical endpoints but also in terms of potential safety concerns of relatively high dose vitamin D supplementation in patients with advanced HF.
In conclusion, no beneficial latency effects of vitamin D supplementation on overall mortality could be demonstrated in patients with advanced HF at high mortality risk. Instead, the disappearance of unfavourable findings in the vitamin D group (higher HRs for hospitalization and for MCS implant) indicates that vitamin D supplementation may have triggered adverse effects on the cardiovascular system.
Conflict of interest
None declared.
Funding
The study was sponsored by our clinic. The Friede Springer Herz Stiftung (Berlin, Germany) and Merck KGaA (Darmstadt, Germany, EMR200109‐616) provided funding for the study. Merck KGaA also provided the study medication, and DiaSorin (Dietzenbach, Germany) provided the 25(OH)D test kits. The funding sources were not involved in the study design, collection, analysis, or interpretation of data or in preparation or submission of the manuscript for publication.
Supporting information
Figure S1. Cumulative incidence of all‐cause mortality in the per‐protocol population by study group.
Figure S2. Cumulative incidence of all‐cause mortality during follow‐up in those patients who survived the active study period.
Acknowledgement
Open access funding enabled and organized by Projekt DEAL.
Zittermann, A. , Ernst, J. B. , Prokop, S. , Fuchs, U. , Berthold, H. K. , Gouni‐Berthold, I. , Gummert, J. F. , and Pilz, S. (2020) A 3 year post‐intervention follow‐up on mortality in advanced heart failure (EVITA vitamin D supplementation trial). ESC Heart Failure, 7: 3754–3761. 10.1002/ehf2.12953.
Clinical Trial Registration: NCT01326650.
[Correction added on 28 SEPT 2020, after first onlinepublication: Projekt Deal funding statement has been added.]
References
- 1. Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta‐analysis of prospective cohort studies. Am J Clin Nutr 2012; 95: 91–100. [DOI] [PubMed] [Google Scholar]
- 2. Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev 2014; 1: CD007470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang Y, Fang F, Tang J, Jia L, Feng Y, Xu P, Faramand A. Association between vitamin D supplementation and mortality: systematic review and meta‐analysis. BMJ 2019: 366 l4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zittermann A, Ernst JB, Prokop S, Fuchs U, Dreier J, Kuhn J, Knabbe C, Birschmann I, Schulz U, Berthold HK, Pilz S, Gouni‐Berthold I, Gummert JF, Dittrich M, Börgermann J. Effect of vitamin D on all‐cause mortality in heart failure (EVITA): a 3‐year randomized clinical trial with 4000 IU vitamin D daily. Eur Heart J 2017; 38: 2279–2286. [DOI] [PubMed] [Google Scholar]
- 5. Zheng Y, Zhu J, Zhou M, Cui L, Yao W, Liu Y. Meta‐analysis of long‐term vitamin D supplementation on overall mortality. PLoS ONE 2013; 8: e82109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Afzal S, Brøndum‐Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ 2014; 349: g6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pilz S, Zittermann A, Trummer C, Theiler‐Schwetz V, Lerchbaum E, Keppel MH, Grübler MR, März W, Pandis M. Vitamin D testing and treatment: a narrative review of current evidence. Endocr Connect 2019; 8: R27–R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mawer EB, Backhouse J, Holman CA, Lumb GA, Stanbury SW. The distribution and storage of vitamin D and its metabolites in human tissues. Clin Sci 1972; 43: 413–431. [DOI] [PubMed] [Google Scholar]
- 9. Martinaityte I, Kamycheva E, Didriksen A, Jakobsen J, Jorde R. Vitamin D stored in fat tissue during a 5‐year intervention affects serum 25‐hydroxyvitamin D levels the following year. J Clin Endocrinol Metab 2017; 102: 3731–3738. [DOI] [PubMed] [Google Scholar]
- 10. Mocanu V, Vieth R. Three‐year follow‐up of serum 25‐hydroxyvitamin D, parathyroid hormone, and bone mineral density in nursing home residents who had received 12 months of daily bread fortification with 125 μg of vitamin D₃. Nutr J 2013; 12: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mocanu V, Stitt PA, Costan AR, Voroniuc O, Zbranca E, Luca V, Vieth R. Long‐term effects of giving nursing home residents bread fortified with 125 microg (5000 IU) vitamin D(3) per daily serving. Am J Clin Nutr 2009; 89: 1132–1137. [DOI] [PubMed] [Google Scholar]
- 12. Schemper M, Smith TL. A note on quantifying follow‐up in studies of failure time. Control Clin Trials 1996; 17: 343–346. [DOI] [PubMed] [Google Scholar]
- 13. Zittermann A, Kuhn J, Dreier J, Knabbe C, Gummert JF, Börgermann J. Vitamin D status and the risk of major adverse cardiac and cerebrovascular events in cardiac surgery. Eur Heart J 2013; 34: 1358–1364. [DOI] [PubMed] [Google Scholar]
- 14. Durup D, Jørgensen HL, Christensen J, Tjønneland A, Olsen A, Halkjær J, Lind B, Heegaard AM, Schwarz P. A reverse J‐shaped association between serum 25‐hydroxyvitamin D and cardiovascular disease mortality: the CopD study. J Clin Endocrinol Metab 2015; 100: 2339–2346. [DOI] [PubMed] [Google Scholar]
- 15. van Dijk SC, Sohl E, Oudshoorn C, Enneman AW, Ham AC, Swart KM, van Wijngaarden JP, Brouwer‐Brolsma EM, van der Zwaluw NL, Uitterlinden AG, de Groot LC, Dhonukshe‐Rutten RA, Lips P, van Schoor NM, Blom HJ, Geleijnse JM, Feskens EJ, Smulders YM, Zillikens MC, de Jongh RT, van den Meiracker AH, Mattace Raso FU, van der Velde N. Non‐linear associations between serum 25‐OH vitamin D and indices of arterial stiffness and arteriosclerosis in an older population. Age Ageing 2015; 44: 136–142. [DOI] [PubMed] [Google Scholar]
- 16. Polak JF, O'Leary DH. Carotid intima‐media thickness as surrogate for and predictor of CVD. Glob Heart 2016; 11: 295–312 e3. [DOI] [PubMed] [Google Scholar]
- 17. Institute of Medicine . Dietary reference intakes: calcium and vitamin D. Washington: DC: National Academies Press; 2011. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Cumulative incidence of all‐cause mortality in the per‐protocol population by study group.
Figure S2. Cumulative incidence of all‐cause mortality during follow‐up in those patients who survived the active study period.


