Abstract
Background:
Obesity in midlife and early late-life is associated with worse normal cognitive aging. Dual-energy x-ray absorptiometry (DEXA) suggests that visceral adipose mass (VAM) plays a predominant role, whereas non-visceral adipose mass (NVAM) and lean muscle mass (LMM) have shown conflicting relationships. It is unknown how longitudinal, cognitive changes in age-sensitive domains like fluid intelligence (FI) correspond to VAM, NVAM, and LMM in women and men. Furthermore, changes over time in blood leukocyte sub-populations may partially or fully account for sex-specific associations.
Methods:
Data on 4,431 late middle-aged, cognitively unimpaired adults (mean=64.5y) was obtained from the UK Biobank prospective cohort across 22 centers. FI scores, blood leukocyte counts, and covariates (age, social class, education) were measured at three 2-year intervals over 6 years. DEXA collection overlapped with these intervals. Sex-stratified growth curves, structural equations, and Preacher-Hayes mediation were used to estimate direct and indirect effects. β-weights were standardized.
Results:
More LMM predicted gains in FI scores among women (β=.130, p<.001) and men (β=.089, p<.001). Conversely, more VAM and NVAM independently predicted FI decline equally among sexes (e.g., NVAM: women: β=−.082, p<.001; men: β=−.076, p<.001). Among women, FI associations were fully mediated by higher eosinophil counts via VAM (λ=30.8%, p=.028) and lower lymphocyte counts via LMM (λ=69.2%, p=.021). Among men, FI associations were partially mediated by lower basophils counts via LMM (λ=4.5%, p=.042) and higher counts via VAM (λ=50%, p=.037).
Conclusion:
The proportion of LMM and VAM equally influenced male FI changes over 6 years, whereas higher LMM among women appeared to more strongly influence FI changes. Leukocyte counts strongly mediated VAM- and LMM-related FI changes in a sex-specific manner, but not for NVAM. For clinical translation, exercise studies in older adults may benefit from assessing sex-specific values of DEXA-based tissue mass, FI, and leukocyte sub-populations to gauge potential cognitive benefits of less VAM and more LMM.
1. Introduction
Changes in cognitive status due to normal aging have been well documented and include poorer performance on tests of episodic memory, executive function, visuospatial skills, and processing speed, with verbal abilities staying relatively intact (Salthouse, 2009; Singh-Manoux et al., 2012). Aging is particularly detrimental for fluid intelligence (Kievit et al., 2014), or FI, a cognitive domain encompassing general reasoning, pattern recognition, and the ability to solve novel problems without task-specific experience (Cattell, 1971; Wechsler, 2011). This age-related decline is due partly to slower processing speed over time among older adults (Zimprich and Martin, 2002), anterior prefrontal cortex atrophy (Kievit et al., 2014; Schretlen et al., 2000), and less frontal white matter integrity.
In parallel with cognitive aging, physical changes such as decreased muscle strength and mass (Goodpaster et al., 2006), as well as increased adiposity (Hughes et al., 2002), typically occur. Excess visceral adiposity induces systemic inflammation, and by extension increased neuroinflammation through vagal afferents (Hosoi et al., 2000; Waise et al., 2015) or blood-brain barrier permeability (Elwood et al., 2017). These changes are similar to age-induced increases in innate immunity and decreased acquired immune activation (Briceño et al., 2016; de Bourcy et al., 2017; Hearps et al., 2012; Seidler et al., 2010). By constrast, lean muscle mass may confer direct anti-inflammatory effects (Beenakker et al., 2013; Fornari et al., 2015) or counteract metabolic dysfunction induced by obesity that also gives rise to increased immune responsiveness (Bekkelund and Jorde, 2017; Fornari et al., 2015). Other studies in older adults, however, have found no pro- or anti-inflammatory associations with lean muscle mass and systemic markers (Rossi et al., 2019; Santoro et al., 2019).
Aging-related changes in body composition may also influence cognitive aging. For example, several studies have linked body mass index (BMI) with cognitive function (Arvanitakis et al., 2018; Dahl et al., 2010; Gunathilake et al., 2016). Results have suggested that higher BMI in late-life is protective against cognitive decline (Arvanitakis et al., 2018), likely reflecting less muscle wasting (i.e., sarcopenia) rather than less adipose mass, whereas midlife obesity is tied to cognitive decline and increased risk of developing dementia (Anstey et al., 2011; Kivimaki et al., 2018). These age-related differences in epidemiological findings for BMI are problematic, and cannot be resolved with anthropometric measures that, while providing a reasonably good estimate of visceral adipose mass cannot distinguish compartment-specific adipose distribution or muscle mass (Gomez-Ambrosi et al., 2012; Rothman, 2008). In contrast, dual-energy x-ray absorptiometry (DEXA) precisely quantifies adipose tissue mass in the subcutaneous and visceral compartments, as well as lean muscle mass (Kennedy et al., 2009) which may be a putative protective factor against normal and pathological aging. For instance, more lean mass was related to less global cognitive impairment among women (Noh et al., 2017) while individuals with Alzheimer’s disease had less lean mass than their cognitively healthy counterparts (Burns et al., 2010).
Few studies have examined the potential role of systemic immune markers underlying relationships between body composition and changes in cognition over time. Obesity is tightly associated with chronic inflammation in adipose tissue that elicits systemic inflammation (e.g., Hotamisligil, 2006). For example, higher BMI has been related to elevated markers of inflammation that in turn mediate cognitive dysfunction, particularly executive function deficits (Gunathilake et al., 2016; Lasselin et al., 2016). Aging may further exacerbate such immune activation, as obesity and aging are related to increased systemic levels of classic pro-inflammatory markers such as C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) (Bartlett et al., 2012; Murray et al., 2015) although these markers are inconsistent predictors of cognitive abilities among older adults (Tegeler et al., 2016; Wichmann et al., 2014). Conversely, more lean muscle mass may be related to higher FI over time in part due to reduced systemic inflammation.
In addition to commonly used pro-inflammatory markers, blood-based leukocyte counts have been used to gauge systemic inflammation (Fest et al., 2018; Leng et al., 2005; Wirth et al., 2018). Higher leukocyte counts have been associated with poorer processing speed (Kao et al., 2011), and changes in lymphocyte subtypes may be related to memory (Serre-Miranda et al., 2015; Wang et al., 2017) and executive function (Serre-Miranda et al., 2015).
The present study examined how changes in FI over 6 years were related to body composition (total lean muscle; total non-visceral adipose mass; visceral adipose mass) and 6-year changes in leukocyte sub-populations on FI among older, cognitively unimpaired adults from the UK Biobank cohort (Sudlow et al., 2015). We focused on FI because it is more adversely impacted by aging relative to other forms of executive function or different cognitive domains (Kievit et al., 2014), and a recent factor analytic study in UK Biobank demonstrated that it was the best performing construct that explains prefrontal-related cognitive variation (Lyall et al., 2016). Our central hypothesis of interest was that more visceral adiposity would be related to: 1) increased mobilization of leukocyte sub-populations over time; 2) increased levels of CRP, a systemic acute-phase cytokine that regulates inflammation (Gabay and Kushner, 1999); and 3) changes in FI via mediation of leukocyte sub-populations and/or CRP levels, presumably by inducing neuroinflammation via vagal afferents or perhaps through obesity-induced permeability of the blood-brain barrier (Gustafson et al., 2007; Ouyang et al., 2014). We similarly examined if non-visceral adiposity and lean muscle mass influenced age-related changes in FI through leukocyte sub-populations and/or CRP. Identifying compartment-specific body composition and FI associations, relative effect sizes, and the relative importance specific leukocyte sub-populations or CRP as potential mechanisms could lead to novel therapeutic interventions to minimize age- and/or obesity-related cognitive decline. Analyses were conducted separately in women and men due to morphometric differences. This stratification also allowed for differential associations with FI to be more precisely estimated.
2. Methods
2.1. Cohort and participants
Participants were sampled from the UK Biobank study (Sudlow et al., 2015). This prospective cohort study follows over a half million individuals, aged 40 to 79 years old, from 22 assessment centers located in the United Kingdom from 2007 to the present day. Each participant had baseline measurements taken between 2007 and 2010, when genetic, behavioral, and biological data were collected. A visit to the assessment center involved six consecutive steps: 1) consent, 2) touchscreen questionnaire, 3) verbal interview, 4) eye measures, 5) physical measures and 6) blood/urine sample collection. The touchscreen questionnaire collected sociodemographic, occupation, lifestyle, early life exposure, cognitive function, and family history of illness data. Informed consent to participate was given at baseline examinations. The UK Biobank protocol was approved by the North West MultiCentre Research Ethics Committee.
2.2. DEXA body composition measurement
A sub-cohort of 4,792 subjects had body composition imaging data collected in 2013–2015. Compartment measurements of body composition – lean muscle mass (LMM) in kg, total non-visceral adipose mass (NVAM) in kg that was predominantly subcutaneous fat, and visceral adipose mass (VAM) in kg – were determined by a trained radiographer delivering a 5-minute, full-body DEXA (General Electric Lunar iDXA, Madison, WI) to each participant while they lay supine (Rothney et al., 2012).
2.3. Measurements of blood chemistry
At separate visits (2008, 2012, and 2014), hematology and plasma samples were collected in 4mL EDTA vacutainers and analyzed within 24 hours of sampling utilizing four Beckman Coulter LH750 instruments (Sheard et al., 2017) to quantify counts in 109 cells/L. Cell populations were distinguished as monocytes, neutrophils, basophils, eosinophils, or lymphocytes. Plasma samples were analyzed for C-reactive protein (CRP) in mg/L at two separate assessments (2008 and 2012). CRP values above 10.0 mg/L were excluded from analyses, as they may indicate an acute infection rather than chronic inflammation (Pearson et al., 2003).
2.4. Measurement of fluid intelligence
Participants completed the Fluid Intelligence Test (FIT) as part of the touch-screen questionnaire at three separate assessments (2008, 2012, and 2014). FI is described as the capacity to solve problems that require logic and FI, independent of acquired knowledge. The FIT score, operationalized as FI, is quantified by how many numerical, logic, and syntactic questions, out of 13 total, participants were able to answer correctly within two minutes (Lyall et al., 2016).
2.5. Covariates
Covariates included age, education, socioeconomic status, APOE4 allele status, tobacco smoking, and weekly alcohol consumption. Age was measured in years at baseline. A categorical variable was used to capture education levels. Categories included the following: College or other higher level qualification; post-secondary or vocational; secondary; or none of the previous education levels listed. Socioeconomic status was based on the participant’s average total household income between 2008 and 2014. Responses were recorded as one of five ordinal categories in British Pounds (“Less than 18,000”, “18,000 to 30,999”, “31,000 to 51,999”, “52,000 to 100,000”, “Greater than 100,000”), where the lowest two categories were classified as lower class, the next two categories were classified as middle class, and the greatest category was classified as upper class. All UK Biobank GWAS data has been processed as described (UK Biobank, 2007; Sudlow et al., 2015). Briefly, APOE haplotype was determined using allele variation on rs429358 and rs7412 (Davies et al., 2014). ε3/ε3 participants were designated the reference group and compared to: 1) a ε2/ε2 and ε2/ε3 group; or 2) a ε2/ε4, ε3/ε4, and ε4/ε4 group. Tobacco smoking responses were recorded as whether the subject had “Never smoked”, “Previously smoked”, or “Currently smokes”. Alcohol consumption responses were recorded as average weekly intake (in pints for beer and cider, glasses for wine and champagne, and measures for liquor).
2.6. Considerations of medical exclusion criteria
Many conditions, particularly in obese midlife to late-life adults, are related to chronic systemic inflammation that could influence leukocyte mobilization and cognitive function. Consequently, to explore if these conditions influenced initial model fitting, we excluded participants with ICD-10 codes reflecting a systemic inflammatory condition in their medical record during any hospital stay since 2007. Examples included most diseases of the digestive system (XI) such as ulcerative colitis (K51), inflammatory bowel disease (K52.3), or diverticulitis (K57); chronic lower respiratory diseases (J40-J47); rheumatoid arthritis (M05); multiple sclerosis (G35); systemic lupus erythematosus (M32); and malignant neoplasms of the digestive organs (C15-C26). Our results did not significantly differ based on these exclusion criteria. For instance, among women we observed that visceral adipose mass was still associated with lymphocyte counts (Final Model: β=.190, p<.001; Medical Exclusion Model: β=.193, p<.001) and this also held true among men (Final Model: β=.104, p=.010; Medical Exclusion Model: β=.103, p=.011). Overall, no associations changed in their significance status. Thus, data from these participants remained in the final models.
Neurological disorders are also characterized by neuroinflammation that might influence cognitive function (Swanson et al., 2018). Thus, on an exploratory basis we also excluded participants who had any cerebrovascular disease (I60-I69) or disorder of the nervous system (G00-G99). Again, our pattern of results did not significantly differ based on these excluded participants. Therefore, data from these participants were included in final models.
2.7. Statistical analysis
2.7.1. Longitudinal modeling
Compared to cross-sectional models, longitudinal computations made from observing variables over time enhance predictive performance and correlation with study outcomes (Shaw et al., 2006). Accordingly, for longitudinally-observed variables, we computed individual, across-time averages and non-linear changes over time. We then integrated these values to derive average levels and the sum of changes for each longitudinally assessed variable (Pruessner et al., 2003). This method has demonstrated superior goodness-of-fits, while minimizing type 1 error, and elucidates relationships between variables more robustly by capturing both within- (Hu and Bentler, 1999; Klinedinst, 2017) and between-subject variation over time (Duncan and Duncan, 2004; Klinedinst, 2017; Preacher et al., 2008).
2.7.2. Structural equation models
Structural equation modelling (SEM) was done using R (3.4.1; R Core Team, 2013). Graphs were prepared in ggplot2 (3.1.1; Wickham, 2016). SEM-based mediation has more statistical power than the standard regression procedure (MacKinnon, 2008). SEM has the additional benefit of easily extending to longitudinal data within a single framework (Preacher et al., 2008), as done in this report.
2.7.3. Variable selection
An empirical model-building approach was employed to select the most salient variables that predicted leukocyte counts and FI. In this backward elimination approach a full, “all variables in” model was built, and the least significant variable was removed one at a time until all variables remaining reached p<.200.
2.7.4. Parameter estimation, ANOVA, uncertainty analysis, and mediation
Two separate group-wise SEMs for women and men were built to comprehensively model the mean and covariance structure (Rosseel, 2012) of body composition, leukocyte sub-population counts, and FI scores. See Figure 1 for a conceptual representation of the model. Standardized parameter estimates (β), which were interpreted as the average potential effects of a variable, were estimated using maximum likelihood. ANOVA is reported as the overall portion of variation in FI scores that is explained (R2). Uncertainty analysis relied upon standard errors and p-values, where results were considered significant at p<.001 (***), p<.010 (**), p<.050 (*), and trending at p<.100 (#). Mediation tested if leukocyte sub-populations due to body composition variation affected FI. Specifically, parameter decomposition was used to distinguish indirect (λ) from direct (β) effects (Gunzler et al., 2013). Mediation results via indirect effects (λ) are reported in Results as the additional effect as a percentage of the direct effect, and in Supplementary Table 2 as unstandardized and standardized beta coefficients. A chi-square difference test was conducted to establish whether the association between LMM and FI among women and men had a meaningfully different effect size. In order to maintain a data-driven analysis and ensure robustness, only participants with all available data were considered. In follow-up analyses, full information maximum likelihood (FIML) missingness imputation for leukocyte or demographic values was tested among participants who had DEXA imaging, to ensure that results were not biased by listwise exclusion. Imputation of DEXA imaging data was not possible, as FIML models would be composed of over 99% imputed data due to the relatively small sample size of participants that underwent DEXA imaging.
Figure 1.
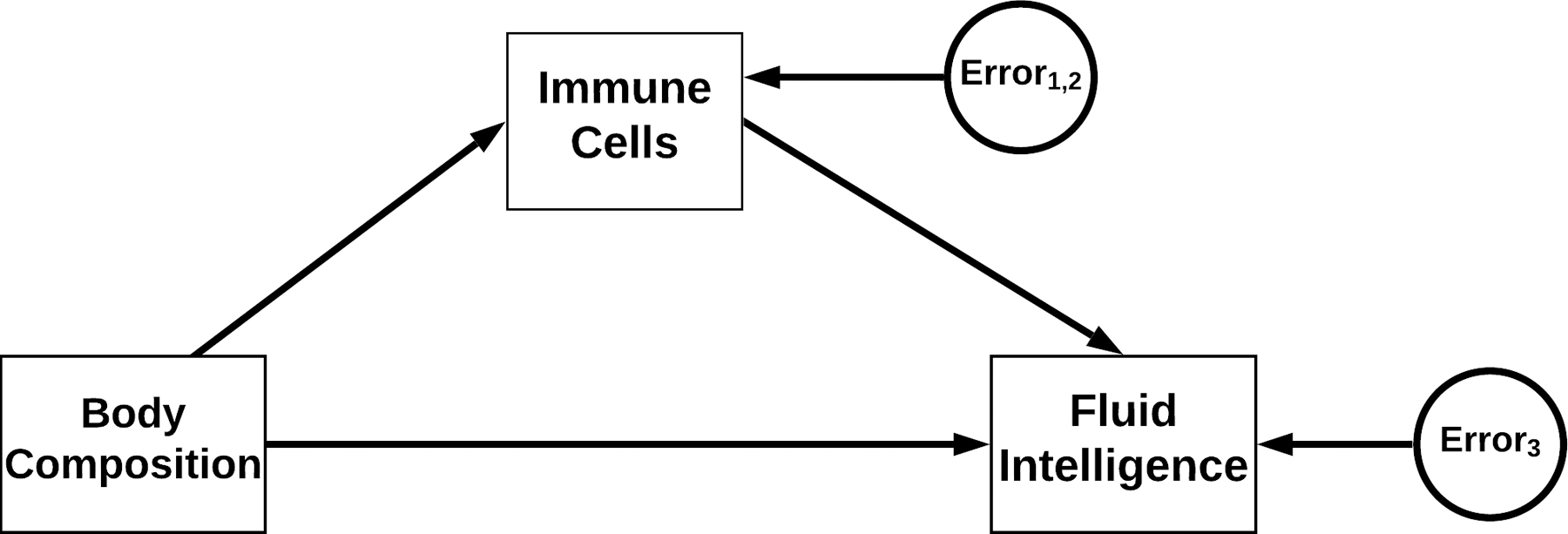
A conceptual diagram of associations and their directionality between body composition, standard differential leukocyte count, and fluid intelligence indices.
3. Results
3.1. Demographics and data summaries
There were a total of 4,431 eligible UK Biobank participants. Supplementary Figure 1 outlines the eligibility criteria used for arriving at the total sample. Table 1 shows baseline sample characteristics stratified by sex. A basic description of the variables used in the models, including their respective ranges, are included in Supplementary Table 1.
Table 1.
Data Summary of UK Biobank Sub-Cohort
| Data, measurement unit | Women | Men |
|---|---|---|
| Sample Size, n | 2298 | 2133 |
| Age, y | 63.70 ± 7.42 | 65.32 ± 7.46 |
| Education Level, No. (%) | ||
| College/other higher level | 1439 (62.62) | 1427 (66.90) |
| Post-secondary/vocational | 368 (16.01) | 415 (19.45) |
| Secondary | 369 (16.06) | 173 (8.11) |
| Other | 122 (5.31) | 118 (5.53) |
| Social Class, No. (%) | ||
| Lower | 1164 (50.65) | 877 (41.11) |
| Middle | 1051 (45.74) | 1155 (54.15) |
| Upper | 83 (3.61) | 101 (4.74) |
| Lean Muscle Mass, kg | 39.75 ± 4.67 | 55.47 ± 6.49 |
| Non-Visceral Adipose Mass, kg | 26.62 ± 9.20 | 24.62 ± 8.44 |
| Visceral Adipose Mass, kg | 0.76 ± 0.57 | 1.67 ± 0.93 |
| Leukocyte Count, billion cells/L | 6.65 ± 1.63 | 6.75 ± 1.63 |
| Fluid Intelligence, total FIT score | 6.72 ± 2.08 | 7.06 ± 2.14 |
Abbreviations: FIT, Fluid Intellect Test.
Values are Mean ± SD unless stated otherwise.
3.2. Path analysis regression outcomes
3.2.1. Main effects of demographics
Within the model, education and social class were associated with higher FI over 6 years among women and men (Figure 2; Figure 3). Age, APOE4 allele status, tobacco smoking, and total alcohol consumption did not significantly load as model covariates and thus were not included in subsequent analyses. The unstandardized and standardized betas, 95% confidence intervals, and p-values for demographics, body composition, and leukocyte measures are reported in Supplementary Table 2 for women and men.
Figure 2.
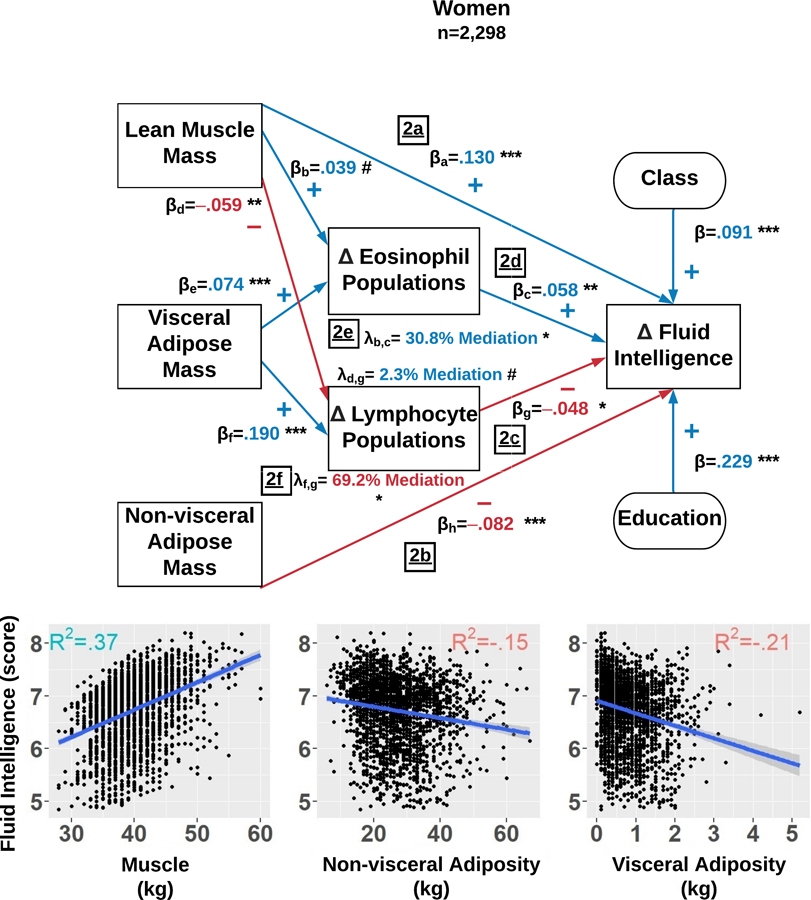
Among women, the structural equation model estimates which factors explain fluid intelligence scores changes over 6 years. The final model simultaneously examined: 1) the direct effects of body composition indices, total lymphocyte count, and total eosinophil count; and 2) the indirect effects of total lymphocytes and eosinophils influencing the associations between body composition and fluid intelligence scores. The Delta symbol (Δ) is defined as the average level and totality of changes in that variable observed over 6 years. The standardized β reflects the average effect size of each path and each path is denoted with a subscript. Each λ reflects the mediation effect resulting from the path analysis and is subscripted to illustrate the paths which compose it. The R2 in each sub-plot represents variance explained in fluid intelligence scores by lean muscle mass (LMM), non-visceral adipose mass (NVAM), and visceral adipose mass (VAM). Mediation percentages are computed with respect to the direct effects. p<.001=***, p<.010 =**, p<.050=*, and p<.100=#.
Figure 3.
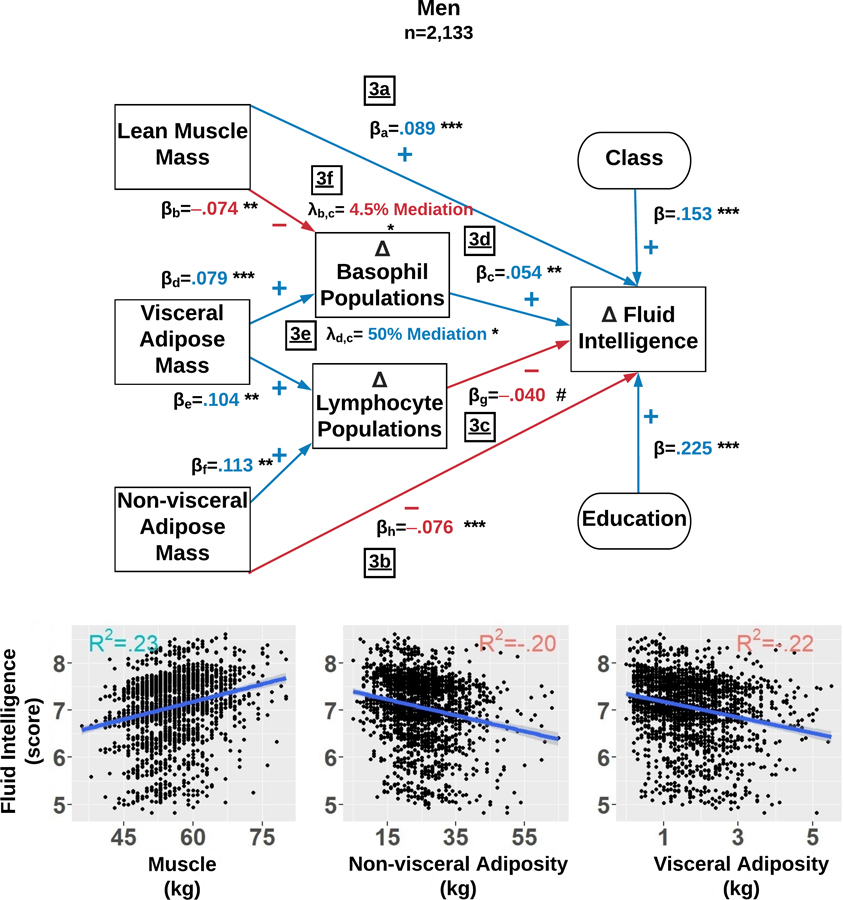
Among men, this structural equation model estimates which factors explain fluid intelligence score changes over 6 years. The final model simultaneously examined: 1) the direct effects of body composition, total lymphocyte count, and total basophil count; and 2) the indirect effects of total lymphocyte count and total basophil count influencing the associations between body composition and fluid intelligence scores. The Delta symbol (Δ) is defined as the average level and totality of changes in that variable observed over 6 years. The standardized β reflects the average effect size of each path and each path is denoted with a subscript. Each λ reflects the mediation effect resulting from the path analysis and is subscripted to illustrate the paths which compose it. The R2 in each sub-plot represents variance explained in fluid intellect scores by lean muscle mass (LMM), non-visceral adipose mass (NVAM), and visceral adipose mass (VAM). Mediation percentages are computed with respect to the direct effects. p<.001=***, p<.010 =**, p<.050=*, and p<.100=#.
3.2.2. Main effects of body composition
We first regressed changes over 6 years in FIT scores on LMM, NVAM, and VAM while controlling for changes over 6 years in serum counts of leukocyte sub-populations. We observed that LMM predicted better FI among women (β=.130, R2=.37, p<.001) and among men (β=.089, R2=.23, p<.001) (Figure 2a and 3a), while NVAM predicted worse FI among both sexes (women: β=−.082, R2=.15, p<.001; men: β=−.076, R2=.20, p<.001) (Figure 2b and 3b). All of VAM’s direct effects were fully mediated by leukocyte sub-populations (see below).
3.2.3. Main effects of leukocyte sub-populations
Independent of body composition, we simultaneously regressed six-year FI changes on six-year changes in serum counts of basophil, eosinophil, neutrophil, lymphocyte, and monocyte populations. Higher lymphocyte counts were related to worse FI in women (β=−.048, p=.016) and marginally in men (β=−.040, p=.057) (Figure 2c and 3c). However, protective-like associations were seen among different leukocyte sub-populations between women and men. Among women, eosinophil counts were positively associated with FI (β=.058, p=.003) (Figure 2d). A similar association was observed among men, but involving basophils instead (β=.054, p=.008) (Figure 3d).
3.2.4. Immune system-related mediation of body composition effects
Lastly, leukocyte sub-population counts were tested as potential mediators of body composition and FI associations. The negative effect of VAM on FI among women was fully mediated by 6-year increases in eosinophils (λ=30.8% of direct effect, R2=.21, p=.028) (Figure 2e) and lymphocyte counts (λ=69.2% of direct effect, R2=.21, p=.020) (Figure 2f). Among men, the negative impact of VAM was partially mediated by increased basophil counts (λ=50% of direct effect, R2=.22, p=.037) (Figure 3e). Basophils also partially mediated LMM and FI associations among men (λ=4.5% of direct effect, p=.042) (Figure 3f).
Figure 4 graphically summarizes, for men and women separately, the relative contributions of predictors and covariates to FI. For example, consider the relationships between VAM, leukocyte counts, and FI among women. Increases in VAM were associated with increased lymphocyte and eosinophil counts, which respectively predicted worse and better FI scores. Thus, VAM appears to influence FI in a dual, mechanistically immunologic manner. However, the overall VAM effect on FI is deleterious due to the influence of higher lymphocyte counts on decreased FI scores (λ=69.2%) vs. higher eosinophil counts on increased FI scores (λ=30.8%).
Figure 4.
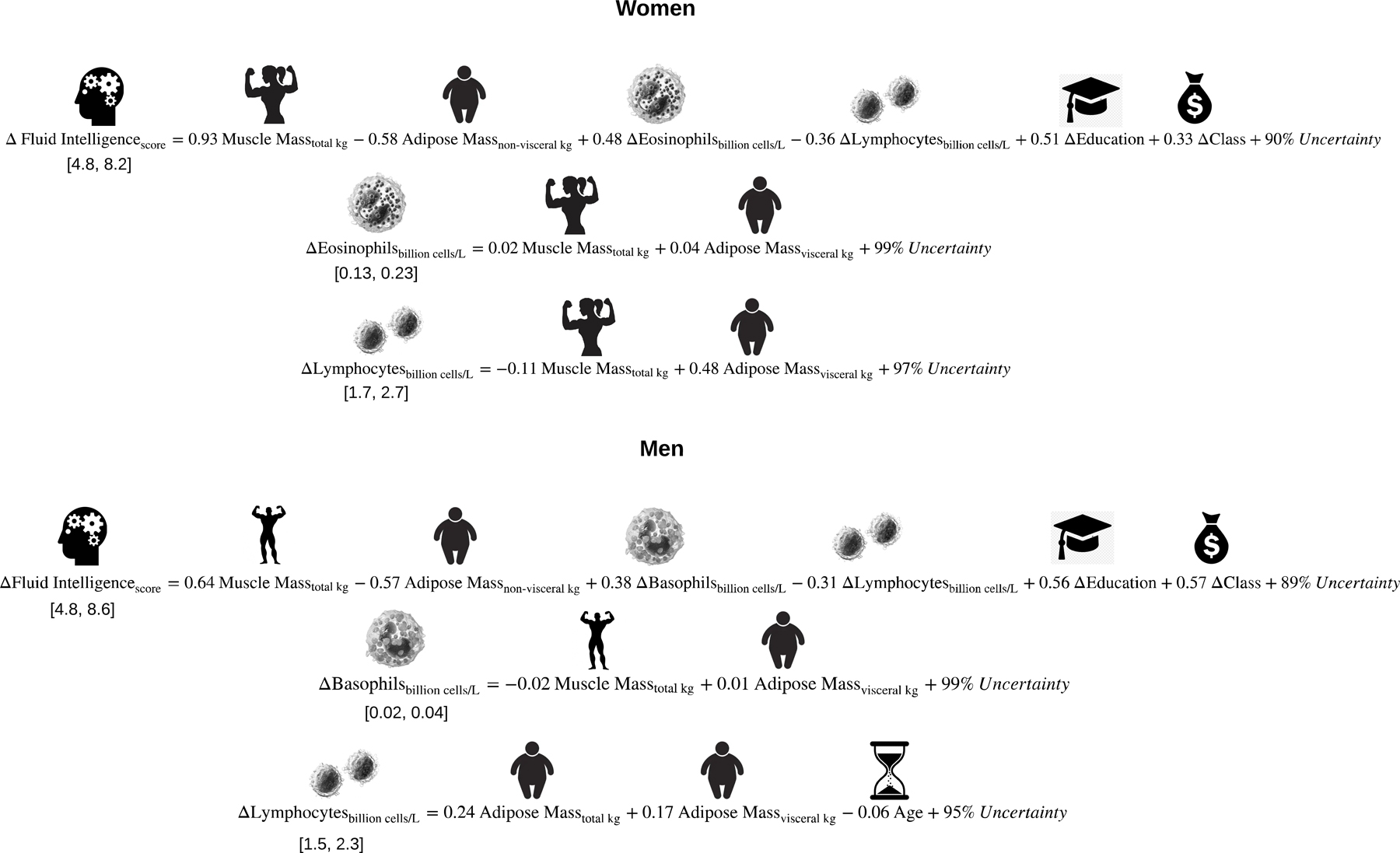
These graphical equations algebraically summarize the models illustrated in Figures 1 and 2. Because the immunologic variables are outcomes that are also nested within the prediction of fluid intelligence, the equation among both women and men that describes fluid intelligence is computed sequentially after the immunology-related equations. The Delta symbol (Δ) is defined as the average level and totality of changes in that variable observed over 6 years. Each coefficient represents the change expected in the outcome if an individual progressed approximately halfway (1.5 standard deviations) along the distribution of that predictor. To offer context for each coefficient, the range of each outcome is listed below the left-hand side of the equation.
3.2.5. Follow-up analyses
To establish whether leukocyte associations were simply representing background inflammatory status, we examined the statistical effects of CRP in two follow-up models for each sex. Given the final models presented above, we first tested if CRP levels predicted FI scores. CRP was not a significant predictor among women (β=−.009, p=.675) nor men (β=−.018, p=.415). It also did not influence final model fit in previously detailed analyses.
In women and men separately, we next examined whether CRP was related to body composition values or leukocyte counts, and if it mediated or moderated associations between these factors and FI scores. Among women, higher CRP was seen with less LMM (β=−.066, p=.005) and more adiposity, including NVAM (β=.248, p<.001), and VAM (β=.214, p<.001). CRP levels were not associated with FI (β=−.009, p=.675). Among men, higher CRP was similarly associated with less LMM (β=−.054, p=.024) and more adiposity such as NVAM (β=.154, p<.001) and VAM (β=.197, p<.001). CRP levels were also not related to FI (β=−.018, p=.414). Importantly, the body composition and leukocyte results remained unchanged when including CRP in models as a covariate or mediator.
To determine whether the effect size for LMM and FI significantly differed between women (β =.130) and men (β=.089), a second model among women was built with the unstandardized β value constrained to the value among men. A chi-squre difference test detected a significant difference (Δχ2(1) = 7.651, p = .005), suggesting that the effect size was larger among women than men.
To establish that results were not biased by listwise exclusion of participants, another follow-up model tested the influence of FIML missingness imputation. Among women who had DEXA imaging, the proportion of missingness was 9.1% and one change in significance status was detected. A trending effect between LMM and eosinophil counts (β=.039, p=.078) became non-significant in the imputed model (β=.036, p=.103). No changes in significance status were detected among men, who had 5.5% proportion of missingness.
4. Discussion
Using longitudinal data from the UK Biobank cohort, the current study tested associations between FI and both DEXA body composition measures and leukocyte sub-populations, as well as independent mediation of FI-body composition association via leukocyte counts. Main effects of total non-visceral adipose mass (i.e., NVAM) on FI were roughly similar for both men and women, while LMM appeared to be a stronger predictor among women versus men. Higher LMM and NVAM were respectively associated with better and worse FI performance over time. Further, leukocyte sub-populations fully mediated VAM associations among women and partially mediated VAM and LMM associations among men.
4.1. Body composition and fluid intelligence
Few studies have directly tested the relationship between LMM and neuropsychological test performance, with one study finding beneficial effects of greater LMM for episodic memory (Sohrabi et al., 2015). Recent work by Spauwen and colleagues (2017) suggests that greater thigh muscle mass may be protective against developing dementia. LMM may be particularly beneficial for preserving cognition among women (Noh et al., 2017). Our results further substantiate these findings, as the effect size observed for LMM was significantly larger for women in comparison to men. Several mechanisms could explain this direct relationship. For example, calorie restricted aged rhesus monkeys maintain more lean muscle mass and evince better set-shifting executive function (Sridharan et al., 2012). The positive relationship between musculature and FI may also reflect more physical activity, which can benefit executive and global cognitive function in aging (Colcombe et al., 2004; Jonasson et al., 2017).
In contrast to the beneficial associations of LMM, more NVAM and VAM were independently related to worse FI scores over six years. Similar findings have implicated greater BMI with deficits in executive function among aged subjects (Stanek et al., 2013), as well as more regional adiposity and poorer global cognition among men (Kanaya et al., 2009). Conversely, reducing total body adipose improves executive function in older adult women (Dao et al., 2013). Adiposity may influence the brain and thus FI due to a number of factors, although we emphasize that our study was correlational in nature. Examples in obese vs. lean rats include increased microglial length, decreased synaptogenesis, and decreased prefrontal cortical volume (Bocarsly et al., 2015).
Finally, we found that adiposity was related to more systemic inflammation, using CRP as a marker, corroborating previous findings fo CRP and IL-6 (Maachi et al., 2004; Murray et al., 2015; Pou et al., 2007). When examining leukocyte sub-populations, we found that lymphocytes, responsible for adaptive immunity, were negatively associated with FI among both sexes. Similarly, cognitive decline in Alzheimer’s and Parkinson’s disease is aligned with changes in lymphocytes, including cytogenetic alterations and elevations in mTOR and PKR kinases (Paccalin et al., 2006; Petrozzi et al., 2002).
4.2. Leukocyte sub-populations and fluid intelligence
Different sub-populations of leukocytes, specific to each sex, were associated with FI, VAM, and LMM. For innate immunity, among women, increased eosinophil populations over 6-years coincided with increased FI. Eosinophils play a role in glucose homeostasis by mediating alternatively activated macrophages, as described in mice (Wu et al., 2011). Similarly, in our study, increased basophil count corresponded to increased FI scores among men. Reduced basophil count has been associated with negative affect (Baek et al., 2016) via the histaminergic system, which could also modulate cognitive domains such as memory (Dere et al., 2010). Additionally, basophils can prevent blood clotting due to their production of heparin (Stone et al., 2010), suggesting less vascular dysfunction may be a viable target.
There are several potential pathways that may underlie our observations. Classically, vagal afferents from the gut can induce neuroinflammation. This pathway seems unlikely, however, because CRP did not mediate tissue mass and FI associations but rather was only related to higher leukocyte counts. Alternatively, leukocytes may more directly impact FI by accessing the brain via circumventricular organs, active transport across the blood-brain barrier, or the blood-brain barrier endothelium. For example, mild systemic inflammation induced by lipopolysaccharide injection has been shown to further activate microglial immune activity in the circumventricular organs (Furube et al., 2018). Peripheral monocytes may also be transported across the blood-brain barrier by the NMDA receptor subunit 1 (Reijerkerk et al., 2010), although monocyte subpopulations were not related to fluid intelligence scores in our study. Changes in blood-brain barrier endothelium and entry of leukocytes could also be affected in part by proinflammatory cytokines such as IL-1β and TNF-α (O’Carroll et al., 2015; Wong et al., 2007). Further, blood-brain barrier integrity is believed to be compromised among individuals with obesity (Gustafson et al., 2007; Ouyang et al., 2014) and type II diabetes (Geng et al., 2018; Janelidze et al., 2017), which may lend credence to some of our leukocyte mediation results observed for visceral adiposity and FI.
4.3. Leukocyte sub-population mediation effects
Leukocyte counts also mediated associations between body morphometry and FI over time. Prior research has indicated proinflammatory markers, specifically CRP, act as mediators between higher BMI and poorer cognitive outcomes (Bourassa and Sbarra, 2017; Gunathilake et al., 2016). However, when we analyzed tissue mass in a compartment-wise manner and controlled for differentiated leukocyte counts, we did not replicate these CRP findings with respect to FI. Among women, we observed that the statistical effect of VAM was fully mediated through changes in eosinophil and lymphocyte populations. Interestingly, VAM was correlated with increased eosinophils and lymphocytes, which respectively predicted better and worse FI, suggesting that VAM is related to mostly deleterious immunologic processes via lymphocytes that may affect FI. Among men, VAM was positively associated with basophil populations, which mediated 50% of VAM’s association with FI.
Other plausible explanations for how leukocytes mediate body composition and FI may implicate the gut-brain axis. Obesity has been associated with lower gut microbial diversity, and the latter has been associated with higher leukocyte counts (Le Chatelier et al., 2013; Turnbaugh et al., 2009) and reduced metabolic capacity (Petersen et al., 2019). Due to communication links between the gut and central nervous system via the vagus nerve (Mayer, 2011), compromised gut microbiota have also been implicated in neurological disorders such as schizophrenia, multiple sclerosis, and Alzheimer’s disease (Cattaneo et al., 2017; Jangi et al., 2016; Nguyen et al., 2019). Future work should examine if specific gut microbial genera and species modulate leukocyte and FI associations, or if gut microbiota separately or synergistically influence cognitive function through vagal afferents.
4.4. Age and fluid intelligence
It is also important to note that the statistical effect of age was non-significant in our models. Kievit and colleagues (2018) and Cornelis et al.(2019) similarly reported weak associations with age and FI in the UK Biobank cohort, despite this link being consistently found in many other cross-sectional and longitudinal studies (Hartshorne and Germine, 2015; Kievit et al., 2014; Rönnlund and Nilsson, 2006). It is plausible that the lack of findings are due to measurements issues, such as practice effects or the follow-up period. Further, our sub-cohort sample was approximately 64 years of age. It may be that more robust effects of age-related decline in FI are observed at earlier points in life. Finally, aging itself may not be deleterious, but rather age-related changes in body morphometry that are related to cognitive decline.
4.5. Strengths and limitations
Several strengths of the study should be noted. First, DEXA partitioned body mass into LMM, NVAM, and VAM, which for the first time gauged their relative associations with FI in the same model. A potential mechanism of action was also tested in this study via the leukocyte sub-population and CRP mediation models. Our findings also did not change after excluding participants with potentially confounding inflammatory or neurological conditions. Lastly, there was adequate sample size to examine the effects of LMM and NVAM on FI among males and females separately.
There are also limitations that should be addressed. For instance, body composition was only measured at one time point and we did not control for ethnicity. At the time of this study, UK Biobank had leukocyte counts and QCed CRP data, where additional cytokine or chemokine markers may have suggested more specific immunologic mechanisms. Listwise deletion was also used for our analytic sample. However, our results followed a similar pattern when using imputation methods. Finally, future research should explore if eosinophils and basophils perform differential functions between women and men relevant or in animal models with regard to FI or other executive function processes.
5. Conclusion
The detrimental relationship between adiposity, health, and cognition has been extensively explored (Abraham et al., 2015; Bocarsly et al., 2015; Kanaya et al., 2009; Stanek et al., 2013) and several studies have established the relationship between LMM and cognition (Sohrabi et al., 2015; Spauwen et al., 2017). However, there is a paucity of research examining how VAM might influence cognition, if LMM may mitigate VAM or NVAM statistical effects, the relative effect sizes of tissue mass in different body compartments when tested simultaneously, or the degree to which body compartment and cognition associations are mediated or moderated by leukocyte sub-populations or a general index of systemic inflammation like CRP. The proportion of adipose and lean mass may be modifiable factors that can elucidate why FI changes with normal aging. Finally, the immune system, both independently as well due to masses of lean muscle and visceral adiposity, may have deleterious and beneficial effects on FI that should be further investigated as potential targets for therapeutics to ameliorate age-related cognitive decline.
Supplementary Material
Supplemental Figure 1. A flow chart diagram indicating the progressive exclusion of participants to derive a final sub-sample with no missingness among data of interest.
Acknowledgements
This study was funded by Iowa State University, NIH R00 AG047282, and AARGD-17-529552. No funding provider had any role in the conception, collection, execution, or publication of this work. This research was conducted using the UK Biobank Resource under Application Number 25057.
Footnotes
Conflict of Interest/Disclosure Statement
The authors have no conflict of interest to report.
References
- Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS, 2015. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation 132, 1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ, Cherbuin N, Budge M, Young J, 2011. Body mass index in midlife and late-life as a risk factor for dementia: A meta-analysis of prospective studies. Obes. Rev 12, e426–e437. [DOI] [PubMed] [Google Scholar]
- Arvanitakis Z, Capuano AW, Bennett DA, Barnes LL, 2018. Body mass index and decline in cognitive function in older Black and White persons. J Gerontol A Biol Sci Med Sci 73, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek JH, Kim HJ, Fava M, Mischoulon D, Papakostas GI, Nierenberg A, Heo JY, Jeon HJ, 2016. Reduced venous blood basophil count and anxious depression in patients with major depressive disorder. Psychiatry Investig 13, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett DB, Firth CM, Phillips AC, Moss P, Baylis D, Syddall H, Sayer AA, Cooper C, Lord JM, 2012. The age-related increase in low-grade systemic inflammation (Inflammaging) is not driven by cytomegalovirus infection. Aging cell 11, 912–915. [DOI] [PubMed] [Google Scholar]
- Beenakker KG, Westendorp RG, de Craen AJ, Slagboom PE, van Heemst D, Maier AB, 2013. Pro-inflammatory capacity of classically activated monocytes relates positively to muscle mass and strength. Aging cell 12, 682–689. [DOI] [PubMed] [Google Scholar]
- Bekkelund SI, Jorde R, 2017. Lean body mass and creatine kinase are associated with reduced inflammation in obesity. Eur. J. Clin. Invest 47, 803–811. [DOI] [PubMed] [Google Scholar]
- Bocarsly ME, Fasolino M, Kane GA, LaMarca EA, Kirschen GW, Karatsoreos IN, McEwen BS, Gould E, 2015. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc. Natl. Acad. Sci. U. S. A 112, 15731–15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa K, Sbarra DA, 2017. Body mass and cognitive decline are indirectly associated via inflammation among aging adults. Brain. Behav. Immun 60, 63–70. [DOI] [PubMed] [Google Scholar]
- Briceño O, Lissina A, Wanke K, Afonso G, von Braun A, Ragon K, Miquel T, Gostick E, Papagno L, Stiasny K, Price DA, Mallone R, Sauce D, Karrer U, Appay V, 2016. Reduced naive CD8+ T-cell priming efficacy in elderly adults. Aging cell 15, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JM, Johnson DK, Watts A, Swerdlow RH, Brooks WM, 2010. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch. Neurol 67, 428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, Ferrari C, Guerra UP, Paghera B, Muscio C, Bianchetti A, Volta GD, Turla M, Cotelli MS, Gennuso M, Prelle A, Zanetti O, Lussignoli G, Mirabile D, Bellandi D, Gentile S, Belotti G, Villani D, Harach T, Bolmont T, Padovani A, Boccardi M, Frisoni GB, 2017. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 49, 60–68. [DOI] [PubMed] [Google Scholar]
- Cattell RB, 1971. Abilities: their structure, growth, and action. Houghton Mifflin, Oxford, England. [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S, 2004. Cardiovascular fitness, cortical plasticity, and aging. Proc. Natl. Acad. Sci. U. S. A 101, 3316–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis MC, Wang Y, Holland T, Agarwal P, Weintraub S, Morris MC, 2019. Age and cognitive decline in the UK Biobank. PLoS One 14, e0213948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl A, Hassing LB, Fransson E, Berg S, Gatz M, Reynolds CA, Pedersen NL, 2010. Being overweight in midlife is associated with lower cognitive ability and steeper cognitive decline in late life. J Gerontol A Biol Sci Med Sci 65, 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao E, Davis JC, Sharma D, Chan A, Nagamatsu LS, Liu-Ambrose T, 2013. Change in body fat mass is independently associated with executive functions in older women: A secondary analysis of a 12-month randomized controlled trial. PLoS One 8, e52831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bourcy CF, Angel CJ, Vollmers C, Dekker CL, Davis MM, Quake SR, 2017. Phylogenetic analysis of the human antibody repertoire reveals quantitative signatures of immune senescence and aging. Proc. Natl. Acad. Sci. U. S. A 114, 1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Zlomuzica A, De Souza Silva MA, Ruocco LA, Sadile AG, Huston JP, 2010. Neuronal histamine and the interplay of memory, reinforcement and emotions. Behav. Brain Res 215, 209–220. [DOI] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC, 2004. An introduction to latent growth curve modeling. Behav. Ther 35, 333–363. [Google Scholar]
- Elwood E, Lim Z, Naveed H, Galea I, 2017. The effect of systemic inflammation on human brain barrier function. Brain. Behav. Immun 62, 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fest J, Ruiter R, Ikram MA, Voortman T, van Eijck CHJ, Stricker BH, 2018. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: A population-based prospective cohort study. Sci. Rep 8, 10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornari R, Francomano D, Greco EA, Marocco C, Lubrano C, Wannenes F, Papa V, Bimonte VM, Donini LM, Lenzi A, Aversa A, Migliaccio S, 2015. Lean mass in obese adult subjects correlates with higher levels of vitamin D, insulin sensitivity and lower inflammation. J. Endocrinol. Invest 38, 367–372. [DOI] [PubMed] [Google Scholar]
- Furube E, Kawai S, Inagaki H, Takagi S, Miyata S, 2018. Brain region-dependent heterogeneity and dose-dependent difference in transient microglia population increase during lipopolysaccharide-induced inflammation. Sci. Rep 8, 2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C, Kushner I, 1999. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med 340, 448–454. [DOI] [PubMed] [Google Scholar]
- Geng J, Wang L, Zhang L, Qin C, Song Y, Ma Y, Chen Y, Chen S, Wang Y, Zhang Z, Yang GY, 2018. Blood-brain barrier disruption induced cognitive impairment is associated with increase of inflammatory cytokine. Front. Aging Neurosci 10, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Ambrosi J, Silva C, Galofre JC, Escalada J, Santos S, Millan D, Vila N, Ibanez P, Gil MJ, Valenti V, Rotellar F, Ramirez B, Salvador J, Fruhbeck G, 2012. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int. J. Obes 36, 286–294. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB, 2006. The loss of skeletal muscle strength, mass, and quality in older adults: The Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci 61, 1059–1064. [DOI] [PubMed] [Google Scholar]
- Gunathilake R, Oldmeadow C, McEvoy M, Inder KJ, Schofield PW, Nair BR, Attia J, 2016. The association between obesity and cognitive function in older persons: How much is mediated by inflammation, fasting plasma glucose, and hypertriglyceridemia? J Gerontol A Biol Sci Med Sci 71, 1603–1608. [DOI] [PubMed] [Google Scholar]
- Gunzler D, Chen T, Wu P, Zhang H, 2013. Introduction to mediation analysis with structural equation modeling. Shanghai archives of psychiatry 25, 390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson DR, Karlsson C, Skoog I, Rosengren L, Lissner L, Blennow K, 2007. Mid-life adiposity factors relate to blood-brain barrier integrity in late life. J. Intern. Med 262, 643–650. [DOI] [PubMed] [Google Scholar]
- Hartshorne JK, Germine LT, 2015. When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychol. Sci 26, 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearps AC, Martin GE, Angelovich TA, Cheng W, Maisa A, Landay AL, Jaworowski A, Crowe SM, 2012. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging cell 11, 867–875. [DOI] [PubMed] [Google Scholar]
- Hosoi T, Okuma Y, Nomura Y, 2000. Electrical stimulation of afferent vagus nerve induces IL-1beta expression in the brain and activates HPA axis. Am J Physiol Regul Integr Comp Physiol 279, R141–R147. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, 2006. Inflammation and metabolic disorders. Nature 444, 860–867. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM, 1999. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling 6, 1–55. [Google Scholar]
- Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA, 2002. Longitudinal changes in body composition in older men and women: Role of body weight change and physical activity. Am J Clin Nutr 76, 473–481. [DOI] [PubMed] [Google Scholar]
- Janelidze S, Hertze J, Nagga K, Nilsson K, Nilsson C, Wennstrom M, van Westen D, Blennow K, Zetterberg H, Hansson O, 2017. Increased blood-brain barrier permeability is associated with dementia and diabetes but not amyloid pathology or APOE genotype. Neurobiol. Aging 51, 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL, Cook S, Tankou S, Stuart F, Melo K, Nejad P, Smith K, Topçuolu BD, Holden J, Kivisäkk P, Chitnis T, De Jager PL, Quintana FJ, Gerber GK, Bry L, Weiner HL, 2016. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 7, 12015–12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson LS, Nyberg L, Kramer AF, Lundquist A, Riklund K, Boraxbekk CJ, 2017. Aerobic exercise intervention, cognitive performance, and brain structure: Results from the Physical Influences on Brain in Aging (PHIBRA) Study. Front. Aging Neurosci 8, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaya AM, Lindquist K, Harris TB, Launer L, Rosano C, Satterfield S, Yaffe K, 2009. Total and regional adiposity and cognitive change in older adults: The Health, Aging and Body Composition (ABC) study. Arch. Neurol 66, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao TW, Chang YW, Chou CC, Hu J, Yu YH, Kuo HK, 2011. White blood cell count and psychomotor cognitive performance in the elderly. Eur. J. Clin. Invest 41, 513–520. [DOI] [PubMed] [Google Scholar]
- Kennedy AP, Shea JL, Sun G, 2009. Comparison of the classification of obesity by BMI vs. dual-energy X-ray absorptiometry in the Newfoundland population. Obesity (Silver Spring) 17, 2094–2099. [DOI] [PubMed] [Google Scholar]
- Kievit RA, Davis SW, Mitchell DJ, Taylor JR, Duncan J, Henson RN, 2014. Distinct aspects of frontal lobe structure mediate age-related differences in fluid intelligence and multitasking. Nat Commun 5, 5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievit RA, Fuhrmann D, Borgeest GS, Simpson-Kent IL, Henson RNA, 2018. The neural determinants of age-related changes in fluid intelligence: a pre-registered, longitudinal analysis in UK Biobank. Wellcome Open Res 3, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivimaki M, Luukkonen R, Batty GD, Ferrie JE, Pentti J, Nyberg ST, Shipley MJ, Alfredsson L, Fransson EI, Goldberg M, Knutsson A, Koskenvuo M, Kuosma E, Nordin M, Suominen SB, Theorell T, Vuoksimaa E, Westerholm P, Westerlund H, Zins M, Kivipelto M, Vahtera J, Kaprio J, Singh-Manoux A, Jokela M, 2018. Body mass index and risk of dementia: Analysis of individual-level data from 1.3 million individuals. Alzheimers Dement 14, 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinedinst BS, 2017. Modeling of biological data using longitudinal intraindividual means integrated with first and second power time-derivatives. Iowa State University. [Google Scholar]
- Lasselin J, Magne E, Beau C, Aubert A, Dexpert S, Carrez J, Laye S, Forestier D, Ledaguenel P, Capuron L, 2016. Low-grade inflammation is a major contributor of impaired attentional set shifting in obese subjects. Brain. Behav. Immun 58, 63–68. [DOI] [PubMed] [Google Scholar]
- Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jorgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clement K, Dore J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T, Bork P, Wang J, Ehrlich SD, Pedersen O, 2013. Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546. [DOI] [PubMed] [Google Scholar]
- Leng S, Xue QL, Huang Y, Semba R, Chaves P, Bandeen-Roche K, Fried L, Walston J, 2005. Total and differential white blood cell counts and their associations with circulating interleukin-6 levels in community-dwelling older women. J Gerontol A Biol Sci Med Sci 60, 195–199. [DOI] [PubMed] [Google Scholar]
- Lyall DM, Cullen B, Allerhand M, Smith DJ, Mackay D, Evans J, Anderson J, Fawns-Ritchie C, McIntosh AM, Deary IJ, Pell JP, 2016. Cognitive test scores in UK Biobank: Data reduction in 480,416 participants and longitudinal stability in 20,346 participants. PLoS One 11, e0154222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maachi M, Pieroni L, Bruckert E, Jardel C, Fellahi S, Hainque B, Capeau J, Bastard JP, 2004. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFalpha, leptin and IL-6 levels in obese women. Int. J. Obes 28, 993–997. [DOI] [PubMed] [Google Scholar]
- MacKinnon D, 2008. Introduction to statistical mediation analysis. Routledge, New York, New York. [Google Scholar]
- Mayer EA, 2011. Gut feelings: the emerging biology of gut-brain communication. Nature reviews. Neuroscience 12, 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray ET, Hardy R, Hughes A, Wills A, Sattar N, Deanfield J, Kuh D, Whincup P, 2015. Overweight across the life course and adipokines, inflammatory and endothelial markers at age 60–64 years: Evidence from the 1946 birth cohort. Int. J. Obes 39, 1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Kosciolek T, Maldonado Y, Daly RE, Martin AS, McDonald D, Knight R, Jeste DV, 2019. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr. Res 204, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh HM, Oh S, Song HJ, Lee EY, Jeong JY, Ryu OH, Hong KS, Kim DH, 2017. Relationships between cognitive function and body composition among community-dwelling older adults: A cross-sectional study. BMC Geriatr 17, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Carroll SJ, Kho DT, Wiltshire R, Nelson V, Rotimi O, Johnson R, Angel CE, Graham ES, 2015. Pro-inflammatory TNFalpha and IL-1beta differentially regulate the inflammatory phenotype of brain microvascular endothelial cells. J. Neuroinflammation 12, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang S, Hsuchou H, Kastin AJ, Wang Y, Yu C, Pan W, 2014. Diet-induced obesity suppresses expression of many proteins at the blood-brain barrier. J. Cereb. Blood Flow Metab 34, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paccalin M, Pain-Barc S, Pluchon C, Paul C, Besson MN, Carret-Rebillat AS, Rioux-Bilan A, Gil R, Hugon J, 2006. Activated mTOR and PKR kinases in lymphocytes correlate with memory and cognitive decline in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord 22, 320–326. [DOI] [PubMed] [Google Scholar]
- Petersen C, Bell R, Klag KA, Lee SH, Soto R, Ghazaryan A, Buhrke K, Ekiz HA, Ost KS, Boudina S, O’Connell RM, Cox JE, Villanueva CJ, Stephens WZ, Round JL, 2019. T cell-mediated regulation of the microbiota protects against obesity. Science 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrozzi L, Lucetti C, Scarpato R, Gambaccini G, Trippi F, Bernardini S, Del Dotto P, Migliore L, Bonuccelli U, 2002. Cytogenetic alterations in lymphocytes of Alzheimer’s disease and Parkinson’s disease patients. Neurol. Sci 23 Suppl 2, S97–98. [DOI] [PubMed] [Google Scholar]
- Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF Jr., Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O’Donnell CJ, Benjamin EJ, Fox CS, 2007. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: The Framingham Heart Study. Circulation 116, 1234–1241. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Wichman AL, Briggs NE, MacCallum RC, 2008. Latent growth curve modeling. Sage. [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH, 2003. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28, 916–931. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2013. R: A Language and Environment for Statistical Computing. Vienna, Austria. [Google Scholar]
- Reijerkerk A, Kooij G, van der Pol SM, Leyen T, Lakeman K, van Het Hof B, Vivien D, de Vries HE, 2010. The NR1 subunit of NMDA receptor regulates monocyte transmigration through the brain endothelial cell barrier. J. Neurochem 113, 447–453. [DOI] [PubMed] [Google Scholar]
- Rönnlund M, Nilsson LG, 2006. Adult life-span patterns in WAIS-R Block Design performance: Cross-sectional versus longitudinal age gradients and relations to demographic factors. Intelligence 34, 63–78. [Google Scholar]
- Rosseel Y, 2012. Lavaan: An R package for structural equation modeling and more. Version 0.5–12 (BETA). J Stat Softw 48, 1–36. [Google Scholar]
- Rossi FE, Lira FS, Silva BSA, Freire A, Ramos EMC, Gobbo LA, 2019. Influence of skeletal muscle mass and fat mass on the metabolic and inflammatory profile in sarcopenic and non-sarcopenic overfat elderly. Aging Clin. Exp. Res 31, 629–635. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, 2008. BMI-related errors in the measurement of obesity. Int. J. Obes 32 Suppl 3, S56–59. [DOI] [PubMed] [Google Scholar]
- Rothney MP, Martin FP, Xia Y, Beaumont M, Davis C, Ergun D, Fay L, Ginty F, Kochhar S, Wacker W, Rezzi S, 2012. Precision of GE Lunar iDXA for the measurement of total and regional body composition in nonobese adults. J. Clin. Densitom 15, 399–404. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, 2009. When does age-related cognitive decline begin? Neurobiol. Aging 30, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro A, Guidarelli G, Ostan R, Giampieri E, Fabbri C, Bertarelli C, Nicoletti C, Kadi F, de Groot L, Feskens E, Berendsen A, Brzozowska A, Januszko O, Kozlowska K, Fairweather-Tait S, Jennings A, Meunier N, Caumon E, Napoli A, Mercatelli D, Battista G, Capri M, Franceschi C, Bazzocchi A, 2019. Gender-specific association of body composition with inflammatory and adipose-related markers in healthy elderly Europeans from the NU-AGE study. Eur. Radiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen D, Pearlson GD, Anthony JC, Aylward EH, Augustine AM, Davis A, Barta P, 2000. Elucidating the contributions of processing speed, executive ability, and frontal lobe volume to normal age-related differences in fluid intelligence. J. Int. Neuropsychol. Soc 6, 52–61. [DOI] [PubMed] [Google Scholar]
- Seidler S, Zimmermann HW, Bartneck M, Trautwein C, Tacke F, 2010. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol 11, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre-Miranda C, Roque S, Santos NC, Portugal-Nunes C, Costa P, Palha JA, Sousa N, Correia-Neves M, 2015. Effector memory CD4(+) T cells are associated with cognitive performance in a senior population. Neurol Neuroimmunol Neuroinflamm 2, e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J, 2006. Intellectual ability and cortical development in children and adolescents. Nature 440, 676–679. [DOI] [PubMed] [Google Scholar]
- Sheard SM, Nichollls R, Froggatt J, 2017. UK Biobank Haematology Data Companion Document
- Singh-Manoux A, Kivimaki M, Glymour MM, Elbaz A, Berr C, Ebmeier KP, Ferrie JE, Dugravot A, 2012. Timing of onset of cognitive decline: Results from Whitehall II prospective cohort study. Br. Med. J 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi HR, Bates KA, Weinborn M, Bucks RS, Rainey-Smith SR, Rodrigues MA, Bird SM, Brown BM, Beilby J, Howard M, Criddle A, Wraith M, Taddei K, Martins G, Paton A, Shah T, Dhaliwal SS, Mehta PD, Foster JK, Martins IJ, Lautenschlager NT, Mastaglia F, Laws SM, Martins RN, 2015. Bone mineral density, adiposity, and cognitive functions. Front. Aging Neurosci 7, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spauwen PJ, Murphy RA, Jonsson PV, Sigurdsson S, Garcia ME, Eiriksdottir G, van Boxtel MP, Lopez OL, Gudnason V, Harris TB, Launer LJ, 2017. Associations of fat and muscle tissue with cognitive status in older adults: The AGES-Reykjavik Study. Age Ageing 46, 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan A, Willette AA, Bendlin BB, Alexander AL, Coe CL, Voytko ML, Colman RJ, Kemnitz JW, Weindruch RH, Johnson SC, 2012. Brain volumetric and microstructural correlates of executive and motor performance in aged rhesus monkeys. Front. Aging Neurosci 4, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek KM, Strain G, Devlin M, Cohen R, Paul R, Crosby RD, Mitchell JE, Gunstad J, 2013. Body mass index and neurocognitive functioning across the adult lifespan. Neuropsychology 27, 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone KD, Prussin C, Metcalfe DD, 2010. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol 125, S73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R, 2015. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 12, e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson A, Wolf T, Sitzmann A, Willette AA, 2018. Neuroinflammation in Alzheimer’s disease: Pleiotropic roles for cytokines and neuronal pentraxins. Behav. Brain Res. 347, 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeler C, O’Sullivan JL, Bucholtz N, Goldeck D, Pawelec G, Steinhagen-Thiessen E, Demuth I, 2016. The inflammatory markers CRP, IL-6, and IL-10 are associated with cognitive function--data from the Berlin Aging Study II. Neurobiol. Aging 38, 112–117. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI, 2009. A core gut microbiome in obese and lean twins. Nature 457, 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waise TMZ, Toshinai K, Naznin F, NamKoong C, Md Moin AS, Sakoda H, Nakazato M, 2015. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem. Biophys. Res. Commun 464, 1157–1162. [DOI] [PubMed] [Google Scholar]
- Wang GY, Taylor T, Sumich A, Merien F, Borotkanics R, Wrapson W, Krӓgeloh C, Siegert RJ, 2017. Associations between immunological function and memory recall in healthy adults. Brain Cogn 119, 39–44. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 2011. WASI-II: Wechsler abbreviated scale of intelligence. PsychCorp
- Wichmann MA, Cruickshanks KJ, Carlsson CM, Chappell R, Fischer ME, Klein BE, Klein R, Tsai MY, Schubert CR, 2014. Long-term systemic inflammation and cognitive impairment in a population-based cohort. J. Am. Geriatr. Soc 62, 1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H, 2016. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York. [Google Scholar]
- Wirth MD, Sevoyan M, Hofseth L, Shivappa N, Hurley TG, Hebert JR, 2018. The Dietary Inflammatory Index is associated with elevated white blood cell counts in the National Health and Nutrition Examination Survey. Brain. Behav. Immun 69, 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D, Prameya R, Dorovini-Zis K, 2007. Adhesion and migration of polymorphonuclear leukocytes across human brain microvessel endothelial cells are differentially regulated by endothelial cell adhesion molecules and modulate monolayer permeability. J. Neuroimmunol 184, 136–148. [DOI] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM, 2011. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich D, Martin M, 2002. Can longitudinal changes in processing speed explain longitudinal age changes in fluid intelligence? Psychol. Aging 17, 690–695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. A flow chart diagram indicating the progressive exclusion of participants to derive a final sub-sample with no missingness among data of interest.


