Abstract
Objective:
To evaluate safety and efficacy of topically administered 0.02% netarsudil ophthalmic solution (Rhopressa™; Aerie Pharmaceutical) in normal and glaucomatous dogs with ADAMTS10-open-angle glaucoma (ADAMTS10-OAG).
Animals studied:
Five normal and 5 glaucomatous Beagle dogs with ADAMTS10-OAG.
Procedures:
In each dog, left or right eye was randomly selected for netarsudil treatment. Contralateral eyes were sham-treated with balanced salt solution (BSS). Following a 1-week baseline period, dogs were treated once daily (q24h) during week 2, and twice daily (q12h) during week 3; week 4 served as washout period. Efficacy was measured by diurnal intraocular pressure (IOP) and pupil diameter. Safety was assessed by routine ophthalmic examination, gonioscopy and pachymetry. Differences in least square means of quantitative outcome measures were compared between netarsudil and BSS sham treated eyes by linear Gaussian model.
Results:
Baseline IOPs were 18.5±0.5mmHg (mean±SEM) in normal and 27.8±1.0 mmHg in OAG dogs. Even though mean IOPs were lower in netarsudil- vs. sham-treated eyes, the overall differences were neither significant nor clinically relevant, regardless of treatment frequency (q24h-normal: sham 16.4±1.1 mmHg vs. treatment 15.6±1.0 mmHg; q24hr-OAG: sham 25.8± 2.3 mmHg vs. treatment 25.7± 2.4 mmHg; q12hr-normal: sham 15.4±0.8 mmHg vs. treatment 14.4±0.8 mmHg; q12hr-OAG: sham 26.3±1.7 mmHg vs. treatment 25.4±1.8 mmHg). Netarsudil administration was well tolerated but resulted in significant, moderate to severe conjunctival hyperemia (p<0.001).
Conclusions:
Once or twice daily administration of netarsudil resulted in marginal and clinically irrelevant IOP decreases in normal and OAG-affected dogs. Except for conjunctival hyperemia, the drug was well tolerated.
Keywords: ADAMTS10, dog, glaucoma, intraocular pressure (IOP), netarsudil, Rhopressa™
1. INTRODUCTION
Glaucoma is an irreversible optic neuropathy and leading cause of incurable vision loss in dogs.1–5 Based on the presence or absence of an identifiable underlying etiology, glaucoma is categorized as either primary or secondary. In contrast to human patients, where normotensive glaucoma is common, all recognized forms of canine glaucoma are associated with increased intraocular pressure (IOP). Unless an underlying cause can be identified and treated, lowering of IOP by medical and surgical means is the only available therapy to slow progression of glaucoma.1 The two main treatment strategies consist of decreasing aqueous humor production by the ciliary body and increasing aqueous humor drainage through conventional and unconventional outflow pathways. In general, topical administered medications represent the first line of therapy for canine glaucoma. Currently, the three most effective and most commonly used classes of topical glaucoma medications are prostaglandin analogues, carbonic anhydrase inhibitors, and beta-adrenergic antagonists.6–16 Because current IOP-lowering glaucoma therapies frequently fail within months with rebounding IOP elevation and blindness, there is a need for more effective treatment options.1 Unfortunately, the emergence of novel compounds has been very slow, and new commercial drugs are optimized for the application in human eyes.
Two Rho kinase inhibitors were recently approved for clinical use in human glaucoma patients: ripasudil in Japan and netarsudil in the United States.17 Rho kinase inhibitors represent the first new class of clinically useful ocular hypotensive drugs for use in human patients since the introduction of prostaglandin analogues in the 1990s.17 They are also the first clinically used compounds that were designed specifically to target cells along the conventional aqueous humor outflow pathway, including the trabecular meshwork, by decreasing cytoskeleton-driven cellular contraction and reducing production of fibrotic extracellular matrix proteins, resulting in lower aqueous humor outflow resistance.17
In 2017, the US Food and Drug Administration approved netarsudil as a 0.02% ophthalmic solution (Rhopressa™; Aerie Pharmaceuticals, Irvine, CA), formerly referred to as AR-1332, for reduction of elevated IOP in human patients with open-angle glaucoma (OAG) or ocular hypertension. Three mechanisms have been identified in preclinical studies by which netarsudil lowers IOP through Rho kinase inhibition and norepinephrine transport inhibition: (1) increase of trabecular outflow facility, (2) decreased production of aqueous humor, and (3) decreased episcleral venous pressure.17–23 To the best of our knowledge, the IOP-lowering effect of Rho kinase inhibitors has not yet been tested in dogs. Because advances in the medical treatment of canine glaucoma are needed, the purpose of this study was to evaluate safety and efficacy of netarsudil in normal and glaucomatous dogs with ADAMTS10-open-angle glaucoma (ADAMTS10-OAG).
2. METHODS
2.1. Animals
A total of 10 purpose-bred adult Beagle dogs, 5 normal and 5 with ADAMTS10-OAG, were used (Table 1). An equal number of males and females were included. Based on availability and OAG-disease state, the normal dogs were younger (11–24 months, median: 18 months) than the glaucomatous animals (32–50 months, median: 32 months). Genotypes were confirmed based on ADAMTS10 gene sequence: glaucomatous dogs were homozygous for the G661R missense mutation which is responsible for OAG in Beagles, while the normal dogs were either carriers of the mutation or homozygous for the wildtype allele.24 The dogs were group-housed in the same environment at the Michigan State University College of Veterinary Medicine with a 12h/12h light/dark cycle (room lights on at 7am and off at 7pm) and fed the same diet. The studies were done in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the Michigan State University Institutional Animal Care and Use Committee (IACUC).
TABLE 1.
List of dogs
| Dog | Group1 | Gender | Age (months) | Netarsudil2 (Rhopressa™) |
|---|---|---|---|---|
| BRI | Normal | F | 11 | OD |
| CHR | Normal | F | 11 | OS |
| HAR | Normal* | M | 24 | OD |
| HOG | Normal* | F | 24 | OD |
| MAT | Normal | M | 18 | OS |
| DAN | Glaucoma | F | 50 | OS |
| FOO | Glaucoma | M | 41 | OD |
| GRI | Glaucoma | M | 32 | OS |
| GEP | Glaucoma | M | 32 | OD |
| GRE | Glaucoma | F | 32 | OS |
ADAMTS10 genotype: Normal, homozygous for wildtype allele; Normal
, heterozygous/carrier of mutant allele; Glaucoma, homozygous for G661R missense mutation.
Contralateral eye in each dog was sham-treated control (balanced salt solution, BSS).
Abbreviations: F, female; M, male; OD, right eye; OS, left eye.
2.2. Study design
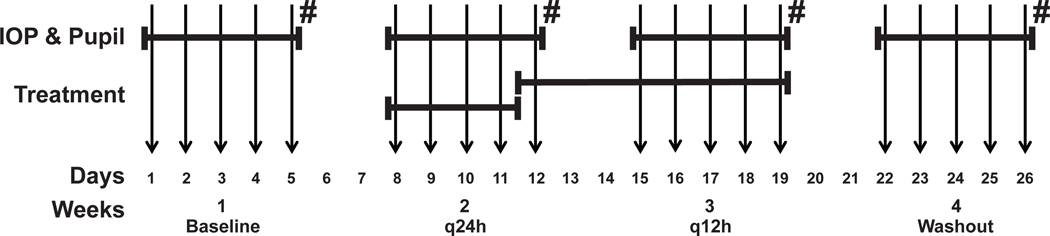
The study was conducted over 4 weeks with data being collected daily from Monday to Friday of each week at 8am, 11:30am, and 3:30pm (Figure 1): (Week 1) baseline data collection, (Week 2) once daily (q24h) netarsudil administration at 8am, (Week 3) twice daily (q12h) netarsudil administration at 8am and 8pm, and (Week 4) drug washout. The washout week was included to verify that IOPs will go back to baseline levels following any potential therapeutic effect, and to control for any learning effect on the data collectors and animals. In each dog, one eye was treated with netarsudil 0.02% ophthalmic solution (Rhopressa™; Aerie Pharmaceuticals) and the fellow eye served as sham-treated control receiving balanced salt solution (BSS; Alcon Laboratories, Inc., Fort Worth, Texas). In the first dog of each the Normal and Glaucoma groups the treatment eye (right eye [OD] vs. left eye [OS]) was chosen randomly by coin toss. Subsequently, OD and OS were alternated in following dogs that were lined up. The order of the dogs was randomized within each group (Normal and Glaucoma) by pulling their names on paper blindly out of a box. Over all 10 dogs, an equal number of OD and OS were treated with netarsudil (Table 1). The investigators collecting the data were masked and did not know which eyes received netarsudil vs. BSS until after data collection and analysis were completed.
FIGURE 1.
Experimental Design. Vertical arrows correspond to days of data collection (Monday through Friday of each week). Diurnal IOP and pupillary diameter were measured every weekday. Treatment with netarsudil and BSS occurred q24h on days 8–11, and subsequently q12h until day 19. Ophthalmic examinations and pachymetry were performed on Fridays (#).
For the first 7 days no medications were administered. Then q24h treatments started at 8am on day 8 (Monday), at the beginning of week 2, and continued for a total of 5 days. After every 8am measurement, 50μl of netarsudil 0.02% ophthalmic solution or BSS were administered on the surface of the designated eyes (Table 1). From day 12 (Friday of week 2), q12h netarsudil and BSS were administered at 8am and 8pm for a total of 8 days, then treatment stopped on Friday evening of week 3 for the last 8 days and washout measurements from Monday to Friday of week 4. In order for the investigators to remain masked, netarsudil and BSS were transferred to numbered sterile microcentrifuge tubes (Fisherbrand; Fisher Scientific, Pittsburgh, PA) and 50 μL of reagent were administered onto the ocular surface at each treatment time point by mechanical pipettor (Satorius Biohit mLINE® Single-Channel Mechanical Pipettor; Sartorius Lab Instruments GmbH & Co. KG, Goettingen, Germany).
2.3. Outcome measures
Data was collected by the same person (KAL) throughout the 20 days to ensure consistency and avoid bias. Measures of efficacy consisted of IOP and pupil diameter; both were measured diurnally Mondays to Fridays of each week at 8am, 11:30am, and 3:30pm. Tonometry was performed with a TonoVet® (Icare Finland Oy, Helsinki, Finland) that was calibrated by the manufacturer within less than 1 year before and after the study. Six readings were taken and averaged at each time point. Readings were only accepted if they were within the range of acceptable standard deviation recommended by the manufacturer (≤2.5 mmHg); these were indicated by no or low bar on the instrument display. Pupil diameter was measured with Jameson calipers (Miltex, Integra York, PA) under scotopic conditions with the room lights turned off (3×10−3 to 8×10−3 lux, measured by ILT1700 Research Radiometer/Photometer; International Light Technologies, Inc., Peabody, MA). A red LED headlight (Quad Tactical; Princeton Tec®, Trenton, NJ) was used to visualize the fundic reflection for outline of the pupil by retroillumination. We elected to measure the vertical pupil diameter based on personal preference. At the timepoints when no netarsudil or BSS was administered, 1 drop of lubricant (OptixCare® Plus Eye Lube; CLCMEDICA, Ontario, Canada) was placed on the cornea following measurements for protection.
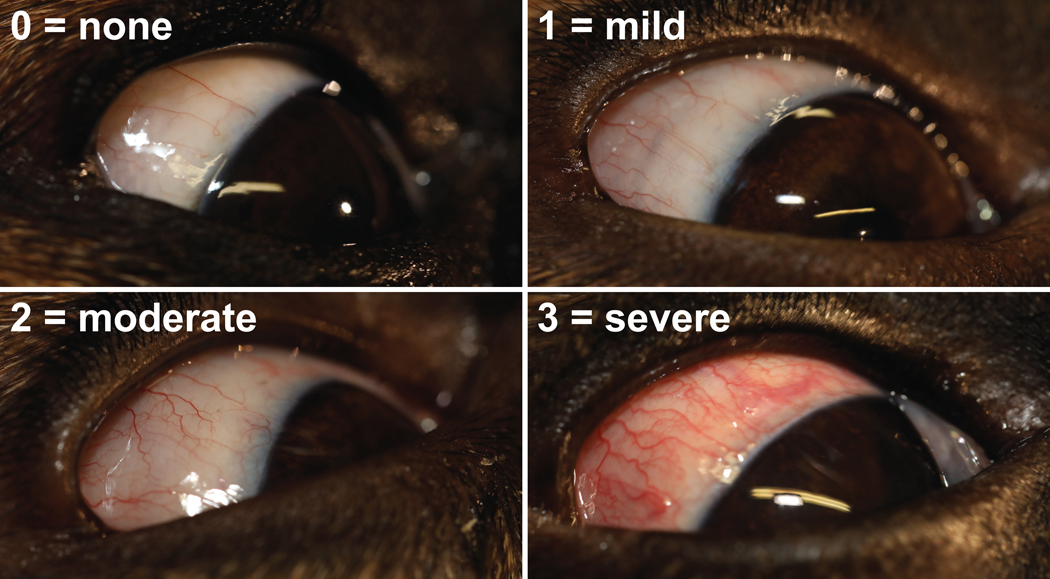
Safety measures included diurnal conjunctival hyperemia, which was assessed at the same time as tonometry and measurement of pupil diameter by application of the Hackett–McDonald Ocular Scoring System: none (score=0), mild (score=1), moderate (score=2), and severe (score=3) (Figure 2).25 Episcleral congestion was not considered since it was not present or minimal at the OAG disease stages selected for this study. Additional safety measures consisted of comfort level, which was assessed throughout the study by severity of blepharospasm, and weekly central corneal thickness (CCT). Latter was measured each Friday afternoon following the 3:30pm data collection time point (Figure 1) by use of a handheld pachymeter (PachPen®; Accutome, Inc., Malvern, PA) following anesthesia (proparacaine HCl 0.5% ophthalmic solution; Akorn, Inc., Buffalo Grove, IL) and lubrication (OptixCare® Plus Eye Lube) of the ocular surface. General ophthalmic examinations were also performed weekly on Friday afternoons following the 3:30pm data collection by slit lamp biomicroscopy (Kowa SL-17 Portable Slit Lamp; Kowa Company, Ltd., Tokyo, Japan) and indirect ophthalmoscopy (Keeler All Pupil II headset; Keeler Instruments, Broomall, PA; Pan Retinal 2.2D condensing lens; Volk Optical, Mentor, OH) in order to detect any potential treatment related adverse effects. These examinations were done by a board-certified veterinary ophthalmologist (AMK). Gonioscopy was performed with a RetCam II (Clarity Medical Systems, Pleasanton, CA) following the conclusion of data collection and following anesthesia (proparacaine HCl 0.5% ophthalmic solution) and lubrication (OptixCare® Plus Eye Lube) of the ocular surface. A generous amount of lubrication serves as a goniolens at the tip of the RetCam camera and allows detailed imaging of the iridocorneal angle.26,27
FIGURE 2:
Photographs of study eyes showing grading of conjunctival hyperemia.
2.4. Statistical analyses
Statistical analyses comparing treatment effects for IOP, pupil size, CCT, and conjunctival hyperemia were performed using a linear Gaussian model that included the effects of dog, treatment, and eye (OD/OS). Dogs were treated as a complete block on which both netarsudil and BSS treatments were applied. Each time point was analyzed separately. Differences in the least square means of treatments were tested using a t-test at p<0.05. The reported outcome measures are the adjusted least square means rather than the arithmetic mean of the raw data. Mean baseline conjunctival hyperemia scores were compared between normal and glaucomatous dogs by analysis of variance (ANOVA).
Power calculations were obtained assuming a two-tailed paired t-test for a minimum mean difference of 5 mmHg in IOP with a sample size of n=5 and alpha=5% (to match this experimental design where dog is the block or pairing factor). The targeted minimum treatment effect of 5 mmHg was set based on reported mean IOP reductions achieved in dogs with currently routinely used topical glaucoma medications and expected IOP variability of the TonoVet® tonometer.6–16 The standard deviation was estimated from the current experiment for each time point and disease condition. This means that there were 120 different standard deviations and as many power values. Computations were implemented using the pwr package of R.28
In order to determine an overall change in IOP over the entire study period that was unrelated to treatment, IOP observations were averaged out for each week, keeping disease status and time of day separate. A Gaussian linear model was fitted including fixed effect of week and random effect of dog. Also unrelated to treatment, baseline CCT was compared between normal and OAG-affected dogs by fitting a Gaussian linear model that included fixed effects of dog, eye and disease status.
3. RESULTS
3.1. Effect of netarsudil on canine IOP and pupil size
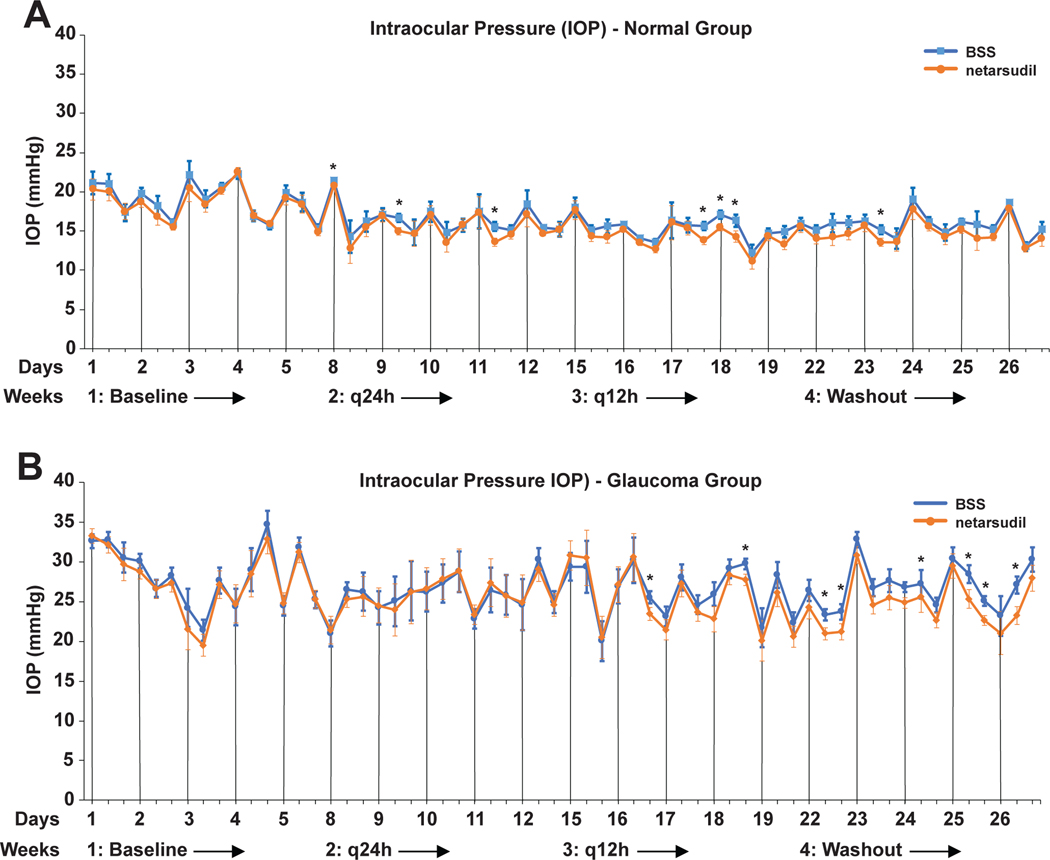
Compared to the BSS sham treatment, the q24h and q12h topical administration of 0.02% netarsudil ophthalmic solution resulted in a marginal, clinically irrelevant IOP reduction in normal and OAG-affected dogs (Figure 3 and Table 2). The q24h administration lead to a significant IOP reduction in only 3/15 time points in normal and 0/15 in OAG-affected dogs. Increasing the frequency of drug administration to q12h resulted in a significant IOP reduction in 3/15 and 2/15 timepoints in normal and OAG dogs, respectively. We also found significant IOP reductions in 1/15 and 5/15 time points during the washout period. Overall, these adjusted intermittent mean IOP reductions varied between 0.6–2.1 mmHg in normal and 1.9–3.2 mmHg in OAG-affected dogs. When looking at individual dogs’ IOP traces, we could not identify animals that responded more to netarsudil than others (data not shown). While both male and female dogs were included in the study, our sample size was too small to determine a gender effect.
FIGURE 3.
Adjusted least square mean IOPs plotted over the entire study time for normal (A) and glaucomatous dogs (B). While there was a trend towards lower IOPs in netarsudil vs. BSS treated eyes, these differences were significant at only few time points with q24h- and q12h-treatment as well as during the washout period. The vertical lines indicate the 8am time points of each day when diurnal data collection was performed. Significance: *, p<0.05.
TABLE 2.
Summary of adjusted least square means of outcome measures
| Week 1 (baseline) | Week 2 (q24h) | Week 3 (q12h) | Week 4 (washout) | |||||
|---|---|---|---|---|---|---|---|---|
| Parameter (means +/− SEM) | Pre-Netarsudil | Pre-BSS | Netarsudil | BSS | Netarsudil | BSS | Post-Netarsudil | Post-BSS |
| IOP (mmHg) | ||||||||
| Normal | 18.4 +/− 0.9 | 18.9 +/− 0.9 | 15.6 +/− 1.0 | 16.4 +/− 1.1 | 14.4 +/− 0.8 | 15.4 +/− 0.8 | 14.8 +/− 0.8 | 15.8 +/− 0.9 |
| OAG | 27.5 +/− 1.6 | 28.2 +/− 1.5 | 25.7 +/− 2.4 | 25.8 +/− 2.3 | 25.4 +/− 1.8 | 26.3 +/− 1.7 | 24.7 +/− 1.3 | 26.9 +/− 1.2 |
| Pupil diameter (mm) | ||||||||
| Normal | 8.2 +/− 0.4 | 8.4 +/−0.4 | 8.3 +/− 0.4 | 8.3+/− 0.4 | 8.1 +/− 0.4 | 8.1 +/− 0.4 | 8.4 +/− 0.4 | 8.5 +/−0.4 |
| OAG | 8.4 +/− 0.4 | 8.2 +/− 0.4 | 8.5 +/− 0.5 | 8.1 +/− 0.5 | 9.0 +/− 0.6 | 8.8 +/− 0.6 | 8.8 +/− 0.5 | 8.4 +/−0.5 |
| Conjunctival hyperemia (score) | ||||||||
| Normal | 0.2 +/− 0.1 | 0.2 +/− 0.1 | 1.8 +/− 0.4 | 0.9 +/− 0.5 | 1.5 +/− 0.4 | 0.8+/− 0.4 | 0.6 +/− 0.2 | 0.9 +/− 0.2 |
| OAG | 1.0 +/− 0.5 | 0.6 +/− 0.4 | 3.4 +/− 0.4 | 1.5 +/− 0.4 | 3.9 +/− 0.6 | 2.2 +/− 0.6 | 2.7 +/− 0.6 | 2.0 +/− 0.6 |
| Central corneal thickness (μm) | ||||||||
| Normal | 554.3+/− 6.9 | 560.8 +/− 7.3 | 540.7 +/− 8.2 | 556.5+/− 8.7 | 573.0 +/− 14.8 | 563.5 +/− 15.7 | 557.5 +/− 10.2 | 559.8 +/− 10.9 |
| OAG | 656.8 +/− 2.6 | 660.2 +/− 2.4 | 641.3 +/− 7.6 | 641.3 +/− 7.1 | 636.5 +/− 10.6 | 636.8 +/− 9.9 | 647.5 +/− 5.7 | 636.7 +/− 5.4 |
Next, we performed a power analysis with the collected data to determine the minimum detectable treatment effect with our group size of 5 dogs. The average power value across all tests was 95%, and 107 of out 120 tests had a power at or above 80% to detect a difference of 5 mmHg or more. Furthermore, only three of the 120 tests had a power below 60%, and the lowest power was 46%. All in all, the experiment was adequately powered to detect the relevant difference of 5 mmHg across the range of timepoints and conditions of this experiment.
Our baseline mean IOPs were 18.5±0.5 mmHg (mean +/− SEM mmHg) for normal and 27.8±1.0 mmHg for OAG-affected dogs. As expected, daily mean IOPs were higher and more variable in OAG-affected compared to normal dogs (Figure 3 and Table 2). In normal dogs we observed a diurnal IOP pattern during weeks 1, 2, and 4 with values highest in the morning; this pattern was less obvious during week 3 (q12h treatment) and into week 4 (washout) (Figure 3). Such a diurnal effect was less obvious in the glaucoma group with more irregular IOP variations throughout the entire study. Regardless of treatment, we found a significant downward trend in average weekly IOP over the entire study period from week 1 to week 4 irrespective of daytime in normal dogs (p<0.01), but only for the 3:30pm time point in OAG-affected dogs (p<0.05).
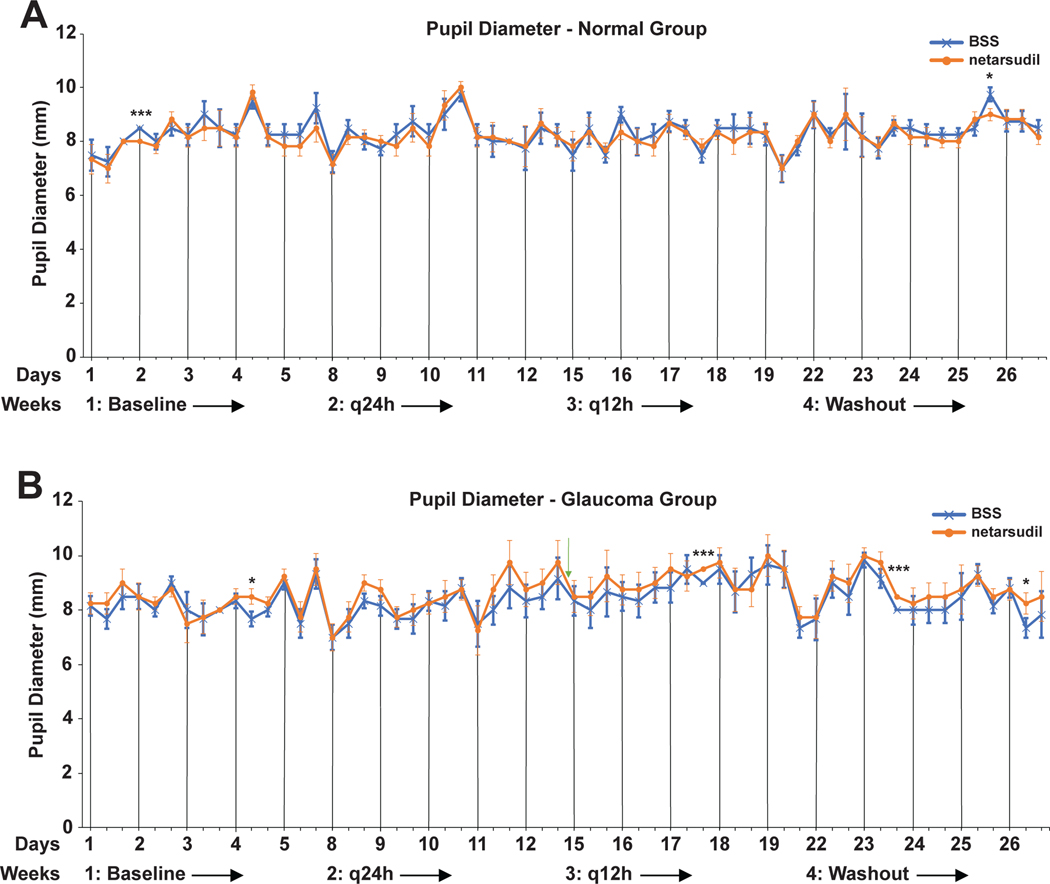
Overall, there was no significant treatment effect of netarsudil on the pupil (Table 2 and Figure 4). Few time points had significantly different mean pupil sizes between netarsudil vs. BSS treatment, but the differences lacked consistency and showed no obvious/clear trend. Therefore, we can conclude that this observation is most likely coincidental.
FIGURE 4:
Adjusted least square mean pupil diameters plotted over the entire study time for normal (A) and glaucomatous dogs (B). The few significant differences between netarsudil vs. BSS treatment are considered coincidental and not a treatment effect. The vertical lines indicate the 8am time points of each day when diurnal data collection was performed. Significance: *, p<0.05; ***, p<0.001.
3.2. Safety of netarsudil in dogs
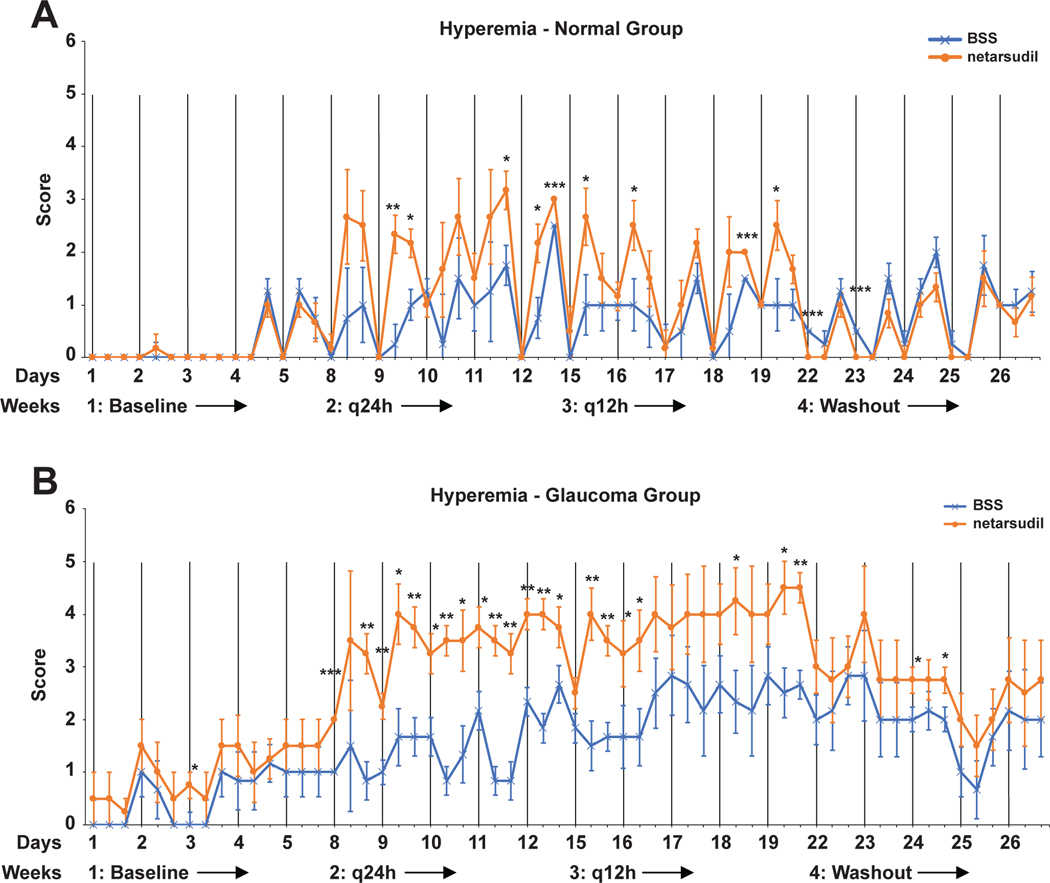
Overall, topical netarsudil was well tolerated by the dogs with no observable signs of ocular discomfort, such as blepharospasm or rubbing following drug administration. Significant, moderate to severe conjunctival hyperemia was observed in all treated eyes (p<0.001) (Figure 5 and Table 2). A diurnal pattern could be observed in normal eyes treated with netarsudil with an increase in conjunctival hyperemia following 8am drop administration. A diurnal pattern was less obvious in the glaucoma group. The mean hyperemia scores were higher in OAG than normal dogs when treated q24h and q12h with either netarsudil or BSS. Conjunctival hyperemia in the BSS sham treated eyes could indicate a potential systemic effect of unilaterally administered netarsudil, or it could occur due to excitement.
FIGURE 5:
Adjusted least square mean conjunctival hyperemia scores plotted over the entire study time for normal (A) and glaucomatous dogs (B). There was a significant increase in conjunctival hyperemia in netarsudil- compared to BSS-treated eyes. Hyperemia was more severe in OAG-affected compared to normal dogs with both netarsudil and BSS treatment. The vertical lines indicate the 8am time points of each day when diurnal data collection was performed. While there was more baseline conjunctival hyperemia in OAG-affected compared to normal dogs, the difference was not significant (p=0.31). Because adjusted least square means rather than the arithmetic means of the raw data are plotted, the conjunctival hyperemia scale extends beyond the 0 to 3 grading. Significance: *, p<0.05; **, p<0.01; ***p<0.001.
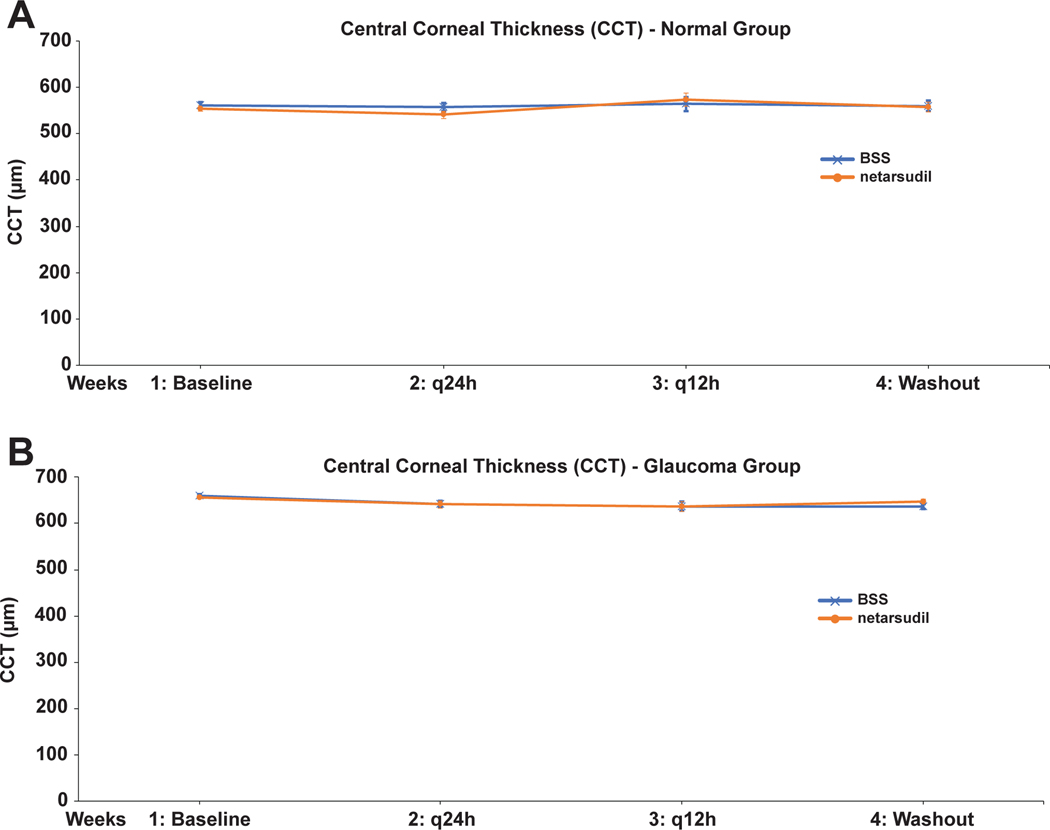
Besides the conjunctival hyperemia, no other treatment-related adverse effects were observed. There were no significant differences in CCT between netarsudil and BSS in normal and OAG-affected dogs, indicating no adverse effects of netarsudil on corneal endothelium (Figure 6 and Table 2). Unrelated to treatment, there was a highly significant difference in CCT between normal and OAG-affected dogs (p<1.8E-8). For example, when comparing baseline measurements, the corneas of the glaucoma dogs were thicker by ~67 μm compared to the normal dogs, most likely due to age- and disease-related effects.29
FIGURE 6:
Adjusted weekly least square mean central corneal thickness (CCT) plotted over the entire study time for normal (A) and glaucomatous dogs (B). There was no detectable effect of netarsudil on corneal thickness.
4. DISCUSSION
Our results show that q24h and q12h topical administration of netarsudil 0.02% ophthalmic solution (Rhopressa™) resulted in a marginal IOP reduction in normal and OAG-affected dogs when compared with BSS sham treatment. We do not consider these IOP reductions clinically relevant when compared to reported steady mean IOP reductions achieved in dogs with currently routinely used topical glaucoma medications, such as the beta-blocker timolol maleate (0–4.1 mmHg reduction in normal and 3.8–5.0 mmHg reduction in OAG-affected dogs),6–10 the carbonic anhydrase inhibitor dorzolamide hydrochloride (3.1–4.1 mmHg reduction in normal and 6.5–16.4 mmHg reduction in OAG-affected dogs),9,11,12 timolol-dorzolamide combination (3.6 mmHg reduction in normal and 6.6–8.4 mmHg reduction in OAG-affected dogs),9,13 and the prostaglandin analogue latanoprost (0.8–3.0 mmHg reduction in normal and 19.6–26.6 mmHg reduction in OAG-affected dogs).14–16
Previous dog studies of netarsudil were limited to in vitro corneal metabolism assays.22 In our study, we evaluated both normal and ADAMTS10-OAG-affected dogs for their IOP responsiveness to netarsudil. ADAMTS10-OAG closely mimics human primary OAG with progressing plaque formation within the trabecular meshwork extracellular space, resulting in gradual decrease in conventional outflow facility and rising IOP with subsequent retinal ganglion cell loss and optic nerve head atrophy.30–35 Another similarity between canine ADAMTS10-OAG and human primary OAG is the IOP increase observed following administration of topical corticosteroids, further suggesting comparable disease mechanisms within the conventional aqueous humor outflow pathways.36
We acknowledge that the use BSS may not have been the ideal sham treatment compared to the Rhopressa™ vehicle (benzalkonium chloride, mannitol, boric acid, and sodium hydroxide in water), but we do not believe that this affected our outcome assessments. The overall mean IOP decrease over time throughout the study period in both netarsudil- and BSS-treated normal eyes was likely based on the dogs’ increasing comfort level with the procedure and data collectors. As the dogs adjusted to the experimental setup, they became calmer and their IOP decreased. We do not know why an overall mean IOP decrease throughout the study period was not present (8am and 11:30am) or less significant (3:30pm) in OAG-affected vs. normal dogs.
Netarsudil 0.02% (Rhopressa™) was developed and optimized for human application. Studies in humans showed stable, long-term IOP reductions between 3.9–6.9 mmHg in patients with primary OAG and ocular hypertension.23,37–40 While these IOP reductions were less effective than those achieved with latanoprost, in patients with lower IOPs of <25–30 mmHg the ocular hypotensive efficacy of netarsudil 0.02% was statistically noninferior to latanoprost and timolol.37–40 These data suggest that topical netarsudil is relatively more effective in patients with lower IOPs, maybe because of the drug’s lowering of episcleral venous pressure.23 In our canine study, we did not observe a higher effectiveness of netarsudil in normotensive vs. hypertensive animals.
We do not know the reasons for the subtherapeutic netarsudil response in dogs. A detailed interspecies comparison of aqueous humor drug concentration and dynamics, including production rate, conventional outflow facility, and episcleral venous pressure, may be helpful.17–20,22,23 In addition to humans, topically administered netarsudil also showed a bigger IOP-lowering effect compared to our dogs in normal animals of two other species: Formosan Rock monkeys and Dutch Belted rabbits. In monkeys and rabbits, q24h topical administration of 0.02% netarsudil solution resulted in a maximal IOP reduction of 5.8±0.3 mmHg and 5.0±0.6 mmHg by day 3 of treatment, respectively.21,22 Clearly, there are species differences in IOP responsiveness to topically administered Rho kinase inhibitors, with netarsudil (USA) and ripasudil (Japan) selected for human application because of best efficacy and least adverse effects compare to a larger number of tested compounds. It is possible that other Rho kinase inhibitors than netarsudil are more effective in dogs.
It remains to be shown if netarsudil has a synergistic effect in canines when combined with other compounds. The q24h-use of a fixed-dose combination of netarsudil 0.02% and latanoprost 0.005% has recently been shown to be superior in reducing IOP in human primary OAG and ocular hypertension patients compared to netarsudil and latanoprost monotherapies and has been approved in 2019 by the U.S. Food and Drug Administration (Rocklatan™, Aerie Pharmaceuticals).17,41,42 We think that there may be the potential that the netarsudil-induced conjunctival hyperemia could result in an increased systemic absorption and less intraocular penetration of other drugs, especially if they are given following netarsudil.
While ADAMTS10-OAG is a well-established and clinically relevant canine glaucoma model for human forms of OAG and is inherited in Beagles, it is not representative of the most common form of canine primary glaucoma affecting a large number of breeds: primary angle-closure glaucoma (PACG).43 While the pathogenesis of canine PACG is still largely unknown, a primary involvement of the trabecular meshwork does not appear to play a major role. Nevertheless, it is possible that a general effect of Rho kinase inhibition on cell contractility within the iridocorneal angle, combined with lower aqueous humor production and lower episcleral venous pressure, could have a beneficial therapeutic effect. Contrary to this notion of a beneficial netarsudil effect on the iridocorneal angle beyond just the trabecular meshwork, we did not find that the iris muscles were affected as assessed by the lack of any detectable change in pupil size in our dogs.
Unrelated to IOP, netarsudil and other Rho kinase inhibitors may have other beneficial effects in the treatment of glaucoma and other ophthalmic conditions. The reduction of cell contraction and fibrosis-related gene expression may be helpful in reducing scar formation over the subconjunctival bleb, a main reason for aqueous humor drainage implant failure in both human and canine patients.1,17 An additional potential positive treatment effect of Rho kinase inhibitors is the demonstrated stimulation of corneal endothelial wound healing with the compound Y27632 in normal dogs following experimental transcorneal freezing.44 Netarsudil and other Rho kinase inhibitors have also been shown to have neuroprotective effects on retinal ganglion cells and may stimulate axonal regeneration, even when given topically on the ocular surface.45–47 Similarly, Rho kinase inhibitors have also been shown to increase ocular blood flow in animal models, including improved perfusion of the optic nerve head, which may contribute to the slowing of glaucomatous optic neuropathy.48–50 The increase in blood flow is based on vasodilation due to the Rho kinase inhibitor-induced relaxation of the vascular smooth muscle cells.51
The topical administration of netarsudil ophthalmic solution was well tolerated by all the dogs with no signs of ocular discomfort such as blepharospasm or pawing at the eyes. We did observe mild to moderate significant conjunctival hyperemia in all netarsudil treated eyes compared to BSS. The hyperemia was stronger in the glaucoma group, possibly because of a disease-related pre-existing redness. A diurnal pattern could be observed in normal eyes treated with netarsudil with an increase in conjunctival hyperemia following 8am drop administration. Interestingly, even the BSS treated eyes developed some increased conjunctival hyperemia, especially in OAG-affected dogs. This finding could potentially be based on a systemic effect of unilaterally administered netarsudil on both eyes, excitement, or tonometry-related ocular surface irritation. The occurrence of conjunctival hyperemia was not surprising since Rho kinase inhibitors increase blood flow via vasodilation, and conjunctival hyperemia has been reported in humans, rabbits, and monkeys treated with netarsudil.22 Conjunctival hyperemia was the most frequent adverse effect in humans with ~50–66% of subjects affected, some with subconjunctival hemorrhage, which we have not observed in the dogs.17,23,37–40 While incidence and severity may decrease with repeated dosing, up to 30% of human study subjects discontinued netarsudil trials because of these adverse events.27,37,39 In rabbits and monkeys, the conjunctival hyperemia was mild and the only observable adverse effect of netarsudil.21,22 Since we selected BSS instead of the vehicle used in Rhopressa™ as a sham-treatment control, we could not determine how much of the conjunctival hyperemia in dogs was caused by the vehicle rather than netarsudil.
We did not observe any other ocular abnormalities in our dogs. In addition to the repeated, detailed ophthalmic examinations, including slit lamp biomicroscopy, we monitored the health of the corneal endothelial cells indirectly by CCT measurements without noticing any changes. As discussed above, Rho kinase inhibitors facilitate corneal endothelial wound healing in dogs.44 We did not observe cornea verticillata in the dogs, which is seen in ~5–26% of human patients after 2–25 weeks of netarsudil treatment.38–40 Cornea verticillata manifests by whorl-shaped microdeposits that collect at the basal level of the corneal epithelium. In human subjects cornea verticillata could only be detected by slit lamp biomicroscopy, did not affect the subjects’ visual acuity, and disappeared in most patients within 13 weeks of netarsudil discontinuation.38–40 It is possible that the 2-week treatment period may have been too short in our dogs for these corneal deposits to develop. Cornea verticillata was not observed in the published rabbit and monkey studies, but the study durations may have also been too short (days), and it is not clear if slit lamp biomicroscopy was performed so that mild corneal changes could have been missed.21,22
In conclusion, with marginal and clinically irrelevant IOP decreases in normal and OAG-affected dogs, the therapeutic usefulness of topical netarsudil ophthalmic solution is inferior to currently routinely used glaucoma medications. While additional studies in other forms of canine glaucoma still need to be performed, netarsudil is unlikely to be a worthwhile treatment option to lower IOP in canine glaucoma patients, especially considering the cost of the medication. Nevertheless, we discussed the potential, IOP-unrelated benefits of Rho kinase inhibitors for glaucoma and other canine ocular disorders and the need for additional studies.
ACKNOWLEDGMENTS
The authors thank Kristin Koehl, Heather Defore, Thoralf Hoelzer, Caroline Ko, and the staff of Michigan State University Campus Animal Resources for their technical assistance. The study was funded by NIH grant R01-EY025752, BrightFocus Foundation, and Michigan State University College of Veterinary Medicine Endowed Research Funds.
Footnotes
CONFLICTS OF INTEREST
There are no conflicts of interest related to this study. Dr. Komáromy received research funding from PolyActiva Pty. Ltd., Melbourne/Australia, while the presented work was conducted.
REFERENCES
- 1.Komáromy AM, Bras D, Esson DW, et al. The future of canine glaucoma therapy. Vet Ophthalmol. 2019;22(5):726–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelatt KN, MacKay EO. Prevalence of the breed-related glaucomas in pure-bred dogs in North America. Vet Ophthalmol. 2004;7(2):97–111. [DOI] [PubMed] [Google Scholar]
- 3.Gelatt KN, MacKay EO. Secondary glaucomas in the dog in North America. Vet Ophthalmol. 2004;7(4):245–259. [DOI] [PubMed] [Google Scholar]
- 4.Strom AR, Hassig M, Iburg TM, Spiess BM. Epidemiology of canine glaucoma presented to University of Zurich from 1995 to 2009. Part 2: secondary glaucoma (217 cases). Vet Ophthalmol. 2011;14(2):127–132. [DOI] [PubMed] [Google Scholar]
- 5.Strom AR, Hassig M, Iburg TM, Spiess BM. Epidemiology of canine glaucoma presented to University of Zurich from 1995 to 2009. Part 1: Congenital and primary glaucoma (4 and 123 cases). Vet Ophthalmol. 2011;14(2):121–126. [DOI] [PubMed] [Google Scholar]
- 6.Gum GG, Larocca RD, Gelatt KN, Mead JP, Gelatt JK. The effect of topical timolol maleate on intraocular pressure in normal beagles and beagles with inherited glaucoma. Prog Vet Comp Ophthalmol. 1991;1(3):141–150. [Google Scholar]
- 7.Wilkie DA, Latimer CA. Effects of topical administration of timolol maleate on intraocular pressure and pupil size in dogs. Am J Vet Res. 1991;52(3):432–435. [PubMed] [Google Scholar]
- 8.Maehara S, Ono K, Ito N, et al. Effects of topical nipradilol and timolol maleate on intraocular pressure, facility of outflow, arterial blood pressure and pulse rate in dogs. Vet Ophthalmol. 2004;7(3):147–150. [DOI] [PubMed] [Google Scholar]
- 9.Plummer CE, MacKay EO, Gelatt KN. Comparison of the effects of topical administration of a fixed combination of dorzolamide-timolol to monotherapy with timolol or dorzolamide on IOP, pupil size, and heart rate in glaucomatous dogs. Vet Ophthalmol. 2006;9(4):245–249. [DOI] [PubMed] [Google Scholar]
- 10.Smith LN, Miller PE, Felchle LM. Effects of topical administration of latanoprost, timolol, or a combination of latanoprost and timolol on intraocular pressure, pupil size, and heart rate in clinically normal dogs. Am J Vet Res. 2010;71(9):1055–1061. [DOI] [PubMed] [Google Scholar]
- 11.Cawrse MA, Ward DA, Hendrix DV. Effects of topical application of a 2% solution of dorzolamide on intraocular pressure and aqueous humor flow rate in clinically normal dogs. Am J Vet Res. 2001;62(6):859–863. [DOI] [PubMed] [Google Scholar]
- 12.Gelatt KN, MacKay EO. Changes in intraocular pressure associated with topical dorzolamide and oral methazolamide in glaucomatous dogs. Vet Ophthalmol. 2001;4(1):61–67. [DOI] [PubMed] [Google Scholar]
- 13.Scardillo A, Pugliese M, De Majo M, Niutta PP, Pugliese A. Effects of topical 0.5% levobunolol alone or in association with 2% dorzolamide compared with a fixed combination of 0.5% timolol and 2% dorzolamide on intraocular pressure and heart rate in dogs without glaucoma. Vet Ther. 2010;11(3):E1–6. [PubMed] [Google Scholar]
- 14.Studer ME, Martin CL, Stiles J. Effects of 0.005% latanoprost solution on intraocular pressure in healthy dogs and cats. Am J Vet Res. 2000;61(10):1220–1224. [DOI] [PubMed] [Google Scholar]
- 15.Gelatt KN, MacKay EO. Effect of different dose schedules of latanoprost on intraocular pressure and pupil size in the glaucomatous Beagle. Vet Ophthalmol. 2001;4(4):283–288. [DOI] [PubMed] [Google Scholar]
- 16.Tofflemire KL, Whitley EM, Allbaugh RA, et al. Comparison of two- and three-times-daily topical ophthalmic application of 0.005% latanoprost solution in clinically normal dogs. Am J Vet Res. 2015;76(7):625–631. [DOI] [PubMed] [Google Scholar]
- 17.Tanna AP, Johnson M. Rho Kinase Inhibitors as a Novel Treatment for Glaucoma and Ocular Hypertension. Ophthalmology. 2018;125(11):1741–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang RF, Williamson JE, Kopczynski C, Serle JB. Effect of 0.04% AR-13324, a ROCK, and norepinephrine transporter inhibitor, on aqueous humor dynamics in normotensive monkey eyes. J Glaucoma. 2015;24(1):51–54. [DOI] [PubMed] [Google Scholar]
- 19.Kiel JW, Kopczynski CC. Effect of AR-13324 on episcleral venous pressure in Dutch belted rabbits. J Ocul Pharmacol Ther. 2015;31(3):146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li G, Mukherjee D, Navarro I, et al. Visualization of conventional outflow tissue responses to netarsudil in living mouse eyes. Eur J Pharmacol. 2016;787:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sturdivant JM, Royalty SM, Lin CW, et al. Discovery of the ROCK inhibitor netarsudil for the treatment of open-angle glaucoma. Bioorg Med Chem Lett. 2016;26(10):2475–2480. [DOI] [PubMed] [Google Scholar]
- 22.Lin CW, Sherman B, Moore LA, et al. Discovery and preclinical development of netarsudil, a novel ocular hypotensive agent for the treatment of glaucoma. J Ocul Pharmacol Ther. 2018;34(1–2):40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazemi A, McLaren JW, Kopczynski CC, Heah TG, Novack GD, Sit AJ. The effects of netarsudil ophthalmic solution on aqueous humor dynamics in a randomized study in humans. J Ocul Pharmacol Ther. 2018;34(5):380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuchtey J, Olson LM, Rinkoski T, et al. Mapping of the disease locus and identification of ADAMTS10 as a candidate gene in a canine model of primary open angle glaucoma. PLoS genetics. 2011;7(2):e1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munger RJ. Veterinary ophthalmology in laboratory animal studies. Vet Ophthalmol. 2002;5(3):167–175. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed IIK, MacKeen LD. A new approach to imaging the angle: The RetCam makes goniography fast, easy, and effective. Glaucoma Today. 2007;5(4):28–30. [Google Scholar]
- 27.Park SA, Sledge D, Monahan C, Bartoe JT, Komáromy AM. Primary angle-closure glaucoma with goniodysgenesis in a Beagle dog. BMC Vet Res. 2019;15(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org/. 2013. [Google Scholar]
- 29.Gilger BC, Whitley RD, McLaughlin SA, Wright JC, Drane JW. Canine corneal thickness measured by ultrasonic pachymetry. Am J Vet Res. 1991;52(10):1570–1572. [PubMed] [Google Scholar]
- 30.Brooks DE, Samuelson DA, Gelatt KN, Smith PJ. Morphologic changes in the lamina cribrosa of beagles with primary open-angle glaucoma. Am J Vet Res. 1989;50(6):936–941. [PubMed] [Google Scholar]
- 31.Gelatt KN, Gum GG, Gwin RM, Bromberg NM, Merideth RE, Samuelson DA. Primary open angle glaucoma: inherited primary open angle glaucoma in the beagle. Am J Pathol. 1981;102(2):292–295. [PMC free article] [PubMed] [Google Scholar]
- 32.Gelatt KN, Gum GG, Mackay EO, Gelatt KJ. Estimations of aqueous humor outflow facility by pneumatonography in normal, genetic carrier and glaucomatous beagles. Vet Comp Ophthalmol. 1996;6(3):148–151. [Google Scholar]
- 33.Gelatt KN, Gwin RM, Peiffer RL Jr., Gum GG. Tonography in the normal and glaucomatous beagle. Am J Vet Res. 1977;38(4):515–520. [PubMed] [Google Scholar]
- 34.Gelatt KN, Peiffer RL Jr., Gwin RM, Gum GG, Williams LW. Clinical manifestations of inherited glaucoma in the beagle. Invest Ophthalmol Vis Sci. 1977;16(12):1135–1142. [PubMed] [Google Scholar]
- 35.Samuelson DA, Gum GG, Gelatt KN. Ultrastructural changes in the aqueous outflow apparatus of beagles with inherited glaucoma. Invest Ophthalmol Vis Sci. 1989;30(3):550–561. [PubMed] [Google Scholar]
- 36.Gelatt KN, Mackay EO. The ocular hypertensive effects of topical 0.1% dexamethasone in beagles with inherited glaucoma. J Ocul Pharmacol Ther. 1998;14(1):57–66. [DOI] [PubMed] [Google Scholar]
- 37.Bacharach J, Dubiner HB, Levy B, Kopczynski CC, Novack GD, Group A-CS. Double-masked, randomized, dose-response study of AR-13324 versus latanoprost in patients with elevated intraocular pressure. Ophthalmology. 2015;122(2):302–307. [DOI] [PubMed] [Google Scholar]
- 38.Serle JB, Katz LJ, McLaurin E, et al. Two phase 3 clinical trials comparing the safety and efficacy of netarsudil to timolol in patients with elevated intraocular pressure: Rho kinase elevated IOP treatment trial 1 and 2 (ROCKET-1 and ROCKET-2). Am J Ophthalmol. 2018;186:116–127. [DOI] [PubMed] [Google Scholar]
- 39.Khouri AS, Serle JB, Bacharach J, et al. Once-daily netarsudil versus twice-daily timolol in patients with elevated intraocular pressure: the randomized phase 3 ROCKET-4 study. Am J Ophthalmol. 2019;204:97–104. [DOI] [PubMed] [Google Scholar]
- 40.Kahook MY, Serle JB, Mah FS, et al. Long-term safety and ocular hypotensive efficacy evaluation of netarsudil ophthalmic solution: Rho kinase elevated IOP treatment trial (ROCKET-2). Am J Ophthalmol. 2019;200:130–137. [DOI] [PubMed] [Google Scholar]
- 41.Asrani S, Robin AL, Serle JB, et al. Netarsudil/latanoprost fixed-dose combination for elevated intraocular pressure: 3-month data from a randomized phase 3 trial. Am J Ophthalmol. 2019; pii: S0002-9394(19)30284-3. doi: 10.1016/j.ajo.2019.06.016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42.Lewis RA, Levy B, Ramirez N, et al. Fixed-dose combination of AR-13324 and latanoprost: a double-masked, 28-day, randomised, controlled study in patients with open-angle glaucoma or ocular hypertension. Br J Ophthalmol. 2016;100(3):339–344. [DOI] [PubMed] [Google Scholar]
- 43.Komáromy AM, Petersen-Jones SM. Genetics of Canine Primary Glaucomas. Vet Clin North Am Small Anim Pract. 2015;45(6):1159–1182, v. [DOI] [PubMed] [Google Scholar]
- 44.Miyagi H, Kim S, Li J, Murphy CJ, Thomasy SM. Topical Rho-associated kinase inhibitor, Y27632, accelerates corneal endothelial regeneration in a canine cryoinjury model. Cornea. 2019;38(3):352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw PX, Sang A, Wang Y, et al. Topical administration of a Rock/Net inhibitor promotes retinal ganglion cell survival and axon regeneration after optic nerve injury. Exp Eye Res. 2017;158:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hensel N, Rademacher S, Claus P. Chatting with the neighbors: crosstalk between Rho-kinase (ROCK) and other signaling pathways for treatment of neurological disorders. Front Neurosci. 2015;9:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto K, Maruyama K, Himori N, et al. The novel Rho kinase (ROCK) inhibitor K-115: a new candidate drug for neuroprotective treatment in glaucoma. Invest Ophthalmol Vis Sci. 2014;55(11):7126–7136. [DOI] [PubMed] [Google Scholar]
- 48.Tokushige H, Waki M, Takayama Y, Tanihara H. Effects of Y-39983, a selective Rho-associated protein kinase inhibitor, on blood flow in optic nerve head in rabbits and axonal regeneration of retinal ganglion cells in rats. Curr Eye Res. 2011;36(10):964–970. [DOI] [PubMed] [Google Scholar]
- 49.Ohta Y, Takaseki S, Yoshitomi T. Effects of ripasudil hydrochloride hydrate (K-115), a Rho-kinase inhibitor, on ocular blood flow and ciliary artery smooth muscle contraction in rabbits. Jpn J Ophthalmol. 2017;61(5):423–432. [DOI] [PubMed] [Google Scholar]
- 50.Sugiyama T, Shibata M, Kajiura S, et al. Effects of fasudil, a Rho-associated protein kinase inhibitor, on optic nerve head blood flow in rabbits. Invest Ophthalmol Vis Sci. 2011;52(1):64–69. [DOI] [PubMed] [Google Scholar]
- 51.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522 Pt 2:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]