Abstract
IMPORTANCE
Research clearly indicates that current approaches to newborn blood spot screening (NBS) education are ineffective. Incorporating NBS education into prenatal care is broadly supported by lay and professional opinion.
OBJECTIVE
To determine the efficacy and effect of prenatal education about newborn screening and use of residual dried blood spots (DBS) in research on parental knowledge, attitudes, and behaviors.
DESIGN, SETTING, AND PARTICIPANTS
A randomized clinical trial of prenatal educational interventions, with outcomes measured by survey at 2 to 4 weeks postpartum. Participants were recruited from obstetric clinics in Salt Lake City, Utah; San Francisco, California; and the Bronx, New York. Eligible women were English- or Spanish-speaking adults and did not have a high-risk pregnancy. A total of 901 women were enrolled. Participants who completed the follow-up survey included 212 women in the usual care group (70% retention), 231 in the NBS group (77% retention), and 221 women in the NBS + DBS group (75% retention). Those who completed the survey were similar across the 3 groups with respect to age, ethnicity, race, education, marital status, income, obstetric history, and language.
INTERVENTIONS
Participants were randomized into 1 of 3 groups: usual care (n = 305), those viewing an NBS movie and brochure (n = 300), and those viewing both the NBS and DBS movies and brochures (n = 296).
MAIN OUTCOMES AND MEASURES
Two to four weeks postpartum, women completed a 91-item survey by telephone, addressing knowledge, attitudes, and behavior with respect to opting out of NBS or DBS for their child.
RESULTS
A total of 901 women (mean age, 31 years) were randomized and 664 completed the follow-up survey. The total correct responses on the knowledge instrument in regard to NBS were 69% in the usual care group, 79% in the NBS group, and 75% in the NBS + DBS group, a significant between-group difference (P < .05). Although all groups showed strong support for NBS, the percentage of women who were “very supportive” was highest in the NBS group (94%), followed by the NBS + DBS group (86%) and was lowest in the usual care group (73%) (P < .001). The interventions were not associated with decisions to decline newborn screening or withdraw residual DBS. Nine women stated that they had declined NBS (all the usual care group; P < .001). With respect to DBS, 5 participants indicated that they contacted the health department to have their child’s sample withdrawn after testing: 3 in the NBS + DBS group and 2 in the usual care group (P = .25).
CONCLUSIONS AND RELEVANCE
Educational interventions can be implemented in the prenatal clinic, using multimedia tools and electronic platforms. Prenatal education is effective in increasing postnatal knowledge and support for these programs. These results are relevant to other contexts in which residual clinical specimens and data are used for research purposes.
CLINICAL TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT02676245
In the United States, newborn blood spot screening (NBS) is conducted by state health departments for the early identification of infants with certain genetic, metabolic, and endocrine disorders. Each year, almost all 4 million neonates born in the United States are screened for more than 30 conditions. Because of the significant benefits for children, in all but Wyoming and the District of Columbia, screening is conducted without parental permission, although most states permit parents to opt out of screening for religious or philosophical reasons.
Parents are key participants in state newborn screening systems. An effective collaboration between professionals and parents is critical for successful screening, diagnosis, and treatment, yet evidence indicates that many parents are poorly informed about NBS. They have expressed a strong preference for more information about it.1,2 With an inadequate understanding of NBS, parents can be adversely affected if they experience a false-positive or indeterminate test result.3–8 Given the continued expansion of NBS and the potential to use DNA sequencing, improving parental education will be increasingly important.9
Although 21 states require parental education, all of them offer information to parents, primarily through printed brochures provided in the newborn nursery.10 However, NBS brochures typically are commingled with a variety of other materials provided to parents after the birth of a child; the hectic postpartum environment and the need to address other health care priorities in newborn and maternal care contribute to the poor efficacy for current educational approaches.1
To address this problem, the American Academy of Pediatrics Task Force on Newborn Screening outlined a national agenda for strengthening state newborn screening systems, which included a call for the development and assessment of new educational tools for parents and professionals.11 There also is a clear consensus that the education of parents in regard to newborn screening and residual specimens should occur as a part of prenatal care, rather than in the postpartum period alone.1,12–18 Pregnancy is potentially a more effective time for this education because of the long period and parents’ eagerness to learn anything relevant to the health of their child.19 Prenatal education about NBS is supported by the American Academy of Pediatrics and the American College of Obstetrics and Gynecology.20 Despite this consensus, prenatal care providers are not addressing this topic.21,22 Furthermore, to our knowledge there have been no rigorous assessments of NBS prenatal education to support these consensus opinions.
An issue closely related to NBS is the retention and use of residual dried blood spots (DBS). After clinical screening is complete, DBS remain on virtually every baby screened and a number of states store these specimens for several purposes, including quality improvement, forensic applications, and biomedical research.10,11 Research with DBS has been conducted with deidentified spots and a waiver of informed permission from parents.
The storage and use of residual samples for research has become a significant controversy in recent years because of public concerns about the lack of parental knowledge and consent for these activities. Two states, Minnesota and Texas, were sued by parents in 2009 for the retention and use of residual DBS without parental permission. Research has shown that, while although parents and the public are generally supportive of research uses of DBS, the public expects that parents will be informed of this practice, when relevant, and will be offered a choice about storage and research use.2,23
The current project was designed to develop evidence-based, multimedia education tools about NBS and retention and use of DBS and to assess the impact of prenatal education about these programs on the knowledge, attitudes, and decisions of parents.
Methods
Pregnant women at approximately 36 weeks’ gestation were recruited at Intermountain Healthcare in Salt Lake City, Utah; Albert Einstein College of Medicine/Montefiore Medical Center in the Bronx, New York; and the University of California, San Francisco, and San Francisco General Hospital obstetrics clinics in San Francisco. These communities were selected to provide a diverse participant population and because these 3 states have similar policies in regard to NBS and residual DBS. All 3 states permit parents to opt out of NBS, and each retains DBS but permits parents to opt out of DBS retention and research use. The study was approved by the institutional review boards at the University of Utah, Intermountain Healthcare, Einstein/Montefiore, University of California, San Francisco, and San Francisco General Hospital (see the protocol in the Supplement).
The educational tools were developed by the Genetic Science Learning Center at the University of Utah. A 6-minute movie was developed to convey information about NBS,24 based on the work of Davis et al.1 A separate 7-minute movie was developed in accordance with our previous research to convey basic information about residual DBS and their potential uses in biomedical research.23,24 The movies were presented on a tablet computer in English or Spanish. Additionally, the Genetic Science Learning Center developed a brochure on DBS and we used a Health Resources and Services Administration brochure on NBS; both were in English and Spanish.24 Videos were validated for content and balanced presentation of information about NBS and DBS by a 13-member external advisory board of NBS experts.
Eligible women were English- or Spanish-speaking adults who did not have a high-risk pregnancy in their own estimation. Women were approached for participation in the prenatal clinics. After providing written consent, participants completed a 21-item survey that included perceived knowledge about NBS and demographic and contact information. Individuals self-reported their classification for race and ethnicity. Attitudes on the types of issues addressed in this research can be influenced by cultural background, education, and experience so we sought to recruit a diverse subject population and to ensure comparability across groups.
Participants were randomized into 1 of 3 groups: a usual care group, who received information about NBS and DBS routinely provided to all parents during the prenatal or postnatal periods in their clinic or birthing facilities; a group who viewed the NBS movie and received the NBS brochure; and a group who viewed both the NBS and DBS movies during 1 clinic visit and received the 2 brochures.
Women randomized to an intervention group were provided a tablet computer to view the movie(s) and the brochure(s) during a waiting time in the clinic. The clinic physicians and staff were familiar with the study but were not directly involved in the interventions. Participants were provided a gift card valued at $20 for completion of this phase of the study.
Two to 4 weeks after the due date of their baby, women were contacted by telephone by a professional survey research firm (blind to randomization) for completion of the postintervention survey. The survey consisted of 91 items addressing knowledge and attitudes about NBS and DBS programs, and participant’s behavior with respect to opting out of either NBS or DBS. The NBS knowledge tool was based on research by Davis et al.1 The DBS knowledge tool was based on our previous work.23
The survey company made 5 attempts to contact participants. On completion of the survey, participants were sent a $40 gift card.
We recruited partners of participants by telephone at the completion of the participant’s survey. Partners completed the same postintervention survey as the mother and were provided an additional $40 gift card.
Our study involved randomizing pregnant women to 1 of 3 educational interventions followed by a postpartum assessment of knowledge, attitudes, and decisions regarding newborn screening and the retention of residual blood spots. We did not initially recognize that educational interventions designed to enhance knowledge but not change health behaviors should be considered a clinical trial under this definition. We now understand that JAMA Pediatrics considers this type of research to be covered by the ICMJE registration policy and registered our study post hoc at clinicaltrials.gov.
Analysis
All analyses were conducted with SPSS version 22. Univariate analysis of variance with Tukey post hoc adjustment was conducted to test the relationship of group assignment (standard, NBS, or NBS + DBS) on knowledge outcome scores. Pearson χ2 or Fisher exact test (if cell count minimums were violated) was used to test group assignment with categorical outcome variables (eg, opting out of NBS, attitude and opinion questions).
Results
Between September 2013 and October 2014, 1247 women were approached in the recruitment sites (Figure). Twenty-eight percent of individuals approached declined to participate and 901 women were enrolled and randomized. The usual care group had 305 participants initially enrolled, the NBS video group had 300 participants, and the NBS + DBSs group had 296. Participants who completed the follow-up survey included 212 women in the usual care group (70% retention), 231 in the NBS group (77% retention), and 221 in the NBS +DBS group (75% retention). Participants who completed the survey were similar across the 3 groups with respect to age, ethnicity, race, education, marital status, income, obstetric history, language, and gestational age.
Figure.
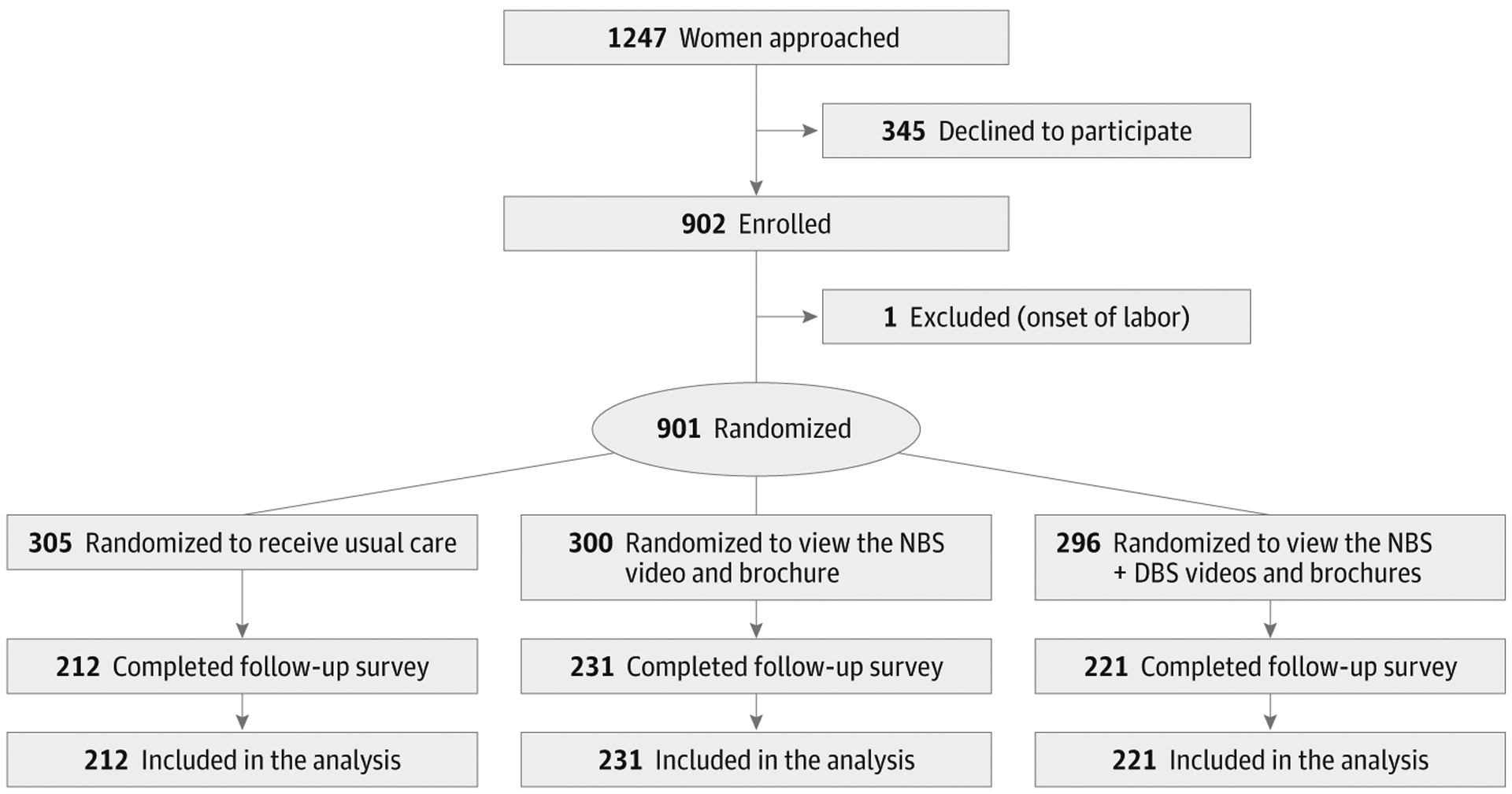
Flow of Participants Through the Newborn Screening Trial
NBS indicates newborn blood spot screening; DBS, dried blood spots.
A summary of the demographic characteristics of the participants is presented in Table 1. The average time between the prenatal intervention and the postpartum survey was 7.2 weeks (SD 3.3).
Table 1.
Participant Characteristics at Enrollment (N = 664)
| Characteristic | No. (%) |
|---|---|
| Age, mean (SD), y | 31.0 (5.6) |
| Weeks enrollment to survey, mean (SD) | 7.2 (3.3) |
| Given birth before | |
| Yes | 361 (54.4) |
| No | 302 (45.6) |
| Race | |
| Black or African American | 112 (17.0) |
| White | 321 (48.6) |
| Asian | 52 (7.9) |
| Multiracial | 39 (5.9) |
| Native Hawaiian or Pacific Islander | 8 (1.2) |
| American Indian or Alaska Native | 3 (0.5) |
| Other | 94 (14.2) |
| Unknown or not reported | 31 (4.7) |
| Ethnicity | |
| Hispanic | 184 (27.9) |
| Non-Hispanic | 475 (72.1) |
| Language | |
| English | 622 (93.7) |
| Spanish | 42 (6.3) |
| Marital status | |
| Married or living with partner | 546 (83.1) |
| Significantly involved with partner but not living together | 44 (6.7) |
| Single/not significantly involved | 67 (10.2) |
| Income, $ | |
| <24 999 | 109 (16.4) |
| 25 000–50 000 | 113 (17.0) |
| 50 001–100 000 | 146 (22.0) |
| 100 001–150 000 | 76 (11.4) |
| >150 000 | 74 (11.1) |
| Not sure/did not answer | 146 (22.0) |
| Education | |
| Less than high school or no formal education | 47 (7.1) |
| High school or GED | 107 (16.1) |
| Some college | 128 (19.3) |
| Associate’s degree, community college, or vocational degree | 62 (9.4) |
| College graduate | 189 (28.5) |
| Professional or graduate degree | 130 (19.6) |
| Enrollment site | |
| California | 255 (38.4) |
| New York | 195 (29.4) |
| Utah | 214 (32.2) |
Abbreviation: GED, general equivalency diploma.
Knowledge Outcomes
We hypothesized that the prenatal educational interventions would increase knowledge at 2 to 4 weeks postpartum. This period was chosen because NBS results are typically available by 2 weeks of age. The total correct responses on our knowledge instrument in regard to NBS increased from 69% in the usual care group to 79% in the NBS group and 75% in the NBS +DBS group. The difference between each group was statistically significant (P < .05).
Knowledge scores on a 20-item survey of facts about DBS showed 42% correct responses for the usual care group, 46% for the NBS group, and 65% for the NBS + DBS group. The difference for the NBS + DBS group compared with the other 2 groups was significant (P < .05).
Attitude Outcomes
A concern in the NBS field is that the education of parents about the state program or DBS practices might jeopardize support for these programs. Our project sought to determine the impact of prenatal education on the attitudes of parents after the delivery of their baby and after their own experience with newborn screening.
Table 2 illustrates the responses to the question “From your experience, and what you understand about newborn screening, how supportive are you of this program?” Although all groups showed strong support for NBS, the percentage of women who were “very supportive” was highest in the NBS group (94%), followed by the NBS + DBS group (86%), and was lowest in the usual care group (73%) (P < .001).
Table 2.
Attitudes and Opinions
| Survey Question | Intervention Group, No. (%) | ||
|---|---|---|---|
| Usual Care (n = 212) |
NBS (n = 231) |
NBS+DBS (n = 221) |
|
| From your experience, and what you understand about newborn screening, how supportive are you of this program? | |||
| Very supportive | 151 (73) | 216 (94) | 189 (86) |
| Moderately supportive | 47 (23) | 12 (5) | 28 (13) |
| A little supportive | 6 (3) | 3 (1) | 3 (1) |
| Not supportive at all | 3 (1) | 0 | 0 |
| I am satisfied with the information I have received about newborn screening | |||
| Completely agree | 46 (22) | 76 (33) | 87 (39) |
| Mostly agree | 81 (38) | 114 (50) | 108 (49) |
| Not sure | 22 (10) | 9 (4) | 6 (3) |
| Mostly disagree | 50 (24) | 27 (12) | 18 (8) |
| Completely disagree | 13 (6) | 4 (2) | 2 (1) |
| I am satisfied with the information I have received about the use of dried blood spots after newborn screening | |||
| Completely agree | 11 (5) | 16 (7) | 82 (37) |
| Mostly agree | 29 (14) | 23 (10) | 100 (45) |
| Not sure | 22 (10) | 34 (15) | 7 (3) |
| Mostly disagree | 58 (27) | 77 (33) | 20 (9) |
| Completely disagree | 92 (43) | 81 (35) | 12 (5) |
| How concerned are you that your state saves the leftover blood spots from babies after testing is done? | |||
| Not at all concerned | 53 (25) | 74 (32) | 93 (42) |
| Not very concerned | 60 (29) | 78 (34) | 62 (28) |
| Somewhat concerned | 60 (29) | 48 (21) | 37 (17) |
| Very concerned | 37 (18) | 29 (13) | 27 (12) |
Abbreviations: DBS, dried blood spots; NBS, newborn blood spot screening.
Table 2 illustrates responses to the statement “I am satisfied with the information I have received about newborn screening.” Women in the NBS + DBS group reported higher satisfaction (88%) (either “completely agree” or “mostly agree”), followed by the NBS group (83%) and then the usual care group (60%) (P < .001). In response to the statement “I have enough information about newborn screening,” 50% of the women in the usual care group responded that they “completely agree” or “mostly agree,” whereas 77% of women in the NBS group and 82% of those in the NBS + DBS group answered similarly (P < .001).
Table 2 illustrates the responses to the statement “I am satisfied with the information I have received about the use of dried bloodspot after newborn screening.” Women in the NBS + DBS group reported higher satisfaction (82%) (“completely agree” or “mostly agree”) than those in the NBS group (17%) and usual care group (19%) (P < .001).
Table 2 includes responses to the question “How concerned are you that your state saves leftover blood spots from babies after testing is done?” Forty-three percent of the NBS + DBS group reported being “not at all concerned,” whereas 32% of the NBS group and 25% of the usual care group reported the same (P = .002).
We asked the question “Do you think it is alright for these leftover blood spots to be used for important research on diseases that affect mothers and babies?” The NBS + DBS group reported higher agreement (72% reported “definitely all right”) than the NBS group (60%) and the usual care group (53%) (P < .001).
The primary use of DBS is for quality assurance activities to maintain and improve test modalities. We asked participants the question “In your opinion, do you think it would be all right for health departments to use leftover blood spots to maintain the quality of newborn screening tests?” Although more than 90% in each group responded “definitely” or “probably alright,” the NBS + DBS group showed stronger support (58% reported “definitely alright”) than either the NBS group (41%) or the usual care group (40%) (P = .004). Also, participants who responded either “probably not alright” or “definitely not alright” constituted 10% of the usual care group, 9% of the NBS group, and 6% of the NBS + DBSs group.
Finally, we asked participants the question “In your opinion, when is the best time to educate parents about newborn screening?” Reponses from all 3 groups were similar, with “early in pregnancy” the response for 46% of women in the usual care group, 47% in the NBS group, and 53% in the NBS +DBS group, and “later in pregnancy” the response of 37% in each group (P = .23).
Partners
Partners of participating women rarely attended the 36-week obstetric visit, so we attempted to recruit them when the women completed the outcome survey. We sought to know whether education of pregnant women would increase knowledge or change attitudes in their partners, perhaps by fostering a discussion of the topic between the partners. Only 106 partners completed the survey. The partners had similar responses to the knowledge and attitude items across the 3 groups, indicating that educational interventions for the pregnant women had no impact on the knowledge or attitudes of their partners with respect to NBS and DBS.
Parental Decisions in Regard to NBS and DBS
Utah, New York, and California permit parents to opt out of newborn screening and DBS retention and use. In our postpartum survey, we asked participants whether they chose not to participate in NBS and whether they had asked the health department to not retain their child’s specimen after testing was complete.
Of the participants, 9 stated that they had declined NBS. However, all of these women were in the usual care group (; P < .001). These participants were asked why they declined NBS, and their answers indicated that they were confused about its nature. We surmised that, in most or all cases, parents had declined other prenatal or postnatal services. With respect to DBS, 5 participants indicated that they contacted the health department to have their child’s sample withdrawn after testing. Three of these women were in the NBS + DBS group and 2 were in the usual care group (; P = .25 [N = 658]).However, a post hoc power analysis showed that our study had low power (16%; α = .05) to detect a difference between groups for the rare occurrence of a request for sample withdrawal.
Comments About the Educational Tools
We asked participants to comment on the educational tools with the question “How helpful did you find the movie on newborn screening for learning about newborn screening?” For the groups who viewed the NBS movie, responses indicated that 83% found the movie “very helpful” or “somewhat helpful.” For the NBS + DBS group, 95% found the movies “very helpful or “somewhat helpful.” Qualitative responses about the movies were almost uniformly positive, with many stating the length was appropriate and the tablet computer was useful.
Discussion
The interventions in the third trimester were effective in increasing knowledge of women about NBS and DBS approximately 7 weeks later, after the birth of their child. This increase in knowledge was consistent across the 3 sites. The most impressive influence was the positive effect on maternal attitudes about the NBS program and the practice of DBS storage and research use. Prenatal education about the NBS program and DBS storage and research use was associated with a significant increase in support of these practices.
The research use of biospecimens obtained for clinical purposes is common in academic medical centers, although this practice can be controversial.25 Often information about secondary research uses of clinical specimens is absent or embedded in general consent agreements for clinical care. In the context of newborn screening, research suggests that parents are supportive of DBS retention and use as long as parents are informed of this practice and they have a choice.26,27 This reflects public attitudes about the secondary use of clinical specimens in other contexts.28,29
The study reported here is important because it demonstrates that more information about NBS and DBS is associated with increased support by new parents. Remarkably, even with modest levels of program knowledge (65% correct in the NBS + DBS group), we observed high levels of program support (86% very supportive in the NBS + DBS group). These data suggest that even if individuals do not remember all the details of the programs, the receipt of information can be reassuring. From a policy perspective, our data indicate that NBS programs can be more transparent about these programs and policies without jeopardizing public support. Indeed, public support is likely to be enhanced.
Our results did not indicate that the refusal rate for either NBS or DBS retention was increased after the educational interventions. However, a modest increase in rate of refusal for NBS would be concerning for health departments, and our study was not large enough to determine the impact on the rate of refusals if educational interventions were routinely implemented across a state population.
These results are relevant to recent changes in public policy. In December 2014, the federal Newborn Screening Saves Lives Reauthorization Act of 2014 was enacted. This legislation stipulated that all US Department of Health and Human Services-funded research using DBS will be considered human subjects research, regardless of whether the spots are deidentified. Furthermore, the legislation prohibits waivers of parental consent. States that retain DBS must now implement an informed permission process for US Department of Health and Human Services-funded research uses of these specimens. Our results indicate that information about this choice could be offered in the context of prenatal care, establishing a foundation for informed consent for DBS use.
An important limitation of this study is that the intervention was conducted by research assistants and not clinic staff. A remaining question is whether this type of intervention can be effectively provided by clinicians in routine clinical care. We designed the intervention to be easily delivered on a tablet computer and presented during waiting times in the clinics. However, routine implementation in the clinic environment would require purchasing and managing the equipment, having time for orienting patients to the tools, answering questions, and maintaining staff commitment to the effort. With the rapid expansion of smartphones and electronic medical records, patients may be able to view these resources on their own devices outside the clinical encounter. Further research to evaluate best practices for implementation of this NBS education during routine obstetric practice is needed to ensure the effective delivery of this important information at a time desired by most couples.
Supplementary Material
Key Points.
Question
Is prenatal parental education about newborn screening and residual dried blood spot retention effective in changing parental knowledge and attitudes?
Findings
Prenatal parental education about newborn blood spot screening and residual dried blood spot retention was effective in increasing support for these important programs but was not associated with the decision to decline newborn blood spot screening or withdraw residual dried blood spot samples.
Meaning
State newborn screening programs should consider prenatal education programs that can be conducted with limited concern about adverse effects on newborn blood spot screening programs. These results are relevant to recent changes in public policy.
Funding/Support:
This research was conducted with National Institutes of Health grant 1R01HD062762.
Role of the Funder/Sponsor: The National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Davis TC, Humiston SG, Arnold CL, et al. Recommendations for effective newborn screening communication: results of focus groups with parents, providers, and experts. Pediatrics. 2006; 117(5 pt 2):S326–S340. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell E, Clark L, Anderson R, Botkin JR. Residual newborn screening samples for research: parental information needs for decision-making. J Spec Pediatr Nurs. 2013;18(2):115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baroni MA, Anderson YE, Mischler E. Cystic fibrosis newborn screening: impact of early screening results on parenting stress. Pediatr Nurs. 1997;23(2):143–151. [PubMed] [Google Scholar]
- 4.Beucher J, Leray E, Deneuville E, et al. Psychological effects of false-positive results in cystic fibrosis newborn screening: a two-year follow-up. J Pediatr. 2010;156(5):771-776, 776.e1. [DOI] [PubMed] [Google Scholar]
- 5.Moran J, Quirk K, Duff AJ, Brownlee KG. Newborn screening for CF in a regional paediatric centre: the psychosocial effects of false-positive IRT results on parents. J Cyst Fibros. 2007;6(3):250–254. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt JL, Castellanos-Brown K, Childress S, et al. The impact of false-positive newborn screening results on families: a qualitative study. Genet Med. 2012;14(1):76–80. [DOI] [PubMed] [Google Scholar]
- 7.Tluczek A, Mischler EH, Bowers B, et al. Psychological impact of false-positive results when screening for cystic fibrosis. Pediatr Pulmonol Suppl. 1991;7:29–37. [DOI] [PubMed] [Google Scholar]
- 8.Waisbren SE, Albers S, Amato S, et al. Effect of expanded newborn screening for biochemical genetic disorders on child outcomes and parental stress. JAMA. 2003;290(19):2564–2572. [DOI] [PubMed] [Google Scholar]
- 9.Howard HC, Knoppers BM, Cornel MC, Wright Clayton E, Sénécal K, Borry P; European Society of Human Genetics; P3G International Paediatric Platform; Human Genome Organisation; PHG Foundation. Whole-genome sequencing in newborn screening? a statement on the continued importance of targeted approaches in newborn screening programmes. Eur J Hum Genet. 2015;23 (12):1593–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis MH, Goldenberg A, Anderson R, Rothwell E, Botkin J. State laws regarding the retention and use of residual newborn screening blood samples. Pediatrics. 2011;127(4):703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics. Serving the family from birth to the medical home: newborn screening: a blueprint for the future—a call for a national agenda on state newborn screening programs. Pediatrics. 2000;106(2 pt 2):389–422. [PubMed] [Google Scholar]
- 12.Campbell ED, Ross LF. Incorporating newborn screening into prenatal care. Am J Obstet Gynecol. 2004;190(4):876–877. [DOI] [PubMed] [Google Scholar]
- 13.Kim S, Lloyd-Puryear MA, Tonniges TF. Examination of the communication practices between state newborn screening programs and the medical home. Pediatrics. 2003;111(2):E120–E126. [DOI] [PubMed] [Google Scholar]
- 14.Larsson A, Therrell BL. Newborn screening: the role of the obstetrician. Clin Obstet Gynecol. 2002; 45(3):697–710. [DOI] [PubMed] [Google Scholar]
- 15.Bonhomme N Integrating consumer perspectives into newborn screening. Genetic Alliance Annual Conference. http://www.resourcerepository.org/documents/1538/integratingconsumerperspectivesintonewbornscreening/. 2009. Accessed July 30, 2009. [Google Scholar]
- 16.Rothwell E, Anderson R, Goldenberg A, et al. Assessing public attitudes on the retention and use of residual newborn screening blood samples: a focus group study. Soc Sci Med. 2012;74(8): 1305–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose NC, Dolan SM. Newborn screening and the obstetrician. Obstet Gynecol. 2012;120(4):908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolan S, Green NS. Newborn screening can readily become part of prenatal care. Am J Obstet Gynecol. 2004;191(6):2180–2181. [DOI] [PubMed] [Google Scholar]
- 19.Diem K Newborn screening—should it be part of prenatal care? Am J Obstet Gynecol. 2004;190(4):874. [DOI] [PubMed] [Google Scholar]
- 20.Committee on Genetics. Newborn screening and the role of the obstetrician-gynecologist. Obstet Gynecol. 2015;125(1):256–260. [DOI] [PubMed] [Google Scholar]
- 21.Faulkner LA, Feuchtbaum LB, Graham S, Bolstad JP, Cunningham GC. The newborn screening educational gap: what prenatal care providers do compared with what is expected. Am J Obstet Gynecol. 2006;194(1):131–137. [DOI] [PubMed] [Google Scholar]
- 22.Hayeems RZ, Miller FA, Little J, et al. Informing parents about expanded newborn screening: influences on provider involvement. Pediatrics. 2009;124(3):950–958. [DOI] [PubMed] [Google Scholar]
- 23.Botkin JR, Rothwell E, Anderson RA, et al. What parents want to know about the storage and use of residual newborn bloodspots. Am J Med Genet A. 2014;164A(11):2739–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genetic Science Learning Center. NBS and DBS movies/brochures. http://gslcutah.org/2013/05/newborn-bloodspot-videos/. Accessed December 2015.
- 25.Grady C. Enduring and emerging challenges of informed consent. N Engl J Med. 2015;372(9):855–862. [DOI] [PubMed] [Google Scholar]
- 26.Botkin JR, Rothwell E, Anderson R, Stark LA, Mitchell J. Public attitudes regarding the use of electronic health information and residual clinical tissues for research. J Community Genet. 2014;5(3):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bombard Y, Miller FA, Hayeems RZ, et al. Citizens’ values regarding research with stored samples from newborn screening in Canada. Pediatrics. 2012;129(2):239–247. [DOI] [PubMed] [Google Scholar]
- 28.Chen DT, Miller FG, Emanuel EJ, et al. Research with stored biological samples. 2005;165:652–655. [DOI] [PubMed] [Google Scholar]
- 29.Wendler D, Emanuel E. The debate over research on stored biological samples: what do sources think? Arch Intern Med. 2002;162(13):1457–1462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


