Abstract
Background:
Hepatorenal dysfunction is a risk factor for mortality in patients with chronic tricuspid regurgitation due to acquired heart disease. Ebstein anomaly is the most common cause of primary tricuspid regurgitation in adults with congenital heart disease, but the prevalence and prognostic implications of hepatorenal dysfunction are unknown in this population.
Objectives:
The purpose of this study was to determine the risk factors and prognostic implications of hepatorenal dysfunction, as measured primarily by the use of model for end-stage liver disease excluding international normalized ratio (MELD-XI score), as well as looking at other associated factors.
Methods:
Retrospective study of adults with Ebstein anomaly that received care at Mayo Clinic, 2003–2018.
Results:
Of 692 patients, median MELD-XI score was 10.2 (9.4–13.3); 53 (8%) died and 3 (0.4%) underwent heart transplant. MELD-XI was an independent predictor of death/transplant (HR 1.32, 1.11–2.06, p<0.001). In the subset of patients with serial MELD-XI scores (n=416), temporal change in MELD-XI score (ΔMELD-XI) was also a predictor of death/transplant. In the subset of patients that underwent tricuspid valve surgery (n=344), a postoperative improvement in MELD-XI score (ΔMELD-XI) was associated with improved long-term survival. Impaired right atrial (RA) reservoir strain and elevated estimated RA pressure were associated with worse baseline MELD-XI and ΔMELD-XI scores.
Conclusions:
Hepatorenal dysfunction is a predictor of mortality in Ebstein anomaly, and RA dysfunction and hypertension are hemodynamic biomarkers that can identify patients at risk for deterioration in hepatorenal function and mortality. These data highlight the prognostic importance of noncardiac organ-system dysfunction, and provides complementary clinical risk stratification metrics for management of these patients.
Keywords: Ebstein anomaly, Hepatorenal dysfunction, Mortality
CONDENSED ABSTRACT
The purpose of this study was to determine the risk factors for, and prognostic implications of hepatorenal dysfunction (as measured by model for end-stage liver disease excluding international normalized ratio, MELD-XI score) in Ebstein anomaly. Of 692 patients, the median MELD-XI score was 10.2 (9.4–13.3), and MELD-XI was an independent predictor of death/transplant. Right atrial reservoir strain and pressure were hemodynamic biomarkers that can identify patients at risk for deterioration in hepatorenal function and mortality. These data highlight the prognostic importance of noncardiac organ-system dysfunction, and provide complementary clinical risk stratification metrics for management of these patients.
Introduction
The cardiovascular system does not function in isolation, but rather, it is intricately connected to other organ-systems, the most important being the kidney and the liver [1, 2]. Because of the complex interaction between the heart, kidney, and liver, the presence of heart failure (cardiac dysfunction) can lead to structural and physiologic dysfunction of the liver and kidney (hepatorenal dysfunction) [3–5]. Tricuspid regurgitation is the most common valvular heart disease associated with hepatorenal dysfunction, and the presence of hepatorenal dysfunction is an independent risk factor for mortality in patients with chronic tricuspid regurgitation and right heart failure due to acquired heart disease [3–7]. As a result, hepatorenal function indices are used for risk stratification in patients undergoing tricuspid valve surgery in the acquired heart disease population [7].
Ebstein anomaly is characterized by primary tricuspid valve dysfunction and right ventricular cardiomyopathy, and it is the most common cause of severe tricuspid regurgitation in patients with congenital heart disease.[8, 9] Chronic tricuspid regurgitation leading to (or exacerbating) right ventricular dysfunction and right heart remodeling sets the stage for right heart failure (systemic venous congestion and low right heart cardiac output) in this population [8–10]. Although the prevalence, hemodynamic determinants, and clinical implications of hepatorenal dysfunction in patients with chronic tricuspid regurgitation due to acquired heart disease have been well described,[3–5] such data are lacking in patients with congenital tricuspid regurgitation. This constitutes an important knowledge gap since the data derived from the acquired heart disease population may not be applicable to the congenital heart disease population because of significant differences in demographic characteristics and disease pathophysiology. The purpose of this study was to determine the risk factors for, and prognostic implications of hepatorenal dysfunction in Ebstein anomaly.
Methods
Study population and study design
We reviewed the MACHD (Mayo Adult Congenital Heart Disease) Registry and identified adults (age >18 years) with Ebstein anomaly that received care at Mayo Clinic Rochester, MN from January 1, 2003 to December 31, 2018. From this cohort, we selected consecutive patients that had at least one comprehensive metabolic panel performed in the outpatient clinic and at least one year follow-up. We excluded patients that were hospitalized 30 days prior to comprehensive metabolic panel, and patients with inadequate echocardiographic images for offline analysis of right and left heart hemodynamics. The Mayo Clinic Institutional Review Board approved the study.
The study objectives were as follows: (1) To identify the baseline clinical risk factors associated with hepatorenal dysfunction, and the relationship between hepatorenal dysfunction at baseline and transplant-free survival during follow-up. For this study objective, we analyzed the data of all patients that met to study inclusion criteria (Cohort #1). (2) To evaluate temporal change in hepatorenal function, and the relationship between temporal changes in hepatorenal function and transplant-free survival during follow-up. For this objective, we analyzed the data of patients that had 2 comprehensive metabolic panels performed at least 24 months apart without any cardiac surgical or transcatheter procedures between both tests (Cohort #2). (3) To determine the extent to which hepatorenal function improved after tricuspid valve surgery, and the relationship between postoperative improvements in hepatorenal function and transplant-free survival. For this objective, we analyzed the data of patients that had preoperative comprehensive metabolic panel (within 3 months prior to surgery) and postoperative comprehensive metabolic panel (at least 12 months after surgery) (Cohort #3).
Assessment of hepatorenal function
Hepatorenal function was assessed using the model for end-stage liver disease excluding international normalized ratio (MELD-XI) score. MELD-XI score was calculated as: 5.11× ln (serum total bilirubin in mg/dL) + 11.76 × ln (serum creatinine in mg/dL) + 9.44.[11] In order to avoid negative scores, a lower limit of total bilirubin and creatinine was set at 1.0 mg/dl.[11] We chose MELD-XI score as the primary measure of hepatorenal function, it allows for accurate assessment of hepatorenal function in all patients including those on vitamin K antagonist.
As secondary measures of hepatorenal function, we calculated the MELD score, MELD score substituting serum albumin for international normalized ratio (MELD-albumin), and MELD score with serum sodium (MELD-Na). MELD score was calculated as 3.8*loge(serum bilirubin [mg/dL]) + 11.2*loge(INR) + 9.6*loge(serum creatinine [mg/dL]) + 6.4.[12] MELD-Na was calculated as MELD + 1.32 * (137-Na) − [0.033*MELD * (137-Na)].[13] MELD-albumin was calculated as 11.2× [ln (1 + (4.1-albumin)] +3.78 × ln (serum total bilirubin in mg/dL) + 9.57 × ln (serum creatinine in mg/dL) + 9.6.43.[7] Of note, MELD score and MELD-Na score were only calculated in patients who were not on vitamin K antagonist and had international normalized ratio data available (n=408). These indices were chosen because of known association with clinical outcomes in prior studies [7, 12, 13].
Other clinical variables
In order to identify the determinants of hepatorenal dysfunction, we collected and analyzed baseline clinical variables at the time of the first comprehensive metabolic panel. The variables analyzed include demographic characteristics, surgical data, and echocardiographic indices of cardiac structure, function, and hemodynamics. All echocardiographic images were reviewed and offline measurements performed. Atrial and ventricular function were assessed using speckle tracking strain imaging, and right atrial (RA) pressure was estimated based on the size and collapsibility of the inferior vena cava.[14–16] Transplant free survival was ascertained through a detailed review of the medical records and Accurint database, which is an institutional proprietary database for verification of mortality data.
Statistical Analysis
Data were presented as mean ± standard deviation, median (interquartile range), counts (%) or estimates (95% confidence interval), and between-group comparisons were performed using chi-square test, t-test and Wilcoxon rank sum test as appropriate. Logistic regression and receiver operating characteristic (ROC) curve were used to determine the optimal cut-off point for MELD-XI to detect patients at risk for death/transplant with the optimal balance between sensitivity and specificity based on Youden index. Time-to-event analyses were performed using the Kaplan Meier method and Cox regression. First, a univariate Cox model was developed using the following variables: patient demographics, type of tricuspid valve surgery, comorbidities, laboratory data, medications, and echocardiographic indices. Next, the variables with p<0.1 on univariate analysis were incorporated into a multivariate regression model using forward stepwise selection, and a p<0.1 was required to remain in the models. We checked the proportional hazard assumptions using Schoenfeld residual, and these assumptions were confirmed by a non-significant relationship between residuals and time. Because some of the patients underwent tricuspid valve surgery during follow-up at different times, tricuspid valve surgery was modeled as a time-dependent covariate in the proportional hazards regression models.
Exploratory analyses were performed to determine whether renal dysfunction was associated with death/transplant (independent of hepatic dysfunction), and whether the other secondary indices of hepatorenal function (MELD, MELD-albumin and MELD-Na) were associated with death/transplant. For each of these exploratory analyses, we substituted MELD-XI score in the multivariate model with estimated GFR, MELD, MELD-albumin and MELD-Na respectively. All statistical analyses were performed with JMP and SAS software (versions 14.0 and 9.4 respectively; SAS Institute Inc, Cary NC).
Results
Cohort #1: All patients
Supplemental Figure 1 shows a flowchart of all patients (Cohort #1), patients with serial MELD-XI scores without cardiac surgical or transcatheter interventions between tests (Cohort #2), and patients with preoperative and postoperative MELD-XI scores (Cohort #3). Cohort #1 comprise of 692 patients that met the study inclusion criteria, and the mean age at the time of baseline MELD-XI score assessment was 40±15 years and 281 (41%) were males. The baseline clinical characteristics and echocardiographic indices are shown in Table 1.
Table 1:
Baseline Characteristics (n=692)
| Age, years | 40±15 |
| Male | 281 (41%) |
| Body mass index, kg/m2 | 27±6 |
| Prior tricuspid valve surgery | 126 (18%) |
| Oxygen saturation at rest (%) | 95±2 |
| Comorbidities | |
| Hypertension | 139 (20%) |
| Diabetes | 32 (5%) |
| History of atrial arrhythmia | 301 (44%) |
| History of ventricular arrhythmia | 32 (5%) |
| Medications | |
| Loop diuretics | 146 (21%) |
| Beta blockers | 167 (24%) |
| ACEI/ARB | 129 (19%) |
| Aldosterone antagonist | 43 (6%) |
| Warfarin | 184 (27%) |
| Direct oral anticoagulant | 31 (5%) |
| Echocardiography | |
| RA reservoir strain, % | 30±11 |
| RV global longitudinal strain | 19±5 |
| TAPSE, mm | 25±9 |
| RV s’, cm/s | 14±6 |
| IVC diameter-expiration, mm | 19±4 |
| IVC diameter-inspiration, mm | 10±3 |
| IVC collapsibility,% | 48±11 |
| Estimated RA pressure, mmHg | 8±4 |
| ≥Moderate TR severity | 506 (77%) |
| TR velocity, m/s | 2.4±0.4 |
| Estimated RVSP, mmHg | 33±8 |
| LA reservoir strain, % | 32±9 |
| LV end-diastolic volume index, ml/m2 | 49±11 |
| LV end-systolic volume index, ml/m2 | 20±7 |
| LV stroke volume index, ml/m2 | 35±7 |
| LV cardiac index, L/min/m2 | 2.6±0.7 |
| LV ejection fraction, % | 59±8 |
| LV global longitudinal strain, % | 22±4 |
| Lateral mitral E/e’ | 7±3 |
ACEI: Angiotensin converting enzyme inhibitor; ARB: Angiotensin II receptor blockers; RA: Right atrium; RV: Right ventricle; TR: Tricuspid regurgitation; LV: Left ventricle; E/e’: ratio of mitral inflow pulsed wave Doppler early velocity to mitral annular tissue Doppler early velocity
Hepatorenal function indices
Table 2 shows laboratory indices of hepatorenal function. The median estimated glomerular filtration rate (GFR) was 85 (69–101) ml/min/1.73m2, and 92 (13%) had estimated GFR was <60 ml/min/1.73m2, consistent with stage III chronic kidney disease. The median MELD-XI score was 10.2 (9.4–13.3), and MELD-XI score correlated with age, sex, atrial arrhythmia history, and right and left heart hemodynamic indices (Supplemental Table 1). However, on multivariate analysis, estimated RA pressure had the strongest correlation with MELD-XI score (standardized β=0.64), followed by, left ventricular stroke volume index (standardized β=0.38), RA reservoir strain (standardized β=0.32), and age (standardized β=0.21) (Table 3).
Table 2:
Hepatorenal Function Indices (n=692)
| Laboratory indices | |
|---|---|
| Hemoglobin, g/dl | 13.4±1.5 |
| Platelet, x109/l (normal 150 – 450) | 224±65 |
| Creatinine, mg/dl (normal 0.6 – 1.1) | 1.1 (0.8–1.3) |
| Estimated GFR, ml/min/1.73m2 | 85 (69–101) |
| NT proBNP, pg/ml | 103 (56–416) |
| Albumin, g/dl (normal 3.5 – 5.0) | 4.1 (3.7–4.6) |
| AST, U/l (normal 8 – 43) | 37 (29–54) |
| ALT, U/l (normal 7 – 45) | 36 (27 –58) |
| ALP, U/l (normal 37 – 98) | 89 (69–136) |
| Total bilirubin, mg/dl (normal 0.1 – 1.0) | 0.9 (0.5–1.6) |
| Direct bilirubin, mg/dl (normal 0 – 0.03) | 0.3 (0.1–0.5) |
| INR(normal <1.2) | 1.3 (1.1–1.6) |
AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP; Alkaline phosphatase; INR: International normalized ratio
Table 3:
Multivariate Model Showing Risk Factors for Hepatorenal Dysfunction (MELD-XI score) (R2=0.37, p<0.001)
| β coefficient±SE | p | |
|---|---|---|
| Age, per year | 0.08±0.02 | 0.006 |
| Male sex | 0.29±0.09 | 0.007 |
| RA reservoir strain, per % | −0.22±0.13 | 0.01 |
| Estimated RA pressure, per 1 mmHg | 0.52±0.16 | <0.001 |
| LV stroke volume index, per 1 ml/m2 | −0.28±0.11 | 0.04 |
RA: Right atrium; LV: Left ventricle; SE: Standard error
Outcomes
The median follow-up was 79 (41–102) months, and during this period, 53 (8%) patients died and 3 (0.4%) underwent heart transplant. The median age at the time of death was 62 (46–70) years, and the cause of death was end-stage heart failure (n=18), arrhythmia/sudden cardiac death (n=6), postoperative death after cardiac surgery (n=5), stroke/intracranial hemorrhage (n=5), malignancy (n=2), sepsis/multi-organ failure (n=6), end-stage renal failure (n=4), mixed etiology (n=3), and unknown etiology (n=4). The indication for heart transplant was end-stage heart failure in all 3 cases. The composite endpoint of death/ transplant occurred in 56 (8%) patients.
The MELD-XI score was associated with death/transplant (HR 1.38, 1.17–2.83, P<0.001) on univariate analysis, and remained an independent risk factor for death/transplant on multivariate analysis (HR 1.32, 1.11–2.06, p<0.001; Table 4). ROC curve analysis show that MELD-XI >11 provided the optimal cut-off point to detect patients at risk for death/transplant (area under the curve 0.72). As compared to low MELD-XI score (≤11), high MELD-XI score (>11) was associated with 3-fold increase in risk of death/transplant (HR 3.16, 1.54–6.47, P<0.001) on multivariate analysis.
Table 4:
Multivariate Model Showing Predictors of Death and/or Transplant
| HR (95%CI) | p | |
|---|---|---|
| MELD-XI score, per unit change | 1.32 (1.11–2.06) | <0.001 |
| Age, per year | 1.03 (1.01–1.05) | 0.03 |
| Loop diuretics | 1.97 (1.08–3.37) | 0.004 |
| Estimated RA pressure, per 1 mmHg | 1.14 (1.06–1.22) | <0.001 |
MELD: Model for end-stage liver disease; RA: Right atrial pressure; HR: Hazard ratio; CI: Confidence interval
Exploratory analyses
Exploratory analyses showed that estimated GFR (HR 1.22, 1.09–2.11, p<0.001 per 10 unit decline in GFR), MELD (HR 1.12, 1.06–2.04, p=0.01), MELD-Na (HR 1.14, 1.04–2.12, p=0.02) and MELD-albumin (HR 1.18, 1.10–1.95, p<0.001) were risk factors for death/transplant.
Cohort #2: Temporal decline in hepatorenal function
A total of 416 patients met the inclusion criteria for this analysis (Supplemental Figure 1). As compared to the rest of the cohort, the patients in cohort #2 lower prevalence of atrial arrhythmia, but otherwise both groups had similar clinical characteristics (Supplemental Table 3). The mean age was 39±13 years, 161 (39%) were males, and 61 (15%) had Ebstein anomaly repair (tricuspid valve surgery) prior to the baseline assessment of MELD-XI score assessment. There was no significant difference in the number of patients that were on renin-angiotensin-aldosterone system antagonists at the time of baseline MELD-XI score assessment as compared to subsequent MELD-XI score assessment (71/416 [17%] vs 68/416 [16%], p=0.8).
The median MELD-XI score was 9.8 (9.4–12.1). Using the same cut-off point derived from the ROC curve analysis of Cohort #1, 337 (81%) of the patients in Cohort #2 had low MELD-XI score (MELD-XI ≤11) while 79 (19%) had high MELD-XI score (MELD-XI >11). The median interval between the baseline and follow-up hepatorenal function assessment was 27 (25–32) months, and during this period, the median MELD-XI score increased from 9.8 (9.4–12.1) to 10.7 (9.8–13.2), and the median change in MELD-XI (ΔMELD-XI) was 0.9 (95% confidence interval 0.5–1.3). ΔMELD-XI was associated with age (β±SE 0.09±0.02, p=0.01) and male sex (β±SE 0.14±0.08, p=0.01), RA reservoir strain (β±SE −0.17±0.98, p=0.04 and estimated RA pressure (β±SE 0.28±0.15, p<0001), but not to tricuspid regurgitation severity. This suggests a more rapid deterioration in hepatorenal function in older patients, males, and in patients with more severe RA dysfunction and RA hypertension. Concordant with the temporal increase in MELD-XI score, we also observed an increase in aspartate aminotransferase (33±4 vs 41±6 U/l, p<0.001) and alanine aminotransferase (36±6 vs 42±7 U/l, p=0.02), and these changes were consistent with deterioration in hepatorenal function. However the temporal change in alkaline phosphatase (82±19 vs 89±24 U/l, p=0.07), platelet count (262±61 vs 248±52 ×109/l, p=0.4) and albumin (4.4±40.3 vs 4.1±0.4 g/dl, p=0.09) did not reach statistical significance.
Of the 337 patients with low MELD-XI score at baseline, 65 (19%) developed high MELD-XI score, while all 79 patients that had high MELD-XI score at baseline also had high score during follow-up (Central Illustration). As compared to the patients that remained in the low MELD-XI score group at the time of follow-up test, those that developed high MELD-XI score had a lower transplant-free survival (Central Illustration). This suggests that temporal change in hepatorenal function provides additional prognostication information beyond that of baseline hepatorenal function alone.
Central illustration: Flowchart and Kaplan Meier curves showing the impact of deterioration in hepatorenal function on survival.
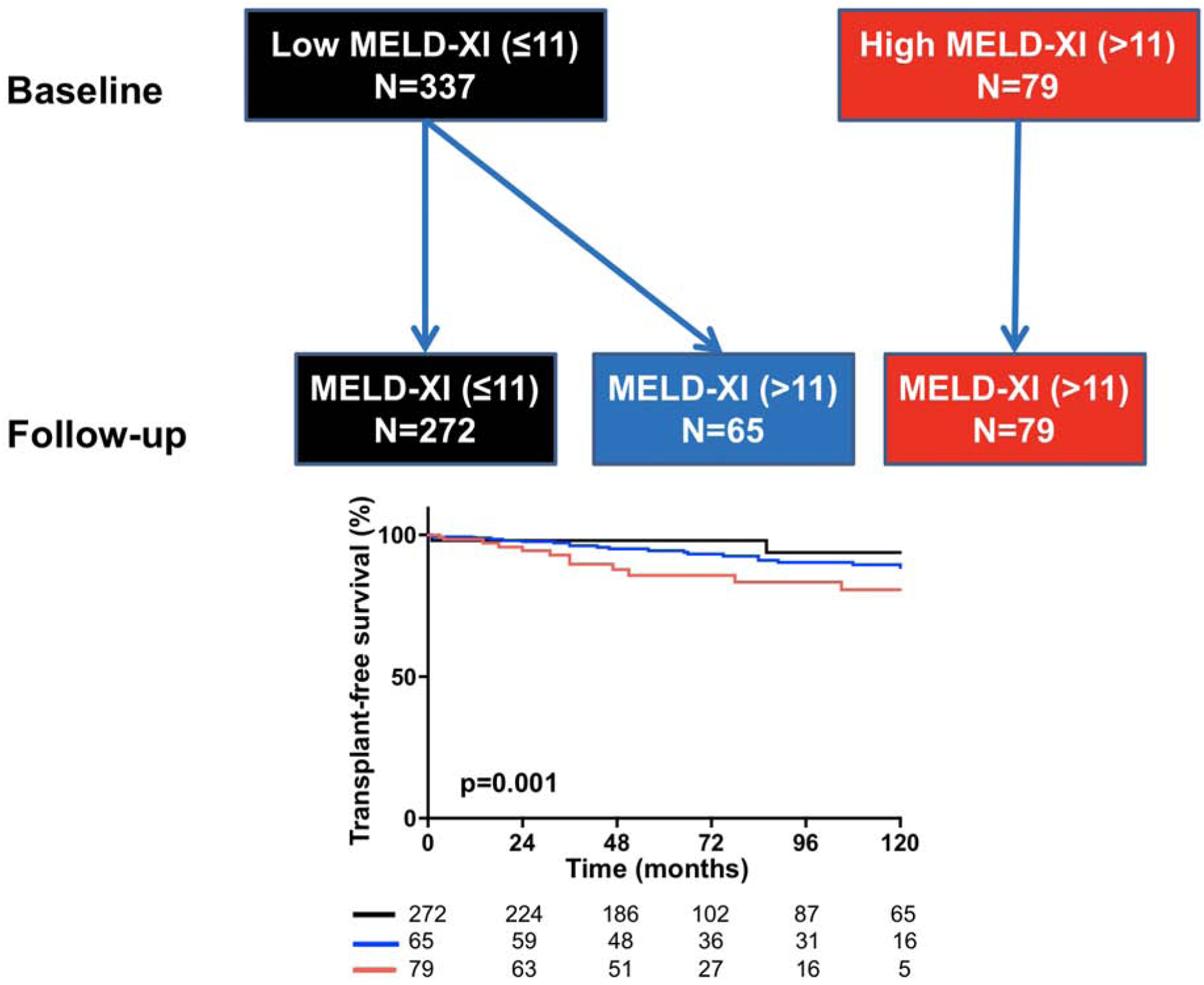
Top: Flowchart showing patients that had low MELD-XI score at baseline and during follow-up (black), patients that moved from low to high MELD-XI score during follow-up (blue), and patients that had high MELD-XI score at baseline and during follow-up (red). Bottom: Compared to the patients that remained in the low MELD-XI class (black), those that developed high MELD-XI score during follow-up (blue) had lower 210-year transplant-free survival (97% vs 86%, p=0.006). Patients with high MELD-XI score at baseline and during follow-up had the lowest survival of the 3 groups (78%). p=0.01 represent curve comparison across all 3 groups using log-rank test.
Cohort #3: Postoperative changes in hepatorenal function
A total of 344 patients met the inclusion criteria for this analysis (Supplemental Figure 1). As compared to the rest of the cohort, the patients in cohort #3 lower RA reservoir strain but otherwise both groups had similar clinical characteristics (Supplemental Table 4). The mean age at the time of baseline MELD-XI score assessment was 41±16 years and 137 (40%) were males. Of the 344 patients, 246 (72%) had tricuspid valve repair while 98 (28%) had tricuspid valve replacement with a bioprosthesis. None of the patients had cavopulmonary connection (1.5 ventricle repair).
The median MELD-XI score was 10.7 (10.1–14.3) at the time of surgery, and 188 (55%) had low MELD-XI score while 156 (45%) had high MELD-XI score. The median interval between the baseline and follow-up hepatorenal function assessment was 15 (12–17) months, and during this period, the median MELD-XI score decreased from 10.7 (10.1–14.3) to 9.6 (9.4–12.7), and the ΔMELD-XI was −1.1 (95% confidence interval −0.6 – −1.7). ΔMELD-XI was associated with preoperative RA reservoir strain (β±SE 0.28±0.11, p<0001) and estimated RA pressure (β±SE −0.33±0.21, p<0001) but not to the type of tricuspid valve surgery (repair vs replacement) or residual tricuspid regurgitation.
Of the 156 patients with high MELD-XI score at baseline, 52 (34%) patients had reduction in postoperative MELD-XI score to be reclassified as low MELD-XI, while all 188 patients that had low MELD-XI at baseline also had low score during follow-up (Figure 1). As compared to the patients that remain in the high MELD-XI score during follow-up, those that had improvement in MELD-XI score had better transplant-free survival (Figure 1). This suggests that tricuspid valve surgery improves hepatorenal function in some patients, and a postoperative improvement in hepatorenal function was associated with improved outcomes.
Figure 1:
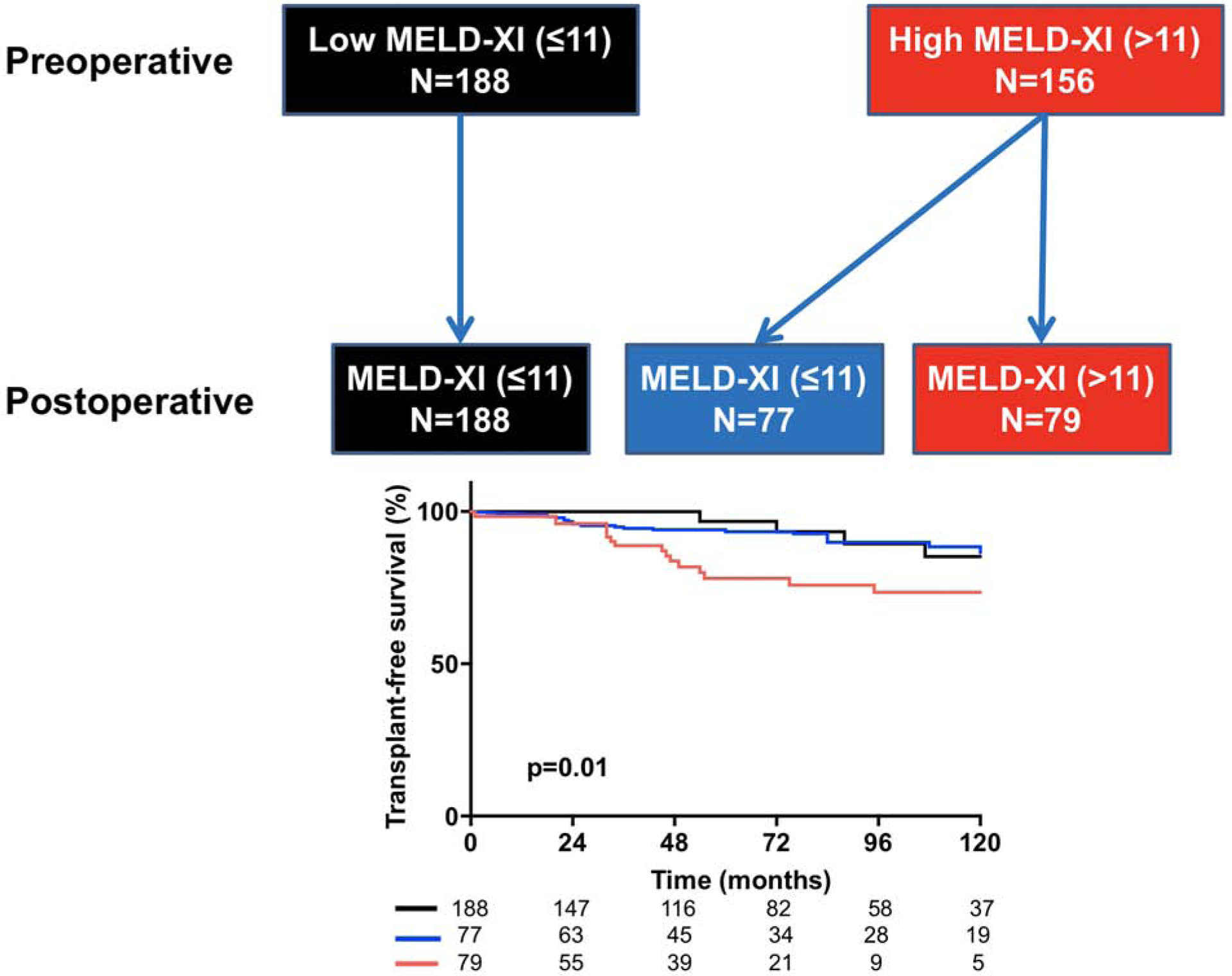
Flowchart and Kaplan Meier curves showing the impact of postoperative change in hepatorenal function on survival. Top: Flowchart showing patients that had high MELD-XI score at preoperative and postoperative assessments (red), patients that postoperative improvement in MELD-XI score, i.e. moved from high to low MELD-XI score after surgery (blue), and patients that had low MELD-XI score at preoperative and postoperative assessments (black). Bottom: Compared to the patients that remained in the high MELD-XI class (red), those that had postoperative reduction in MELD-XI score (blue) had better 10-year transplant-free survival (71% vs 86%, p=0.001). However 10-year transplant-free survival was similar between the patients with postoperative improvement in MELD-XI score (blue) and those that remained in the low MELD-XI group (black) (86% vs 84%, p=0.7). p=0.04 represent curve comparison across all 3 groups using log-rank test.
Discussion
There is a complex pathophysiologic interaction between the heart, kidney, and liver, and as a result, a disease process in one organ-system can lead to structural and functional changes in the other organ-systems.[1, 2] Hepatorenal dysfunction is a well-known complication in patients with chronic tricuspid regurgitation and right heart failure due to acquired heart disease, and it is a risk factor for mortality.[3–7] However similar outcome data are lacking in patients with chronic tricuspid regurgitation due to congenital heart disease. Based on a comprehensive analysis of clinical, hemodynamic, and laboratory data of a large cohort of adults with Ebstein anomaly, we report the following results: (1) The hemodynamic risk factors for hepatorenal dysfunction (as measured by MELD-XI score) were RA dysfunction, RA hypertension, and low stroke volume, and hepatorenal dysfunction was an independent predictor of mortality. (2) Temporal deterioration in hepatorenal function was associated with reduced transplant-free survival, and thus provides additional prognostication information beyond that of baseline hepatorenal function alone. (3) Tricuspid valve surgery improved hepatorenal function in a third of patients, and a postoperative improvement in hepatorenal function was associated with improved outcomes.
The MELD score (and its subsequent modifications such as MELD-XI) was originally designed for risk stratification and prioritization of organ allocation in patients with end-stage liver disease.[7, 12, 13] It provides a composite metric of hepatorenal function, and it is currently used for risk stratification and prognostication in patients with different types of cardiac pathologies.[3–7, 17] The MELD-XI score has been studied in adults with congenital heart disease, and it is associated with mortality after cardiac surgery.[18, 19] With regards to specific congenital heart disease diagnosis, the available data are limited to studies conducted in patients with Fontan palliation, which is a unique physiology characterized by chronic systemic venous congestion and low cardiac output.[20] Patients with Ebstein anomaly, especially those with advanced right heart dysfunction, also have systemic venous congestion and low cardiac output, placing them at risk for hepatorenal dysfunction.
Although previous studies have established the prognostic implications of hepatorenal dysfunction in patients with congenital heart disease,[18–21] the current study provides several novel results that can improve clinical practice in this population. First, this study showed that RA dysfunction, increased estimated RA pressure, and low stroke volume were associated with hepatorenal dysfunction at baseline, and can also predict temporal deterioration in hepatorenal function during follow-up. Hence they can be used as hemodynamic biomarkers to identify patients at risk for deterioration in hepatorenal function and mortality during follow-up, thus enabling the clinician to consider an early referral for tricuspid valve surgery if significant tricuspid regurgitation is present or heart transplantation if conventional cardiac surgery is not feasible. Another important novel finding is that postoperative improvement in hepatorenal function indices after tricuspid valve surgery occurred in some patients (especially those with less severe RA dysfunction and hypertension at the time of surgery), and was associated with improved outcome. Conversely, patients without postoperative improvement in hepatorenal function were at higher risk for mortality during follow-up and perhaps should be considered for heart transplant.
The severity of tricuspid regurgitation at baseline, type of tricuspid valve surgery, and residual tricuspid regurgitation after surgery were not associated with hepatorenal function. This suggests that, while tricuspid regurgitation is the primary lesion responsible for right heart volume overload, the deleterious effect of chronic volume overload is likely mediated through RA remodeling and dysfunction. This will, in turn, result in systemic venous congestion with reduced cardiac output, compromised end-organ perfusion and ultimately injury to the liver and the kidney. This is consistent with the pathophysiologic mechanisms for cardiorenal syndrome and cardiac cirrhosis already described in patients with chronic heart failure [1, 2].
Limitations
This is a retrospective single center study, and as a result it is subject to the limitations inherent in this type of study design. The association between hepatorenal function and mortality could have been influenced by temporal changes in medical therapy, effect modification from comorbidities, or other potential confounders that were not controlled for in the regression models. We did not have liver and kidney imaging data to determine whether hepatorenal function as measured by MELD-XI correlated with structural changes in these organs. We relied on echocardiographic estimation of RA pressure as a measure of RA hypertension; however the correlation between estimated RA pressure and invasively measured RA pressure has not been studied in this population.
Conclusions
Hepatorenal dysfunction at baseline or temporal decline in hepatorenal function during follow-up are risk factors for mortality, and hence can help identify high risk patients that may benefit from early referral for tricuspid valve surgery or heart transplant since guideline directed medical therapy has not been shown to be effective in this population.. The presence of RA dysfunction and low stroke volume are hemodynamic biomarkers that can identify patients at risk for deterioration in hepatorenal function and mortality. The patients without postoperative improvement in hepatorenal function indices have higher risk for mortality, and perhaps should be closely monitored to determine optimal time for transplant evaluation. These data highlight the prognostic importance of noncardiac organ-system dysfunction, and provides complementary clinical risk stratification metrics for management of these patients. Further studies are required to determine whether integrating hepatorenal function indices into clinical decision making will result in improved long-term survival in this population.
Supplementary Material
Clinical Perspectives.
Competency in Medical Knowledge: Ebstein anomaly is the most common congenital cause of primary tricuspid regurgitation in adults. Hepatorenal dysfunction, as measured using the model for end-stage liver disease excluding international normalized ratio (the MELD-XI score), is a predictor of mortality that complements right atrial dysfunction, hypertension and low stroke volume, which are markers of hemodynamic dysfunction in patients with Ebstein anomaly. Translational Outlook: Further studies are needed to determine whether incorporating indices of hepatorenal dysfunction into clinical management strategies can improve long-term survival in patients with Ebstein anomaly.
Funding:
Dr. Egbe is supported by National Heart, Lung, and Blood Institute (NHLBI) grant K23 HL141448. The MACHD Registry is supported by the Al-Bahar Research grant.
Abbreviations:
- RA
right atrium
- MELD
model for end-stage liver disease
- GFR
glomerular filtration rate
- ROC
receiver operating characteristic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: none
References
- 1.Xanthopoulos A, Starling RC, Kitai T, Triposkiadis F. Heart Failure and Liver Disease: Cardiohepatic Interactions. JACC Heart Fail. 2019. February;7(2):87–97. [DOI] [PubMed] [Google Scholar]
- 2.Rangaswami J, Bhalla V, Blair JEA, Chang TI, Costa S, Lentine KL, Lerma EV, Mezue K, Molitch M, Mullens W, Ronco C, Tang WHW, McCullough PA; American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Clinical Cardiology. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation. 2019. April 16;139(16):e840–e878. [DOI] [PubMed] [Google Scholar]
- 3.Maeder MT, Holst DP, Kaye DM. Tricuspid regurgitation contributes to renal dysfunction in patients with heart failure. J Card Fail. 2008. December;14(10):824–30. [DOI] [PubMed] [Google Scholar]
- 4.Aziz TM, Saad RA, Burgess MI, Campbell CS, Yonan NA. Clinical significance of tricuspid valve dysfunction after orthotopic heart transplantation. J Heart Lung Transplant. 2002. October;21(10):1101–8. [DOI] [PubMed] [Google Scholar]
- 5.Lau GT, Tan HC, Kritharides L. Type of liver dysfunction in heart failure and its relation to the severity of tricuspid regurgitation. Am J Cardiol. 2002. December 15;90(12):1405–9. [DOI] [PubMed] [Google Scholar]
- 6.Allen LA, Felker GM, Pocock S, McMurray JJ, Pfeffer MA, Swedberg K, Wang D, Yusuf S, Michelson EL, Granger CB; CHARM Investigators. Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail. 2009. February;11(2):170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Liu YX, Seto WK, Wu MZ, Yu YJ, Lam YM, Au WK, Chan D, Sit KY, Ho LM, Tse HF, Yiu KH. Prognostic Value of Hepatorenal Function By Modified Model for End-stage Liver Disease (MELD) Score in Patients Undergoing Tricuspid Annuloplasty. J Am Heart Assoc. 2018. July 13;7(14):e009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holst KA, Connolly HM, Dearani JA. Ebstein’s Anomaly. Methodist Debakey Cardiovasc J. 2019. Apr-Jun;15(2):138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qureshi MY, Sommer RJ, Cabalka AK. Tricuspid Valve Imaging and Intervention in Pediatric and Adult Patients With Congenital Heart Disease. JACC Cardiovasc Imaging. 2019. April;12(4):637–651. [DOI] [PubMed] [Google Scholar]
- 10.Fujioka T, Kühn A, Sanchez-Martinez S, Bijnens BH, Hui W, Slorach C, Roehlig C, Mertens L, Vogt M, Friedberg MK. Impact of Interventricular Interactions on Left Ventricular Function, Stroke Volume, and Exercise Capacity in Children and Adults With Ebstein’s Anomaly. JACC Cardiovasc Imaging. 2019. May;12(5):925–927. [DOI] [PubMed] [Google Scholar]
- 11.Heuman DM, Mihas AA, Habib A, Gilles HS, Stravitz RT, Sanyal AJ, Fisher RA. MELD-XI: a rational approach to “sickest first” liver transplantation in cirrhotic patients requiring anticoagulant therapy. Liver Transpl. 2007. January;13(1):30–7. [DOI] [PubMed] [Google Scholar]
- 12.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001. February;33(2):464–70. [DOI] [PubMed] [Google Scholar]
- 13.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008. September 4;359(10):1018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010. July;23(7):685–713; quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 15.Akazawa Y, Fujioka T, Kühn A, Hui W, Slorach C, Roehlig C, Mertens L, Vogt M, Friedberg MK. Right Ventricular Diastolic Function and Right Atrial Function and Their Relation With Exercise Capacity in Ebstein Anomaly. Can J Cardiol. 2019. December;35(12):1824–1833. [DOI] [PubMed] [Google Scholar]
- 16.Egbe AC, Connolly HM, Miranda WR, Scott CG, Borlaug BA. Prognostic implications of inferior vena cava haemodynamics in ambulatory patients with tetralogy of Fallot. ESC Heart Fail. 2020. June 26;7(5):2589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MS, Kato TS, Farr M, Wu C, Givens RC, Collado E, Mancini DM, Schulze PC. Hepatic dysfunction in ambulatory patients with heart failure: application of the MELD scoring system for outcome prediction. J Am Coll Cardiol. 2013. June 4;61(22):2253–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams ED, Jackson NJ, Young T, DePasquale EC, Reardon LC. Prognostic utility of MELD-XI in adult congenital heart disease patients undergoing cardiac transplantation. Clin Transplant. 2018. June;32(6):e13257. [DOI] [PubMed] [Google Scholar]
- 19.Konno R, Tatebe S, Sugimura K, Satoh K, Aoki T, Miura M, Suzuki H, Yamamoto S, Sato H, Terui Y, Miyata S, Adachi O, Kimura M, Saiki Y, Shimokawa H. Prognostic value of the model for end-stage liver disease excluding INR score (MELD-XI) in patients with adult congenital heart disease. PLoS One. 2019. November 19;14(11):e0225403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assenza GE, Graham DA, Landzberg MJ, Valente AM, Singh MN, Bashir A, Fernandes S, Mortele KJ, Ukomadu C, Volpe M, Wu F. MELD-XI score and cardiac mortality or transplantation in patients after Fontan surgery. Heart. 2013. April;99(7):491–6. [DOI] [PubMed] [Google Scholar]
- 21.Dimopoulos K, Diller GP, Koltsida E, Pijuan-Domenech A, Papadopoulou SA, Babu-Narayan SV, Salukhe TV, Piepoli MF, Poole-Wilson PA, Best N, Francis DP, Gatzoulis MA. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008. May 6;117(18):2320–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


