Abstract
Background
In cryptococcal meningitis phase 2 clinical trials, early fungicidal activity (EFA) of Cryptococcus clearance from cerebrospinal fluid (CSF) is used as a surrogate endpoint for all-cause mortality. The Food and Drug Administration allows for using surrogate endpoints for accelerated regulatory approval, but EFA as a surrogate endpoint requires further validation. We examined the relationship between rate of CSF Cryptococcus clearance (EFA) and mortality through 18 weeks.
Methods
We pooled individual-level CSF data from 3 sequential cryptococcal meningitis clinical trials conducted during 2010–2017. All 738 subjects received amphotericin + fluconazole induction therapy and had serial quantitative CSF cultures. The log10-transformed colony-forming units (CFUs) per mL CSF were analyzed by general linear regression versus day of culture over the first 10 days.
Results
Mortality through 18 weeks was 37% for EFA > = 0.60 (n = 170), 36% for 0.40–0.59 (n = 182), 39% for 0.30–0.39 (n = 112), 35% for 0.20–0.29 (n = 87), and 50% for those with EFA < 0.20 CFU/mL/day (n = 187). The hazard ratio for 18-week mortality, comparing those with EFA < 0.20 to those with EFA > = 0.20, was 1.60 (95% confidence interval, 1.25, 2.04; P = .002). The lowest EFA group had lower median CD4 T-cell counts (P < .01) and lower proportion of patients with CSF pleocytosis (P < .001).
Conclusions
EFA is associated with all-cause mortality in cryptococcal meningitis. An EFA threshold of > = 0.20 log10 CFU/mL/day was associated with similar 18-week mortality (37%) compared to 50% mortality with EFA < 0.20. This EFA threshold may be considered a target for a surrogate endpoint. This builds upon existing studies to validate EFA as a surrogate endpoint.
Keywords: cryptococcus, meningitis, cryptococcal meningitis, early fungicidal activity, surrogate endpoint
In cryptococcal meningitis trials, early fungicidal activity (EFA) reflecting Cryptococcus cerebrospinal fluid (CSF) clearance is used as a surrogate endpoint. We examined the relationship between EFA and survival in 738 Africans receiving amphotericin-based therapy: EFA < 0.20 log10 CFU/mL/day has decreased 18-week survival.
Cryptococcal meningitis causes 15% of AIDS-related deaths globally [1]. Despite this significant disease burden, Cryptococcus remains a neglected disease [2]. Antifungal therapies exist, but less toxic, more effective therapies are needed. The most recent US Food and Drug Administration (FDA) approval for antifungals effective for Cryptococcus date back to 1997 for Ambisome® and 1990 for fluconazole [3, 4].
One hindrance in the search for new therapeutic approaches is the length of time that clinical trials can take to reach a conclusion due to the use of all-cause mortality as a primary endpoint. To decrease the time required to determine the effectiveness of new treatments, the FDA allows for using surrogate endpoints. FDA defines a “surrogate endpoint’ to mean a marker, such as a laboratory measurement, radiographic image, physical sign, or other measure, that is not itself a direct measurement of clinical benefit, and a) is known to predict clinical benefit and could be used to support traditional approval of a drug or biological product; or b) is reasonably likely to predict clinical benefit and could be used to support the accelerated approval of a drug or biological product. From a regulatory perspective, endpoints are grouped as validated surrogated endpoints, reasonably likely surrogated endpoints, or candidate surrogated endpoints.
With neglected diseases, surrogate endpoints are critical to decrease costs and accelerate development of new therapies. In tuberculosis, a validated surrogate endpoint used for accelerated FDA approval has been “time to sputum culture conversion to negative.” In cryptococcal meningitis, a surrogate endpoint used in phase II trials has been early fungicidal activity (EFA). EFA is a quantitative measure of the rate of fungal Cryptococcus yeast clearance from the cerebrospinal fluid (CSF). Several studies have reported a relationship between EFA and all-cause mortality through 10 weeks when analyzing pooled individual-level or group-level data [5–7]. A limitation has been the need to pool data from different treatment regimens and across different countries.
We sought to investigate the association between EFA and all-cause mortality using a large cohort of cryptococcal meningitis research participants enrolled in sequential clinical trials. Participants in these sequential trials received the same background induction regimen of amphotericin B deoxycholate and fluconazole. We examined the relationship between EFA and survival through 18 weeks.
METHODS
Study Population
Three sequential clinical trials were pooled in this analysis: the “Cryptococcal Optimal ART Timing” (COAT; n = 162) trial [8], the “Adjunctive Sertraline for the Treatment of HIV-associated Cryptococcal Meningitis” (ASTRO-CM; n = 179) phase 2 pilot trial [9], and ASTRO-CM randomized trial (n = 397) [10]. Participants were Ugandan adults living with human immunodeficiency virus (HIV) who presented to Mulago National Referral Hospital, Kampala or Mbarara Regional Referral Hospital, between November 2010 and June 2017 and who were diagnosed with cryptococcal meningitis by CSF cryptococcal antigen. The COAT trial additionally included participants from GF Jooste Hospital in Cape Town, South Africa.
CSF Quantitative Cultures and EFA Calculation
During the COAT trial, CSF quantitative cultures were performed on days 1, 7, 14, and additionally as needed for intracranial pressure control [11]. During the ASTRO-CM trials, cultures were performed on days 1, 3, 7, 10–14, and additionally as needed for intracranial pressure control. In brief, quantitative Cryptococcus CSF cultures were performed with a series of five 1:10 serial dilutions on the CSF, as previously described [12]. The colony-forming units (CFUs) per mL of CSF were analyzed by general linear regression on log10 CFU/mL versus day of CSF culture. The rate of fungal clearance from the CSF in units of log10CFU/mL/day was calculated from the slope of the fit line for each patient [5, 12]. To minimize survival bias, we limited the contributions of quantitative cultures to the first 10 days of antifungal therapy.
Statistical Analysis
We divided subject EFA measurements into 4 groups of: 0.40 log10 CFU/mL per day or greater, 0.30 to 0.39, 0.20 to 0.29, and < 0.20 or less. These breakpoints were chosen for ease of interpretation in 0.10 log10 CFU/mL/day increments. A further analysis was performed with additional breakpoints above 0.40 (of 0.40 to 0.59 and 0.60 or greater). For the 4 EFA groups, we plotted the cumulative survival against time from cryptococcal meningitis diagnosis, with a total follow-up time of 18 weeks and compared the survival curves with a log-rank test. We used Cox-regression to adjust for risk factors associated with cryptococcal mortality or factors differentially distributed across EFA groups. With the parabolic risk of mortality by CD4 T-cell count [13], adjustment for CD4 utilized 2 indicator variables of: CD4 < 50 cells/μL or CD4 ≥ 100 cells/μL. Association between EFA group and other markers or demographic data was examined via Kruskall-Wallis and χ 2 tests.
RESULTS
We analyzed data from 738 participants with a calculable EFA within the first 10 days of meningitis diagnosis. Overall, 1249 individuals were screened during the studies used for this analysis. Of these, 56 were not enrolled, 133 had an initial sterile culture, 135 died prior to day 10 with <2 LPs performed, and 189 had had <2 LPs despite surviving to day 10, leaving 738 participants. A full breakdown of these decision points is given in a consort diagram (Supplemental Figure 1). Of the 738 participants, 62% (461/738) were ART-naive with a median [P25, P75] CD4 count of 16 [6, 49] cells/μL. Mortality at 10 weeks was 36% (262/738) and 40% (297/738) by 18 weeks. Complete demographic and outcome information separated by EFA group is available in Table 1.
Table 1.
Participant Characteristics and Outcomes by Early Fungicidal Activity (EFA) Group
| EFA Group, log10 CFU/mL/day | |||||
|---|---|---|---|---|---|
| ≥0.40 | 0.30–0.39 | 0.20–0.29 | ≤0.19 | P-value | |
| N | 352 | 112 | 87 | 187 | |
| Age, years | 35 [29, 41] | 36 [30, 41] | 35 [30, 38] | 35 [30, 40] | .73 |
| Men | 203 (58%) | 71 (63%) | 50 (58%) | 127 (68%) | .11 |
| Prior cryptococcosis | 30 (9%) | 6 (5%) | 12 (14%) | 22 (12%) | .13 |
| Receiving ART | 131 (37%) | 35 (31%) | 41 (47%) | 70 (37%) | .15 |
| TB diagnosis at baseline | 20 (5.7%) | 8 (7.1%) | 8 (9.2%) | 16 (8.6%) | .52 |
| Glasgow coma scale < 15 | 140 (40%) | 43 (38%) | 36 (41%) | 68 (37%) | .87 |
| Hemoglobin, g/dL | 11.7 [10.4, 13.1] | 11.6 [9.5, 13.5] | 11.8 [10.4, 13.2] | 11.8 [10.0, 13.5] | .82 |
| CD4 count, cells/μL | 20 [8, 56] | 16 [6, 51] | 14 [6, 46] | 11 [6, 35] | <.01 |
| CSF quantitative culture, CFU/mL | 87 000 [4 600, 425 000] | 112 000 [8 000, 430 000] | 43 000 [1 000, 290 000] | 54 000 [3 000, 250 000] | .03 |
| CSF pressure, >25 cmH 2 O | 191 (65%) | 49 (50%) | 42 (58%) | 99 (61%) | .08 |
| CSF ≥ 5 white cells/μL | 164 (47%) | 54 (51%) | 34 (40%) | 55 (30%) | <.001 |
| 18-week mortality | 129 (37%) | 44 (39%) | 31 (36%) | 93 (50%) | .02 |
Data are n (%) or median [P25, P75]. P-values from Kruskall-Wallis test for medians or χ 2 test for proportions.
Abbreviations: ART, antiretroviral therapy; CFU, colony-forming units; CSF, cerebrospinal fluid; EFA, early fungicidal activity; HIV, human immunodeficiency virus; TB, tuberculosis.
In assessing EFA versus 18-week survival time, participants were divided into groups by EFA, producing the following groupings: ≥0.40 (n = 352), 0.30 to 0.39 (n = 112), 0.20 to 0.29 (n = 87), and < 0.20 (n = 187) log10CFU/mL/day. Participants with EFA < 0.20 had the lowest median CD4 T-cell counts (median 11 cells/μL; P < .01) and lowest proportion with CSF pleocytosis (30% with ≥ 5 white cells/μL CSF; P < .001).
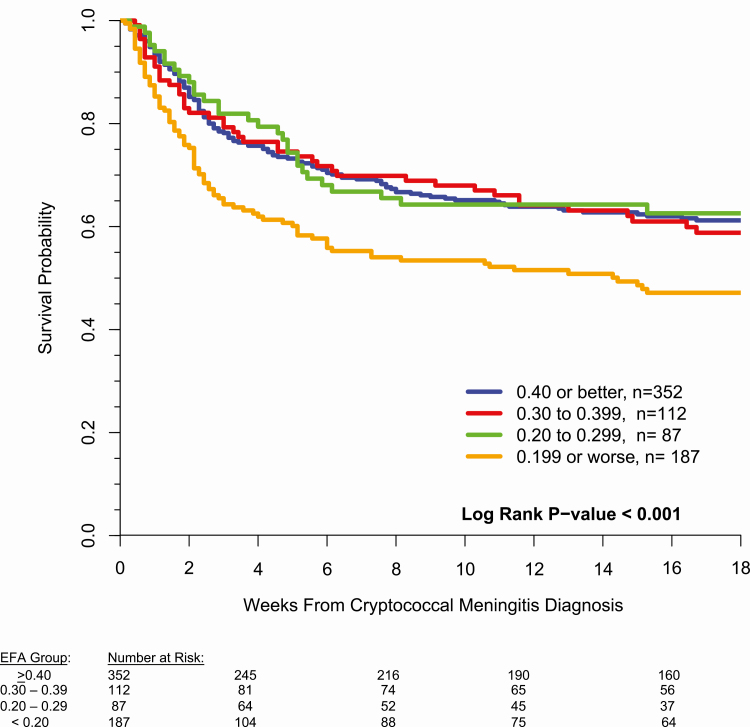
The 18-week mortality rates were 37%, 39%, 36%, and 50% for the EFA > = 0.40, 0.30 to 0.39, 0.20 to 0.29, and < 0.20 groups, respectively. Compared to those with EFA ≥ 0.20, there was increased 18-week mortality when EFA was <0.20 log10CFU/mL/day (Figure 1, hazard ratio = 1.60; 95% confidence interval [CI], 1.25 to 2.04; P = .0002). When adjusting for baseline Glasgow coma scale, hemoglobin, CSF quantitative culture, CSF pleocytosis (white cells ≥ 5/μL), biological sex, and cohort, EFA less than 0.20 log10CFU/mL/day remained significantly associated with 18-week survival (adjusted hazard ratio = 1.83; 95% CI, 1.40 to 2.40; P < .0001). Additionally when adjusting for CD4 cell count using indicator variables (CD4 1–24, 25–49, 50–99 [13], CD4 ≥ 100 cells/μL), increased hazard of 18-week mortality remained present for those with EFA < 0.20 log10CFU/mL/day (hazard ratio = 1.71; 95% CI, 1.30 to 2.26; P < .0001).
Figure 1.
EFA of Cryptococcus CSF clearance rate values demonstrating a difference in survival for those with an EFA of 0.20 or less log10 CFU/mL/day among 738 persons living with HIV with culture-positive cryptococcal meningitis. Abbreviations: CFU, colony-forming units; CSF, cerebrospinal fluid; EFA, early fungicidal activity; HIV, human immunodeficiency virus.
Even when examining higher EFAs by further separating out the ≥ 0.40 EFA group, mortality did not improve further with the highest CSF yeast clearance. Mortality through 18 weeks was 37% for EFA ≥ 0.60 (n = 170), 36% for EFA 0.40–0.59 (n = 182), 39% for EFA 0.30–0.39 (n = 112), and 36% for EFA 0.2–0.29 (n = 87). Thus, even as the EFA increased to ≥ 0.60 log10CFU/mL/day, 18-week survival did not further improve as compared to EFAs 0.20 to 0.59 log10CFU/mL/day. There was no inoculum effect with amphotericin B combination therapy as EFA was not correlated with baseline CSF quantitative culture log10 CFU/mL (r = −0.003, P = .93).
DISCUSSION
We have observed that EFA is independently associated with survival through 18 weeks, with increased mortality when the rate of CSF Cryptococcus clearance was lower than 0.20 log10CFU/mL/day. Overall, analysis of EFA has become an important component of early-phase clinical trials investigating new treatment options for cryptococcal meningitis, serving as a surrogate marker for all-cause mortality. In this study, we expand on the existing reports of EFA as a surrogate marker using a robust participant cohort. Those with an EFA greater than 0.20 log10CFU/mL/day, regardless of measurement, had a similar 18-week survival of 63% compared to 50% survival with EFA < 0.20. Our data suggest that target EFA for new antifungal therapies should be above 0.20 log10CFU/mL/day as an EFA surrogate endpoint.
This study builds on 2 prior analyses that reported that EFA was a surrogate endpoint for 10-week survival [6, 7]. Bicanic and colleagues pooled individual level data from 4 trials from South Africa, Thailand, and Uganda among subjects receiving amphotericin-based combination therapy or fluconazole therapy [6]. Conversely, Montezuma-Rusca and colleagues compared the reported group mean EFA versus 10-week survival of 9 studies [7]. Notably, Montezuma-Rusca combined different publications utilizing different statistical techniques for estimating EFA (linear regression vs linear mixed-effects model), yet there remained a correlation between 10-week survival and EFA. Our study, using different cohorts—not included in the prior publications, validates these earlier findings.
There are some limitations to this study. When looking at factors affecting patient survival after cryptococcal meningitis, it is key to remember that this is a complex issue with EFA being just one of multiple factors contributing to mortality. Other risk factors for mortality such as altered mental status, anemia, high peripheral white blood cell count, high C-reactive protein, very low or high CD4 T-cell counts, poor cerebral oxygenation, and immune reconstitution inflammatory syndrome are less directly dependent on antifungal therapy [14–20]. Although we do control for many of these in our analysis, the fact remains that EFA does not perfectly predict patient outcome as antifungal therapy alone does not address all of the factors contributing to death in HIV-related cryptococcal meningitis. Although EFA estimates are limited to those who survived to receive 2 lumbar punctures, EFA is a quantitative marker of microbiologic activity of an antifungal regimen. When adjusting for other factors associated with mortality, the hazard ratio minimally changed, strongly supporting that EFA is an independent risk factor for mortality. Whether EFA is also associated with mortality in HIV-negative cryptococcosis is unknown.
Recognizing this potential prognostic feature of EFA may be important when considering clinical trial cessation or continuation in the early stages of a trial. Future studies may benefit from examining the average EFA of trial participants while on a study treatment. Those resulting in EFA measurements <0.20 are unlikely to increase mean length of survival and should be terminated early, whereas those with better EFAs could potentially lead to survival consistent with current regimens. Given that the goal in using EFA as a surrogate marker is to decrease the time needed to determine the efficacy of novel antifungal agents in treating cryptococcal meningitis, adding this new facet to our understanding EFA’s role in patient outcome would accelerate the time to interpretable results. EFA should be a surrogate endpoint for accelerated regulatory approval of new antifungal therapies in cryptococcal meningitis, a neglected disease.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Professor Tom Harrison and colleagues for developing the quantitative culture methodology for cryptococcosis and Professor Tihana Bicanic for teaching our meningitis research team the quantitative culture technique in November 2010.
Disclaimer. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of this report. The opinions, findings and conclusions expressed in this manuscript reflect those of the authors alone.
Financial support. This research was supported by the National Institute of Neurologic Diseases and Stroke and Fogarty International Center ((R01NS086312, K01TW010268, R25TW009345, K43TW010718), the National Institute of Allergy and Infectious Diseases (U01AI089244, T32AI055433), United Kingdom Medical Research Council / DfID / Wellcome Trust Global Clinical Trials (M007413/1), and Grand Challenges Canada (S4–0296–01). G. M. was supported by the Wellcome Trust (098316 and 203135/Z/16/Z), the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (NRF) of South Africa (grant 64787), NRF incentive funding (UID: 85858) and the South African Medical Research Council through its Tuberculosis and Human Immunodeficiency Virus Collaborating Centres Programme with funds received from the National Department of Health (RFA SAMRC-RFA-CC: TB/HIV/AIDS-01–2014).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodrigues ML. Funding and innovation in diseases of neglected populations: the paradox of cryptococcal meningitis. PLoS Negl Trop Dis 2016; 10:e0004429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saag MS, Powderly WG, Cloud GA, et al. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. The NIAID mycoses study group and the AIDS clinical trials group. N Engl J Med 1992; 326:83–9. [DOI] [PubMed] [Google Scholar]
- 4. Hamill RJ, Sobel JD, El-Sadr W, et al. Comparison of 2 doses of liposomal amphotericin B and conventional amphotericin B deoxycholate for treatment of AIDS-associated acute cryptococcal meningitis: a randomized, double-blind clinical trial of efficacy and safety. Clin Infect Dis 2010; 51:225–32. [DOI] [PubMed] [Google Scholar]
- 5. Bicanic T, Meintjes G, Wood R, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis 2007; 45:76–80. [DOI] [PubMed] [Google Scholar]
- 6. Bicanic T, Muzoora C, Brouwer AE, et al. Independent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis 2009; 49:702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Montezuma-Rusca JM, Powers JH, Follmann D, Wang J, Sullivan B, Williamson PR. Early fungicidal activity as a candidate surrogate endpoint for all-cause mortality in cryptococcal meningitis: a systematic review of the evidence. PLoS One 2016; 11:e0159727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boulware DR, Meya DB, Muzoora C, et al. ; COAT Trial Team Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rhein J, Morawski BM, Hullsiek KH, et al. ; ASTRO-CM Study Team Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis 2016; 16:809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rhein J, Huppler Hullsiek K, Tugume L, et al. ; ASTRO-CM team Adjunctive sertraline for HIV-associated cryptococcal meningitis: a randomised, placebo-controlled, double-blind phase 3 trial. Lancet Infect Dis 2019; 19:843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rolfes MA, Hullsiek KH, Rhein J, et al. The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis 2014; 59:1607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dyal J, Akampurira A, Rhein J, et al. ; ASTRO-CM Trial Team Reproducibility of CSF quantitative culture methods for estimating rate of clearance in cryptococcal meningitis. Med Mycol 2016; 54:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tugume L, Rhein J, Hullsiek KH, et al. ; COAT and ASTRO-CM teams HIV-associated cryptococcal meningitis occurring at relatively higher CD4 counts. J Infect Dis 2019; 219:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jarvis JN, Bicanic T, Loyse A, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 2014; 58:736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lofgren S, Hullsiek KH, Morawski BM, et al. ; COAT and ASTRO-CM Trial Teams Differences in immunologic factors among patients presenting with altered mental status during cryptococcal meningitis. J Infect Dis 2017; 215:693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tugume L, Morawski BM, Abassi M, et al. Prognostic implications of baseline anaemia and changes in haemoglobin concentrations with amphotericin B therapy for cryptococcal meningitis. HIV Med 2017; 18:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boulware DR, Meya DB, Bergemann TL, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med 2010; 7:e1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Musubire AK, Meya DB, Rhein J, et al. ; COAT and ASTRO trial teams Blood neutrophil counts in HIV-infected patients with cryptococcal meningitis: association with mortality. PLoS One 2018; 13:e0209337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diehl JW, Hullsiek KH, Okirwoth M, et al. Cerebral oximetry for detecting high-mortality risk patients with cryptococcal meningitis. Open Forum Infect Dis 2018; 5:ofy105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rhein J, Hullsiek KH, Evans EE, et al. Detrimental outcomes of unmasking cryptococcal meningitis with recent ART initiation. Open Forum Infect Dis 2018; 5:ofy122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.