Abstract
Background
Cohort studies have reported a high prevalence of musculoskeletal, neurologic, auditory, and visual complications among Ebola virus disease (EVD) survivors. However, little is known about the host- and disease-related predictors of these symptoms and their etiological mechanisms.
Methods
The presence and patterns of 8 cardinal symptoms that are most commonly reported following EVD survival were assessed in the 326 EVD survivors who participated in the ongoing longitudinal Liberian EVD Survivor Study. At quarterly study visits, symptoms that developed since acute EVD were recorded and blood was collected for biomarkers of inflammation and immune activation.
Results
At baseline (mean 408 days from acute EVD), 75.5% of survivors reported at least 1 new cardinal symptom since surviving EVD, which in 85.8% was rated as highly interfering with life. Two or more incident symptoms were reported by 61.0% of survivors, with pairings of joint pain, headache, or fatigue the most frequent. Women were significantly more likely than men to report headache, while older age was significantly associated with musculoskeletal and visual symptoms. In analyses adjusted for multiple comparisons, no statistically significant association was found between any symptom and 26 markers of inflammation and immune activation. Symptom frequency remained largely unchanged during study follow-up.
Conclusions
Post-EVD complications occur in a majority of survivors and remain present more than 4 years after acute infection. An association between markers of inflammation and immune activation and individual symptoms was not found, suggesting an alternative etiology for persistent post-EVD symptomatology.
Keywords: Ebola virus disease, survivors, inflammation, post-EVD complications
Post-EVD complications were reported in more than 75% of survivors and remain present more than 4 years after acute infection. An association between inflammation and immune activation markers and individual symptoms was not found, suggesting an alternative etiology for persistent post-EVD symptomatology.
An estimated 17 000 people survived the 2014–2016 West African outbreak of Ebola virus, more than 10 times the number of Ebola virus disease (EVD) survivors from all previously recorded outbreaks combined [1]. Reports from observational cohorts of EVD survivors in Sierra Leone, Guinea, and Liberia, as well as from earlier outbreaks in Central Africa, describe a constellation of musculoskeletal, neurologic, visual, and auditory disturbances that, along with general fatigue, are most commonly reported by survivors [2–11].
These cardinal complications appear to be highly prevalent, experienced by 75% to 90% of survivors in the weeks and months after recovery [2, 5, 7–10]. Among 329 patients attending a dedicated EVD survivors clinic in Liberia, 90% reported at least 1 post-EVD symptom during a clinic visit over the year following acute EVD, most commonly, arthralgias, headache, and ocular problems [12]. PREVAIL III, a longitudinal study of 966 Liberian EVD survivors, compared their reported symptoms with those reported from a control group of close contacts over a period that corresponded to the second year following survivor recovery from EVD [4]. Survivors were significantly more likely to report headache and muscle and joint pain than controls, which is congruent with prior studies, but also were more likely to report urinary frequency and memory loss. Overall, in PREVAIL III, symptoms tended to decline during study follow-up.
While these studies and others have identified the most common post-EVD symptoms and their prevalence, they have largely considered symptoms in isolation and less often as co-occurring complexes. In addition, there are few data regarding host- and disease-related predictors of these symptoms and the potential mechanisms that drive them [5, 9]. To address these gaps and to examine the role of inflammation and immune activation as potential mechanisms underlying these clinical complications, we established a longitudinal cohort of Liberian EVD survivors in whom select markers of inflammation and immune activation were measured and compared between EVD survivors with and without cardinal symptoms, as well as household contacts of EVD survivors from neighboring Sierra Leone.
METHODS
Study Participants and Setting
The ongoing longitudinal Liberian Ebola Survivor Study is an observational cohort study based at the Eternal Love Winning Africa (ELWA) Hospital, outside Monrovia, Liberia, of individuals with a history of prior EVD as evidenced by a discharge certificate from an Ebola treatment unit (ETU) verified by photo identification who are at least 5 years of age and willing and able to consent to participation. Participants were recruited from June 2015 through June 2016 via community-based efforts including messaging by Ebola survivor associations and Monrovia-area Ebola survivor clinics. Data and specimens from 50 household contacts of EVD survivors without a history of EVD and no serologic evidence of Ebola virus infection participating in a separate longitudinal study of EVD survivors and contacts in Sierra Leone served as controls. Institutional review board approval was obtained from the University of North Carolina, Tulane University, the University of Liberia, and the Sierra Leone Ethics and Scientific Review Committee. All participants provided written informed consent with assent obtained from minors aged <18 years along with consent from their parent/guardian.
Study Visits
Every 3 months participants are administered surveys conducted in Liberian English by research associates trained in Good Clinical Practice and Collaborative Institutional Training Initiative ethics and compliance certified. Surveys included a symptom assessment, which is an expansion of the Wahler physical symptoms inventory, with cardinal symptoms described by survivors from earlier outbreaks of Ebola. Participants were asked if they experienced a listed symptom (at baseline visit: since discharge from the ETU and at follow-up: since last study visit) and whether the symptom existed before acute EVD. Only symptoms that emerged since ETU discharge were further characterized, including if present at the time of the interview and, if so, the severity. The severity of a symptom was assessed by the degree that it interfered in the participant’s life. Originally, the response options included a Likert scale of 1, does not interfere at all; 2, interferes very little; 3, interferes somewhat; and 4, interferes very much. However, in August 2017, the survey was modified as some participants struggled to differentiate between the middle 2 options and was rephrased using more commonly used terms for the same concepts of severity to 1, does not interfere at all; 2, interferes small; and 3, interferes plenty. For the purposes of the analyses, in the coding of the original 4 options, the middle 2 choices were collapsed.
Participants received $30 for completion of the study visit. Males aged ≥18 years were offered the option of providing semen for Ebola RNA polymerase chain reaction (PCR) testing, as described previously [13, 14].
Blood Specimen Collection
Plasma and serum were collected from EVD survivors at each study visit and stored at −30ºC until analysis. For the controls, serum was collected and stored at −30ºC until analysis.
Semen Collection
Semen was collected every 3 months from a subset of male participants and tested in real time as described previously for the presence of Ebola virus RNA by real-time qualitative reverse-transcriptase PCR (RT-PCR) using the GeneXpert Ebola test [13–15].
Markers of Inflammation and Immune Activation Biomarker Assays
Serum samples were analyzed at the University of North Carolina Center for AIDS Research HIV/STD Laboratory Core in Chapel Hill for 31 biomarkers; 28 were measured on Luminex MagPix using R&D Systems (Minneapolis, MN) kits: a 23-plex assay for CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CD163, CD25 (interleukin [I-2), CXCL1 (Fractalkine), CXCL10 (IP-10), CXCL8 (IL-8), CXCL9 (MIG), G-CSF, granzyme B, interferon (IFN)-γ, IL-1α, IL-10, IL-12p70, IL-17A, IL-1ra, IL-2, IL-4, IL-5, IL-6, tumor necrosis factor (TNF) R1, TNF-α; a 3-plex assay for IL-1β, GM-CSF, TNF-RII; and single-plex assays for CRP and ferritin. Enzyme-linked immunosorbent assay kits were used for D-dimer (ThermoFisher, Waltham, MA) and soluble CD8 (sCD8, MyBioSource, San Diego, CA). Lipopolysaccharide was measured using a Limulus Amebocyte Lysate Chromogenic Endpoint Assay (Hycult Biotech, Wayne, PA). Soluble CD8 was performed on single freeze–thaw samples, while all other analytes were measured after 2 freeze thaws. All assays were performed in duplicate.
Statistical Analyses
For the analysis of host- and disease-related predictors of post-EVD symptoms of any severity, 3 groups of EVD survivors were considered: individuals who experienced a cardinal symptom, individuals who did not experience the symptom of interest but experienced a different cardinal symptom, and asymptomatic individuals without any of the cardinal symptoms. Analysis of variance (ANOVA) was used to test for the association between age and symptoms. For time from ETU discharge, Kruskal–Wallis tests were performed as some of the assumptions for ANOVA were violated. For gender and semen RNA status, Fisher exact tests were conducted.
Markers of inflammation and immune activation in blood collected during the baseline visit were correlated with the following baseline cardinal symptoms: joint pain, headache, fatigue, muscle pain, vision problems, feet numbness, hand numbness, and hearing loss. In cases where blood was not available from a baseline visit, symptoms from the earliest visit when blood was collected were used for correlation with these markers. To test associations between individual inflammatory markers and symptoms, logistic regression analysis was performed where individual markers were included as a covariate. For these regression models, only 2 groups of EVD survivors were considered: individuals who experienced the cardinal symptom and individuals who did not experience the cardinal symptom. For markers with left or right truncations due to the detection limits, we imputed truncated values by the corresponding detection limits but added another dummy variable that indicates the truncation status whose effect was treated and tested as part of the association. In order to preserve type 1 error, a permutation-based method was used to adjust for multiple testing (Supplementary Methods).
For the associations between individual inflammatory markers and symptoms where the raw P value was < .05, an exploratory analysis was performed to compare the biomarker levels between EVD survivors with the symptom of interest, EVD survivors who did not experience the symptom of interest, and uninfected household contacts without prior EVD. Kruskal–Wallis tests were performed as some of the assumptions for ANOVA were violated.
Data processing and analysis was performed using SAS/STAT software, Version 9.4 of the SAS System for Windows, and RStudio, Version 1.1.456.
RESULTS
Participant Characteristics
A total of 326 participants were enrolled in the cohort. The mean participant age is 31.5 years (range, 5.1–68.6), and just over half (55.2%) are male. The mean time from ETU discharge to study entry (baseline) is 408 days (range, 51–614) and the mean time on study is 1002 days (range, 549–1107 days). The median number of study visits is 7 (range, 3–9 visits). Of the male participants, 149 provided semen for Ebola virus RNA PCR analysis, and 13 (8.8%) have at least 1 specimen with detected RNA. Twelve participants have been lost to follow-up, including 1 who died and 11 who have moved out of the area or country.
Post-EVD Symptoms at Baseline Visit and During Study Follow-up
At baseline, 246 of the 326 participants (75.5%) reported at least 1 cardinal symptom that developed after acute EVD and was present at the visit, most commonly joint pain, followed by headache and then fatigue (Table 1). Overall, 211 (64.7% of all participants and 85.8% of those with any cardinal symptom) had a symptom that was rated by the participant as highly interfering with life; of these, joint pain, headache, and fatigue were also the most common.
Table 1.
Frequency of Incident Post-Ebola Virus Disease Cardinal Symptoms in the Liberian Ebola Virus Disease Survivor Cohort
| Symptom | All | Female | Male | |||
|---|---|---|---|---|---|---|
| Children, N = 18 | Adults, N = 128 | Children, N = 14 | Adults, N = 166 | |||
| N (%) | N (%) | N (%) | ||||
| Fatigue | Not present | 225 (69.2) | 14 (77.8) | 81 (63.2) | 9 (64.3) | 121 (73.3) |
| Interferes some | 24 (7.4) | 0 (0.0) | 12 (9.4) | 0 (0.0) | 12 (7.3) | |
| Interferes a lot | 76 (23.4) | 4 (22.2) | 35 (27.3) | 5 (35.7) | 32 (19.4) | |
| Numbness in feet | Not present | 272 (83.7) | 17 (94.4) | 101 (78.9) | 13 (92.9) | 141 (85.5) |
| Interferes some | 11 (3.4) | 0 (0.0) | 7 (5.5) | 0 (0.0) | 4 (2.4) | |
| Interferes a lot | 42 (12.9) | 1 (5.6) | 20 (15.6) | 1 (7.1) | 20 (12.1) | |
| Numbness in hands | Not present | 285 (87.4) | 18 (100.0) | 111 (86.7) | 12 (85.7) | 144 (86.7) |
| Interferes some | 10 (3.1) | 0 (0.0) | 6 (4.7) | 0 (0.0) | 4 (2.4) | |
| Interferes a lot | 31 (9.5) | 0 (0.0) | 11 (8.6) | 2 (14.3) | 18 (10.8) | |
| Headache | Not present | 220 (67.5) | 14 (77.8) | 73 (57.0) | 11 (78.6) | 122 (73.5) |
| Interferes some | 19 (5.8) | 0 (0.0) | 9 (7.0) | 0 (0.0) | 10 (6.0) | |
| Interferes a lot | 87 (26.7) | 4 (22.2) | 46 (36.0) | 3 (21.4) | 34 (20.5) | |
| Hearing loss | Not present | 314 (96.3) | 18 (100.0) | 125 (97.6) | 14 (100.0) | 157 (94.6) |
| Interferes some | 3 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (1.8) | |
| Interferes a lot | 9 (2.8) | 0 (0.0) | 3 (2.3) | 0 (0.0) | 6 (3.6) | |
| Joint pain | Not present | 198 (60.7) | 16 (88.9) | 65 (50.8) | 11 (78.6) | 106 (63.9) |
| Interferes some | 17 (5.2) | 0 (0.0) | 8 (6.3) | 0 (0.0) | 9 (5.4) | |
| Interferes a lot | 111 (34.0) | 2 (11.1) | 55 (42.9) | 3 (21.4) | 51 (30.7) | |
| Muscle pain | Not present | 282 (86.5) | 18 (100.0) | 109 (85.8) | 14 (100.0) | 141 (84.9) |
| Interferes some | 2 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.2) | |
| Interferes a lot | 41 (12.6) | 0 (0.0) | 18 (14.2) | 0 (0.0) | 23 (1.4) | |
| Vision problems | Not present | 266 (81.6) | 15 (83.3) | 105 (82.0) | 14 (100.0) | 132 (79.5) |
| Interferes some | 5 (1.5) | 0 (0.0) | 4 (3.1) | 0 (0.0) | 1 (0.6) | |
| Interferes a lot | 55 (16.9) | 3 (16.7) | 19 (14.8) | 0 (0.0) | 33 (19.9) |
The response options presented to participants included does not interfere (not present), interferes small (interferes some), interferes plenty (interferes a lot). Children defined as age <18 years.
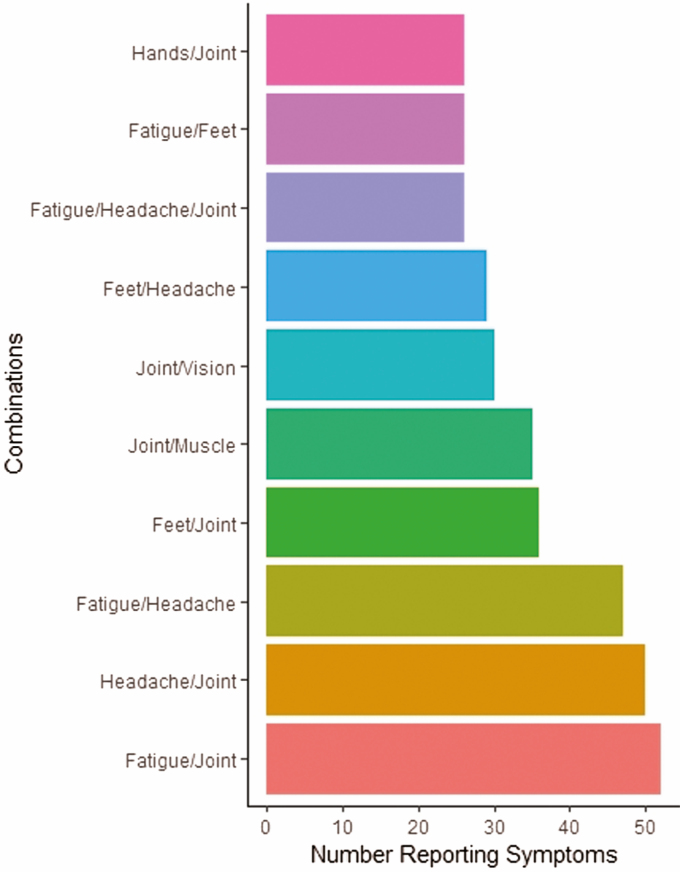
Of the 246 with any cardinal symptom at baseline, 150 (61.0%) reported multiple symptoms including 66 (26.8%) with 2 and 84 (34.1%) with 3 or more. The most frequently reported constellations of symptoms were pairings among joint pain, headache, and fatigue (Figure 1).
Figure 1.
Combinations of cardinal symptoms reported at baseline (N = 326; only those combinations that at least 25 people reported are shown).
Women were significantly more likely than men to report experiencing headache, including a highly interfering headache (Table 2). Women were less likely than men to report baseline hearing problems, but the number of participants with this symptom was small. The mean ages of those with a baseline report of joint, muscle, and vision symptoms, including those that were highly interfering, were significantly greater than those without these symptoms.
Table 2.
Predictors of Cardinal Symptoms of Any Severity Reported at Baseline
| Symptom 0 = Not Present 1 = Present | N | Gender | Age | Time from Ebola Treatment Unit | |||||
|---|---|---|---|---|---|---|---|---|---|
| % M (N) | % F (N) | P Value (Raw)a | Mean (SD) | P Value (Raw)b | Mean (SD) | P Value (Raw)c | |||
| Fatigue | Asx | 80 | 31.28 (56) | 16.44 (24) | .0077 | 28.56 (12.47) | .0038 | 398.75 (113.44) | .1269 |
| 0 | 145 | 41.34 (74) | 48.63 (71) | 33.78 (11.26) | 402.15 (95.19) | ||||
| 1 | 100 | 27.37 (49) | 34.93 (51) | 30.72 (11.11) | 425.37 (103.21) | ||||
| Numbness in feet | Asx | 80 | 31.28 (56) | 16.44 (24) | .0068 | 28.56 (12.47) | .0218 | 398.75 (113.44) | .0760 |
| 0 | 192 | 54.75 (98) | 64.38 (94) | 32.22 (11.50) | 405.89 (101.33) | ||||
| 1 | 53 | 13.97 (25) | 19.18 (28) | 33.67 (10.46) | 432.42 (87.80) | ||||
| Numbness in hands | Asx | 80 | 31.11 (56) | 16.44 (24) | .0041 | 28.56 (12.47) | .0193 | 398.75 (113.44) | .5125 |
| 0 | 205 | 55.56 (100) | 71.92 (105) | 32.18 (11.04) | 412.84 (102.04) | ||||
| 1 | 41 | 13.33 (24) | 11.64 (17) | 34.12 (12.33) | 403.02 (82.89) | ||||
| Headache | Asx | 80 | 31.11 (56) | 16.44 (24) | .0022 | 28.56 (12.47) | .0300 | 398.75 (113.44) | .0068 |
| 0 | 140 | 42.78 (77) | 43.15 (63) | 32.31 (11.62) | 395.49 (99.45) | ||||
| 1 | 106 | 26.11 (47) | 40.41 (59) | 32.76 (10.81) | 431.95 (94.98) | ||||
| Hearing loss | Asx | 80 | 31.11 (56) | 16.44 (24) | .0018 | 28.56 (12.47) | .0129 | 398.75 (113.44) | .0043 |
| 0 | 234 | 63.89 (115) | 81.51 (119) | 32.28 (11.29) | 415.86 (97.13) | ||||
| 1 | 12 | 5.00 (9) | 2.05 (3) | 36.84 (10.10) | 320.33 (95.33) | ||||
| Joint pain | Asx | 80 | 31.11 (56) | 16.44 (24) | .0078 | 28.56 (12.47) | .0002 | 398.75 (113.44) | .1731 |
| 0 | 118 | 33.89 (61) | 39.04 (57) | 30.07 (11.12) | 423.15 (102.93) | ||||
| 1 | 128 | 35.00 (63) | 44.52 (65) | 34.75 (10.96) | 400.19 (94.33) | ||||
| Muscle pain | Asx | 80 | 31.11 (56) | 16.55 (24) | .0053 | 28.56 (12.47) | .0017 | 398.75 (113.44) | .0070 |
| 0 | 202 | 55.00 (99) | 71.03 (103) | 31.65 (11.60) | 419.63 (99.81) | ||||
| 1 | 43 | 13.89 (25) | 12.41 (18) | 36.37 (8.74) | 369.98 (85.52) | ||||
| Vision problems | Asx | 80 | 31.11 (56) | 16.44 (24) | .0044 | 28.56 (12.47) | .0014 | 398.75 (113.44) | .0149 |
| 0 | 186 | 50.00 (90) | 65.75 (96) | 31.46 (11.25) | 420.44 (95.89) | ||||
| 1 | 60 | 18.89 (34) | 17.81 (26) | 35.73 (10.76) | 382.57 (103.84) |
The P value threshold for significance (P = .00361) was obtained through a permutation-based method. Significant values compared with this threshold are in bold. 0 = Ebola virus disease (EVD) survivor without that specific symptom. 1 = EVD survivor with that specific symptom.
Abbreviations: Asx, asymptomatic survivors, those without any cardinal symptom; SD, standard deviation.
aFisher exact test.
bAnalysis of variance.
cKruskal–Wallis test.
Men with Ebola virus RNA detected in their semen (at baseline or at any subsequent time point) were no more likely to report baseline cardinal symptoms compared with other men, although there were trends for a greater proportion of semen RNA-positive men reporting vision problems (P = .04) and a smaller proportion of these men reporting fatigue (P = .04). However, both were no longer significant after adjusting for multiple comparisons (Supplementary Table 2).
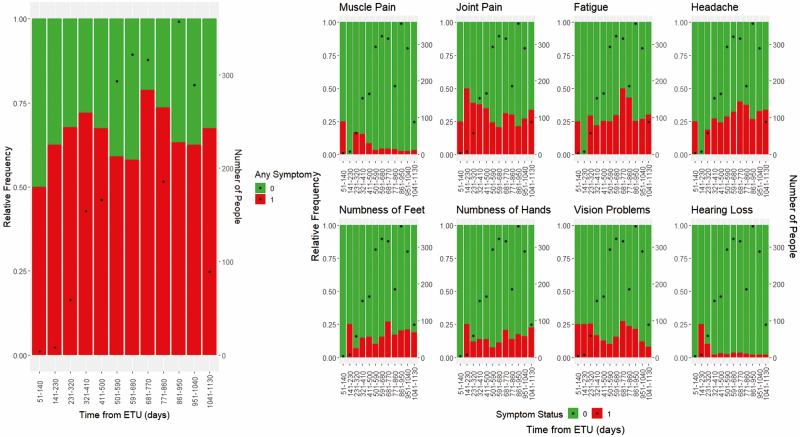
Over the course of study follow-up, the proportion of participants reporting any cardinal symptom changed little (Figure 2).
Figure 2.
Cardinal symptom frequency over time. Abbreviation: ETU, Ebola treatment unit.
Markers of Inflammation and Immune Activation
Levels of the 31 markers of inflammation and immune activation measured at baseline were generally low among both EVD survivors and uninfected controls. For 17 markers, more than half of the values were below the manufacturer’s limit of assay detection.
Association Between Inflammation and Immune Activation Markers and Post-EVD Symptoms
Initial logistic regression models controlling for age, sex, and time from ETU discharge found no statistically significant associations between inflammatory markers and symptoms after adjusting for multiple comparisons. Analyses evaluating symptoms by severity also did not reveal a significant difference in levels of biomarkers in EVD survivors with a severe symptom compared with any other group (data not shown).
Exploratory analyses comparing biomarker levels between uninfected controls, EVD survivors with the symptom of interest, and EVD survivors without the symptom of interest were performed for biomarker/symptom combinations where the raw P values from the logistic regression models were < .05. Among EVD survivors, those with headache had higher D-dimer levels, those with muscle pain had higher IFN-γ levels, and those with vision problems had higher CRP levels than EVD survivors without those symptoms, respectively. Uninfected controls had higher values of these biomarkers than both EVD survivors with and without the symptom of interest (Supplementary Table 1). In contrast, certain inflammatory marker levels were lower in EVD survivors reporting a cardinal symptom, including MIP-1α and lower extremity numbness, TNF-α and headache, IL-6 and hearing loss, and IL-8 and vision problems, compared with EVD survivors without those symptoms. Uninfected controls had higher levels of TNF-α and IL-8 compared with both EVD survivors with and without the symptoms of interest but had lower levels of MIP-1α and IL-6 than EVD survivors without the symptom of interest (Supplementary Table 1). After adjusting for multiple comparisons, none of these associations remained statistically significant (Supplementary Table 1).
There was no significant difference in the levels of any of the biomarkers between men who had evidence of Ebola virus PCR-positive semen and those who tested negative at the time of the first blood draw (data not shown).
DISCUSSION
In this cohort of 326 Ebola survivors, complications of acute EVD were highly prevalent 1 year after recovery, were often co-occurring, and were largely sustained during approximately 3 years of follow-up. An association between cardinal post-EVD symptoms and markers of inflammation and immune activation was not observed.
Our finding of persistence of symptoms stands in contrast with prior studies describing a decrease in the prevalence of post-EVD symptoms. In a retrospective review of the outpatient medical records of 329 patients attending the ELWA Ebola Survivor Clinic, de St. Maurice and colleagues found a decrease over time in recording of most symptoms including musculoskeletal complaints, visual disturbances, headache, depression, and weight loss [12]. A decline in symptoms, with the exception of uveitis, was also reported by the PREVAIL III study. In that study, symptoms waned over the course of a year in both the EVD survivors as well as uninfected controls. These disparate results may reflect differential methodology, such as the questions used to detect symptoms and the duration of follow-up. In addition, studies of EVD survivors that are clinic-based may observe declines in symptoms if treatment (eg, pain medication) is made available to participants.
We found associations between female gender and headache, as well as age and joint, muscle, and vision-related complaints. These findings are of interest given data that suggest a higher prevalence of headache in post-pubescent girls and women compared with males and the common occurrence of musculoskeletal and vision problems during aging [16]. It is notable that the mean age difference between those with and without musculoskeletal and vision issues was relatively small, approximately 4 years. These data suggest that symptoms experienced during convalescence from acute EVD may accentuate or accelerate those that may tend to occur in the absence of prior EVD.
While putative inflammatory and immune activation pathways have been posited as potential pathogenic mechanisms underlying these somatic complaints [16–18], in this study, the largest analysis of inflammation and post-EVD complications to date, we did not find a significant association between the presence of a cardinal symptom and any among a comprehensive array of biomarkers of inflammation or immune activation. Concomitant medications were reviewed to ensure antiinflammatory effects of nonsteroidal anti-inflammatory drugs (NSAIDs), and steroid medications were considered. Of 326 participants, none were taking steroid medications and only 1 was taking an NSAID.
Persistence of viral antigens in compartments such as the eye, central nervous system, or genital tract could drive immune responses that result in post-EVD symptoms [13, 17, 18]. While CRP levels were greater in survivors with vision problems compared with survivors without vision problems after controlling for age, sex, and time from ETU to enrollment, this did not meet the threshold for statistical significance after accounting for multiple comparisons. Further, we found no association between seminal RNA and symptoms, and there was no significant difference in levels of biomarkers between men with and without seminal RNA detected.
Our study has a number of strengths, including a relatively large sample size with few lost to follow-up, a longitudinal design, and criteria for post-EVD symptoms that were well-defined. However, there are limitations that should be considered when interpreting these findings. Foremost, the prevalence of the symptoms of interest were examined only in EVD survivors and not controls without a history of EVD. Therefore, background rates of these complaints are unknown. While our focus on only cardinal symptoms that emerged since acute EVD and were not present before this illness was designed to minimize the inclusion of symptoms unrelated to EVD, the extent to which some of the reported complaints were independent of EVD cannot be determined. In addition, our study may be subject to selection bias if those with symptoms were more likely to consent to participate. Recruitment for this cohort was independent of a survivor clinic, and many who entered the study stated a motivation to participate was access to testing of their genital fluid for Ebola virus RNA. Participants recruited in Liberia and Sierra Leone were all individuals from these areas; however, specific ethnicities from these EVD survivors and seronegative EVD controls were not recorded. The sites of recruitment in the 2 countries are in close geographical proximity (approximately 170 miles), and it is unlikely that there are population differences that could affect comparisons of the levels of inflammatory markers in the 2 groups. Last, the sample size may be inadequate to detect associations that are more modest. In particular, the analysis of the relationship between seminal Ebola virus and inflammation is limited by the small number of men who had Ebola RNA detected in their semen. In addition, as mentioned, cellular markers of inflammation were not evaluated.
In conclusion, symptoms commonly reported by survivors of acute EVD were highly prevalent, persistent 1 to 4 years after recovery, and were usually regarded as highly interfering with life. Almost two-thirds of the survivors studied experienced 2 or more symptoms, most commonly joint pain, headache, and fatigue. Symptomatology was influenced by gender and age but not time from ETU discharge. There was no association between markers of inflammation and immune activation with the most commonly reported symptoms. Continued study of EVD survivors to determine the natural history of these symptoms, as well as investigations dedicated to identifying their pathogenic mechanisms including cellular markers of inflammation, will be integral to developing interventions that can reduce their impact on health and quality of life.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the many Ebola virus disease survivors who have contributed to this study.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grants R01AI123535, R01AI135105 to W. F. and D. W.; K23 AI121516 to W. F.; K24 DA037101 to D. W.; and AI085073 to K. K.) and the National Institute of Environmental Health Sciences at the National Institutes of Health (grant T32ES007018 to K. K.).
Potential conflicts of interest. D. H. received grants from the Bill & Melinda Gates Foundation; W. F. and D. W. received grant support from the National Institutes of Health. K. D. received funding from the Bill & Melinda Gates foundation outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Situation report Ebola virus disease. 10 June 2016. Accessed 15 April 2019. [Google Scholar]
- 2. Clark DV, Kibuuka H, Millard M, et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect Dis 2015; 15:905–12. [DOI] [PubMed] [Google Scholar]
- 3. Epstein L, Wong KK, Kallen AJ, Uyeki TM. Post-Ebola signs and symptoms in U.S. survivors. N Engl J Med 2015; 373:2484–6. [DOI] [PubMed] [Google Scholar]
- 4. Group PIS, Sneller MC, Reilly C, et al. A longitudinal study of Ebola sequelae in Liberia. N Engl J Med 2019; 380:924–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mattia JG, Vandy MJ, Chang JC, et al. Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross-sectional study. Lancet Infect Dis 2016; 16:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mohammed H, Vandy AO, Stretch R, et al. Sequelae and other conditions in Ebola virus disease survivors, Sierra Leone, 2015. Emerg Infect Dis 2017; 23(1): 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nanyonga M, Saidu J, Ramsay A, Shindo N, Bausch DG. Sequelae of Ebola virus disease, Kenema District, Sierra Leone. Clin Infect Dis 2016; 62:125–6. [DOI] [PubMed] [Google Scholar]
- 8. Qureshi AI, Chughtai M, Loua TO, et al. Study of Ebola virus disease survivors in Guinea. Clin Infect Dis 2015; 61:1035–42. [DOI] [PubMed] [Google Scholar]
- 9. Rowe AK, Bertolli J, Khan AS, et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidémies à Kikwit. J Infect Dis 1999; 179(Suppl 1):S28–35. [DOI] [PubMed] [Google Scholar]
- 10. Tiffany A, Vetter P, Mattia J, et al. Ebola virus disease complications as experienced by survivors in Sierra Leone. Clin Infect Dis 2016; 62:1360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilson HW, Amo-Addae M, Kenu E, Ilesanmi OS, Ameme DK, Sackey SO. Post-Ebola syndrome among Ebola virus disease survivors in Montserrado County, Liberia 2016. Biomed Res Int 2018; 2018:1909410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de St. Maurice A, Ervin E, Orone R, et al. Care of Ebola survivors and factors associated with clinical sequelae–Monrovia, Liberia. Open Forum Infect Dis 2018; 5(10): ofy239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fischer WA, Brown J, Wohl DA, et al. Ebola virus ribonucleic acid detection in semen more than two years after resolution of acute Ebola virus infection. Open Forum Infect Dis 2017; 4(3): ofx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Loftis AJ, Quellie S, Chason K, et al. Validation of the Cepheid GeneXpert for detecting Ebola virus in semen. J Infect Dis 2017; 215:344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pettitt J, Higgs ES, Adams RD, Jahrling PB, Hensley LE. Use of existing diagnostic reverse-transcription polymerase chain reaction assays for detection of Ebola virus RNA in semen. J Infect Dis 2016; 213:1237–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peterlin BL, Gupta S, Ward TN, Macgregor A. Sex matters: evaluating sex and gender in migraine and headache research. Headache 2011;51:839–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacobs M, Rodger A, Bell DJ, et al. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet 2016;388:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Varkey JB, Shantha JG, Crozier I, et al. Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med 2015;372:2423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.