Abstract
Background
Human immunodeficiency virus (HIV) infection and antiretroviral therapy (ART) are associated with bone loss leading to increased fracture rate among persons with HIV (PWH). We previously showed long-acting antiresorptive zoledronic acid (ZOL) prevented ART-induced bone loss through 48 weeks of therapy and here investigate whether protection persisted.
Methods
We randomized 63 nonosteoporotic, treatment-naive adult PWH initiating ART to ZOL (5 mg) versus placebo in a double-blinded, placebo-controlled, phase IIb trial. Here we analyzed the long-term outcome data (144 weeks). Plasma bone turnover markers and bone mineral density (BMD) were quantified at weeks 0, 12, 24, 48, 96, and 144. Primary outcome was change in bone resorption marker C-terminal telopeptide of collagen (CTx). Repeated-measures analyses using mixed linear models were used to estimate and compare study endpoints.
Results
At 96 weeks, mean CTx was 62% lower with ZOL relative to placebo (n = 46; CTx = 0.123 vs 0.324 ng/mL; P < .001); at 144 weeks a 25% difference between arms was not statistically significant. At 48 weeks, lumbar spine BMD with ZOL was 11% higher than placebo (n = 60; P < .001) and remained 9–11% higher at 96 (n = 46) and 144 (n = 41; P < .001) weeks. 144 weeks after ZOL infusion, BMD did not change at the lumbar spine (P = .22) but declined at the hip (P = .04) and femoral neck (P = .02).
Conclusions
A single dose of ZOL administered at ART initiation blunts bone resorption and BMD loss at key fracture-prone anatomical sites in treatment-naive PWH for 3 years. A multicenter randomized phase III clinical trial validating these results in a larger population is needed.
Clinical Trials Registration
Keywords: antiretroviral therapy, bone loss, zoledronic acid, bone mineral density, fracture prevention
In a phase IIb clinical trial of viremic, nonosteoporotic persons with HIV initiating antiretroviral therapy, we demonstrate that a single dose of zoledronic acid durably blunts bone loss at key fracture-prone anatomical sites through a 3-year follow-up period.
As persons with human immunodeficiency virus (HIV; PWH) are aging, the long-term complications of chronic HIV infection are increasingly relevant and burdensome [1, 2]. A common metabolic sequela of longstanding HIV infection is bone loss, with the prevalence of osteopenia and osteoporosis in PWH ranging from 48–55% and 10–34%, respectively [3]. This is accompanied by a 2- to 9-fold higher fracture prevalence in PWH compared with the general population [4, 5]. Additionally, PWH appear to be at greater risk for fracture earlier in life compared with their HIV-seronegative counterparts [6, 7]. The excessive burden of fragility bone disease is projected to increase as PWH are aging [8], underscoring the urgent need for HIV-specific preventive and therapeutic strategies to improve bone health in this high-risk population.
Mechanisms underlying HIV-associated bone disease are likely multifactorial, mediated by direct and indirect effects of the virus, host, immune system, and antiretroviral therapy (ART) [9]. Antiretroviral therapy induces bone mineral density (BMD) loss of 2–6%, often within the first 1–2 years of therapy [10]; these effects, although varied in magnitude are universal to all ART regimens. Even with tenofovir alafenamide, a formulation introduced to minimize toxicities associated with tenofovir disoproxil fumarate (TDF), an approximate 1% BMD loss at 48 weeks was observed in relatively healthy PWH (median CD4 T-cell counts; 404 [283–550] cells/μL) [11–13]. More concerning, however, close to one-third of patients in these studies sustained BMD loss of more than 3% at the hip and/or lumbar spine.
Our group recently demonstrated that bone resorption is positively associated with the magnitude of immune reconstitution in PWH initiating ART [14]. Using an animal model of immunodeficiency, we observed that forcing aggressive homeostatic reconstitution by adoptive transfer of low numbers of T cells resulted in profound bone resorption and loss of BMD that was prevented by administration of zoledronic acid (ZOL), a potent long-acting antiresorptive [15]. In a subsequent human study, we showed in a double-blinded, placebo-controlled phase IIb clinical trial that ZOL prevented ART-induced bone effects through the first 48 weeks of ART initiation [16]. In this long-term follow-up study, we investigate whether the skeletal protection observed earlier with ZOL persisted through 96 and 144 weeks.
METHODS
Trial Design
Details of the methods and design have been previously described [16]. This phase IIb clinical trial was conducted at the Grady Infectious Diseases Program Clinic in Atlanta, Georgia, between January 2010 and January 2015. All subjects provided written informed consent, and the study was approved by the Institutional Review Board of Emory University. The study was registered at ClinicalTrials.gov (NCT01228318).
Objectives
The primary objective was to investigate whether ZOL ameliorates ART-induced bone resorption in the study population. Secondary objectives included ZOL’s impact on BMD outcomes at key fracture-prone anatomical sites and safety and clinical measures.
Participants
Viremic (HIV-1 RNA >1000 copies/mL) treatment-naive PWH aged 30 to 50 years who were planning ART initiation, had no history of bone or active immunological disease, and were in generally good health were eligible for the study. Patients who had osteoporosis (t score < −2.5), prior or current use of antiresorptives, recent (within 6 months) or planned invasive dental procedures, serious systemic illness, or who were pregnant or breastfeeding were excluded.
Randomization
Treatment assignments were stratified according to screening HIV-1 RNA (<100 000 or ≥100 000 copies/mL), age (30–39 or 40–49 years), and sex and were generated using a pseudo-random-number generator with permuted blocks for each of the 8 levels of stratification.
Interventions
At entry, participants initiated ART per standard of care with standard doses of atazanavir/ritonavir + tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) [17]. Antiretroviral therapy change was allowed after study entry in the case of drug intolerance or virologic failure. On the same day of ART initiation, participants also received a single intravenous infusion of ZOL (5 mg/100 mL ready-to-infuse solution) if assigned to the ZOL arm or a single infusion of placebo (220 mg mannitol and 24 mg sodium citrate in a 100-mL ready-to-infuse solution) if assigned to the placebo arm. Serum vitamin D3 and calcium levels were monitored at each study visit, and replacement therapy was initiated when levels were insufficient or deficient (≤29 ng/dL).
Follow-up
Study outcomes were assessed at baseline and at study weeks 12–144. The study was unblinded when the last enrolled participant completed the visit at 24 weeks. Clinical and safety laboratory tests were performed at weeks 2 and 12 and every 3 months thereafter.
Outcome Measures
Blood samples and plasma were processed and stored until analysis by commercial enzyme-linked immunosorbent assays to quantify plasma C-terminal telopeptide of collagen (CTx) and osteocalcin, which are sensitive and specific markers of bone resorption and bone formation, respectively. Bone mineral density was assessed using a Lunar prodigy scanner (GE Lunar) dual-energy X-ray absorptiometry machine and Encore Software, version 2010 13.31, at Emory University Hospital. Osteopenia was defined as t scores between −1.0 and −2.5, and osteoporosis as t scores of −2.5 or less per World Health Organization criteria [18]. Clinical and safety laboratory tests were performed at a Clinical Laboratory Improvement Amendments adherent laboratory. Safety reports were generated by the Data Coordinating Center every year and reviewed by an independent medical safety monitor.
Sample Size and Power Considerations
Pilot data from a study of treatment-naive PWH on therapy for 24 weeks with lopinavir/ritonavir + TDF/FTC [14] formed the basis for estimating sample size as previously described [16].
Statistical Analysis
The primary analyses of the data were performed on an intention-to-treat basis. Repeated-measures analyses of CTx, osteocalcin, and BMD measurements were performed with a means model via the SAS MIXED Procedure (version 9.4; SAS Institute) providing separate estimates of the means by time on study and treatment arm. The same model was used to analyze percentage change from baseline for CTx, osteocalcin, and BMD. Each model included 3 predictors (treatment arm, time on study, and the statistical interaction between treatment arm and time on study). A compound-symmetric variance-covariance form in repeated measurements was assumed for each outcome, and robust estimates of the standard errors of parameters were used to perform statistical tests and construct 95% confidence intervals (CIs) [19]. Specific statistical tests were done within the framework of the mixed-effects linear model. All statistical tests were 2-sided and unadjusted for multiple comparisons. A value of P < .05 indicated statistical significance. The study was designed as a fixed-sample size study and no formal interim analyses were performed for safety and efficacy. Statistical stopping boundaries were not established as an aid to early stopping of the study. Monthly data-quality checks and data queries were generated when creating analytic data sets used to generate monthly safety reports and monthly summary statistics. Each of 36 solicited adverse events was counted only once per patient as the most severe level reported across the 6 study visits during the 144-week follow-up period and compared between treatment arms with a chi-square or Fisher’s exact test. Statistical analyses were limited to the 20 most commonly reported adverse events after excluding those symptoms reported as mild.
RESULTS
Demographic and Clinical Characteristics
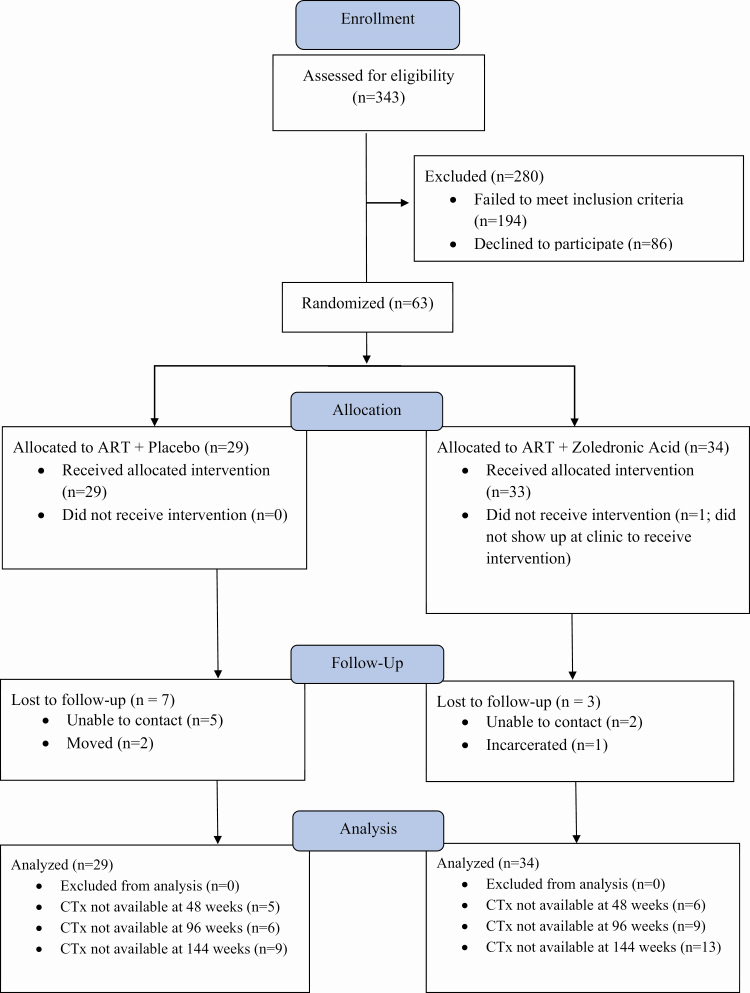
A total 343 patients were assessed for eligibility (Figure 1), and 63 were randomized to receive either ZOL (n = 34) or active placebo (n = 29). Demographic and clinical characteristics were comparable between the 2 study arms (Table 1).
Figure 1.
CONSORT diagram. The progress through the phases (enrollment, intervention allocation, follow-up, and data analysis) of a double-blind, randomized controlled trial in nonosteoporotic, viremic, ART-naive adults with HIV comparing a single zoledronic acid (5 mg) infusion at the time of ART initiation with active placebo infusion is shown. One patient in the ART + placebo arm had CTx data but outside the clinical visit window at 96 (92–105) and 144 (141–153) weeks. CTx was missing for an additional 12 patients at 144 weeks, but for the patients not lost to follow-up bone mineral density outcome data were available for analysis. Abbreviations: ART, antiretroviral therapy; CONSORT, Consolidated Standards of Reporting Trials; CTx, C-terminal telopeptide of collagen; HIV, human immunodeficiency virus.
Table 1.
Baseline Demographic and Clinical Characteristics by Treatment Arm
| Characteristic | ART + PL (n = 29) | ART + ZOL (n = 34) |
|---|---|---|
| Age, mean (SD), years | 39.4 (6.9) | 39.7 (6.6) |
| Sex, n (%) | ||
| Male | 23 (79.3) | 27 (79.4) |
| Female | 6 (20.7) | 7 (20.6) |
| Race, n (%) | ||
| White | 3 (10.3) | 7 (20.6) |
| Black | 26 (89.7) | 27 (79.4) |
| History of smoking, n (%) | ||
| Yes | 26 (89.7) | 24 (70.6) |
| No | 3 (10.3) | 10 (29.4) |
| Current smoking, n (%) | ||
| Yes | 23 (79.3) | 19 (55.9) |
| No | 6 (20.7) | 15 (44.1) |
| No. of cigarettes smoked per day (in patients with history of smoking), mean (SD) | 7.6 (4.5) | 7.5 (6.0) |
| Years of cigarette smoking (in patients with history of smoking), mean (SD) | 13.4 (8.1) | 13.9 (9.6) |
| Alcohol use in past 30 days, n (%) | ||
| Daily | 1 (3.4) | 2 (5.9) |
| 5–6 times/wk | 0 (0) | 1 (2.9) |
| 3–4 times/wk | 1 (3.4) | 4 (11.8) |
| 1–2 times/wk | 8 (27.6) | 3 (8.8) |
| 2–3 times/mo | 2 (6.9) | 2 (5.9) |
| Once/month | 3 (10.3) | 8 (23.5) |
| Never | 14 (48.3) | 14 (41.2) |
| Baseline osteopenia in any area,a n (%) | ||
| Yes | 10 (34.5) | 7 (21.9) |
| No | 19 (65.6) | 25 (78.1) |
| Baseline lumbar spine BMD, mean (SD) | ||
| g/cm3 | 1.23 (0.14) | 1.29 (0.14) |
| t score | 0.16 (1.16) | 0.67 (1.22) |
| History of bone fracture, n (%) | ||
| Yes | 5 (17.2) | 10 (29.4) |
| No | 24 (82.8) | 24 (70.6) |
| HIV-1 RNA, mean (SD), log10 copies/mL | 4.81 (0.96) | 5.26 (0.44) |
| CD4+ count, mean (SD), cells/µL | 155 (145) | 102 (69) |
| Serum calcium, mean (SD), mg/dL | 9.3 (0.4) | 9.1 (0.4) |
| Serum vitamin D, mean (SD), ng/mL | 27.8 (10.0) | 28.1 (11.7) |
Data are presented as n (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; BMD, bone mineral density; HIV, human immunodeficiency virus; PL, active placebo; SD, standard deviation; ZOL, zoledronic acid.
aPatients with osteoporosis were not enrolled in the study. Baseline dual-energy X-ray absorptiometry measurements for 2 patients were performed with a different machine and were not included in the analyses.
Zoledronic Acid Blunted ART-induced Bone Resorption
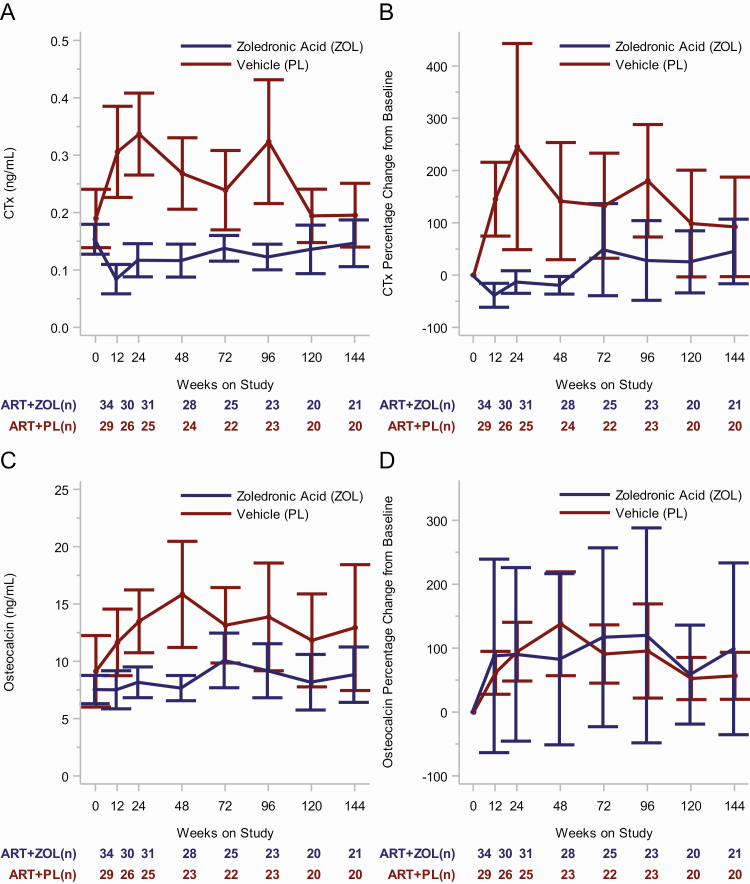
CTx in the treatment arms changed in significantly different ways (ie, different temporal patterns over time) during the 144 weeks of follow-up (P < .001, test for interaction between time on study and treatment arm). Mean CTx was similar in both treatment arms at randomization (0.154 vs 0.190 ng/mL for ZOL vs placebo, respectively; P = .22) but became significantly lower in the ZOL arm at 12 weeks, 24 weeks, and 48 weeks (all P < .001) as previously reported [19]. This trend continued through the 144 weeks of follow-up, with the mean CTx lower in the ZOL compared with the placebo arm at 72 weeks (n = 47; 0.138 vs 0.239 ng/mL; P = .007), 96 weeks (n = 46; 0.123 vs 0.324 ng/mL; P < .001), 120 weeks (n = 40; 0.136 vs 0.194 ng/mL; P = .07), and 144 weeks (n = 41; 0.147 vs 0.196 ng/mL; P = .17) (Figure 2A). Treatment with ZOL led to a 73%, 65%, and 57% reduction in mean bone resorption relative to placebo at 12, 24, and 48 weeks [19]. Whereas the ZOL treatment arm had a 62% reduction in mean bone resorption at 96 weeks (CTx mean difference, 0.201 ng/mL; 95% CI, 0.090–0.312 ng/mL), a 25% difference between the treatment arms at 144 weeks was not statistically significant (CTx mean difference, 0.049 ng/mL; 95% CI, −0.020 to 0.118 ng/mL). The CTx mean percentage increases from baseline to 12, 24, 48, 72, 96, 120, and 144 weeks were 145%, 244%, 140%, 130%, 190%, 108%, and 102%, respectively, in the placebo arm. The CTx mean percentage decreases from baseline to 12, 24, 48, and 72 weeks were 39%, 13%, 20%, and 49%, respectively, with an increase from baseline of 27% at 96 weeks (P = .47), 25% at 120 weeks (P = .40), and 45% at 144 weeks (P = .15) in the ZOL arm (Figure 2B; Table 2; Supplementary Tables 1 and 2). CTx results from multivariable repeated-measures analyses and post hoc subgroup analyses stratified by race, sex, and other clinical and demographic variables are summarized in Supplementary Table 3 and in the Supplementary Text.
Figure 2.
Longitudinal change in bone resorption outcomes by treatment arm. A, Model-based mean longitudinal changes in CTx by treatment arm and weeks on study. B, Model-based mean CTx percentage change from baseline by treatment arm and weeks on study. C, Model-based mean longitudinal changes in osteocalcin by treatment arm and weeks on study. D, Model-based mean osteocalcin percentage change from baseline by treatment arm and weeks on study. For each of the 4 panels, the vertical bars are the 95% confidence intervals and the numbers below time points signify the number of subjects in each treatment group at each time interval. Abbreviations: ART, antiretroviral therapy; CTx, C-terminal telopeptide of collagen; PL, active placebo; ZOL, zoledronic acid.
Table 2.
Baseline-Adjusted Means at 144 Weeks of Follow-up for Bone Resorption and Bone Mineral Density Outcomes by Treatment Arm
| Variable and Treatment | No. | Adjusted Mean (95% CI)a | Mean Difference (95% CI) | P |
|---|---|---|---|---|
| CTx, ng/mL | ||||
| ART + ZOL | 21 | 0.196 (.140, .251) | 0.049 (−.020, .119) | .1604 |
| ART + PL | 20 | 0.146 (.105, .187) | … | |
| Osteocalcin, ng/mL | ||||
| ART + ZOL | 21 | 12.966 (7.472, 18.459) | 4.160 (−1.851, 10.172) | .1741 |
| ART + PL | 20 | 8.805 (6.365, 11.246) | … | |
| Lumbar spine, g/cm2 | ||||
| ART + ZOL | 29 | 1.306 (1.258, 1.354) | −0.129 (−.199, −.057) | .0005 |
| ART + PL | 20 | 1.177 (1.125, 1.230) | … | |
| Lumbar spine, t score | ||||
| ART + ZOL | 29 | 0.780 (.368, 1.193) | −1.089 (−1.685, −.472) | .0005 |
| ART + PL | 20 | −0.298 (−.743, .146) | … | |
| Lumbar spine, z score | ||||
| ART + ZOL | 29 | 0.054 (−.338, .446) | −1.033 (−1.594, −.471) | .0004 |
| ART + PL | 20 | −0.979 (−1.380, −.577) | … | |
| Hip, g/cm2 | ||||
| ART + ZOL | 29 | 1.074 (1.035, 1.112) | −0.063 (−.128, .002) | .0556 |
| ART + PL | 20 | 1.010 (.958, 1.062) | … | |
| Hip, t score | ||||
| ART + ZOL | 29 | 0.018 (−.304, .341) | −0.487(−1.016, .041) | .0705 |
| ART + PL | 20 | −0.469 (−.888, −.050) | … | |
| Hip, z score | ||||
| ART + ZOL | 29 | −0.713 (−1.035, −.391) | −0.534 (−1.004, −.065) | .0259 |
| ART + PL | 20 | −1.247 (−1.589, −.906) | … | |
| Femoral neck, g/cm2 | ||||
| ART + ZOL | 29 | 1.058 (1.016, 1.100) | −0.046 (−.112, .020) | .1712 |
| ART + PL | 20 | 1.012 (.961, 1.064) | … | |
| Femoral neck, t score | ||||
| ART + ZOL | 29 | 0.064 (−.286, .414) | −0.357 (−.899, .185) | .1956 |
| ART + PL | 20 | −0.293 (−.707, .121) | … | |
| Femoral neck, z score | ||||
| ART + ZOL | 29 | −0.571 (−.868, −.274) | −0.362 (−.827, .1026) | .1261 |
| ART + PL | 20 | −0.934 (−1.291, −.576) | … |
Abbreviations: ART, antiretrovial therapy; CI, confidence interval; CTx, C-terminal telopeptide of collagen; PL, active placebo; ZOL, zoledronic acid.
aAdjusted mean defined as the predicted response value obtained by fitting the regression equation for each treatment arm at the mean baseline value for the 2 treatment arms and estimated using analysis of covariance at 144 weeks for each outcome.
Bone Formation Was Not Adversely Impacted by Zoledronic Acid
Osteocalcin in the 2 treatment groups changed in similar ways (similar temporal patterns over time) during follow-up (P = .35, test for interaction between time on study and treatment group). A compensatory increase in osteocalcin was observed in the placebo arm but not in the ZOL arm (P = .007) when testing the time-averaged differences between the 2 treatment groups. Mean difference in osteocalcin between the arms pooled over the 144-week follow-up period was −4.2 ng/mL (95% CI, −10.2 to −1.9 ng/mL) (Figure 2C). While mean differences in osteocalcin at 12, 24, and 48 weeks differed significantly [19], they did not significantly differ at 96 or 144 weeks (P = .08 and P = .18, respectively). Respective mean differences at 96 and 144 weeks were −4.7 ng/mL (95% CI, −10.0 to 0.6 ng/mL) and −4.1 ng/mL (95% CI, −10.1 to 1.9 ng/mL). In the placebo arm, the osteocalcin mean percentage increases from baseline to 96 and 144 weeks were 95% (95% CI, 21–169%) and 55% (95% CI, 18–92%), respectively. The osteocalcin mean percentage increases from baseline to 96 and 144 weeks did not change in the ZOL arm: 113% (95% CI, −44% to 271%) and 91% (95% CI, −29% to 211%), respectively (Figure 2D; Supplementary Tables 4 and 5).
Zoledronic Acid Prevented Antiretroviral Therapy–Induced Bone Mineral Density Loss
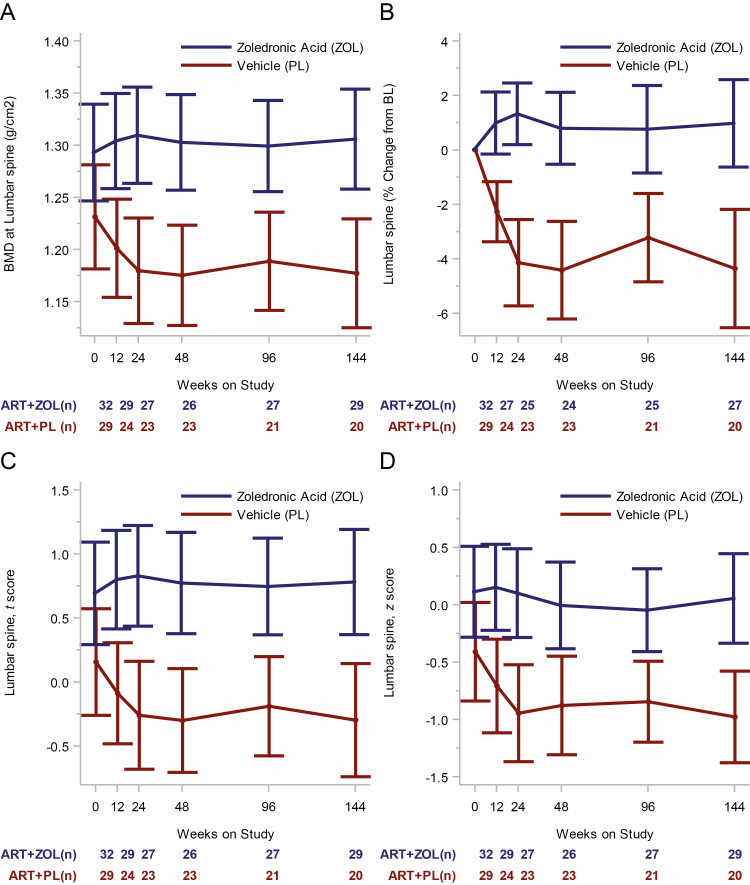
Lumbar spine BMD in the treatment arms changed in significantly different ways (ie, different temporal patterns over time) during the 144 weeks of follow-up (P < .001, test for interaction between time on study and treatment arm). Mean lumbar spine BMD was similar in both treatment arms at randomization (P = .08) but became significantly higher in the ZOL arm at 12, 24, and 48 weeks [19], and this effect persisted through 96 weeks (1.299 vs 1.189 g/cm2; P < .001) and 144 weeks (1.306 vs 1.177 g/cm2; P < .001) (Figure 3A). Zoledronic acid significantly increased lumbar spine BMD at 12, 24, and 48 weeks [19] and, relative to placebo, led to a mean difference in lumbar spine BMD at 96 and 144 weeks of 0.111 g/cm2 (9% increase) and 0.129 g/cm2 (11% increase), respectively. Bone mineral density at the lumbar spine did not change from baseline to 144 weeks in the ZOL arm (mean percentage increase of 1.0%; 95% CI, −0.61% to 2.61%; P = .22) but decreased by −4.3% (95% CI, −6.48% to −2.15%; P < .001) in the placebo arm (Figure 3B; Supplementary Tables 6–9). In the ZOL arm, the hip and the femoral neck BMD was preserved up to week 48; however, a small but significant decline from baseline by 144 weeks was observed at these sites—for the hip BMD: mean decline, 0.016 g/cm2; (95% CI, 0.001–0.031; P = .04; for the femoral neck BMD: mean decline, 0.021 g/cm2; 95% CI, 0.003–0.041; P = .02 (Supplementary Figure 1; Supplementary Tables 10–14). At baseline, the number of particpants with osteopenia (T-score between −1.0 and −2.5) in any area in the placebo arm was 10 (34.5%) and was 7 (21.9%) in the ZOL arm. No participant was osteoporotic (T-score ≤ −2.5) at baseline. The 29 participants in the ZOL arm who completed the week-144 visit maintained their baseline BMD status (number with osteopenia, 6; 21%). By contrast, of the 20 participants who completed the week-144 visit in the placebo arm, 8 (40%) were osteopenic (T-score between −1.0 and −2.5) and 1 participant developed osteoporosis.
Figure 3.
Longitudinal change in lumbar spine BMD outcomes by treatment arm and weeks on study. A, Model-based mean longitudinal changes in BMD at the lumbar spine by treatment arm and weeks on study. B, Model-based mean BMD at the lumbar spine percentage change from BL by treatment arm and weeks on study. C, Model-based mean longitudinal changes in lumbar spine t scores by treatment arm and weeks on study. D, Model-based mean longitudinal changes in lumbar spine z scores by treatment arm and weeks on study. For each of the 4 panels, the vertical bars are the 95% confidence intervals, and the numbers below time points signify the number of subjects in each treatment group at each time interval. Abbreviations: ART, antiretroviral therapy; BL, baseline; BMD, bone mineral density; PL, active placebo; ZOL, zoledronic acid.
Zoledronic Acid Treatment Did Not Impact the Rate of Virologic Suppression or Immunologic Response
Supplementary Figure 2A summarizes the cumulative initial virologic suppression by treatment arm (P = .34, log-rank test). By 144 weeks, initial virologic suppression was 91% in the ZOL arm and 100% in the placebo arm. CD4+ T-cell counts in the 2 treatment arms increased over time (P < .001). Neither the pattern of change (P = .60) nor the difference between treatment arms was significant (P = .20) (Supplementary Figure 2B; Supplementary Table 15).
Serious Adverse Effects, Adverse Effects, and Laboratory Toxicities
Over the 144 weeks of follow-up, no serious adverse effects (SAEs) were reported to be possibly or definitively related to ZOL treatment. Serious adverse effects were similar between the ZOL and placebo arms. Supplementary Table 16 summarizes patient-reported adverse effects by treatment arm. Supplementary Table 17 summarizes laboratory toxicities. There were no statistically significant differences between treatment arms for the incidence of any grade 3 or higher laboratory toxicities during 144 weeks of follow-up.
DISCUSSION
In this study, we extend our findings reported previously through the first 48 weeks following ART initiation [19]. We demonstrate that ART-associated bone resorption and BMD loss extend beyond the first 2 years of therapy, and that a single dose of ZOL given at the time of therapy initiation durably blunts these effects.
Prior studies have shown the vast majority of ART-associated bone loss occurs 1–2 years after therapy initiation, with subsequent stabilization in BMD thereafter [20]. In this report, ART initiation led to a surge in bone resorption in patients randomized to the placebo arm, peaking at 24 weeks and persisting through 96 weeks. ZOL ameliorated this increase resorption, resulting in a 62% reduction in mean bone resorption at 96 weeks, and 9–11% higher lumbar spine BMD at 96–144 weeks, relative to placebo. Current guidance for the management of bone disease in HIV provides basic recommendations for all PWH and those specifically diagnosed with osteoporosis [10]. For PWH initiating ART, it is recommended to avoid certain antiretrovirals known to be detrimental to bone (ie, TDF and protease inhibitors) [10]. Our findings suggest that additional strategies to minimize the risk of ART-induced skeletal deterioration may be warranted, such as prophylactic antiresorptive therapy, and that the benefits of such intervention may extend beyond the first 2 years following ART initiation. Such preemptive therapy may be of particular benefit to PWH with multiple risk factors for fragility bone disease or with low BMD at the onset of ART.
The rate of loss in BMD experienced by PWH initiating ART is similar to that seen during the rapid phase of bone loss in postmenopausal osteoporosis [21]. However, the pathogenesis of bone loss in these groups differs starkly and, furthermore, HIV-specific demographic and clinical characteristics require special consideration. First, bone loss and fragility fracture in the setting of HIV infection occur at a much younger age, with data [6] suggesting at least a decade earlier than HIV-seronegative individuals [3, 6]. Second, the skeletal health of PWH is compromised at baseline (pre-ART) due to a variety of intrinsic and extrinsic factors [9, 22]. Intrinsically, effects of HIV on inflammation, the adaptive immune dysregulation, and disruption in the integrity of the immunoskeletal interphase have been well described [23–26]. Direct viral effects on bone were recently reported by Raynaud-Messina et al [27]. Extrinsically, traditional risk factors for low BMD are highly prevalent in PWH, further compounding the risk of fragility bone disease in this population [28]. Finally, initiation of ART paradoxically worsens bone health [14, 29]. These considerations underscore the pressing need to develop HIV-specific preventive and therapeutic strategies for alleviating bone disease in this high-risk population. Further, an epidemic of other metabolic comorbidities and aging add urgency to this clinical priority for PWH [8, 30].
The role of calcium/vitamin D supplementation in improving skeletal health in PWH remains unclear and may depend on baseline 25-hydroxyvitamin D levels, ART status, and dosing of supplementation [23–25]. Our data suggest that the use of bisphosphonates to reduce ART-induced bone loss in PWH is promising. While we observed a significant decrease in lumbar spine BMD of PWH initiating ART in the placebo arm at 144 weeks, no change was observed in lumbar spine BMD in the ZOL arm; however, significant increases in hip and femoral neck BMD were found in ZOL-treated PWH at this time point. Zoledronic acid has also been studied in ART-treated PWH with low BMD and was shown to be superior to TDF-switching at increasing BMD [26]. A study of ART-treated men with HIV with low BMD at baseline who received 2 annual doses of ZOL (4 mg) demonstrated favorable effects on bone turnover and BMD that persisted over nearly 2 years [31].
Although the sample size was relatively small, ZOL did not suppress bone formation in our study population as has been previously reported in other studies [28]. This is important because prolonged use of bisphosphonates may cause low bone turnover that has been associated with poorly remodeled bone susceptible to microcracks [32]. Further, ZOL at a single dose was safe and well tolerated, and we observed comparable virologic suppression and magnitude of CD4+ T-cell reconstitution between treatment arms.
Our phase IIb clinical trial was a proof-of-concept study conducted at a single site and therefore has several limitations. Generalizability of our findings is limited by the small sample size and homogeneous study population (eg, predominantly African-American men). The 144-week study duration could not evaluate the impact of our intervention on long-term bone outcomes such as fracture.
In conclusion, a single infusion of ZOL at the time of ART initiation blunted ART-induced bone resorption and prevented bone loss in nonosteoporotic PWH. Importantly, preserved BMD was observed at key fracture-prone anatomical sites through week 144, during which time ZOL was well tolerated. These data provide a robust framework for the design and execution of larger confirmatory phase 3, multicenter, randomized clinical trials investigating the efficacy of antiresorptive therapy in mitigating ART-induced bone loss in PWH.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. I. O. and M. N. W. conceived the original idea with contributions from J. L. L.; I. O. and L. F. C. wrote the first draft with contributions from M. N. W., K. A. E., and L. W.; K. T. performed all laboratory assays and contributed to data analysis; K. A. E. and L. W. were responsible for data management and statistical analysis; J. L. L., C. D. L., A. F., C. A. M., and A. N. S. were part of the investigating team and were involved with outcomes and safety monitor and manuscript development. All authors read the current version of the manuscript.
Acknowledgments. The authors are grateful to all the patients who participated in this study. They also thank Allison Ross Eckard in the Departments of Pediatrics and Medicine, Division of Infectious Diseases, at the Medical University of South Carolina, and Albert Anderson, Department of Medicine, at Emory University for service as the medical safety monitor.
Disclaimer. The study was sponsored by the the National Institutes of Health (NIH), and zoledronic acid (ZOL) and its active placebo were provided by Novartis. The NIH and Novartis did not play any additional role in the study other than financial support and furnishing of ZOL and placebo, respectively.
Financial support. Research reported in this publication was supported by the National Institute on Aging (NIA) (award number R01AG040013). Additional support was provided by (NIA award number U54AG062334) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (award numbers R01AR059364, R01AR070091, and R01AR068157 to M. N. W. and I. O.). M. N. W. is also supported by a grant from the Biomedical Laboratory Research and Development Service of the VA Office of Research and Development (grant number 5I01BX000105). L. F. C is also supported by the National Center for Advancing Translational Sciences (NCATS) of the NIH (award numbers UL1TR002378 and TL1TR002382). K. T. is also supported by the National Heart, Lung, and Blood Institute of the NIH (award number K01HL131333). C. A. M. is also supported by NCATS of the NIH (award numbers ULTR002378 and KL2TR002381). A. N. S is also supported by the National Institute of Allergy and Immunology (NIAID) of the NIH (award number K23AI114407). C. D. L. is also supported by the NIAID of the NIH (award number K23AI124913). The authors gratefully acknowledge services provided by the Emory Center for AIDS Research, funded though NIAID (P30AI050409), and the Georgia Clinical and Translational Science Alliance, funded though the NCATS (UL1TR000454).
Potential conflicts of interest. A. N. S. reports a research grant to Emory University from Gilead, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Samji H, Cescon A, Hogg RS, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aberg JA. Aging, inflammation, and HIV infection. Top Antivir Med 2012; 20:101–5. [PMC free article] [PubMed] [Google Scholar]
- 3. Moran CA, Weitzmann MN, Ofotokun I. Bone loss in HIV infection. Curr Treat Options Infect Dis 2017; 9:52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab 2008; 93:3499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prieto-Alhambra D, Güerri-Fernández R, De Vries F, et al. . HIV infection and its association with an excess risk of clinical fractures: a nationwide case-control study. J Acquir Immune Defic Syndr 2014; 66:90–5. [DOI] [PubMed] [Google Scholar]
- 6. Gonciulea A, Wang R, Althoff KN, et al. . An increased rate of fracture occurs a decade earlier in HIV+ compared with HIV- men. AIDS 2017; 31:1435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharma A, Shi Q, Hoover DR, et al. . Increased fracture incidence in middle-aged HIV-infected and HIV-uninfected women: updated results from the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr 2015; 70:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smit M, Brinkman K, Geerlings S, et al. ; ATHENA Observational Cohort Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 15:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ofotokun I. Deciphering how HIV-1 weakens and cracks the bone. Proc Natl Acad Sci USA 2018; 115:2551–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown TT, Hoy J, Borderi M, et al. . Recommendations for evaluation and management of bone disease in HIV. Clin Infect Dis 2015; 60:1242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sax PE, Wohl D, Yin MT, et al. ; GS-US-292-0104/0111 Study Team Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015; 385:2606–15. [DOI] [PubMed] [Google Scholar]
- 12. Mills A, Crofoot G Jr, McDonald C, et al. . Tenofovir alafenamide versus tenofovir disoproxil fumarate in the first protease inhibitor-based single-tablet regimen for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr 2015; 69:439–45. [DOI] [PubMed] [Google Scholar]
- 13. Mills A, Arribas JR, Andrade-Villanueva J, et al. ; GS-US-292-0109 Study Team Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis 2016; 16:43–52. [DOI] [PubMed] [Google Scholar]
- 14. Ofotokun I, Titanji K, Vunnava A, et al. . Antiretroviral therapy induces a rapid increase in bone resorption that is positively associated with the magnitude of immune reconstitution in HIV infection. AIDS 2016; 30:405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ofotokun I, Titanji K, Vikulina T, et al. . Role of T-cell reconstitution in HIV-1 antiretroviral therapy-induced bone loss. Nat Commun 2015; 6:8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ofotokun I, Titanji K, Lahiri CD, et al. . A single-dose zoledronic acid infusion prevents antiretroviral therapy-induced bone loss in treatment-naive HIV-infected patients: a phase IIb trial. Clin Infect Dis 2016; 63:663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lennox JL, Landovitz RJ, Ribaudo HJ, et al. ; ACTG A5257 Team Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Intern Med 2014; 161:461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement 2000; 17:1–45. [PubMed] [Google Scholar]
- 19. Diggle PJ, Liang K, Zeger SL.. Analysis of longitudinal data. Oxford: Clarendon Press, 1994:68–77. [Google Scholar]
- 20. Bolland MJ, Wang TK, Grey A, Gamble GD, Reid IR. Stable bone density in HAART-treated individuals with HIV: a meta-analysis. J Clin Endocrinol Metab 2011; 96:2721–31. [DOI] [PubMed] [Google Scholar]
- 21. Finkelstein JS, Brockwell SE, Mehta V, et al. . Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 2008; 93:861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weitzmann MN, Ofotokun I. Physiological and pathophysiological bone turnover—role of the immune system. Nat Rev Endocrinol 2016; 12:518–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yin MT, RoyChoudhury A, Bucovsky M, et al. . A randomized placebo-controlled trial of low- versus moderate-dose vitamin D3 supplementation on bone mineral density in postmenopausal women with HIV. J Acquir Immune Defic Syndr 2019; 80:342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Overton ET, Chan ES, Brown TT, et al. . Vitamin D and calcium attenuate bone loss with antiretroviral therapy initiation: a randomized trial. Ann Intern Med 2015; 162:815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yin MT, Chan ES, Brown TT, et al. . Vitamin D does not modulate immune-mediated bone loss during ART initiation. Antivir Ther 2019. doi: 10.3851/IMP3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoy JF, Richardson R, Ebeling PR, et al. ; ZEST Study Investigators Zoledronic acid is superior to tenofovir disoproxil fumarate-switching for low bone mineral density in adults with HIV. AIDS 2018; 32:1967–75. [DOI] [PubMed] [Google Scholar]
- 27. Raynaud-Messina B, Bracq L, Dupont M, et al. . Bone degradation machinery of osteoclasts: An HIV-1 target that contributes to bone loss. Proc Natl Acad Sci USA 2018; 115:E2556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Black DM, Delmas PD, Eastell R, et al. ; HORIZON Pivotal Fracture Trial Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356:1809–22. [DOI] [PubMed] [Google Scholar]
- 29. Grant PM, Kitch D, McComsey GA, et al. . Low baseline CD4+ count is associated with greater bone mineral density loss after antiretroviral therapy initiation. Clin Infect Dis 2013; 57:1483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong C, Gange SJ, Moore RD, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Multimorbidity among persons living with human immunodeficiency virus in the United States. Clin Infect Dis 2018; 66:1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bolland MJ, Horne AM, Briggs SE, et al. . Effects of intravenous zoledronate on bone turnover and bone density persist for at least 11 years in HIV-infected men. J Bone Miner Res 2019; 34:1248–53. [DOI] [PubMed] [Google Scholar]
- 32. Shane E. Evolving data about subtrochanteric fractures and bisphosphonates. N Engl J Med 2010; 362:1825–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.