Abstract
Theoretical models distinguish between neural responses elicited by distal threats and those evoked by more immediate threats1–3. Specifically, slower, “cognitive” fear responses involve a network of brain regions including ventral hippocampus (vHPC) and medial prefrontal cortex (mPFC), while immediate, “reactive” fear defensive responses rely on regions such as the periaqueductal gray (PAG)4,5. It is unclear, however, how anxiety and its neural substrates relate to these distinct defensive survival circuits. We tested whether individual differences in trait anxiety would impact escape behavior and neural responses to slow and fast attacking predators: conditions designed to evoke “cognitive” and “reactive” fear, respectively. Behaviorally, we found that trait anxiety was not related to escape decisions for fast threats, but individuals with higher trait anxiety escaped earlier during slow threats. Functional MRI showed that when subjects faced slow threats, trait anxiety positively correlated with activity in the vHPC, mPFC, amygdala and insula. Further, the strength of the functional coupling between the vHPC and mPFC was correlated with the degree of trait anxiety. These findings suggest that anxiety plays little or no role in escape under conditions of proximal threat. Instead, anxiety affects “cognitive” fear circuits that are involved in volitional strategic escape.
Anxiety is often described as an enduring, conscious state of apprehension. Theoretical work6–8 proposes that anxiety is an emotional state independent from fear, which is instead evoked when a threat is increasingly proximal, and which ought to be minimally influenced by the anxiety state of the organism3,9. While this is generally well recognized in the non-human animal literature, researchers in the field of human affective neuroscience have paid relatively little attention to the question of whether anxiety and fear have different associated neural circuitry, and under what conditions anxiety might influence defensive behaviors in ecological scenarios. Moreover, recent advances have distinguished different classes of defensive responses which rely on distinct neural circuits, and which may complicate the theoretical relationship between fear and anxiety4,5.
Non-human animal research has shown that anxiety states involve a well-defined set of neural circuits10. The vHPC and mPFC are of particular interest as they have repeatedly been shown to be recruited during the regulation and representation of anxiety provoking features of the environment11–14. The vHPC has input into the mPFC and it appears to be the interaction between these regions that drives anxiety related behaviors12. More recently, CA1 cells in the vHPC have been shown to exhibit stable representations of anxiety provoking environments and these cells drive avoidance behaviors15.
In humans, functional magnetic resonance imaging (fMRI) has been employed in conjunction with “active escape” paradigms, the goal of which is to evade an artificial predator with the capacity to chase, capture and shock the subject. Studies have shown that when an artificial predator is distant, increased activity is observed in the ventromedial prefrontal cortex (vmPFC)4. However, as the artificial predator moves closer, a switch to enhanced activation in the midbrain PAG is observed4. More recently, using a novel escape decision task, work from our lab has supported a similar “cognitive” and “reactive” fear differentiation of defensive survival circuits, by showing that fast escape decisions are associated with activity in the PAG5, a region shown previously to be involved in reactive flight4, while slower escape decisions rely on the vHPC, posterior cingulate cortex and mPFC5, a circuit implicated in behavioral flexibility and internal risk assessment16.
The vHPC-mPFC anxiety circuit therefore overlaps with the “cognitive” fear circuit recruited during these slower escape decisions3, but appears to be independent from “reactive” fear regions that are involved with threat under limited time constraints. In general, these “reactive” fear areas (e.g. PAG) have limited interaction with higher level cortical brain regions, thus are unlikely to be implicated in anxiety. Thus, it is possible that while anxiety plays no role during imminent threat (when “reactive” fear circuits are recruited), it may be important within “cognitive” fear circuits, and subsequently affect defensive behavior in the face of less imminent threats.
In order to provide evidence for this possibility, a critical question is whether individual differences in levels of trait anxiety will selectively affect “cognitive” fear circuits during defensive decision making, or whether “reactive” fear circuits are also influenced by the trait anxiety of the individual. Moreover, it is equally important to determine whether there are commensurate changes in survival behaviors and decision making as a result of differences in trait anxiety, as would be expected if anxiety has an ethological origin6.
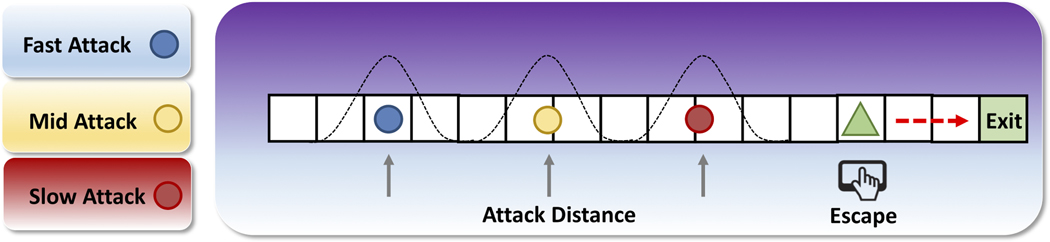
To address these questions, we reanalyzed behavioral and neural data collected in our previously published study5, along with previously unanalyzed trait anxiety data (the Spielberger State-Trait Anxiety Inventory; STAI-Y17). In each trial of the behavioral task, participants passively earned money while they encountered virtual predators of three colors, each representing different attack distances (Figure 1a). These attack distances were drawn from Gaussian distributions that were unique to the particular predator type. Fast attack predators (i.e. far or early attacking) were characterized by the virtual predator quickly switching from slow approach to fast attack velocity, therefore requiring the subject to make quick escape decisions. On the other hand, slow attack predators (i.e. close or late attacking) slowly approached for longer time periods, resulting in larger buffer zones and more time to contemplate escape. (It is important to emphasize that “fast” and “slow” here describe the timing of the predator attack, not the speed of the predators.) The goal of the task was to try and successfully escape, while at the same time maximizing the amount of money earned by fleeing as late as possible (i.e. at the shortest distance from the predator, or flight initiation distance, FID).
Figure 1.
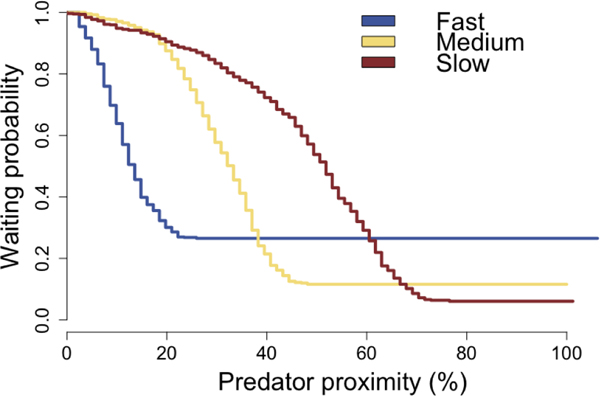
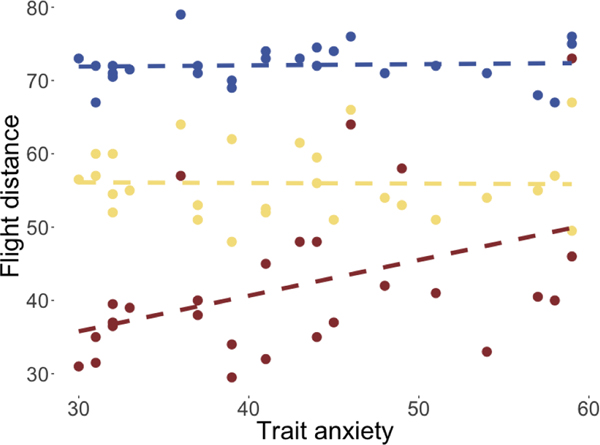
Flight initiation distance paradigm and behavioral results.</p>(a) Predator escape paradigm. In each trial, participants were presented with a cue indicating the predator type. The predator would appear on the left side of the runway, and slowly move toward the participant (green triangle). Participants passively accrued money while they waited, but at any time could press a button to begin their escape toward the exit. The predator would speed up (attack) at a random distance drawn from the respective Gaussian distributions shown above. If participants were caught by the predator, they would receive a mild electric shock and lose any money accrued on that trial.</p>(b) Kaplan-Meier survival curves for each predator type, as a function of predator proximity. Curves reflect pooled data from all subjects.</p>(c) Flight initiation distance for each predator type, as a function of STAI-Y scores. Each dot corresponds to a single subject’s median FID in one condition. Dashed lines show the linear fit to the data.
Subjects performed this task while undergoing functional magnetic resonance imaging (fMRI) in order to assess the relative contributions of the “reactive fear” and “cognitive fear” networks to their escape decisions, and whether behavior or brain activity in these circuits varied as a function of trait anxiety. Given the theoretical and neural differentiation between “reactive fear” and “cognitive fear”, we hypothesized that individuals with high trait anxiety would show preferential activity in the “cognitive fear” circuitry, but not the “reactive fear” circuitry. We also hypothesized that individuals scoring higher in trait anxiety would make earlier escape decisions, but only when there is sufficient time to assess threat.
To test the hypothesis that trait anxiety would affect escape decisions, we estimated a mixed effects linear regression model, with subjects’ median FIDs as the dependent variable, and predator type and STAI-Y scores1 as the independent variables (Table 1). Relative to the fast predator type, we observed the expected effects of the medium (β = −17.88, SE = 2.23, p < 0.001) and slow (β = −52.22, SE = 2.26, p < 0.001) predator types. Importantly, we observed a significant interaction effect between the slow predator type and STAI-Y scores (β = 0.57, SE = 0.05, p < 0.001), suggesting that trait anxiety and FID were related, but only for the slow predator condition (see Figure 1c)2.
Table 1.
Linear regression of predator type and STAI-Y scores on flight initiation distance.
|
Dependent variable: |
|
|---|---|
| Flight initiation distance | |
| Medium predator | −17.879*** (−22.252, −13.507) |
| Slow predator | −52.219*** (−56.646, −47.792) |
| STAI-Y | −0.010 (−0.212, 0.192) |
| Medium predator:STAI-Y | 0.072 (−0.029, 0.173) |
| Slow predator:STAI-Y | 0.567*** (0.465, 0.668) |
| Constant | 72.239*** (63.395, 81.083) |
| Observations | 1,691 |
| Log Likelihood | −5,892.115 |
| Akaike Inf. Crit. | 11,800.230 |
| Bayesian Inf. Crit. | 11,843.690 |
Note:
p<0.1;
p<0.05;
p<0.01
Note that because participants were given electrical stimulation when they were caught by the virtual predator, in order to obviate interference it was necessary to exclude these trials from the imaging analysis reported below. For consistency, the behavioral analysis above also excluded unsuccessful escape trials. However, unsuccessful escape trials still contain information about subjects’ tolerance to predator distance. To ensure that the analyses above were not biased by this possibility, we adopted a technique from survival analysis, which allowed us to take into account the unsuccessful trials as censored data. To appropriately prepare the data for this analysis (which is more commonly used to model time-based responses rather than distance-based responses) we transformed the dependent variable of FID by subtracting FID from the maximum FID, then normalizing this by the maximum FID. This new dependent variable can be thought of as predator proximity, expressed as a percentage. The Kaplan-Meier estimated survival curves (i.e. probability of waiting as a function of predator proximity) for each predator are shown in Figure 1b.
To control for the potential effect of data censoring, we repeated the analysis of behavioral data using a mixed effects Cox regression model on the probability of flight responses over time, which took into account predator type and participant heterogeneity. This model again revealed the expected effects of the medium (β = −0.98, SE = 0.29, z = −3.34, p < 0.001) and slow (β = −3.09, SE = 0.3, z = −10.43, p < 0.001) predator types. Importantly, it also again revealed a significant interaction effect between the slow predator type and STAI-Y scores (β = 0.05, SE = 0.01, z = 6.74, p < 0.001). This effect had a hazard ratio of 1.05, equivalent to a 5% increase in chance of fleeing per unit increase of STAI-Y.
The results above provide clear evidence that trait anxiety influences subjects’ propensity to escape earlier when given enough time to prepare an escape. However, it is unclear whether this should negatively affect their economic performance in the task. To test this, we performed a two-way repeated measures ANOVA, with predator type and STAI-Y scores as independent variables, and subjects’ cumulative total earnings as the dependent variable. Given that subjects could earn more money in the slow predator condition, we first standardized reward scores for each predator type. There was no significant effect of predator type on standardized earnings (F(2,52) = 0.34, p = 0.667, ε = 0.81), but we observed a significant main effect of STAI-Y scores on total earnings (F(1,26) = 4.32, p = 0.048, = .09), suggesting that subjects with higher STAI-Y scores had poorer economic performance in the task, across all predator types. There was no interaction effect of STAI-Y scores and predator type (F(2,52) = 0.36, p = 0.656, ε = 0.81).
Although economic gain is an index of performance in this task, it could be argued that the more ecologically important performance measure is escape success. Notably, subjects’ economic performance and proportion of escape trials were not significantly correlated across all predator types (r(26) = .09, p = .643)3. To test whether trait anxiety was related to how frequently subjects successfully escaped the predators, we again performed a two-way repeated measures ANOVA, with predator type and STAI-Y scores as independent variables, and the proportion of successful escape trials as the dependent variable. While there were no main effects of STAI-Y scores (F(1,26) = 0.23, p = 0.633) or predator type (F(2,52) = 1.89, p = 0.175, ε = 0.53), the ANOVA revealed a significant interaction effect between STAI-Y scores and predator type (F(2,52) = 4.46, p = 0.031, ε = 0.68, = 15). Simple effects analyses (one-way repeated measures ANOVAs within each predator type) revealed a significant effect only for the slow predator type (F(1,26) = 5.49, p = 0.027, = . 17), but not for the fast (F(1,26) = 2.12, p = 0.158) or medium predator (F(1,26) = 0.39, p = 0.536). This suggested that, similar to the analysis of FID above, STAI-Y score was positively related to escape success only in the slow predator condition. Overall, these results show that subjects with higher trait anxiety tended to more successfully escape predators, but that this also negatively impacted how much money they earned in the task (a summary of performance measures can be found in the Supplementary materials, in Table 5).
We next tested our hypothesis that only during slow attack would we see a positive correlation between trait anxiety and activity in the “cognitive fear” circuitry. For this analysis we excluded unsuccessful escape trials due to the interference of the electric stimulation on BOLD response (mean trials excluded were 6.88, 3.71, and 3.37, per subject, out of 23, 24 and 25, for the fast, medium and slow predator types, respectively). We focused on the 2 seconds prior to participants’ flight initiation responses, which allowed us to examine the neural activity in anticipation of the escape response (detailed methodology of the base fMRI analysis can be found in5). We first contrasted the slow attacking predator condition with the fast attacking predator condition. We then used participants’ STAI-Y score scores as 2nd level regressors for this contrast, such that any significant increase in activity would indicate positive modulation by trait anxiety for the slow predator condition (for a similar analysis using contrasts for the slow and fast predators against a control condition, see Supplementary Materials).
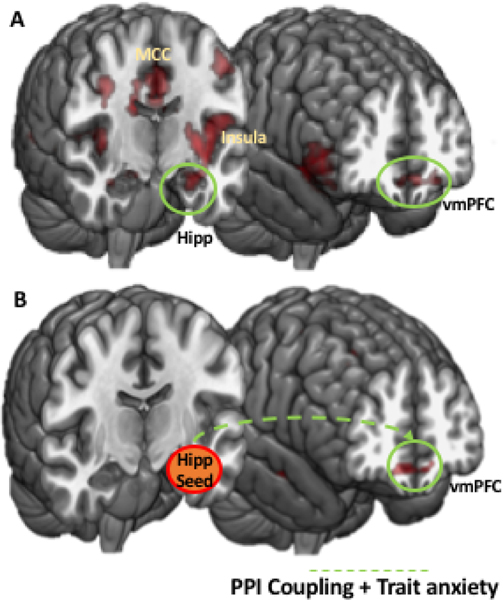
After thresholding and correction, we observed significant BOLD responses in regions including amygdala, hippocampus, vmPFC and midcingulate cortex (Figure 2A, Table 2). This was consistent with our hypothesis, and supported the behavioral findings whereby STAI-Y score exclusively influences escape decisions when the threat is distant (in the case of the slow attacking predator), but not when the threat is imminent (in the case of the fast attacking predators). A visualization of BOLD response as a function of trait anxiety for select regions is shown in Figure 4 in the Supplemental materials.
Figure 2.
A) Neural activity associated with STAI-Y score scores for the slow versus fast predator contrast. B) PPI coupled brain areas modulated by STAI-Y score. vmPFC, ventromedial prefrontal cortex; Hipp, hippocampus; MCC, mid-cingulate cortex.
Table 2.
Activation table for 2nd level STAI-Y score correlation for the slow versus fast predator contrast.
| Brain Region | Left/Right | Cluster Size | T-score | MNI coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Hippocampus | L | 60 | 5.32 | −15 | −27 | −6 |
| Postcentral Gyrus | L | 209 | 4.91 | −45 | −18 | 54 |
| Medial Prefrontal Cortex | L | 63 | 4.70 | −3 | 51 | −14 |
| Insula | L | 94 | 4.53 | −40 | 8 | −3 |
| Insula | R | 107 | 4.74 | 36 | 6 | −6 |
| Amygdala | R | 15 | 4.93 | 22 | 0 | −20 |
Note: p<0.05, FDR corrected
To assess the the interaction of brain regions involved in escape decisions, we performed a generalized psychophysiological interaction (gPPI) analysis18. Given the theoretical and empirically demonstrated involvement of the vHPC in cognitive fear and anxiety4,19, and because of its exhaustive bidirectional anatomical connections with the amygdala and its nuclei, as well as its functional role in fear, stress and emotion15,20,21, we chose vHPC as an independent seed region (see Supplemental materials for a corresponding analysis using the entire hippocampus as a seed region). A corresponding structural ROI was obtained using the WFU Pickatalas. This first level gPPI analysis on the slow versus fast predator contrast is reported in5, and will not be reported here for brevity. We then added STAI-Y score as a regressor in a second level analysis. STAI-Y score significantly modulated the functional coupling between vHPC seed, bilateral mPFC, right IFG, the left insula (Figure 2, Table 6 in Supplemental materials). Overall, this suggests that these macrocircuits are those that facilitated the impact of STAI-Y score on escape decisions in the slow predator condition.
Our results provide evidence that trait anxiety can influence escape decisions, but only under conditions of relatively prolonged threat, compared to more imminent threats2,6,22. This disassociation implies that trait anxiety selectively affects decisions of different ethnological classes, distinguished by the amount of time afforded for reflection and cognitive strategizing. The notion of a dichotomous mapping between temporally proximal threats and fear, and temporally distal threats and anxiety is not new. For example, rodents’ defensive behavior differs when threat is distal versus when it is immediate23, and anxiolytic drugs appear to only affect the former8. Likewise, previous models of threat evaluation have suggested that both anxious and non-anxious individuals will respond similarly to proximal threats, but individuals with high anxiety will exhibit differential behavior to more distal threats1. However, this is the first empirical study to show that trait anxiety selectively impacts escape decisions in humans under this specific class of threat.
The interpretation that trait anxiety affects only “cognitive” fear behavior was supported with our neuroimaging results. These results showed that brain areas previously indicated to be involved with behavioral flexibility and information processing aspects of fear responses (including hippocampus, amygdala, mPFC and insula4,5,16) covaried with trait anxiety. However, areas associated with “reactive” fear - the PAG, superior colliculus, mid-cingulate cortex and central nucleus of the amygdala4,5,24 - were not significantly affected by variability in anxiety. Notably, these findings strongly support theories based on defensive distance25, whereby defensive responses to immediate threats and dangers map onto low-level brain areas such as the PAG, whereas responses to physically or psychologically distal or anticipated threats map to higher-level areas such as the PFC24,26. Our findings extend these theories by providing a clear disassociation of the effects of trait anxiety on one circuit over the other, with accompanying behavioral effects, in an ecologically relevant paradigm.
These seed-based functional coupling results are consistent with previous non-human animal studies showing functional interactions between the ventral and dorsal hippocampus and vmPFC in anxiety provoking environments11,12,27. For example, local field potential recordings in rodents have shown that there is synchrony in theta oscillatory activity between vHPC and mPFC, and that this synchrony is increased in anxiogenic environments12. In addition, single unit recordings have shown that cells in mPFC have stronger anxiety-related firing patterns when phased-locked with local field potentials in the vHPC11. Using magnetoencephalography, others have corroborated these non-human animal findings in humans28. Our results parallel both the human and non-human animal evidence for functional coupling between the vHPC and mPFC, and further consolidate the characterization of this interaction with a different brain imaging method.
The specific nature of the coupling between the vHPC and mPFC has garnered some previous discussion. For example, because vHPC-mPFC connections are unidirectional29, it has been suggested that the vHPC primes mPFC to represent anxiety-related features of the environment, possibly using memories of threats to estimate threat probability28. MPFC has efferent projections to amygdala and PAG, and these connections have been suggested to be the downstream areas responsible for the initiation of defensive behavioral responses24,30, and the inhibition of exploratory behaviors11. To complement this, vHPC also has direct projections to BLA, BNST, and the lateral hypothalamic area (LHA), which can also facilitate anxiety responses15.
In light of the results from our study, it is possible that vHPC may encode the previously learned threat context (i.e. the predator condition), and relay this information to the mPFC where it influences strategic decision making. Our results suggest that the observed increase in connectivity between vHPC and mPFC in trait anxious individuals may reflect a priming mechanism which lowered the threshold for escape responses, resulting in earlier escape decisions10. However, for the fast predator condition, this slow, deliberative priming is not sufficient, and thus the initiation of behavioral responses appears to bypass this connection. One compelling question is whether trait anxiety merely interacts with this vHPC-mPFC mechanism, or whether it can be fully identified with information processing between these subregions. While we speculatively provide this neural mechanism for trait anxiety - which is also supported by the non-human animal literature - we emphasize that this requires causal corroboration, perhaps in the form of pharmaceutical manipulations in humans. Another further piece of evidence that would provide compelling support for such a mechanism would be trial-by-trial prediction of flight initiation distance using brain activity in vHPC-mPFC (an approach that our design lacked appropriate power for).
Notably, we did not observe modulation of BLA, BNST or LHA, by trait anxiety. One likely possibility is that these areas are involved in longer-term anxiety responses, requiring the recruitment of corticotropin-releasing hormone23, and that our slow predator condition was not adequately protracted to cause these responses. Given that BLA and amygdala have strong inputs to vHPC31,32, another possibility is that these areas are more commonly recruited during fear learning (which we did not examine), and imbue the encoding of environmental stimuli with emotional salience (e.g.33). Indeed, most empirical evidence of the increased involvement of the amygdala in trait anxious individuals has come from learning paradigms and studies of fear conditioning (e.g.34). Thus, trait anxiety is likely to affect both the encoding of threats, as well as their retrieval from memory, potentially via different neural substrates. This latter point may be of critical import for many clinical anxiety disorders (such as post traumatic stress disorder), where threats have already been learned. One further possibility, as suggested previously28, is that vHPC is specifically involved in threat memory retrieval only when there is approach-avoidance conflict35–37, as in the case with the trade-off between reward and threat of shock in our task.
Previous research has also suggested the possibility that mPFC representation of the environment depends of the strength of vHPC input: moderate input appropriately signals the aversiveness of specific features, but strong input decreases discriminative capability, leading to generalized anxiety responses11. In our study we were not able to evaluate individuals’ abilities to discriminate between different levels of threat, but this would be a promising avenue for future research. In particular, this might suggest that populations with clinical anxiety disorders may exhibit increased coupling between vHPC and mPFC across threat levels, and consequently faster escape decisions for all predator conditions.
The impact of trait anxiety on escape decisions could influence survival outcomes in at least two important ways38,39. Firstly, if individuals with high trait anxiety escape predators earlier, they expedite other behaviors, like foraging, and thus may accrue less primary rewards. Our results support this idea by showing that those with higher trait anxiety earned less total reward in our task. On the other hand, it could be argued that a more survival-relevant performance metric is successful escape - additional reward is irrelevant if caught by a predator. Our results also showed that individuals with higher trait anxiety made a higher proportion of successful escape decisions. However, unlike reward, which was affected across all predator conditions, individuals with higher trait anxiety only made a higher proportion of successful escape decisions within the slow predator condition, in line with the idea that trait anxiety only affects flight decisions under these contexts. One possible explanation for this difference may have been that trait anxiety also affected escape responses in the medium and fast predator conditions, but to a lesser, non-significant degree. This is especially possible considering that there is some individual variability both in trait anxiety and in performance in general, and thus our specification of “cognitive” and “reactive” fear classes will not have perfectly divided performance in these individuals. A series of experiments spanning a large range of predator conditions and reward contingencies may be able to address this issue with more clarity, and perhaps reveal population level differences in how trait anxiety influences performance. Ultimately, both the accrual of reward and successful escape are important factors for survival, and differences in trait anxiety appear to arbitrate between these, depending on threat context.
Coexisting with a disassociation of anxiety and fear based on defensive distance is a disassociation based on defensive direction26. The “direction” of this construct refers to approach / avoidance, and theoretical work proposes that fear drives avoidance of danger, while anxiety drives approach toward danger26. In our experimental design, an approach avoidance conflict existed between reward and the threat of shock. Because the slow predator condition allowed individuals to earn greater reward, this condition may have elicited greater relative anxiety. Under the defensive direction framework, we may have expected participants with higher trait anxiety to endure longer in this condition. However, we found that individuals scoring higher in trait anxiety escaped earlier, which speaks against defensive direction as a potential explanation for our behavioral results. It would be of interest however, for future experiments to more closely examine how defensive direction and trait anxiety relate to each other (see Supplementary Materials for an analysis including a measure of behavioral inhibition).
Previous studies have also found evidence that anxiety can affect decision making. For example, individuals with higher dispositional anxiety are more likely to be more risk-averse in tasks such as the balloon analogue risk task40. Our study makes an important contribution to this literature by situating individuals in an ecological setting, where the effect of anxiety can be seen as a plausible adaptive role, rather than a straightforward deficit in decision making. As such, our findings support evolutionary accounts of anxiety disorders41,42. While it is important to note that our current findings do not generalize to populations with clinical anxiety disorders, such as post-traumatic stress disorder, our hope is that future research will capitalize on the distinctions between threat contexts in order to better diagnose and treat these disorders. One potential avenue, for example, would be to tailor treatments and interventions based on individual differences in threat categorization.
Overall, this study provides strong empirical support for the notion that trait anxiety affects behavior only when there is sufficient time to appropriately cognize a threat, and not when threats require an immediate reactive response. These behavioral results were borne out in an ecologically relevant paradigm, and were complemented with neural data which suggest that previously learned threat contexts more heavily influence strategic decision making in trait anxious individuals. The present study provides a complement to previous work describing the contexts under which “reactive” fear defensive responses manifest3–5, and the behavioral and neural signatures of these responses, and in combination, point to the importance of examining different ecological classes of threat in future work.
Methods
30 subjects were recruited according to the guidelines of the Columbia University Institutional Review Board after providing informed consent. This sample size was chosen consistent with previous studies using similar designs4,43. Data from one subject was lost due to computer error. One additional subject was excluded due to excessive movement during the scan. Our final sample consisted of 28 subjects (17 female, age = 25.4 ± 7.3 years).
Stimuli, apparatus and procedure
This article constitutes an independent analysis of data from a previously published study5, with detailed methods reported here for completeness. Participants completed a computer-based task while in an fMRI scanner. The goal of the task was to earn as much money as possible while avoiding being caught by a virtual predator. Prior to the beginning of each trial, participants were presented with a 2 second cue indicating one of three different predator types that would be present in the upcoming trial. The participants were then shown a two-dimensional runway (90 units distance), with an triangle icon representing the position of the participant toward the right of the runway (at 80 units distance), and a circle icon representing the position of a predator at the left side of the runway (at 1 unit distance). This predator had two distinct modes of movement. In “approach” mode, the predator would proceed rightward along the runway at 4 units per second. At a randomly chosen distance (i.e. the attack distance) the predator would switch to “chase” mode, at which point it would advance at 10 units per second. These attack distances were randomly sampled from one of three Gaussian distributions, with means of 25, 40, 50 (standard deviations: 20, 20, 20; for the “slow”, “medium”, and “fast” predator types, respectively 4). Participants would passively gain money at a rate of 2 cents per second while they remained on the runway, and at any time could press a button to begin an escape toward the right side of the runway at 2 units per second. Notably, if participants did not respond prior to the predator reaching its attack distance, it was not possible for them to escape. This prevented participants from merely relying on their reaction time by responding after the predator switched modes. If participants escaped successfully, they would earn the monetary reward accumulated during that trial. If they failed to escape successfully (i.e. were caught by the predator) participants were given a mildly aversive electric shock (the shock magnitude was calibrated to each individual prior to testing), and the monetary reward earned in that trial would be forfeit. Thus, to perform this task optimally, participants had to learn the distributions of attack distances for each of the predator types, and respond as late as possible, provided the distance between them and the predator (i.e. the FID) was sufficient for a successful escape. Prior to the beginning of this main task, participants completed a brief, 8 trial practice session to familiarize themselves with the paradigm. (The attack distances of the predators were drawn from different distributions to those used in the main task.) Participants then completed 96 trials of the main task. After 48 trials, the predator-color cue was re-assigned in order to maintain the attentional demands of the task. Participants also performed a matching control condition for each predator type, without the risk of shock or the incentive of monetary reward, but otherwise identical to the main task. After completion of the computer task, subjects were asked to complete a series of personality questionnaires that included the trait subscale of the Spielberger State-Trait Anxiety Inventory, Form Y17 and the behavioral inhibition/activation scale (BIS/BAS)44(see Supplementary materials for an analysis of BIS scores). The computer task was programmed in Cogent with Matlab. Data collection and analysis were not performed blind to the conditions of the experiments.
All fMRI data were acquired using a GE Discovery MR750 3.0 T scanner with 32-channel headcoil. The imaging session consisted of two function scans, each twenty minutes, as well as a high-resolution anatomical T1-weighted image (1 mm isotropic resolution) collected at the beginning of each scan session. For functional imaging, interleaved T2*-weighted gradient-echo echo planar imaging (EPI) sequences were used to produce 45 3-mm-thick oblique axial slices (TR = 2 s., TE = 25 ms, flip angle = 77, FOV = 192 × 192 mm, matrix = 64 × 64). Each functional run began with five volumes (1000 msecs) before the first stimulus onset. These volumes were discarded before entering analysis to allow for magnetic field equilibration. Participants viewed the screen via a mirror mounted on the head coil, and a pillow and foam cushions were placed inside the coil to minimize head movement. Electric stimulation was delivered using a BIOPAC STM100C.
Data analysis
All statistical analyses for the behavioral data were carried out in R45, using the packages ‘ezANOVA’46, ‘coxme’47, and ‘lme4’48. Prior to analyses, data were tested for normality and equal variances using Shapiro-Wilk and Mauchly’s sphericity test, respectively. Where appropriate, log transformations of data were performed to account for non-normality, and Greenhouse–Geisser corrections were performed to account for violations of sphericity, with the correction factor values (ε) and original degrees of freedom reported. Partial eta-squared effect sizes are reported only for significant analyses. Where appropriate, we corrected for multiple comparisons using Holm-Bonferroni. All tests were two-tailed unless otherwise specified. We used an alpha level of .05 for all statistical tests5.
Analysis of fMRI data was carried out using scripted batches in SPM8 software (Welcome Trust Centre for Neuroimaging, London, UK) implemented in Matlab 7 (The MathWorks Inc., Natick MA). Structural images were subjected to the unified segmentation algorithm implemented in SPM8, yielding discrete cosine transform spatial warping coefficients used to normalize each individual’s data into MNI space. Functional data were first corrected for slice timing difference, and subsequently realigned to account for head movements. Normalized data were finally smoothed with a 6-mm FWHM Gaussian kernel.
Preprocessed images were subjected to a two-level general linear model using SPM8. The first level contained the following regressors of interest, each convolved with the canonical two-gamma hemodynamic response function: a 2 s box-car function for the onset of the trial (during predator type cue presentation), a 4–8 s (duration jittered) box-car function from the onset to 2 s prior to participants’ flight decisions, a 2 s boxcar function for the time prior to participants’ flight decisions, and a 4–8 s (duration jittered) box-car function for the remainder of the trial. Mean-centered STAI-Y scores ratings were included as orthogonal regressors. In addition, nuisance regressors consisted of motion parameters determined during preprocessing, their first temporal derivative and discrete cosine transform-based temporal low frequency drift regressors with a cutoff of 192 s. Beta maps were used to create linear contrast maps, which were then subjected to second-level, random-effects one-sample t-tests. In addition, a flexible factorial model was used to examine the main effects of predator type. The resulting statistical maps were thresholded at p < 0.05, and we corrected for multiple comparisons using false discovery rate correction (FDR whole brain corrected)49.
After whole-brain analyses, a hypothesis-driven region of interest (ROI) analysis was performed. These regions were chosen based on results from a previous study using the same behavioral task (see5).
The functional connectivity analysis was performed for the response phase (escape decision) using a generalized psychophysiological interactions (gPPI) approach18. vHPC was chosen as the seed region for subsequent PPI analysis due to its functional role in fear, stress and emotion4,19 and its empirically demonstrated involvement in our previous study5. (See Supplementary Materials for a similar analysis that includes the dorsal hippocampus.) In the PPI model, regressors of interest included the 3 predator conditions (slow/medium/fast), their corresponding control conditions, and the PPI terms for the above mentioned 6 conditions. Using the gPPI toolbox18, a first level connectivity analysis was carried out based on the PPI term of the direct comparison between the two predator conditions (slow versus fast attacking predator). (A similar connectivity analysis based on the PPI term of the comparison between the slow predator and control condition can be found in the Supplementary Materials.) As a second level analysis, STAI-Y scores were then introduced as a co-variate to examine how trait anxiety alters the strength of the PPI with respect to the seed regions.
Data and code availability
Behavioral data and accompanying code for all behavioral analyses and figures can be found on the Open Science Framework (https://osf.io/c4qbr/). FMRI data and analysis code are available from the corresponding author on reasonable request.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Mental Health (NIMH) grant 2P50MH094258 (D.M.), funds from The Tianqiao and Chrissy Chen Foundation P2026052 (D.M.).
Footnotes
Competing financial interests
All authors declare no competing interests.
We also collected data on the behavioral inhibition/activation scale. For an analysis of this scale, see “Behavioral inhibition and flight initiation distance” in the Supplemental materials.
It is important to note that participants had a larger time window in which to respond in the slow predator condition, thus, the variance in escape distances was not equal across predator types. For details, and a control analysis taking into account the differences in variance, see “Variability in flight initiation distance” in the Supplemental materials. Importantly, controlling for these variance differences resulted in no changes to our findings
Economic performance and proportion of escape trials were not significantly correlated within the slow predator condition (r(26) = −.31, p = .108)
Note that these predator types differed only in their mean attack distance, and not actually the speed of their attack.
Post-hoc power analyses are available from the authors by request.
References
- 1.Mathews A & Mackintosh B. A cognitive model of selective processing in anxiety. Cogn. Ther. Res 22, 539–560 (1998). [Google Scholar]
- 2.Mobbs D, Hagan CC, Dalgleish T, Silston B & Prevost C. The ecology of human fear: survival optimization and the nervous system. Front. Neurosci 9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mobbs D. The ethological deconstruction of fear(s). Curr. Opin. Behav. Sci. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mobbs D et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Sci. 317, 1079–1083 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi S et al. How cognitive and reactive fear circuits optimize escape decisions in humans. Proc. Natl. Acad. Sci (2018). DOI 10.1073/pnas.1712314115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mobbs D & Kim JJ Neuroethological studies of fear, anxiety, and risky decision-making in rodents and humans. Curr. Opin. Behav. Sci 5, 8–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeDoux J. Rethinking the emotional brain. Neuron 73, 653–676 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanchard RJ, Yudko EB, Rodgers RJ & Blanchard DC Defense system psychopharmacology: an ethological approach to the pharmacology of fear and anxiety. Behav. Brain Res 58, 155–165 (1993). [DOI] [PubMed] [Google Scholar]
- 9.Fanselow MS & Lester LS A functional behavioristic approach to aversively motivated behavior: predatory imminence as a determinant of the topography of defensive behavior In Bolles RC & Beecher M (eds.) Evolution and Learning, chap. 10, 185–211 (Lawrence Erlbaum Associates, 1988). [Google Scholar]
- 10.Calhoon GG & Tye KM Resolving the neural circuits of anxiety. Nat. Neurosci 18, 1394 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adhikari A, Topiwala MA & Gordon JA Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity. Neuron 71, 898–910 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adhikari A, Topiwala MA & Gordon JA Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 65, 257–269 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padilla-Coreano N et al. Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron 89, 857–866 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benoit RG, Davies DJ & Anderson MC Reducing future fears by suppressing the brain mechanisms underlying episodic simulation. Proc. Natl. Acad. Sci 113, E8492–E8501 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jimenez JC et al. Anxiety cells in a hippocampal-hypothalamic circuit. Neuron (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNaughton N & Corr PJ Survival circuits and risk assessment. Curr. Opin. Behav. Sci 24, 14–20 (2018). [Google Scholar]
- 17.Spielberger C, Gorsuch R, Lushene R, Vagg P & Jacobs G. Manual for the state-trait anxiety inventory (palo alto, ca, consulting psychologists press). Inc (1983). [Google Scholar]
- 18.McLaren DG, Ries ML, Xu G & Johnson SC A generalized form of context-dependent psychophysiological interactions (gppi): a comparison to standard approaches. Neuroimage 61, 1277–1286 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McHugh S, Deacon R, Rawlins J & Bannerman DM Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav. Neurosci 118, 63 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Fanselow MS & Dong H-W Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moser M-B & Moser EI Functional differentiation in the hippocampus. Hippocampus 8, 608–619 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Lima SL & Dill LM Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool 68, 619–640 (1990). [Google Scholar]
- 23.Davis M, Walker DL, Miles L & Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacol. 35, 105–135 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graeff FG Neuroanatomy and neurotransmitter regulation of defensive behaviors and related emotions in mammals. Braz. J. Med. Biol. Res 27, 811–829 (1994). [PubMed] [Google Scholar]
- 25.Blanchard RJ & Blanchard DC An ethoexperimental analysis of defense, fear, and anxiety In McNaughton N & Andrews G (eds.) Otago conference series, No. 1. Anxiety, 124–133 (University of Otago Press, 1990). [Google Scholar]
- 26.McNaughton N & Corr PJ A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci. & Biobehav. Rev 28, 285–305 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Young CK & McNaughton N. Coupling of theta oscillations between anterior and posterior midline cortex and with the hippocampus in freely behaving rats. Cereb. Cortex 19, 24–40 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Khemka S, Barnes G, Dolan RJ & Bach DR Dissecting the function of hippocampal oscillations in a human anxiety model. J. Neurosci 37, 6869–6876 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parent MA, Wang L, Su J, Netoff T & Yuan L-L Identification of the hippocampal input to medial prefrontal cortex in vitro. Cereb. Cortex 20, 393–403 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vertes RP Differential projections of the infralimbic and prelimbic cortex in the rat. Synap. 51, 32–58 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Felix-Ortiz AC et al. Bla to vhpc inputs modulate anxiety-related behaviors. Neuron 79, 658–664 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Felix-Ortiz AC & Tye KM Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci. 34, 586–595 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malvaez M et al. Basolateral amygdala rapid glutamate release encodes an outcome-specific representation vital for reward-predictive cues to selectively invigorate reward-seeking actions. Sci. Reports 5, 12511 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Indovina I, Robbins TW, Nunez-Elizalde AO, Dunn BD & Bishop SJ Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron 69, 563–571 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bach DR et al. Human hippocampus arbitrates approach-avoidance conflict. Curr. Biol 24, 541–547 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito R & Lee AC The role of the hippocampus in approach-avoidance conflict decision-making: evidence from rodent and human studies. Behav. Brain Res 313, 345–357 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Oehrn CR et al. Human hippocampal dynamics during response conflict. Curr. Biol 25, 2307–2313 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Mathews A. Why worry? the cognitive function of anxiety. Behav. Res. Ther 28,455–468 (1990). [DOI] [PubMed] [Google Scholar]
- 39.Perkins PJ, AM & Corr. Anxiety as an adaptive emotion In Parrott G (ed.) The Positive Side of Negative Emotions, 37 (The Guilford Press, 2014). [Google Scholar]
- 40.Maner JK et al. Dispositional anxiety and risk-avoidant decision-making. Pers. Individ. Differ 42, 665–675 (2007). [Google Scholar]
- 41.Meacham F & Bergstrom T, C. Adaptive behavior can produce maladaptive anxiety due to individual differences in experience. Evol. Medicine, Public Heal 2016, 270–285 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marks I. f. & Nesse RM Fear and fitness: An evolutionary analysis of anxiety disorders. Ethol. Sociobiol 15, 247–261 (1994). [Google Scholar]
- 43.Mobbs D et al. From threat to fear: the neural organization of defensive fear systems in humans. J. Neurosci 29, 12236–12243 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carver CS & White TL Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: Thebis/bas scales. J. Pers. Soc. Psychol 67, 319 (1994). [Google Scholar]
- 45.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: (2013). [Google Scholar]
- 46.Lawrence MA ez: Easy Analysis and Visualization of Factorial Experiments (2016). R package version 44–0. [Google Scholar]
- 47.Therneau TM coxme: Mixed Effects Cox Models (2015). R package version 22–5. [Google Scholar]
- 48.Bates D, Mächler M, Bolker B & Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw 67, 1–48 (2015). DOI 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 49.Genovese CR, Lazar NA & Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15, 870–878 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Gray JA The psychophysiological basis of introversion-extraversion. Behav. Res. Ther 8, 249–266 (1970). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Behavioral data and accompanying code for all behavioral analyses and figures can be found on the Open Science Framework (https://osf.io/c4qbr/). FMRI data and analysis code are available from the corresponding author on reasonable request.