Abstract
Objective: To determine the levels of s-IgA in saliva of caries patients and healthy controls, and to evaluate whether there is a correlation between it and caries by meta-analysis. Methods: The PubMed, MEDLINE, EMBASE, Web of Science, Cochrane Library, Scopus, Chinese National Knowledge Infrastructure, Wanfang Data, Chongqing VIP database for Chinese Technical Periodicals, and China BioMedical Literature Services System databases were searched initially in April 2020 and repeated in August 2020. Two independent evaluators screened the literature and extracted the data according to the inclusion and exclusion criteria. R 4.0.2 software was used for meta-analysis. I2 test was commonly reflected the heterogeneity. Subgroup analysis and meta-regression analysis explore the sources of heterogeneity. Sensitivity analysis, funnel diagram, Begg’s rank correlation, and Egger’s linear regression were used to determine the possibility of publication bias. Results: The study was reviewed according to the project guidelines for optimal reporting (PRISMA) based on meta-analysis. A total of 30 case–control studies were included, with a total sample size of 1545 patients, including 918 caries patients and 627 healthy controls. Salivary s-IgA levels in caries patients were significantly lower than those in healthy controls (SMD = −0.49, 95%CI: [−0.94; −0.03], P=0.03). In addition, the results of subgroup analysis showed that the significant decrease of salivary s-IgA level was correlated with children patients, mixed dentition and Asian people (children: SMD = −0.45, 95%CI: [−0.89; −0.01], P=0.04; mixed dentition: SMD = −0.61, 95%CI: [−1.24; 0.03], P=0.06; Asian: SMD = −0.62, 95%CI: [−1.17; −0.08], P=0.02). The funnel diagram included in the study was symmetrically distributed, and the sensitivity analysis confirmed the robustness of the results. Conclusion: Salivary s-IgA levels in caries patients were significantly lower than in healthy controls. It has also been demonstrated that salivary s-IgA may be used as an alternative measure to identify subjects at risk of caries susceptibility, suggesting that salivary s-IgA may be a protective factor for dental caries.
Keywords: case-control study, Dental caries, Meta-analysis, s-IgA, Salivary
Introduction
Caries remains a serious public health problem in most parts of the world, about 10 percent of the world’s people suffering from this disease [1]. It is a kind of disease that the bacteria in the dental plaque ferment the sugar in the food to produce the acid, cause the tooth hard tissue demineralization dissolves and produce the chronic progressive destruction [2]. Saliva contains a variety of components, including electrolytes, enzymes, and antimicrobial peptides such as immunoglobulin A, lactoferrin, and lysozyme [3]. Saliva is involved in a variety of physiological functions, including lubricating the mouth, wetting food and swallowing, protecting the oral mucosa from dryness, participating in immune defense, and playing a key role in regulating the ecological balance of oral flora [4]. In particular, salivary secretory immunoglobulin A (s-IgA) not only mediates the humoral immune response to regulate caries activity but also interferes with the formation of caries causing microbial adhesion to the tooth surface and biofilm [5]. Studies in recent years have shown that salivary s-IgA plays an important role in local immunity by binding to caries causing microbial surface molecules such as adhesion to prevent microbial adhesion to the surface of tooth hard tissue [6]. It can reduce the hydrophobicity of the surface of bacteria and directly destroy Streptococcus mutans toxin and glucosyltransferase (GTF), making them inactivated [7]. Directly combine with the cariogenic microorganism to form an immune complex, which is more beneficial to remove [7]. Salivary s-IgA acts together with complement lysozyme to dissolve bacteria [7]. Regulate the phagocytic function of mucosa polynuclear leukocytes and phagocytes.
The locally specific immune response mediated by s-IgA is species-specific and can induce cross-reactive immunity to enhance the original local immunity [8]. Salivary s-IgA, combined with specific epitopes of cariogenic bacteria, resulted in a locally specific immune response. In recent years, several studies have assessed the relationship between aggregate, nonspecific, and specific levels of s-IgA in saliva and caries, but the results from different studies vary widely. One theory is that salivary s-IgA inhibits the process of bacterial adhesion to the tooth surface, neutralizes certain enzymes and bacterial toxins of cariogenic bacteria, and synergizes with other salivary proteins such as lactoferrin or lysozyme, which may have caries-preventing effects [7,9,10]. In contrast, another theory suggests that differences in salivary s-IgA levels have nothing to do with caries susceptibility. People with higher salivary s-IgA levels have more dental caries than those with lower levels, suggesting a protein concentration-effect [10,11].
A previous meta-analysis found that the salivary s-IgA levels of patients with dental caries were higher than that of healthy controls, but the results of the study were highly heterogeneous and did not explore the source of the heterogeneity [12]. As time goes by, the results of most studies in recent years have shown the opposite conclusions of the previous meta-analysis. To further explore the relationship between salivary s-IgA levels and caries, we conducted the present study. We excluded noncompliant literature and reported an updated meta-analysis of all studies of salivary s-IgA levels, and performed subgroup analysis, sensitivity, and meta-regression analysis to explore heterogeneity sources. Publication bias was identified by using the funnel graph method, Begg’s rank correlation, and Egger’s linear regression. Therefore, by collecting relevant literature on salivary s-IgA levels and caries before June 2020, meta-analysis was conducted in this paper to compare the differences in salivary s-IgA levels between caries-free groups and dental caries patients. Provide help for early clinical intervention and prevention of dental caries patients, and provide a reference basis for a screening of dental caries susceptible population.
Materials and methods
Protocol and registration
The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) checklist was used as a guide for conducting and reporting the present study (Supplementary Table S1) [13]. This updated meta-analysis was registered on the International Prospective Register of Systematic Reviews (PROSPERO) database (registry number: CRD42018112317; http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018112317).
Selection criteria
Inclusion criteria
(1) A case–control study of salivary secretory immunoglobulin A measurements in caries and control cases published inside and outside China as of August 2020. (2) Without language limitation. (3) Clinical investigation of both case (caries group) and control (caries-free group). (4) Salivary levels of s-IgA were measured in both the case and control groups. (5) Caries was diagnosed by the World Health Organization (WHO) according to DMFT/dmft or DMFS/dmfs index as the standard.
Exclusion criteria
(1) There are no specific data report or the data are too small, which is not conducive to the analysis of literature; 2) Repeat study; 3) Only case group (caries group), no control literature; 4) Animal experiments and in vitro studies; 5) A history of congenital malformations and systemic diseases; 6) Literature types included case report, review, systematic evaluation, etc.
Search strategy
Through domestic databases: Chinese National Knowledge Infrastructure (CNKI), Wanfang Data, Chongqing VIP database for Chinese Technical Periodicals (VIP), and China BioMedical Literature Services System (SinoMed); Foreign databases: PubMed, MEDLINE, Embase, Scopus, Web of Science, Cochrane Library. The search process is completed by two evaluators independently under the guidance of professionals. Subjects and free word searches were performed using the following keywords: ‘Dental Caries’, ‘Immunoglobulin A, Secretory’, ‘Immunoglobulin A’, ‘Saliva’. According to the references found after the literature take the manual search, to prevent the omission of important literature. The retrieval strategy is shown in the Table 1.
Table 1. Search strategy.
| No. | Query | Results |
|---|---|---|
| #12 | Search #3 AND #8 AND #11 | 360 |
| #11 | Search #9 OR #10 | 61724 |
| #10 | ((Saliva [Title/Abstract]) OR (Salivas [Title/Abstract])) OR (spittle [Title/Abstract]) | 45519 |
| #9 | Search “Saliva”[Mesh] | 41626 |
| #8 | Search #4 OR #5 OR #6 OR #7 | 68162 |
| #7 | (((Immunoglobulin A [Title/Abstract]) OR (IgA [Title/Abstract])) OR (IgA1 [Title/Abstract])) OR (IgA2 [Title/Abstract]) | 55797 |
| #6 | Search “Immunoglobulin A” [Mesh] | 37402 |
| #5 | ((((((((Immunoglobulin A, Secretory [Title/Abstract]) OR (Secretory IgA [Title/Abstract])) OR (IgA, Exocrine [Title/Abstract])) OR (Exocrine IgA [Title/Abstract])) OR (SIgA [Title/Abstract])) OR (IgA, Secretory [Title/Abstract])) OR (Secretory Immunoglobulin A [Title/Abstract])) OR (Colostral IgA [Title/Abstract])) OR (IgA, Colostral [Title/Abstract]) | 6131 |
| #4 | Search “Immunoglobulin A, Secretory” [Mesh] | 5611 |
| #3 | Search #1 OR #2 | 62212 |
| #2 | (((((((((((((((((Dental Caries [Title/Abstract]) OR (Dental Decay [Title/Abstract])) OR (“Caries, Dental” [Title/Abstract])) OR (“Decay, Dental” [Title/Abstract])) OR (Carious Dentin [Title/Abstract])) OR (Carious Dentins [Title/Abstract])) OR (“Dentin, Carious” [Title/Abstract])) OR (“Dentins, Carious” [Title/Abstract])) OR (Dental White Spot [Title/Abstract])) OR (“White Spots, Dental” [Title/Abstract])) OR (White Spots [Title/Abstract])) OR (“Spot, White” [Title/Abstract])) OR (“Spots, White” [Title/Abstract])) OR (White Spot [Title/Abstract])) OR (Dental White Spots [Title/Abstract])) OR (“White Spot, Dental” [Title/Abstract])) OR (caries [Title/Abstract])) OR (tooth caries [Title/Abstract]) | 47252 |
| #1 | Search “Dental Caries” [Mesh] | 46062 |
Source: PubMed; Searched on August 8, 2020
Literature screening and data extraction
Repeated references were deleted by using the EndNote X9 software. Literature and data will be selected and extracted by two reviewers independently. If there is any disagreement, it will be decided by discussion or by the third evaluator. We reviewed each literature using the exclusion and inclusion criteria. If the same author uses the same data again in another study, only one study will be included. In the literature screening, the title of the article was read first, and the abstract was read further after excluding irrelevant literature to determine whether to include or not. Then read the full text in detail, culling irrelevant articles. The data were extracted into pre-designed forms as follows: first author’s name, publication date, country, age, sample size, caries index (dmft and/or DMFT), s-IgA content, saliva extraction time, saliva type, s-IgA measurement type, and statistical methods and results. After data extraction, the two data were checked, inconsistencies were extracted again, and data analysis was conducted after confirmation. If the included studies provided only the median and extreme values of salivary s-IgA levels, they could be converted to mean and standard deviation according to the methods provided by Wan et al [14]. If the original article does not provide any important data for the above characteristics, we are attempting to send an email to the author to obtain available information.
Quality assessment
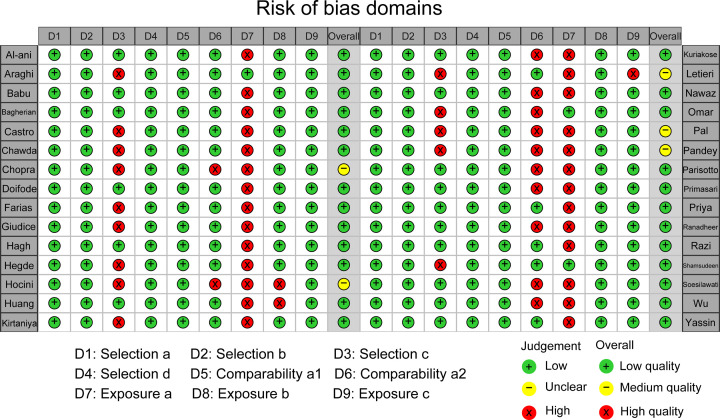
The included studies were assessed using the case–control study quality assessment criteria recommended by the Newcastle–Ottawa scale (NOS) criteria [15]. At present, this was the only accepted quality assessment tool in the case–control study, see the supplementary document. Supplementary Table S4 lists the NOS case–control study quality assessment table. The scale allows for the evaluation of case–control, cohort, and observational studies. The scale provides a standardized method for quality evaluation, with a total of 8 items, including the selection of research objects, comparability of study groups, and exposure or outcome ascertainment. The stars are used to answer the question, and with a maximum score of 9 for each study. The quality evaluation was conducted by two researchers independently at the same time, and a third party would decide the differences after discussion. The quality of the literature was determined by stars number: 0–3 stars were of low quality, 4–6 stars were of medium quality, and 7–9 stars were of high quality [16].
Statistical analysis
Data were analyzed by a third researcher using R 4.0.2 software. Mean concentration, standard deviation, and sample size of salivary s-IgA were used for statistical analysis. Due to the different units of salivary s-IgA concentration in each study, all the measured results were converted to μg/ml. Values from the baseline were used in studies evaluating baseline and follow-up. For the evaluation of low, medium, and high caries patients, we used the combined subgroup method to obtain the salivary s-IgA value. Subgroup analyses were also performed, dividing the study into region differences (Asia, Europe, North America, South America, Africa), age differences (adults, ≥18; children, <18), dentition type (permanent dentition, mixed dentition, deciduous dentition), salivary s-IgA detection methods (enzyme-linked immunosorbent assay (ELISA), Immunoturbidimetry, radioimmunoassay (RIA), noncompetitive biotin-avidin enzyme-linked immunosorbent assay (NABA)). Continuous variable data were used as the effective index, and the results were expressed as mean difference (MD) or standardized mean difference (SMD) effect quantity and 95% confidence interval (CI). Heterogeneity of the included results was analyzed by the χ2 test (test level: α = 0.01), and the quantitative judgment of heterogeneity was made by combining I2 [17]. When I2>50%, indicating that there was significant heterogeneity among the studies included in the meta-analysis, and the random effect model was used for statistical analysis of the summary results of the present study. Sensitivity analysis was used to evaluate the reliability of the overall effect at all levels and to find out the factors affecting the inter-study heterogeneity as far as possible. To determine the source of heterogeneity, stratified subgroup analysis, and random effect meta-regression were performed by age, dentistry, region, and salivary s-IgA assays to assess potential confounders in the present study. Begg’s [18] and Egger’s [19] tests and funnel charts were used to assess the potential publication bias in the included studies.
Results
Inclusion literature screening
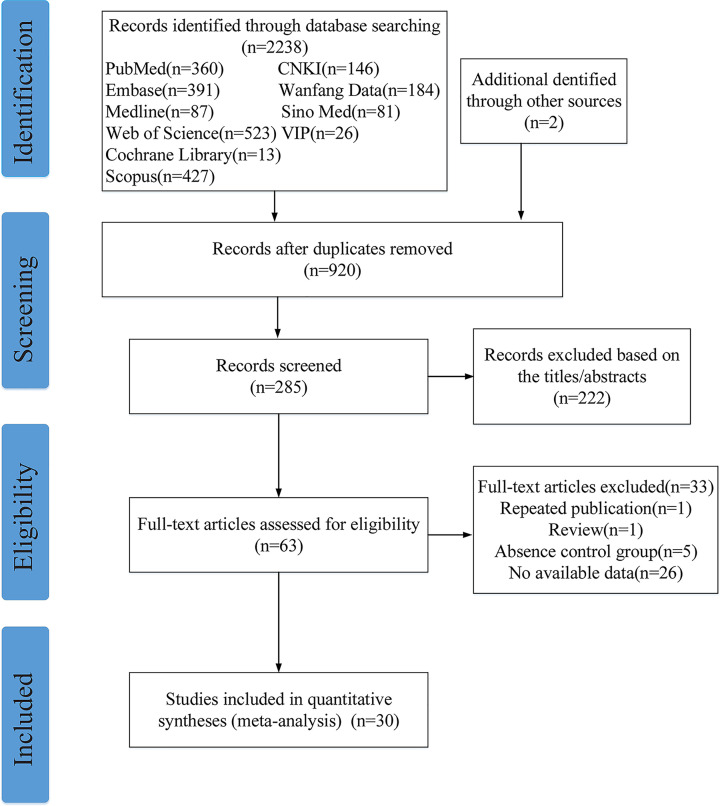
EndNote X9 literature management software was used to merge the retrieval results, and 1320 duplicate results were removed, leaving 920 for study. After reading the title and abstract, 222 studies were excluded for reasons including reviews, in vitro studies or animal experiments, indicators that did not include salivary s-IgA levels, duplicate reports, incorrect or incomplete data, and unreasonable study design.
About 63 studies were screened by two reviewers independently and blind, and 30 studies were finally included [5]. The screening process is shown in Figure 1. The total sample size of the included study was 1545 cases, 918 cases in the caries group, and 627 cases in the control group. Information on the included studies is shown in Table 2. The included studies were published between 1993 and 2020. All studies were case–control and reported the relationship between salivary s-IgA levels and dental caries in different age groups and different dentition period. Table 3 shows the nature of saliva collected in each study, collection time, the method for detecting s-IgA, and the basic characteristics of statistical analysis. Figure 2 shows the evaluation results of 30 studies in the NOS case-control study quality evaluation form.
Figure 1. Flow chart showing the study selection.
Table 2. Characteristics of included studies.
| Author and year | Country | Subject | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Caries-active | Caries-free | ||||||||
| Age (year) | Sample size | Caries index (n) | s-IgA level (μg/ml) | Age (year) | Sample size | Caries index | s-IgA level (μg/ml) | ||
| Al-ani 2020 [20] | U.S.A. | 18–70 (38.36 ± 13.20) | All: 28 Male: 13 Female: 15 |
High caries risk (28) | All: 0.207 ± 0.133 Male: 0.2414 ± 0.14817 Female: 0.17699 ± 0.11394 |
18–70 (38.84 ± 12.16) | All: 32 Male: 14 Female: 18 |
Low caries risk | All: 0.238 ± 0.139 Male: 0.26016 ± 0.17057 Female: 0.21996 ± 0.11159 |
| Araghi 2018 [21] | IRN | 20–40 | All: 30 Low caries: 20 High caries: 10 |
DMFT <5 (20) DMFT ≥5 (10) |
All: 0.1472 ± 0.071 Low caries: 0.1328 ± 0.0509 High caries: 0.1760 ± 0.0968 |
20–40 | 10 | DMFT = 0 | 0.0939 ± 0.0239 |
| Babu 2017 [22] | IND | 8–12 (10.00 ± 1.33) | 20 | DMFT = 3.8 ± 1.00 | 158.55 ± 30.24 | 8–12 (8.9 ± 0.71) | 20 | DMFT = 0 | 183.80 ± 19.37 |
| Bagherian 2013 [23] | IRN | 3–5.8 (5.08 ± 0.73) | 45 | dmft = 9.3 ± 3.6 | 1961.4 ± 1000.7 | 3–5.83 (4.92 ± 1.02) | 45 | dmft = 0 | 1484.5 ± 811.6 |
| Castro 2016 [24] | CHL | 25 ± 3 | 20 | DMFT = 3.9 ± 0.7 | 26.80 ± 2.5 | 24 ± 2 | 20 | DMFT = 0 | 50.65 ± 7.5 |
| Chawda 2011 [25] | IND | 4–8 | All: 20 Low caries: 10 High caries: 10 |
DMFT+dmft = 1-5 (10) DMFT+dmft = 6-10 (10) |
All: 176.45 ± 40.692 Low caries: 186.6 ± 48.4 High caries: 166.3 ± 30.4 |
4–8 | 10 | DMFT+dmft = 0 | 243.6 ± 48.7 |
| Chopra 2011 [26] | IND | 24–45 | 88 | DMFT > 1 | 774 ± 473 | 24–45 | 14 | DMFT = 0 | 727 ± 409 |
| Doifode 2011 [27] | IND | 8–10 (8.73 ± 0.46) | 15 | dfs = 19.00 ± 8.52 DMFS = 1.60 ± 2.03 |
89.8 ± 15.6 | 8–10 (9.13 ± 0.35) | 15 | dfms = 0 DMFT = 0 |
107.4 ± 15.2 |
| Farias 2003 [28] | BRA | 1–3.9 (3.14 ± 0.75) | 20 | dmfs = 16.4 ± 8.9 | 50.4 ± 45.0 | 1–3.9 (3.29 ± 0.59) | 20 | dmft = 0 | 32.5 ± 21.0 |
| Giudice 2019 [29] | ITA | 4–16 | 39 | DMFT+dmft = 0.25 ± 0.24 | 218.0 ± 129.0 | 4–16 | 20 | DMFT+dmft = 0 | 167.0 ± 45.0 |
| Hagh 2013 [30] | IRN | 19–4 (26.8 ± 5.61) | 15 | DMFT = 5.26 ± 3.04 | 6.02 ± 0.76 | 19–4 (28.5 ± 7.07) | 25 | DMFS = 0 | 12.32 ± 1.99 |
| Hegde 2013 [5] | IND | 20–30 | All: 60 Low caries: 20 High caries: 40 |
DMFT = 1–5 (20) DMFT = 6–10 (20) DMFT ≥ 10 (20) |
All: 1905.10 ± 1016.57 High caries: 1384.6 ± 609.14 Low caries: 2946.10 ± 858.10 |
20–30 | 20 | DMFT = 0 | 3155.1 ± 489.3 |
| Hocini 1993 [31] | FRA | 20-63 (34.3 ± 10.7) | All: 21 Male: 11 Female: 10 |
DMFT > 10 | All: 34.2 ± 20.9 Male: 35.53 ± 18.3 Female: 32.7 ± 24.37 |
22–64 (38.5 ± 11.8) | All: 22 Male: 13 Female: 9 |
DMFT = 0 | All: 31.4 ± 36.1 Male: 36.15 ± 45.61 Female: 24.58 ± 14.33 |
| Huang 2006 [32] | CHN | 4–6 | 45 | dmft ≥ (4–6) | 1195.7 ± 538.2 | 4–6 | 45 | dmft = 0 | 2272.0 ± 673.1 |
| Kirtaniya 2009 [33] | IND | 6–14 | All: 35 Low caries: 20 High caries: 15 |
DMFT+deft = 1.7 ± 0.48 (10) DMFT+deft = 3.6 ± 0.52 (10) DMFT+deft = 8.8 ± 3.49 (15) |
All: 0.3819 ± 0.1224 Low caries: 0.4095 ± 0.1015 High caries: 0.345 ± 0.141 |
6–14 | 11 | DMFT+deft = 0 | 0.49 ± 0.142 |
| Kuriakose 2013 [34] | IND | 3–5 | 17 | dmft > 5 | 99.6 ± 28.3 | 3–5 | 17 | dmft = 0 | 151.5 ± 22.2 |
| Letieri 2019 [35] | BRA | 2–5 (3.0 ± 1.0) | 23 | dmfs = 10.2 | 32.94 ± 32.16 | 2–5 (3.7 ± 1.2) | 23 | dmfs = 0 | 25.40 ± 15.44 |
| Nawaz 2019 [36] | PAK | 33 ± 12.58 | All: 28 Low caries: 29 High caries: 29 |
DMFT = 0–5 (29) DMFT > 5 (29) |
All: 32.165 ± 8.0561 Low caries: 34.64 ± 6.37 High caries: 29.69 ± 8.88 |
30 ± 7.3 | 29 | DMFT = 0 | 7.67 ± 8.23 |
| Omar 2012 [37] | EGY | 4–6 | 35 | dmft = 1–3 (11); dmft = 4–6 (13);dmft > 6 (11) | All: 0.7514 ± 0.3946 | 4–6 | 10 | dmft = 0 | 0.81 ± 0.38 |
| Pal 2013 [38] | IND | 9.5 ± 2.45 | All: 30 Low caries: 15 High caries: 15 |
DMFT = 6.600 ± 2.098 DMFT = 2.200 ± 0.941 |
All: 167.635 ± 31.8001 Low caries: 189.47 ± 25.99 High caries: 145.80 ± 19.94 |
10.00 ± 2.59 | 15 | DMFT = 0 | 215.4 ± 26.71 |
| Pandey 2018 [39] | IND | 5–14 (9.36 ± 2.37) | All: 40 Low caries: 20 High caries: 20 |
DMFT = 2.32 ± 0.86 (20) DMFT = 6.74 ± 2.16 (20) |
All: 164.315 ± 32.0691 Low caries: 186.10 ± 24.70 High caries: 142.53 ± 22.4 |
5–14 (10.2 ± 2.35) | 20 | DMFT = 0 | 214.8 ± 27.56 |
| Parisotto 2011 [40] | U.S.A. | 3–4 | 17 | dmfs ≥ 3 (dmft = 3.10 ± 2.6) | 181.97 ± 34.18 | 3–4 | 23 | dmfs = 0 | 132.22 ± 19.03 |
| Primasari 2019 [41] | IDN | 1.65 ± 0.44 | All: 34 Low caries: 26 High caries: 8 |
deft = 1–3 (14); deft = 4–5 (18); deft = 6–8 (8) | All: 0.602 ± 0.424 Low caries: 0.6287 ± 0.4344 High caries: 0.514 ± 0.405 |
1.35 ± 0.39 | 34 | dmft = 0 | 0.702 ± 0.421 |
| Priya 2013 [42] | IND | 7–12 | 15 | DMFT/dmft ≥ 3 | 130.7 ± 15.5 | 7–12 | 15 | DMFT/dmft = 0 | 119.0 ± 15.8 |
| Ranadheer 2011 [43] | IND | 8–12 | 20 | DMFT/dmft ≥ 3 | 117.6 ± 18.5 | 8–12 | 20 | DMFT/dmft = 0 | 75.85 ± 24.8 |
| Razi 2020 [44] | IND | 12–15 | All: 20 Male: 10 Female: 10 |
DMFT ≥ 10 | All: 85.0 ± 14.3 Male: 91.8 ± 8.3 Female: 79.1 ± 16.3 |
12–15 | All: 20 Male: 10 Female:10 |
DMFT = 0 | All: 106.3 ± 28.5 Male: 118.2 ± 34.3 Female: 94.3 ± 15.3 |
| Shamsudeen 2008 [45] | IND | 3–6 | 10 | dmft ≥ 5 | 2211 ± 778.68 | 3–6 | 10 | dmft = 0 | 2300.0 ± 432.0 |
| Soesilawati 2019 [46] | IDN | 6–9 | All: 48 Low caries: 40 High caries: 8 |
dmft = 1 (15); dmft = 2 (3); dmft = 3 (3); dmft = 4 (19); dmft = 5 (8) |
All: 295.63 ± 183.57 Low caries: 320.30 ± 187.75 High caries: 172.25 ± 94.80 |
6–9 | 12 | dmft = 0 | 545.83 ± 90.30 |
| Wu 2002 [47] | CHN | 4–5 | 20 | dmft ≥ 5 | 52.44 ± 13.23 | 4–5 | 20 | dmft = 0 | 75.73 ± 22.15 |
| Yassin 2016 [48] | IRQ | 7–10 | All: 30 Male: 11 Female: 19 |
DMFT/dmft ≥ 5 | All: 1091.2 ± 287.8 Male: 1037.7 ± 242.0 Female: 1122.2 ± 313.3 |
7–10 | All: 30 Male: 12 Female: 18 |
DMFT/dmft = 0 | All: 1285.8 ± 281.0 Male: 1305.2 ± 291.7 Female: 1272.9 ± 281.4 |
DMFT/dmft index: the sum of decayed missing and filled permanent/deciduous teeth; DMFS/dmfs index: the sum of decayed missing and filled permanent/deciduous surfaces; Low caries: DMFT/dmft = 1–5; High caries: DMFT/dmft > 5.
Table 3. Characteristics of included studies and quality score.
| First Author and Year | Saliva collection | Time | Amount of saliva | Detection method | Statistic test and P-value | Quality score |
|---|---|---|---|---|---|---|
| Al-ani 2020 [20] | Unstimulated saliva | Missing data | 5–10 ml | ELISA | Mann–Whitney and Kruskal–Wallis tests, P=0.388 | 8 |
| Araghi 2018 [21] | Unstimulated saliva | 10:00–12:00 (a.m.) | Missing data | Immunoturbidimetry | ANOVA test, P=0.046 | 8 |
| Babu 2017 [22] | Unstimulated saliva | 10:00–12:00 (a.m.) | 2 ml | ELISA | T test, P=0.0399 | 8 |
| Bagherian 2013 [23] | Unstimulated saliva | 10:00–11:00 (a.m.) | Missing data | ELISA | Pearson test, P=0.015 | 8 |
| Castro 2016 [24] | Unstimulated saliva | 09:00–11:00 (a.m.) | 15 ml | ELISA | Student’s t test, P=0.001 | 7 |
| Chawda 2011 [25] | Unstimulated saliva | 10:00–12:00 (a.m.) | 0.5 ml | Immunoturbidimetry | ANOVA test, P=0.001 | 7 |
| Chopra 2011 [26] | Unstimulated saliva | 09:00–11:00 (a.m.) | Missing data | ELISA | ANOVA test, P>0.05 | 6 |
| Doifode 2011 [27] | Unstimulated saliva | Missing data | Missing data | Immunoturbidimetry | T test, P=0.012 | 8 |
| Farias 2003 [28] | Unstimulated saliva | 08:00–11:00 (a.m.) | 1.5 ml | Immunoturbidimetry | U Mann–Whitney, P<0.05 | 7 |
| Giudice 2019 [29] | Unstimulated saliva | 10:00–11:00 (a.m.) | Missing data | ELISA | Mann–Whitney, P=0.175 | 7 |
| Hagh 2013 [30] | Unstimulated saliva | 09:00–12:00 (a.m.) | Missing data | ELISA | Mann–Whitney and Kruskal–Wallis tests, P=0.009 | 8 |
| Hegde 2013 [5] | Unstimulated saliva | 10:00–11:00 (a.m.) | Missing data | Immunoturbidimetry | One-way ANOVA test, P<0.001 | 7 |
| Hocini 1993 [31] | Unstimulated saliva | Missing data | Missing data | ELISA | Mann–Whitney U test, P>0.05 | 5 |
| Huang 2006 [32] | Unstimulated saliva | 10:00–11:00 (a.m.) | 2 ml | RIA | Group t test, P<0.05 | 7 |
| Kirtaniya 2009 [33] | Unstimulated saliva | 09:00–12:00 (a.m.) | Missing data | ELISA | P<0.01 | 7 |
| Kuriakose 2013 [34] | Unstimulated saliva | 08:00–09:00 (a.m.) | 5 ml | ELISA | Unpaired t test, P=0.001 | 7 |
| Letieri 2019 [35] | Unstimulated saliva | 08:00–10:00 (a.m.) | 1 ml | ELISA | Mann–Whitney U test, P<0.03 | 6 |
| Nawaz 2019 [36] | Unstimulated saliva | Missing data | 3 ml | ELISA | ANOVA test, P<0.001 | 7 |
| Omar 2012 [37] | Unstimulated saliva | 09:00–11:00 (a.m.) | 3 ml | ELISA | Low and high caries group, Pearson test, P<0.01 | 7 |
| Pal 2013 [38] | Unstimulated saliva | 10:00–11:00 (a.m.) | 4 ml | ELISA | ANOVA test, P<0.001 | 6 |
| Pandey 2018 [39] | Unstimulated saliva | 10:00–11:00 (a.m.) | 5 ml | ELISA | Chi-square test, P=0.001 | 6 |
| Parisotto 2011 [40] | Unstimulated saliva | Missing data | 250 μl | ELISA | Wilcoxon signed-rank test, P=0.0118 | 7 |
| Primasari 2019 [41] | Unstimulated saliva | 09:00–11:00 (a.m.) | 2 ml | ELISA | Kruskal–Wallis ANOVA test, P=0.227 | 7 |
| Priya 2013 [42] | Unstimulated saliva | Missing data | 2–3 ml | ELISA | Independent sample t test, P=0.05 | 8 |
| Ranadheer 2011 [43] | Unstimulated saliva | Missing data | Missing data | ELISA | Schefft test, P=0.05 | 7 |
| Razi 2020 [44] | Unstimulated saliva | Missing data | 5 ml | ELISA | Independent t test, P=0.015 | 8 |
| Shamsudeen 2008 [45] | Unstimulated saliva | 10:00–11:00 (a.m.) | Missing data | Immunoturbidimetry | Student’s t test, P>0.05 | 8 |
| Soesilawati 2019 [46] | Unstimulated saliva | 10:00–12:00 (a.m.) | Missing data | ELISA | Mann–Whitney U test, P<0.001 | 7 |
| Wu 2002 [47] | Unstimulated saliva | 09:00–10:00 (a.m.) | 2 ml | NABA | Group t test, P<0.05 | 7 |
| Yassin 2016 [48] | Unstimulated saliva | Missing data | Missing data | Immunoturbidimetry | Group t test, P=0.01 | 8 |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; NABA, noncompetitive avidin-biotin immunoenzymatic assay; RIA, radioimmunoassay.
Figure 2. Risk of bias assessment for included studies.
Reviewers’ judgment of the risk of bias for each item for each of the 30 studies included in the meta-analysis.
The correlation between salivary s-IgA level and caries
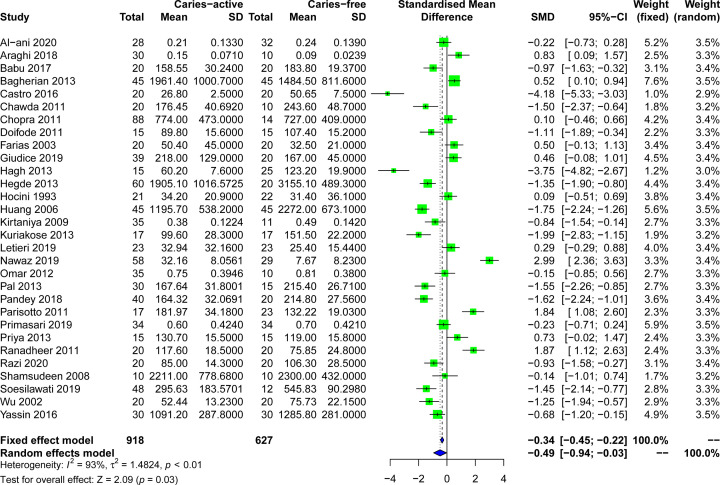
A total of 30 studies reported salivary s-IgA levels in caries patients. The overall results of the correlation between salivary s-IgA levels and caries were shown in Figure 3. Salivary s-IgA levels in caries patients (mean ± SD) ranged from 2211.00 ± 778.68 [45] to 0.1472 ± 0.0710 μg/ml [21], while those in healthy controls ranged from 3155.10 ± 489.30 [5] to 0.0939 ± 0.0239 μg/ml [21]. Due to the heterogeneity of I2>50% in the study, the random effect model (P<0.001) was adopted for data consolidation analysis. The negative correlation between salivary s-IgA levels and caries was determined in this meta-analysis (SMD = −0.49, 95%CI: [−0.94; −0.03], P=0.03; Figure 3).
Figure 3. Forest plots of salivary s-IgA levels in patients with dental caries and control groups.
Subgroup analysis
To explore the source of heterogeneity of differences in salivary s-IgA levels, we based on region differences, age differences, dentition type, and salivary s-IgA detection methods were sub-group analysis (Table 4). First, the subgroup analysis based on region (Table 4 and Supplementary Figure S1), due to the obvious heterogeneity (I2>50%), the random-effects model was adopted, showing that the salivary s-IgA levels of the Asian caries group were significantly lower than that of the healthy control group (SMD = −0.62, 95%CI: [−1.17; −0.08], P=0.02), and people in other regions did not show significant differences. Based on the age subgroup analysis (Table 4 and Supplementary Figure S2), due to the obvious heterogeneity, the random-effects model was adopted, showing that the salivary s-IgA levels of the children’s caries group were significantly lower than that of the healthy control group (SMD = −0.45, 95%CI: [−0.89; −0.01], P=0.04), and adults did not show significant differences. However, the number of studies on adults was limited, so further research is needed to confirm this finding. Based on the subgroup analysis of dentition (Table 4 and Supplementary Figure S3), due to the obvious heterogeneity, the random-effects model was used to show that the salivary s-IgA levels of caries patients with mixed dentition were lower than that of the healthy control group (SMD = −0.61, 95%CI: [−1.24; 0.03], P=0.06), but the difference was not significant. There was no difference in salivary s-IgA levels of caries patients with permanent dentition and deciduous dentition compared with healthy controls. The subgroups were ELISA, immunoturbidimetry, RIA, and NABA, but showed that salivary s-IgA levels have no significant difference between dental caries patients and healthy controls (Table 4 and Supplementary Figure S4). Subgroup analysis showed that age and region might be the source of salivary s-IgA levels heterogeneity.
Table 4. Subgroup analysis of salivary s-IgA levels in dental caries patients.
| Subgroups | N | SMD | SMD (95%CI) | Z | P | Model | Heterogeneity | |
|---|---|---|---|---|---|---|---|---|
| I2 | P | |||||||
| Salivary s-IgA | ||||||||
| Area | ||||||||
| North America | 100 | 0.79 | −1.24; 2.81 | 0.77 | 0.44 | Random | 95.1% | <0.001 |
| Asia | 1172 | −0.62 | −1.17; −0.08 | 2.26 | 0.02 | Random | 93.9% | <0.001 |
| South America | 126 | −1.06 | −3.27; 1.14 | 0.95 | 0.34 | Random | 96.4% | <0.001 |
| Europe | 102 | 0.30 | −0.11; 0.70 | 1.46 | 0.14 | Fixed | 0% | 0.363 |
| Africa | 45 | −0.15 | −0.85; 0.56 | 0.42 | 0.68 | Random | NA | NA |
| Combined | 1545 | −0.49 | −0.94; −0.03 | 2.11 | 0.03 | Random | 93.6% | <0.001 |
| Age | ||||||||
| ≥18 | 492 | −0.64 | −1.92; 0.64 | 0.99 | 0.32 | Random | 96.7% | <0.001 |
| <18 | 1053 | −0.45 | −0.89; −0.01 | 2.00 | 0.04 | Random | 90.4% | <0.001 |
| Combined | 1545 | −0.49 | −0.94; −0.03 | 2.11 | 0.03 | Random | 93.6% | <0.001 |
| Dentition periods | ||||||||
| Deciduous dentition | 513 | −0.23 | −0.91; 0.44 | 0.68 | 0.50 | Random | 92.2% | <0.001 |
| Mixed dentition | 500 | −0.61 | −1.24; 0.03 | 1.86 | 0.06 | Random | 90.3% | <0.001 |
| Permanent dentition | 532 | −0.66 | −1.81; 0.48 | 1.15 | 0.25 | Random | 96.4% | <0.001 |
| Combined | 1545 | −0.49 | -0.94; -0.03 | 2.11 | 0.03 | Random | 93.6% | <0.001 |
| Detection method | ||||||||
| ELISA | 1135 | −0.39 | −0.97; 0.19 | 1.33 | 0.18 | Random | 94.4% | <0.001 |
| Immunoturbidimetry | 300 | −0.49 | −1.16; 0.17 | 1.45 | 0.15 | Random | 85.5% | <0.001 |
| RIA | 90 | −1.75 | −2.24; −1.26 | 7.08 | <0.001 | Random | NA | NA |
| NABA | 40 | −1.25 | −1.94; −0.57 | 3.66 | <0.001 | Random | NA | NA |
| Combined | 1545 | −0.49 | −0.94; −0.03 | 2.11 | 0.03 | Random | 93.6% | <0.001 |
CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; N, number of studies; NA, not available; NABA, noncompetitive avidin-biotin immuno enzymatic assay; RIA, radioimmunoassay; SMD, standardized mean difference.
Salivary s-IgA level and the different gender of dental caries patients
The results of the correlation between salivary s-IgA levels and gender were shown in Figure 4. Because of the limited number of studies on gender differences (only four studies were included) [20,31,44,48], and the heterogeneity in the studies was less than 50% (I2<50%), the fixed effects model was used (P>0.05). When comparing the salivary s-IgA levels of male caries patients and healthy controls (Figure 4A), we found that the salivary s-IgA levels of male caries patients were significantly lower than that of healthy controls (SMD = −0.46, 95%CI: [−0.87; −0.04], P=0.025). Similar results were found in women (Figure 4A). The salivary s-IgA levels of the caries group were lower than that of the healthy control group, but the difference was not statistically significant (SMD = −0.38, 95%CI: [−0.77; 0.00], P=0.05).
Figure 4. Forest plots of salivary s-IgA levels in different gender patients with dental caries and control groups.
(A) Forest plot of the relationship between salivary s-IgA levels and male group. (B) Forest plot of the relationship between salivary s-IgA levels and female group.
The relationship between the saliva s-IgA level and the severity of dental caries in patients with dental caries
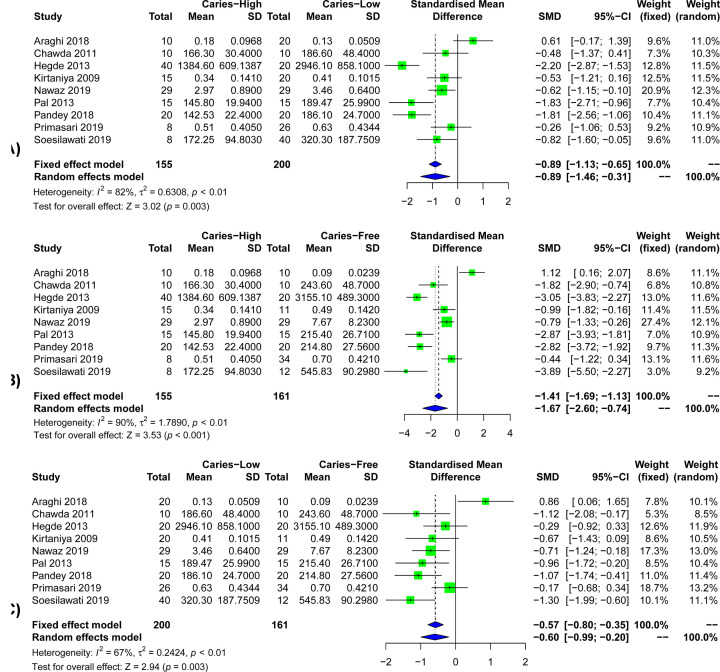
The results of the correlation between salivary s-IgA levels and the severity of dental caries were shown in Figure 5. Because of the limited number of studies on differences in the severity of caries (only nine studies were included) [21,25,5,33,36,38,39,41,46], and the heterogeneity in the studies was greater than 50% (I2>50%), a random-effects model was used (P<0.001). The results show that when comparing the salivary s-IgA levels of patients with high dental caries and patients with low dental caries (Figure 5A), we found that the salivary s-IgA levels of patients with high dental caries were significantly lower than those of patients with low dental caries (SMD = −0.89, 95%CI: [−1.46; −0.31], P=0.003). When comparing the salivary s-IgA levels of patients with high caries and healthy controls (Figure 5B), the salivary s-IgA levels of patients with high caries were significantly lower than those of healthy controls (SMD = −1.67, 95%CI: [−2.60; −0.74], P<0.001). When comparing the salivary s-IgA levels of patients with low caries and healthy controls (Figure 5C), the salivary s-IgA levels of patients with mild caries were significantly lower than those of healthy controls (SMD = −0.60, 95%CI: [−0.99; −0.20], P=0.003).
Figure 5. Forest plots of salivary s-IgA levels in different caries degree patients and control groups.
(A) Forest plots of salivary s-IgA levels in high caries group and low caries group. (B) Forest plots of salivary s-IgA levels in high caries group and healthy control group. (C) Forest plots of salivary s-IgA levels in low caries group and healthy control group.
Sensitivity analysis and publication bias
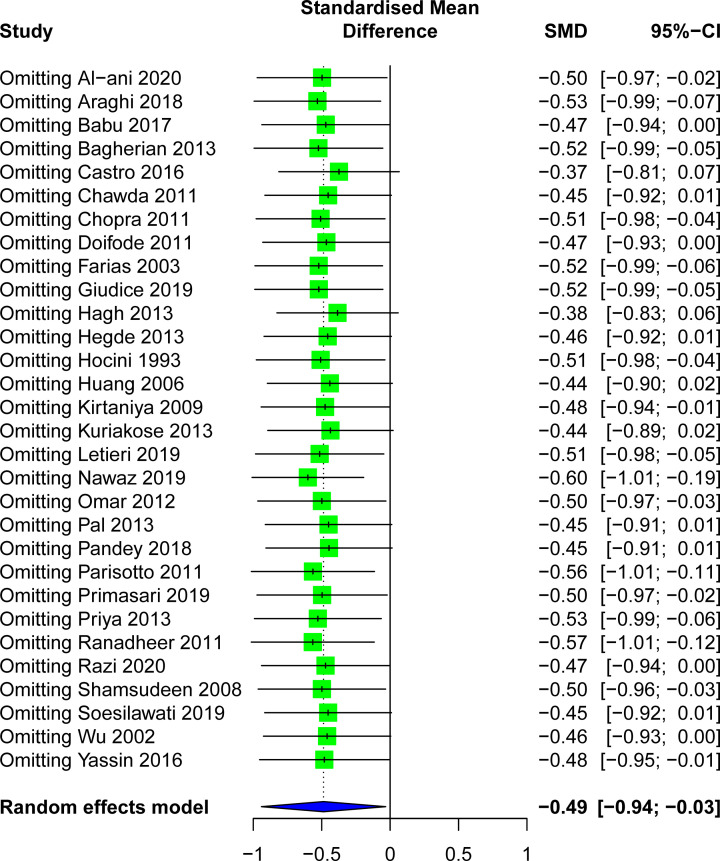
A sensitivity analysis was performed to avoid heterogeneity. Figure 6 shows 14 studies with acceptable heterogeneity (I2 = 46%). The [22,25,27,5,32–34,38,39,44–48] caries-free healthy control group included 265 study subjects and the caries group included 410 study subjects, and the combined meta-analysis of caries activity was performed at lower levels of s-IgA (SMD = −1.23, 95%CI: [−1.48; −0.99], P<0.001). In the sensitivity analysis, the removal of any individual study did not change the overall statistical significance, indicating that the meta-analysis was relatively stable and reliable (Figure 7). Salivary s-IgA levels in caries patients and healthy controls were analyzed for publication bias. The magnitude of the effect was concentrated around the overall effect, with large samples concentrated at the top and small samples distributed around the bottom in an inverted funnel shape, indicating that publication bias had a small effect. The results showed that the effect of publication bias was small (Figure 8).
Figure 6. Forest plot for the salivary s-IgA levels between caries-active patients and healthy controls of 14 articles remained after sensitivity test.
Figure 7. Sensitivity analyses for the association between salivary s-IgA levels and dental caries.
Figure 8. Assessment publication bias for the association between salivary s-IgA concentration and dental caries.
Random-effects meta-regression analysis
Meta-regression analysis was used to find the source of heterogeneity. Age, dentition periods, region, and salivary s-IgA measurement type were used as covariates, but none of the factors showed significant confounding bias as a source of risk factors (Table 5).
Table 5. Meta-regression analysis coefficients for salivary s-IgA levels in the examined group of studies.
| Variables | Coefficient (SE) | 95% Confidence interval | P |
|---|---|---|---|
| Region | −0.06 (0.37) | −0.83; 0.71 | 0.86 |
| Age | −0.45 (1.09) | −2.71; 1.81 | 0.68 |
| Dentition Periods | 0.55 (0.63) | −0.74; 1.84 | 0.38 |
| Detection method | −0.45 (0.44) | −1.35; 0.45 | 0.31 |
Discussion
After reading the full text, according to the inclusion and exclusion criteria, 1 review, 1 republished study, 5 studies excluding caries-free control, and 26 studies that could not extract available data were excluded from this systematic evaluation. Finally, 30 case–control studies with 918 caries active patients and 627 healthy controls to explore the relationship between salivary s-IgA concentration and caries. About 16 of the studies reported lower levels of s-IgA in saliva from caries patients compared with healthy controls without caries, and 22 of the studies showed statistical differences. In general, our meta-analysis shows that the concentration of s-IgA in the saliva of caries patients was significantly lower than that of the normal control group (SMD = −0.49, 95%CI: [−0.94; −0.03], P=0.03), suggesting that that low concentration of saliva salivary s-IgA is associated with an increased risk of caries and may be used as a potential biomarker for screening caries susceptible population in the future.
Our results were inconsistent with previous meta-analysis results [12]. They found that the levels of salivary s-IgA in caries patients were significantly higher than those in healthy controls, and the increased salivary s-IgA levels were positively correlated with the occurrence of caries. However, the results of our study showed that the degree of caries activity was negatively correlated with salivary s-IgA concentration. Included in our meta-analysis, however, another 18 studies, including before 12 in previous meta-analysis study, two of them were not included in the present study. The reasons were as follows: Saliva is irritant [49]; lack of a control group [50]. So was excluded in our meta-analysis. We have extended the previous meta-analysis to show the correlation between salivary s-IgA levels and dental caries and conducted subgroup analysis, sensitivity analysis, and meta-regression analysis to find the source of heterogeneity.
The most important humoral antibody of oral mucosal specific immunity is s-IgA [51,52]. One typical s-IgA is mainly composed of two IgA monomers (i.e. dimer IgA), one J chain and one secretory component (SC). S-IgA and J chains are synthesized by mucosal epithelial lamina propria or salivary gland plasma cells, and SC, as dimer IgA specific receptor, is located on the epithelial cell membrane and synthesized by epithelial cells. When IgA binds SC segments, its structure is more compact and is not easy to be enzymatically hydrolyzed, which is conducive to the high antibody activity of IgA on the mucosal surface and in the exocrine secretion [53]. The main function of s-IgA is to block or prevent pathogenic bacteria from adhering to the mucosal epithelial cells or the surface of teeth. S-IgA cannot activate complement but can activate the complement system by the alternative pathway in the polymerization state. S-IgA has no bactericidal effect and the effect of promoting the formation of tonic complement fragment and lacks a direct conditioning effect, but IgA-mediated antibody-dependent cytotoxicity can be found in the human body [7].
Salivary s-IgA is a relatively stable anti-inflammatory immunoglobulin, which can maintain its activity in the environment of oral proteolytic enzymes for a long time [54,55]. The potential link between caries and saliva s-IgA levels remains to be elaborated. One possible mechanism is that saliva s-IgA prevents pathogens from adhering to the tooth surface. Salivary s-IgA inhibits microbial colonization and neutralizes microbial enzymes or toxins [56–58]. Salivary s-IgA antibody can prevent the colonization of Streptococcus mutans by neutralizing glycosyltransferase (GTF), thus reducing the rate of GTF binding to Streptococcus mutans pili, thus inhibiting the development of dental caries [59,60]. Another potential mechanism is that salivary s-IgA can synergize and promote the bacteriostasis of the lactoferrin and peroxidase system, which can also explain the correlation between salivary s-IgA and caries [61–63].
During the occurrence and development of dental caries, the host’s immune defense response is also very important. Most of the people included in the present study were children (1053). The decrease of s-IgA in the saliva of children with dental caries may be due to the challenge of various microorganisms in the oral cavity in their childhood. Their immune system or lymphatic system is developing or immature, and they will swallow saliva repeatedly, resulting in s-IgA decreased [33]. Besides, the feeding method of the child and the level of s-IgA in the mother’s saliva and breast milk will also affect the level of s-IgA in the oral saliva [64]. In the formation of dental caries, cariogenic microorganisms need to overcome the host’s nonspecific defense barriers (cleaning mechanism, swallowing and saliva flow, etc.), and then must escape the recognition of soluble immune or nonimmune host molecules in host secretions [7]. The above processes were all related to the formation of dental caries. Salivary s-IgA can bind to the surface antigens of microorganisms in saliva to make them agglutinate, thereby promoting their rapid elimination and preventing the occurrence of dental caries [10]. However, some studies have suggested that microorganisms can protect themselves from host immune attack by forming biofilms and reducing the expression of antigens [65,66], which will cause higher levels of s-IgA in the saliva of healthy people than patients with caries. Dental caries was caused by the imbalance between enamel demineralization induced by bacterial biofilms and oral defenses including immune and inflammatory responses. The level of s-IgA in the saliva of caries patients was significantly lower than that of healthy people, but the activation mode of salivary s-IgA and the protective mechanism against caries still need further study.
The results of Primasari et al. [41] and Al-ani et al. [20] found that there was no difference in salivary s-IgA levels between caries-free people and caries patients, which was contradictory with the results of the present study. The results obtained by the above studies may be due to the small sample size and different criteria for judging dental caries. Besides, it is recommended that salivary s-IgA levels be measured in children over the age of 6, whose immune system is considered complete [46]. Jafarzadeh et al. [67] found that salivary s-IgA levels increase with age before the age of 60, and slightly decrease in the 61–70 age group. The reasons described above may cause conflicting results with this study. Fidalgo et al [12] systematic review and meta-analysis have reported the opposite results. First, it may be because the saliva requirements were not selected. They included contains both nonirritating saliva and irritating saliva. The current commonly used saliva source has irritant parotid gland fluid, irritant whole saliva, and nonirritating whole saliva. Nonirritating whole saliva is more reasonable to analyze because the interaction between antibacterial substances and bacteria in the saliva is mostly occurred in nonirritating whole saliva [68]. Also, subjects’ emotions, inflammation, infection, systemic diseases, medication, age, and the interaction of various substances in saliva can all affect the experimental results, and the above factors should be avoided as far as possible. This was why the present study only included articles that collected nonirritating saliva.
Although the results of most studies included in the present study indicate that the saliva s-IgA concentration of caries patients is significantly lower than that of the normal control group. Considering other factors that may influence the relationship between salivary s-IgA levels and dental caries, we conducted subgroup analysis based on age, region, type of dentition, and salivary s-IgA detection method. Subgroup analysis revealed a more consistent association between salivary s-IgA levels and caries in children. Salivary s-IgA levels tended to be lower in children's caries patients than in healthy controls, but the heterogeneity of the results was significant. This also reminds us that it may be that children were usually unable to maintain oral health properly and were one of the most vulnerable to other oral diseases. In addition to age, other important factors could affect the levels of s-IgA in saliva, including saliva flow rate, smoking, pregnancy, and other stress factors [7,69,70]. Therefore, the level of saliva s-IgA is of great importance to the prevention of dental caries in children. Studies on different regions showed that the correlation between the levels of salivary s-IgA in Asian caries patients was more significant than in other regions. It may be related to the economic level, social background, and dietary factors. Studies in other regions did not show relevance, possibly due to the small number of studies and subjects included, and the inconsistent criteria for determining dental caries. We have made correction according to the reviewer’s comments. Besides, the saliva s-IgA level of dental caries patients in the mixed dentition group compared with the primary dentition and permanent dentition group was different from that of the control group, but the difference was not statistically significant (P=0.06). This was consistent with what we have previously obtained in the subgroup of different age patients. It further shows that salivary s-IgA concentration has a better correlation in children. Furthermore, other potential confounders were analyzed by using meta-regression, but none of them were related to salivary s-IgA levels, suggesting that these factors were unlikely to explain the differences in salivary s-IgA levels between caries patients and healthy controls.
The salivary s-IgA detection method also results in some differences [61]. At present, the commonly used methods for the determination of s-IgA concentration in saliva include immunoturbidimetry, RIA, and ELISA. The accuracy of the immunoturbidimetric method is poor and the error of manual measurement is large; RIA is time-consuming and requires the use of radioisotopes but is highly sensitive. The NABA method uses a biotin-labeled antibody to replace the enzyme-labeled antibody in the ordinary ELISA method. Due to the higher affinity between biotin and avidin, the stability of the experiment is improved and the reaction time is shortened. However, the operation process is more complicated and is used less. ELISA is the leading technology in clinical immunoassays with specificity, sensitivity, ease of operation, and stability of reagents. More importantly, it does not pose a threat to the environment. Although the salivary s-IgA assay used was not an exclusion criterion, the included major studies used confidence methods to evaluate s-IgA. All included studies indicated the use of commercial kits under the manufacturer’s instructions.
We further included the gender grouping of the four studies and found that in both the male group and the female group, the s-IgA concentration in the saliva of caries patients was significantly lower than that of people without caries. The relationship between gender and salivary s-IgA levels was rarely reported. The results of the present study also showed that gender did not affect on the difference in saliva s-IgA levels between caries patients and healthy controls. Similarly, the nine included studies were grouped according to the degree of caries, and the results showed that the salivary s-IgA concentration of patients with high caries was significantly lower than that of patients with low caries, and the salivary s-IgA concentration of patients with high caries was significantly lower than that of the caries-free group. The salivary s-IgA concentration of patients with low caries was significantly lower than that of the caries-free group. This indicates that the concentration of salivary s-IgA was related to the degree of caries, and the concentration of salivary s-IgA was also dose-related with the severity of caries.
Of course, our meta-analysis also has some limitations. Due to language limitations, our study was unable to include all available data covering salivary s-IgA and caries. The included studies were case–control studies, demonstrating a possible association between salivary s-IgA and caries, but not a causal relationship between salivary s-IgA and caries. Other types of study designs have yet to prove whether low salivary s-IgA levels play a role in the development of caries, or whether caries induces low salivary s-IgA expression in the population. Our adjustment for the potential confounders included in the study was limited. Although we used the subgroup analysis and meta-regression analysis to find the source of the heterogeneity, other sources of heterogeneity may still affect the results, such as saliva flow rate, smoking, pregnancy, and body fat composition and other factors, such as stress can affect the accuracy of the results. But there were a few research reports on those clinical data, so no meta-regression analysis was conducted.
The conclusions are drawn from this meta-analysis still need us to further verify the specific protective mechanism of s-IgA against caries in animal or cell experiments. Using the protective effect of saliva s-IgA, we can further develop vaccines to prevent caries. In susceptible people, the body is induced to produce specific and non-specific s-IgA, and the content of s-IgA in the saliva is increased, thereby achieving the effect of preventing dental caries [71]. It is also possible to make a protective agent containing s-IgA and apply it on the surface of the teeth of patients with a high incidence of dental caries, or to use mouthwash or toothpaste containing s-IgA to weaken or eliminate potential cariogenic factors in a local area. Besides, the study of Choonharuangdej et al. [72] showed that the group with higher IgA concentration in gingival crevicular fluid had less severe periodontitis. Therefore, the concentration of GCF-s-IgA antibody may also be related to the occurrence and development of periodontitis. The research on s-IgA and endodontic and periodontal pathologies is also an important direction worthy of our further research and exploration.
Conclusion
In summary, the present study showed that the levels of salivary s-IgA in caries patients were lower than that in healthy control group, indicating that the decrease of salivary s-IgA levels was closely related to the progress of caries. The levels of salivary s-IgA can be used as a valuable biomarker to evaluate the clinical status of caries patients. To confirm this finding and determine whether salivary s-IgA has clinical value in the treatment of caries patients, larger studies and better study designs are needed in the future.
Supplementary Material
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- NABA
noncompetitive avidin-biotin immunoenzymatic assay
- NOS
Newcastle–Ottawa scale
- RIA
radioimmunoassay
- SMD
standardized mean difference
Data Availability
The data used to support the findings of this study are included within the article.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the grants from National Natural Science Foundation of China [grant number 81760194].
Author Contribution
Zeyu Wu contributed to the data curation, formal analysis, methodology, software, and draft writing. Yi Gong contributed to the formal analysis, methodology, software, and draft writing. Chen Wang and Jing Lin contributed to the investigation and software. Jin Zhao contributed to the validation, conception, and design.
References
- 1.Vos T., Abajobir A.A., Abate K.H., Abbafati C., Abbas K.M., Abd-Allah F. et al. (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet North Am. Ed. 390, 1211–1259 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selwitz R.H., Ismail A.I. and Pitts N.B. (2007) Dental caries. Lancet (London, England) 369, 51–59 10.1016/S0140-6736(07)60031-2 [DOI] [PubMed] [Google Scholar]
- 3.Edgar W.M., O'Mullane D.M. and Dawes C.(eds). (2004) Saliva and oral health, 4th ednpp. 1–11, British Dental Association, London, http://wrigleyoralhealth.com/content/docs/SHL_S_OH_A5_2015_FINAL.pdf [Google Scholar]
- 4.Dodds M., Roland S., Edgar M. and Thornhill M. (2015) Saliva A review of its role in maintaining oral health and preventing dental disease. BDJ Team 2, 11–13, https://www.nature.com/articles/bdjteam2015123 10.1038/bdjteam.2015.123 [DOI] [Google Scholar]
- 5.Mithra H., Darshana D., Chitharanjan S. and Aditya S. (2013) Correlation between dental caries and salivary immunoglobulin in adult Indian population: An in vivo study. J. Restor. Dent. 1, 22–25 [Google Scholar]
- 6.Hemadi A.S., Huang R., Zhou Y. and Zou J. (2017) Salivary proteins and microbiota as biomarkers for early childhood caries risk assessment. Int. J. Oral. Sci. 9, e1–e8 10.1038/ijos.2017.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamont R.J., Hajishengallis G.N., Koo H.M. and Jenkinson H.F.(eds). (2020) Oral microbiology and immunology 75, 2nd ednpp. 214–217, John Wiley & Sons, http://www.asmscience.org/content/book/10.1128/9781555818906 [Google Scholar]
- 8.Brandtzaeg P. (2013) Secretory IgA: Designed for Anti-Microbial Defense. Front. Immunol. 4, 222 10.3389/fimmu.2013.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gornowicz A., Tokajuk G., Bielawska A., Maciorkowska E., Jabłoński R., Wójcicka A. et al. (2014) The assessment of sIgA, histatin-5, and lactoperoxidase levels in saliva of adolescents with dental caries. Med. Sci. Monit. 20, 1095–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynge Pedersen A.M. and Belstrøm D. (2019) The role of natural salivary defences in maintaining a healthy oral microbiota. J. Dent. 80, S3–S12 10.1016/j.jdent.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 11.Tulunoglu O., Demirtas S. and Tulunoglu I. (2006) Total antioxidant levels of saliva in children related to caries, age, and gender. Int. J. Paediatr. Dent. 16, 186–191 10.1111/j.1365-263X.2006.00733.x [DOI] [PubMed] [Google Scholar]
- 12.Fidalgo T.K., Freitas-Fernandes L.B., Ammari M., Mattos C.T., de Souza I.P. and Maia L.C. (2014) The relationship between unspecific s-IgA and dental caries: a systematic review and meta-analysis. J. Dent. 42, 1372–1381 10.1016/j.jdent.2014.07.011 [DOI] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G. and Prisma Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan X., Wang W., Liu J. and Tong T. (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stang A. (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 16.Tang S., Wang Y., Gong X. and Wang G. (2015) A Meta-Analysis of Maternal Smoking during Pregnancy and Autism Spectrum Disorder Risk in Offspring. Int. J. Environ. Res. Public Health 12, 10418–10431 10.3390/ijerph120910418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J.P. and Thompson S.G. (2002) Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 18.Begg C.B. and Mazumdar M. (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101, https://www.jstor.org/stable/2533446 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 19.Egger M., Davey Smith G., Schneider M. and Minder C. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-ani A., MacDonald D.A. and Ahmad M. (2020) Salivary sIgA and PRAP-1 Protein in Relation to Dental Caries: A Comparative Study. J. Adv. Oral. Res. 11, 71–76 10.1177/2320206820913746 [DOI] [Google Scholar]
- 21.Haeri-Araghi H., Zarabadipour M., Safarzadeh-Khosroshahi S. and Mirzadeh M. (2018) Evaluating the relationship between dental caries number and salivary level of IgA in adults. J. Clin. Exp. Dent. 10, e66–e69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagadesh Babu B., Venugopal Reddy N., Thimma Reddy B.V., Daneswari V. and Puppala N. (2017) Comparitive evaluation of salivary IgA levels and dental caries in obese and non-obese children. Int. J. Adv. Res. 5, 766–772 [Google Scholar]
- 23.Bagherian A. and Asadikaram G. (2012) Comparison of some salivary characteristics between children with and without early childhood caries. Indian J. Dent. Res. 23, 628–632 10.4103/0970-9290.107380 [DOI] [PubMed] [Google Scholar]
- 24.Castro R.J., Herrera R. and Giacaman R.A. (2016) Salivary protein characteristics from saliva of carious lesionfree and high caries adults. Acta Odontol. Latinoam. 29, 178–185, http://www.scielo.org.ar/pdf/aol/v29n2/v29n2a11.pdf [PubMed] [Google Scholar]
- 25.Chawda J.G., Chaduvula N., Patel H.R., Jain S.S. and Lala A.K. (2011) Salivary SIgA and dental caries activity. Indian Pediatr. 48, 719–721 10.1007/s13312-011-0113-y [DOI] [PubMed] [Google Scholar]
- 26.Chopra M., Jadhav S., Venugopalan A., Hegde V. and Chopra A. (2012) Salivary immunoglobulin A in rheumatoid arthritis (RA) with focus on dental caries: a cross-sectional study. Clin. Rheumatol. 31, 247–250 10.1007/s10067-011-1796-0 [DOI] [PubMed] [Google Scholar]
- 27.Doifode D. and Damle S. (2011) Comparison of salivary IgA levels in caries free and caries active children. Int. J. Clin. Dent. Sci. 2, 10–14, https://www.edentj.com/index.php/ijcds/article/view/196 [Google Scholar]
- 28.de Farias D.G. and Bezerra A.C. (2003) Salivary antibodies, amylase and protein from children with early childhood caries. Clin. Oral Investig. 7, 154–157 10.1007/s00784-003-0222-7 [DOI] [PubMed] [Google Scholar]
- 29.Lo Giudice G., Nicita F., Militi A., Bertino R., Matarese M., Currò M. et al. (2019) Correlation of s-IgA and IL-6 Salivary with Caries Disease and Oral Hygiene Parameters in Children. Dent J. (Basel) 8, 3 10.3390/dj8010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golpasand Hagh L., Zakavi F., Ansarifar S., Ghasemzadeh O. and Solgi G. (2013) Association of dental caries and salivary sIgA with tobacco smoking. Aust. Dent. J. 58, 219–223 10.1111/adj.12059 [DOI] [PubMed] [Google Scholar]
- 31.Hocini H., Iscaki S., Bouvet J.P. and Pillot J. (1993) Unexpectedly high levels of some presumably protective secretory immunoglobulin A antibodies to dental plaque bacteria in salivas of both caries-resistant and caries-susceptible subjects. Infect. Immun. 61, 3597–3604 10.1128/IAI.61.9.3597-3604.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang H.H., Yu H., Zhang L., Liu H., Niu Y.M. and Wang N.Q. (2006) Correlation between immunochemical level and patient with caries. West China J. Stomatol. 24, 77–78 [PubMed] [Google Scholar]
- 33.Kirtaniya B.C., Chawla H.S., Tiwari A., Ganguly N.K. and Sachdev V. (2009) Natural prevalence of antibody titres to GTF of S. mutans in saliva in high and low caries active children. J. Indian Soc. Pedod. Prev. Dent. 27, 135–138 10.4103/0970-4388.57092 [DOI] [PubMed] [Google Scholar]
- 34.Kuriakose S., Sundaresan C., Mathai V., Khosla E. and Gaffoor F.M. (2013) A comparative study of salivary buffering capacity, flow rate, resting pH, and salivary Immunoglobulin A in children with rampant caries and caries-resistant children. J. Indian Soc. Pedod. Prev. Dent. 31, 69–73 [DOI] [PubMed] [Google Scholar]
- 35.Letieri A., Freitas-Fernandes L.B., Valente A., Fidalgo T. and de Souza I. (2019) Longitudinal Evaluation of Salivary Iga-S in Children with Early Childhood Caries Before and After Restorative Treatment. J. Clin. Pediatr. Dent. 43, 239–243 10.17796/1053-4625-43.4.3 [DOI] [PubMed] [Google Scholar]
- 36.Nawaz A., Batool H., Kashif M., Abbas A., Shahzad F., Tahir R. et al. (2019) Immune profiling of saliva in patients with and without dental caries. Bangladesh J. Med Sci. 18, 536–539 10.3329/bjms.v18i3.41622 [DOI] [Google Scholar]
- 37.Omar O.M., Khattab N.M. and Rashed L.A. (2012) Glucosyltransferase B, immunoglobulin a, and caries experience among a group of Egyptian preschool children. J. Dent. Child. (Chic.) 79, 63–68 [PubMed] [Google Scholar]
- 38.Pal S., Mitra M., Mishra J., Saha S. and Bhattacharya B. (2013) Correlation of total salivary secretory immunoglobulin A (SIgA) and mutans specific SIgA in children having different caries status. J. Indian Soc. Pedod. Prev. Dent. 31, 270–274 [DOI] [PubMed] [Google Scholar]
- 39.Pandey S., Goel M., Nagpal R., Kar A., Rapsang E. and Matani P. (2018) Evaluation of Total Salivary Secretory Immunoglobulin A and Mi/fans-specific SIgA among Children having Dissimilar Caries Status. J. Contemp. Dent. Pract. 19, 651–655 [PubMed] [Google Scholar]
- 40.Parisotto T.M., King W.F., Duque C., Mattos-Graner R.O., Steiner-Oliveira C., Nobre-Dos-Santos M. et al. (2011) Immunological and microbiologic changes during caries development in young children. Caries Res. 45, 377–385 10.1159/000330230 [DOI] [PubMed] [Google Scholar]
- 41.Primasari A., Octiara E. and Yanti N. (2019) Risk factor of secretory immunoglobulin A and salivary lysozyme level in children aged under 3 years to severe early childhood caries. IOP Conference Series: Earth and Environmental Science, vol. 305, p. 012001, IOP Publishing, https://iopscience.iop.org/article/10.1088/1755-1315/305/1/012001/meta [Google Scholar]
- 42.Geetha Priya P.R., Asokan S., Karthick K., Reddy N.V. and Rao V.A. (2013) Effect of dental treatments on salivary immunoglobulin A of children with and without dental caries: a comparative study. Indian J. Dent. Res. 24, 394 10.4103/0970-9290.118004 [DOI] [PubMed] [Google Scholar]
- 43.Ranadheer E., Nayak U.A., Reddy N.V. and Rao V.A. (2011) The relationship between salivary IgA levels and dental caries in children. J. Indian Soc. Pedod. Prev. Dent. 29, 106–112 10.4103/0970-4388.84681 [DOI] [PubMed] [Google Scholar]
- 44.Razi M.A., Qamar S., Singhal A., Mahajan A., Siddiqui S. and Mohina Minz R.S. (2020) Role of natural salivary defenses in the maintenance of healthy oral microbiota in children and adolescents. J. Family Med. Prim. Care 9, 1603–1607 10.4103/jfmpc.jfmpc_1134_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shifa S., Muthu M.S., Amarlal D. and Rathna Prabhu V. (2008) Quantitative assessment of IgA levels in the unstimulated whole saliva of caries-free and caries-active children. J. Indian Soc. Pedod. Prev. Dent. 26, 158–161 [DOI] [PubMed] [Google Scholar]
- 46.Soesilawati P., Notopuro H., Yuliati Y., Ariani M.D. and Alwino Bayu Firdauzy M. (2019) The role of salivary sIgA as protection for dental caries activity in Indonesian children. Clin. Cosmet. Investig. Dent. 11, 291–295 10.2147/CCIDE.S194865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao-xia W., Shu-jie L., Jing-ming L. and Zhenghong Z. (2004) Investigation of relationship between secretory immunoglobulin A, Salivary peroxidase and caries in children. J. Modern Stomatol. 18, 199–201 [Google Scholar]
- 48.Yassin H N. (2016) Comparison of Immunoglobulin IgA Level in the Stimulated Saliva of Caries-Free and Caries-Active Children Aged 7-10 Years. J. Baghdad College Dentistry 28, 155–158, https://jcodental-uobaghdad-edu.org/index.php/jbcd/article/view/1443 10.12816/0031124 [DOI] [Google Scholar]
- 49.Thaweboon S., Thaweboon B., Nakornchai S. and Jitmaitree S. (2008) Salivary secretory IgA, pH, flow rates, mutans streptococci and Candida in children with rampant caries. Southeast Asian J. Trop. Med. Public Health 39, 893–899, https://pdfs.semanticscholar.org/1c60/cea6d406df020d15a2c3ecdee2b8ba5ee031.pdf [PubMed] [Google Scholar]
- 50.Sikorska M.H., Mielnik-Blaszczak M. and Kapeć E. (2002) The relationship between the levels of SigA, lactoferrin and alpha(1) proteinase inhibitor in saliva and permanent dentition caries in 15-year-olds. Oral. Microbiol. Immunol. 17, 272–276 10.1034/j.1399-302X.2002.170502.x [DOI] [PubMed] [Google Scholar]
- 51.Herich R. (2017) Is the role of IgA in local immunity completely known? Food Agr. Immunol. 28, 223–237 10.1080/09540105.2016.1258547 [DOI] [Google Scholar]
- 52.Abbas A.K. and Lichtman A.H. (2015) Basic immunology: functions and disorders of the immune system, 5th edn, pp. 153–170, Elsevier Health Sciences, https://www.elsevier.com/books/basic-immunology/abbas/978-0-323-39082-8 [Google Scholar]
- 53.Johansson M. and Lycke N.Y. (2003) Immunology of the human genital tract. Curr. Opin. Infect. Dis. 16, 43–49 10.1097/00001432-200302000-00008 [DOI] [PubMed] [Google Scholar]
- 54.Ahl T. and Reinholdt J. (1991) Detection of immunoglobulin A1 protease-induced Fab alpha fragments on dental plaque bacteria. Infect. Immun. 59, 563–569 10.1128/IAI.59.2.563-569.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee S.R., Kwon H.K., Song K.B. and Choi Y.H. (2004) Dental caries and salivary immunoglobulin A in Down syndrome children. J. Paediatr. Child Health 40, 530–533 10.1111/j.1440-1754.2004.00457.x [DOI] [PubMed] [Google Scholar]
- 56.Gregory R.L., Hobbs L.C., Kindle J.C., VanTo T. and Malmstrom H.S. (1990) Immunodominant antigens of Streptococcus mutans in dental caries-resistant subjects. Hum. Antibodies Hybridomas 1, 132–136 10.3233/HAB-1990-1303 [DOI] [PubMed] [Google Scholar]
- 57.Legler D.W., McGhee J.R., Lynch D.P., Mestecky J.F., Schaefer M.E., Carson J. et al. (1981) Immunodeficiency disease and dental caries in man. Arch. Oral. Biol. 26, 905–910 10.1016/0003-9969(81)90150-3 [DOI] [PubMed] [Google Scholar]
- 58.Cogulu D., Sabah E., Kutukculer N. and Ozkinay F. (2006) Evaluation of the relationship between caries indices and salivary secretory IgA, salivary pH, buffering capacity and flow rate in children with Down's syndrome. Arch. Oral. Biol. 51, 23–28 10.1016/j.archoralbio.2005.06.001 [DOI] [PubMed] [Google Scholar]
- 59.Kilian M. and Russell M.W. (2015) Microbial evasion of IgA functions. Mucosal Immunology, 4th edn, pp. 455–469, Academic Press, https://www.sciencedirect.com/science/article/pii/B9780124158474000227 [Google Scholar]
- 60.Shivakumar K.M., Vidya S.K. and Chandu G.N. (2009) Dental caries vaccine. Indian J. Dent. Res. 20, 99–106, http://www.ijdr.in/text.asp?2009/20/1/99/49066 10.4103/0970-9290.49066 [DOI] [PubMed] [Google Scholar]
- 61.Rudney J.D. and Smith Q.T. (1985) Relationships between levels of lysozyme, lactoferrin, salivary peroxidase, and secretory immunoglobulin A in stimulated parotid saliva. Infect. Immun. 49, 469–475 10.1128/IAI.49.3.469-475.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jentsch H., Beetke E. and Göcke R. (2004) Salivary analyses and caries increment over 4 years: an approach by cluster analysis. Clin. Oral Investig. 8, 156–160 10.1007/s00784-004-0263-6 [DOI] [PubMed] [Google Scholar]
- 63.Law V., Seow W.K. and Townsend G. (2007) Factors influencing oral colonization of mutans streptococci in young children. Aust. Dent. J. 52, 93–159 10.1111/j.1834-7819.2007.tb00471.x [DOI] [PubMed] [Google Scholar]
- 64.Palmeira P. and Carneirosampaio M. (2016) Immunology of breast milk. Rev. Assoc. Med. Bras. 62, 584–593 10.1590/1806-9282.62.06.584 [DOI] [PubMed] [Google Scholar]
- 65.Sanui T. and Gregory R.L. (2009) Analysis of Streptococcus mutans biofilm proteins recognized by salivary immunoglobulin A. Oral. Microbiol. Immunol. 24, 361–368 10.1111/j.1399-302X.2009.00523.x [DOI] [PubMed] [Google Scholar]
- 66.Khatoon Z., McTiernan C.D., Suuronen E.J., Mah T.F. and Alarcon E.I. (2018) Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 4, e01067 10.1016/j.heliyon.2018.e01067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jafarzadeh A., Sadeghi M., Karam G.A. and Vazirinejad R. (2010) Salivary IgA and IgE levels in healthy subjects: relation to age and gender. Braz. Oral. Res. 24, 21–27 10.1590/S1806-83242010000100004 [DOI] [PubMed] [Google Scholar]
- 68.Benderli Y., Erdilek D., Koray F., Telci A. and Turan N. (2000) The relation between salivary IgA and caries in renal transplant patients. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 89, 588–593 10.1067/moe.2000.105144 [DOI] [PubMed] [Google Scholar]
- 69.Allard R.H. (2000) Tobacco and oral health: attitudes and opinions of European dentists; a report of the EU working group on tobacco and oral health. Int. Dent. J. 50, 99–102 10.1002/j.1875-595X.2000.tb00806.x [DOI] [PubMed] [Google Scholar]
- 70.Vellappally S., Fiala Z., Smejkalová J., Jacob V. and Somanathan R. (2007) Smoking related systemic and oral diseases. Acta Medica. (Hradec. Kralove) 50, 161–166 10.14712/18059694.2017.76 [DOI] [PubMed] [Google Scholar]
- 71.Boyaka P.N. (2017) Inducing Mucosal IgA: A Challenge for Vaccine Adjuvants and Delivery Systems. J. Immunol. (Baltimore, Md.: 1950) 199, 9–16 10.4049/jimmunol.1601775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choonharuangdej S., Chutinet A. and Kuphasuk Y. (2011) Crevicular Porphyromonas gingivalis-specific immunoglobulin A levels in healthy and periodontitis-affected Thai cohorts. J. Investig Clin. Dent. 2, 43–50 10.1111/j.2041-1626.2010.00039.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are included within the article.