There is little debate that residual neuromuscular blockade (often defined as a threshold train-of-four [TOF] value less than 0.9 or 0.951) is associated with postoperative upper airway muscle dysfunction. This is evident from pharmaco-physiological interaction studies in volunteers in which small quantities of neuromuscular blocking agent are infused into awake patients, a study design that removes the confounding effects of surgery and anesthesia. The consistent observation is that minimal neuromuscular blockade (TOF-ratio 0.7 to 0.95) leads to upper airway dilator muscles dysfunction2, partial supralaryngeal airway obstruction during inspiration2,3, impaired swallowing4, and increased risk of food displacement into the laryngeal inlet5 (Figure 1).
Figure 1:
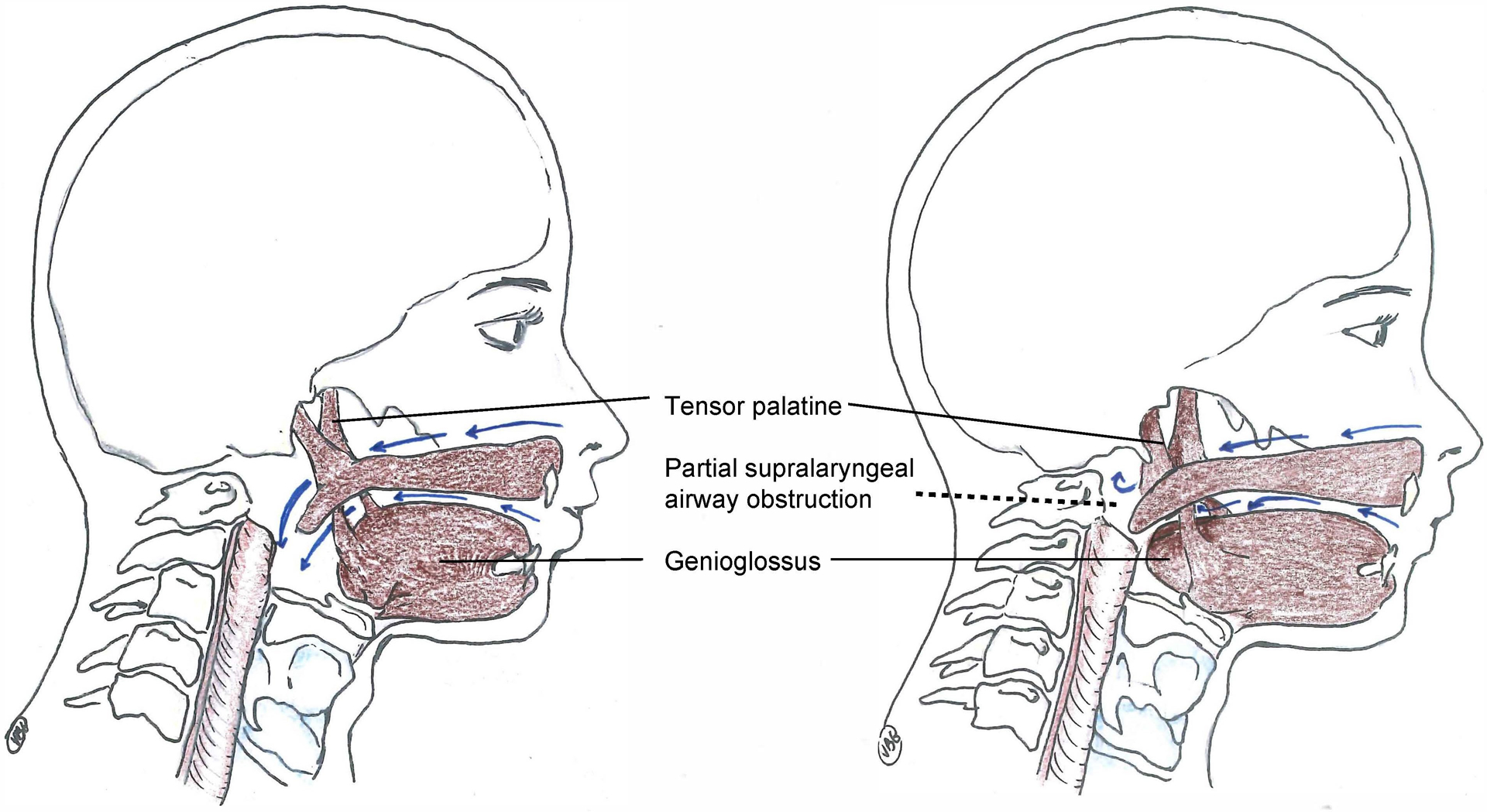
Pharmaco-physiology of upper airway failure during residual neuromuscular blockade.
Minimal residual neuromuscular blockade (train-of-four ratio 0.5–0.95) affects upper airway dilator muscle function which increases the vulnerability of the supralaryngeal airway to collapse during inspiration. The left panel shows a patient with full recovery of neuromuscular transmission. During inspiration, physiological coactivation of the tensor palatine and genioglossus muscles, which occur about 100 milliseconds before the contraction of the respiratory pump muscle (diaphragm), prevent negative inspiratory pressure related upper airway collapse. The right panel demonstrates the pharmaco-physiology during minimal neuromuscular blockade: the negative pressure generated by the diaphragm leads to a complete or partial collapse of the retronasal or retroglossal airway. These effects of residual neuromuscular transmission blockade can be treated with the application of non-invasive ventilation.
The data indicating a high vulnerability of the upper airway function to the use of neuromuscular blocking agents in volunteers translate to patients after surgery: high doses of non-depolarizing neuromuscular blocking agents and incomplete recovery of the TOF-ratio are associated with clinical indicators of postoperative upper airway obstruction and hypoxia6. In addition, patients with residual neuromuscular blockade require postoperative intensive care unit admission more frequently7. For this reason, many clinicians routinely use acetylcholine esterase inhibitors to reverse residual neuromuscular blockade at the end of surgery, an approach that can further impair respiratory muscle function when these reversal drugs are given despite full recovery from neuromuscular transmission block. Quantitative assessment of neuromuscular function continues to be poorly utilized leading to imprecise dosing of acetylcholine esterase inhibitors. Inappropriate administration of neostigmine in the absence of neuromuscular blockade has been shown to impair airway dilator muscle activity, increase airway collapsibility, and produce a restrictive pattern of ventilation8,9.
Although encapsulating agents such as sugammadex intrinsically possess no properties that reduce postoperative pulmonary complications, they do provide faster and more complete reversal of neuromuscular blockade10. In contrast to the acetylcholine esterase inhibitor neostigmine, encapsulating agents do not affect upper airway muscle function and they allow for complete reversal11.
In this month’s issue of Anesthesia & Analgesia, Krause and colleagues present important new information suggesting that the use of encapsulating drugs may reduce postoperative airway failure, indicated by a lower utilization of postoperative non-invasive ventilation12. The transition from neostigmine to sugammadex was near-complete, 99.97% (3419/3420) of patients in the pre-sugammadex group received neostigmine and 99.41% (3873/3896) of patients in the post-sugammadex group received sugammadex. Capitalizing on this rapid transition from neostigmine to sugammadex, the authors used an interrupted time series design to analyze the consequences of this change in practice. The incidence of postoperative reintubation or requirement for non-invasive ventilation was 6.1% (209/3420) in the pre-sugammadex group and 4.2% (164/3896) in the post-sugammadex group. Interrupted time series analysis using segmented logistic regression modeling with adjustment for potential confounders was used to show that the transition to sugammadex was associated with reduced odds of re-intubation or initiation of non-invasive ventilation.
Interrupted time series analysis: A valid method to examine the consequences of a change in clinical practice
Overall, the authors are to be applauded for applying sophisticated methods to evaluate the effects of their quality improvement study. We provide additional advice that may be helpful to improve the quality of future studies (Table 1).
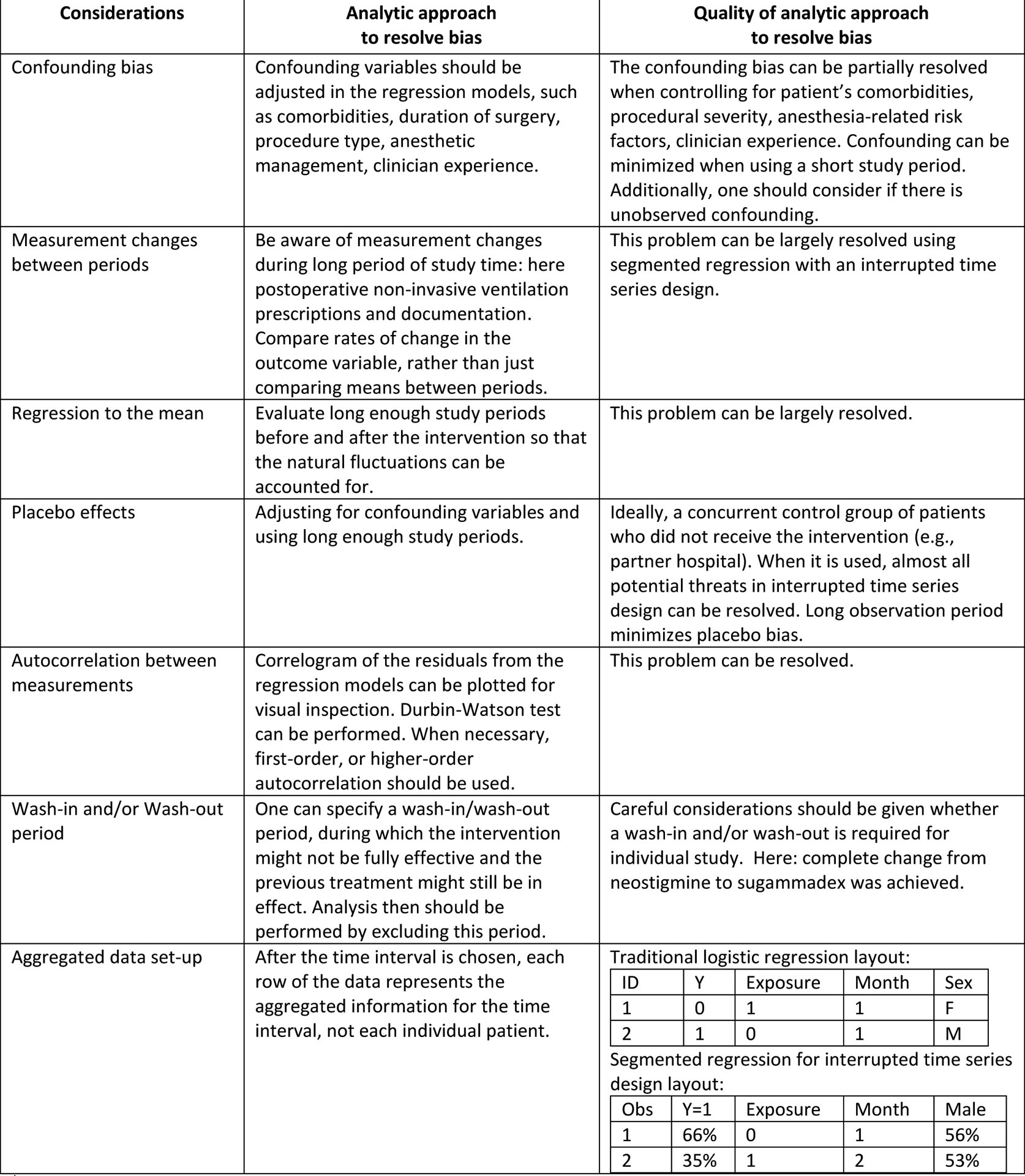
Table 1.
Considerations for studies using segmented regression analysis with an interrupted time series design.
|
This table was generated based on Mascha and Sessler 2019 and Wagner, et al. 2002.
Krause and colleagues utilized an interrupted time series design, which is quasi-experimental approach, to evaluate the longitudinal effects of an intervention (institutional change from neostigmine to sugammadex) on a variable of interest (reintubation or initiation of non-invasive ventilation) over time13. The following methodological issues may impact the interpretation of interrupted time series designs: confounding bias, provider learning effects, between-period measurement change, regression to the mean, and placebo effects (Table 1)14. The authors handled these challenges well. To reduce confounding bias, the authors included characteristics suspected to change between study periods using a pre-specified confounder model. Since the outcome was studied from August 15 2015 to May 12 2017, the authors were likely to have avoided significant measurement changes between periods, which need to be considered as a consequence of other practice changes. The authors also evaluated the rate of change in the outcome over time, which effectively avoid the caveats of solely studying means between periods that can be confounded by measurement changes. The authors also successfully overcame threats to validity related to regression to the mean (the observation that a high-incidence period tends to be followed by a low-incidence period due to chance alone14), placebo effects (providers knowing they are being studied and adjusting patient care accordingly), and learning effects (significant improvements that may have occurred due to clinician experience) by using long enough study periods13.
In general, segmented regression analysis with an interrupted time series design requires a different data set-up than traditional models. Specifically, instead of using individual-level covariates, all data should be aggregated within equally spaced chosen time intervals (week, month, or quarter). While the characteristics of patients are shown in the first row of the data, in segmented regression, all patients from month 1 were summarized in the first row of the data (Table 1). Krause and colleagues used individual-level data, which may have confounded the time series results13. The authors also failed to assess for autocorrelation, the idea that observations made closer together tend to be more similar than observations separated by a long duration of time.14 When this correlation is significant, failing to account for the correlation will lead to overestimation of the effect of interest, resulting in the Type I error.
What can clinicians learn from this quality improvement study?
The shortcomings of before-after studies aside, this investigation has several notable quality features. The before and after study periods included the same calendar months, thereby decreasing the impact of seasonal changes in flu risk and staffing that may affect the initiation of non-invasive ventilation. The wash out period was short (three months), decreasing the likelihood that unmeasured variables such as changes in clinical practice, surgical techniques, or anesthetic practice differed between study periods. The study was large, including 7,316 patients. Lastly, the research question was assessed in a “real-world” situation without the constraints of strict inclusion criteria and idealized study conditions, improving study generalizability.
It is important to note that the authors, in fact, observed changes in postoperative airway failure – a subtype of postoperative respiratory failure. Krause and colleagues comment that the incidence of reintubation amongst all patients was 0.8% and the incidence of initiation of non-invasive ventilation was 4.9%. The reduction in pulmonary complications observed in this study was driven by changes in non-invasive ventilation whereas the authors did not find a difference in reintubation requirement. The initiation of new non-invasive ventilation for 1 in 20 patients is substantial; the manuscript could be strengthened by including the criteria used for initiation of non-invasive ventilation, which would help readers understand this rate.
The article is not without limitations. Routine screening for sleep apnea with alerts and perioperative management guidance for high-risk patients has been associated with lower requirement for postoperative mechanical ventilation15. A conservative interpretation of study results includes the caveat that the increased institutional focus on sleep apnea during the study period may have decreased the incidence of non-invasive ventilation in the post-sugammadex period. The percentage of patients without documented peripheral nerve stimulation reduced from 7.9% with neostigmine to 3.3% with sugammadex. This is perhaps in part also related to an increased institutional focus on monitoring neuromuscular blockade, which may improve accurate dosing of reversal agents, and reduce postoperative respiratory failure. An additional limitation is the absence of quantitative neuromuscular blockade monitoring. Without quantitative monitoring in the post-anesthesia care unit, it is impossible to conclude that recovery of neuromuscular transmission mediates the decrease in postoperative airway failure observed following introduction of sugammadex. In addition, 87.7% (6,418 / 7,316) of patients received neostigmine or sugammadex with a documented train-of-four count of 4/4. The study may not be generalizable to patients reversed at a deeper level of neuromuscular blockade, or those clinicians who don’t use neuromuscular transmission monitoring.
In conclusion, the present analysis shows that immediate postoperative airway failure is lower with the encapsulating agent sugammadex than with the acetylcholine esterase inhibitor neostigmine. The change in practice from neostigmine to sugammadex had beneficial effects on postoperative respiratory function that need to be further validated in future studies. A reduction in postoperative residual blockade has been associated with reduced odds of intensive care unit admission, but no change in hospital costs7. Accordingly, there is insufficient evidence to conclude these beneficial effects on postoperative respiratory function translate to cost reduction.
Glossary of Terms:
- TOF
train-of-four
Footnotes
Conflicts of Interest: Brandon Togioka received an investigator initiated studies grant from Merck and Co. This grant supported a randomized controlled trial that compared moderate neuromuscular blockade reversal with sugammadex against neostigmine. The primary outcome was a composite of postoperative pulmonary complications, of which one component was upper airway obstruction (NCT02861131). Matthias Eikermann received an investigator initiated grant to study provider variability in the use of neuromuscular blocking agents, and to validate a residual neuromuscular blockade prediction tool (NCT03585348 and NCT03585400).
References:
- 1.Blobner M, Hunter JM, Meistelman C, et al. Use of a train-of-four ratio of 0.95 versus 0.9 for tracheal extubation: an exploratory analysis of POPULAR data. Br J Anaesth. 2019. October 10 pii: S0007–0912(19)30654-3. doi: 10.1016/j.bja.2019.08.023.. [DOI] [PubMed] [Google Scholar]
- 2.Herbstreit F, Peters J, Eikermann M. Impaired upper airway integrity by residual neuromuscular blockade: increased airway collapsibility and blunted genioglossus muscle activity in response to negative pharyngeal pressure. Anesthesiology. 2009;110(6):1253–1260. [DOI] [PubMed] [Google Scholar]
- 3.Eikermann M, Vogt FM, Herbstreit F, et al. The predisposition to inspiratory upper airway collapse during partial neuromuscular blockade. Am J Respir Crit Care Med. 2007;175(1):9–15. [DOI] [PubMed] [Google Scholar]
- 4.Sundman E, Witt H, Olsson R, Ekberg O, Kuylenstierna R, Eriksson LI. The incidence and mechanisms of pharyngeal and upper esophageal dysfunction in partially paralyzed humans: pharyngeal videoradiography and simultaneous manometry after atracurium. Anesthesiology. 2000;92(4):977–984. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson LI, Sundman E, Olsson R, et al. Functional assessment of the pharynx at rest and during swallowing in partially paralyzed humans: simultaneous videomanometry and mechanomyography of awake human volunteers. Anesthesiology. 1997;87(5):1035–1043. [DOI] [PubMed] [Google Scholar]
- 6.Murphy GS, Szokol JW, Marymont JH, Greenberg SB, Avram MJ, Vender JS. Residual neuromuscular blockade and critical respiratory events in the postanesthesia care unit. Anesth Analg. 2008;107(1):130–137. [DOI] [PubMed] [Google Scholar]
- 7.Grabitz SD, Rajaratnam N, Chhagani K, et al. The Effects of Postoperative Residual Neuromuscular Blockade on Hospital Costs and Intensive Care Unit Admission: A Population-Based Cohort Study. Anesth Analg. 2019;128(6):1129–1136. [DOI] [PubMed] [Google Scholar]
- 8.Herbstreit F, Zigrahn D, Ochterbeck C, Peters J, Eikermann M. Neostigmine/glycopyrrolate administered after recovery from neuromuscular block increases upper airway collapsibility by decreasing genioglossus muscle activity in response to negative pharyngeal pressure. Anesthesiology. 2010;113(6):1280–1288. [DOI] [PubMed] [Google Scholar]
- 9.Kent NB, Liang SS, Phillips S, et al. Therapeutic doses of neostigmine, depolarising neuromuscular blockade and muscle weakness in awake volunteers: a double-blind, placebo-controlled, randomised volunteer study. Anaesthesia. 2018;73(9):1079–1089. [DOI] [PubMed] [Google Scholar]
- 10.Haerter F, Simons JC, Foerster U, et al. Comparative Effectiveness of Calabadion and Sugammadex to Reverse Non-depolarizing Neuromuscular-blocking Agents. Anesthesiology. 2015;123(6):1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eikermann M, Zaremba S, Malhotra A, Jordan AS, Rosow C, Chamberlin NL. Neostigmine but not sugammadex impairs upper airway dilator muscle activity and breathing. Br J Anaesth. 2008;101(3):344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krause M, McWilliams SK, Bullard KJ, et al. Neostigmine versus sugammadex for reversal of neuromuscular blockade and effects on re-intubation for respiratory failure or newly initiated non-invasive ventilation—An interrupted time series design. Anesth Analg. 2019. November 5. doi: 10.1213/ANE.0000000000004505.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. [DOI] [PubMed] [Google Scholar]
- 14.Mascha EJ, Sessler DI. Segmented Regression and Difference-in-Difference Methods: Assessing the Impact of Systemic Changes in Health Care. Anesth Analg. 2019;129(2):618–633. [DOI] [PubMed] [Google Scholar]
- 15.Raub D, Santer P, Nabel S, et al. BOSTN Bundle Intervention for Perioperative Screening and Management of Patients With Suspected Obstructive Sleep Apnea: A Hospital Registry Study. Anesth Analg. 2019, July 2. doi: 10.1213/ANE.0000000000004294. [DOI] [PubMed] [Google Scholar]


