Abstract
Context
Oral glucose tolerance test (OGTT)-related hypoglycemia is common in pancreatic-insufficient cystic fibrosis (PI-CF), but its mechanistic underpinnings are yet to be established.
Objective
To delineate the mechanism(s) underlying OGTT-related hypoglycemia.
Design and Setting
We performed 180-minute OGTTs with frequent blood sampling in adolescents and young adults with PI-CF and compared results with those from a historical healthy control group. Hypoglycemia (Hypo[+]) was defined as plasma glucose <65 mg/dL. We hypothesized that CF-Hypo[+] would demonstrate impaired early phase insulin secretion and persistent late insulin effect compared with control-Hypo[+], and explored the contextual counterregulatory response.
Main Outcome Measure
OGTT 1-hour and nadir glucose, insulin, C-peptide, and insulin secretory rate (ISR) incremental areas under the curve (AUC) between 0 and 30 minutes (early) and between 120 and 180 minutes (late), and Δglucagon120-180min and Δfree fatty acids (FFAs)120-180min were compared between individuals with CF and control participants with Hypo[+].
Results
Hypoglycemia occurred in 15/23 (65%) patients with CF (43% female, aged 24.8 [14.6-30.6] years) and 8/15 (55%) control participants (33% female, aged 26 [21-38] years). The CF-Hypo[+] group versus the control-Hypo[+] group had higher 1-hour glucose (197 ± 49 vs 139 ± 53 mg/dL; P = 0.05) and lower nadir glucose levels (48 ± 7 vs 59 ± 4 mg/dL; P < 0.01), while insulin, C-peptide, and ISR-AUC0-30 min results were lower and insulin and C-peptide, and AUC120-180min results were higher (P < 0.05). Individuals with CF-Hypo[+] had lower Δglucagon120-180min and ΔFFA120-180min compared with the control-Hypo[+] group (P < 0.01).
Conclusions
OGTT-related hypoglycemia in PI-CF is associated with elevated 1-hour glucose, impaired early phase insulin secretion, higher late insulin exposure, and less increase in glucagon and FFAs. These data suggest that hypoglycemia in CF is a manifestation of islet dysfunction including an impaired counterregulatory response.
Keywords: cystic fibrosis, hypoglycemia, glucose tolerance, pancreatic insufficiency, insulin secretion, glucose counterregulation
Glucose homeostasis is often abnormal in individuals with pancreatic-insufficient cystic fibrosis (PI-CF), existing on a continuum from early glucose intolerance to impaired glucose tolerance to cystic fibrosis-related diabetes (CFRD) based on oral glucose tolerance test (OGTT) criteria that include 1-hour as well as 2-hour glucose measurements (1). While CFRD is associated with deteriorating pulmonary and nutritional status and significantly increased mortality (2-5), early glucose abnormalities too are clinically relevant as they are associated with worse pulmonary function prior to the development of diabetes (6-8). Consequently, annual OGTTs are recommended by the CF Foundation beginning by 10 years of age (9). Though seemingly incongruent with progression to CFRD, postprandial and OGTT-related hypoglycemia are reported in up to 45% of patients with CF but as high as 64% in those with PI-CF (10-15).
The mechanisms underlying hypoglycemia in CF and the relationship of hypoglycemia to the onset of CFRD remain poorly defined (16, 17). Recent studies suggest dysregulated insulin secretion and impaired counterregulatory response are operative (15, 18), but these studies have lacked a control group for comparisons to the response observed in individuals without CF who experience reactive OGTT-related hypoglycemia. While other studies have not demonstrated a relationship between hypoglycemia occurring at 2 hours during a standard OGTT and incidence of CFRD (10, 14), these reports are limited by the 2-hour observation period and inclusion of individuals with impaired glucose tolerance, who by definition cannot experience hypoglycemia by the 2-hour glucose, and lack of stratification for early glucose intolerance (defined as an elevated 1-hour glucose) (19, 20).
We hypothesized that the reactive hypoglycemia following OGTT in PI-CF is a manifestation of both impaired early phase insulin secretion and altered counterregulatory responses. To test these hypotheses, we compared islet cell hormonal responses during an extended OGTT in individuals with PI-CF with hypoglycemia to responses obtained from a control group without CF and a control group with PI-CF without hypoglycemia. Confirmation of these hypotheses would support growing evidence that postprandial hypoglycemia is an early indicator of islet dysfunction in CF that precedes progression to CFRD.
Methods
Individuals aged 12 to 30 years with CF, diagnosed by sweat test or cystic fibrosis transmembrane regulator mutation analysis, and pancreatic insufficiency (PI), defined by need for enzyme replacement therapy based on clinical symptoms and/or fecal elastase <200 µg/g, and due for annual OGTT, were recruited for an extended OGTT study from March 2019 through October 2019. Data on most recent forced expiratory volume in 1 second, cystic fibrosis transmembrane regulator modulator use, and genotype were extracted from the medical record. Individuals with a history of organ transplant, CFRD with fasting hyperglycemia (glucose ≥126 mg/dL), or receiving treatment with rapid-acting insulin, or requiring systemic glucocorticoids in the 4 weeks preceding the study visit were excluded. Non-CF control participants were individuals without diabetes from a previously reported study (21). Initial recruitment goals were developed based on power calculations for identifying differences in individuals with CF-Hypo[+] versus those with CF-Hypo[–]; however, the prevalence of CF-Hypo[+] was significantly higher than anticipated, hampering comparisons. In order to include normative data, we used a sample of convenience.
After a 12-hour overnight fast and baseline blood sampling at t = –10 minutes and 0 minutes using an indwelling intravenous catheter, participants ingested 1.75 g/kg of glucose solution (max 75 g) over a 5-minute period starting at t = 0. Additional blood samples were obtained at t = 10, 20, and 30 minutes, and then every subsequent 15 minutes until 240 minutes in individuals with PI-CF and every subsequent 30 minutes until 180 minutes in control participants. Testing in individuals with PI-CF only was terminated for symptomatic hypoglycemia (glucose <65 mg/dL) or any glucose ≤50 mg/dL.
This study was approved by the Institutional Review Boards of the University of Pennsylvania (Penn) and Children’s Hospital of Philadelphia (CHOP). Written informed consent and assent (as appropriate for age) were obtained from all participants.
Biochemical analyses
Plasma glucose was measured in duplicate using an YSI 2300 glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH) or Nova StatStrip glucometer (Data Sciences International, St Paul, MN) that demonstrates a high level of agreement (22). Plasma insulin, C-peptide, and glucagon (23) were measured in duplicate by double-antibody radioimmunoassays (Millipore, Billerica, MA). Free fatty acids (FFAs) were measured spectrophotometrically using an enzymatic colorimetric assay (Fujifilm WAKO Diagnostics, Mountain View, CA). For control participants, previously measured glucose and insulin utilized the same assay methodology (as previously reported (21), and stored plasma aliquots were used to assay C-peptide, glucagon, and FFAs concomitantly with those from the individuals with CF.
Definitions
Research OGTTs were used to define glucose tolerance among study participants: - CFRD: 2-hour glucose ≥200 mg/dL- Impaired Glucose Tolerance (IGT): 2-hour glucose 140–199 mg/dL - Early Glucose Intolerance (EGI): 1-hour glucose ≥155 mg/dL and 2-hour glucose <140 mg/dL- Normal Glucose Tolerance (NGT): 1-hour glucose ≤155 mg/dL and 2-hour glucose <140 mg/dL.Hypoglycemia (Hypo[+]) was defined as glucose <65 mg/dL.
Calculations
Insulin secretory rates (ISRs) were calculated using the oral minimal model as described by Breda et al (24, 25) and derived by parametric deconvolution of C-peptide kinetics as estimated using a 2-compartment model (26). Insulin sensitivity (SI) was estimated based on the model of Dalla Man using a “differential equation” approach (27). Incremental areas under the curve (AUCs) were calculated for 0 to 30 minutes and 120 to 180 minutes to assess early responses and late effects during the OGTTs. For individuals whose test was terminated early, the AUC120-180min was calculated as the last 60 minutes relative to the time of study termination (eg, an individual concluding at 150 minutes would be AUC90-150min). Absolute change in glucagon (Δglucagon120-180min) and FFA (ΔFFA120-180min) over the last 60 minutes of the test was derived from the final measurement minus the antepenultimate measurement. FFA minimal model calculations were performed according to the methods of Boston and Moate to examine dynamics of FFA suppression, nadir, and rebound as estimates of lipolysis (28).
ISR, SI, and FFA parameter estimation was performed using in WinSAAM software 3.0.8 (University of Pennsylvania, New Bolton Center, Kennett Square, Pennsylvania).
Statistical analyses
Continuous data are reported as mean ± standard deviation or median (min-max), unless otherwise noted. Categorical data are presented as percentage. Hypoglycemia (Hypo[+]) was defined as glucose <65 mg/dL and used to assign participants to exposure groups for analysis. Given the small sample size, regardless of normality, continuous variables were compared using the nonparametric Mann-Whitney U test and categorical data by the Fisher exact test. Regression analyses were also performed to further investigate the relationships of (a) insulin-AUC120-180min and glucose-AUC120-180min and (b) Δglucagon and ΔFFA while adjusting for baseline glucagon and FFA, respectively. Significance was considered at P ≤ 0.05 (2-tailed). All statistical analyses were performed in STATA 15 software (StataCorp LP, College Station, Texas). Given the availability of control data to only 180 minutes, data from individuals with CF were analyzed to180 minutes.
Results
Individuals with PI-CF (n = 23) were of comparable gender distribution and age to the non-CF control group (n = 15) (Table 1). Body weight, and consequently body mass index, were lower in the group with PI-CF than in the control group (P < 0.05 for both comparisons; Table 1). The CF-Hypo[–] group had worse glucose tolerance (3/8 CFRD, 4/8 IGT, 1/8 EGI), compared with CF-Hypo[+] group (6/15 IGT, 7/15 EGI, and 2/15 NGT) (italics in Table 1; P < 0.05 by the Fisher exact test).
Table 1.
Participant Characteristics
| CF | Overall n = 23 | Control | Overall n = 15 | P Values (CF vs Control) | ||||
|---|---|---|---|---|---|---|---|---|
| Hypo[+] n = 15 | Hypo[–] n = 8 | Hypo[+] n = 8 | Hypo[–] n = 7 | Overall | Hypo[+] | |||
| Female, n (%) | 7 (47) | 3 (37.5) | 10 (43) | 2 (25) | 3 (43) | 5 (33) | 0.54 | 0.33 |
| Age | 25.7 (14.6-30.6) | 23.6 (19.2-29.4) | 24.8 (14.6-30.6) | 25.5 (21-35) | 26 (23-38) | 26 (21-38) | 0.08 | 0.37 |
| Height (cm) | 164.7 (158.7-182.2) | 171.2 (161-187) | 165 (158.7-186.9) | 169.5 (155.5-181) | 170 (155.5-186.2) | 170 (155.5-186.2) | 0.69 | 0.63 |
| Weight (kg) | 61.9 (47.4-78.3) | 60.5 (46.4-79.4) | 61.9 (46.4-79.4) | 72.6 (55.6-87.2) | 69.4 (57.6-87.9) | 70.8 (55.6-87.9) | 0.02 | 0.09 |
| BMI-z | 0.15 (–1.9 to 1.2) | 0.12 (–2.4 to 0.51) | 0.12 (–2.44 to 1.23) | 0.44 (–0.43 to 1.2) | 0.66 (–0.3 to 1.2) | 0.61 (–0.43 to 1.22) | 0.03 | 0.15 |
| Fasting PG (mg/dL) | 88.8 (63.5-113.5) | 95.1 (79.3-106.8) | 91 (63.5-113.5) | 86.5 (75.9-107) | 91.2 (86.2-98.9) | 88.9 (75.9-107) | 0.57 | 0.69 |
| OGTT 1-hour PG (mg/dL) | 196.5 (113-272.5) | 210.9 (159-265) | 201.5 (113-272.5) | 139.8 (69.6-211.5) | 133.4 (109-193) | 136.8 (69.6-211.5) | <0.01 | 0.02 |
| OGTT 2-hour PG (mg/dL) | 121.1 (47.2-197) | 192.3 (112-266) | 145.9 (47.2-266) | 95.5 (60.4-145) | 105.1 (88.9-127.5) | 99.9 (60.4-145) | <0.01 | 0.11 |
| Normal glucose tolerance | 2 (13) | - | 2 (9) | 5 (62) | 6 (86) | 11 (73) | ||
| Early glucose intolerance | 7 (47) | 1 (13) | 8 (35) | 2 (25) | 1 (14) | 3 (20) | ||
| Impaired glucose tolerance | 6 (40) | 4 (50) | 10 (43) | 1 (13) | - | 1 (7) | ||
| Diabetes | - | 3 (37) | 3 (13) | - | - | - | ||
| FEV 1 % predicted | 89 (48-128) | 74 (32-96) | 86 (32-128) | |||||
| Genotype, n (%) delF508 heterozygous | 6 (40) | 2 (25) | 8 (35) | |||||
| Genotype, n (%) delF508 homozygous | 6 (40) | 5 (63) | 11 (48) | |||||
| Modulator therapy, n (%) | 9 (60) | 6 (75) | 15 (65) |
Data are expressed as median (minimum-maximum). Comparisons were performed between the CF and Control Overall and Hypo [+] subgroups. Italics represent P < 0.05 for CF-Hypo [+] versus CF-Hypo [–].
Abbreviations: BMI, body mass index; CF, cystic fibrosis; FEV1, forced expiratory volume in 1 second; Hypo, hypoglycemia; OGTT, oral glucose tolerance test; PG, plasma glucose.
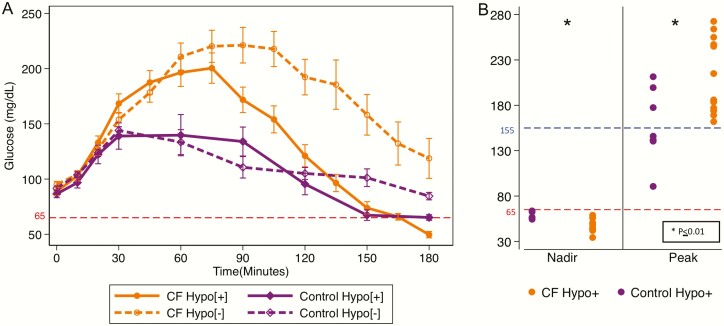
Hypoglycemia occurred in 15 (65%) individuals with PI-CF and 8 (55%) control participants by 180 minutes. The 1-hour glucose was higher (197 ± 49 vs 139 ± 53 mg/dL; P = 0.05), and the nadir glucose was lower (48 ± 7 vs 59 ± 4 mg/dL; P < 0.01) in CF-Hypo[+] group compared with the control-Hypo[+] group (Fig. 1A,B). In addition, glucose concentrations at 90 to 180 minutes and glucose-AUC120-180min were lower in the CF-Hypo[+] group versus the CF-Hypo[–] group (all P < 0.05; Fig. 1A and Table 2). For Hypo[+], nadir glucose occurred most commonly at 180 minutes for both participants with CF (range 135-180 minutes) and control participants (range 90-180 minutes). Additionally, 3 of the 8 control-Hypo[+] participants spontaneously recovered by 180 minutes while no individuals with CF-Hypo[+] self-resolved in the 180-minute time frame (P = 0.01).
Figure 1.
(A) Plasma glucose in response to ingestion of 1.75 g/kg of glucose solution (max 75 g) over a 5-minute period starting at t = 0 during the oral glucose tolerance test (OGTT). Data are expressed as mean ± standard error of the mean. Average glucose at 90 to 180 minutes is higher in the CF-Hypo[–] group versus the CF-Hypo[+] group. Average glucose concentration at 90 to 180 minutes and glucose area under the curve120-180min were lower in the CF-Hypo[+] group versus CF-Hypo[–] group. (B) Left: Nadir plasma glucose for control-Hypo[+] group and CF-Hypo[+] group. Right: Peak plasma glucose for control-Hypo[+] and CF-Hypo[+] groups. The 1-hour glucose was higher (197 ± 49 vs 139 ± 53 mg/dL; P = 0.05), and the nadir glucose was lower (48 ± 7 vs 59 ± 4 mg/dL; P < 0.01) in the CF-Hypo[+] group compared with the control-Hypo[+] group. CF, cystic fibrosis; Hypo, hypoglycemia.
Table 2.
OGTT Responses During the First 30 Minutes and the Last 60 Minutes of Observation Post-Ingestion
| CF | Control | P Values (CF vs Control) | ||||||
|---|---|---|---|---|---|---|---|---|
| Hypo[+] | Hypo[–] | Overall | Hypo[+] | Hypo[–] | Overall | Overall | Hypo+ | |
| Glucose AUC 0-30min | 3574.5 (2842.5-4719.5) | 3641.5 (2998.5- 3903.4) | 3606.5 (2842.5- 4719.5) | 3258.1 (2484.2- 4102.5) | 3419.3 (3304.4- 3769.4) | 3387.8 (2484.2- 4102.5) | 0.2 | 0.25 |
| Insulin AUC 0-30min (μU ∙min/mL) | 372.5 (175.75-774.5) | 308.5 (91.5- 583.75) | 372.5 (91.5-774.5) | 891.4 (619.3-2270) | 904.8 (424.8-1242.3) | 904.8 (424.8-2270) | <0.01 | <0.01 |
| C-peptide AUC 0-30min (ng∙min/dL) | 71.9 (43.2-93.1) | 57.2 (32.8- 75.2) | 67.6 (32.8-93.1) | 107.6 (48.3-130.5) | 97.8 (55.1-114.4) | 99.7 (48.3-130.5) | <0.01 | 0.01 |
| ISR AUC 0-30min (μU/mL) | 2056 (1227-3789.5) | 1559.6 (788- 2685.2) | 1786.1 (788-3789.5) | 4046.6 (2504.3- 5220.2) | 3863.8 (738.3-4231.2) | 3990.7 (738.3- 5220.2) | <0.01 | <0.01 |
| Glucose AUC 120-180min | –320.6 (–1253.3 to 4101.8) | 2580.8 (108- 9079.5) | 1088.3 (–1253.3 to 9079.5) | –797 (1386.3 to 523.5) | 145 (–565.3 to 2136.2) | –565.3 (–1386.3 to 2136.2) | 0.02 | 0.04 |
| Insulin AUC 120-180min (μu ∙min/mL) | 1015.5 (510-3395.3) | 1497.8 (354.8- 2670.8) | 1046.3 (354.8- 3395.3) | 450.8 (10.5-2160) | 894 (246-1197) | 703.5 (10.5-2160) | <0.01 | <0.01 |
| C-peptide AUC 129-180min (ng∙min/dL) | 260.3 (174.5-634.2) | 297 (139.6- 599.7) | 285.9 (139.6-634.2) | 114.3 (24-300.6) | 189 (62-318.6) | 135 (24-318.6) | <0.01 | <0.01 |
| ΔGlucagon 120-180min (pg/mL) | –5 (–214 to 21) | –4.5 (–26 to 7) | –5 (–214 to 21) | 10.5 (–1 to 39) | –11 (–22 to 19) | 3 (–22 to 9) | <0.01 | <0.01 |
| ΔFFA 120-180min (mM) | 0.007 (–0.06 to 0.35) | –0.009 (–0.036 to 0.033) | –0.003 (–0.062 to 0.353) | 0.34 (–0.008 to 0.42) | 0.025 (–0.018 to 0.83) | 0.064 (–0.018 to 0.829) | <0.01 | 0.01 |
Data are expressed as median (minimum-maximum). Comparisons in this table include rank-sum testing performed between the CF and Control Overall and Hypo [+] subgroups. Italics represent P < 0.05 for CF-Hypo [+] vs CF-Hypo [–].
Abbreviations: AUC, area under the curve; CF, cystic fibrosis; FFA, free fatty acid; Hypo, hypoglycemia; ISR, insulin secretory rate.
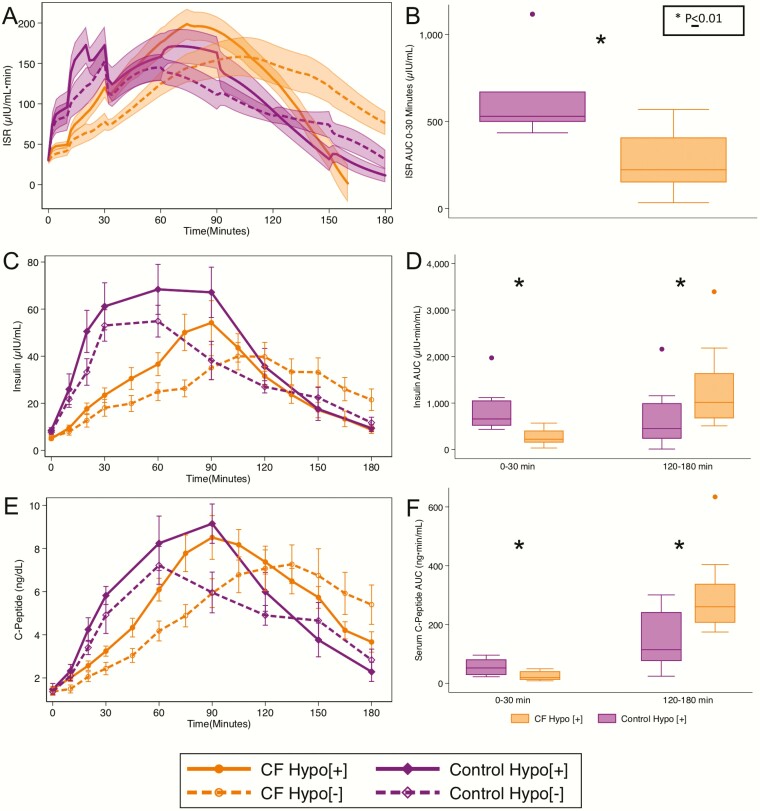
Early phase insulin-AUC0-30 min, C-peptide-AUC0-30 min, and ISR-AUC0-30 min were lower in individuals with CF-Hypo[+] compared with control-Hypo[+] participants (P = 0.01; Table 2; Fig. 2A,B,C,D). Meanwhile, insulin and C-peptide AUC120-180min were higher in the CF-Hypo[+] group compared with the control-Hypo[+] participants (P < 0.05; Table 2; Fig. 2E,F). The CF-Hypo[+] group did not differ from the control-Hypo[+] group in insulin sensitivity (SI: 12.4 ± 5.5 vs 8.7 ± 5.8 [10–4 min-1 per μU/mL]). CF-Hypo[+] participants and CF-Hypo[–] participants did not differ in insulin-AUC0-30 min or ISR-AUC0-30 min (P = 0.3 and 0.1, respectively). The C-peptide-AUC0-30 min was higher in the CF-Hypo[+] group (P = 0.03). No difference in insulin and C-peptide AUC120-180min between the CF-Hypo[+] and CF-Hypo[–] groups was found. Not unexpectedly, insulin-AUC120-180min and glucose-AUC120-180min were positively correlated in individuals with PI-CF, but this relationship did not differ between CF-Hypo[+] and CF-Hypo[–] groups (29).
Figure 2.
(A) Insulin secretory rate (ISR), (C) plasma insulin, and (E) C-peptide in response to the OGTT. (B) ISR AUC0-30min, (D) insulin AUC0-30min & 120-180min, and (F) C-peptide AUC0-30min & 120-180min as measures of early responses and late effects during the OGTT. Data are means ± SE (A, C, E), and box plots represent the median, upper and lower quartiles, minimum and maximum except outliers (whiskers), and outliers (dots). Note that apparent discrepancy in median insulin AUC120-180min, for the CF-Hypo[+] group compared with the control-Hypo[+] group in the line graph versus box plot is related to the termination time for individuals with CF. AUC, area under the curve; CF, cystic fibrosis; Hypo, hypoglycemia; OGTT, oral glucose tolerance test; SE, standard error.
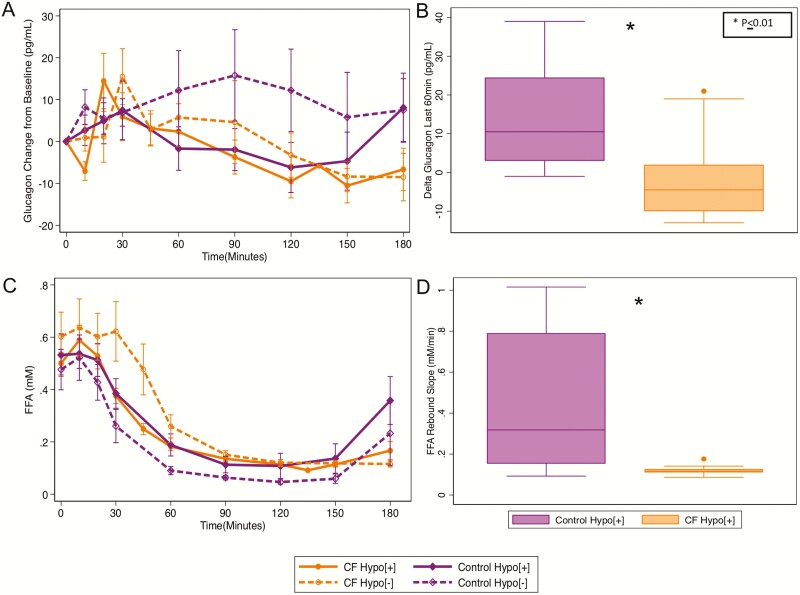
The CF-Hypo[+] group had less Δglucagon over the last 60 minutes (P < 0.01) than the control-Hypo[+] group (Table 2, Fig. 3A,B). This finding persisted in a regression model with adjustment for glucagon 60 minutes before hypoglycemia. Similarly, individuals with CF-Hypo[+] had less ΔFFA120-180min (P < 0.01) and a decreased FFA rebound (0.12 ± .03 vs 0.45 ± 0.35 mM/min; P = 0.02) than individuals in the control-Hypo[+] group (Table 2, Fig. 3C,D). This finding persisted in a regression model with adjustment for FFA 60 minutes prior to hypoglycemia. Neither FFA suppression nor FFA nadir differed in the CF-Hypo[+] versus the control-Hypo[+] groups.
Figure 3.
(A) Glucagon and (C) free fatty acids (FFA) in response to the OGTT. (B) ΔGlucagon120-180min and (D) FFA rebound slope as measures of the counterregulatory response during OGTT-related hypoglycemia. Hypo, hypoglycemia; OGTT, oral glucose tolerance test.
Insulin AUC120-180min and Δglucagon120-180min were negatively correlated (rho = –0.52; P = 0.01) overall in the combined group of CF and control-Hypo[+]; however, no apparent relationship for CF-Hypo[+] was independently found as multiple individuals with CF had no glucagon response in the last 60 minutes even at the lower insulin AUC. Results were similar for C-peptide (data not shown).
Higher insulin AUC120-180min was associated with less ΔFFA120-180min overall (rho = –0.67; P < 0.01); however, again no apparent relationship for CF-Hypo[+] (rho = –0.28, P = 0.3) was found. Results were similar for C-peptide (data not shown).
Higher Δglucagon120-180min was associated with higher ΔFFA120-180min, approaching significance (rho = 0.36; P = 0.09); however, for the CF-Hypo[+] group, the correlation is negative and not significant (rho = –0.18; P = 0.51) while for the control-Hypo[+] group, the correlation was positive and not significant (rho = 0.58; P = 0.12).
Discussion
These results demonstrate that the reactive hypoglycemia following OGTT in individuals with PI-CF is associated with impaired early phase insulin secretion, late post-OGTT exposure to insulin, and impaired glucagon and FFA counterregulatory responses compared with non-CF control participants. These are the first data examining OGTT-related or postprandial hypoglycemia in CF to include a non-CF control group that clearly delineates the presence of both impaired islet β- and α-cell responses consistent with dysregulated islet function contributing to reactive hypoglycemia in PI-CF.
We previously presented evidence of early glucose dysregulation, loss of early phase insulin secretion, and higher later-phase insulin secretion in individuals with PI-CF who experienced hypoglycemia during a mixed-meal tolerance test (18). In the present prospective study of hypoglycemia, we compare insulin secretion responses during an OGTT to those of healthy individuals with OGTT-related hypoglycemia. We show that, as with mixed-meal tolerance test, OGTT-related hypoglycemia is not only common in PI-CF but is associated with higher glucose excursion during the first hour post-ingestion, lower ISR in the first 30 minutes, and higher late insulin concentration. Additionally, while our previous study showed no difference in postprandial glucagon between those with PI-CF who developed hypoglycemia and those who did not (18), we now demonstrate that individuals with PI-CF with OGTT-related hypoglycemia have less increase in glucagon and FFAs and more severe hypoglycemia when compared with healthy controls. Additionally, this study had the opportunity to compare the degree of hypoglycemia between control participants and individuals with CF. While the frequency of hypoglycemia in control participants was similar to those with individuals with CF, the glucose nadir was significantly lower in CF. Furthermore, we defined Hypo[+] as individuals who experienced hypoglycemia at any point during OGTT, including those who spontaneously recovered, in order to interrogate counterregulatory mechanisms. Spontaneous recovery occurred in 3/8 (37.5%) control-Hypo[+] participants and in 0/15 (0%) individuals with CF-Hypo[+]. Thus, 5/15 (33.3%) individuals in the control group experienced persistent hypoglycemia, which is consistent with prior literature (30, 31).
Other studies investigating hypoglycemia in CF have concluded that hypoglycemia is not insulin mediated, given absolute lower insulin concentrations in CF-Hypo[+] individuals compared with CF-Hypo[–] individuals (15, 17). These studies have included individuals with pancreatic-sufficient CF who exhibit normal early phase insulin secretion (32) and are at much lower risk for experiencing postprandial hypoglycemia or future CFRD, potentially confounding interpretation. Our study restricted inclusion to individuals with PI-CF and demonstrates those with CF-Hypo[+] have significantly higher insulin and C-peptide AUC in the last 60 minutes of testing compared with the control-Hypo[+] group. This later insulin exposure, in combination with the lower early phase insulin secretion in CF-Hypo[+] supports our hypothesis and prior study conclusion that β-cell secretory impairment is operative in CF OGTT-related hypoglycemia. CF-Hypo[–] and CF-Hypo[+] groups did not differ significantly in early insulin secretion or late compensatory insulin secretion, although the early phase C-peptide response was lower. Comparisons between these groups are potentially confounded by worse glucose tolerance in the CF-Hypo[–] group such that, relative to glucose, exposure to insulin secretion was even further impaired. These data, nevertheless, support the hypothesis that once β-cell secretory impairment progresses in severity, individuals with CF no longer experience hypoglycemia as a result of the dysregulated islet cell function.
Individuals with PI-CF are considered to have global islet dysfunction that also includes lower alpha-cell secretion (32), an impairment that suggests inadequate glucagon secretion may contribute to the development of postprandial hypoglycemia (15). In the present study, individuals with CF-Hypo[+] did not experience the late increase in glucagon observed in the control-Hypo[+] group. Whether this impairment arises from a primary alpha-cell defect (33), intra-islet insulin suppression of glucagon secretion (34), or potential alterations in incretin secretion/sensitivity (with lower glucose-dependent insulinotropic polypeptide [GIP] in PI-CF33) remains unclear. Interestingly, our control participants with higher late insulin and C-peptide tended to have less increase in glucagon, a finding that suggests a direct role for insulin-mediated inhibition of glucagon responses. This relationship was not present in individuals with CF-Hypo[+] in whom glucagon responses were lower regardless of insulin status. In our prior study, we demonstrated lower glucagon-like peptide-1 and higher GIP in individuals with CF-Hypo[+] compared with those with CF-Hypo[–], which may confound this relationship (18). In contrast, Aitken et al did not find a difference in glucagon-like peptide-1 or GIP between those with and without hypoglycemia (15).
Finally, we have shown that individuals with CF-Hypo[+] have persistent FFA suppression despite the presence of hypoglycemia. Insulin is well recognized to suppress lipolysis (35), and suppressed FFAs are a prominent feature of hyperinsulinism (even in the absence of an elevated plasma insulin concentration). In contrast, catecholamines and, to a lesser extent, glucagon stimulate lipolysis to generate FFAs (36). With persistent or severe insulin-induced hypoglycemia, catecholamines can overcome the suppressive effect of insulin to increase FFA. Aitken et al observed increased epinephrine levels from 165 to 180 minutes post-OGTT in individuals with CF who experienced hypoglycemia compared with those who did not (15). While this study did not measure FFAs or include a non-CF control group, the suppressed FFA reported here at the time of hypoglycemia suggest an inadequate epinephrine response in addition to glucagon secretion impairment superimposed upon persistent insulin action.
The study strengths consist of frequent blood collection, mathematical modeling of the ISR, inclusion of only individuals with pancreatic insufficiency, and inclusion of historical lean control participants of similar age and gender distribution who participated in a study using similar extended OGTT methodology; these control participants enable comparisons with “normal” reactive hypoglycemia. A number of limitations are worth acknowledging. Despite inclusion criteria of age >12 years, only one youth, a 14-year-old male with EGI and who experienced hypoglycemia, was included. While no similarly aged adolescents were represented in the control group, group differences were unlikely attributable to the inclusion of 1 adolescent with CF. The sample size was relatively small and, specifically, few individuals with CF and NGT participated. Furthermore, comparisons between individuals with CF-Hypo[+] and individuals with CF-Hypo[–] were hampered by the frequency of hypoglycemia among individuals with CF. This cross-sectional study was unable to determine if hypoglycemia is an intermediary along the continuum of progressive insulin secretion defects and if hypoglycemia episodes wane as worsening glucose tolerance and CFRD emerge. Lastly, concerns with glucagon assays have arisen, specifically regarding the ability to adequately assess suppressed glucagon concentrations. The radioimmunoassay utilized in this study has high sensitivity and specificity for glucagon over oxyntomodulin and glicentin compared with other methodologies (23), and has been shown in prior studies to successfully detect suppressed glucagon in response to both oral (37) and intravenous challenges (32, 38, 39). Unfortunately, we do not have catecholamine or incretin data for the present study to further characterize the difference in hypoglycemia between groups.
In summary, data from this study draw attention to the early β-cell secretory defect that (a) is noted in individuals with PI-CF with reactive hypoglycemia and (b) causes increased glucose excursion and possible late compensatory relative hyperinsulinism. The study also provides further evidence for broader islet dysfunction with impaired α-cell glucagon response. Of additional clinical relevance, given the high rate of significant hypoglycemia by 3 hours post glucose load, patients should be encouraged to have a meal following their annual OGTT to reduce the occurrence of hypoglycemia. At this time, we recommend larger, longitudinal studies to inform a more complete appreciation of the causal mechanisms and to evaluate the risk for CFRD progression in individuals experiencing hypoglycemia, specifically in individuals with PI-CF.
Acknowledgments
We would like to thank the individuals with CF for their participation; the nursing staff of the Penn & CHOP Center for Human Phenomic Science for their care of participants and technical assistance; Heather Collins, PhD, of the University of Pennsylvania Diabetes Research Center Radioimmunoassay and Biomarkers Core for performance of the immunoassays; and Huong-Lan Nguyen of the Human Metabolism Resource of the University of Pennsylvania Institute for Diabetes, Obesity & Metabolism for laboratory assistance.
Financial Support: This work was supported by the Cystic Fibrosis Foundation grants KILBER18B0, KILBER19D0 (to M.J.K.), Pediatric Endocrine Society (to M.J.K), Public Health Services Research grants R01 DK97830 (to A.K. and M.R.R), NCATS UL1TR001878 (Penn and CHOP Center for Human Phenomic Science), P30 DK19525 (University of Pennsylvania Diabetes Research Center), and the Human Metabolism Resource of the University of Pennsylvania Institute for Diabetes, Obesity & Metabolism.
Author Contributions: M.J.K. designed and conducted the study, researched and analyzed data, and wrote the manuscript. C.H. and S.S. participated in the conduct of the study, researched and analyzed data, and reviewed/edited the manuscript. D.S. researched and analyzed data and reviewed/edited the manuscript. M.C. researched data and reviewed/edited the manuscript. C.K. participated in the conduct of the study, researched data, and reviewed/edited the manuscript. D.H. contributed to the design, researched data, and revised the manuscript critically for important intellectual content. R.C.R., M.R.R., and A.K. contributed to the design and conduct of the study, researched and analyzed data, and wrote the manuscript. M.J.K. and A.K. are the guarantors of this work and, as such, take full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
Affirmation of Originality and Authorship: The work submitted for publication is original and has not been published other than as an abstract or preprint in any language or format and has not been submitted elsewhere for print or electronic publication consideration. Each person listed as authors participated in the work in a substantive manner and is prepared to take public responsibility for it.
Glossary
Abbreviations
- AUC
area under the curve
- CF
cystic fibrosis
- CFRD
cystic fibrosis-related diabetes
- FFA
free fatty acid
- GIP
glucose-dependent insulinotropic polypeptide
- Hypo[+]
hypoglycemia
- ISR
insulin secretory rate
- OGTT
oral glucose tolerance test
- PI
pancreatic insufficiency
- Si
insulin sensitivity
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Nyirjesy SC, Sheikh S, Hadjiliadis D, et al. β-Cell secretory defects are present in pancreatic insufficient cystic fibrosis with 1-hour oral glucose tolerance test glucose ≥155 mg/dL. Pediatr Diabetes. 2018;19(7):1173-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG, Morgan WJ. Epidemiology of cystic fibrosis-related diabetes. J Pediatr. 2005;146(5):681-687. [DOI] [PubMed] [Google Scholar]
- 3. Koch C, Rainisio M, Madessani U, et al. ; Investigators of the European Epidemiologic Registry of Cystic Fibrosis . Presence of cystic fibrosis-related diabetes mellitus is tightly linked to poor lung function in patients with cystic fibrosis: data from the European Epidemiologic Registry of Cystic Fibrosis. Pediatr Pulmonol. 2001;32(5):343-350. [DOI] [PubMed] [Google Scholar]
- 4. Lanng S, Thorsteinsson B, Nerup J, Koch C. Influence of the development of diabetes mellitus on clinical status in patients with cystic fibrosis. Eur J Pediatr. 1992;151(9):684-687. [DOI] [PubMed] [Google Scholar]
- 5. Lewis C, Blackman SM, Nelson A, et al. Diabetes-related mortality in adults with cystic fibrosis. Role of genotype and sex. Am J Respir Crit Care Med. 2015;191(2):194-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med. 2000;162(3 Pt 1):891-895. [DOI] [PubMed] [Google Scholar]
- 7. Leclercq A, Gauthier B, Rosner V, et al. Early assessment of glucose abnormalities during continuous glucose monitoring associated with lung function impairment in cystic fibrosis patients. J Cyst Fibros. 2014;13(4):478-484. [DOI] [PubMed] [Google Scholar]
- 8. Brodsky J, Dougherty S, Makani R, Rubenstein RC, Kelly A. Elevation of 1-hour plasma glucose during oral glucose tolerance testing is associated with worse pulmonary function in cystic fibrosis. Diabetes Care. 2011;34(2):292-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moran A, Brunzell C, Cohen RC, et al. ; CFRD Guidelines Committee . Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the cystic fibrosis foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33(12):2697-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Radike K, Molz K, Holl RW, Poeter B, Hebestreit H, Ballmann M. Prognostic relevance of hypoglycemia following an oral glucose challenge for cystic fibrosis-related diabetes. Diabetes Care. 2011;34(4):e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Battezzati A, Battezzati PM, Costantini D, et al. Spontaneous hypoglycemia in patients with cystic fibrosis. Eur J Endocrinol. 2007;156(3):369-376. [DOI] [PubMed] [Google Scholar]
- 12. Haliloglu B, Gokdemir Y, Atay Z, et al. Hypoglycemia is common in children with cystic fibrosis and seen predominantly in females. Pediatr Diabetes. 2017;18(7):607-613. [DOI] [PubMed] [Google Scholar]
- 13. Hirsch IB, Janci MM, Goss CH, Aitken ML. Hypoglycemia in adults with cystic fibrosis during oral glucose tolerance testing. Diabetes Care. 2013;36(8):e121-e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mannik LA, Chang KA, Annoh PQK, et al. Prevalence of hypoglycemia during oral glucose tolerance testing in adults with cystic fibrosis and risk of developing cystic fibrosis-related diabetes. J Cyst Fibros. 2018;17(4):536-541. [DOI] [PubMed] [Google Scholar]
- 15. Aitken ML, Szkudlinska MA, Boyko EJ, Ng D, Utzschneider KM, Kahn SE. Impaired counterregulatory responses to hypoglycaemia following oral glucose in adults with cystic fibrosis. Diabetologia. 2020;63(5):1055-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moheet A, Ode KL. Hypoglycaemia in patients with cystic fibrosis- harbinger of poor outcomes or innocent bystander? J Cyst Fibros. 2018;17(4):428-429. [DOI] [PubMed] [Google Scholar]
- 17. Armaghanian N, Brand-Miller JC, Markovic TP, Steinbeck KS. Hypoglycaemia in cystic fibrosis in the absence of diabetes: a systematic review. J Cyst Fibros. 2016;15(3):274-284. [DOI] [PubMed] [Google Scholar]
- 18. Kilberg MJ, Sheikh S, Stefanovski D, et al. Dysregulated insulin in pancreatic insufficient cystic fibrosis with post-prandial hypoglycemia. J Cyst Fibros. 2020;19(2):310-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheikh S, Putt ME, Forde KA, Rubenstein RC, Kelly A. Elevation of one hour plasma glucose during oral glucose tolerance testing. Pediatr Pulmonol. 2015;50(10):963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ode KL, Frohnert B, Laguna T, et al. Oral glucose tolerance testing in children with cystic fibrosis. Pediatr Diabetes. 2010;11(7):487-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rickels MR, Goeser ES, Fuller C, et al. Loss-of-function mutations in ABCA1 and enhanced β-cell secretory capacity in young adults. Diabetes. 2015;64(1):193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rabiee A, Magruder JT, Grant C, et al. Accuracy and reliability of the Nova StatStrip® glucose meter for real-time blood glucose determinations during glucose clamp studies. J Diabetes Sci Technol. 2010;4(5):1195-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bak MJ, Albrechtsen NW, Pedersen J, et al. Specificity and sensitivity of commercially available assays for glucagon and oxyntomodulin measurement in humans. Eur J Endocrinol. 2014;170(4):529-538. [DOI] [PubMed] [Google Scholar]
- 24. Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes. 2001;50(1): 150-158. [DOI] [PubMed] [Google Scholar]
- 25. Toffolo G, Breda E, Cavaghan MK, Ehrmann DA, Polonsky KS, Cobelli C. Quantitative indexes of beta-cell function during graded up&down glucose infusion from C-peptide minimal models. Am J Physiol Endocrinol Metab. 2001;280(1): E2-10. [DOI] [PubMed] [Google Scholar]
- 26. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41(3):368-377. [DOI] [PubMed] [Google Scholar]
- 27. Dalla Man C, Caumo A, Cobelli C. The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng. 2002;49(5):419-429. [DOI] [PubMed] [Google Scholar]
- 28. Boston RC, Moate PJ. NEFA minimal model parameters estimated from the oral glucose tolerance test and the meal tolerance test. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R395-R403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubenstein RC. Kilberg_supplemental figure.pdf. June 2020. doi: 10.6084/m9.figshare.12416435.v1 [DOI]
- 30. Jung Y, Khurana RC, Corredor DG, et al. Reactive hypoglycemia in women. Results of a health survey. Diabetes. 1971;20(6):428-434. [DOI] [PubMed] [Google Scholar]
- 31. Fariss BL. Prevalence of post-glucose-load glycosuria and hypoglycemia in a group of healthy young men. Diabetes. 1974;23(3):189-191. [DOI] [PubMed] [Google Scholar]
- 32. Sheikh S, Gudipaty L, De Leon DD, et al. Reduced β-cell secretory capacity in pancreatic-insufficient, but not pancreatic-sufficient, cystic fibrosis despite normal glucose tolerance. Diabetes. 2017;66(1):134-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Edlund A, Pedersen MG, Lindqvist A, Wierup N, Flodström-Tullberg M, Eliasson L. CFTR is involved in the regulation of glucagon secretion in human and rodent alpha cells. Sci Rep. 2017;7(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cooperberg BA, Cryer PE. Insulin reciprocally regulates glucagon secretion in humans. Diabetes. 2010;59(11):2936-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jensen MD, Caruso M, Heiling V, Miles JM. Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes. 1989;38(12):1595-1601. [DOI] [PubMed] [Google Scholar]
- 36. Coppack SW, Jensen MD, Miles JM. In vivo regulation of lipolysis in humans. J Lipid Res. 1994;35(2):177-193. [PubMed] [Google Scholar]
- 37. Teff KL, Rickels MR, Grudziak J, Fuller C, Nguyen HL, Rickels K. Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes. 2013;62(9):3232-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rickels MR, Mueller R, Teff KL, Naji A. {beta}-Cell secretory capacity and demand in recipients of islet, pancreas, and kidney transplants. J Clin Endocrinol Metab. 2010;95(3):1238-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gudipaty L, Rosenfeld NK, Fuller CS, Gallop R, Schutta MH, Rickels MR. Effect of exenatide, sitagliptin, or glimepiride on β-cell secretory capacity in early type 2 diabetes. Diabetes Care. 2014;37(9):2451-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.