Abstract
Neighborhoods are where we live, learn, work, pray, and play. Growing evidence indicates that neighborhoods are an important determinant of health. The built features of our neighborhoods, such as the ways in which the streets are designed and connected and the availability of green spaces and transit stops, as well as the social features, such as the trust among neighbors and the perceptions of safety, may influence health through multiple pathways, such as access to important resources, psychosocial stress, and health behaviors. In particular, the extant literature consistently documents an association between neighborhood features and renal-associated conditions, such as cardiovascular disease, hypertension, diabetes, and obesity. There is also some evidence suggesting an association between neighborhood poverty and ESKD. The link between neighborhood and earlier stages of CKD, however, has been less clear, with most studies documenting no association. It may be that the neighborhood measures used in previous studies do not capture features of the neighborhood important for earlier stages of disease development and progression. It may also be that our current biomarkers (e.g., eGFR) and urine protein are not able to pick up very early forms of renal damage because of the kidney’s overall high reserve capacity. This paper critically reviews the state of the literature on neighborhood and renal disease, with recommendations for neighborhood measures in future research. Neighborhoods are designed, built, and informed by policy, and thus, they are amenable to intervention, making them a potentially powerful way to improve renal health and reduce health inequalities at the population level.
Keywords: Chronic Kidney Disease, Health Behavior, Health Status Disparities, Kidney, Failure Chronic, Poverty, Renal Insufficiency, Chronic, Residence Characteristics, Socioeconomic Factors, Stress, Psychological
Introduction
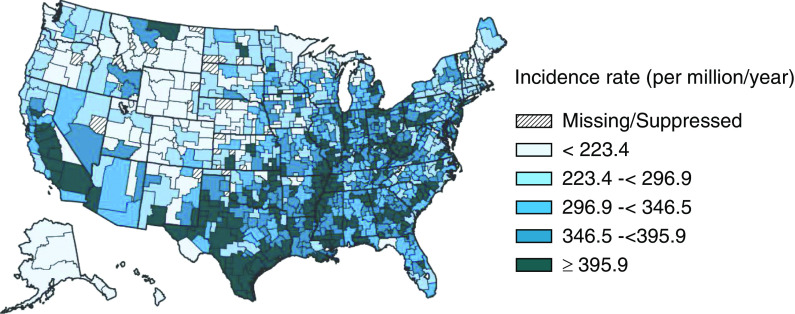
The geographic variation in ESKD in the United States has been reported for decades (1,2) and can still be documented using the US Renal Data System (USRDS) (Figure 1). Since the early descriptive reports, a growing literature has indicated the importance of place in the development and progression of several major renal-associated conditions, such as cardiovascular diseases, hypertension, diabetes, and obesity (3–6). Reports also suggest an association between county- and neighborhood-level racial and economic characteristics and incident ESKD (7), hemodialysis survival (8), placement on transplant waiting list (9–11), and ESKD mortality (8). This link between context and disease has been shown to be important for population health overall and for racial and socioeconomic inequalities in health (12–14).
Figure 1.
Map of the standardized incidence rate of ESKD by health service area in the US population, 2012–2016. It is standardized to the age-sex-race distribution of the 2011 United States population. Special analyses exclude unknown age, sex, health service area, and unknown/other race. Values for cells with ten or fewer patients are suppressed. The data reported here have been supplied by the US Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US Government. Data source: Special analyses, USRDS ESKD Database (61).
The State of the Literature on Neighborhoods and Renal Diseases
At the core of the study of the association between place and health are neighborhoods—where we live, learn, work, pray, and play. Features of our neighborhoods can influence health through access to important resources, psychosocial stress, and health behaviors (3). Although individual socioeconomic status (SES; e.g., education and income) is related to neighborhood quality, neighborhoods represent much more that is important for kidney health (i.e., the structural and functional well-being of the kidney in which the kidney has age-appropriate structure and function) than the composite of individual SES (3). Indeed, neighborhood features are related to incident diabetes and hypertension, independent of individual-level SES (15,16).
A recent review (17) outlined four general categories of neighborhood features that may be important for kidney health and health inequalities: sociodemographic context, the built and physical environment, the social environment, and health care resources (Figure 2). The sociodemographic context generally includes the social, economic, and racial/ethnic composition of the neighborhood. It is not the composition, per se, that drives disease development and progression. Rather, these measures are proxies for the variation in public and private investment and socially accepted behavioral norms (i.e., norms around nutritional diet and exercise) that are important for health. For example, neighborhood racial segregation (i.e., the sorting of different racial groups into neighborhoods of unequal quality through historical and contemporary policies and practices) is associated with incident cardiovascular disease (14). Further, moving to neighborhoods with less racial segregation is associated with improvements in cardiovascular health, such as decreases in BP (6).
Figure 2.
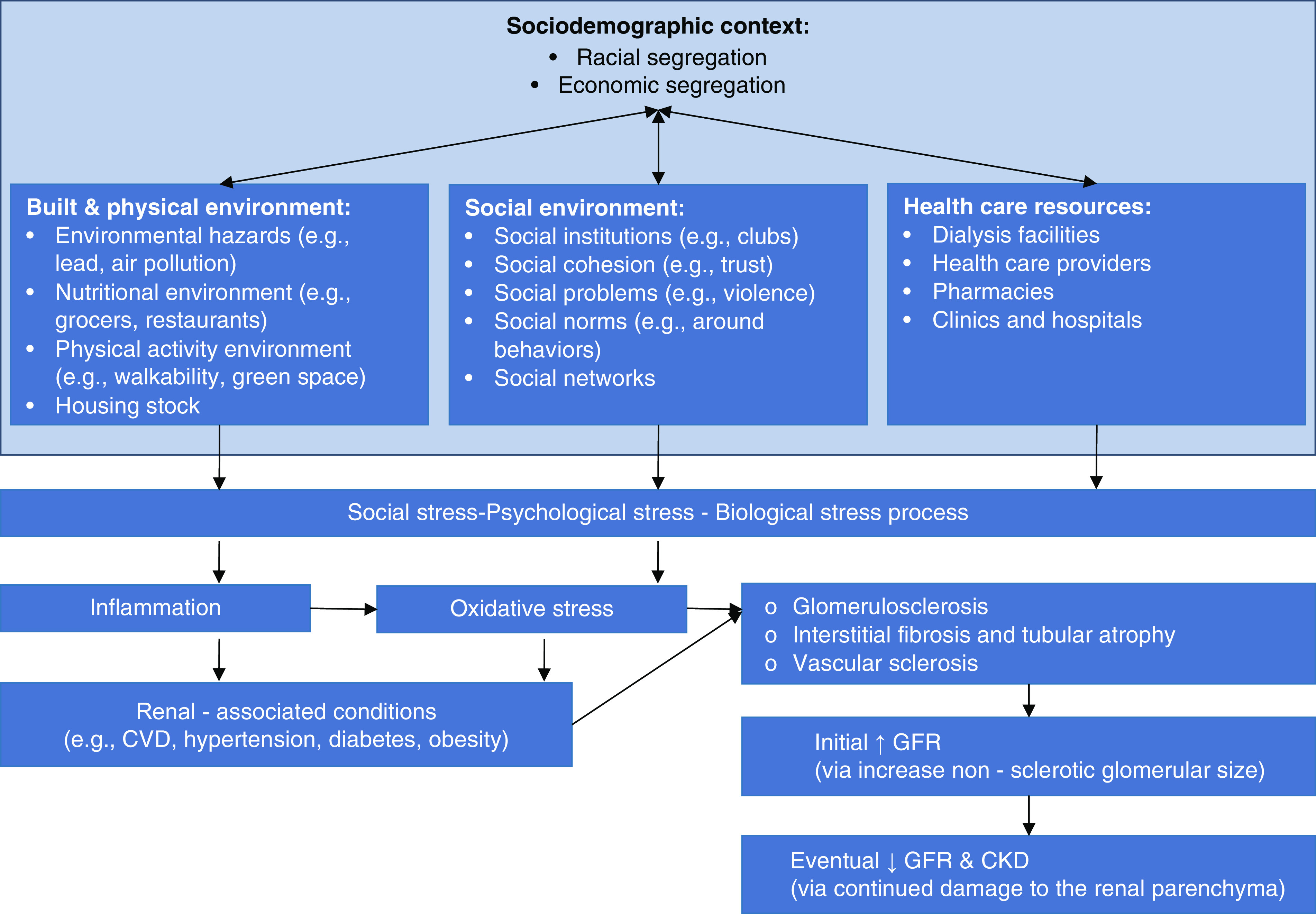
Conceptual model linking neighborhood context to renal pathology. Although not covered in the discussion, others have outlined the link between social, psychologic, and biologic stress and the kidney (62). CVD, cardiovascular disease.
Regarding sociodemographic context, some have examined the link between neighborhood SES and kidney function and damage. In general, this literature suggests little or no association between neighborhoods and renal health, particularly at earlier stages of CKD. For example, some report an association between neighborhood poverty and incident ESKD using the USRDS (15), whereas others report no association between neighborhood (16) and county (17) poverty and low eGFR (16) or incident ESKD using the Reasons for Geographic Differences in Stroke (REGARDS) cohort (17). When a more comprehensive SES index composed of multiple items, such as median household income, mean home value, and percentage in professional occupations, is used, neighborhood SES has been related to increases in serum creatinine (18) and decreases in eGFR to <45 ml/min per 1.73 m2 over time (19)—but these results are not consistent across age, race, or sex (18–20). Some have also examined neighborhood SES over the life course, including childhood and adulthood; however, results suggest that neither childhood county SES (19) nor early adulthood neighborhood SES (19) nor the cumulative childhood and adulthood residential SES (21) is related to later adulthood declines in eGFR. Consistent with these reports, a recent study suggested that duration of residence in the southeast United States is associated with incident ESKD but not baseline albuminuria or an eGFR<60 ml/min per 1.73 m2 (22). It may be that neighborhood racial and economic segregation better captures the resources and constraints important for the development and progression of CKD. Although no studies to our knowledge examine neighborhood segregation and measures of renal health, one study has shown an association between county-level segregation and mortality for patients on dialysis (8). In other words, black patients on dialysis who live in counties where black and white residents live apart from each other experience a greater risk of mortality compared with black patients who live in counties that are less segregated (regardless of whether the patient himself/herself lives in a neighborhood in the county that is composed of predominantly black residents). Future research on neighborhood segregation should ask: “Do black patients living in neighborhoods with predominantly black residents who are segregated from other neighborhoods experience faster CKD progression compared with other patients?” Although only a crude proxy for neighborhood segregation, there is evidence that neighborhood racial composition is associated with CKD-related measures. For example, neighborhoods with higher proportions of black residents did have high-quality dialysis facilities (23,24); however, black patients did not necessarily access these facilities (23,24).
The built and physical environment comprises neighborhood features ranging from housing to walkability (using map data on street connectivity) to local businesses (using data from public sources, such as the business census and private databases) to pollution (using data public data from the Environmental Protection Agency [EPA]) (25). These features have been associated with renal-associated conditions, including obesity, diabetes, and hypertension, as well as the social inequalities in these conditions (26–30). Although there is a dearth of empirical work linking these types of neighborhood features to renal health, evidence from other literatures suggests a link. For example, evidence from the environmental sciences links neighborhood SES and racial composition (using census data) to hazardous waste siting and industrial pollution (using data from the EPA) (31). Numerous pollutants are related to CKD (32–34). Further, environmental lead, for example, is associated with increases in BP and oxidative stress in endothelial cells and vascular smooth muscle cells (35,36), which intimately relate to kidney function and damage. Neighborhood food quality and availability, as another example, are linked to obesity and diabetes (26,37,38), and they are thought to be linked to CKD and ESKD (39). Dietary protein and sodium can have a marked effect on intraglomerular pressure, glomerular hyperfiltration, and proinflammatory gene expression (40–42).
The social environment includes features that capture the social connections among its residents as well as the social effect of the context upon its residents. This literature, which generally uses surveys asking people how they feel about their neighborhoods, has yielded mixed results, even for renal-associated conditions. For example, neighborhood social cohesion (e.g., trust among neighbors, etc.) was associated with lower hypertension (27,43) and diabetes (26) prevalence but was not associated lower body mass index (44). A different aspect of the social environment, neighborhood social problems (e.g., physical disorder and violence), was not associated with either hypertension prevalence (45) or body mass index (44). There are not many studies linking social context to measures of renal diseases. However, researchers recently reported that social problems, but not social cohesion, were associated with annualized declines in eGFR only for black adults (46).
Neighborhood health care resources include dialysis centers but extend to the density and type of hospitals, community health centers, and health care providers and the density of health-related resources, such as pharmacies and dispensaries, and urgent care facilities. These features of the neighborhood mark access to health care resources (47) but also mark public and private investments in prevention and disease management care that may correlate with other investments in health. For example, reports have documented “pharmacy deserts” in neighborhoods with mostly poor or black residents (48). Similarly, neighborhood poverty is related to a decreased likelihood of being on a transplant waitlist (9).
A puzzle remains in that, although neighborhood characteristics are generally related to renal-associated diseases, RRT-associated outcomes (i.e., access to transplant and dialysis facility quality), and ESKD, they have not been shown to be consistently related to earlier stages of CKD (2,19,16,20,49,50). This inconsistency may be due to measurement limitations: (1) a focus on neighborhood SES particularly at a single time point in the life course and (2) a reliance on eGFR alone in studies with healthy and/or younger adults. Much of what we know about neighborhoods and CKD is on the basis of information from a handful of datasets (Table 1). There are three broad categories of datasets with information (or the potential for information linkage) on both neighborhoods and kidney health, each with strengths and limitations.
(1) Demographic studies are those with samples drawn from the noninstitutionalized population using complex stratified sampling approaches such that they, when weighted, represent a certain area, such as the nation. A strength of these data is that they are representative of populations. However, most of these datasets may have only basic blood-based renal biomarkers, ESKD information through linkage to USRDS, and kidney disease mortality through linkage to the National Death Index. Further, for those datasets, such as the National Health and Nutrition Examination Survey, that include urine albumin-creatinine ratio, the data are available only in cross-section, meaning that it is not possible to examine incidence.
(2) Epidemiologic studies are those with samples drawn from the noninstitutionalized population in a specific number of places using various sampling methods. These data are not sampled nor weighted to be statistically representative of any particular population. However, there are important strengths of these data, including that they use an observational design to assess potential causal associations. Further, most are longitudinal with both blood- and urine-based biomarkers at multiple time points. They also often sample those without clinical presentation of certain diseases in order to capture disease development so that associations can be made with both prevalence and importantly, incidence.
(3) Clinical studies are those with patients who were recruited specifically because they have some type of kidney disease. Generally, these studies have numerous urine- and blood-based biomarkers over specific time intervals. A major strength of these studies is that there is a deep interrogation of clinical, molecular, and pathology information. In addition to clinical studies, there are clinical data available for linkage to neighborhood information. The USRDS has been used in numerous studies to document the link between place and ESKD. Furthermore, there is tremendous potential in linking the clinical data available through electronic health records, both local and national, to neighborhood data (51,52). This method allows for monitoring of acute and chronic health conditions in real time in relation to neighborhood. At the national level, the Department of Veterans Affairs has a large integrated electronic health record including over 9 million veterans in the United States who receive care through the Department of Veterans Affairs. These data have been recently used to examine geographic clustering of rapid eGFR decline (53) and have the potential to be used to examine numerous neighborhood features in relation to disease progression.
Table 1.
Demographic, epidemiologic, and clinical datasets used to study the association between residential context and kidney health
| Datasets | Sample Size | Years | Locations | Age at Baseline, yr | Spatial Unita | Kidney Measures | |||||
| Urine Protein-Creatinine Ratio, Urine Albumin-Creatinine Ratiob | Serum Creatinine | Serum Cystatin C | Chronic Kidney Disease Mortality | End Stage Kidney Disease | Apolipoprotein L1 | ||||||
| Demographic studies and datasets | |||||||||||
| Americans’ Changing Lives | 3617 | 1986–2019 | US | 25–84 | Tract | IP | IP | X | X | IP | |
| Health and Retirement Study | 25,444 | 1992–2019c | US | 51–61 | Tract | X | X | X | X | ||
| National Health and Nutrition Examination Survey, Wave III | 33,994 | 1988–1994 | US | 0–90 | Addressd | X | X | X | X | ||
| National Health and Nutrition Examination Survey, Continuous | Approximately 5000/yr | 1999–2020c | US | 0–90 | Addressd | X | X | X | X | ||
| National Longitudinal Study of Adolescents | 12,105 | 1994–2018 | US | 14–18 | Block | X | IP | IP | IP | ||
| National Vital Statistics System, Mortality Data | All US | 1968–2017c | US | All US | County | X | |||||
| Epidemiologic studies and databases | |||||||||||
| Atherosclerosis Risk in Communities | 15,792 | 1987–2019 | Multiple US | 45–64 | Tract | X | X | P | X | X | |
| Coronary Risk Development in Young Adults | 5115 | 1985–2016 | Multiple US | 18–30 | Block | X | X | X | P | P | X |
| Dallas Heart Study | 3500 | 2000–2009 | Dallas, TX | 30–65 | Block | X | X | X | P | P | X |
| Jackson Heart Study | 6500 | 2000–2012 | Jackson, MS | 35–94 | Tract | X | X | X | X | X | X |
| Multi-Ethnic Study of Atherosclerosis | 6814 | 2000–2018 | Multiple US | 45–84 | Tract | X | X | X | X | X | X |
| Reasons for Geographic Differences in Stroke | 28,878 (4) | 2003–2016 | Multiple US | 45–98 | Block | X | X | X | X | X | X |
| US Veterans Administration Database | All VA | 1977–c | US | All VA | County | X | X | X | X | X | X |
| Clinical studies and databases | |||||||||||
| Chronic Renal Insufficiency Cohort | 5499 | 2003–2018 | Multiple US | 21–74 | Tract | X | X | X | X | X | X |
| Clinical Phenotyping Resource and Biobank Core | 1645 | 2009–2019 | Multiple US | 30–64e | Tract | X | X | X | X | ||
| Cure Glomerulonephropathy | 2400 | 2014–2020 | Multiple US | IP | Tract | X | X | X | X | X | |
| Nephrotic Syndrome Study Network | 2561 | 2010–2019 | Multiple US | 0–80 | Tract | X | X | X | X | X | |
| US Renal Data System | All ESKD | 1978–c | US | All ESKD | Zip code | X | X | ||||
Clinical measures are not necessarily available for all waves of data. The years available include a range of temporal data collection efforts. For example, some studies collect information every 2 years, whereas others collect data every 6 years. US, United States; IP, data collection in progress; P, data possibly available; VA, US Department of Veterans Affairs.
Represents the most spatially granular unit available.
Information generally available on either urine albumin-creatinine ratio or urine protein-creatinine ratio.
Represents ongoing data collection efforts into the indefinite future.
Geospatial data are available to researchers through Census Research Data Centers.
Interquartile range available only.
Table 2.
Clinical studies and databases with renal histopathology and residential context information
| Clinical Studies and Databases | Years | Locations | Sample Size | Demographic Characteristics | Biopsy Scored? | ||
| Age, yr, Mean ± Standard Deviation | White, % | Women, % | |||||
| Aging Kidney Anatomy Study: Healthy Living Kidney Donors (63–65) | 2011–2021 | Multiple US | Adults: 2453a | Adults: 44±12 | 94 | 59 | Complete |
| Aging Kidney Anatomy Study: Tumor Nephrectomy Patients (65) | 2011–2021 | Multiple US | Adults: 780a | Adults: 64±12 | NR | 36 | Complete |
| Boston Kidney Biopsy Cohort (66) | 2006–2016 | Boston, MA | Adults: 676 | Adults: 52±17 | 64 | 53 | Complete |
| Clinical Phenotyping and Resource Biobank Core (Patients with Renal Disease) (67) | 2009–2018 | Multiple US | Adults: 1374; peds: 271 | Adults: 55±20; peds: 11±4 | 52 | 52 | In progress |
| Cure Glomerulonephropathy Consortium (68) | 2014–2020 | Multiple US | Adults: 1680a; peds: 720 | Adults: 46±17; peds: 10±4 | 67 | 43 | In progress |
| Nephrotic Syndrome Study Network (69,70) | 2010–2019 | Multiple US, Canada | Adults: 355; peds: 313 | Adults: 46±16; peds: 6±5 | 54 | 41 | In progress |
Each scored database contains pathology kidney data including glomerular size, percentage glomerulosclerosis, percentage of interstitial fibrosis and tubular atrophy, measures for vascular disease, and clinical indicators of renal function (e.g., creatinine, creatine-based eGFR, and urinary protein/albumin). Some databases have more extensive kidney pathology data as well as additional clinical data, genomic data, and molecular data. US, United States; peds, pediatric patients.
Recruitment is ongoing; sample size reflects current enrollment.
Improving Measurement of Neighborhoods in Studies of CKD and ESKD
Although efforts are underway, there are currently no known published studies using demographic data to examine the link between residential context and kidney health. There are several studies, however, using epidemiologic data, including the Atherosclerosis Risk in Communities (19–21), the Cardiovascular Health Study (18), the Multi-Ethnic Study of Atherosclerosis (46), and the REGARDS (16,17,22,54), as well as the US Department of Veterans Affairs patient database (53). We provide Table 1 as a companion to the detailed tables in a recent report (55), in which the authors outline numerous types of administrative data available for linkage to demographic, epidemiologic, and clinical datasets. In the published studies, the focus has been on neighborhood SES. We recommend drawing from the larger epidemiology literature on neighborhood context and renal-associated diseases in several ways.
First, we recommend examining racial and economic segregation, which may better capture the clustering within cities that guides public and private investment compared with measures that focus only on the racial composition or SES of one’s immediate neighborhood. Racial segregation has been consistently associated with numerous renal-associated conditions (14), and recent reports suggest that moving to less segregated neighborhoods is associated with improvements in cardiovascular health (6). With the continued rise of socioeconomic inequality in the United States, we also recommend examining economic segregation in connection with racial segregation.
Second, we recommend examining neighborhood context across the life course rather than solely at a single point. Although some have reported inconsistent associations between county SES in childhood and neighborhood SES throughout adulthood and eGFR (18–21). we suggest building on these studies to include age-period-cohort analysis. In this type of analysis, researchers can examine the association between neighborhood context and kidney health markers among specific birth cohorts while disentangling potential effect of historical periods (e.g., the Civil Rights Era) and chronological age (56,57).
Third, we recommend innovative approaches to modeling multiple features of the neighborhood context together. It may also be that different features of the neighborhood context interact with each other. Research suggests that social context, for example, amplifies the association between environmental pollutants and renal-associated diseases (58–60). These complex associations may clarify why neighborhood SES alone is not related to measures of kidney function and damage.
Fourth, we recommend considering the ways in which “neighborhood” is conceptualized for measurement. In reality, neighborhoods are cohesive areas of towns and cities; the cohesive element varies and may be, for example, built (e.g., planned housing development) or sociohistorical (e.g., Chicago’s Bronzeville). However, in the reality of most CKD research, it is not possible to use these conceptualizations of neighborhood. Therefore, many use census tracts (i.e., census-based administrative residential areas of roughly 4000 people), zip codes (i.e., US Postal Service–based administrative areas used for efficient mail delivery; generally much larger than tracts), or even county. In some cases, researchers must use a single approach to neighborhood because it is all that is available in the dataset. This is the case with regard to zip code in the USRDS. Many epidemiologic datasets use census tract as the proxy for neighborhood. Which proxy for neighborhood is used depends on the exposure under study (e.g., racial composition, SES, parks, dialysis centers, or grocery stores) and may affect the interpretation of the results.
Many neighborhood features have become recognized as important determinants of health and health inequalities. Although there is robust empirical literature linking neighborhood features and renal-associated conditions, the literature is inconsistent in relation to kidney function and damage. In this review, we recommend expanding the study of neighborhoods and renal diseases in the following ways: (1) examining racial and economic segregation, which may better capture the clustering within cities that guide public and private investment compared with measures that focus only on the racial composition or SES of one’s immediate neighborhood; (2) examining neighborhood context across the life course rather than solely at a single point; (3) using innovative approaches to model multiple features of the neighborhood context together (for example, including interaction effects between features, like social context, environmental pollutants and renal-associated diseases); and (4) considering the ways in which “neighborhood” is conceptualized for measurement, understanding, for example, that measures like zip code may not be an appropriate proxy for neighborhood.
Expanding our approach to the studying of neighborhood and renal health can clarify mechanisms and point to important primary as well as secondary and tertiary intervention points because residential context is shaped by policy, guided by public and private investment and disinvestment, and thus, amenable to change.
Disclosures
L.H. Mariani reports personal fees from Reata Pharmaceuticals, outside the submitted work. All remaining authors have nothing to disclose.
Funding
This work was supported by Department of Health and Human Services, National Institutes of Health, National Institute on Diabetes and Digestive and Kidney Disorders grant 5K01DK106322 (to M.T. Hicken).
Author Contributions
M.T. Hicken and C.J. Lapedis conceptualized the study; M.T. Hicken and C.J. Lapedis were responsible for data curation; M.T. Hicken provided supervision; M.T. Hicken and C.J. Lapedis wrote the original draft; M.T. Hicken was responsible for funding acquisition and visualization; and M.T. Hicken, J. Hodgin, B.J. Jang, C.J. Lapedis, and L.H. Mariani reviewed and edited the manuscript.
References
- 1.United States Renal Data System : 2017 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2017 [Google Scholar]
- 2.McClellan AC, Plantinga L, McClellan WM: Epidemiology, geography and chronic kidney disease. Curr Opin Nephrol Hypertens 21: 323–328, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Diez Roux AV, Mair C: Neighborhoods and health. Ann N Y Acad Sci 1186: 125–145, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Lim S, Harris TG: Neighborhood contributions to racial and ethnic disparities in obesity among New York City adults. Am J Public Health 105: 159–165, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ludwig J, Sanbonmatsu L, Gennetian L, Adam E, Duncan GJ, Katz LF, Kessler RC, Kling JR, Lindau ST, Whitaker RC, McDade TW: Neighborhoods, obesity, and diabetes—a randomized social experiment. N Engl J Med 365: 1509–1519, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kershaw KN, Robinson WR, Gordon-Larsen P, Hicken MT, Goff DC Jr., Carnethon MR, Kiefe CI, Sidney S, Diez Roux AV: Association of changes in neighborhood-level racial residential segregation with changes in blood pressure among black adults: The CARDIA study. JAMA Intern Med 177: 996–1002, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volkova N, McClellan W, Klein M, Flanders D, Kleinbaum D, Soucie JM, Presley R: Neighborhood poverty and racial differences in ESRD incidence. J Am Soc Nephrol 19: 356–364, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimmel PL, Fwu CW, Eggers PW: Segregation, income disparities, and survival in hemodialysis patients. J Am Soc Nephrol 24: 293–301, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM: Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol 20: 1333–1340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patzer RE, Amaral S, Klein M, Kutner N, Perryman JP, Gazmararian JA, McClellan WM: Racial disparities in pediatric access to kidney transplantation: Does socioeconomic status play a role? Am J Transplant 12: 369–378, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saunders MR, Cagney KA, Ross LF, Alexander GC: Neighborhood poverty, racial composition and renal transplant waitlist. Am J Transplant 10: 1912–1917, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Morenoff JD, House JS, Hansen BB, Williams DR, Kaplan GA, Hunte HE: Understanding social disparities in hypertension prevalence, awareness, treatment, and control: The role of neighborhood context. Soc Sci Med 65: 1853–1866, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kershaw KN, Diez Roux AV, Burgard SA, Lisabeth LD, Mujahid MS, Schulz AJ: Metropolitan-level racial residential segregation and black-white disparities in hypertension. Am J Epidemiol 174: 537–545, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kershaw KN, Osypuk TL, Do DP, De Chavez PJ, Diez Roux AV: Neighborhood-level racial/ethnic residential segregation and incident cardiovascular disease: The multi-ethnic study of atherosclerosis. Circulation 131: 141–148, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrity BH, Kramer H, Vellanki K, Leehey D, Brown J, Shoham DA: Time trends in the association of ESRD incidence with area-level poverty in the US population. Hemodial Int 20: 78–83, 2016 [DOI] [PubMed] [Google Scholar]
- 16.McClellan WM, Newsome BB, McClure LA, Howard G, Volkova N, Audhya P, Warnock DG: Poverty and racial disparities in kidney disease: The REGARDS study. Am J Nephrol 32: 38–46, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crews DC, Gutiérrez OM, Fedewa SA, Luthi JC, Shoham D, Judd SE, Powe NR, McClellan WM: Low income, community poverty and risk of end stage renal disease. BMC Nephrol 15: 192, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merkin SS, Diez Roux AV, Coresh J, Fried LF, Jackson SA, Powe NR: Individual and neighborhood socioeconomic status and progressive chronic kidney disease in an elderly population: The Cardiovascular Health Study. Soc Sci Med 65: 809–821, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Shoham DA, Vupputuri S, Diez Roux AV, Kaufman JS, Coresh J, Kshirsagar AV, Zeng D, Heiss G: Kidney disease in life-course socioeconomic context: The Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis 49: 217–226, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Merkin SS, Coresh J, Diez Roux AV, Taylor HA, Powe NR: Area socioeconomic status and progressive CKD: The Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis 46: 203–213, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Shoham DA, Vupputuri S, Kaufman JS, Kshirsagar AV, Diez Roux AV, Coresh J, Heiss G: Kidney disease and the cumulative burden of life course socioeconomic conditions: The Atherosclerosis Risk in Communities (ARIC) study. Soc Sci Med 67: 1311–1320, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plantinga L, Howard VJ, Judd S, Muntner P, Tanner R, Rizk D, Lackland DT, Warnock DG, Howard G, McClellan WM: Association of duration of residence in the southeastern United States with chronic kidney disease may differ by race: The REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort study. Int J Health Geogr 12: 17, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saunders MR, Lee H, Maene C, Schuble T, Cagney KA: Proximity does not equal access: Racial disparities in access to high quality dialysis facilities. J Racial Ethn Health Disparities 1: 291–299, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H, Caldwell JT, Maene C, Cagney KA, Saunders MR: Racial/ethnic inequities in access to high-quality dialysis treatment in Chicago: Does neighborhood racial/ethnic composition matter? [published online ahead of print February 5, 2020]. J Racial Ethn Health Disparities doi: 10.1007/s40615-020-00708-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perdue WC, Stone LA, Gostin LO: The built environment and its relationship to the public’s health: The legal framework. Am J Public Health 93: 1390–1394, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christine PJ, Auchincloss AH, Bertoni AG, Carnethon MR, Sánchez BN, Moore K, Adar SD, Horwich TB, Watson KE, Diez Roux AV: Longitudinal associations between neighborhood physical and social environments and incident type 2 diabetes mellitus: The multi-ethnic study of atherosclerosis (MESA). JAMA Intern Med 175: 1311–1320, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaiser P, Diez Roux AV, Mujahid M, Carnethon M, Bertoni A, Adar SD, Shea S, McClelland R, Lisabeth L: Neighborhood environments and incident hypertension in the multi-ethnic study of atherosclerosis. Am J Epidemiol 183: 988–997, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diez Roux AV, Auchincloss AH, Franklin TG, Raghunathan T, Barr RG, Kaufman J, Astor B, Keeler J: Long-term exposure to ambient particulate matter and prevalence of subclinical atherosclerosis in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 167: 667–675, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Brook RD: Cardiovascular effects of air pollution. Clin Sci (Lond) 115: 175–187, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Booth KM, Pinkston MM, Poston WS: Obesity and the built environment. J Am Diet Assoc 105[Suppl 1]: S110–S117, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Bullard RD, Mohai P, Saha R, Wright B: Toxic Wastes and Race at Twenty: 1987-2007, Cleveland, OH, United Church of Christ Racial Justice Ministry Team, 2007 [Google Scholar]
- 32.Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, Weaver V: Blood cadmium and lead and chronic kidney disease in US adults: A joint analysis. Am J Epidemiol 170: 1156–1164, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z: Associations of ambient coarse particulate matter, nitrogen dioxide, and carbon monoxide with the risk of kidney disease: A cohort study. Lancet Planet Health 1: e267–e276, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z: Particulate matter air pollution and the risk of incident CKD and progression to ESRD. J Am Soc Nephrol 29: 218–230, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zachariah JP, Wang Y, Penny DJ, Baranowski T: Relation between lead exposure and trends in blood pressure in children. Am J Cardiol 122: 1890–1895, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ni Z, Hou S, Barton CH, Vaziri ND: Lead exposure raises superoxide and hydrogen peroxide in human endothelial and vascular smooth muscle cells. Kidney Int 66: 2329–2336, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Morland K, Diez Roux AV, Wing S: Supermarkets, other food stores, and obesity: The atherosclerosis risk in communities study. Am J Prev Med 30: 333–339, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Auchincloss AH, Mujahid MS, Shen M, Michos ED, Whitt-Glover MC, Diez Roux AV: Neighborhood health-promoting resources and obesity risk (the multi-ethnic study of atherosclerosis). Obesity (Silver Spring) 21: 621–628, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutiérrez OM: Contextual poverty, nutrition, and chronic kidney disease. Adv Chronic Kidney Dis 22: 31–38, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalantar-Zadeh K, Fouque D: Nutritional management of chronic kidney disease. N Engl J Med 377: 1765–1776, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Hostetter TH, Meyer TW, Rennke HG, Brenner BM: Chronic effects of dietary protein in the rat with intact and reduced renal mass. Kidney Int 30: 509–517, 1986 [DOI] [PubMed] [Google Scholar]
- 42.Tovar-Palacio C, Tovar AR, Torres N, Cruz C, Hernández-Pando R, Salas-Garrido G, Pedraza-Chaverri J, Correa-Rotter R: Proinflammatory gene expression and renal lipogenesis are modulated by dietary protein content in obese Zucker fa/fa rats. Am J Physiol Renal Physiol 300: F263–F271, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Mujahid MS, Diez Roux AV, Morenoff JD, Raghunathan TE, Cooper RS, Ni H, Shea S: Neighborhood characteristics and hypertension. Epidemiology 19: 590–598, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Mujahid MS, Diez Roux AV, Shen M, Gowda D, Sánchez B, Shea S, Jacobs DR Jr., Jackson SA: Relation between neighborhood environments and obesity in the multi-ethnic study of atherosclerosis. Am J Epidemiol 167: 1349–1357, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Mujahid MS, Diez Roux AV, Cooper RC, Shea S, Williams DR: Neighborhood stressors and race/ethnic differences in hypertension prevalence (the Multi-Ethnic Study of Atherosclerosis). Am J Hypertens 24: 187–193, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hicken MT, Katz R, Crews DC, Kramer HJ, Peralta CA: Neighborhood social context and kidney function over time: The multi-ethnic study of atherosclerosis. Am J Kidney Dis 73: 585–595, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hao H, Lovasik BP, Pastan SO, Chang HH, Chowdhury R, Patzer RE: Geographic variation and neighborhood factors are associated with low rates of pre-end-stage renal disease nephrology care. Kidney Int 88: 614–621, 2015 [DOI] [PubMed] [Google Scholar]
- 48.Qato DM, Daviglus ML, Wilder J, Lee T, Qato D, Lambert B: ‘Pharmacy deserts’ are prevalent in Chicago’s predominantly minority communities, raising medication access concerns. Health Aff (Millwood) 33: 1958–1965, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Shoham DA, Vupputuri S, Kaufman JS, Kshirsagar AV, Diez Roux AV, Coresh J, et al. G: Kidney disease and the cumulative burden of life course socioeconomic conditions: The Atherosclerosis Risk in Communities (ARIC) Study. Soc Sci Med 67: 1311–1320, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez RA, Hotchkiss JR, O’Hare AM: Geographic information systems and chronic kidney disease: Racial disparities, rural residence and forecasting. J Nephrol 26: 3–15, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siegel SD, Brooks MM, Gbadebo BM, Laughery JT: Using geospatial analyses of linked electronic health records and tobacco Outlet data to address the social determinants of smoking. Prev Chronic Dis 16: E152, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laranjo L, Rodrigues D, Pereira AM, Ribeiro RT, Boavida JM: Use of electronic health records and geographic information systems in public health surveillance of type 2 diabetes: A feasibility study. JMIR Public Health Surveill 2: e12, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bowe B, Xie Y, Xian H, Lian M, Al-Aly Z: Geographic variation and US county characteristics associated with rapid kidney function decline. Kidney Int Rep 2: 5–17, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanner RM, Gutiérrez OM, Judd S, McClellan W, Bowling CB, Bradbury BD, Safford MM, Cushman M, Warnock D, Muntner P: Geographic variation in CKD prevalence and ESRD incidence in the United States: Results from the reasons for geographic and racial differences in stroke (REGARDS) study. Am J Kidney Dis 61: 395–403, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hicken MT, Gipson DS: Matching the genotype in resolution: Innovative ways of phenotype capture. Semin Nephrol 35: 279–290, 2015 [DOI] [PubMed] [Google Scholar]
- 56.Elder GH Jr.: The life course as developmental theory. Child Dev 69: 1–12, 1998 [PubMed] [Google Scholar]
- 57.Yang Y, Land KC: Age-Period-Cohort Analysis New Models, Methods, and Empirical Applications, Boca Raton, FL, Taylor, Francis, CRC Press, 2013 [Google Scholar]
- 58.Hicken MT, Adar SD, Hajat A, Kershaw KN, Do DP, Barr RG, Kaufman JD, Diez Roux AV: Air pollution, cardiovascular Outcomes, and social disadvantage: The multi-ethnic study of atherosclerosis. Epidemiology 27: 42–50, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hicken MT, Gee GC, Morenoff J, Connell CM, Snow RC, Hu H: A novel look at racial health disparities: The interaction between social disadvantage and environmental health. Am J Public Health 102: 2344–2351, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hicken M, Gragg R, Hu H: How cumulative risks warrant a shift in our approach to racial health disparities: The case of lead, stress, and hypertension. Health Aff (Millwood) 30: 1895–1901, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.United States Renal Data System : USRDS 2000 Annual Data Report: Atlas of CKD and ESKD in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2000 [Google Scholar]
- 62.Bruce MA, Beech BM, Sims M, Brown TN, Wyatt SB, Taylor HA, Williams DR, Crook E: Social environmental stressors, psychological factors, and kidney disease. J Investig Med 57: 583–589, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elsherbiny HE, Alexander MP, Kremers WK, Park WD, Poggio ED, Prieto M, Lieske JC, Rule AD: Nephron hypertrophy and glomerulosclerosis and their association with kidney function and risk factors among living kidney donors. Clin J Am Soc Nephrol 9: 1892–1902, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Denic A, Mathew J, Lerman LO, Lieske JC, Larson JJ, Alexander MP, Poggio E, Glassock RJ, Rule AD: Single-nephron glomerular filtration rate in healthy adults. N Engl J Med 376: 2349–2357, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Denic A, Mathew J, Nagineni VV, Thompson RH, Leibovich BC, Lerman LO, Lieske JC, Alexander MP, Augustine JJ, Kremers WK, Rule AD: Clinical and pathology findings associate consistently with larger glomerular volume. J Am Soc Nephrol 29: 1960–1969, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Srivastava A, Palsson R, Kaze AD, Chen ME, Palacios P, Sabbisetti V, Betensky RA, Steinman TI, Thadhani RI, McMahon GM, Stillman IE, Rennke HG, Waikar SS: The prognostic value of histopathologic lesions in native kidney biopsy specimens: Results from the Boston Kidney Biopsy Cohort Study. J Am Soc Nephrol 29: 2213–2224, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.George M. O'Brien Kidney Translational Core Center: Demographic Characteristics C-PROBE, 2018. Available at: https://kidneycenter.med.umich.edu/c-probe-core/demographics. Accessed June 12, 2018
- 68.Mariani LH, Bomback AS, Canetta PA, Flessner MF, Helmuth M, Hladunewich MA, Hogan JJ, Kiryluk K, Nachman PH, Nast CC, Rheault MN, Rizk DV, Trachtman H, Wenderfer SE, Bowers C, Hill-Callahan P, Marasa M, Poulton CJ, Revell A, Vento S, Barisoni L, Cattran D, D’Agati V, Jennette JC, Klein JB, Laurin L-P, Twombley K, Falk RJ, Gharavi AG, Gillespie BW, Gipson DS, Greenbaum LA, Holzman LB, Kretzler M, Robinson B, Smoyer WE, Guay-Woodford LM; CureGN Consortium : CureGN study rationale, design, and methods: Establishing a large prospective observational study of glomerular disease. Am J Kidney Dis 73: 218–229, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gadegbeku CA, Gipson DS, Holzman LB, Ojo AO, Song PX, Barisoni L, Sampson MG, Kopp JB, Lemley KV, Nelson PJ, Lienczewski CC, Adler SG, Appel GB, Cattran DC, Choi MJ, Contreras G, Dell KM, Fervenza FC, Gibson KL, Greenbaum LA, Hernandez JD, Hewitt SM, Hingorani SR, Hladunewich M, Hogan MC, Hogan SL, Kaskel FJ, Lieske JC, Meyers KE, Nachman PH, Nast CC, Neu AM, Reich HN, Sedor JR, Sethna CB, Trachtman H, Tuttle KR, Zhdanova O, Zilleruelo GE, Kretzler M: Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int 83: 749–756, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barisoni L, Gimpel C, Kain R, Laurinavicius A, Bueno G, Zeng C, Liu Z, Schaefer F, Kretzler M, Holzman LB, Hewitt SM: Digital pathology imaging as a novel platform for standardization and globalization of quantitative nephropathology. Clin Kidney J 10: 176–187, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]