Abstract
Since the detection of the first case of COVID-19 in Chile on March 3rd, 2020, a total of 513188 cases, including ~14302 deaths have been reported in Chile as of November 2nd, 2020. Here, we estimate the reproduction number throughout the epidemic in Chile and study the effectiveness of control interventions especially the effectiveness of lockdowns by conducting short-term forecasts based on the early transmission dynamics of COVID-19. Chile’s incidence curve displays early sub-exponential growth dynamics with the deceleration of growth parameter, p, estimated at 0.8 (95% CI: 0.7, 0.8) and the reproduction number, R, estimated at 1.8 (95% CI: 1.6, 1.9). Our findings indicate that the control measures at the start of the epidemic significantly slowed down the spread of the virus. However, the relaxation of restrictions and spread of the virus in low-income neighborhoods in May led to a new surge of infections, followed by the reimposition of lockdowns in Greater Santiago and other municipalities. These measures have decelerated the virus spread with R estimated at ~0.96 (95% CI: 0.95, 0.98) as of November 2nd, 2020. The early sub-exponential growth trend (p ~0.8) of the COVID-19 epidemic transformed into a linear growth trend (p ~0.5) as of July 7th, 2020, after the reimposition of lockdowns. While the broad scale social distancing interventions have slowed the virus spread, the number of new COVID-19 cases continue to accrue, underscoring the need for persistent social distancing and active case detection and isolation efforts to maintain the epidemic under control.
Author summary
In context of the ongoing COVID-19 pandemic, Chile has been one of the hardest-hit countries in Latin America, struggling to contain the spread of the virus. In this manuscript, we employ renewal equation to estimate the reproduction number (R) for the early ascending phase of the COVID-19 epidemic and by July 7th, 2020 to guide the magnitude and intensity of interventions required to combat the COVID-19 epidemic. We also estimate the instantaneous reproduction number throughout the epidemic in Chile. Moreover, we generate short-term forecasts based on the epidemic trajectory using phenomenological models, and assess counterfactual scenarios to understand any additional resources required to contain the virus’ spread. Our results indicate early sustained transmission of SARS-CoV-2. However, the initial control measures at the start of the epidemic significantly slowed down the spread of the virus. The easing of COVID-19 restrictions in April led to a new wave of infections, followed by the re-imposition of lockdowns in Greater Santiago and other municipalities. Most recent estimates of reproduction number indicate a decline in the virus transmission. While broad-scale social distancing interventions have slowed the virus spread, the number of new COVID-19 cases continue to accrue, underscoring the need for persistent social distancing efforts.
Introduction
The coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was declared a global pandemic by the World Health Organization (WHO) on March 11th, 2020 [1, 2]. This highly contagious unprecedented virus has impacted government and public institutions, strained the health care systems, restricted people in their homes, and caused country-wide lockdowns resulting in a global economic crisis [3–5]. Moreover, as of November 2nd, 2020, nearly 46 million COVID-19 cases in 213 countries and territories have been reported, including more than 1.2 million deaths [6]. The social, economic, and psychological impact of this pandemic on much of the world’s population is profound [7–13].
Soon after its initial rapid spread in China, the first case of novel coronavirus beyond China was reported in Thailand on January 13th, 2020 [14]. The first case in the USA was not identified until January 20th, 2020 followed by the detection of the first cases in the European territory on January 24th, 2020 [15, 16]. The COVID-19 pandemic has since spread to every continent except the Antarctica. While some countries like New Zealand and Australia have steadily suppressed the COVID-19 spread, reporting less than 150 cases per day as of November 2nd, 2020, other countries like Brazil, India, and the USA still struggle to contain the increasing number of cases [17]. Subsequently, considerable COVID-19 outbreaks have occurred in Latin America since late February 2020.
The WHO declared Latin America the new epicenter of the COVID-19 on May 22nd, 2020 [18]. Latin America has paid a high toll during the COVID-19 pandemic, with some of the worlds’ highest death rates [19–21]. While home to less than 10% of the world population, Latin America accounts for about one-third of all reported global deaths (~370 thousand) [6]. Several socioeconomic, demographic, and political factors make control of the pandemic in Latin America particularly challenging [22–25]. Most countries in the region are now facing the stark social and economic costs imposed by large-scale non-pharmaceutical interventions while largely failing to control the epidemic’s spread [13, 24, 26]. Despite these unique conditions, the region has received relatively little attention from researchers globally [19]. As of November 1st, 2020, the highest number of cases have been reported in Brazil (5,516,658), followed by Argentina (1,157,179), Colombia (1,063,151), Mexico (918,811), Peru (900,180) and Chile (510,256) [17, 27]. Adjusted by population, Chile’s COVID-19 outbreak is among the worst globally, with more than 26,000 cases and 980 deaths per million inhabitants [28].
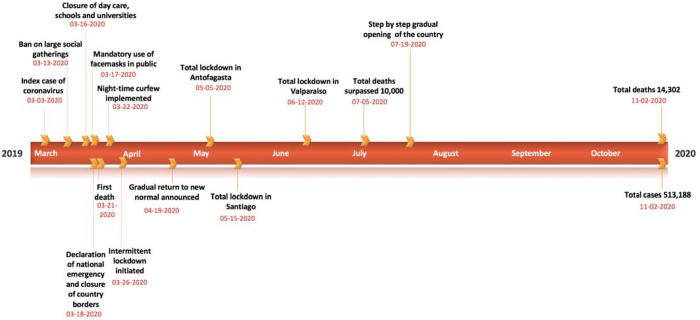
The first case of SARS-CoV-2 in Chile was identified on March 3rd, 2020. While the initial cases were imported from southeast Asia and Europe, the COVID-19 case counts have expanded in this country, placing Chile in phase 4 of the pandemic on March 25th, 2020 [28, 29]. Chile was the fifth country in Latin America after Brazil, Mexico, Ecuador and Argentina to report COVID-19 cases. The first six imported cases were reported in Talca and in the capital of Chile, Santiago [28]. However, since the early phase of the outbreak, Chile has employed an agile public health response by announcing a ban on public health gatherings of more than 500 people on March 13th, 2020, when the nationwide cumulative case count reached 44 reported cases [30].
Moreover, the Chilean government announced the closure of all daycares, schools, and universities on March 16th, 2020. These closures were followed by the announcement to close country borders on March 18th, 2020, and the declaration of national emergency on the same date, accompanied by several concrete interventions to further contain the outbreak in the region [31]. In particular, these included a night-time curfew in Chile starting on March 22nd, 2020, and localized lockdowns (i.e., intermittent lockdowns at the municipality level depending on total cases and case growth) starting on March 28th, 2020 in two municipalities in Southern Chile and seven municipalities in Santiago [32]. These initial containment strategies kept the COVID-19 case counts lower than regional peers; Brazil, Peru, and Ecuador until the end of April 2020. However, the government started to ease the COVID-19 restrictions in late April by reopening the economy under the “Safe Return” plan, including the televised opening of some businesses and stores, as new infections had reduced between 350–500 per day by the end of April, implying an only apparent flattening of the COVID-19 curve [33–35]. Moreover, imposing and lifting lockdowns in small geographical areas (municipalities) proved unsuccessful in areas with high interdependencies such as the Greater Santiago [36]. This strategy resulted in a new wave of infections; with the virus spreading from more affluent areas of Chile to more impoverished, crowded communities, forcing the government to reimpose lockdown measures in Santiago in mid-May (Figure 1) [23, 37, 38]. By mid-July, the government implemented the “step by step” strategy, considering five stages of gradual opening, at the municipality level, based on the periodic monitoring of epidemiological and health system indicators. The case counts continued to increase, averaging ~4943 cases per day in June 2020, and started to decline thereafter. The mid-June peak of infections resulted in intensive care units (ICU) reaching saturation levels of 89% nationally and 95% in the Metropolitan Region [39]. Thus far, Chile has accumulated 513,188 reported cases including 14,302 deaths as of November 2nd, 2020. The majority (~52%) of COVID-19 cases are concentrated in Region Metropolitana (mostly in Chile’s capital, Santiago), with 297,423 reported cases, followed by 30,498 cases in Valparaiso located in coastal central Chile, and 30,934 cases in Biobio located in southern Chile [40, 41]. Moreover, the crude case fatality rate in Chile (~2.8%) resonates with the global average case fatality rate (2.6%) [17, 42].
Figure 1:
Timeline of the milestones COVID-19 pandemic in Chile as of November 2nd, 2020.
In this study, we estimate the transmission potential of COVID-19, including the effective reproduction number, R, during the early transmission phase of the COVID-19 epidemic in Chile and around the mid of the epidemic, by July 7th, 2020. We also estimate the instantaneous reproduction number throughout the epidemic in Chile. The reproduction number can guide the magnitude and intensity of control interventions required to combat the COVID-19 outbreak [43, 44]. We examine the effectiveness of control interventions in Chile (see Table 1) on the transmission rate. To do this, we conduct short-term forecasts using phenomenological growth models calibrated using the early trajectory of the epidemic and by the mid of the epidemic (as of July 7th, 2020) [45] to anticipate additional resources required to contain the epidemic. These phenomenological growth models are useful in capturing the epidemic’s empirical patterns, especially when the epidemiological data are limited, and significant uncertainty exists around infectious disease epidemiology [46]. These models provide a starting point for forecasting the epidemic size and characterizing the temporal changes in the reproduction number during the epidemic [47].
Table 1:
Timeline of the implementation of the social distancing interventions in Chile as of November 2nd, 2020.
| Date | Control interventions |
|---|---|
| March 13th, 2020 | Ban on large social gatherings implemented in Chile [30] |
| March 16th, 2020 | |
| Mandatory quarantine of high-risk individuals returning from Iran, China, West Europe and South Korea | |
| March 18th, 2020 | |
| Telework implemented | |
| March 19th, 2020 | Closure of mall and department stores with the exception of supermarkets, pharmacies, banks and grocery stores |
| March 21st, 2020 | Closure of non-essential business including theatres, restaurant, bars and Gyms |
| March 22nd, 2020 | Night time curfew implemented [32] |
| March 26th, 2020 | Intermittent lockdown initiated (implemented at municipality level) [32] |
| April 8th, 2020 | Orders on mandatory use of facemasks in public transport [49] |
| April 17th, 2020 | Orders on mandatory use of facemasks in all public spaces [60] |
| April 30th, 2020 | First shopping mall is reopened in Santiago and then closed the next day [50] |
| May 5th, 2020 | Total lockdown in Antofagasta [31] |
| May 15th, 2020 | Total lockdown imposed in all municipalities of Santiago [51] |
| June 12th, 2020 | Total lockdown in Valparaiso [31] |
| July 19th, 2020 | Step by step gradual reopening of the country |
Methods
COVID-19 incidence and testing data
We obtained updates on the daily series of new COVID-19 cases as of November 2nd, 2020, from the publicly available data from the GitHub repository created by the Chile’s government [27]. Incidence case data by the date of reporting per day, confirmed by PCR (polymerase chain reaction) tests from March 3rd–November 2nd, 2020, were analyzed. The daily testing and positivity rates available from April 9th–November 2nd, 2020, were also analyzed.
Models
We utilize two phenomenological growth models, the generalized growth model (GGM) and the generalized logistic growth model (GLM) that have been validated by deriving short-term forecasts for multiple infectious diseases in the past, including SARS, pandemic Influenza, Ebola, and Dengue [52, 53].
Generalized growth model (GGM)
We generate short term forecasts using the generalized growth model (GGM) that characterizes the early ascending phase of the epidemic by estimating two parameters: (1) the intrinsic growth rate, r; and (2) a dimensionless “deceleration of growth” parameter, p. This model allows to capture a range of epidemic growth profiles by modulating parameter p. The GGM model is given by the following differential equation:
In this equation C′(t) describes the incidence curve over time t, C(t) describes the cumulative number of cases at time t and p∈[0,1] is a “deceleration of growth” parameter. This equation becomes constant incidence over time if p=0 and an exponential growth model for cumulative cases if p =1. Whereas if p is in the range 0< p <1, then the model indicates sub-exponential growth dynamics [54, 55].
Generalized logistic growth model (GLM)
The generalized logistic growth model (GLM) is an extension of the simple logistic growth model that captures a range of epidemic growth profiles, including sub-exponential (polynomial) and exponential growth dynamics. GLM characterizes epidemic growth by estimating (i) the intrinsic growth rate, r (ii) a dimensionless “deceleration of growth” parameter, p and (iii) the final epidemic size, k0. The final epidemic size is sensitive to small variations in the deceleration of growth parameters [56] and would vary as the epidemic progresses. The deceleration parameter modulates the epidemic growth patterns, including the sub-exponential growth (0< p <1), constant incidence (p =0) and exponential growth dynamics (p =1). The GLM model is given by the following differential equation:
Where describes the incidence over time t and the cumulative number of cases at time t is given by C(t) [45]. This simple logistic growth type model typically supports single peak epidemics in the number of new infections followed by a burnt-out period, unless external driving forces such as the seasonal variations in contact patterns exist. This model can underestimate the peak timing and the duration of outbreaks. This model can also underestimate the case incidence before the inflection point has occurred [45, 47, 53, 57].
Calibration of the GGM and GLM model
We calibrate the GGM and the GLM model to the daily incidence curve by dates of reporting in Chile using time series data from March 3rd–March 30th, 2020, and from May 9th – July 7th, 2020, respectively (Figure 2). The period from March 3rd–March 30th, 2020, includes the initial interventions made by the Chilean government, whereas the period from May 9th-July 7th, 2020, comprises the reimposition of lockdowns after a brief reopening of society under the “new normal” (Figure 1).
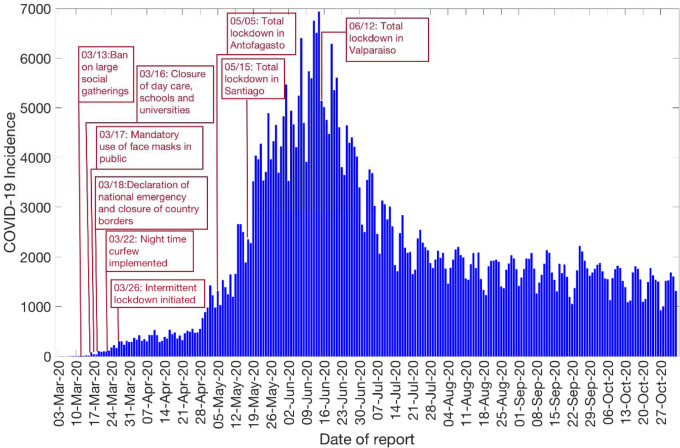
Figure 2:
Daily incidence curve for all COVID-19 confirmed cases in Chile as of November 2nd, 2020 (9).
Model parameters are estimated by a non-linear least-square fitting of the model solution to the incidence data by the date of reporting. This is achieved by searching for the set of model parameters that minimizes the sum of squared differences between the observed data yti = yt1, yt2, …. ytn and the corresponding mean incidence curve given by : where corresponds to the set of parameters of the GGM model and corresponds to the set of parameters of the GLM model. In both cases, the objective function for the best fit solution of is given by:
where ti is the time stamp at which the time series data are observed and n is the total number of data points available for inference. The initial condition is fixed to the first observation in the data set. This way, gives the best fit to the time-series data . Next, we utilize a parametric bootstrapping approach assuming a negative binomial error structure for the GGM and GLM model to derive uncertainty in the parameters obtained by non-linear least-square fit of the data as previously described [54, 58]. The variance is assumed to be three times the mean for GGM and 96 times the mean for the GLM. The model confidence intervals of parameters and the 95% prediction intervals of model fit are also obtained using the parametric bootstrap approach [54].
Reproduction number, R, from case incidence using GGM
The reproduction number, R, is defined as the average number of secondary cases generated by a primary case at time t during the outbreak. This is crucial to identify the intensity of interventions required to contain an epidemic [59–61]. Estimates of effective R indicate if the disease transmission continues (R>1) or if the active disease transmission ceases (R<1). Therefore, in order to contain an outbreak, we need to maintain R<1. We estimate the reproduction number by calibrating the GGM to the epidemic’s early growth phase (27 days) [55]. We model the generation interval of SARS-CoV-2, assuming gamma distribution with a mean of 5.2 days and a standard deviation of 1.72 days [62]. We estimate the growth rate parameter, r, and the deceleration of growth parameter, p, as described above. The progression of local incidence cases li at calendar time ti is simulated from the calibrated GGM model. Then in order to estimate the reproduction number, we apply the discretized probability distribution of the generation interval denoted by ρi to the renewal equation as follows [43, 44, 63]:
The numerator represents the total new cases li, and the denominator represents the total number of cases that contribute to generating the new cases li at time ti. Hence, Rt, represents the average number of secondary cases generated by a single case at time t. Next, we derive the uncertainty bounds around the curve of Rt directly from the uncertainty associated with the parameter estimates (r, p) obtained from the GGM. We estimate Rt for 300 simulated curves assuming a negative binomial error structure where the variance is assumed to be three times the mean [54].
Reproduction number, R, from case incidence using GLM
In order to estimate the reproduction number by July 7th, 2020 (after the reimposition of lockdowns in Santiago and Valparaiso), we calibrate the GLM from May 9th – July 7th, 2020 [55]. Next, we model the generation interval [62], estimate the model parameters (r, p, k0) from GLM and the reproduction number from the renewal equation as described above [43, 44, 63]. The uncertainty bounds around the curve of Rt are derived directly from the uncertainty associated with the parameter estimates (r,p, k0). We estimate Rt for 300 simulated curves assuming a negative binomial error structure [54] where the variance is assumed to be 96 times of the mean calculated by averaging mean to variance ratio calculated from the data (by binning data points and calculating directly from the data itself).
Instantaneous reproduction number, R, using the Cori method
We estimate R by the ratio of number of new infections generated at time t (It), to the total infectiousness of infected individuals at time t, given by :
In this equation, ws represents the infectivity profile of the infected individual, which depends on the time since infection (s), but is independent of the calendar time (t) [66, 67].
More specifically, ws is defined as a probability distribution describing the average infectiousness profile of an individual after infection. Distribution of ws is affected by individual biological factors such as symptom severity or pathogen shedding. The equation indicates the sum of infection incidence up to time step t − 1, weighted by the infectivity function ws. The distribution of the generation time can be utilized to approximate the infectivity profile, ws, however, since the time of infection is rarely observed, it becomes difficult to measure the distribution generation time [64]. Hence, time of symptom onset is usually used to estimate the distribution of serial interval, which is defined as the time interval between the dates of symptom onset among two successive cases in a transmission chain [68]. The infectiousness of a case is a function of the time since infection. This quantity is proportional to ws if we set the timing of infection in the primary case as the time zero of ws and assume that the generation interval equals the SI. The SI was assumed to follow a gamma distribution with a mean of 5.2 days and a standard deviation of 1.72 days [62]. Analytical estimates of Rt were obtained within a Bayesian framework using EpiEstim R package in R language [68]. Rt was estimated at 7-day intervals. We reported the median and 95% credible interval (CrI).
3. Results
Case incidence data
The Ministry of Health Chile reported a total of 481,342 COVID-19 cases as of November 2nd, 2020 [27]. The epidemic curve showed an increasing trajectory from April-June 2020 and declined thereafter. On average, ~443 (SD: 133.6) new cases per day were reported in April 2020, ~2697 (SD:1342) new cases per day were reported in May 2020 and ~4943 (SD:972.2) new cases per day were reported in June 2020, the maximum number of cases reported per day during the epidemic. The per-day cases declined starting July, with ~2456 (SD:581) new cases reported per day in July 2020, ~1808 (SD:258) new cases per day reported in August 2020, ~1706 (SD:294) new cases per day reported in September 2020, and ~1521 (SD:275) new cases per day reported in October 2020. Figure 2 shows the daily incidence data of all confirmed cases in Chile as of November 2nd, 2020.
Initial growth dynamics and estimate of the reproduction number using GGM
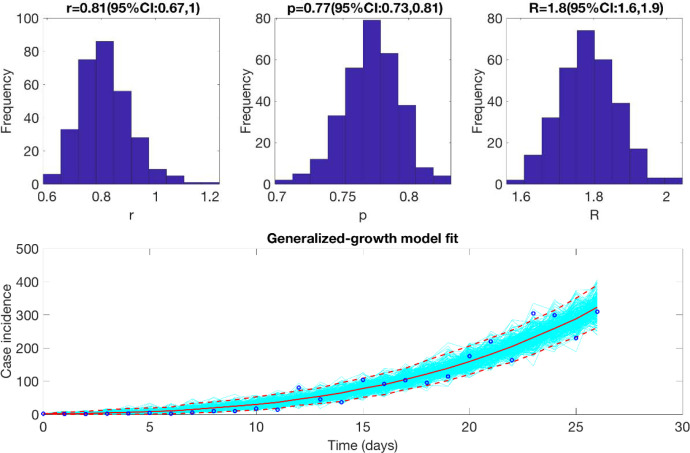
We estimate the reproduction number for the first 27 epidemic days incorporating the effects of the social distancing interventions, as explained in Table 1 and Figure 1. The incidence curve displays sub-exponential growth dynamics with the scaling of growth parameter (deceleration of growth parameter), p, estimated at 0.77 (95% CI: 0.73, 0.81) and the intrinsic growth rate, r, estimated at 0.81 (95% CI: 0.67, 1.0). During the early transmission phase the reproduction number was estimated at 1.8 (95% CI: 1.6, 1.9) (Figure 3).
Figure 3:
Reproduction number with 95% CI estimated using the GGM model. The estimated reproduction number of the COVID-19 epidemic in Chile as of March 28th, 2020, is 1.8 (95% CI: 1.6, 1.9).
Growth dynamics and estimate of reproduction number using GLM by July 7, 2020
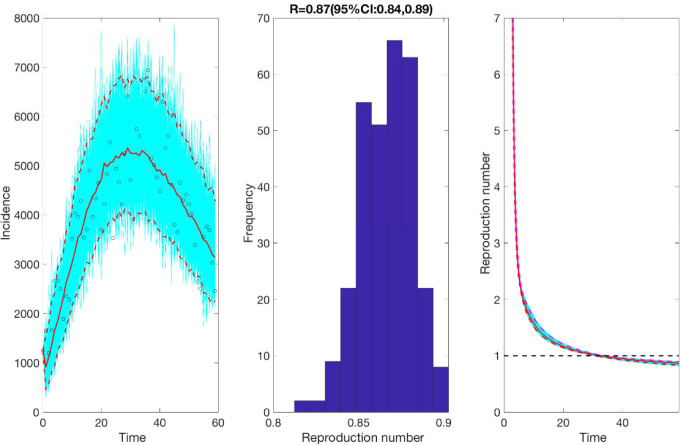
We also estimate the reproduction number from May 9th- July 7th, 2020, incorporating the effects of the reimplementation of localized lockdowns in Santiago, Antofagasta, and Valparaíso. The incidence curve displays a nearly linear growth trend with the deceleration of growth parameter, p, estimated at 0.51 (95% CI: 0.47, 0.56). The deceleration parameter in the GLM model helps modulate the trajectory of the epidemic, depicting a linear growth trend. The intrinsic growth rate, r, was estimated at 22 (95% CI: 13, 31) and the final epidemic size, k0, estimated at 3.4 e+05 (95% CI: 3.1 e+05, 3.7 e+05). The reproduction number was estimated at 0.87 (95% CI: 0.84, 0.89) as of July 7th, 2020 (Figure 4).
Figure 4:
Reproduction number with 95% CI estimated by calibrating the GLM model from May 9th-July 7th, 2020. The estimated reproduction number of the COVID-19 epidemic in Chile as of July 7th, 2020, is 0.87 (95% CI: 0.84, 0.89).
Estimate of instantaneous reproduction number using Cori method
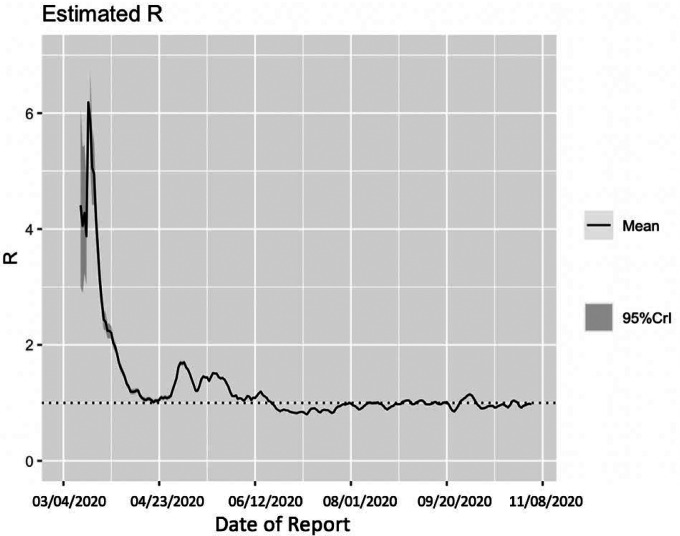
Utilizing the Cori method based on a sliding weekly window, we observe that the reproduction number peaked on March 16th, 2020, with an estimate of R~ 6.19 (95% CrI= 5.84, 7.08). The reproduction number declined thereafter and reached ~1.00 (95% CrI: 0.99, 1.04) on April 17th, 2020. From April 18th-June 18th, 2020 the reproduction number fluctuated between 1.01–1.75. This was followed by a decline in the reproduction number to less than 1.0 between June 19th-August 9th, 2020. Since then, the reproduction number has fluctuated around 1.0 with the most recent estimate of R ~ 0.96 (95% CrI: 0.95, 0.98) (Figure 5).
Figure 5:
Estimate of instantaneous reproduction number (R) for the COVID-19 epidemic in Chile as of November 2nd, 2020 using the Cori method. The most recent estimate of R~ 0.96 (95% CrI: 0.95, 0.98) as of November 2nd, 2020. Black solid line represents the mean R and the gray shaded region represents the 95% credible interval around mean R.
Assessing the impact of social distancing interventions
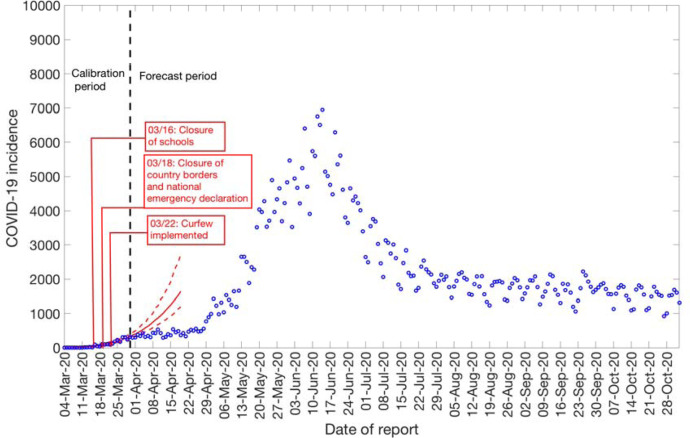
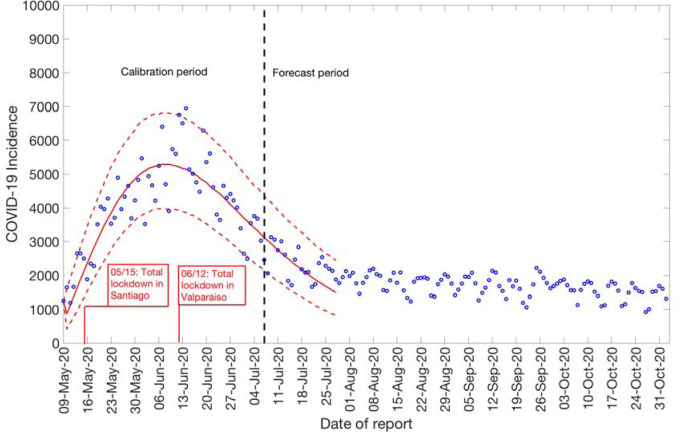
To assess the impact of social distancing interventions in Chile given in Table 1, we generated a 20-day ahead forecast for Chile based on the daily incidence curve until March 30th, 2020. The 28-day calibration period of the GGM model yields an estimated growth rate, r, at 0.8 (95% CI: 0.6, 1.0) and a deceleration of growth parameter, p, at 0.8 (95% CI: 0.7, 0.8), indicating early sub-exponential growth dynamics. The 20-day ahead forecast suggested that the early social distancing measures significantly slowed down the early spread of the virus in Chile, whose effect is noticeable about two weeks after implementing an intervention, as shown in Figure 6. A case resurgence was observed in Chile in mid-May 2020. As a consequence of this case resurgence, a total lockdown was imposed in Greater Santiago (representing ~52% of total COVID-19 cases during the epidemic) on May 15th, 2020. The quarantine in Santiago was gradually eased from August 17, 2020, and was lifted on September 28, 2020, as a part of the move to phase three of a five-step plan of deconfinement that would allow movement on regional transportation and reopening of non-essential businesses and schools [31, 69, 70]. We generated a 20-day ahead forecast based on the daily incidence curve from May 9th-July 7th, 2020. The 60-day calibration of the GLM model yields an estimated scaling of the growth parameter, p, at 0.52 (95% CI: 0.47, 0.57), representing an almost linear growth pattern. The 20-day ahead average forecast utilizing the GLM model showed that Chile could accumulate ~45,160 cases (95% CI: 27,934–67,600) between July 8th-July 27th, 2020 (Figure 7). Our forecast results approximate closely the ~46798 cases reported between July 8th-July 27th, 2020 by the Ministry of Health, Chile.
Figure 6:
20-days ahead forecast of the COVID-19 epidemic in Chile by calibrating the GGM model until March 30th, 2020. Blue circles correspond to the data points; the solid red line indicates the best model fit, and the red dashed lines represent the 95% prediction interval. The vertical black dashed line represents the time of the start of the forecast period.
Figure 7:
20-days ahead forecast of the COVID-19 epidemic in Chile by calibrating the GLM model from May 9th-July 7th, 2020. Blue circles correspond to the data points; the solid red line indicates the best model fit, and the red dashed lines represent the 95% prediction interval. The vertical black dashed line represents the time of the start of the forecast period. (96.2 is variance)
COVID-19 Testing rates and positivity rate
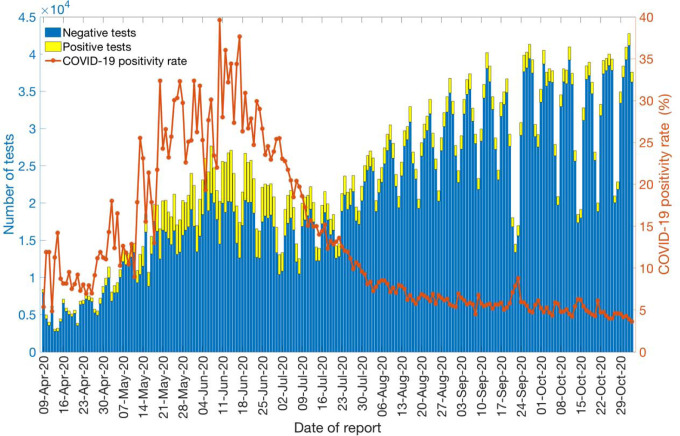
Daily testing and positivity rates for the time period April 9th–November 2nd, 2020, by the reporting date are shown in Figure 8. The total number of tests performed for this time period were 4,325,617, amongst which 10.9% (47,597) had positive results. The average number of daily tests was estimated at ~5,460 for April 2020 and ~12,959 for May 2020, a 137% increase. The testing rate in Chile further increased in June 2020, testing on average ~17,578 individuals per day, followed by a slight decline in July 2020, testing on average 16587 individuals per day. However, the testing rates continued to increase in August (average ~26,079 tests per day), September (average ~29,663 tests per day), and October (average ~31,821 tests per day), indicating an expanding testing capacity of the country. The positivity rate (percentage of positive tests among the total number of tests) has fluctuated from a monthly average of ~9.07% (SD: 2.3) in April 2020 to a monthly average of ~4.87% (SD: 0.65) in October 2020.
Figure 8:
Laboratory results for the COVID-19 tests conducted in Chile as of November 2nd, 2020. The blue color represents the negative test results, and the yellow color represents the positive test results. The solid orange line represents the positivity rate of COVID-19 in Chile.
4. Discussion
The estimates of the early transmission potential in Chile for the first 27 days of the epidemic indicate sustained local transmission in the country with the estimate of reproduction number R at ~1.8 (95% CI: 1.6, 1.9) which is also in accordance with the estimate of the reproduction number obtained from the Cori method (R~2.2 95% CrI (2.14, 2.28)). The estimates of R from our analysis agree with the estimates of R retrieved from studies conducted in the surrounding Latin American countries including Peru and Brazil [71, 72]. Other countries including Korea, South Africa and Iran also exhibit similar estimates of R that lie in the range of 1.5–7.1 [73–80]. In contrast, some other countries including Singapore and Australia have reported much lower estimates of R (R <1) that can be correlated with the implementation of early strict social distancing interventions in these countries [81, 82].
The initial deceleration of the growth parameter in Chile indicates a sub-exponential growth pattern (p~0.8), consistent with sub-exponential growth patterns of COVID-19 that have been observed in Singapore (p~0.7), Korea (p~0.76) and other Chinese provinces excluding Hubei (p~0.67) [78, 81, 83]. In contrast, studies conducted in Peru, a Latin American country, and Iran have reported a nearly exponential growth pattern of the COVID-19 whereas an exponential growth pattern has been reported in China [72, 75, 83].
Although the initial transmission stage of COVID-19 in Chile has been attributed to multiple case importations, Chile quickly implemented control measures against the COVID-19 epidemic, including border closures on March 18th, 2020, to prevent further case importations. The 20-day ahead forecast of our GGM model calibrated to 28 days suggest that the social distancing measures, including closure of schools, universities and day cares, have helped slow down the early virus spread in the country by reducing population mobility (Table 1, Figure 1, Figure 6) [84]. The commixture of interventions, including localized lockdowns, night-time curfew, school closures, and the ban on social gatherings in Chile, can probably be attributed to preventing the disease trend from growing exponentially during the early growth phase, as has occurred elsewhere [3, 4]. However, the significant increase in case incidence observed in mid-May can probably be attributed to the relaxation of social distancing measures and reopening of society in late April, in the context of the “Safe Return” plan [31]. As the virus reached the lower-income neighborhoods in Chile, the pandemic quickly exploded [23, 38, 39, 85]. While the COVID-19 case incidence exhibited a relative stabilization in case trajectory for April 2020 (with an average of ~443 cases per day), highlighting the positive effects of early quarantine and lockdowns, the reopening of society and early economic reactivation in late April 2020 probably resulted in the surge of cases resulting in an acceleration of the epidemic with estimates of R higher than 1.0. The total lockdown comprised of stay-at-home orders imposed in Greater Santiago (which accounted for about 77% of cases in the country) on May 15th showed an effect in slowing the virus’s transmission. Similar lockdowns were imposed in Antofagasta on May 5th and in Valparaíso on June 12th, though these regions together represent only ~10% of cases in Chile [31]. The deceleration of growth parameter, p, has been estimated at ~0.51 (95% CI: 0.47, 0.56) after the reimposition of lockdowns and social distancing measures in May, consistent with a linear incidence growth trend, indicating deceleration of the epidemic.
Moreover, we estimated a reproduction number, R, of ~0.87 (95% CI: 0.84, 0.89) in early July, indicating a decline in transmission of the virus consistent with the stay-at-home orders. This reproduction number corresponds to the instantaneous reproduction number estimated during the course of the epidemic utilizing the Cori method, which also indicates a decrease in disease transmission with R~0.8 as of early July. The instantaneous reproduction number has fluctuated around ~1 since early August with the most recent estimate of reproduction number, R~0.9 as of November 2nd, 2020. The 20-day ahead forecast calibrating data to the GLM model (from May 9th-July 7th, 2020) has reasonably indicated a declining trend in case incidence. The forecast results also imply that approximately ~45,160 cases (95% CI: 27,934–67,600) could be observed in Chile from July 8th-July 27th, 2020. The actual case count by for this time period, as reported by the Chilean government indicated 46,798 cases, closely approximating our mean GLM forecast, falling within the 95% PI. Therefore, based on the most recent estimates of R (Figure 5), it can be implied that maintaining social distancing measures, limiting social gatherings, and reducing population mobility have served as essential ways to containing the spread of the virus [86, 87].
Though the number of reported cases in Latin America remains low compared to the United States, official data for many Latin American countries are incomplete. However, Chile has tested a higher percentage of its residents than any other Latin American nation, lending confidence to its reliability [88]. Chile’s testing capabilities have been greatly expanded during the pandemic, in part from a coordinated effort lead by the Ministry of Science to include testing from public and private laboratories. For instance, the average number of COVID-19 tests performed in Chile per day per thousand people is 1.52 compared to the neighboring South American country, Peru (~0.2 tests per thousand people) [89]. The average positivity rate for the whole span of the epidemic in Chile is estimated at ~12.98%. However, the average monthly positivity rate of COVID-19 in Chile is estimated at ~5.90% and ~4.88% for September and October, respectively, compared to ~20.09% in May 2020. The high positivity rate at the beginning of the epidemic indicates that the government failed to cast a wide enough net to test the masses early in the pandemic, and there were probably many more active cases than those detected by epidemiological surveillance, underestimating the epidemic growth curve [90–92]. The most recent lower testing rates indicate that Chile is testing a comparatively larger segment of the population. This positivity rate for Chile is also consistent with the positivity rate obtained from India, the United States, Canada, and Germany that exhibit moderately high positivity rates (4–8%) for COVID-19, indicating overall limited testing in these countries [89, 93]. In comparison, some countries like Mexico and the Czech Republic exhibit very high positivity rates (30–51%) [89]. Other countries like Denmark, New Zealand, Australia, Singapore, and South Korea have reached very low positivity rates (0–3%) by testing the masses with South Korea’s large testing capacity combined with a strategy that tracks infected people via cell phones [88, 89, 94]. Moreover, studies suggest there is asymptomatic transmission of SARS-CoV-2 [66, 95, 96], which means we could have underestimated our estimates based on the daily incidence’s growth trend from symptomatic cases [97–99]. On the other hand, preliminary results of a study have shown the relative transmission of asymptomatic cases in Santiago to be almost ~3% [100]. While our study highlights the effectiveness of broad-scale social distancing and control interventions in Chile, it also underscores the need for persistent isolation and social distancing measures to stomp all active disease transmission chains in Chile. In the absence of pharmacological interventions and considering the advent of second waves in Asia and Europe, non-pharmacological interventions such as the ones implemented in Chile are the available options for countries to address the pandemic before large segments of the population are immunized with effective and safe vaccines. In this scenario, real-time metrics that characterize the transmission dynamics and control are crucial to face the future challenges that the pandemic will impose.
This study has some limitations. First, our study analyzes cases by the dates of reporting while it is ideal to analyze the cases by the dates of onset or after adjusting for reporting delays. On the other hand, a substantial fraction of the COVID-19 infections exhibits very mild or no symptoms at all, which may not be reflected by data [101, 102]. Moreover, the data are not stratified by local and imported cases; therefore, we assumed that all cases contribute equally to the transmission dynamics of COVID-19. Finally, the extent of selective underreporting, and its impact on these results is difficult to assess.
5. Conclusions
In this study, we estimate the transmission potential of SARS-CoV-2 in Chile. Our current findings point to sustained transmission of SARS-CoV-2 in the early phase of the outbreak, with our estimate of the reproduction number at R~1.8. The COVID-19 epidemic in Chile followed an early sub-exponential growth trend (p ~0.8) which has transformed into an almost linear growth trend (p ~0.5) as of July 7th, 2020. The most recent estimate of reproduction number, R, is ~0.9 as of November 2nd, 2020, indicating a decline in the virus transmission in Chile. The implementation of lockdowns and apt social distancing interventions have indeed slowed the spread of the virus. However, the number of new COVID-19 cases continue to accumulate, underscoring the need for persistent social distancing and active contact tracing efforts to maintain the epidemic under control.
Funding:
G.C. is partially supported from NSF grants 1610429 and 1633381 and R01 GM 130900. EU is partially funded by the ANID Millennium Science Initiative [grant NCN17_081]
Footnotes
Availability of data and materials
The datasets used and analyzed during the current study are available from Base de Datos COVID-19 repository, http://www.minciencia.gob.cl/covid19.
Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.WHO. WHO Director-General’s opening remarks at the media briefing on COVID-19–11 March 2020 World Health Organization; 2020. [April 23]. Available from: https://bit.ly/2A8aCIO. [Google Scholar]
- 2.Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet. 2020;395(10223):514–23. doi: 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Campbell H, Kulkarni D, Harpur A, Nundy M, Wang X, et al. The temporal association of introducing and lifting non-pharmaceutical interventions with the time-varying reproduction number (R) of SARS-CoV-2: a modelling study across 131 countries. The Lancet Infectious Diseases. 2020. Epub October 22. doi: 10.1016/S1473-3099(20)30785-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker PGT, Whittaker C, Watson OJ, Baguelin M, Winskill P, Hamlet A, et al. The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science (New York, NY). 2020;369(6502):413–22. doi: 10.1126/science.abc0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flaxman S, Mishra S, Gandy A, Unwin HJT, Mellan TA, Coupland H, et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584(7820):257–61. doi: 10.1038/s41586-020-2405-7 [DOI] [PubMed] [Google Scholar]
- 6.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. The Lancet Infectious Diseases. 2020;20(5):533–4. doi: 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bank W. The Economy in the Time of Covid-19 LAC Semiannual Report. Washington, DC: World Bank:2020. April 12 Report No. [Google Scholar]
- 8.Baek C, Mccrory PB, Messer T, Mui P. Unemployment effects of stay-at-home orders: Evidence from high frequency claims data. The Review of Economics and Statistics. 2020:1–72. [Google Scholar]
- 9.Rojas FL, Jiang X, Montenovo L, Simon KI, Weinberg BA, Wing C. Is the Cure Worse than the Problem Itself? Immediate Labor Market Effects of COVID-19 Case Rates and School Closures in the U.S. National Bureau of Economic Research; 2020;(0898–2937). doi: 10.3386/w27127 [DOI] [Google Scholar]
- 10.Gupta S, Montenovo L, Nguyen TD, Rojas FL, Schmutte IM, Simon KI, et al. Effects of Social Distancing Policy on Labor Market Outcomes. National Bureau of Economic Research Working Paper Series. 2020;No. 27280. doi: 10.3386/w27280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfefferbaum B, North CS. Mental Health and the Covid-19 Pandemic. New England Journal of Medicine. 2020;383(6):510–2. doi: 10.1056/NEJMp2008017 [DOI] [PubMed] [Google Scholar]
- 12.De Girolamo G, Cerveri G, Clerici M, Monzani E, Spinogatti F, Starace F, et al. Mental Health in the Coronavirus Disease 2019 Emergency—The Italian Response. JAMA Psychiatry. 2020;77(9):974–6. doi: 10.1001/jamapsychiatry.2020.1276 [DOI] [PubMed] [Google Scholar]
- 13.Asahi K, Undurraga EA, Valdes R, Wagner R. The effect of COVID-19 on the economy: evidence from an early adopter of localized lockdowns. medRxiv. 2020:2020.09.21.20198887. doi: 10.1101/2020.09.21.20198887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okada P, Buathong R, Phuygun S, Thanadachakul T, Parnmen S, Wongboot W, et al. Early transmission patterns of coronavirus disease 2019 (COVID-19) in travellers from Wuhan to Thailand, January 2020. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2020;25(8). Epub 2020/03/05. doi: 10.2807/1560-7917.Es.2020.25.8.2000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spiteri G, Fielding J, Diercke M, Campese C, Enouf V, Gaymard A, et al. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro surveillance 2020;25(9):2000178. doi: 10.2807/1560-7917.ES.2020.25.9.2000178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First Case of 2019 Novel Coronavirus in the United States. New England Journal of Medicine. 2020;382(10):929–36. doi: 10.1056/NEJMoa2001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. Situation Reports Coronavirus World Health Organization; 2020. [March 5]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. [Google Scholar]
- 18.Taylor L. How South America became the new centre of the coronavirus pandemic. NewScientist. 2020. June 10. [Google Scholar]
- 19.COVID-19 in Latin America: a humanitarian crisis. The Lancet. 2020;396(10261):1463. doi: 10.1016/S0140-6736(20)32328-X [DOI] [PubMed] [Google Scholar]
- 20.Undurraga EA, Chowell G, Mizumoto K. Case fatality risk by age from COVID-19 in a high testing setting in Latin America: Chile, March-May, 2020. Infectious Disease Poverty. 2020;(In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munayco C, Chowell G, Tariq A, Undurraga EA, Mizumoto K. Risk of death by age and gender from CoVID-19 in Peru, March-May, 2020. Aging (Albany NY). 2020;12(14):13869–81. Epub 2020/07/21. doi: 10.18632/aging.103687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor L. How Latin America is fighting covid-19, for better and worse. BMJ. 2020;370:m3319. doi: 10.1136/bmj.m3319 [DOI] [PubMed] [Google Scholar]
- 23.Gil M, Undurraga E. CoVID-19 has exposed how ‘the other half’ (still) lives. Bulletin of Latin American Research. 2020;In press. [Google Scholar]
- 24.De Souza WM, Buss LF, Candido DdS, Carrera J-P, Li S, Zarebski AE, et al. Epidemiological and clinical characteristics of the COVID-19 epidemic in Brazil. Nature Human Behaviour. 2020;4(8):856–65. doi: 10.1038/s41562-020-0928-4 [DOI] [PubMed] [Google Scholar]
- 25.Garcia PJ, Alarcón A, Bayer A, Buss P, Guerra G, Ribeiro H, et al. COVID-19 Response in Latin America. The American Journal of Tropical Medicine and Hygiene. 2020;103(5):1765–72. doi: 10.4269/ajtmh.20-0765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.González-Bustamante B. Evolution and early government responses to COVID-19 in South America. World Development. 2021;137:105180. doi: 10.1016/j.worlddev.2020.105180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Government of Chile M. [2020 April 25];MinScience/ Data-COVID19. 2020 [cited. ]. Available from: https://github.com/MinCiencia/Datos-COVID19.
- 28.MOH. Cifras Oficiales COVID-19 2020. Available from: https://www.gob.cl/coronavirus/cifrasoficiales/.
- 29.Chile Gd. Chile enters the Stage 4 Coronavirus transmission scenario and President Piñera announces the closure of the borders and secures the supply chain. Gob.cl: 2020. March 16 Report No. [Google Scholar]
- 30.Cambero F. Chile bans large public events over coronavirus fears, ahead of planned protests. Rueters. 2020. March 13. [Google Scholar]
- 31.(MINSAL) MdS. Plan de acción por coronavirus 2020. [cited 2020 July 1]. Available from: https://www.gob.cl/coronavirus/plandeaccion/.
- 32.MOH. Dispone medidas sanitarias que indica por brote de COVID-19. Norms 1143498, 1143591, 1746958, 1143651,1143645 Biblioteca del Congreso Nacional de Chile2020 [April 24]. Available from: https://www.leychile.cl/N?i=1143498&f=2020-04-04&p=.
- 33.Wires N. Chile’s capital goes into strict lockdown after Covid-19 surge. France 24 2020. May 17. [Google Scholar]
- 34.Prensa Presidencia GC. “Presidente Piñera presenta Plan Retorno Seguro: “Hoy es tiempo de poner a Chile por delante”.” 2020. [cited 2020 June 10]. Available from: https://prensa.presidencia.cl/comunicado.aspx?id=150453.
- 35.Tercera L. 55% of the stores open and lines to enter: This was the reopening of the Mall Apumanque under strict protocol. La Tercera; 2020. April 30. [Google Scholar]
- 36.Li Y, Undurraga EA, Zubizarreta JR. Effectiveness of Localized Lockdowns in the SARS-CoV-2 Pandemic. medRxiv. 2020:2020.08.25.20182071. doi: 10.1101/2020.08.25.20182071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beaubien J. How Chile Ended Up With One Of The Highest COVID-19 Rates. npr; 2020. July 2. [Google Scholar]
- 38.Bennett M. All things equal? Heterogeneity in policy effectiveness against COVID-19 spread in chile. World development. 2021;137:105208-. Epub 2020/09/24. doi: 10.1016/j.worlddev.2020.105208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canals M, Cuadrado C, Canals A, Yohannessen K, Lefio LA, Bertoglia MP, et al. Epidemic trends, public health response and health system capacity: the Chilean experience in four months of the COVID-19 pandemic. Rev Panam Salud Publica. 2020;44:e99–e. doi: 10.26633/RPSP.2020.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gob.cl. Official figures COVID-19 2020. [cited 2020 April 24]. Available from: https://www.gob.cl/coronavirus/cifrasoficiales/#reportes.
- 41.Government of Chile M. COVID-19 data. Github; 2020. [Google Scholar]
- 42.MOH. Cifras Oficiales COVID-19’, Ministry of Health, Plan de Acción Coronavirus COVID-19 2020. [April 2]. Available from: https://www.gob.cl/coronavirus/cifrasoficiales/.
- 43.Nishiura H, Chowell G. Early transmission dynamics of Ebola virus disease (EVD), West Africa, March to August 2014. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2014;19(36). Epub 2014/09/19. [DOI] [PubMed] [Google Scholar]
- 44.Nishiura H, Chowell G. The Effective Reproduction Number as a Prelude to Statistical Estimation of Time-Dependent Epidemic Trends. Springer, editor2009. 103–12 p. [Google Scholar]
- 45.Shanafelt DW, Jones G, Lima M, Perrings C, Chowell G. Forecasting the 2001 Foot-and-Mouth Disease Epidemic in the UK. Ecohealth; 2018;15(2):338–47. Epub 2017/12/13. doi: 10.1007/s10393-017-1293-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roosa K, Lee Y, Luo R, Kirpich A, Rothenberg R, Hyman JM, et al. Short-term Forecasts of the COVID-19 Epidemic in Guangdong and Zhejiang, China: February 13–23, 2020. Journal of clinical medicine. 2020;9(2):596. doi: 10.3390/jcm9020596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chowell G, Hincapie-Palacio D, Ospina J, Pell B, Tariq A, Dahal S, et al. Using Phenomenological Models to Characterize Transmissibility and Forecast Patterns and Final Burden of Zika Epidemics. Public Library of Science Currents. 2016;8:ecurrents.outbreaks.f14b2217c902f453d9320a43a35b583. doi: 10.1371/currents.outbreaks.f14b2217c902f453d9320a43a35b9583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pública MdIyS. Declares status of constitutional exception of catastrophe, by public calamity, in the territory of Chile Biblioteca del Congreso Nacional de Chile. 2020. [April 22]. Available from: https://www.leychile.cl/Navegar?idNorma=1143580&idVersion=2020-03-26&idParte=.
- 49.Regan H, Yeung J, Renton A, Woodyatt A, Wagner Meg. Chile mandates face masks on public and private transportation. CNN; 2020. April 6. [Google Scholar]
- 50.Mostrado E. Among applause: Mayor Lavín launches pilot plan to open the Apumanque mall. elm strador. 2020. April30. [Google Scholar]
- 51.Chile A. Total quarantine in Santiago: what time, when does it start and until what day does it extend. Tikitakas. 2020. May 15. [Google Scholar]
- 52.Chowell G, Tariq A, Hyman JM. A novel sub-epidemic modeling framework for short-term forecasting epidemic waves. BioMed Central Medicine. 2019;17(1):164. doi: 10.1186/s12916-019-1406-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pell B, Kuang Y, Viboud C, Chowell G. Using phenomenological models for forecasting the 2015 Ebola challenge. Epidemics. 2018;22:62–70. Epub 2016/12/04. doi: 10.1016/j.epidem.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 54.Chowell G. Fitting dynamic models to epidemic outbreaks with quantified uncertainty: A primer for parameter uncertainty, identifiability, and forecasts. Infectious Disease Modelling. 2017;2(3):379–98. doi: 10.1016/j.idm.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viboud C, Simonsen L, Chowell G. A generalized-growth model to characterize the early ascending phase of infectious disease outbreaks. Epidemics. 2016;15:27–37. doi: 10.1016/j.epidem.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chowell G, Sattenspiel L, Bansal S, Viboud C. Mathematical models to characterize early epidemic growth: A review. Physics of Life Reviews. 2016;18:66–97. doi: 10.1016/j.plrev.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chowell G, Luo R, Sun K, Roosa K, Tariq A, Viboud C. Real-time forecasting of epidemic trajectories using computational dynamic ensembles. Epidemics. 2020;30:100379. doi: 10.1016/j.epidem.2019.100379 [DOI] [PubMed] [Google Scholar]
- 58.Chowell G, Ammon CE, Hengartner NW, Hyman JM. Transmission dynamics of the great influenza pandemic of 1918 in Geneva, Switzerland: Assessing the effects of hypothetical interventions. Journal of Theoretical Biology. 2006;241(2):193–204. doi: 10.1016/j.jtbi.2005.11.026 [DOI] [PubMed] [Google Scholar]
- 59.Chowell G, Abdirizak F, Lee S, Lee J, Jung E, Nishiura H, et al. Transmission characteristics of MERS and SARS in the healthcare setting: a comparative study. BioMed Central Medicine. 2015;13(1):210. doi: 10.1186/s12916-015-0450-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson RM, May RM. Infectious Diseases of Humans. Oxford, editor. Oxford Univeristy Press; 1991. [Google Scholar]
- 61.Nishiura H, Chowell G, Heesterbeek H, Wallinga J. The ideal reporting interval for an epidemic to objectively interpret the epidemiological time course. J R Soc Interface. 2010;7(43):297–307. Epub 2009/07/01. doi: 10.1098/rsif.2009.0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ganyani T, Kremer C, Chen D, Torneri A, Faes C, Wallinga J, et al. Estimating the generation interval for coronavirus disease (COVID-19) based on symptom onset data, March 2020. Eurosurveillance. 2020;25(17):2000257. doi: doi: 10.2807/1560-7917.ES.2020.25.17.2000257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paine S, Mercer G, Kelly P, Bandaranayake D, Baker M, Huang Q, et al. Transmissibility of 2009 pandemic influenza A(H1N1) in New Zealand: effective reproduction number and influence of age, ethnicity and importations. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2010;15(24). Epub 2010/06/26. [PubMed] [Google Scholar]
- 64.Fraser C. Estimating Individual and Household Reproduction Numbers in an Emerging Epidemic. PLOS ONE. 2007;2(8):e758. doi: 10.1371/journal.pone.0000758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chong KC, Zee BCY, Wang MH. Approximate Bayesian algorithm to estimate the basic reproduction number in an influenza pandemic using arrival times of imported cases. Travel medicine and infectious disease. 2018;23:80–6. Epub 2018/04/14. doi: 10.1016/j.tmaid.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 66.He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nature Medicine. 2020;26(5):672–5. doi: 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 67.Wallinga J, Teunis P. Different Epidemic Curves for Severe Acute Respiratory Syndrome Reveal Similar Impacts of Control Measures. American Journal of Epidemiology. 2004;160(6):509–16. doi: 10.1093/aje/kwh255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cori A, Ferguson NM, Fraser C, Cauchemez S. A New Framework and Software to Estimate Time-Varying Reproduction Numbers During Epidemics. American Journal of Epidemiology. 2013;178(9):1505–12. doi: 10.1093/aje/kwt133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gardaworld. Chile: Authorities lift COVID-19 lockdown measures in Santiago from September 28 /update 34 Gardaworld; 2020. [cited 2020 November 2]. Available from: https://www.garda.com/crisis24/news-alerts/384111/chile-authorities-lift-covid-19-lockdown-measures-in-santiago-from-september-28-update-34. [Google Scholar]
- 70.Gradaworld. Chile: Authorities to gradually lift lockdown restrictions in central Santiago from August 17 /update 24 Gradaworld; 2020. [cited 2020 November 2]. Available from: https://www.garda.com/crisis24/news-alerts/368666/chile-authorities-to-gradually-lift-lockdown-restrictions-in-central-santiago-from-august-17-update-24. [Google Scholar]
- 71.Felix FHC, Fontenele JB. Instantaneous R calculation for COVID-19 epidemic in Brazil. medRxiv. 2020:2020.04.23.20077172. doi: 10.1101/2020.04.23.20077172 [DOI] [Google Scholar]
- 72.Munayco CV, Tariq A, Rothenberg R, Soto-Cabezas GG, Reyes MF, Valle A, et al. Early transmission dynamics of COVID-19 in a southern hemisphere setting: Lima-Peru: February 29th-March 30th, 2020. Infectious Disease Modelling. 2020. doi: 10.1016/j.idm.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Read JM, Bridgen JR, Cummings DA, Ho A, Jewell CP. Novel coronavirus 2019-nCoV: early estimation of epidemiological parameters and epidemic predictions. medRxiv. 2020:2020.01.23.20018549. doi: 10.1101/2020.01.23.20018549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. The Lancet. 2020;395(10225):689–97. doi: 10.1016/S0140-6736(20)30260-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muniz-Rodriguez K, Fung IC-H, Ferdosi SR, Ofori SK, Lee Y, Tariq A, et al. Transmission potential of COVID-19 in Iran. medRxiv. 2020:2020.03.08.20030643. doi: 10.1101/2020.03.08.20030643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mizumoto K, Kagaya K, Chowell G. Early epidemiological assessment of the transmission potential and virulence of coronavirus disease 2019 (COVID-19) in Wuhan City: China, January-February, 2020. medRxiv. 2020:2020.02.12.20022434. doi: 10.1101/2020.02.12.20022434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hwang J, Park H, Kim S-H, Jung J, Kim N. Basic and effective reproduction numbers of COVID-19 cases in South Korea excluding Sincheonji cases. medRxiv. 2020:2020.03.19.20039347. doi: 10.1101/2020.03.19.20039347 [DOI] [Google Scholar]
- 78.Shim E, Tariq A, Choi W, Lee Y, Chowell G. Transmission potential and severity of COVID-19 in South Korea. International Journal of Infectious Diseases. 2020;93:339–44. doi: 10.1016/j.ijid.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mbuvha R, Marwala T. Bayesian Inference of COVID-19 Spreading Rates in South Africa. medRxiv. 2020:2020.04.28.20083873. doi: 10.1101/2020.04.28.20083873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Masjedi H, Rabajante JF, Bahranizadd F, Zare MH. Nowcasting and Forecasting the Spread of COVID-19 in Iran. medRxiv. 2020:2020.04.22.20076281. doi: 10.1101/2020.04.22.20076281 [DOI] [Google Scholar]
- 81.Tariq A, Lee Y, Roosa K, Blumberg S, Yan P, Ma S, et al. Real-time monitoring the transmission potential of COVID-19 in Singapore, March 2020. medRxiv. 2020:2020.02.21.20026435. doi: 10.1101/2020.02.21.20026435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Price DJ, Shearer FM, Meehan MT, McBryde E, Moss R, Golding N, et al. Early analysis of the Australian COVID-19 epidemic. medRxiv. 2020:2020.04.25.20080127. doi: 10.1101/2020.04.25.20080127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roosa K, Lee Y, Luo R, Kirpich A, Rothenberg R, Hyman JM, et al. Real-time forecasts of the COVID-19 epidemic in China from February 5th to February 24th, 2020. Infectious Disease Modelling. 2020;5:256–63. doi: 10.1016/j.idm.2020.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cuadrado C, Monsalves MJ, Gajardo J, Bertoglia MP, Najera M, Alfaro T, et al. Impact of small-area lockdowns for the control of the COVID-19 pandemic. medRxiv. 2020:2020.05.05.20092106. doi: 10.1101/2020.05.05.20092106 [DOI] [Google Scholar]
- 85.Contesse J. Responding to COVID-19 Without Public Trust. The Regulatory Review. 2020. June 2. [Google Scholar]
- 86.France-Presse A. Chile prolongs Santiago lockdown as daily virus deaths rise. The Jakarta Post. 2020. June 4. [Google Scholar]
- 87.Chang S, Pierson E, Koh PW, Gerardin J, Redbird B, Grusky D, et al. Mobility network models of COVID-19 explain inequities and inform reopening. Nature. 2020. doi: 10.1038/s41586-020-2923-3 [DOI] [PubMed] [Google Scholar]
- 88.Thomson E, Sanders P. Chile Charts New Path With Rolling Lockdowns, Immunity Cards. Bloomberg. 2020. April 22. [Google Scholar]
- 89.Oxford Uo. Our World in Data: Oxford Martin School; 2020. [cited 2020 June 28]. Available from: https://ourworldindata.org/coronavirus/country/peru?country=~PER#how-many-tests-are-performed-each-day.
- 90.Li R, Pei S, Chen B, Song Y, Zhang T, Yang W, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science (New York, NY). 2020;368(6490):489–93. Epub 2020/03/18. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bhatia S, Imai N, Cuomo-Dannenburg G, Baguelin M, Boonyasiri A, Cori A, et al. Report 6: Relative sensitivity of international surveillance. Imperial College London COVID-19 Response Team: 2020 February 21. Report No. [Google Scholar]
- 92.Asahi K, Undurraga EA, Wagner R. Benchmarking the CoVID-19 pandemic across countries and states in the U.S.A. under heterogeneous testing. medRxiv. 2020:2020.05.01.20087882. doi: 10.1101/2020.05.01.20087882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Project CT. The COVID-19 tracking project 2020. [May 1]. Available from: https://covidtracking.com/about-project.
- 94.Buchanan L, Lai KKR, McCann A. U.S. Lags in Coronavirus Testing After Slow Response to Outbreak. The NewYork Times; 2020. March 17. [Google Scholar]
- 95.Y L, S F, S F. The contribution of pre-symptomatic infection to the transmission dynamics of COVID-2019 [version 1; peer review: 3 approved]. Wellcome Open Research. 2020;(5):58. doi: 10.12688/wellcomeopenres.15788.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moghadas SM, Fitzpatrick MC, Sah P, Pandey A, Shoukat A, Singer BH, et al. The implications of silent transmission for the control of COVID-19 outbreaks. Proceedings of the National Academy of Sciences. 2020;117(30):17513–5. doi: 10.1073/pnas.2008373117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wei WE, Li Z, Chiew CJ, Yong SE, Toh MP, Lee VJ. Presymptomatic Transmission of SARS-CoV-2 — Singapore, January 23–March 16, 2020. 2020. April 1 Report No. [DOI] [PMC free article] [PubMed]
- 98.Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2020;25(10):2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mayorga L, García Samartino C, Flores G, Masuelli S, Sánchez MV, Mayorga LS, et al. Detection and isolation of asymptomatic individuals can make the difference in COVID-19 epidemic management. medRxiv. 2020:2020.04.23.20077255. doi: 10.1101/2020.04.23.20077255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Desarrollo Ud. UDD study seeks to determine Covid-19 prevalence in the Metropolitan Region. School of Medicine, German clinic development university; 2020. May 25. [Google Scholar]
- 101.Tindale L, Coombe M, Stockdale JE, Garlock E, Lau WYV, Saraswat M, et al. Transmission interval estimates suggest pre-symptomatic spread of COVID-19. medRxiv. 2020:2020.03.03.20029983. doi: 10.1101/2020.03.03.20029983 [DOI] [Google Scholar]
- 102.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324(8):782–93. doi: 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]