Abstract
Chitin contributes to the rigidity of the insect cuticle and serves as an attachment matrix for other cuticular proteins. Deficiency of chitin results in abnormal embryos, cuticular structural defects and growth arrest. When chitin is not turned over during molting, the developing insect is trapped inside the old cuticle. Partial deacetylation of cuticular chitin is also required for proper laminar organization of the cuticle and vertical pore canals, molting, and locomotion. Thus, chitin and its modifications strongly influence the structure of the exoskeleton as well as the physiological functions of the insect.
Internal tendons and specialized epithelial cells called “tendon cells” that arise from the outer layer of epidermal cells provide attachment sites at both ends of adult limb muscles. Membrane processes emanating from both tendon and muscle cells interdigitate extensively to strengthen the attachment of muscles to the extracellular matrix (ECM). Protein ligands that bind to membrane-bound integrin complexes further enhance the adhesion between muscles and tendons. Tendon cells contain F-actin fiber arrays that contribute to their rigidity. In the cytoplasm of muscle cells, proteins such as talin and other proteins provide attachment sites for cytoskeletal actin, thereby increasing integrin binding and activation to mechanically couple the ECM with actin in muscle cells. Mutations in integrins and their ligands, as well as depletion of chitin deacetylases, result in defective locomotion and muscle detachment from the ECM. Thus, chitin in the cuticle and chitin deacetylases strongly influence the shape and functions of the exoskeleton as well as locomotion of insects.
Keywords: Chitin, cuticle, insects, deacetylation, molting, locomotion, muscle attachment
1. INTRODUCTION
Insect cuticles are extracellular matrices (ECM) secreted by the underlying epidermal cells. They serve as physical and chemical barriers protecting the insect from dehydration, mechanical injury and predation. A major component of insect cuticles is the unbranched carbohydrate polymer, chitin, consisting entirely of β−1–4 linked N-acetylglucosamines. Chitin bundles (18–25 chains in each bundle [1, 2]) in the cuticle are embedded in a matrix composed of a large assortment of structural proteins containing a chitin-binding domain known as Rebers & Riddiford (R & R) consensus motif(s) [3] and another class of proteins with chitin binding domain 14 (CBM14) with 1 or 3 such binding domains [4]. The cuticle-associated α-chitin with antiparallel chains is laminar, highly crystalline, water impermeable, rigid, and is tightly attached to the epidermal cell. On the other hand, the peritrophic matrix (PM)-associated chitin is hydrated, lattice-like, with pores that are permeable to small enzyme proteins and smaller molecules and is detached from the midgut epithelial cells. It is bound to hydrophilic proteins with a variable number of peritrophin-A (CBM14) motif(s). Its precise crystalline structure is not clear but is thought to be β-chitin [5]).
The arrangement of proteins surrounding the chitin bundles in the insect cuticle is not known precisely. Isolation of thicker chitin-protein fibers with diameters of 50–250 nm and lengths of 300 nm [2] from insect cuticles indicate higher order organization of this polymer. In many arthropods the cuticle is mineralized to further enhance its mechanical properties, such as hardness and ability to withstand stress and cracks. The ratio of chitin to protein as well the type of the R & R motif present in the cuticular proteins seems to vary especially between hard and soft cuticles [6–8]. Chitin content, thickness of the cuticle, the extent of protein cross-linking and tanning influence properties of the cuticle. Interestingly, cuticular chitin is also partially deacetylated. In the following, we look at the role of the content and organization of chitin in the cuticle and the enzymes involved in chitin metabolism that influence the structural properties of cuticle and physiological functions of the insect.
1.1. Chitin and Chitosan Content of Cuticle
The chitin content of cuticle is generally in the range of 20–40% of dry weight but varies widely from one developmental stage to another of the same insect and among insects of different orders [1, 9]. The amount of chitin can be as low as 2% or as high as 45% with the remaining being mostly protein, phenolics and minerals in some cases. These values are derived from chemical analyses of castoff cuticles or by solid state NMR. Eggshells also contain trace amounts of chitin [10]. Anywhere from 5 to 20% of the N-acetylglucosamine residues in naturally occurring chitin are deacetylated. The nature and organization of the chitin in insect cuticles vary widely depending on its anatomical location, developmental stage and the physiological function of the organ. Thus, the serosal cuticle that covers the developing embryo, larval cuticle, pupal cuticle, tracheal cuticle and cuticles associated with adult structures such as the pronotum, forewing, and hindwing exhibit widely divergent properties. The cuticle of beetles continues to grow during adult stage, becoming thicker and depositing cuticular layers with distinctly different structural and mechanical properties compared to the layers deposited before adult emergence [11]. In general, the soft cuticles secreted by serosal cells, young larvae and those associated with flexible structures such as the hind wings used in flight are thin and flexible while those associated with protective structures such as pronotum, elytron and head skeleton are thick and rigid.
1.2. Arrangement of Chitin in the Cuticle
The epithelial cells that cover the entire body plan of insects is the source of cuticular chitin. Some of these epithelial cells invaginate to form other structures such as the appendages or the alimentary canal to become specialized structures that offer further protection or mechanical support for internal organs and muscle attachment sites. Thus, the foregut and hindgut should be considered extensions of the exoskeleton. They are derived from ectodermal cells and do deposit chitin containing cuticular linings. Nascent chitin chains made by insect epidermal cells spontaneously associate with one another to form crystallites. A solubilized in vitro chitin synthesizing system using membrane preparations from midgut tissue of Tribolium castaneum with UDP-N-acetylglucosamine as the activated substrate indicated that the nascent chitin chains formed by membrane-bound chitin synthase oligomers spontaneously self-assemble and crystallize as nanofibrils with a diameter of ~3 nm and length of 300 nm [12]. In the crystallites of chitin chain aggregates from several orders of insects and arthropods, the mean diameter had a narrow distribution with a mean of 2.8 nm (Fig. 1) [1]. In vivo, the chitin crystallites may further associate with cuticular proteins to become extended microfibrils (50–250 nm in diameter) [2, 12]. Chitin crystals exist predominantly in the orthorhombic α-form in which the linear chains are antiparallel to the neighboring chains, which are stabilized by both intra-chain and interchain hydrogen bonds [13, 14]. More recent synchrotron X-ray diffraction measurements have refined this structure further and reveal a three-dimensional network of H-bonded sheets. In this structure, there are two distinct conformations of C6–O6 bond of the hydroxymethyl groups leading to maximization of H-bonding potential in which all carbonyl and amino groups of the C2-acetamido groups are in-volved, greatly increasing the stability of this polymer [15, 16]. The other two allomorphs of chitin, β and γ, occur in specialized chitin-containing structures, but not in insect cuticles and will not be considered here.
Fig. (1).
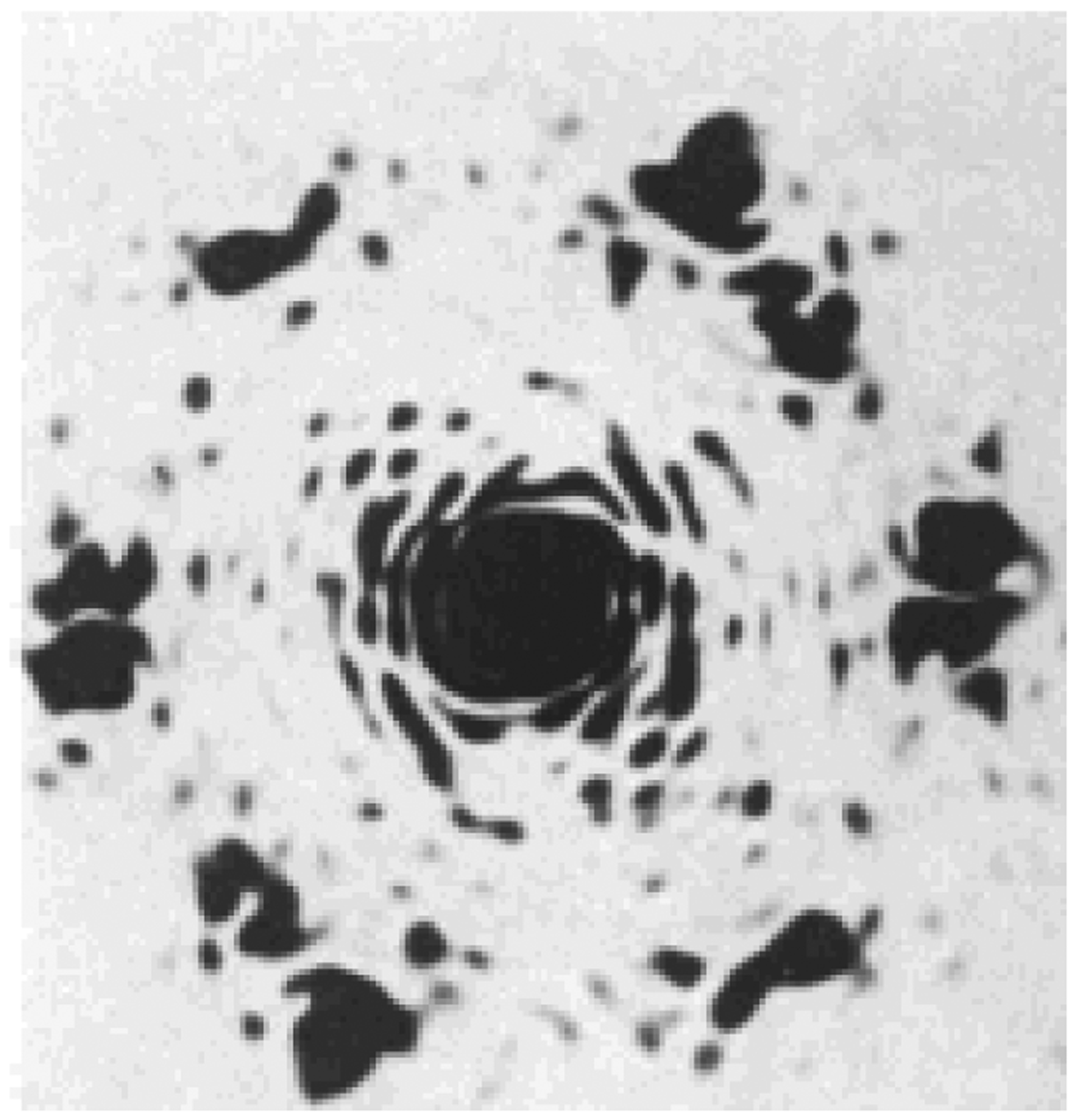
Optical diffraction patterns from the electron micrographs showing the chitin crystallites in exocuticle from Oryctes rhinoceros, pharate adult femur. Six hexagonally arranged spots may be seen showing the near-perfect hexagonal packing of the chitin crystallites, A. C. Neville, et al. (1976) [1].
The α-chitin crystallites that are secreted into space immedi-ately above the epidermal cells are further organized into a composite structure of chitin and proteins, which appear as an alternating arrangement of electron-dense and electron-lucent layers (laminae) when viewed with polarized light microscope or a transmission electron microscope (TEM). Fig. 2 illustrates the higher orders of chitin chain organization in the exocuticle of a lobster [2]. Within each layer that is parallel to the surface of the epidermal cell, the chitin crystallites are thought to be arranged side by side, all pointing in the same direction. The laminae can be stacked vertically in several configurations. The helicoidal stack (a twisted plywood-like structure), which is common, consists of laminar sheets that are turned with respect to each other by a constant small anticlockwise angle. Such an arrangement of plies of chitin laminae display isotropic stress response when subjected to external, in-plane stresses [2]. In this arrangement, the chitin fibers assume a parabolic appearance in oblique sections [17]. A second type of stacking involves laminae that are arranged in bundles of orthogonal stacks, sometimes with a short transitional helicoidal stack. There are also examples of laminae stacked vertically with respect to the epidermal surface (e.g. the gula cuticle of Pachnoda marginata), and even interlocking stacks [2, 18–20]. Often there are distinct differences between the packing of laminae in the exocuticle versus the endocuticle. In cuticles of several beetles including T. castaneum, the laminae appear to be more closely packed in the exocuticle (the layer that is deposited early in the instar and before eclosion) than in the endocuticle which is deposited later (typically after molting) [11, 20]. A comparison of elytral cuticle from 40 beetles representing 24 families indicates substantial variations in total thickness and widths of individual cuticular layers. In endocuticles of several beetle elytra there are stacks of parallel laminae that form thick bundles (macrofibers; also known as “balken cuticle”). These stacks are further arranged orthogonally with a small transitional helicoidal region in between stacks [11, 20, 21].
Fig. (2). Microstructure of lobster cuticle.
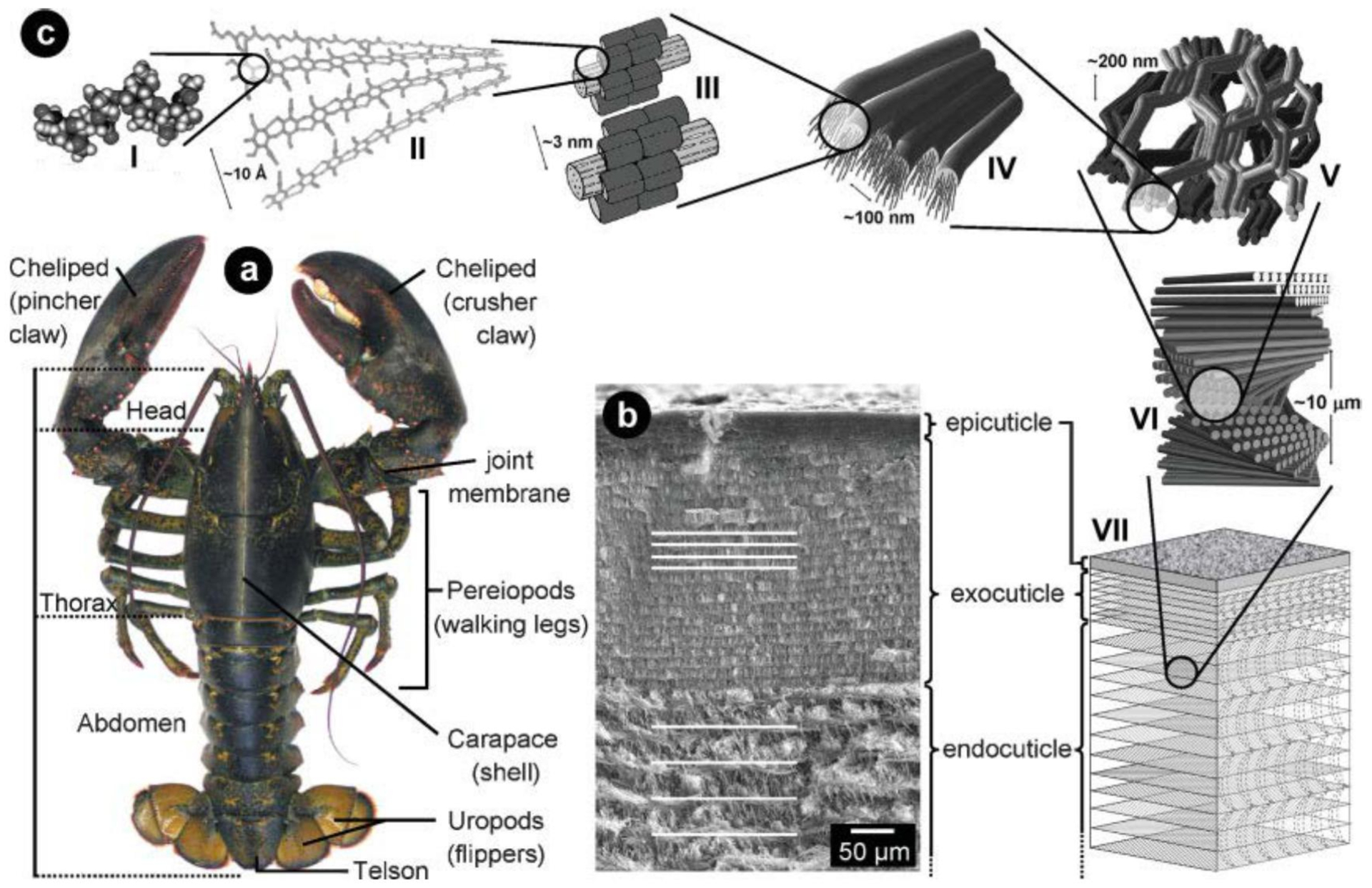
a) Morphology of the American lobster Homarus americanus. b) SEM image of a cross-section through the cuticle, showing the epi-, exo-, and endocuticle. c) Hierarchical organization, starting with the N-acetyl-glucosamine molecules (I) forming anti-parallel chains of a-chitin (II). 18 to 25 chitin molecules wrapped with proteins form nanofibrils (III), which cluster to form chitin-protein fibers (IV) that are arranged in horizontal planes, where the long axes of the fibers are all oriented in the same direction (V). The chitin protein fibers form the typical twisted plywood structure (VI) of the three-layered cuticle (VII). From Fabritius et al., (2009) [2]. With permission from Professor Raabe.
1.3. Pore Canals and Pore Canal Fibers
Another structural feature seen in many cuticles is the presence of pore canals that traverse either a vertical path or a twisted ribbon-like path perpendicular to the epidermal surface. The pore canals also contain a central column of vertically oriented long chitin fibers in a tube with an oval (or circular) cross section [2, 11]. TEM analysis of the pore canals in T. castaneum elytral cuticle has revealed the complex structure of the pore canals. (Fig. 3). In addition to the central thick chitin fibers, the pore canals are lined by a circular (or oval) colonnade of smaller chitin fibers that seem to reinforce the walls of the pore canals.
Fig. (3). Ultrastructure of elytral dorsal cuticle of the pharate adult of T. castaneum.
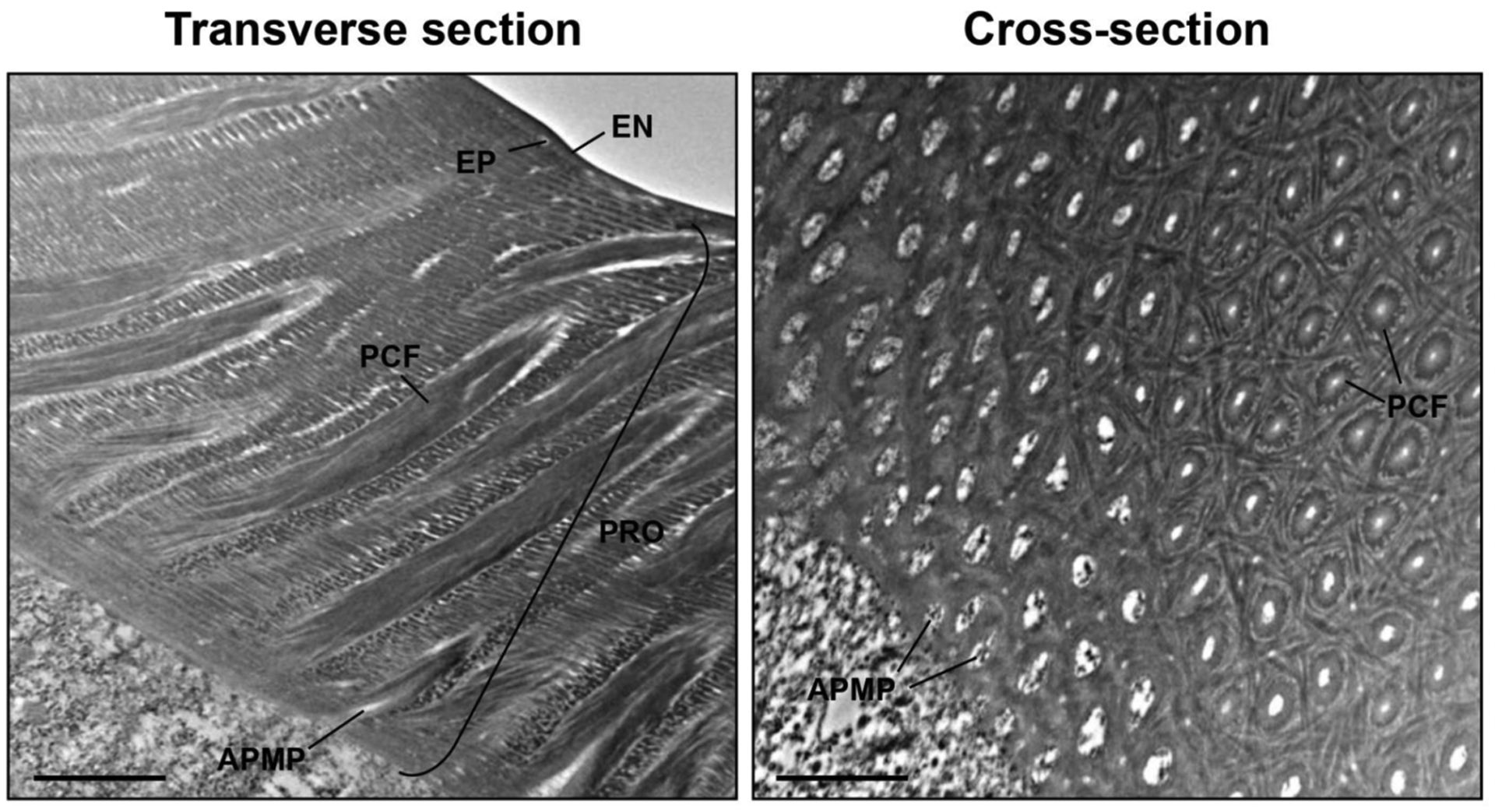
Ultrastructure of rigid elytral dorsal cuticle from pharate adults (5-day-old pupae) was analyzed by TEM. EN, envelope; EP, epicuticle; PRO, procuticle; PCF, pore canal fiber; APMP, apical plasma membrane protrusion. Scale bar = 1 μm.
Interestingly, the pore canals are located directly above apical plasma membrane protrusions (APMPs) into the cuticle giving rise to the notion that the pore canals are assembled in this location with the chitin fibers being made by chitin synthases (CHSs) located on the APMPs. The pore canals may have a two-fold function; to transport material needed for the assembly of the outermost layers of the cuticle such as the envelope and/or epicuticle; and for mechanical reinforcement of the cuticle. The vertically oriented pore canals can be thought to act as “stitches” designed to withstand transverse load stress, leading to less deformation/delamination and capable of opposing crack propagation [18]. The distribution density of the pore canals also seems to vary among different types of cuticle. Typically, the larval cuticles have fewer pore canals per unit surface area compared to the adult cuticle. They are also narrower and seem to follow a helicoidal path suggesting that they traverse the laminar cuticle following their helicoidal stacking (Fig. 4). The combination of vertical pore canals and the horizontal laminae appear to be designed to maximize the resistance to load/sheer stress in multiple directions using the least amount of composite material. Within the cuticle, the distribution of the matrix proteins is not random. Individual cuticular proteins exhibit unique distribution patterns in the laminae and inside the pore canals [6, 7, 21–23] indicating specific roles for these proteins in organization of the cuticular layers and pore canals.
Fig. (4). Ultrastructure of larval body and adult elytral cuticles of T. castaneum.
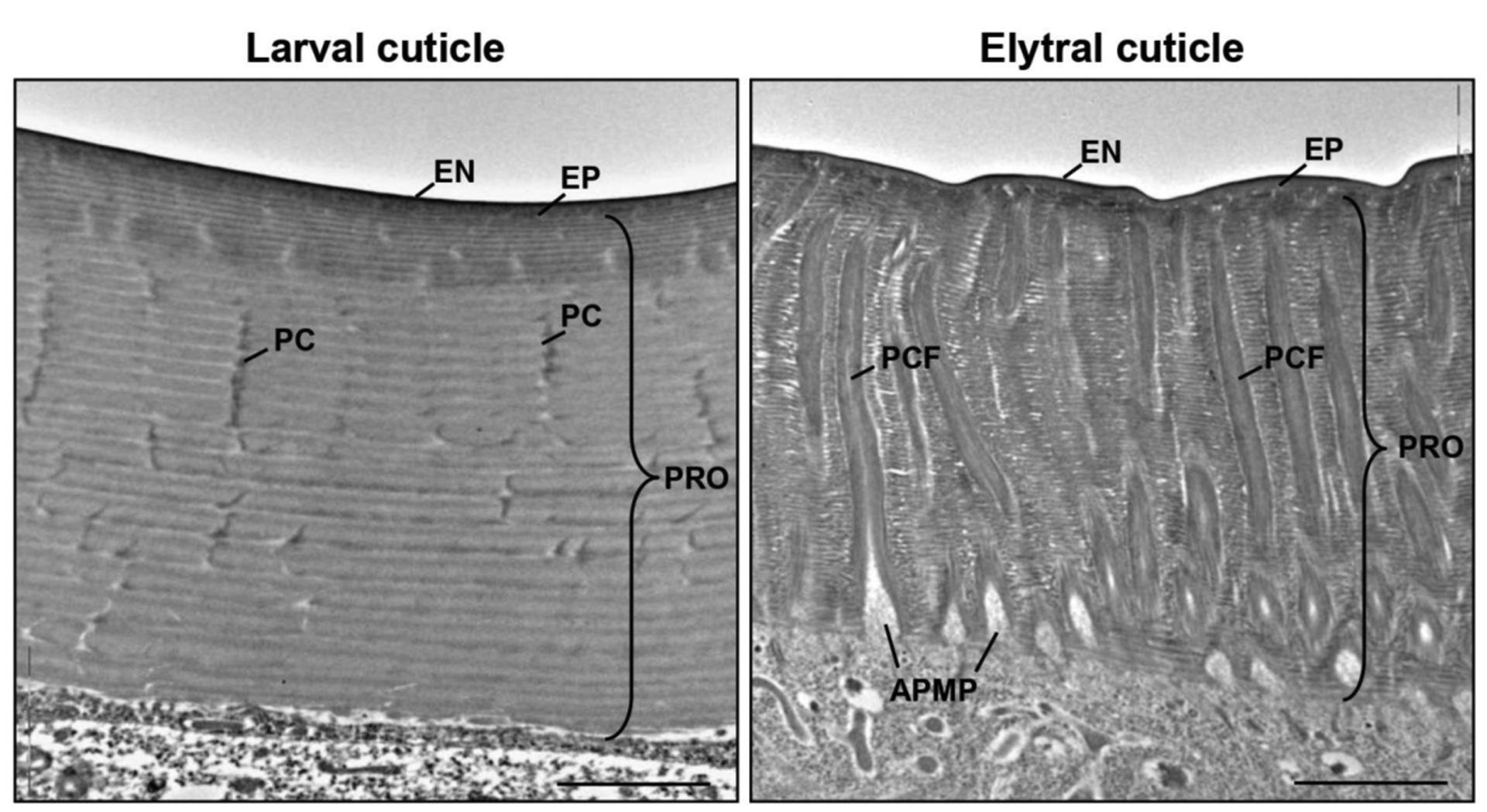
Both larval and elytral dorsal cuticle are composed of distinct layers including the envelope, epicuticle and procuticle. The procuticle consists of a number of horizontal, chitinous laminae. In addition, there are numerous pore canals running transverse to the laminae in elytral cuticle, as well as to the apical plasma membrane. The canals extend from the apical plasma membrane to the epicuticle region and contain a core of pore canal fibers. EN, envelope; EP, epicuticle; PRO, procuticle; PC, pore canal; PCF, pore canal fiber; APMP, apical plasma membrane protrusion. Scale bar = 2 μm. From Noh et al., (2014) [21].
1.4. Specialization in the Enzymes that Synthesize Chitin
A detailed study of genes encoding CHS in insects belonging to different orders indicated the presence of two paralogous genes in insect genomes [23–27]. Expression studies indicated that one Chs gene, Chs-A, was expressed specifically in cuticle forming tissues during the period when cuticle is being deposited. Interestingly, this gene was not expressed in midgut tissue, which instead expresses the paralogous Chs-B gene. Thus, there is specialization in the functions of the two forms of CHS, one designed for the production of cuticular chitin and the other for the production of PM-associated chitin. It is noteworthy that the chitin products of these two CHS associate with different groups of proteins and the chitin-protein composite matrices have different properties, most important being hydrophilicity and permeability [28,29].
1.5. Role of Chitin Synthase on Cuticle Structure and Function
The genes responsible for cuticular integrity were initially identified by screening mutants affecting cuticle patterning [30]. Mutations in a gene termed krotzkopf verkehrt (kkv) resulted in embryos with severely disrupted head cuticle. This mutation was later identified as a nonsense mutation in a gene encoding a protein homologous to chitin synthases characterized from sheep blowfly, mosquito, and tobacco hornworm [31–33] leading to a truncated CHS [34]. The depletion of transcripts for Chs-A by the administration of dsRNA resulted in reduced chitin content in the cuticle as detected by gold-labeled wheat germ agglutinin (Fig. 5) [34,35].
Fig. (5). Wheat germ agglutinin (WGA)-gold labeling TEM of dorsal elytral cuticle of T. castaneum.
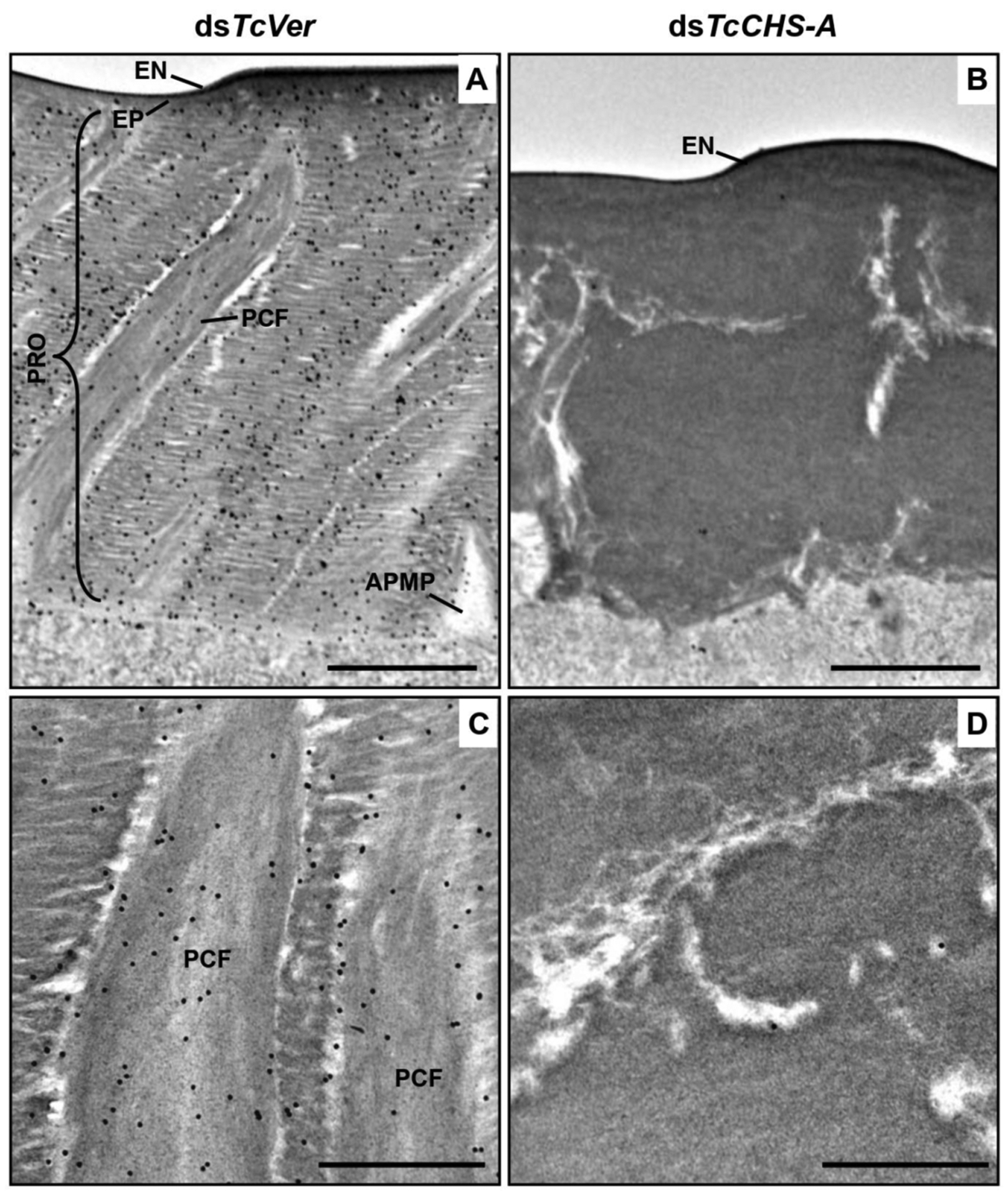
Ultra-thin sections (~90 nm) of pharate adults (5 d-old pupae) that were injected with dsTcVer (200 ng per insect) in late instar larvae were incubated with gold-labeled (10 nm) WGA to detect chitin in the dorsal side of elytral cuticle. dsRNA for chitin synthase-A (dsTcChs-A), which is required for cuticular chitin synthesis, was injected as a negative control. Chitin was detected in both horizontal laminae and vertical PCFs of elytral cuticle from dsTcVer-treated insects (A and C). Few or no gold particles were observed in TcChs-A-deficient insects (B and D). EN, envelope; EP, epicuticle; PRO, procuticle; PCF, pore canal fibers; APMP, apical plasma membrane protrusion. Scale bar in A and B = 1 μm and C and D = 500 nm. From Noh et al., (2015) [22].
The effect of depletion of CHS-A protein has been studied in several insects. In Drosophila melanogaster, mutants homozygous for the kkv gene exhibit a “blimp” embryonic phenotype upon devitellinization and failure of eggs to hatch [34, 35]. In several insects, depletion of either one or both of the alternatively spliced Chs-A transcripts either by oral administration or injection of Chs-A dsRNA results in a variety of effects, including molting arrest, mortality, loss of fecundity, failure of egg hatch, defective cuticle, greatly reduced chitin content, reduction in ovary size, degeneration of oocytes, and loss of embryonic desiccation resistance [26–28, 34, 35, 37–44]. Similar results were obtained when the insects were treated with the “chitin inhibitor”, diflubenzuron or the chitin synthase inhibitor nikkomycin [35, 45–47]. Thus, most of the effects described above could be explained as the consequence of reduced synthesis of chitin in the target tissues. CHS-A is the sole contributor to cuticle-associated chitin. A cuticle, and by implication, chitin is essential at all stages of development to protect the insect from mechanical injury and predation not only from the outside, but also internally as a component of cuticular linings of the foregut and the hindgut. The midgut, likewise, is protected from microbes and abrasive agents in the diet by the chitinous PM that prevents food from directly contacting the midgut lining cells. The most vulnerable periods in the life of an insect are the periods when the cuticle and/or the PM are being remodeled during each molt. Appropriately, the requirement for the type of cuticle or PM is tailored to the needs of the particular developmental stage and/or the anatomical location of the tissue.
1.6. The Chitinous Serosal Cuticle Protects the Egg from Desiccation
While the chorion of the eggshell protects the embryo during the early period after oviposition, even the eggshell has small amounts of chitin, suggesting a role for chitin even in the unfertilized egg [10, 48]. The production of a serosal cuticle containing chitin is critical for the prevention of desiccation and correlates with the expression of Chs-A transcript in Aedes aegypti [40]. Earlier during embryonic development, the serosal cells secrete a cuticle that completely covers the enlarging embryo and makes the egg resistant to dehydration stress. A comparative analysis of egg resistance to desiccation (ERD) in three different species of mosquitoes indicated a direct correlation between the chitin content of whole eggs and ERD and an inverse correlation with egg volume as well as eggshell surface density (weight/area) [49]. The serosal cuticle is multi-laminate, as are the larval and adult cuticles. The serosal cuticle seems to require the same assortment of enzymes and protein factors needed for chitin synthesis and its assembly into laminae as larval and adult cuticle. For example, a specific alternatively spliced transcript of Chs-A as well as the proteins, knickkopf, retroactive, and a laccase are required for the maturation of a laminar serosal cuticle [40–43, 47, 49].
1.7. Isoforms of CHS-A Exhibit Developmental and Tissue-specific Expression
A large majority of the Chs-A genes characterized by insects belonging to different orders have alternative exons [24–27, 50, 51], the exceptions being the water flea, Daphnea pulex and the pea aphid, Acyrthosiphon pisum. Two pairs of alternate exons have been described for the Chs-A gene, one near the 5’-end and the other close to the 3’-end. The alternate exon set closer the 5’-end has been described only in lepidopteran insects including Ostrinia furnacalis, Bombyx mori and Manduca sexta. In the well-studied case of O. furnacalis, the Chs-A gene gives rise to two transcripts differing in length due to the use of an alternate promoter located within the second intron, leading to the production of a protein that is missing nine N-terminal amino acids [50,51]. Treatment of fifth instar larvae of this insect with 20-hydroxyecdysone resulted in an earlier increase in transcripts with a specific combination of alternate exons indicating hormonal control of splicing [51]. In the putative promoter region, there are hormone response elements, consistent with the differential hormonal response. The other pair of alternate exons near the 3’-end code for two alternate stretches of exactly 59 amino acids that included a transmembrane segment. The sequences of the two encoded segments, while being similar, are easily distinguishable from the alternative form and show evolutionary conservation across several orders of insects indicating a biological necessity for the two alternate forms of this protein. Further, the relative abundance of these two isoforms of proteins varies during various developmental stages and in different tissues especially the trachea [24, 27, 53,54].
1.8. Chs-B Specializes in the Synthesis of PM-associated Chitin
The paralogous gene, Chs-B, is specifically expressed in the midgut tissue, but not in the epidermal tissues that secrete a cuticle [24, 53–56]. Down-regulation of Chs-B transcripts resulted in the loss of PM-associated chitin, growth failure, loss of integrity of the PM and mortality [38, 57–59]. It also led to increased susceptibility to chitin synthesis inhibitors and calcofluor [60]. These results indicate that CHS-B is uniquely involved in the synthesis of PM-associated chitin.
1.9. Deposition of Insect Cuticle is a Precisely Orchestrated Process
During each molt, insects synthesize a new cuticle and partially digest and shed the cuticle from the previous developmental stage as the exuvium. This process is crucial for growth and development, because the old cuticle is a physical constraint to insect growth. Ecdysis involves both the synthesis of chitin in the new procuticle as well as degradation of chitin (and protein) in the innermost parts of the old cuticle. The synthesis of the new cuticle begins with the deposition of a new outermost envelope layer above the epidermal cell, followed shortly by the appearance of the epicuticular layer [11, 61–64]. Neither the envelope nor the epicuticle has any detectable chitin and, therefore, is not expected to contribute to the rigidity of the cuticle (Fig. 6) [2, 11]. Later, the exocuticle followed by the endocuticle begins to form and takes on the appearance of alternating electron-dense and electron-lucent laminae visible in TEM images. The number of laminae in those cuticles varies depending on the stage of development as well as the location of the epidermal cells in the body plan. The endocuticle of beetles formed after adult eclosion is brick-like, Balken cuticle with unidirectional stacks of chitin. The cuticle is thick in areas such as the pronotum, leg cuticle, the elytral dorsal cuticle and the ventral abdomen, where rigidity is needed to protect against abrasive stress. Presumably, the higher overall content of chitin in the newly formed, thicker composite cuticle accounts for the greater rigidity compared to the cuticle of the earlier stage(s). However, the cuticle is thin in areas such as dorsal abdomen, hindwing and the elytral ventral cuticle of T. castaneum (Fig. 6) [11, 21].
Fig. (6). Schematic diagram of the ultrastructure of elytral cuticle of T. castaneum.
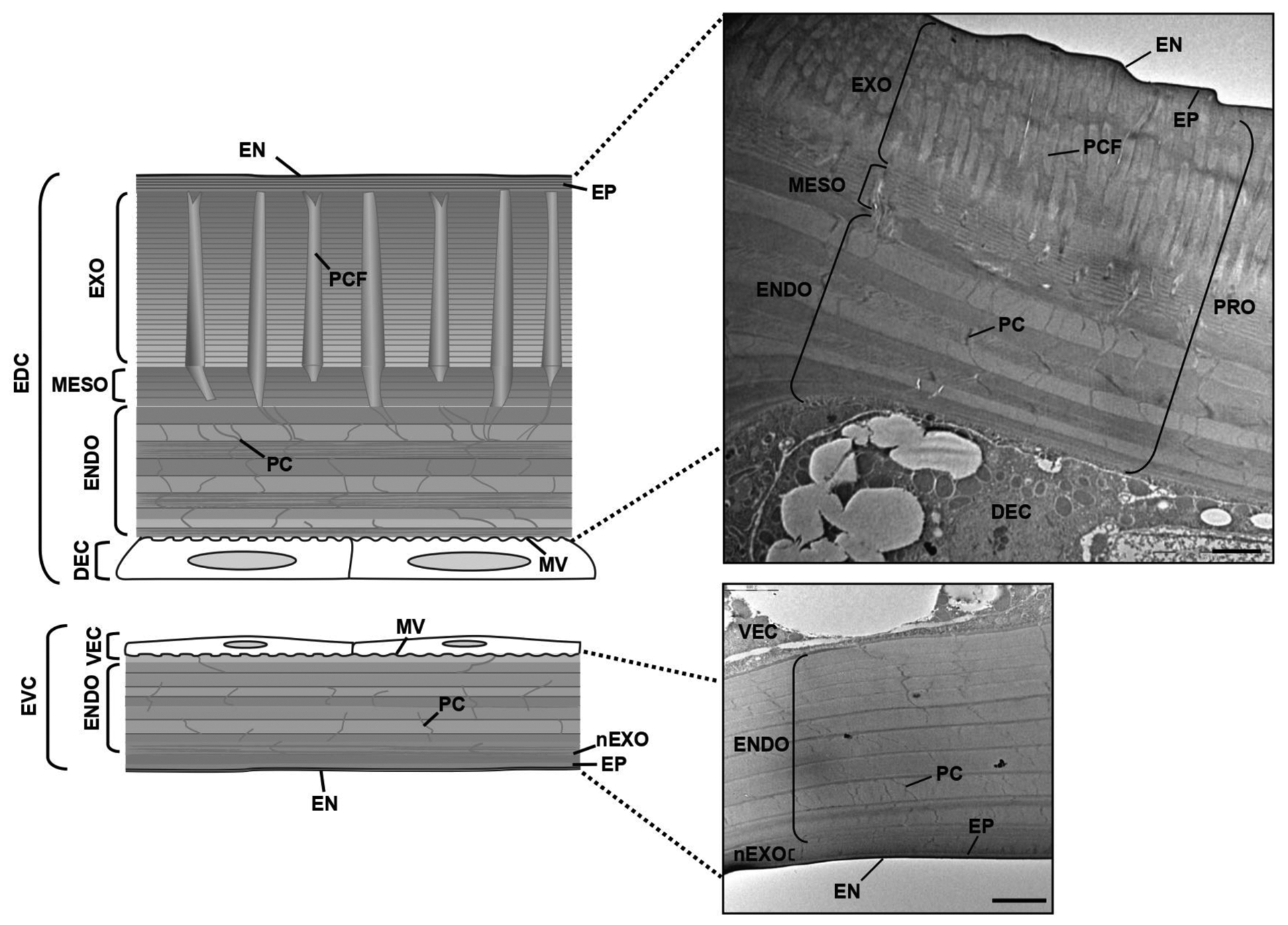
Ultrastructure of the rigid elytral dorsal cuticle (EDC) and flexible elytral ventral cuticles (EVC) of 10 d-old T. castaneum adult were analyzed by TEM. EN, envelope; EP, epicuticle; EXO, exocuticle; MESO, mesocuticle; ENDO, endocuticle; PC, pore canal; PCF, pore canal fiber; DEC, dorsal epidermal cell; VEC, ventral epidermal cell. Scale bar = 2 μm.
Significant differences in the chitin content have been reported between the rigid veins and the membranous portions of the wing in the cicada, Magicicada cassini [65]. The wing membrane of the locust, Schistocerca gregaria apparently lacks chitin [66]. The authors suggest that the wing consists of only the epicuticle, indicating that the chitin content can be tailored to match the function of the tissue. A comparative analysis of the cuticle architecture of several beetles and weevils with or without flight ability indicated that there is a trade-off between stability and weight requirements among them. Thus, a light-weight elytron with a minimal amount of hemolymph space between the dorsal and ventral elytral cuticles facilitates flight in some beetles, whereas weevils lacking flight can afford to add weight to their elytron and abdomen by having many compact chitinous layers as a defensive strategy [20].
1.10. Turnover of Chitin in the Old Cuticle
The digestion of the chitin in the old cuticle involves the participation of several enzymes and proteins. These include an assortment of proteolytic enzymes and chitinolytic enzymes present in the molting fluid that accumulates between the old cuticle and the new cuticle [67]. The chitin in the tanned old cuticle is embedded in a matrix of highly cross-linked proteins and is bound/protected by chitin binding proteins such as Knickkopf (KNK) [42], two families of cuticular proteins analogous to peritrophins with one family with a single chitin binding domain (CPAP1) and a second family with three chitin binding domains (CPAP3) [68–73]. The role of the proteins with R & R chitin binding motif has not been investigated extensively. Down regulation of CPAP1-H or Knickkopf proteins has been shown to result in a substantial decrease in chitin content by chemical/enzymatic analyses or by staining of elytra with fluorescently labeled chitin binding protein probes [42, 70]. The elytra from these chitin-depleted insects were fragile. In the case of KNK depletion, the reduction in chitin content could be reversed by simultaneous down regulation of a chitinase present in the newly forming cuticle indicating that KNK indeed offered protection from chitinolytic enzymes (Fig. 7) [42]. Several of these proteins including KNK and CPAP’s have been shown to bind tightly to chitin or chitosan [42, 71, 73]. These proteins may have to be stripped or degraded first to expose the core chitin crystallites in the old cuticle during the molting process. Some of the chymotrypsin-like enzymes involved in cuticle turnover during adult morphogenesis of T. castaneum have been identified by dsRNA-mediated downregulation of specific chymotrypsin-like enzymes suspected of having a role in molting by virtue of their presence in molting fluid [74]. Other enzymes were predicted by proteomics and transcriptomics analyses to have such a role [75–77]. A particularly exhaustive study of genes involved in molting was carried out by Zhang et al. [77]. These proteases were identified in molting fluid from B. mori by proteomic analysis and the corresponding orthologs were identified in the T. castaneum genome. RNAi of these genes in T. castaneum confirmed that downregulation of transcripts for many of these proteins interfered with the molting process. Thus, removal of the specific (or a majority of) proteins from the old cuticle is an essential process in the shedding of the old cuticle, which remains attached to the new cuticle. It appears that the expression of chitinolytic enzymes alone is not sufficient for shedding of old cuticle.
Fig. (7). TcKnk prevents degradation of chitin by molting fluid chitinases.
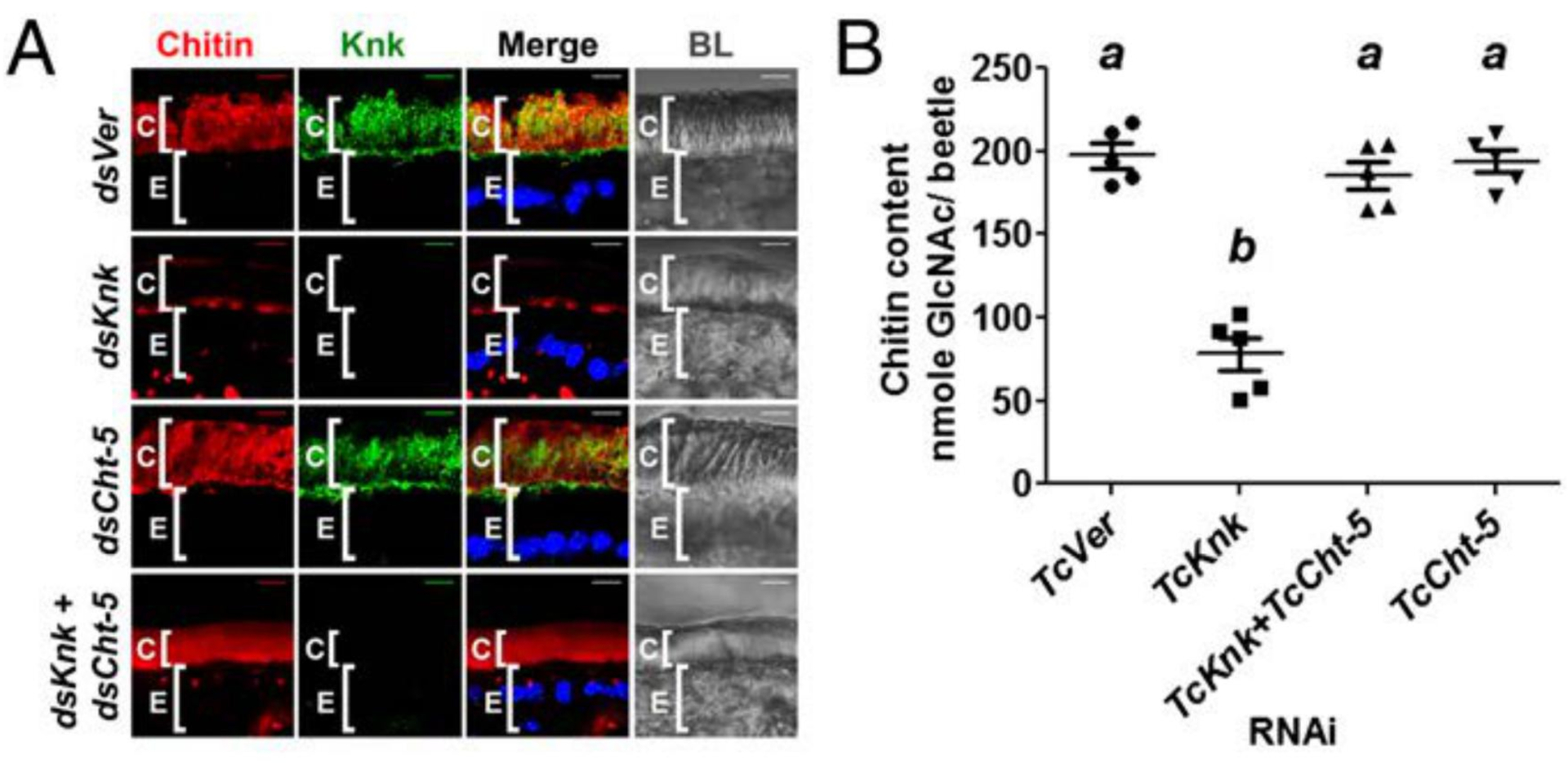
(A) Chitin depletion resulting from TcKnk RNAi was rescued after double RNAi for TcKnk and TcCht-5 (dsKnk + dsCht-5). Chitin, red; TcKnk, green; DAPI, blue; C, cuticle; E, epithelial cells. Scale bars = 5 μm. (B) Analysis of total chitin content by a modified Morgan-Elson assay. Data are reported as mean ± SE (n = 5 each). Statistical significance was computed with Student’s t-test. Means identified by different letters (a and b) are significantly different at P < 0.05. Modified from Chaudhari et al. [42].
1.11. Lytic Polysaccharide Monooxygenases (LPMOs) Assist in Cuticular Chitin Degradation
The digestion of crystalline chitin may also require the participation of a lytic polysaccharide monooxygenase (LPMO). Originally thought of as chitin binding proteins that assisted in hydrolysis of chitin, this class of proteins has now been classified as enzymes that have their own auxiliary oxidative cleavage of glycosidic bonds in many polysaccharides. They oxidize the C1, C4 or both carbon atoms of the sugars that participate in the α- or β−1,4 linkages of polysaccharides and require Cu(II) ions, an electron donor, and a source of oxygen. They are classified in the CAZy families named AA9-AA11 and AA13-AA16 with different carbohydrate substrate preferences [78–82]. The LPMOs can act on crystalline chitin and do not require water, which is a constraint for chitinolytic enzymes belonging to families 18 and 20 glycosylhydrolases (GH18 and GH20). In the process, new ends are generated in the substrate that are accessed by GH18 and GH20 enzymes. Once the individual chitin chain (or other carbohydrate chains) have become open i.e. decrystallized from the multi-chain, anti-parallel-chitin crystallites, the chitinolytic enzymes can digest the layers of chitin that are exposed to the molting fluid. This leads to the loosening of the cuticle from the newly formed envelope layer of the underlying epidermal cell.
1.12. Chitinolytic Enzymes Assist in Digestion of Old Cuticle
Two chitinases belonging to GH18 are important for molting. The chitinases implicated in degradation of chitin belong to groups I and group II [83–85]. Depletion of the transcripts for these chitinases by RNAi results in molting defects and failure to remove the old cuticle from the newly deposited cuticle (Fig. 8) [77, 86–88]. In addition, in lepidopteran insects, an additional chitinase belonging to group h has been found to coordinate chitin degradation in the cuticle [84, 121].
Fig. (8). Ultrastructure of pupal cuticle in TcCht-5-deficient pharate adult.
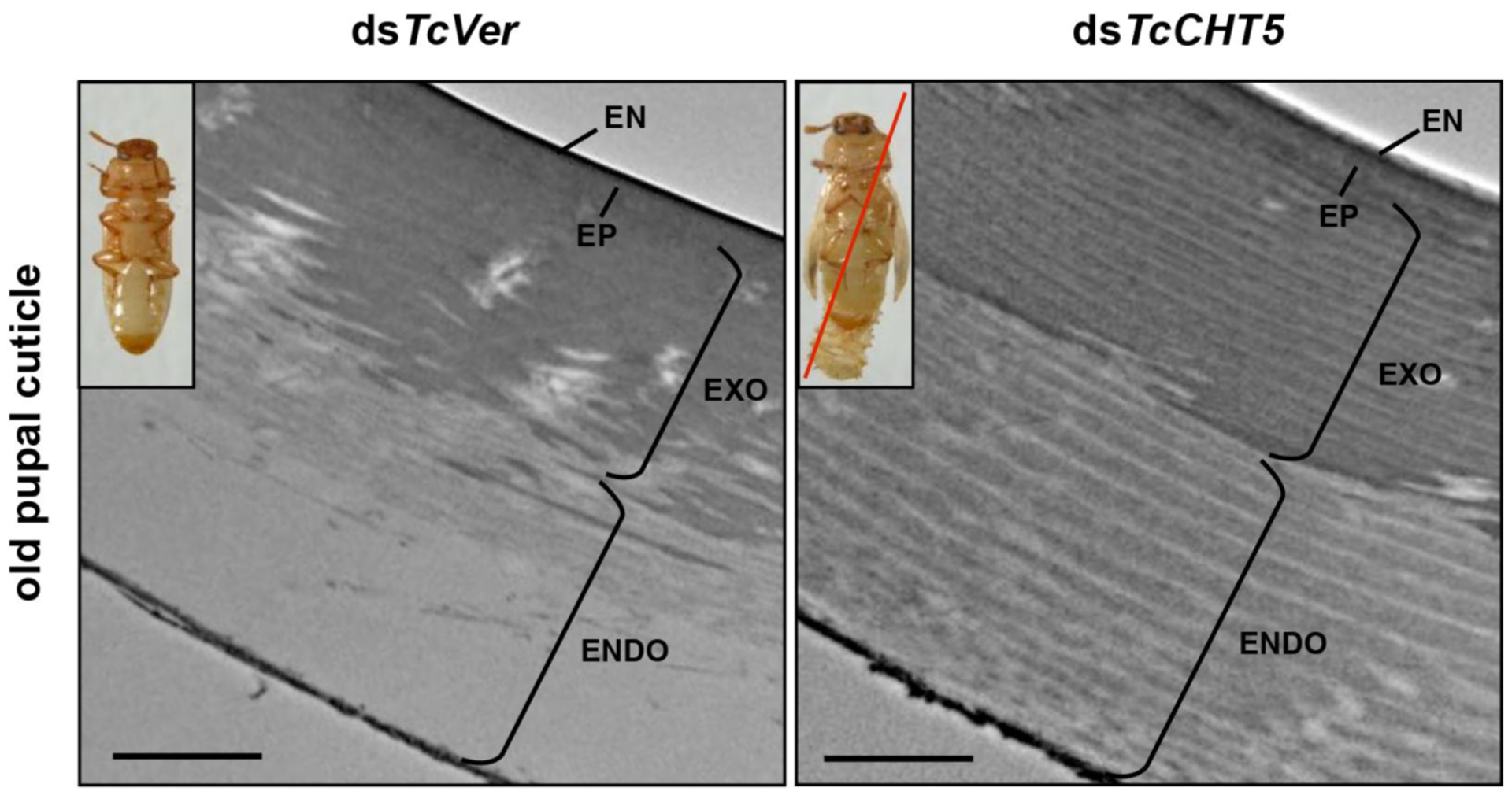
Ultrastructure of pupal cuticle of pharate adults (5-day-old pupae) that have been treated with dsTcCht-5 or dsTcVer was analyzed by TEM. The endocuticle of dsTcVer-control insects was degraded during adult eclosion. In contrast, TcCht-5-deficient insects caused failure of turnover the chitinous endocuticle layer, which led to lethal pupal-adult molting phenotype (inset in right panel). EN, envelope; EP, epicuticle; EXO, exocuticle; ENDO, endocuticle. Scale bar = 1 μm.
Similarly, RNAi of genes encoding GH20 enzymes also affects molting and leads to mortality [89–92]. Their function is to degrade chito-oligosaccharides released by the action of GH18 chitinases on chitin to the monomeric N-acetylglucosamines. In their absence, the accumulation of chitotriose and chito-tetraose results in inhibition of the activities of GH18 chitinases as a result of competitive inhibition at the substrate-binding site [93, 94]. The net result is the failure to digest chitin in the old cuticle and trapping of the insect in the old cuticle.
1.13. Role of Chitin Deacetylases in Chitin Organization in the Cuticle
While deacetylation of N-acetylglucosamines in naturally occurring chitin is well established, the precise distribution of glucosamine (GlcN) residues (random, alternating, or clustered at the non-reducing end or the reducing end) in the polymer is also unknown. Deacetylation is probably mediated by a family of proteins with chitin deacetylase (CDA)-like domains belonging to carbohydrate esterase family 4 (CE-4) [95, 96]. Chitin deacetylation is most likely coupled with chitin synthesis, with the nascent chains being the preferred substrates for CDAs [97]. In insects, the CDA family appears to have undergone expansion by acquiring additional protein domains including either a six-cysteine-containing chitin-binding domain belonging to the CBM14 family or a low-density lipoprotein receptor A (LDLa) domain or both [98–101]. Thus, some insect genomes have as many as nine genes encoding CDAs divided into as many as five groups. Additional complexity arises from the use of alternative splicing of some CDA transcripts giving rise to additional isozymes. There is tissue-specific expression as well as developmental regulation of their expression. Some of the insect CDAs appear to be essential for insect molting and survival. Mutations in two CDA-like genes of D. melanogaster, named serpentine and vermiform, resulted in abnormally shaped dorsal tracheal trunks during embryonic development [102, 103]. Similarly, depletion of transcripts for Cda1 or Cda2 during larval, pupal and adult molts of T. castaneum, Locusta migratoria, Choristoneura fumiferana and Leptinotarsa decemlineata results in molting arrest and mortality as well as the loss of laminar organization of the cuticle and pore canals integrity [104–109]. Overexpression of a CDA from Spodoptera frugiperda and S. exigua that is expressed in the gut resulted in increased susceptibility to a baculovirus [110]. The normal organization of cuticle consists of chitin-protein containing laminae that are parallel to the cell surface, interspersed with vertically oriented pore canals. The cuticle assumes abnormal shapes when chitin biosynthetic enzymes or chitin modifying enzymes such as CDA are deficient. Further, when the levels of some isoforms of CDA proteins are depleted by RNAi, the insects also exhibit defective locomotion [104, 109]. How chitin deacetylase family proteins affect the shape and architecture of insect cuticle as well as its locomotion has not been explored in detail till now.
1.14. Group I Chitin Deacetylases are Essential for the Maintenance of Cuticle Integrity in T. castaneum.
The T. castaneum genome contains nine genes with CDA domains. These genes have been divided into five groups based on phylogenetic analysis and the presence of different protein domains [98, 101]. Group I enzymes contain three identifiable domains including a chitin binding domain (CAZy, CBM family 14), a low-density lipoprotein receptor A (LDLa) domain and carbohydrate esterase family 4 (CE-4) (CDA-like) domain. CDA1 and CDA2, which belong to this group, share extensive sequence similarities and are important for molting and cuticle organization in numerous insects [104–109]. The depletion of transcripts for these two genes in T. castaneum by administration of either dsRNA alone or both together in the prepupal stages results in failure to molt to the adult stage and mortality as pharate adults (Fig. 9A). Analyses of the elytra by TEM indicate variable defects in cuticle arrangement including loss of the parallel laminar arrangement [109], helicoidal organization [106], reduction in chitin and cuticle thickness [107] or loss of structural integrity of the pore canals that traverse the length of the procuticle as shown in Fig. 10 [109]. The effects are more pronounced when both transcripts are depleted at the same time.
Fig. (9). Loss of function phenotypes produced by RNAi for TcCDAs.
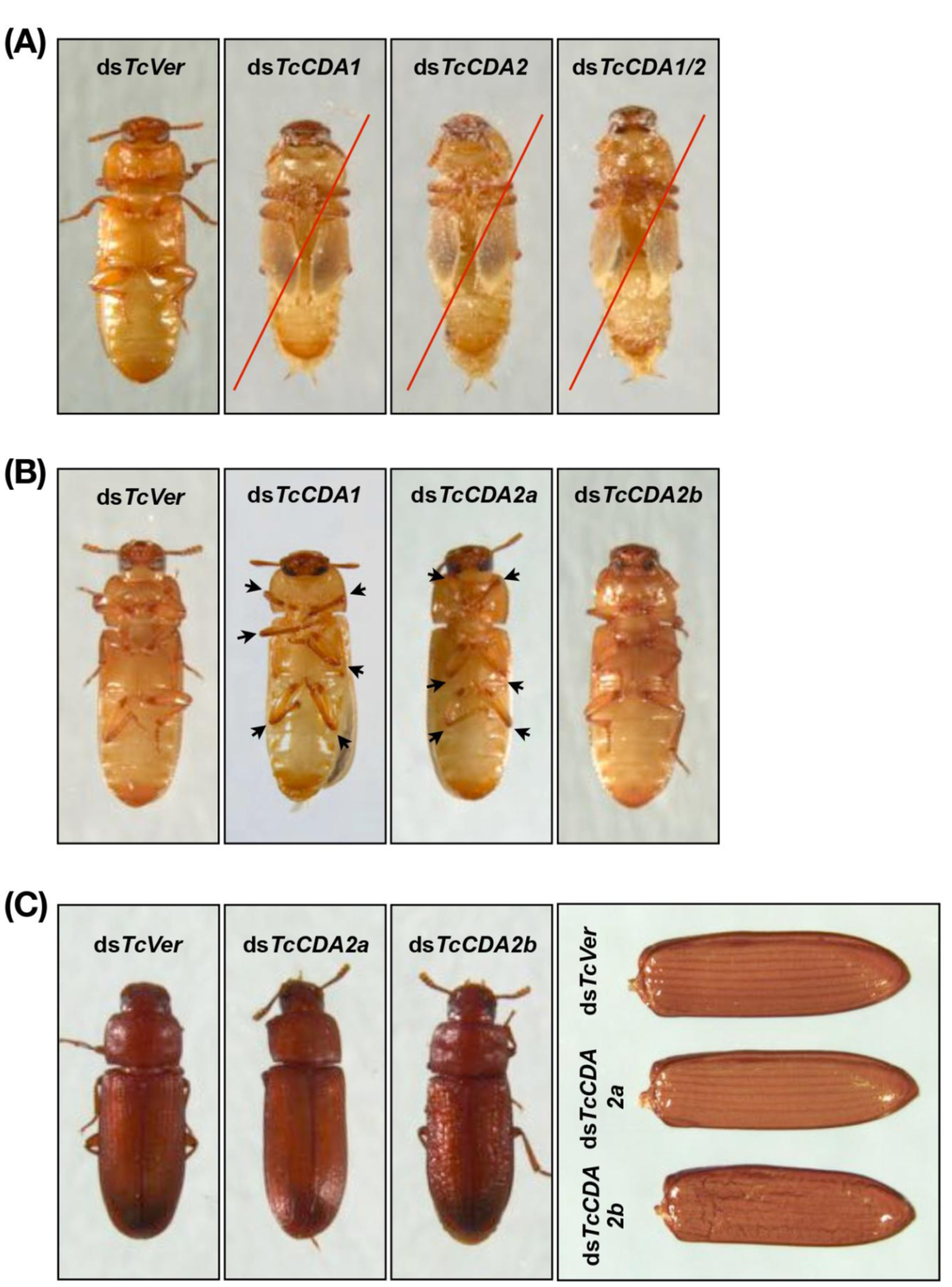
Insects treated with dsTcCDA1, dsTcCDA2 or dsTcCDA1/2 (200 ng per each) failed adult eclosion. They did not shed their old pupal cuticle and died (red lines). (B) RNAi of TcCDA2a or TcCDA2b did not prevent adult eclosion. However, the resulting TcCDA2a-deficient adults were unable to move their femoral-tibial joints (arrows), while the dsTcCDA2b-treated adults exhibited roughened and wrinkled elytra and pronotum (C). Note that ~60% pupae treated with 100 pg of dsTcCDA1 did molt to adults. However, the resulting adults exhibit a defect in movement of femoral-tibial joints (arrows in B) similar to that observed in TcCDA2a-deficient adults.
Fig. (10). Ultrastructure of rigid elytral dorsal cuticles after RNAi for TcCDAs.
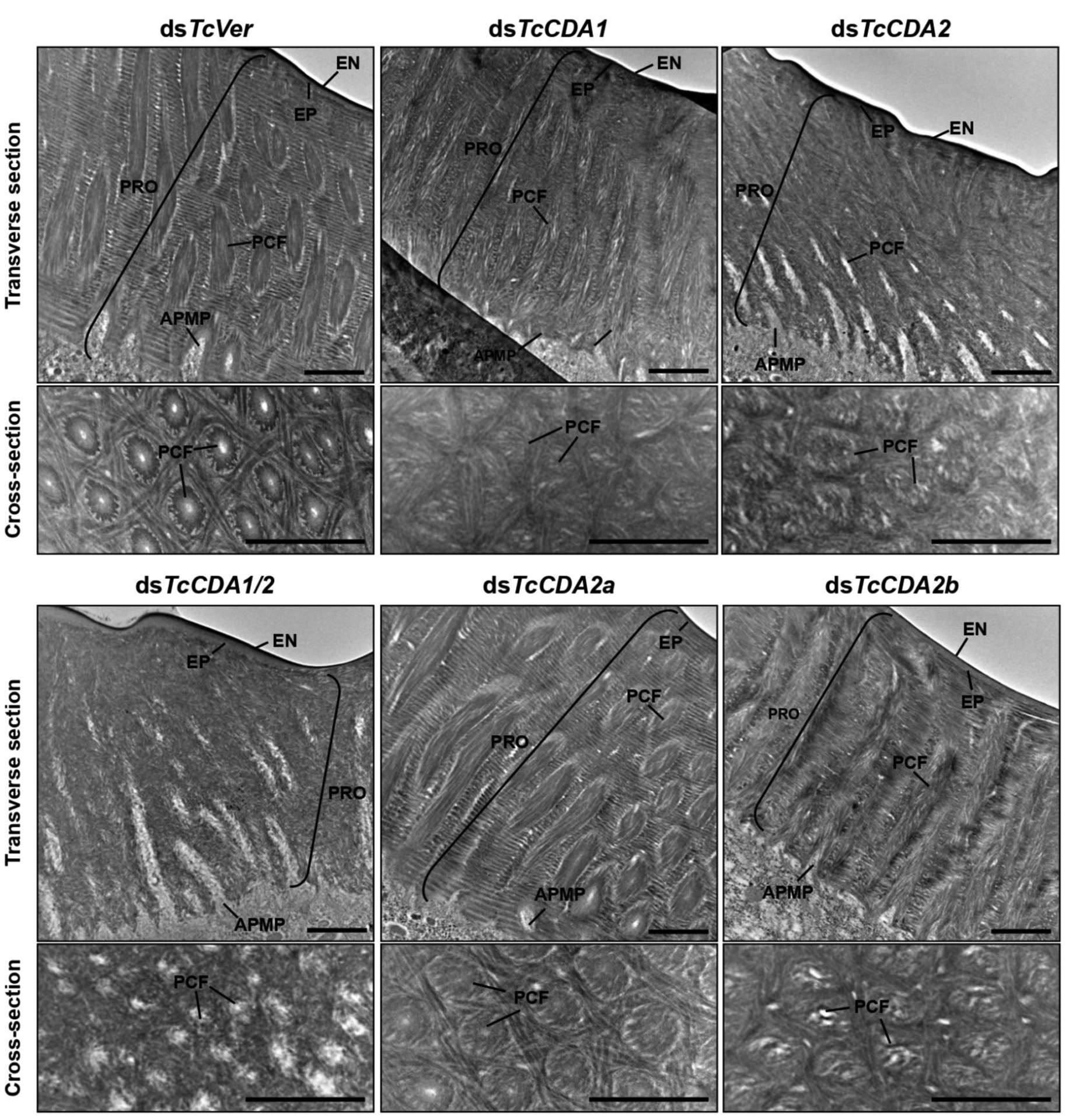
Ultrastructure of elytral dorsal cuticle from pharate adults (5-day-old pupae) that had been treated with dsTcCDA1, dsTcCDA2, dsTcCDA1/2, dsTcCDA2a, dsTcCDA2b or dsTcVer was analyzed by TEM. Cross-sections were made parallel to the epidermal cell surface. EN, envelope; EP, epicuticle; PRO, procuticle; PCF, pore canal fiber; APMP, apical plasma membrane protrusion. Scale bar = 1 μm.
1.15. Depletion of TcCDA2a Results in Defective Locomotion
The gene encoding T. castaneum TcCDA2 has two alternative exons for exon 3, leading to the production of two isozyme forms of this protein, TcCDA2a and TcCDA2b. The depletion of specific alternatively spliced transcripts of TcCDA2 by RNAi using dsRNA specific for exon 3a or exon 3b at the prepupal stage does not result in lethality at the adult molt transition (Fig. 9B). The elytra do have the chitin-protein laminae and vertical pore canals, though some minor alterations in cuticular architecture can be discerned (Fig. 10). Depletion of only the CDA2a transcript results in adults that exhibit walking defects and the inability to bend/unbend femoral/tibial joints (Fig. 9B). The depletion of TcCDA2b transcript results in wrinkled elytra (Fig. 9C) [104]. While the structural defects in elytra, such as wrinkling, is consistent with the observable defects in the cuticle, the inability to move limb joints was not easily explained.
1.16. Mutations that Result in Defective Locomotion
One possible explanation for defective locomotion is that extra cellular matrix (ECM) defects result in defective attachment of the insect cuticle with muscles of limbs. Several mutations in D. melanogaster that exhibit defective cuticle attachment to muscles have been described. Among them are myospheroid (βPS integrin), Thrombospondin, Tiggrin, fondue and talin mutants, which lead to the detachment of muscles from the ECM or from neighboring muscles [111–115]. These genes encode for either integrins, ligands of integrins or extracellular proteins that promote the adhesion of membrane-bound integrins on tendon cells with their cognate interacting partner integrins on muscle cell membranes. Integrins are responsible for the adhesion of cells and the strength of such adhesion is determined by the number of partner integrins on opposing cell types. The strength of integrin binding interactions is further modulated by ligands secreted by either muscle or tendon cells into the extracellular space. Intracellular or membrane-associated proteins such as Talin can further influence this process by interacting with the cytosolic tails of integrins and concentrating the integrin molecules at adhesion sites and by interactions with cytoskeletal actin network [116].
1.17. Muscle and Tendons Develop Coordinately During Adult Limb Morphogenesis
The development of D. melanogaster adult muscles occurs in coordination with the development of internal tendons in all leg segments [117]. These internal tendons are derived from a specialized group of epithelial domains of tendon precursors. These cells invaginate the leg disc and develop in close association with leg muscle founder cells that fuse with fusion-competent myoblasts. The tendons and myotubes develop in a coordinated fashion and regulate each other’s development. Muscle cells are closely associated with tendon cells during normal leg development at the pupal stage and this association is disrupted in tendon cells of mutants that express a defective form of Stripe, a transcription factor that regulates tendon development in dorsal femur of D. melanogaster (Fig. 11) [117]. These tendon-associated muscle cells become precursors of syncytial muscle fibers and give rise to myotubes that grow into myofibers and eventually attach to apodemes (muscle attachment sites) that differentiate from the leg epithelium. This attachment completes a physical connection between the leg cuticle and the internal tendon via muscle fibers capable of conveying the power of muscle contraction to the exoskeleton.
Fig. (11). Disrupting apodeme development affects myoblast spatial organization.
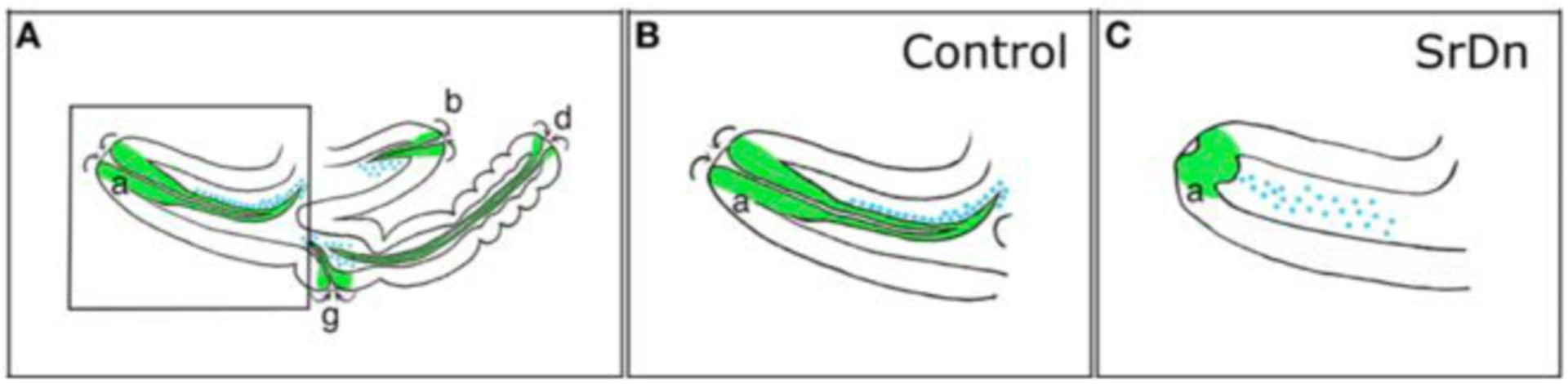
Drawings of sagittal views at 5 h after pupae formation (APF) of a whole wild-type leg disc (A) with a focus on the tibia levator tendon/apodeme (a) of the dorsal femur segment (B) and a leg disc for which apodeme invagination in the dorsal femur was affected (C). Only some apodemes (green) and their associated myoblasts (blue) are represented. Note that their invagination (curved arrows) goes onto form a lumen. (a) tibia levator tendon in dorsal femur (associated muscle: tibia levator muscle), (b) tibia depressor tendon in ventral femur (associated muscle: tibia depressor muscle), (d) long tendon in tarsus (associated muscles: long tendon muscle 1, tarsus reductor muscles 1 and 2), (g) tarsus levator tendon in dorsal tibia (associated muscle: tarsus levator muscle). From Soler et al. (2016) [117]. With permission from Dr. Soler.
1.18. Tendon Cells and Muscle Cell Membranes Interdigitate to form Tight Connections
To determine whether cuticle plays any role in muscle attachment to the cuticle, we analyzed TEM sections T. castaneum leg segments at the 10-d old adult stage. At muscle attachment sites (apodemes), we looked for interactions between the leg epithelial cells and muscle cells. As shown in Fig. 12 top panels, the apodeme cells below the coxal cuticle form numerous interdigitations of membrane processes emanating from both the adjoining epidermal and muscle cells. We found similar interactions between apodemes and muscles cells in D. melanogaster thoracic muscle attachment sites (Fig. 12 lower panel).
Fig. (12). Morphology of muscle attachement sites in T. castaneum and D. melanogaster.
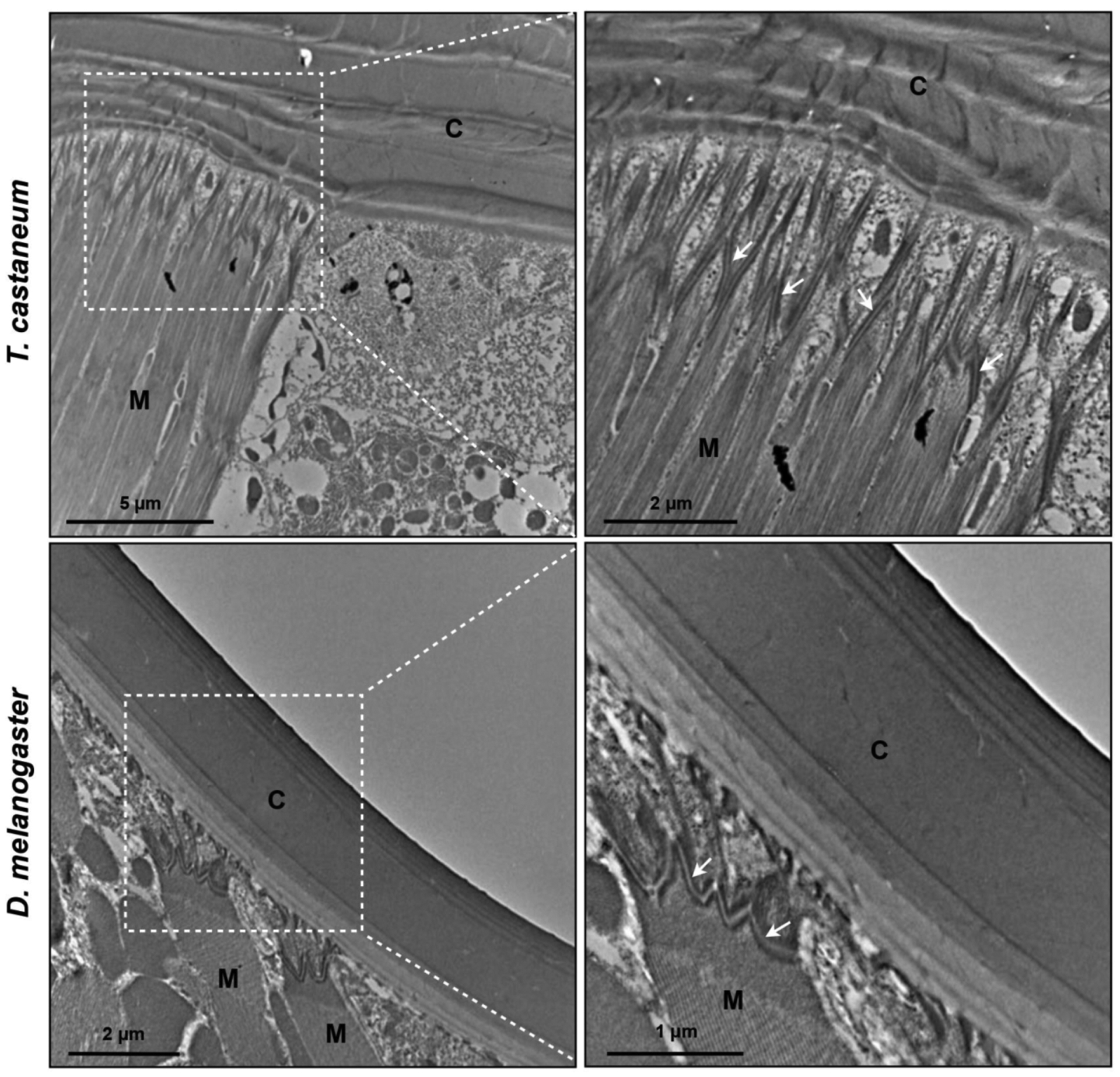
Morphology and interaction between the apodeme cells and muscle cells of the leg segments from 10-day-old T. castaneum adult and the dorsal thoracic cuticle in mature D. melanogaster adult were analyzed by TEM. Arrows indicate interdigitations between apodemes and muscle cell. C, cuticle; M, muscle.
1.19. Loss of Proteins that Stabilize Adhesion Results in Muscle Detachment
Mutations involving subunits of integrins that promote tendon-muscle adhesions result in the detachment of muscles and compromise locomotion. Loss of a coagulation protein, Fondue, that accumulates at muscle-tendon junctions and muscle-muscle junctions, results in defective locomotion, detached muscles, as well as a loss of membrane interdigitation between adjacent tendon and muscle cells (Fig. 13) [114]. Other proteins that accumulate at these muscle attachment sites include Tiggrin, Larval serum protein 1ɣ and Thrombospondin. Mutations or loss of any of these proteins leads to varying degrees of muscle detachment, decreased locomotion, and lethality at various stages.
Fig. (13). Loss of Fon alters cuticle integrity, tendon cell cytoarchitecture, and ECM accumulation.
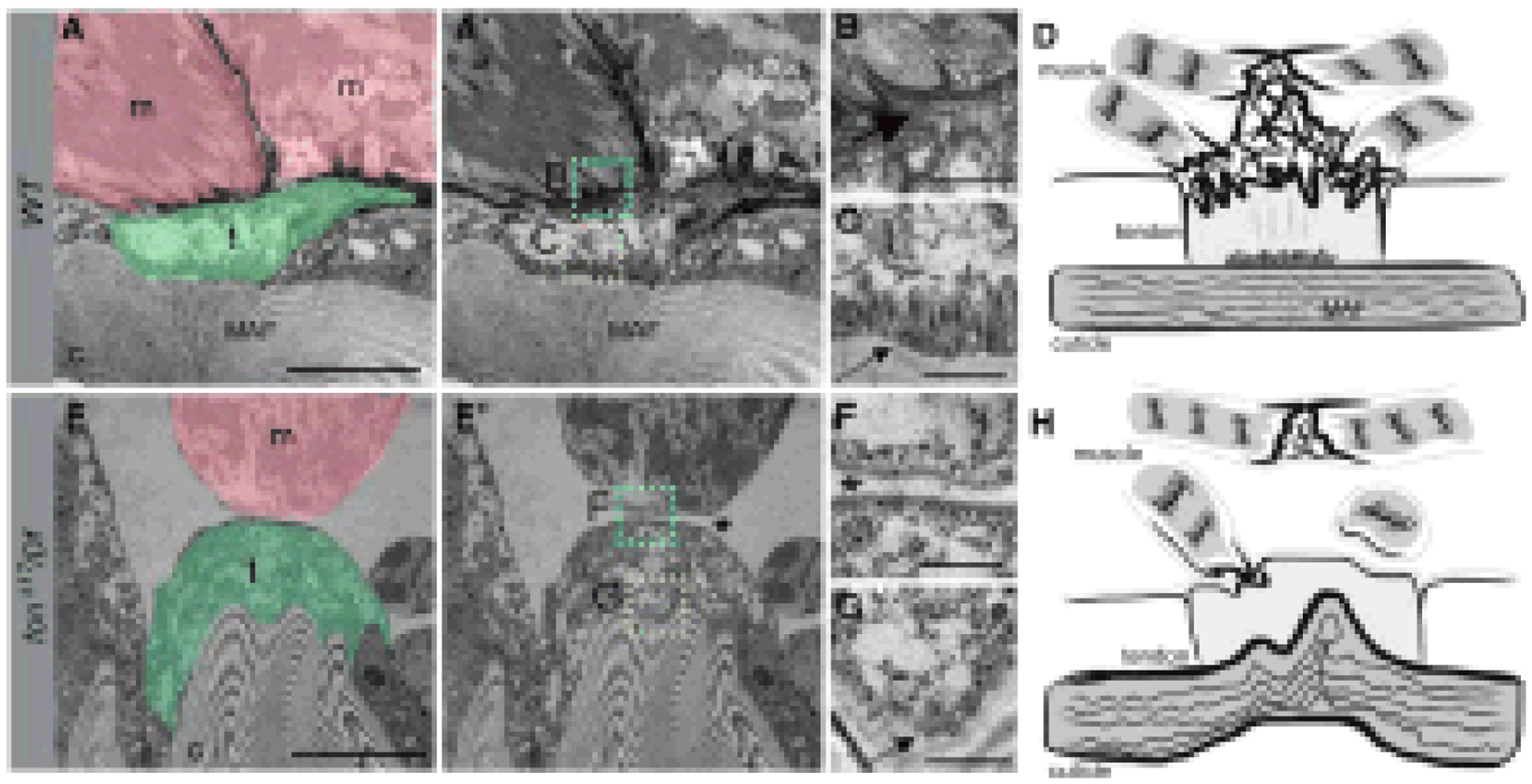
(A-C and E-G) TEMs of an indirect muscle-tendon attachment site in WT (A-C) or fonD17/Df mutants (E-G). (A and A’) Muscles (m; pink) are interlaced with tendon cells (t; green) at control myotendinous junctions. The insets correspond to high magnification images in B (cyan) and C (yellow). (B) An electron-dense ECM is observed between the sarcolemma and tendon cell membranes (large arrow). (C) Apical junctions present at the base of tendon cells (small arrow) are associated with muscle attachment fibers (A and A’) that extend into the cuticle. (D) Generalized schematic representation of WT muscle attachment. (E and E’) The detached muscle (m; pink) in this fon mutant remains close to the tendon cell (t; green), which is attached to a highly convoluted cuticle (c). The insets correspond to close-up images in F (cyan) and G (yellow). (F) There is a loss of electron-dense ECM material and membrane interdigitation between the muscle and tendon cell (asterisk). (G) Note the absence of tendon cell junctional complexes at the base of the tendon cell (G, small arrow). (H) Illustration reveals the dramatic loss of muscle attachment, ECM accumulation, and morphological abnormalities associated with mutations in fon. Bars, 10 μm for A and E; 1 μm for B, C, F, and G. From Green et al. [114].
1.20. F-actin and Microtubule Arrays Complete the Tight Linkage Between the ECM and Muscles
The D. melanogaster tendon cells contain numerous stable F-actin arrays that overlap with arrays of microtubules that extend apicobasally and constitute cytoskeletal arrays that connect with the cuticle on the apical side and with muscle tips on the basal side [118]. Thus, tendon cells act as bridge cells that connect two extracellular matrices (cuticle and the basal matrix of tendon cells). On the basal side, membrane processes of tendon cells interdigitate with similar propcesses emanating from tips of muscle cells [114, 118–120]. The F-actin-containing cytoskeletal arrays may strengthen the tendon cells to withstand stress caused by the contraction of attached muscles and to convey force to the rigid cuticle, thus aiding locomotion of the insect. We anticipate that factors that affect the attachment of the tendon cell F-actin-microtubule arrays to the chitin in the cuticle may interfere with locomotion. Our finding that chitin deacetylases influence locomotion indicates a role for this protein in maintaining the rigidity of tendon cells and their ability to attach to muscles that underlie them.
CONCLUSION
We conclude that the cuticle plays an essential role in determining the shape as well as the mechanical and chemical properties of the exoskeleton, which is critical for the survival and function of the whole animal, including locomotion. Many of the genes involved in chitin metabolism are critical for the structural integrity of the exoskeleton, which covers the entire body plan and therefore, influences the development and survival of the insect. One of the unexplored areas is how the exoskeleton is tied to muscle function. CDA deficiency and/or insufficient deacetylation of chitin in the cuticle near muscle attachment sites is likely to interfere with the adhesion of muscles with tendons. This, in turn, may lead to muscle fiber detachment starting from the extracellular cuticular matrix all the way to where the muscle fibers attach to internal tendons. Thus, the ability to convey the power of muscle contraction to the exoskeleton is affected, leading to defective locomotion. Additional studies are needed to elucidate the exact nature of the interaction between chitin/chitosan and integrin and/or other components that are critical for muscle attachment to the cuticle
FUNDING
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2018R1A2B6005106) to YA and Basic Science Research Program through the NRF funded by the Ministry of Education (NRF-2015R1A6A3A04060323) to MYN.
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].Neville AC, Parry DA, Woodhead-Galloway J. The chitin crystallite in arthropod cuticle. J Cell Sci 1976; 21(1): 73–82. [DOI] [PubMed] [Google Scholar]
- [2].Fabritius HO, Sachs C, Triguero PR, Roobe D. Influence of structural principles on the mechanics of a biological fiber-based composite material with hierarchical organization: the exoskeleton of the lobster Homarus americanus. Adv Mater 2009; 21: 391–400. 10.1002/adma.200801219 [DOI] [Google Scholar]
- [3].Rebers JE, Willis JH. A conserved domain in arthropod cuticular proteins binds chitin. Insect Biochem Mol Biol 2001; 31(11): 1083–93. 10.1016/S0965-1748(01)00056-X [DOI] [PubMed] [Google Scholar]
- [4].Jasrapuria S, Arakane Y, Osman G, Kramer KJ, Beeman RW, Muthukrishnan S. Genes encoding proteins with peritrophin A-type chitin-binding domains in Tribolium castaneum are grouped into three distinct families based on phylogeny, expression and function. Insect Biochem Mol Biol 2010; 40(3): 214–27. 10.1016/j.ibmb.2010.01.011 [DOI] [PubMed] [Google Scholar]
- [5].Rudall KM, Kenchington W. The chitin system. Biol Rev Camb Philos Soc 1973; 49: 597–636. 10.1111/j.1469-185X.1973.tb01570.x [DOI] [Google Scholar]
- [6].Zhou Y, Badgett MJ, Bowen JH, Vannini L, Orlando R, Willis JH. Distribution of cuticular proteins in different structures of adult Anopheles gambiae. Insect Biochem Mol Biol 2016; 75: 45–57. 10.1016/j.ibmb.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vannini L, Willis JH. Localization of RR-1 and RR-2 cuticular proteins within the cuticle of Anopheles gambiae. Arthropod Struct Dev 2017; 46(1): 13–29. 10.1016/j.asd.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dittmer NT, Hiromasa Y, Tomich JM, et al. Proteomic and transcriptomic analyses of rigid and membranous cuticles and epidermis from the elytra and hindwings of the red flour beetle, Tribolium castaneum. J Proteome Res 2012; 11(1): 269–78. 10.1021/pr2009803 [DOI] [PubMed] [Google Scholar]
- [9].Kramer KJ, Muthukrishnan S. Chitin metabolism in insects Comprehensive Molecular Insect Science. Oxford, UK: Elsevier; 2005; pp. 111–44. 10.1016/B0-44-451924-6/00051-X [DOI] [Google Scholar]
- [10].Moreira MF, Dos Santos AS, Marotta HR, et al. A chitin-like component in Aedes aegypti eggshells, eggs and ovaries. Insect Biochem Mol Biol 2007; 37(12): 1249–61. 10.1016/j.ibmb.2007.07.017 [DOI] [PubMed] [Google Scholar]
- [11].Noh MY, Muthukrishnan S, Kramer KJ, Arakane Y. Development and ultrastructure of the rigid dorsal and flexible ventral cuticles of the elytron of the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol 2017; 91: 21–33. 10.1016/j.ibmb.2017.11.003 [DOI] [PubMed] [Google Scholar]
- [12].Cohen E Chitin biochemistry: synthesis, hydrolysis and inhibition. Adv Insect Physiol 2010; 38: 5–74. 10.1016/S0065-2806(10)38005-2 [DOI] [Google Scholar]
- [13].Carlstrom D The crystal structure of alpha-chitin (poly-N-acetyl-D-glucosamine). J Biophys Biochem Cytol 1957; 3(5): 669–83. 10.1083/jcb.3.5.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Minke R, Blackwell J. The structure of alpha-chitin. J Mol Biol 1978; 120(2): 167–81. 10.1016/0022-2836(78)90063-3 [DOI] [PubMed] [Google Scholar]
- [15].Sikorski P, Hori R, Wada M. Revisit of alpha-chitin crystal structure using high resolution X-ray diffraction data. Biomacromolecules 2009; 10(5): 1100–5. 10.1021/bm801251e [DOI] [PubMed] [Google Scholar]
- [16].Beckham GT, Crowley MF. Examination of the α-chitin structure and decrystallization thermodynamics at the nanoscale. J Phys Chem B 2011; 115(15): 4516–22. 10.1021/jp200912q [DOI] [PubMed] [Google Scholar]
- [17].Bouligand Y Twisted fibrous arrangements in biological materials and cholesteric mesophases. Tissue Cell 1972; 4(2): 189–217. 10.1016/S0040-8166(72)80042-9 [DOI] [PubMed] [Google Scholar]
- [18].Cheng L, Wang LY, Karlsson AM. Mechanics-based analysis of selected features of the exoskeletal microstructure of Popillia japonica. J Mater Res 2009; 24: 3253–67. 10.1557/jmr.2009.0409 [DOI] [Google Scholar]
- [19].Barbakadze N, Enders S, Gorb S, Arzt E. Local mechanical properties of the head articulation cuticle in the beetle Pachnoda marginata (Coleoptera, Scarabaeidae). J Exp Biol 2006; 209(Pt 4): 722–30. 10.1242/jeb.02065 [DOI] [PubMed] [Google Scholar]
- [20].van de Kamp T, Riedel A, Greven H. Micromorphology of the elytral cuticle of beetles, with an emphasis on weevils (Coleoptera: Curculionoidea). Arthropod Struct Dev 2016; 45(1): 14–22. 10.1016/j.asd.2015.10.002 [DOI] [PubMed] [Google Scholar]
- [21].Noh MY, Kramer KJ, Muthukrishnan S, Kanost MR, Beeman RW, Arakane Y. Two major cuticular proteins are required for assembly of horizontal laminae and vertical pore canals in rigid cuticle of Tribolium castaneum. Insect Biochem Mol Biol 2014; 53: 22–9. 10.1016/j.ibmb.2014.07.005 [DOI] [PubMed] [Google Scholar]
- [22].Noh MY, Muthukrishnan S, Kramer KJ, Arakane Y. Tribolium castaneum RR-1 cuticular protein TcCPR4 is required for formation of pore canals in rigid cuticle. PLoS Genet 2015; 11(2)e1004963 10.1371/journal.pgen.1004963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mun S, Noh MY, Dittmer NT, et al. Cuticular protein with a low complexity sequence becomes cross-linked during insect cuticle sclerotization and is required for the adult molt. Sci Rep 2015; 5: 10484 10.1038/srep10484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Arakane Y, Hogenkamp DG, Zhu YC, et al. Characterization of two chitin synthase genes of the red flour beetle, Tribolium castaneum, and alternate exon usage in one of the genes during development. Insect Biochem Mol Biol 2004; 34(3): 291–304. 10.1016/j.ibmb.2003.11.004 [DOI] [PubMed] [Google Scholar]
- [25].Ashfaq M, Sonoda S, Tsumuki H. Developmental and tissue-specific expression of CHS1 from Plutella xylostella and its response to chlorfluazuron. Pestic Biochem Physiol 2007; 89: 20–30. 10.1016/j.pestbp.2007.02.004 [DOI] [Google Scholar]
- [26].Wang Y, Fan HW, Huang HJ, et al. Chitin synthase 1 gene and its two alternative splicing variants from two sap-sucking insects, Nilaparvata lugens and Laodelphax striatellus (Hemiptera: Delphacidae). Insect Biochem Mol Biol 2012; 42(9): 637–46. 10.1016/j.ibmb.2012.04.009 [DOI] [PubMed] [Google Scholar]
- [27].Yang WJ, Xu KK, Cong L, Wang JJ. Identification, mRNA expression, and functional analysis of chitin synthase 1 gene and its two alternative splicing variants in oriental fruit fly, Bactrocera dorsalis. Int J Biol Sci 2013; 9(4): 331–42. 10.7150/ijbs.6022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Muthukrishnan S, Merzendorfer H, Arakane Y, Yang Q. Chitin metabolic pathways in insects and their regulation Extracellular composite matrices in arthropods. Switzerland: Springer; 2016; pp. 31–65. 10.1007/978-3-319-40740-1_2 [DOI] [Google Scholar]
- [29].Merzendorfer H Insect chitin synthases: a review. J Comp Physiol B 2006; 176(1): 1–15. 10.1007/s00360-005-0005-3 [DOI] [PubMed] [Google Scholar]
- [30].Jürgens G, Wieschaus E, Nüsslein-Volhard C, Kluding H. Mutations affecting the pattern of the larval cuticle inDrosophila melanogaster: II. Zygotic loci on the third chromosome. Wilehm Roux Arch Dev Biol 1984; 193(5): 283–95. 10.1007/BF00848157 [DOI] [PubMed] [Google Scholar]
- [31].Tellam RL, Vuocolo T, Johnson SE, Jarmey J, Pearson RD. Insect chitin synthase cDNA sequence, gene organization and expression. Eur J Biochem 2000; 267(19): 6025–43. 10.1046/j.1432-1327.2000.01679.x [DOI] [PubMed] [Google Scholar]
- [32].Ibrahim GH, Smartt CT, Kiley LM, Christensen BM. Cloning and characterization of a chitin synthase cDNA from the mosquito Aedes aegypti. Insect Biochem Mol Biol 2000; 30(12): 1213–22. 10.1016/S0965-1748(00)00100-4 [DOI] [PubMed] [Google Scholar]
- [33].Zhu YC, Specht CA, Dittmer NT, Muthukrishnan S, Kanost MR, Kramer KJ. Sequence of a cDNA and expression of the gene encoding a putative epidermal chitin synthase of Manduca sexta. Insect Biochem Mol Biol 2002; 32(11): 1497–506. 10.1016/S0965-1748(02)00070-X [DOI] [PubMed] [Google Scholar]
- [34].Ostrowski S, Dierick HA, Bejsovec A. Genetic control of cuticle formation during embryonic development of Drosophila melanogaster. Genetics 2002; 161(1): 171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Moussian B, Schwarz H, Bartoszewski S, Nüsslein-Volhard C. Involvement of chitin in exoskeleton morphogenesis in Drosophila melanogaster. J Morphol 2005; 264(1): 117–30. 10.1002/jmor.10324 [DOI] [PubMed] [Google Scholar]
- [36].Noh MY, Muthukrishnan S, Kramer KJ, Arakane Y. A chitinase with two catalytic domains is required for organization of the cuticular extracellular matrix of a beetle. PLoS Genet 2018; 14(3)e1007307 10.1371/journal.pgen.1007307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tian H, Peng H, Yao Q, et al. Developmental control of a lepidopteran pest Spodoptera exigua by ingestion of bacteria expressing dsRNA of a non-midgut gene. PLoS One 2009; 4(7)e6225 10.1371/journal.pone.0006225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Arakane Y, Muthukrishnan S, Kramer KJ, et al. The Tribolium chitin synthase genes TcCHS1 and TcCHS2 are specialized for synthesis of epidermal cuticle and midgut peritrophic matrix. Insect Mol Biol 2005; 14(5): 453–63. 10.1111/j.1365-2583.2005.00576.x [DOI] [PubMed] [Google Scholar]
- [39].Zhang J, Liu X, Zhang J, et al. Silencing of two alternative splicing-derived mRNA variants of chitin synthase 1 gene by RNAi is lethal to the oriental migratory locust, Locusta migratoria manilensis (Meyen). Insect Biochem Mol Biol 2010; 40(11): 824–33. 10.1016/j.ibmb.2010.08.001 [DOI] [PubMed] [Google Scholar]
- [40].Rezende GL, Martins AJ, Gentile C, et al. Embryonic desiccation resistance in Aedes aegypti: presumptive role of the chitinized serosal cuticle. BMC Dev Biol 2008; 8: 82 10.1186/1471-213X-8-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jacobs CG, Braak N, Lamers GE, van der Zee M. Elucidation of the serosal cuticle machinery in the beetle Tribolium by RNA sequencing and functional analysis of Knickkopf1, Retroactive and Laccase2. Insect Biochem Mol Biol 2015; 60: 7–12. 10.1016/j.ibmb.2015.02.014 [DOI] [PubMed] [Google Scholar]
- [42].Chaudhari SS, Arakane Y, Specht CA, et al. Knickkopf protein protects and organizes chitin in the newly synthesized insect exoskeleton. Proc Natl Acad Sci USA 2011; 108(41): 17028–33. 10.1073/pnas.1112288108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Souza-Ferreira PS, Mansur JF, Berni M, et al. Chitin deposition on the embryonic cuticle of Rhodnius prolixus: the reduction of CHS transcripts by CHS-dsRNA injection in females affects chitin deposition and eclosion of the first instar nymph. Insect Biochem Mol Biol 2014; 51: 101–9. 10.1016/j.ibmb.2013.12.004 [DOI] [PubMed] [Google Scholar]
- [44].Ye C, Jiang YD, An X, et al. Effects of RNAi-based silencing of chitin synthase gene on moulting and fecundity in pea aphids (Acyrthosiphon pisum). Sci Rep 2019; 9(1): 3694 10.1038/s41598-019-39837-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mansur JF, Figueira-Mansur J, Santos AS, et al. The effect of lufenuron, a chitin synthesis inhibitor, on oogenesis of Rhodnius prolixus. Pestic Biochem Physiol 2010; 98: 59–67. 10.1016/j.pestbp.2010.04.013 [DOI] [Google Scholar]
- [46].Bansal R, Mian MAR, Mittapalli O, Michel AP. Characterization of a chitin synthase encoding gene and effect of diflubenzuron in soybean aphid, Aphis glycines. Int J Biol Sci 2012; 8(10): 1323–34. 10.7150/ijbs.4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chaudhari SS, Noh MY, Moussian B, et al. Knickkopf and retroactive proteins are required for formation of laminar serosal procuticle during embryonic development of Tribolium castaneum. Insect Biochem Mol Biol 2015; 60: 1–6. 10.1016/j.ibmb.2015.02.013 [DOI] [PubMed] [Google Scholar]
- [48].Arakane Y, Specht CA, Kramer KJ, Muthukrishnan S, Beeman RW. Chitin synthases are required for survival, fecundity and egg hatch in the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol 2008; 38(10): 959–62. 10.1016/j.ibmb.2008.07.006 [DOI] [PubMed] [Google Scholar]
- [49].Farnesi LC, Menna-Barreto RF, Martins AJ, Valle D, Rezende GL. Physical features and chitin content of eggs from the mosquito vectors Aedes aegypti, Anopheles aquasalis and Culex quinquefasciatus: Connection with distinct levels of resistance to desiccation. J Insect Physiol 2015; 83: 43–52. 10.1016/j.jinsphys.2015.10.006 [DOI] [PubMed] [Google Scholar]
- [50].Qu M, Yang Q. A novel alternative splicing site of class A chitin synthase from the insect Ostrinia furnacalis - gene organization, expression pattern and physiological significance. Insect Biochem Mol Biol 2011; 41(12): 923–31. 10.1016/j.ibmb.2011.09.001 [DOI] [PubMed] [Google Scholar]
- [51].Qu M, Yang Q. Physiological significance of alternatively spliced exon combinations of the single-copy gene class A chitin synthase in the insect Ostrinia furnacalis (Lepidoptera). Insect Mol Biol 2012; 21(4): 395–404. 10.1111/j.1365-2583.2012.01145.x [DOI] [PubMed] [Google Scholar]
- [52].Moussian B, Tång E, Tonning A, et al. Drosophila Knickkopf and Retroactive are needed for epithelial tube growth and cuticle differentiation through their specific requirement for chitin filament organization. Development 2006; 133(1): 163–71. 10.1242/dev.02177 [DOI] [PubMed] [Google Scholar]
- [53].Hogenkamp DG, Arakane Y, Zimoch L, et al. Chitin synthase genes in Manduca sexta: characterization of a gut-specific transcript and differential tissue expression of alternately spliced mRNAs during development. Insect Biochem Mol Biol 2005; 35(6): 529–40. 10.1016/j.ibmb.2005.01.016 [DOI] [PubMed] [Google Scholar]
- [54].Zimoch L, Hogenkamp DG, Kramer KJ, Muthukrishnan S, Merzendorfer H. Regulation of chitin synthesis in the larval midgut of Manduca sexta. Insect Biochem Mol Biol 2005; 35(6): 515–27. 10.1016/j.ibmb.2005.01.008 [DOI] [PubMed] [Google Scholar]
- [55].Bolognesi R, Arakane Y, Muthukrishnan S, Kramer KJ, Terra WR, Ferreira C. Sequences of cDNAs and expression of genes encoding chitin synthase and chitinase in the midgut of Spodoptera frugiperda. Insect Biochem Mol Biol 2005; 35(11): 1249–59. 10.1016/j.ibmb.2005.06.006 [DOI] [PubMed] [Google Scholar]
- [56].Kumar NS, Tang B, Chen X, Tian H, Zhang W. Molecular cloning, expression pattern and comparative analysis of chitin synthase gene B in Spodoptera exigua. Comp Biochem Physiol B Biochem Mol Biol 2008; 149(3): 447–53. 10.1016/j.cbpb.2007.11.005 [DOI] [PubMed] [Google Scholar]
- [57].Kato N, Mueller CR, Fuchs JF, Wessely V, Lan Q, Christensen BM. Regulatory mechanisms of chitin biosynthesis and roles of chitin in peritrophic matrix formation in the midgut of adult Aedes aegypti. Insect Biochem Mol Biol 2006; 36(1): 1–9. 10.1016/j.ibmb.2005.09.003 [DOI] [PubMed] [Google Scholar]
- [58].Agrawal S, Kelkenberg M, Begum K, et al. Two essential peritrophic matrix proteins mediate matrix barrier functions in the insect midgut. Insect Biochem Mol Biol 2014; 49: 24–34. 10.1016/j.ibmb.2014.03.009 [DOI] [PubMed] [Google Scholar]
- [59].Kelkenberg M, Odman-Naresh J, Muthukrishnan S, Merzendorfer H. Chitin is a necessary component to maintain the barrier function of the peritrophic matrix in the insect midgut. Insect Biochem Mol Biol 2015; 56: 21–8. 10.1016/j.ibmb.2014.11.005 [DOI] [PubMed] [Google Scholar]
- [60].Zhang X, Zhang J, Zhu KY. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol Biol 2010; 19(5): 683–93. 10.1111/j.1365-2583.2010.01029.x [DOI] [PubMed] [Google Scholar]
- [61].Locke M, Huie P. Apolysis and the turnover of plasma membrane plaques during cuticle formation in an insect. Tissue Cell 1979; 11(2): 277–91. 10.1016/0040-8166(79)90042-9 [DOI] [PubMed] [Google Scholar]
- [62].Leopold RA, Newman SM, Helgeson G. A comparison of cuticle deposition during the pre- and posteclosion stages of the adult weevil, Anthonomus grandis Boheman (Coleoptera: Curculionidae). Int J Insect Morphol Embryol 1992; 21: 37–62. 10.1016/0020-7322(92)90004-7 [DOI] [Google Scholar]
- [63].Moussian B, Seifarth C, Müller U, Berger J, Schwarz H. Cuticle differentiation during Drosophila embryogenesis. Arthropod Struct Dev 2006; 35(3): 137–52. 10.1016/j.asd.2006.05.003 [DOI] [PubMed] [Google Scholar]
- [64].Sobala LF, Adler PN. The gene expression program for the formation of wing cuticle in Drosophila. PLoS Genet 2016; 12(5)e1006100 10.1371/journal.pgen.1006100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Gullion JD, Gullion T. Solid-state NMR study of the cicada wing. J Phys Chem B 2017; 121(32): 7646–51. 10.1021/acs.jpcb.7b05598 [DOI] [PubMed] [Google Scholar]
- [66].Smith CW, Herbert R, Wootton RJ, Evans KE. The hind wing of the desert locust (Schistocerca gregaria Forskål). II. Mechanical properties and functioning of the membrane. J Exp Biol 2000; 203(Pt 19): 2933–43. [DOI] [PubMed] [Google Scholar]
- [67].Reynolds SE, Samuels RI. Physiology and biochemistry of insect moulting fluid Adv Insect Physiol 1996; 26: 157–232. 10.1016/S0065-2806(08)60031-4 [DOI] [Google Scholar]
- [68].Barry MK, Triplett AA, Christensen AC. A peritrophin-like protein expressed in the embryonic tracheae of Drosophila melanogaster. Insect Biochem Mol Biol 1999; 29(4): 319–27. 10.1016/S0965-1748(99)00004-1 [DOI] [PubMed] [Google Scholar]
- [69].Behr M, Hoch M. Identification of the novel evolutionary conserved obstructor multigene family in invertebrates. FEBS Lett 2005; 579(30): 6827–33. 10.1016/j.febslet.2005.11.021 [DOI] [PubMed] [Google Scholar]
- [70].Jasrapuria S, Specht CA, Kramer KJ, Beeman RW, Muthukrishnan S. Gene families of cuticular proteins analogous to peritrophins (CPAPs) in Tribolium castaneum have diverse functions. PLoS One 2012; 7(11)e49844 10.1371/journal.pone.0049844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Qu M, Ren Y, Liu Y, Yang Q. Studies on the chitin/chitosan binding properties of six cuticular proteins analogous to peritrophin 3 from Bombyx mori. Insect Mol Biol 2017; 26(4): 432–9. 10.1111/imb.12308 [DOI] [PubMed] [Google Scholar]
- [72].Pesch YY, Riedel D, Behr M. Obstructor A organizes matrix assembly at the apical cell surface to promote enzymatic cuticle maturation in Drosophila. J Biol Chem 2015; 290(16): 10071–82. 10.1074/jbc.M114.614933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Petkau G, Wingen C, Jussen LCA, Radtke T, Behr M. Obstructor-A is required for epithelial extracellular matrix dynamics, exoskeleton function, and tubulogenesis. J Biol Chem 2012; 287(25): 21396–405. 10.1074/jbc.M112.359984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Broehan G, Arakane Y, Beeman RW, Kramer KJ, Muthukrishnan S, Merzendorfer H. Chymotrypsin-like peptidases from Tribolium castaneum: a role in molting revealed by RNA interference. Insect Biochem Mol Biol 2010; 40(3): 274–83. 10.1016/j.ibmb.2009.10.009 [DOI] [PubMed] [Google Scholar]
- [75].Qu M, Ma L, Chen P, Yang Q. Proteomic analysis of insect molting fluid with a focus on enzymes involved in chitin degradation. J Proteome Res 2014; 13(6): 2931–40. 10.1021/pr5000957 [DOI] [PubMed] [Google Scholar]
- [76].Liu HW, Wang LL, Tang X, et al. Proteomic analysis of Bombyx mori molting fluid: Insights into the molting process. J Proteomics 2018; 173: 115–25. 10.1016/j.jprot.2017.11.027 [DOI] [PubMed] [Google Scholar]
- [77].Zhang J, Lu A, Kong L, Zhang Q, Ling E. Functional analysis of insect molting fluid proteins on the protection and regulation of ecdysis. J Biol Chem 2014; 289(52): 35891–906. 10.1074/jbc.M114.599597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Vaaje-Kolstad G, Bøhle LA, Gåseidnes S, et al. Characterization of the chitinolytic machinery of Enterococcus faecalis V583 and high-resolution structure of its oxidative CBM33 enzyme. J Mol Biol 2012; 416(2): 239–54. 10.1016/j.jmb.2011.12.033 [DOI] [PubMed] [Google Scholar]
- [79].Vaaje-Kolstad G, Westereng B, Horn SJ, et al. An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 2010; 330(6001): 219–22. 10.1126/science.1192231 [DOI] [PubMed] [Google Scholar]
- [80].Courtade G, Aachmann FL. Chitin-active lytic polysaccharide monooxygenases Adv Exp Med Biol. Singapore: Springer; 2019; pp. 115–29. [DOI] [PubMed] [Google Scholar]
- [81].Sabbadin F, Hemsworth GR, Ciano L, et al. An ancient family of lytic polysaccharide monooxygenases with roles in arthropod development and biomass digestion. Nat Commun 2018; 9(1): 756 10.1038/s41467-018-03142-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Filiatrault-Chastel C, Navarro D, Haon M, et al. AA16, a new lytic polysaccharide monooxygenase family identified in fungal secretomes. Biotechnol Biofuels 2019; 12: 55 10.1186/s13068-019-1394-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zhu Q, Arakane Y, Banerjee D, Beeman RW, Kramer KJ, Muthukrishnan S. Domain organization and phylogenetic analysis of the chitinase-like family of proteins in three species of insects. Insect Biochem Mol Biol 2008; 38(4): 452–66. 10.1016/j.ibmb.2007.06.010 [DOI] [PubMed] [Google Scholar]
- [84].Tetreau G, Cao X, Chen YR, et al. Overview of chitin metabolism enzymes in Manduca sexta: Identification, domain organization, phylogenetic analysis and gene expression. Insect Biochem Mol Biol 2015; 62: 114–26. 10.1016/j.ibmb.2015.01.006 [DOI] [PubMed] [Google Scholar]
- [85].Nakabachi A, Shigenobu S, Miyagishima S. Chitinase-like proteins encoded in the genome of the pea aphid, Acyrthosiphon pisum. Insect Mol Biol 2010; 19(Suppl. 2): 175–85. 10.1111/j.1365-2583.2009.00985.x [DOI] [PubMed] [Google Scholar]
- [86].Zhu Q, Arakane Y, Beeman RW, Kramer KJ, Muthukrishnan S. Functional specialization among insect chitinase family genes revealed by RNA interference. Proc Natl Acad Sci USA 2008; 105(18): 6650–5. 10.1073/pnas.0800739105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Xi Y, Pan PL, Ye YX, Yu B, Xu HJ, Zhang CX. Chitinase-like gene family in the brown planthopper, Nilaparvata lugens. Insect Mol Biol 2015; 24(1): 29–40. 10.1111/imb.12133 [DOI] [PubMed] [Google Scholar]
- [88].Li D, Zhang J, Wang Y, et al. Two chitinase 5 genes from Locusta migratoria: molecular characteristics and functional differentiation. Insect Biochem Mol Biol 2015; 58: 46–54. 10.1016/j.ibmb.2015.01.004 [DOI] [PubMed] [Google Scholar]
- [89].Hogenkamp DG, Arakane Y, Kramer KJ, Muthukrishnan S, Beeman RW. Characterization and expression of the β-N-acetylhexosaminidase gene family of Tribolium castaneum. Insect Biochem Mol Biol 2008; 38(4): 478–89. 10.1016/j.ibmb.2007.08.002 [DOI] [PubMed] [Google Scholar]
- [90].Liu F, Liu T, Qu M, Yang Q. Molecular and biochemical characterization of a novel β-N-acetyl-D-hexosaminidase with broad substrate-spectrum from the Aisan corn borer, Ostrinia furnacalis. Int J Biol Sci 2012; 8(8): 1085–96. 10.7150/ijbs.4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Rong S, Li DQ, Zhang XY, et al. RNA interference to reveal roles of β-N-acetylglucosaminidase gene during molting process in Locusta migratoria. Insect Sci 2013; 20(1): 109–19. 10.1111/j.1744-7917.2012.01573.x [DOI] [PubMed] [Google Scholar]
- [92].Yang WJ, Xu KK, Yan X, Li C. Knockdown of β-N-acetylglucosaminidase 2 impairs molting and wing development in Lasioderma serricorne (Fabricius). Insects 2019; 10(11): 396 10.3390/insects10110396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Fukamizo T, Kramer KJ. Mechanism of chitin oligosaccharide hydrolysis by the binary enzyme chitinase system in insect molting fluid. Insect Biochem 1985; 15: 1–7. 10.1016/0020-1790(85)90037-X [DOI] [Google Scholar]
- [94].Fukamizo T, Kramer KJ. Mechanism of chitin hydrolysis by the binary chitinase system in insect molting fluid. Insect Biochem 1985; 15: 141–5. 10.1016/0020-1790(85)90001-0 [DOI] [Google Scholar]
- [95].Zhao Y, Park RD, Muzzarelli RA. Chitin deacetylases: properties and applications. Mar Drugs 2010; 8(1): 24–46. 10.3390/md8010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Grifoll-Romero L, Pascual S, Aragunde H, Biarnés X, Planas A. Chitin deacetylases: structures, specificities, and biotech applications. Polymers (Basel) 2018; 10(4): 10 10.3390/polym10040352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kafetzopoulos D, Thireos G, Vournakis JN, Bouriotis V. The primary structure of a fungal chitin deacetylase reveals the function for two bacterial gene products. Proc Natl Acad Sci USA 1993; 90(17): 8005–8. 10.1073/pnas.90.17.8005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Dixit R, Arakane Y, Specht CA, et al. Domain organization and phylogenetic analysis of proteins from the chitin deacetylase gene family of Tribolium castaneum and three other species of insects. Insect Biochem Mol Biol 2008; 38(4): 440–51. 10.1016/j.ibmb.2007.12.002 [DOI] [PubMed] [Google Scholar]
- [99].Campbell PM, Cao AT, Hines ER, East PD, Gordon KHJ. Proteomic analysis of the peritrophic matrix from the gut of the caterpillar, Helicoverpa armigera. Insect Biochem Mol Biol 2008; 38(10): 950–8. 10.1016/j.ibmb.2008.07.009 [DOI] [PubMed] [Google Scholar]
- [100].Xi Y, Pan PL, Ye YX, Yu B, Zhang CX. Chitin deacetylase family genes in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Insect Mol Biol 2014; 23(6): 695–705. 10.1111/imb.12113 [DOI] [PubMed] [Google Scholar]
- [101].Tetreau G, Dittmer NT, Cao X, et al. Analysis of chitin-binding proteins from Manduca sexta provides new insights into evolution of peritrophin A-type chitin-binding domains in insects. Insect Biochem Mol Biol 2015; 62: 127–41. 10.1016/j.ibmb.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Luschnig S, Bätz T, Armbruster K, Krasnow MA. serpentine and vermiform encode matrix proteins with chitin binding and deacetylation domains that limit tracheal tube length in Drosophila. Curr Biol 2006; 16(2): 186–94. 10.1016/j.cub.2005.11.072 [DOI] [PubMed] [Google Scholar]
- [103].Wang S, Jayaram SA, Hemphälä J, et al. Septate-junction-dependent luminal deposition of chitin deacetylases restricts tube elongation in the Drosophila trachea. Curr Biol 2006; 16(2): 180–5. 10.1016/j.cub.2005.11.074 [DOI] [PubMed] [Google Scholar]
- [104].Arakane Y, Dixit R, Begum K, et al. Analysis of functions of the chitin deacetylase gene family in Tribolium castaneum. Insect Biochem Mol Biol 2009; 39(5–6): 355–65. 10.1016/j.ibmb.2009.02.002 [DOI] [PubMed] [Google Scholar]
- [105].Quan G, Ladd T, Duan J, et al. Characterization of a spruce budworm chitin deacetylase gene: stage- and tissue-specific expression, and inhibition using RNA interference. Insect Biochem Mol Biol 2013; 43(8): 683–91. 10.1016/j.ibmb.2013.04.005 [DOI] [PubMed] [Google Scholar]
- [106].Yu R, Liu W, Li D, et al. Helicoidal organization of chitin in the cuticle of the migratory locust requires the function of the chitin deacetylase2 enzyme (LmCDA2). J Biol Chem 2016; 291(47): 24352–63. 10.1074/jbc.M116.720581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Yu RR, Liu WM, Zhao XM, et al. LmCDA1 organizes the cuticle by chitin deacetylation in Locusta migratoria. Insect Mol Biol 2019; 28(3): 301–12. 10.1111/imb.12554 [DOI] [PubMed] [Google Scholar]
- [108].Wu JJ, Chen ZC, Wang YW, Fu KY, Guo WC, Li GQ. Silencing chitin deacetylase 2 impairs larval-pupal and pupal-adult molts in Leptinotarsa decemlineata. Insect Mol Biol 2019; 28(1): 52–64. 10.1111/imb.12524 [DOI] [PubMed] [Google Scholar]
- [109].Noh MY, Muthukrishnan S, Kramer KJ, Arakane Y. Group I chitin deacetylases are essential for higher order organization of chitin fibers in beetle cuticle. J Biol Chem 2018; 293(18): 6985–95. 10.1074/jbc.RA117.001454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Jakubowska AK, Caccia S, Gordon KH, Ferré J, Herrero S. Down-regulation of a chitin deacetylase-like protein in response to baculovirus infection and its application for improving baculovirus infectivity. J Virol 2010; 84(5): 2547–55. 10.1128/JVI.01860-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Leptin M, Bogaert T, Lehmann R, Wilcox M. The function of PS integrins during Drosophila embryogenesis. Cell 1989; 56(3): 401–8. 10.1016/0092-8674(89)90243-2 [DOI] [PubMed] [Google Scholar]
- [112].Brown NH. Null mutations in the alpha PS2 and beta PS integrin subunit genes have distinct phenotypes. Development 1994; 120(5): 1221–31. [DOI] [PubMed] [Google Scholar]
- [113].Brower DL, Bunch TA, Mukai L, et al. Nonequivalent requirements for PS1 and PS2 integrin at cell attachments in Drosophila: genetic analysis of the alpha PS1 integrin subunit. Development 1995; 121(5): 1311–20. [DOI] [PubMed] [Google Scholar]
- [114].Green N, Odell N, Zych M, et al. A common suite of coagulation proteins function in Drosophila muscle attachment. Genetics 2016; 204(3): 1075–87. 10.1534/genetics.116.189787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Bunch TA, Graner MW, Fessler LI, et al. The PS2 integrin ligand tiggrin is required for proper muscle function in Drosophila. Development 1998; 125(9): 1679–89. [DOI] [PubMed] [Google Scholar]
- [116].Klapholz B, Brown NH. Talin - the master of integrin adhesions. J Cell Sci 2017; 130(15): 2435–46. 10.1242/jcs.190991 [DOI] [PubMed] [Google Scholar]
- [117].Soler C, Laddada L, Jagla K. Coordinated development of muscles and tendon-like structures: early interactions in the Drosophila leg. Front Physiol 2016; 7: 22 10.3389/fphys.2016.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Alves-Silva J, Hahn I, Huber O, Mende M, Reissaus A, Prokop A. Prominent actin fiber arrays in Drosophila tendon cells represent architectural elements different from stress fibers. Mol Biol Cell 2008; 19(10): 4287–97. 10.1091/mbc.e08-02-0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Reedy MC, Beall C. Ultrastructure of developing flight muscle in Drosophila. II. Formation of the myotendon junction. Dev Biol 1993; 160(2): 466–79. 10.1006/dbio.1993.1321 [DOI] [PubMed] [Google Scholar]
- [120].Fernandes JJ, Celniker SE, VijayRaghavan K. Development of the indirect flight muscle attachment sites in Drosophila: role of the PS integrins and the stripe gene. Dev Biol 1996; 176(2): 166–84. 10.1006/dbio.1996.0125 [DOI] [PubMed] [Google Scholar]
- [121].Qu M, Sun S, Liu Y, Deng X, Yang J, Yang Q. Insect group II chitinase OfChtII promotes chitin degradation during larva-pupa molting. Insect Sci 2020; ••• 10.1111/1744-7917.12791 [DOI] [PubMed] [Google Scholar]


