ABSTRACT
Cysteinylation is a post-translational modification (PTM) that occurs when a cysteine residue on a protein forms a disulfide bond with a terminal cysteine molecule. This PTM has been found in the hinge region of several recombinant therapeutic IgG2 antibodies, but the impact of cysteinylation on the safety and immunogenicity of therapeutics remains unclear. In this study, we characterized recombinant and endogenous IgG2 antibodies to quantify their levels of hinge cysteinylation, if present. To the best of our knowledge, this is the first study to identify and quantify hinge cysteinylation in endogenous IgG2 antibodies from healthy human serum. We used anti-IgG2 immunopurification of human serum to specifically enrich for endogenous IgG2 antibodies, and then subjected the resulting samples to Lys-C peptide mapping coupled with targeted mass spectrometry techniques. Using this analytical workflow, we found that all healthy human serum samples tested (N = 10) contained quantifiable levels of hinge cysteinylation (0.8 ± 0.3%) in their endogenous human IgG2s (IgG2-A isoform). These findings demonstrate that hinge cysteinylation in therapeutic IgG2s, at least up to a certain level, is well tolerated in humans and pose minimal safety or immunogenicity risks.
KEYWORDS: IgG2, endogenous antibodies, cysteinylation, disulfide, hinge region, human serum
Introduction
Since the approval of the first therapeutic monoclonal antibody (mAb) Orthoclone OKT3 in 1986,1 mAbs have developed into a multibillion-dollar market.2 These therapeutic agents are generally well tolerated3 and have been used in the treatment of an extensive range of diseases, including cancer, autoimmune disorders, and infectious diseases.4 The molecules, however, are often susceptible to fragmentation,5–7 aggregation,8–10 and other undesirable post-translational modifications (PTM)9,11–13 during manufacturing and storage. These product quality changes in mAbs can potentially alter their biochemical and biophysical properties, and, depending on their impact on bioactivity, pharmacokinetics, safety, and/or immunogenicity,14 it may be critical to control a particular product quality attribute during production and shelf-life.
Cysteinylation involves the addition of a terminal cysteine onto a cysteine residue in a protein via a disulfide bond. In therapeutic mAbs, this PTM may arise from free cysteine molecules present in cell culture media forming covalent adducts via nucleophilic free thiols.12,15 Previous studies have shown that cysteinylation can occur in the hinge regions of recombinant IgG2 antibodies.15,16 The hinge regions of IgG2 antibodies equilibrate between three structural isoforms IgG2-A, IgG2-B, and IgG2-A/B17,18 (Figure 1) with estimated in vivo relative abundances of 7.2%, 21.8%, and 70.8%, respectively.19 Notably, Kita et al. reported that cysteinylation was only found in the hinge region of IgG2-A isoform in recombinant IgG2 mAbs.15 In this case, two terminal cysteines are incorporated into a reduced hinge disulfide bond of the IgG2-A isoform as shown in Figure 2; mono-cysteinylation, where only one terminal cysteine is incorporated, was not found in the hinge region of IgG2-A isoform.
Figure 1.
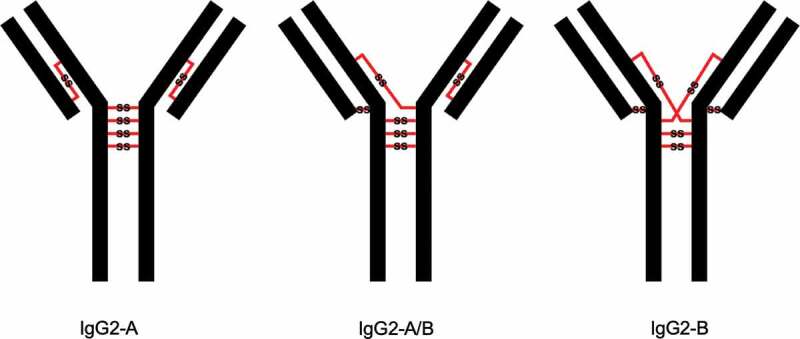
Three structural isoforms of IgG2 (A, A/B, and B) and their associated disulfide connections depicted with red lines. Modified and used with permission of American Chemical Society, from ref.;15 permission conveyed through Copyright Clearance Center, Inc
Figure 2.
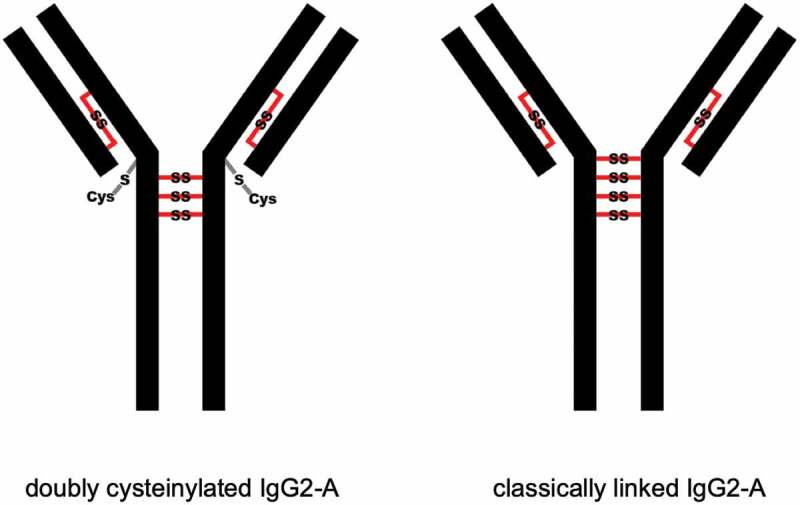
Depictions of doubly cysteinylated and classically linked IgG2-A forms. “Cys” denotes a terminal cysteine molecule that is disulfide bonded to a cysteine on the antibody. Modified and used with permission of American Chemical Society, from ref.;15 permission conveyed through Copyright Clearance Center, Inc
Little information has been reported about the safety or immunogenicity risk of hinge cysteinylation in therapeutic IgG2s to patients. We are not aware of any clinically relevant data (in-house or publicly available) where IgG2 material with hinge cysteinylation was dosed in humans. Initiating a clinical trial expressly for the purpose of testing the impact of cysteinylation poses ethical challenges and is prohibitively expensive. An elegant alternative to a clinical study would be to examine more closely whether or not hinge cysteinylation is present on endogenous human IgG2s; this type of approach has been successfully applied to IgG PTMs such as thioether linkages,20 cysteine racemization,21 trisulfides,22 free thiols,23 and IgG2 disulfide isoforms.18 Evidence that healthy humans are continuously and chronically exposed to hinge cysteinylation on IgG2s would mitigate the safety and immunogenicity risk of hinge cysteinylation in therapeutic IgG2 doses.
Prior proteomics efforts to survey PTMs on endogenous human immunoglobulins, including several that employ non-reduced digestions,18,20,22 have not yielded a positive identification of cysteinylation.14,18,20–22,24 This might be because these studies had a broad focus on all IgG subclasses, a narrow set of in-scope PTMs, and/or limited sensitivity of untargeted analysis for detecting potentially low levels of cysteinylation. In our study, we selectively enriched IgG2s from human serum and used a targeted mass spectrometry approach to quantify the endogenous levels of hinge cysteinylation. The enrichment of IgG2 from healthy human serum samples was accomplished using immunopurification, also referred to as pulldowns, with an anti-IgG2 (human specificity) antibody. The purified IgG2 was then digested under non-reducing conditions and analyzed using a parallel reaction monitoring (PRM) approach. Using this analytical workflow, we present the first reported evidence that quantifiable levels of cysteinylation exist in the hinge region of endogenous human IgG2s.
Results
Doubly cysteinylated IgG2-A in recombinant IgG2 (mAb1)
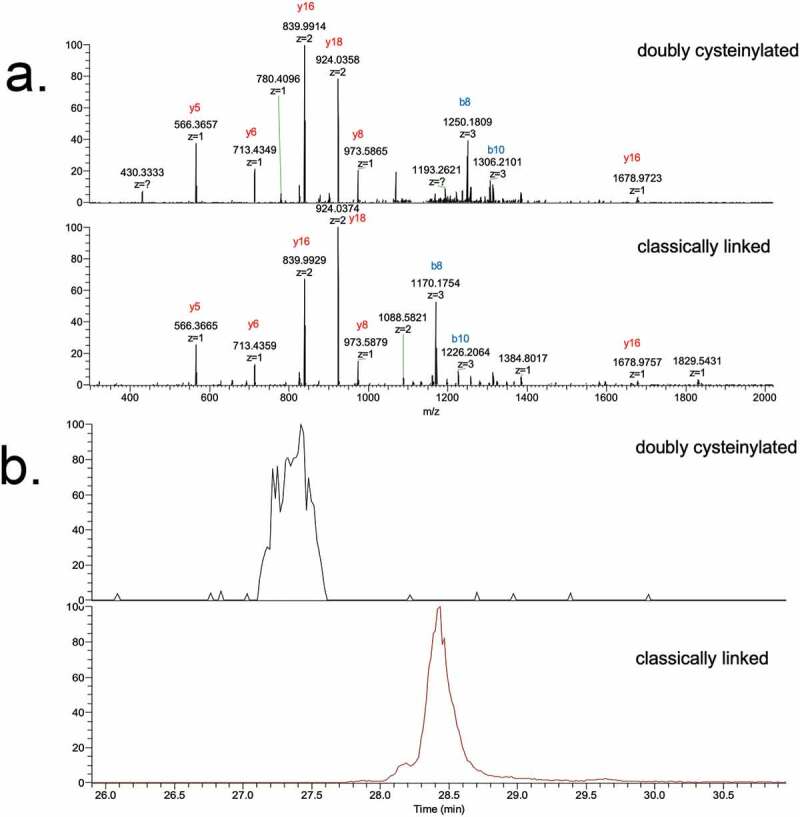
Genentech recombinant IgG2 mAb1 was recently found to contain cysteinylation in the hinge region; mAb1 produced via two processes, Y and Z, served as positive and negative controls in this study. Hinge cysteinylation in mAb1-Y was characterized via a bottom-up examination of the Lys-C dipeptides corresponding to doubly cysteinylated and classically linked IgG2-A forms. Masses consistent with a 5+ charge state of the doubly cysteinylated (1119.1233 m/z, monoisotopic) and classically linked (1071.1189 m/z, monoisotopic) hinge Lys-C dipeptides were observed in mAb1-Y single ion monitoring (SIM) experiments (Figure 3). The corresponding extracted ion chromatograms (XIC) showed that the doubly cysteinylated dipeptide eluted approximately 1 minute earlier than the classically linked dipeptide, an observation that is consistent with the former being more hydrophilic due to the presence of two terminal cysteine residues.
Figure 3.
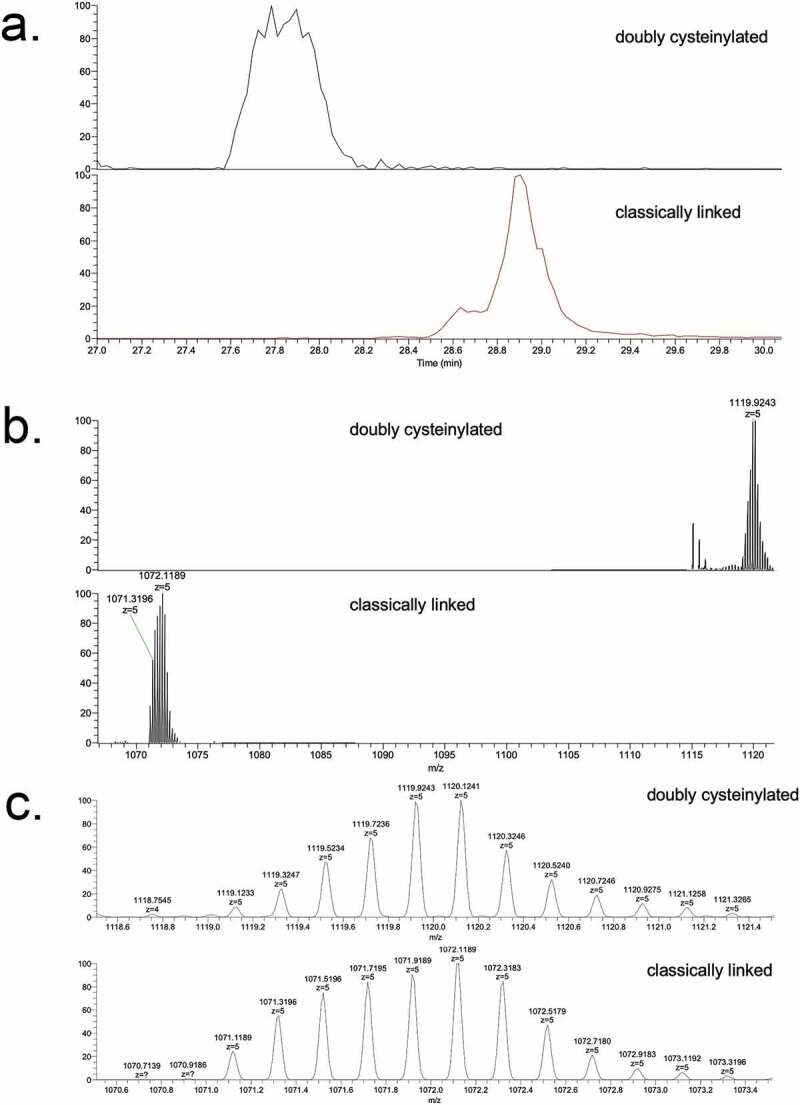
SIM experiments of mAb1-Y Lys-C peptide maps. (a) Extracted ion chromatograms of doubly cysteinylated and classically linked IgG-A hinge Lys-C dipeptides (10 ppm mass tolerance for extraction). (b) Corresponding MS1 spectra using Orbitrap detection (60 000 resolution). (c) Corresponding MS1 spectra that are zoomed-in on the mass features of interest
These identifications were verified using tandem mass spectra from PRM experiments with collision-induced dissociation (CID) fragmentation. The observed y-product ion series (y5 to y18) corroborated the majority of the backbone amino acid sequence, while the b8 and b10-product ions distinguished the doubly cysteinylated from the classically linked hinge dipeptide (Figure 4(a,b)). The mass difference between the b8-product ions corresponding to the doubly cysteinylated (1249.5121 m/z, 3+, monoisotopic) and classically linked (1169.5091 m/z, 3+, monoisotopic) dipeptides was 240.0009 Da, which is in good agreement with the mass of two additional terminal cysteines (Figure 4(c)). The b8 and b10-product ions of the doubly cysteinylated dipeptide confirm that the cysteinylation modification occurs on one of the four hinge interchain disulfide bonds. However, the cysteinylation modification could not be further localized due to the lack of additional fragmentation ions.
Figure 4.
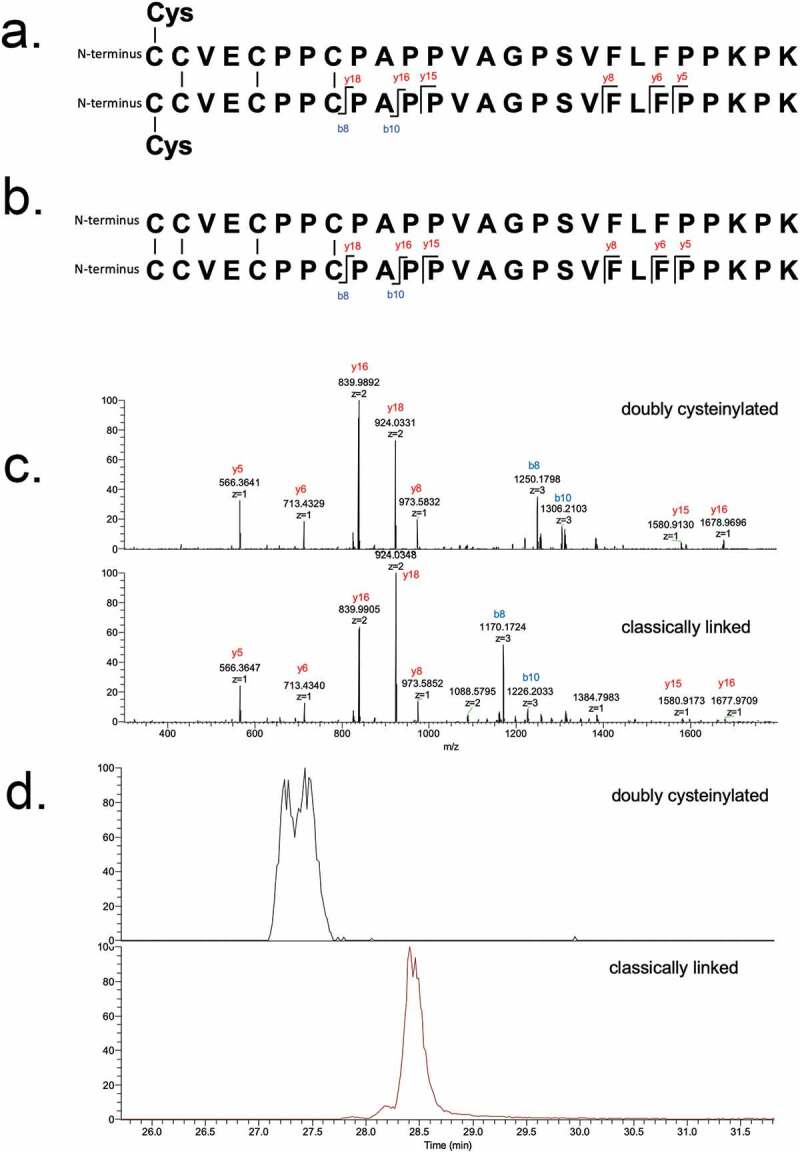
PRM experiments of mAb1-Y Lys-C peptide maps. Sequences of (a) doubly cysteinylated and (b) classically linked IgG2-A hinge Lys-C dipeptides annotated with b- and y-ion fragmentation sites. (c) Corresponding annotated PRM spectra using CID fragmentation and Orbitrap detection (30 000 resolution, 10 ppm mass tolerance for assignments). (d) Corresponding extracted ion chromatograms of precursor/b8 product ion transitions (10 ppm mass tolerance for extraction)
The XIC peak areas of the precursor/b8-product ion transitions belonging to the doubly cysteinylated and classically linked dipeptides were quantified, after which the relative abundance of the doubly cysteinylated IgG2-A form (%2Cys) was determined using eq 1 (see Materials and Methods). In this manner, mAb1-Y was determined to contain 4.3% doubly cysteinylated IgG2-A form. Peak splitting was observed in the XIC peak corresponding to the doubly cysteinylated dipeptide in mAb-Y (Figure 4(d)), although the reason for this heterogeneity is unclear at this time. mAb1-Z was subjected to a similar analysis and yet the doubly cysteinylated IgG2-A form was below the detection limit (detection limit is discussed in a following section).
Qualification of analytical workflow
The anti-IgG2 immunoaffinity purification method used in this study was qualified by first confirming IgG2 enrichment. A cocktail sample composed of recombinant IgG1, IgG2, and IgG4 molecules was subjected to Protein G pulldowns and anti-IgG2 pulldowns. While IgG1, IgG2, and IgG4 molecules were present in the Protein G pulldowns, only IgG2 was present in the anti-IgG2 pulldowns, demonstrating specificity of the pulldown method (Figure 5). Then, the %2Cys of a 0.3% doubly cysteinylated IgG2-A control sample (prepared by co-mixing mAb1-Y and mAb1-Z) was determined before and after anti-IgG2 immunoaffinity purification using PRM analysis; the %2Cys levels in the control sample were the same, irrespective of whether the sample had been immunopurified, indicating no attributable bias to the %2Cys quantitation from the pulldown. The same 0.3% doubly cysteinylated IgG2-A control sample was subjected to a repeatability analysis (9 injection replicates) and the results are summarized in Table 1. Using the standard deviation from repeatedly analyzing this 0.3% doubly cysteinylated IgG2-A sample as an estimate for noise, signal-to-noise (S/N) for this control sample was determined by dividing the signal (0.3%) by the noise (0.03%) to yield a S/N of 10. These results suggest that the limit of quantitation (LOQ, S/N = 10) with PRM analysis was <0.3% doubly cysteinylated IgG2-A and that the limit of detection (LOD, S/N = 3), accordingly, was <0.1% doubly cysteinylated IgG2-A. Finally, a set of IgG2 standards with % doubly cysteinylated IgG2-A levels ranging from 0.3% to 1.7% were prepared by co-mixing mAb1-Y and mAb1-Z. We chose to focus our linearity experiments on a narrow range near the limit of quantitation in anticipation that %2Cys in endogenous IgG2s, if present, would likely be at low levels. The %2Cys in these co-mix standards were measured using the PRM analysis and showed a linear relationship between observed %2Cys and theoretical %2Cys (R2 > 0.99, Figure 6).
Figure 5.
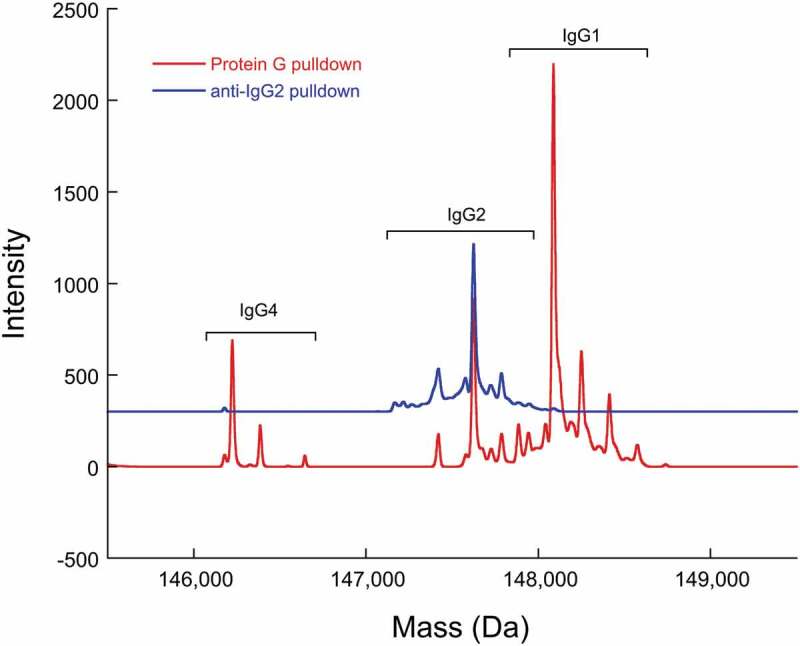
Deconvoluted mass spectra of a recombinant IgG cocktail sample (including IgG1, IgG2, and IgG4 antibodies) that has been subjected to Protein G pulldown or anti-IgG2 pulldown. The heterogeneity within each recombinant IgG subclass is predominantly attributable to Fc glycosylation
Table 1.
PRM repeatability analysis with a 0.3% doubly cysteinylated IgG2-A control sample (prepared by co-mixing mAb1-Y and mAb1-Z). Nine replicates in total were analyzed
| Statistic | %2Cys |
|---|---|
| Mean | 0.3% |
| Standard deviation | 0.03% |
| S/N | 10 |
Figure 6.
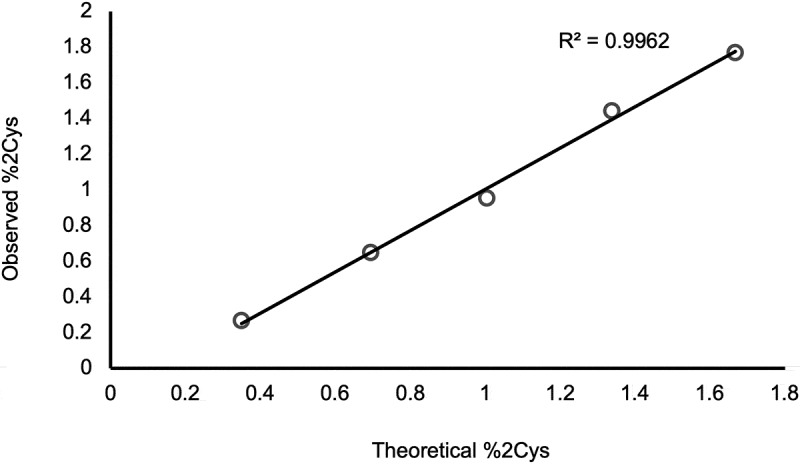
PRM analysis of doubly cysteinylated IgG2 standards (prepared by co-mixing mAb1-Y and mAb1-Z) showing a linear response
Doubly cysteinylated IgG2-A in endogenous human IgG2
Next, we interrogated endogenous IgG2s that were affinity purified from healthy human serum using the same approach as described above for recombinant mAb1. SIM experiments of the endogenous IgG2s lacked the necessary specificity because there was an interfering ion; the interference was likely attributable to a species carried over from the complex biological matrix (data not shown). However, the additional specificity afforded by PRM experiments of the endogenous IgG2s yielded high-quality spectra (Figure 7(a)) and expected XIC peak retention times (Figure 7(b)), providing compelling evidence for the presence of doubly cysteinylated IgG2-A in human serum. Doubly cysteinylated IgG2-A was present in all 10 human serum samples that we analyzed via PRM, ranging from 0.5% to 1.3% and averaging 0.8 ± 0.3% (Table 2). These data suggest that healthy humans are chronically exposed to low (but detectable) levels of IgG2 hinge cysteinylation.
Figure 7.
PRM experiments of endogenous IgG2 Lys-C peptide maps. (a) PRM spectra using CID fragmentation and Orbitrap detection (30 000 resolution, 10 ppm mass tolerance for assignments). (b) Corresponding extracted ion chromatograms of precursor/b8 product ion transitions (10 ppm mass tolerance for extraction). Depicted here is data of endogenous IgG2s affinity purified from a single human subject, as an example (in total 10 human subject samples were analyzed)
Table 2.
Relative abundances of doubly cysteinylated IgG2-A form (%2Cys) in endogenous IgG2s purified from healthy human serum. XICdoubly cysteinylated and XICclassically linked are the extracted ion chromatogram peak areas of the precursor/product ion transitions for the doubly cysteinylated and classically linked IgG2-A Lys-C dipeptides, respectively. Demographic information corresponding to each human subject is included. There was no obvious correlation between hinge cysteinylation levels and age, gender or race, granted the sample size was small
| Gender | Race | Age | XICclassically linked | XICdoubly cysteinylated | %2Cys |
|---|---|---|---|---|---|
| Male | White | 52 | 21214823 | 96558 | 0.5 |
| Male | Hispanic | 66 | 15023572 | 139367 | 0.9 |
| Male | White | 59 | 9312008 | 78597 | 0.8 |
| Male | Black | 58 | 15631974 | 94021 | 0.6 |
| Male | White | 44 | 17710870 | 159266 | 0.9 |
| Female | White | 44 | 25823084 | 160364 | 0.6 |
| Female | Hispanic | 63 | 17255965 | 204560 | 1.2 |
| Female | White | 63 | 23764328 | 142695 | 0.6 |
| Female | White | 58 | 15456256 | 72583 | 0.5 |
| Female | Black | 63 | 17672827 | 229390 | 1.3 |
| Average | 0.8 | ||||
| Standard Deviation | 0.3 | ||||
Discussion
Manufacturing process changes can result in different levels of hinge cysteinylation in recombinant IgG2 therapeutics (e.g., mAb1-Y vs. mAb1-Z), but the exact process levers that lead to these differences are not fully understood. Meanwhile, gaps in our knowledge regarding IgG2 hinge cysteinylation make it difficult to accurately interpret its risk to safety and immunogenicity for patients. In an attempt to address these gaps without conducting a separate clinical study, this study examined hinge cysteinylation on endogenous IgG2s purified from healthy human serum. This is a viable strategy for hinge cysteinylation because this PTM occurs in a conserved region of the IgG2 framework; for modifications occurring in variable regions, the amino acid sequence heterogeneity would likely preclude similar analyses with endogenous IgGs. IgG2s from 10 healthy human serum samples were selectively purified, subjected to Lys-C digestion, and analyzed using targeted mass spectrometry. Hinge cysteinylation was quantifiable in endogenous IgG2s from all 10 human serum samples, ranging from 0.5% to 1.3% doubly cysteinylated IgG2-A. To our knowledge, this is the first time that the hinge cysteinylation modification has been reported in endogenous human IgG2s.
Previous proteomics studies have been completed that surveyed the post-translational modification landscape of endogenous IgGs.14,20–22,24 Some of the aforementioned studies used reduced peptide mapping workflows, which are not amenable for detecting cysteinylation,21,24 while others used non-reduced peptide mapping workflows,18,20,22 and yet hinge cysteinylation modification in IgG2s was not reported. Two major differences between our experiments and prior efforts include: (1) IgG2 enrichment vs. pan-IgG enrichment, and (2) targeted mass spectrometry (i.e., PRM) vs. untargeted mass spectrometry (i.e., data-dependent acquisition). We can reasonably expect that IgG2 enrichment with anti-IgG2 pulldown was responsible for an estimated 4.5-fold boost in sensitivity for our workflow relative to a pan-IgG pulldown such as protein A or G purification since IgG2 typically comprises only 22% of total IgG content (1/0.22 = 4.5).25 Additionally, the specificity from targeted mass spectrometry experiments enabled high confidence identifications and quantitation. Given that our %2Cys results were only modestly above our estimated LOQ and the fact that we observed specificity issues with SIM analysis, identification and quantitation of hinge cysteinylation in endogenous IgG2s would have been difficult without the boosts in sensitivity and specificity introduced by the current workflow.
The identification of cysteinylation in endogenous IgG2s is novel, but should not be surprising considering that S-cysteinylation occurs naturally in other endogenous serum proteins. The concentration of S-cysteinylated proteins in human serum ranges from 60 to 150 µM.26,27 The specific serum proteins that have been identified with S-cysteinylation include but are not limited to human serum albumin27–29 and low-density lipoprotein apolipoprotein B.30 Although the mechanism for cysteinylation of endogenous IgG2s is currently unclear, there is precedence of S-cysteinylation on other endogenous serum proteins.
There are important implications of our findings with regards to the product quality of recombinant IgG2 therapeutics. Since we have established that healthy human beings (N = 10) are chronically exposed to low levels of hinge cysteinylation in IgG2s, the question that remains is: how does this endogenous exposure to hinge cysteinylation compare to the dosed exposure introduced by IgG2 therapeutics? It is helpful to express both the endogenous exposure and dosed exposure in units of doubly cysteinylated IgG2-A concentration in serum (i.e., µg/mL) to facilitate a proper comparison. As summarized in Table 3, we aggregated published values for endogenous IgG serum concentrations, relative abundances of IgG2 and IgG2-A together with the endogenous %2Cys levels determined in this study to arrive at an endogenous exposure in healthy humans of 1.5 µg doubly cysteinylated IgG2-A/mL of serum. The dosed exposure will depend on parameters specific to a particular IgG2 therapeutic (i.e., dosage, %IgG2-A, and %2Cys). For illustrative purposes, if we assume a hypothetical scenario where a recombinant IgG2 therapeutic with %2Cys and %IgG2-A content of 0.5% and 25%, respectively, is dosed at 250 mg (Table 3), then we would arrive at a dosed exposure of 0.125 µg doubly cysteinylated IgG2-A/mL of serum. In this scenario, the additional doubly cysteinylated IgG2-A that is introduced by the therapeutic dose would be 1/12th of the endogenous exposure that the patient already chronically experiences and, therefore, poses minimal safety or immunogenicity risks to the patient. We anticipate that biopharmaceutical development teams can perform similar calculations and comparisons to endogenous exposure as delineated above using parameters specific to their IgG2 therapeutic to perform an informed risk assessment for hinge cysteinylation.
Table 3.
Comparison of endogenous exposure and dosed exposure (hypothetical) to doubly cysteinylated IgG2-A. Included are the mean values for parameters relevant to the exposure calculations
| Endogenous | Dosed therapeutic (hypothetical) | |||
|---|---|---|---|---|
| Parameter | Mean value | Ref | Mean value | Ref |
| IgG serum concentration | 11.8 mg/mL | ref.31 | 0.1 mg/mL* | n/a |
| %IgG2 | 22% | ref.25 | 100% | n/a |
| %IgG2-A (of IgG2) | 7.2% | ref.19 | 25% | n/a |
| %2Cys (of IgG2-A) | 0.8% | This study | 0.5% | n/a |
| Doubly cysteinylated IgG2-A exposure in serum | 1.5 µg/mL | 0.125 µg/mL | ||
*Assuming a hypothetical IgG2 therapeutic dose of 250 mg and an average human serum volume of 2.5L.
It is important to discuss some limitations of the current study. First, we limited our discussion of hinge cysteinylation to the IgG2-A isoform because we did not detect hinge cysteinylation in the other isoforms. However, non-reduced Lys-C digestion of IgG2-A/B and IgG2-B isoforms yield larger peptides than the IgG2-A isoform, and it is plausible that our analytical workflow was not suitable for detecting low-level modifications on these larger IgG2-A/B and IgG2-B Lys-C peptides. Second, our Lys-C dipeptide tandem mass spectra did not have sufficient b- and y-ion coverage to localize the cysteine modification to the exact hinge interchain disulfide bond. Detailed characterization of recombinant IgG2 hinge peptides (augmented with Edman degradation) by Kita et al. did unequivocally identify the most N-terminal hinge interchain disulfide bond as the sole site for cysteinylation,15 but we cannot exclude the possibility of site heterogeneity for hinge cysteinylation on mAb1 and endogenous IgG2s at this time. Third, our relative quantitation approach hinges upon “label-free” comparisons between PRM transitions belonging to the doubly cysteinylated and classically linked dipeptides. The gold standard for quantitation from PRM experiments is inclusion of an isotopically labeled internal standard, which would compensate for variability in sample preparation and LC-MS/MS analysis. We did not have isotopically labeled standards prepared for our studies. Instead, we attempted to minimize quantitation bias by choosing the b8-product ions as part of the transitions for both the doubly cysteinylated and classically linked dipeptides, and we compared the PRM quantitation of mAb1-Y with the SIM “label-free” quantitation of mAb1-Y. The SIM quantitation of cysteinylation levels for mAb1-Y (8.9%) was higher than the PRM quantitation (4.3%), suggesting that our %2Cys in endogenous IgG2s are likely underestimated. Even though we are likely underestimating %2Cys in this study, the endogenous exposure to hinge cysteinylation reported here from endogenous human IgG2s serves as an appropriate worst-case benchmark when comparing to dosed exposures from IgG2 therapeutics.
In conclusion, we identified and quantified hinge cysteinylation in endogenous human IgG2s for the first time. The presence of hinge cysteinylation in endogenous IgG2s purified from healthy humans implies that this modification is safely tolerated in healthy humans up to a certain level. The implications of our findings with respect to product quality of IgG2 therapeutics were discussed. This study highlights the importance of interrogating endogenous levels of “novel” PTMs present in recombinant therapeutics. The rationale and approach used in this study can be extended to other PTMs that have knowledge gaps (with regards to safety and immunogenicity) in order to facilitate a better-informed risk assessment of the quality attribute of interest.
Materials and methods
In-house mAb1-Y and mAb1-Z used in this study denote mAb1 (a recombinant IgG2 antibody) that was produced using two different manufacturing processes, processes Y and Z, respectively, and these two materials contained different levels of doubly cysteinylated hinge IgG2-A. mAb1-Y and mAb1-Z contain 4.3% and non-detectable levels of the doubly cysteinylated IgG2-A form, respectively (determined using targeted PRM analysis as described later). Two recombinant IgG1 and IgG4 molecules were produced at Genentech and were used to demonstrate IgG2 specificity and efficiency of pulldown methods. Human serum from 10 random healthy individuals was obtained from BioIVT (Westbury, NY) under an Institutional Review Board-approved protocol (WIRB protocol# 20161665). Human serum was frozen less than 2 hours after collection from subjects using vacutainers and kept at −70°C until analysis. Anti-IgG2 (human specificity) murine IgG1 antibodies (clone: HP6002) were obtained from BioLegend (San Diego, CA). Tosylactivated MyOne Dynabeads and Protein G Dynabeads were purchased from Thermo Fisher Scientific (Waltham, MA). Endoproteinase Lys-C was obtained from Fujifilm Wako (Osaka, Japan). Other reagents (L-Methionine, guanidine hydrochloride ULTROL grade, N-ethylmaleimide) were obtained from Millipore Sigma (Gillingham, UK).
IgG2 affinity purification
Anti-IgG2 antibodies were conjugated to tosylactivated Dynabeads according to the manufacturer’s recommended instructions. Human serum or recombinant mAb sample (36 µL) was mixed with 14 µL of equilibration buffer (20 mM sodium phosphate, 150 mM sodium chloride, pH 7.4) and added to 3 mg of anti-IgG2 conjugated magnetic beads. Following a 90 min incubation at room temperature (gentle rocking), the beads were pulled down with Dynamag-2 (Thermo Fisher Scientific, Waltham, MA) and iteratively washed with two volumes of 180 µL phosphate wash buffer (equilibration buffer plus 3 mM ethylenediaminetetraacetic acid and 0.005% polysorbate 20) and one volume of 180 µL MilliQ water before eluting with 50 µL 1% formic acid.
Non-reduced Lys-C digestion
Prior to the Lys-C digestion, free sulfhydryl groups were capped by treating 25 µg of purified mAb1 or endogenous IgG2 with 0.6 mM N-ethylmaleimide in 4 M guanidine hydrochloride, 10 mM methionine, 50 mM sodium acetate, pH 5.5 (100 µL total reaction volume) and incubating the mixture for 2 hours at 37°C. Free sulfhydryl alkylation protects against undesired nucleophilic side reactions and prevents disulfide shuffling. The denatured and alkylated sample was then diluted twofold in 100 mM 3-(N-morpholino)propanesulfonic acid buffer pH 7.2. Digestion was initiated by adding 10 µg Lys-C enzyme and incubating for 2 hours at 37°C. After this initial incubation, the digestion was supplemented with an additional 10 µg of Lys-C and incubated for an additional 2 hours at 37°C, after which the reaction was acidified and quenched with 10% formic acid to a final concentration of 1% formic acid.
Targeted LC-MS/MS analysis
Approximately 10 µg of non-reduced Lys-C digested samples was separated on an Agilent 1290 Infinity UHPLC system (Santa Clara, CA) equipped with an Acquity UHPLC peptide CSH C18 column (150 x 2.1 mm, 1.7 µm particle size, 130 Å pore size, Waters, Milford, MA). The column was equilibrated at 77°C with 99% mobile phase A (0.1% formic acid in water) and 1% mobile phase B (0.1% formic acid in acetonitrile). Peptides were eluted off the column using a flowrate of 0.2 mL/min and an initial 5-minute linear gradient from 1% to 13% B followed by 35-minute linear gradient from 13% to 35% B before regenerating the column for the subsequent injection. The LC eluent was coupled to a Thermo Scientific Orbitrap Fusion mass spectrometer (Waltham, MA) with an IonMax NG electrospray ionization source. Important mass spectrometer source settings include a spray voltage of 3700 V and an ion transfer temperature of 325°C.
SIM scans were acquired by isolating ions centered around 1119.7281 m/z (doubly cysteinylated IgG2-A hinge peptide, MH5+, 5th isotope) and 1071.9241 m/z (classically linked IgG2-A hinge peptide, MH5+, 5th isotope) with an isolation width of 10 m/z and Orbitrap detection at 60,000 resolution. PRM scans were acquired by isolating the same ions as in the SIM scans, but with an isolation width of 3 m/z, subjecting the ions to CID fragmentation (35% normalized collision energy), and Orbitrap detection at 30,000 resolution. Thermo Xcalibur 3.0 software was used to collect and process the data. The percentage of doubly cysteinylated peptides (% 2Cys) in a given sample was calculated from the PRM experiments using the following equation:
| (1) |
where XICdoubly cysteinylated and XICclassically linked are the extracted ion chromatogram peak areas of the precursor/product ion transitions (Table 4) for the doubly cysteinylated and classically linked IgG2-A Lys-C dipeptides, respectively.
Table 4.
Parent/b8-product ion transitions used for %2Cys quantitation of PRM experiments. The three b8-product ions listed represent the second, third, and fourth isotopes
| Parent ion |
b8-product ion |
||||||
|---|---|---|---|---|---|---|---|
| Lys-C IgG2-A hinge dipeptide | Monoisotopic mass | m/z | z | Isolation width | m/z | z | Mass tolerance |
| Classically linked | 5350.5911 Da | 1071.9241 | +5 | 3 Da | 1169.8431, 1170.1743, 1170.5077 | +3 | 10 ppm |
| Doubly cysteinylated | 5590.6160 Da | 1119.9241 | +5 | 3 Da | 1249.8461, 1250.1804, 1250.5151 | +3 | 10 ppm |
Intact LC/MS of Protein G versus anti-IgG2 pulldowns
In order to verify IgG2 enrichment with the anti-IgG2 affinity purification procedure, a cocktail sample composed of equal molar ratios of recombinant IgG1, IgG2 (mAb1-Z), and IgG4 antibodies was subjected to anti-IgG2 affinity purification or Protein G affinity purification. The Protein G affinity purification was performed with the same equilibration, wash, and elution steps as the anti-IgG2 affinity purification (described above) with the exception that Protein G Dynabeads were used instead of anti-IgG2 Dynabeads. After affinity purification, approximately 1 µg of eluted cocktail sample was loaded onto a Waters H-Class UHPLC system equipped with a PLRP-S column (150 x 2.1 mm, 8 µm particle size, 1000 Å pore size, Agilent, Santa Clara, CA). The column was equilibrated at 70°C with 80% mobile phase A (0.1% formic acid in water) and 20% mobile phase B (0.1% formic acid in acetonitrile). Proteins were eluted off the column using a flowrate of 0.3 mL/min and an initial 1-minute linear gradient from 20% to 30% B followed by 7-minute linear gradient from 30% to 42% B before regenerating the column for the subsequent injection. The LC eluent was coupled to an ABSciex 5600 Q-TOF (Framingham, MA) with a DuoSpray Turbo electrospray ionization source. Important mass spectrometer source settings include a spray voltage of 5000 V, capillary temperature of 350°C, collisional energy (CE) of 15 V, and a declustering potential (DP) of 275 V. Intact mass spectra was collected using Analyst TF 1.7.1 software and deconvoluted using PeakView 2.2.0.11391 software (BioTool kit add-on).
Acknowledgments
The authors would like to thank Y. Diana Liu for sharing optimized method conditions for non-reduced peptide mapping; and Bing Zhang, Tomasz Baginski, and Galahad Deperalta for providing mAb1-Y and mAb1-Z materials, as well sharing pertinent information regarding mAb1 hinge cysteinylation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Smith SL. Ten years of orthoclone OKT3 (Muromonab-CD3): a review. J Transpl Coord. 1996;6(3):109–9. doi: 10.1177/090591999600600304. [DOI] [PubMed] [Google Scholar]
- 2.Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC.. Monoclonal antibody successes in the clinic. Nat Biotechnol. 2005;23(9):1073–78. doi: 10.1038/nbt0905-1073. [DOI] [PubMed] [Google Scholar]
- 3.Newsome BW, Ernstoff MS. The clinical pharmacology of therapeutic monoclonal antibodies in the treatment of malignancy; have the magic bullets arrived? Br J Clin Pharmacol. 2008;66(1):6–19. doi: 10.1111/j.1365-2125.2008.03187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brekke OH, Sandlie I. Therapeutic antibodies for human diseases at the dawn of the twenty-first century. Nat Rev Drug Discov. 2003;2(1):52–62. doi: 10.1038/nrd984. [DOI] [PubMed] [Google Scholar]
- 5.Vlasak J, Ionescu R. Fragmentation of monoclonal antibodies. mAbs. 2011;3(3):253–63. doi: 10.4161/mabs.3.3.15608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goswami S, Wang W, Arakawa T, Ohtake S. Developments and challenges for MAb-based therapeutics. Antibodies. 2013;2(4):452–500. doi: 10.3390/antib2030452. [DOI] [Google Scholar]
- 7.Shire SJ. Stability of monoclonal antibodies (mAbs). Monoclonal Antibodies: meeting the challenges in manufacturing, formulation, delivery and stability of final drug product. Cambridge (UK): Woodhead Publishing; 2015. p. 45–92. [Google Scholar]
- 8.Cromwell MEM, Hilario E, Jacobson F. Protein aggregation and bioprocessing. Aaps J. 2006;8(3):1–8. doi: 10.1208/aapsj080366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goulet DR, Atkins WM. Considerations for the design of antibody-based therapeutics. J Pharm Sci. 2019;109(1):74–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vázquez-Rey M, Lang DA. Aggregates in monoclonal antibody manufacturing processes. Biotechnol Bioeng. 2011;108(7):1494–508. doi: 10.1002/bit.23155. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins N. Modifications of therapeutic proteins: challenges and prospects. Cytotechnology. 2007;53:121–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y, Wang D, Mason B, Rossomando T, Li N, Liu D, Cheung JK, Xu W, Raghava S, Katiyar A, et al. Structure, heterogeneity and developability assessment of therapeutic antibodies. mAbs. 2019;11(2):239–64. doi: 10.1080/19420862.2018.1553476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Ponniah G, Zhang HM, Nowak C, Neill A, Gonzalez-Lopez N, Patel R, Cheng G, Kita AZ, Andrien B. In vitro and in vivo modifications of recombinant and human IgG antibodies. mAbs. 2014;6(5):1145–54. doi: 10.4161/mabs.29883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck A, Liu H. Macro- and micro-heterogeneity of natural and recombinant IgG antibodies. Antibodies. 2019;8(1):18. doi: 10.3390/antib8010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kita A, Ponniah G, Nowak C, Liu H. Characterization of cysteinylation and trisulfide bonds in a recombinant monoclonal antibody. Anal Chem. 2016;88(10):5430–37. doi: 10.1021/acs.analchem.6b00822. [DOI] [PubMed] [Google Scholar]
- 16.Neill A, Nowak C, Patel R, Ponniah G, Gonzalez N, Miano D, Liu H. Characterization of recombinant monoclonal antibody charge variants using OFFGEL fractionation, weak anion exchange chromatography, and mass spectrometry. Anal Chem. 2015;87(12):6204–11. doi: 10.1021/acs.analchem.5b01452. [DOI] [PubMed] [Google Scholar]
- 17.Wypych J, Li M, Guo A, Zhang Z, Martinez T, Allen MJ, Fodor S, Kelner DN, Flynn GC, Liu YD, et al. Human IgG2 antibodies display disulfide-mediated structural isoforms. J Biol Chem. 2008;283(23):16194–205. doi: 10.1074/jbc.M709987200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dillon TM, Ricci MS, Vezina C, Flynn GC, Liu YD, Rehder DS, Plant M, Henkle B, Li Y, Deechongkit S, et al. Structural and functional characterization of disulfide isoforms of the human IgG2 subclass. J Biol Chem. 2008;283(23):16206–15. doi: 10.1074/jbc.M709988200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu YD, Chen X, Van Enk JZ, Plant M, Dillon TM, Flynn GC. Human IgG2 antibody disulfide rearrangement in vivo. J Biol Chem. 2008;283(43):29266–72. doi: 10.1074/jbc.M804787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Schenauer MR, McCarter JD, Flynn GC. IgG1 thioether bond formation in vivo. J Biol Chem. 2013;288(23):16371–82. doi: 10.1074/jbc.M113.468397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, Flynn GC. Cysteine racemization on igg heavy and light chains. J Biol Chem. 2013;288(48):34325–35. doi: 10.1074/jbc.M113.506915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu S, Wen D, Weinreb PH, Sun Y, Zhang L, Foley SF, Kshirsagar R, Evans D, Mi S, Meier W, et al. Characterization of trisulfide modification in antibodies. Anal Biochem. 2010;400(1):89–98. doi: 10.1016/j.ab.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Huh JH, White AJ, Brych SR, Franey H, Matsumura M. The identification of free cysteine residues within antibodies and a potential role for free cysteine residues in covalent aggregation because of agitation stress. J Pharm Sci. 2013;102(6):1701–11. doi: 10.1002/jps.23505. [DOI] [PubMed] [Google Scholar]
- 24.Schmid I, Bonnington L, Gerl M, Bomans K, Thaller AL, Wagner K, Schlothauer T, Falkenstein R, Zimmermann B, Kopitz J, et al. Assessment of susceptible chemical modification sites of trastuzumab and endogenous human immunoglobulins at physiological conditions. Commun Biol. 2018;1(1). doi: 10.1038/s42003-018-0032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroeder HW, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125(3):197–218. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Giuseppe D, Frosali S, Priora R, Di Simplicio FC, Buonocore G, Cellesi C, Capecchi PL, Pasini FL, Lazzerini PE, Jakubowski H, et al. The effects of age and hyperhomocysteinemia on the redox forms of plasma thiols. J Lab Clin Med. 2004;144(5):235–45. doi: 10.1016/j.lab.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Rossi R, Giustarini D, Milzani A, Dalle-Donne I. Cysteinylation and homocysteinylation of plasma protein thiols during ageing of healthy human beings. J Cell Mol Med. 2009;13:3131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carballal S, Radi R, Kirk MC, Barnes S, Freeman BA, Alvarez B. Sulfenic acid formation in human serum albumin by hydrogen peroxide and peroxynitrite. Biochemistry. 2003;42(33):9906–14. doi: 10.1021/bi027434m. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima F, Shibata T, Kamiya K, Yoshitake J, Kikuchi R, Matsushita T, Ishii I, Giménez-Bastida JA, Schneider C, Uchida K. Structural and functional insights into S-thiolation of human serum albumins. Sci Rep. 2018;8(1):1–12. doi: 10.1038/s41598-018-19610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sotgia S, Carru C, Pinna GA, Deiana L, Zinellu A. D-penicillamine Interferes with S-homocysteinylation and S-cysteinylation of LDL apolipoprotein B. J Clin Pharmacol. 2011;51(12):1728–32. doi: 10.1177/0091270010385933. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Quintela A, Alende R, Gude F, Campos J, Rey J, Meijide LM, Fernandez-Merino C, Vidal C. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol. 2008;151(1):42–50. doi: 10.1111/j.1365-2249.2007.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


