Abstract
The objective of this study was to determine the influence of biochar obtained from exothermic production of lodgepole pine (Pinus contorta) and quaking aspen (Populus tremuloides) on sheep performance and diet digestibility and on preference for a ration enriched with this carbon-based material. Twenty-four lambs were housed in individual pens and assigned to one of three treatment groups (eight animals per group), where they received: 1) a 60:40 ration of alfalfa:barley (Control), 2) an isoenergetic and isonitrogenous ration with alfalfa, barley, and 2% biochar (BC), and 3) a simultaneous offer of the Control and BC rations (Choice). Lambs were exposed to two consecutive feeding periods (Period 1: 13 d and Period 2: 21 d), representing time intervals where the evolution of intake, animal performance, and rumen parameters were assessed; in vivo digestibility was determined during the last 5 d of the study. Ration intake did not differ among groups of lambs (P > 0.10), although during some days in Period 2, intake was greater for the BC and Choice groups than for the Control group (P < 0.05). Lambs in Choice had a lower preference for BC than for the Control ration (Period 1: P < 0.05; Period 2: P < 0.10), although they incorporated a substantial amount of BC (39 and 40%, for Periods 1 and 2, respectively) into their diets. No differences in body weight gains (ADG) or gain-to-feed ratios were found among groups of animals (P > 0.10), although dry matter digestibility and digestible dry matter intake was greater for lambs in the BC group than for lambs in Control group (P < 0.05). The ruminal concentration of the volatile acid acetate in Period 2 was greater for BC than for Choice (P < 0.05). During the same period, the concentration of valerate and ruminal pH values were greater in BC than in Control (P < 0.05). Thus, the addition of biochar to grain-based diets enhanced diet digestibility and influenced some ruminal parameters in lambs. Nevertheless, these positive effects were not reflected in significant improvements on ADG or feed conversion efficiencies. Lambs offered choices between Control and BC rations formed a diet with concentrations of biochar of ~1.2%, suggesting that these animals would tolerate such levels without reductions in ration palatability.
Keywords: diet selection, digestibility, feed efficiency, feed intake, lamb
Introduction
Biochars are pyrolysis-based products intended for use as a soil amendment to increase soil fertility, with positive effects on field water holding capacity, pH, cation exchange capacity, nutrient availability, and fertilizer use efficiency (Atkinson et al., 2010; Manyà, 2012; Knox, 2018). The ability of biochars to bind to different chemicals (Thies and Rilling, 2012) and to adsorb microorganisms (Leng, 2014) and toxins (Toth and Dou, 2016) make these substances appealing for their use in animal feeding systems to enhance animal performance and reduce environmental impact. For instance, some studies have shown that the addition of biochar to ruminant diets (~1% of the diet) lead to reductions in methane emissions in vitro (Hansen et al., 2012; Leng et al., 2012a; Saleem et al., 2018) and in vivo (Leng et al., 2012b, Winders et al., 2019), which attenuates environmental impacts and increases the efficiency of nutrient use by ruminants (Silivong and Preston, 2015). The mechanism underlying this process has been attributed to the electrical conductivity and electron-buffering capacity of carbonaceous materials such as biochars (Yu et al., 2015), which enhance the redox reactions that take place in the rumen, increasing the energy conversion efficiency in livestock (Leng et al., 2013). Interactions of biochar surfaces with rumen microorganisms that enhance the efficiency of energy production and utilization have also been discussed (Leng et al., 2012a,b). In addition, since most biochars are alkaline in nature, they may buffer rumen pH, resulting in improved livestock weight gains under high-energy-containing diets (Kammann et al., 2017).
Despite all the aforementioned benefits, much of the scientific information available on biochars and livestock feeding comes from materials produced in Eurasia and Oceania or from in vitro studies. Information about in vivo utilization of biochars produced from the combustion of resources in North America (e.g., trees such as lodgepole pine, aspen, or olive) and fed to sheep are limited, particularly when feeding high concentrate diets (Winders et al., 2019). In addition, it is unknown whether livestock can self-select biochar given the potential benefits of this substance on ruminant nutrition and productivity. If ruminants learn to ingest biochar, then they will select the product based on their specific and dynamic needs, which may enhance their nutrition and welfare. Thus, the objective of this study was to determine the impact of biochar obtained from the exothermic production of lodgepole pine (Pinus contorta) and quaking aspen (Populus tremuloides) on sheep performance and diet digestibility and on preference for a ration enriched with this carbon-based material.
Materials and Methods
Animals and management
The study was conducted at the Green Canyon Ecology Center, Utah State University, located in Logan, UT (41°44′76″N; −111°50′3.80″W) between 28 May and 24 July 2018, according to experimental procedures approved by the Utah State University Institutional Animal Care and Use Committee (approval no. 10032). Throughout the study, lambs had free access to culinary water and trace mineral salt blocks.
Twenty-four 3-mo-old Finn-Columbia-Polypay-Suffolk crossbred lambs of both sexes with an average initial body weight (BW) of 37.9 ± 0.8 kg (mean ± SEM) were individually penned outdoors under a protective roof in individual adjacent pens with dirt floors, measuring 2.4 × 3.6 m. All animals were orally dosed against gastrointestinal parasites (Albendazole [Spectrum Pharmaceuticals, Cambridge, MA] at 7.5 mg/kg BW) and vaccinated with Clostridium perfringens type C and D toxoid (Zoetis Animal Health, Fairfield, NJ). Lambs were fed ad libitum amounts of alfalfa pellets and 300 g of rolled barley (as-fed basis)/animal/day as their basal diet for 14 d as an adaptation period to their pens and feeding conditions.
Biochar production
Biomass from whole trees, including limbs and needles, of lodgepole pine (Pi. contorta) and quaking aspen (Po. tremuloides) were pyrolyzed by commercial biochar producers Biochar Solutions Inc. and Confluence Energy (Kremmling, CO) through their proprietary two-stage process. In the first stage of the process, the material was carbonized in an oxygen limited environment at temperatures between 500 and 700 °C for less than 1 min. In the second stage, the material was held in a hot gas environment with no oxygen for up to 14 min at temperatures between 300 and 550 °C, and then cooled to ambient temperature. The preparation process results in two size fractions of biochar of different particle size: 1) approximately 80% of material is 1.5 cm long by 1 cm wide by 0.5 cm thick, and about 20% of the material is a fine dust fraction in the order of 10 to 100 s of microns. Given this wide distribution in size, the material used for the study was biochar that passed through a 1-mm screen.
It is noted that biochar is not an approved feed ingredient in the United States, although chemical analyses support its safety for use as a feed ingredient. The biochar was characterized by Control Labs, Watsonville, CA, using the International Biochar Initiative Laboratory tests for certification program. Methods 3050B/6020 (US EPA, 1996, 1998a) were used to measure concentration of arsenic, cobalt, molybdenum, sodium, and selenium in the biochar, which were all nondetectable with detection minimums of 0.46, 0.46, 0.46, 459.1, and 0.92 mg/kg, respectively. Method 7471 (US EPA, 1998b) was used to measure concentration of mercury, which was also nondetectable with a detection minimum of 0.001 mg/kg. Cadmium and lead concentration were detected at 0.2 and 2.0 mg/kg (detection minimum 0.18; methods 3050B/6020; US EPA, 1996, 1998a). The biochar had 92.6% dry matter (DM), and a composition of 82.7% carbon, 0.6% total nitrogen, and 99.3% organic matter on a DM basis (ASTM D1762-84), with a pH value of 8.36.
Treatments and experimental design
Lambs were randomly assigned to three treatment groups (N = 8 lambs per group) and offered ad libitum amounts of the following rations: 1) Control, a basal diet of alfalfa hay and barley (60:40; Table 1); 2) Biochar (BC), a isocaloric and isonitrogenous basal diet containing alfalfa, barley and 2% biochar (Table 1), and 3) Choice (CH), lambs had a simultaneous offer of ad libitum amounts of the Control and BC rations. The two rations used in the study were designed to minimally affect crude protein (CP) and digestible energy (DE) concentration (Table 1). Rations were ground to a particle size of 1 to 3 mm to reduce sorting and they were prepared by mixing the ingredients into batches that were fed to animals during periods of 7 to 10 d, when new batches were made.
Table 1.
Nutritional characteristics (% of DM) of the rations used in the study
| Control | BC1 | |
|---|---|---|
| Ingredient2 | g/kg, as-fed basis | |
| Alfalfa | 60 | 54.5 |
| Barley | 40 | 43.5 |
| Biochar | — | 2 |
| Composition of the diets | DM basis | |
| CP, % | 15.6 ± 0.23 | 14.8 ± 0.4 |
| ADF, % | 27.7 ± 1.3 | 26.3 ± 0.8 |
| NDF, % | 34.8 ± 0.7 | 33.4 ± 0.4 |
| ADL, % | 6.4 ± 0.3 | 6.7 ± 0.3 |
| NDFD (30 h),4 % | 13.5 ± 0.7 | 12.7 ± 0.5 |
| NDFD (30 h)_NDF,5 % | 38.9 ± 1.9 | 37.9 ± 1.2 |
| DE,6 Mcal/kg | 3.05 | 3.05 |
1BC, diet containing biochar.
2All ingredients were ground to a particle size of 2 to 4 mm.
3SEM composited across two periods (P1 and P2).
4In vitro NDF digestibility at 30 h.
5NDFD as a percentage of NDF.
6Calculated based on values obtained from NRC (1985).
Lambs were randomly distributed across groups and pens, considering the variation of gender (female and wethers) and weight, resulting in a uniform distribution of animals within each treatment group. Lambs in the Control, BC, and CH groups had initial BW of 37.9 ± 1.5; 38.0 ± 1.4; and 37.8 ± 1.4 kg, respectively.
Exposure to the rations involved a period of familiarization, and then two periods of measurements (Periods 1 and 2). Intake was measured on a daily basis during Periods 1 and 2, whereas rumen fluid and blood samples were collected at the end of each of these periods. Samples of refusals and feces were collected during the last 5 d of Period 2 for fecal output and digestibility estimates (see below).
Familiarization period
All lambs were exposed to a familiarization period, where the Control and BC groups received their respective diets in ad libitum amounts from 0700 to 1600 for eight consecutive days (12 June to 19 June 2018). The amounts offered were adjusted every morning, so that animals had at least 20% of refusal in their feeders on the ensuing day at 0700. The Choice group was offered the BC diet on even-numbered days and the Control diet on odd-numbered days. Refusals were collected at 1600 and weighed, and no other feed was offered until the following day.
Periods of measurements
The three groups received their respective diets in ad libitum amounts from 0700 to 1600, as described for the familiarization period. Rations were offered in two separate wooden containers for the Control and BC groups. For the CH group, rations were also presented in two separate containers, but each container had a different ration (Control and BC) and the placement of the feed in each container (left vs. right) was randomized across days. The amounts offered for each ration were adjusted every morning, so that animals had at least 20% of refusal in their feeders on the ensuing day at 0700 to ensure that lambs consistently selected a ration rather than an eating position.
To assess the evolution of intake, animal performance, and rumen parameters across time, there were two periods of exposure to the diets. Period 1 occurred from 20 June to 2 July 2018 (13 d), and Period 2 occurred from 3 July to 23 July (21 d).
Measurements
Intake
Daily ration intake was measured by the difference between the amounts of ration offered and refused. Dry matter intake (DMI) was expressed as g DM consumed/kg BW. For the Choice group, ration preference by lamb was estimated as the daily proportion of the DMI, calculated for each ration (Control and BC), relative to the total amount of DMI.
Animal growth and feed efficiency
Lambs were weighed, with a previous liquid and solid fast period of 12 h. Average daily weigh gains (ADG) were estimated as the weight gained during a period divided by the number of days that elapsed during that period. The animals were weighed the day before the familiarization period had started (11 June 2018) and at the end of Periods 1 (3 July 2019) and 2 (24 July 2019). Intake and gain data were used to estimate feed conversion efficiency as gain-to-feed ratio (kg of BW gain:kg of DM intake) for Periods 1 and 2 of the study.
Nutritional analyses
Samples (50 g) of each of the rations were taken on a daily basis and then composited by period. During the last 5 d of the study (Period 2; 19 July to 23 July), representative (20% of the amount retrieved) daily samples of refusals were collected, as well as daily fecal grab samples (20 to 30 g DM) from each lamb in the study, retrieved between 0800 and 0900. These samples were used to determine fecal DM output and in vivo digestibility during that period (see below).
Samples of feed, orts, and feces from each lamb were placed in plastic seal top bags, labeled and immediately stored in a freezer at −20 °C. Samples were subsequently freeze dried (Free Zone 18 Liters, Labconco Corporation, Kansas City, MO) at −60 °C until two consecutive weights did not differ in a 24-h period, and subsequently ground to pass the 1-mm screen of a Wiley mill (model 4; Thomas Scientific Swedesboro, NJ). Feed, orts, and fecal samples were then composited by lamb over the 5-d sampling period.
All composited samples were analyzed for DM, CP, acid detergent fiber (ADF), neutral detergent fiber (NDF), and acid detergent lignin (ADL) contents. DM was determined by drying the samples at 105 °C for 3 h in a forced-air drying oven (Shreve et al., 2006). CP was calculated by analyzing the N concentration of the samples using a Leco FP-528 nitrogen combustion analyzer (AOAC, 2000; method 990.03) and applying the 6.25 conversion factor (Mossé, 1990). NDF contents were measured according to Van Soest et al. (1991; Procedure A), and ADF contents were measured according to AOAC (1990; Method 973.18). ADL was determined following the procedures described by Robertson and Van Soest (1981). Composited samples for rations across periods were also analyzed for NDF digestibility at 30 h (NDFD 30h) using the DaisyII system (Damiran et al., 2008). The DE of the rations was calculated according to NRC (1985).
Fecal DM output and in vivo digestibility
Fecal DM output (FO) during Period 2 was determined using the concentration of an internal marker, ADL, in the ration consumed and in feces (Van Soest, 1994). Fecal output was then determined using the following formula: FO (g/d) = [DMI (g/d) × ADL in feed (g/g)]/ADL in feces (g/g)] (Cochran and Galyean, 1994). Once fecal output was determined, dry matter digestibility (DMD) was calculated for each lamb as follows: DMD (%) = {[DMI (g/d) − FO (g/d)]/DMI (g/d)} × 100] (Cochran and Galyean, 1994).
Daily intakes and fecal outputs of NDF, ADF, and N during Period 2 were used to calculate their apparent total tract digestibility (NDFD, ADFD, and ND, respectively). Digestible DMI (DDMI) was calculated as the product of DMI (g/d) and DMD.
The nitrogen excreted through the feces (g/lamb) was calculated by multiplying the fecal output by the nitrogen concentration in feces.
Rumen and blood analyses
During the last day of each sampling period, rumen fluid was collected with a stomach tube and a vacuum pump at 3 h after food distribution in the morning. The first 30 to 40 mL of rumen fluid collected from each lamb was discarded to avoid salivary contamination. Ruminal fluid was strained through four layers of cheesecloth, and its pH was immediately measured (pH meter no. 44, Beckman Instruments, Palo Alto, CA). Samples of 18 mL were added to vials containing 2 mL of 6 M HCl and stored at −20 °C before analyses for volatile fatty acids (VFA). Before analyses, samples were centrifuged at 20,000 × g for 20 min. Concentrations of VFA were determined using a gas chromatograph (Perkin ElmerAutoSystem gas chromatograph, Bridgeport Avenue, Shelton, CT) on a Restek column packed with Stabilwax-DA. Nitrogen was used as a carrier gas at 150 kPa. The oven temperature was 125 °C, and the detector, injector, and column temperature were 100 °C.
Blood samples (without EDTA added; Becton Dickinson Vacutainer System; Becton Dickinson and Company, Franklin Lakes, NJ; 10-mL serum vacutainer tubes) were collected via jugular venous puncture during the last day of each sampling period 3 h after food distribution in the morning. Samples were allowed to clot for 45 min before being centrifuged (1,500 rpm for 15 min). The serum was extracted, placed in 1.5-mL microcentrifuge tubes, and immediately submitted to the Utah Veterinary Diagnostic Laboratory (Logan, UT) for blood urea nitrogen (BUN) analyses. The assay was performed with a Siemens Dimension Xpand Plus analyzer (Siemens Healthcare Diagnostics, Newar, DE) using Siemens urea nitrogen flex reagent, in an enzymatic method, which uses urease enzyme in a bichromatic rate technique.
Statistical analyses
Ingestive, performance, and physiological response variables were analyzed as a split-plot in time design with lambs (random factor) as the whole plot nested within Group (Control, BC, and Choice) as the whole-plot factor. Day (response variables: intake, digestibility, fecal output) or Period (response variables: ADG, efficiency, rumen, and blood parameters) were the split-plot factors (repeated measures). The mixed model formulation was as follows: , where Yijk = response variable, µ = mean, αi = fixed effect of Group, βi = fixed effect of time, (αβ)ij = interaction between Group and time, ηk(i) = whole-plot error, and εk(ij) = split-plot error (Littell et al., 1998; Wang and Goonewardene, 2004).
Separate analyses were conducted for the CH group to estimate lamb intake, where animal was the whole plot, ration (Control or BC) was the whole-plot factor, and day was the split-plot factor (repeated measure). Percentage of preference for each ration [(intake of a ration/total ration intake) × 100] was analyzed with day and period and their interaction as fixed factors and lamb and period × lamb as the random factors. Radial smoothing of the repeated measures on each lamb at each period was applied. The 95% CI of the estimated percentage was used to determine the range in which the true average proportion selected could vary. A preference was considered when the CI for the ration was greater than a theoretical mean of 50% (i.e., equal or indifferent preference), and when it did not include 50%.
All analyses were computed using the Mixed procedure of SAS (SAS Inst., Inc. Cary, NC; Version 9.4 for Windows). Least squares means (LSMeans) were compared pairwise using the Least Significant Difference test when F-ratios were significant (P < 0.05) and reported along with their standard errors (SEM). A tendency was considered when 0.10 > P > 0.05.
To adjust the temporal autocorrelation observed in data measured on time, covariance structures were tested to fit the models, based on Akaike’s information criterion, where the smallest value represents the best model adjusted.
The normal distribution of data was tested by Kolmogorov–Smirnov test (P > 0.05). Data were transformed when necessary (preference, feed efficiency) by the logarithmic function to meet homogeneity of variance assumptions, and back transformed to report LSMeans.
Results
Diet quality
The average composition of the rations used in the study is presented in Table 1. Rations (Control and BC) had similar concentrations of CP, fiber, and lignin, and were isocaloric.
Familiarization period
During the 8-d familiarization period, there were no differences in ration intake among groups of lambs (33.7 [Control], 34.5 [BC], and 30.2 g/kg BW [Choice]; SEM = 2.7 g/kg BW; P = 0.465; 1.3 [Control], 1.3 [BC], and 1.2 kg/d [Choice]; SEM = 0.1 kg/d; P = 0.342), and no Group × Day interaction (P > 0.10) was detected, although ration intake increased across days for all groups (Day effect; P < 0.0001).
Ration intake, animal growth, and feed efficiency
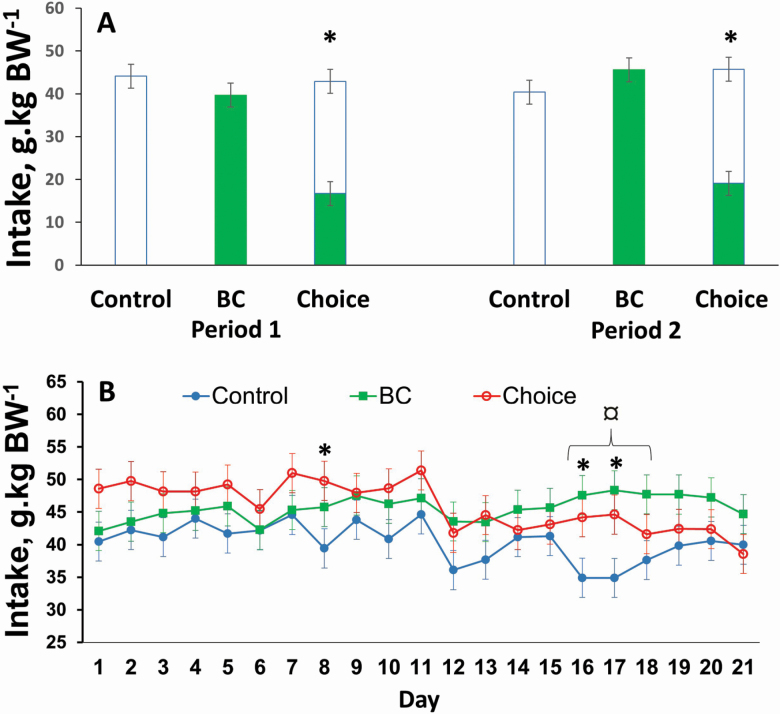
There were no differences in average ration intake for the three groups of lambs tested, either during Period 1 (Group effect; P = 0.545; Day effect; P = 0.014; Group × Day; P = 0.944; Figure 1A) or during Period 2 (Group effect; P = 0.186; Day effect; P = 0.001; Figure 1A). A Group × Day interaction (P = 0.045) was detected for Period 2 given that during some days, the Control group ate less than the BC and/or the Choice groups (P < 0.05; Figure 1B).
Figure 1.
(A) Average intake by three groups of lambs offered three different rations: 1) Control: alfalfa:barley (60:40), 2) BC: control containing 2% of biochar, and 3) Choice: lambs had a choice between Control and BC. Lambs were fed the rations during two consecutive periods (Periods 1 and 2). *Lambs in Choice ate more Control than BC ration (P < 0.05). (B) Daily intake by the three groups of lambs during Period 2, where a Group × Day interaction was detected (P = 0.045). *Choice had greater ration intake than Control (P < 0.05). ¤BC had greater ration intake than Control (P < 0.05). Values are means for eight animals per group. Vertical bars represent ±SEM.
Lambs in the choice group ate on average more Control than BC ration during both periods of the study (Ration effect; P = 0.01 and P = 0.04; Ration × Day; P = 0.729 and P = 0.106, for Periods 1 and 2, respectively; Figure 1A).
Preference for BC was on average 39 ± 4% (with 95% CI of [29, 50%]) and 40 ± 4% (with 95% CI of [30, 51%]) for Periods 1 and 2, respectively. The estimated difference from 50% was −10 ± 4% with 95% CI of (−19, −1%) for Period 1, indicating significantly lower preference than 50% (P = 0.038). The estimated difference was −9 ± 4% with 95% CI (−18, 0%) with a trend for a lower preference than 50% (P = 0.051).
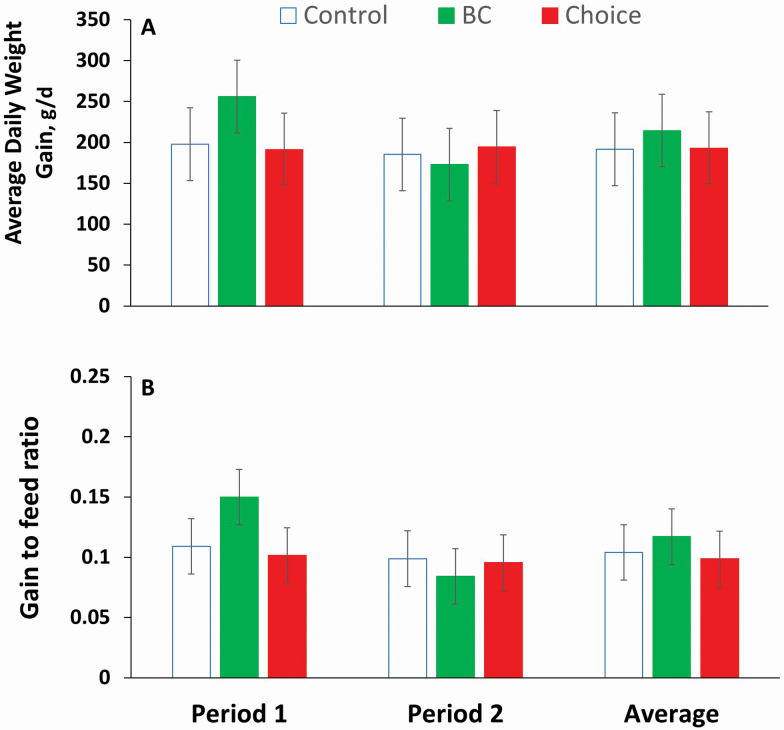
No differences were detected in ADG among groups of lambs for any of the periods assayed or for the two combined periods (Group effect; P = 0.839; Period effect; P = 0.392; Group × Period; P = 0.576; Figure 2A). Likewise, gain-to-feed ratios did not differ among groups of lambs (Group effect; P = 0.502; Group × Period; P = 0.334; Figure 2B). Nevertheless, ADG and feed conversion efficiencies during Period 1 were 23 and 28% greater, respectively, for the BC than for the Control group (Figures 2A and 2B).
Figure 2.
(A) Average daily weight gains and (B) gain-to-feed rations by three groups of lambs offered three different rations: 1) Control: alfalfa:barley (60:40), 2) BC: Control containing 2% of biochar, and 3) Choice: Lambs had a choice between Control and BC. Values are means for eight animals per group. Vertical bars represent ±SEM.
Fecal DM output and in vivo digestibility
No differences in fecal output (P = 0.359), excreted N (P = 0.643), N (P = 0.278), or NDF (P = 0.275) digestibilities were detected among groups of lambs (Table 2). However, lambs in the BC group had greater levels of DMD than the Control group (P = 0.013), and DMD in BC tended (P = 0.07) to be greater than in the Choice group (Table 2). Likewise, the BC group had greater DDMI than the Control group (P = 0.003; Table 2). The greatest values of ADFD were observed for lambs consuming the Control diet (P = 0.009; Table 2). No Day effect or Group × Day interactions (P > 0.10) were detected for fecal output or any of the digestibility estimates.
Table 2.
Dry matter, NDF, ADF, and N digestibilities, intake and fecal output by three groups of lambs (N = 8) fed three different rations: (1) Control: alfalfa:barley (60:40), (2) BC: Control containing 2% of biochar, and (3) Choice: Lambs had a choice between Control and BC
| Item | Control | BC | Choice | SEM1 | P-value2 |
|---|---|---|---|---|---|
| DMD, % | 57.0b | 60.7a | 58.5ab | 0.80 | 0.013 |
| NDFD, % | 26.8 | 29.3 | 26.5 | 1.30 | 0.275 |
| ADFD, % | 26.6a | 21.2b | 19.6b | 1.50 | 0.009 |
| ND,3 % | 53.0 | 58.4 | 53.4 | 2.58 | 0.278 |
| DMI,4 g.kg BW−1.d−1 | 41.9 | 43.3 | 44.5 | 2.19 | 0.692 |
| DMI,5 kg/d | 1.87 | 1.93 | 1.85 | 0.19 | 0.960 |
| DDMI, g.kg BW−1.d−1 | 19.5b | 26.4a | 22.8ab | 1.24 | 0.003 |
| Fecal output, g.kg BW−1.d−1 | 15.0 | 17.1 | 16.2 | 1.03 | 0.359 |
| Excreted N, g kg BW−1 d−1 | 0.36 | 0.39 | 0.40 | 0.03 | 0.643 |
1Standard error of the group mean.
2Fixed effect of group.
3ND, nitrogen digestibility.
4Average intake over two consecutive periods.
5Average intake over two consecutive periods.
a,bMeans in a row with different letters differ (P < 0.05).
Blood and rumen determinations
During Period 2, an increment in the ruminal concentrations of the VFAs acetate (Group × Period; P = 0.014) and propionate (Group × Period; P = 0.031) was observed for the group BC relative to Period 1 (Table 3). In contrast, the group Choice revealed a tendency for the opposite pattern (acetate: P = 0.102; propionate: P = 0.061), and no differences between periods were observed in the Control group for these variables (P > 0.10; Table 3). The concentration of acetate in Period 2 was greater for BC than for Choice (P = 0.040) and it was similar between BC and Control groups (P = 0.132) during the same period. A decline in the concentration of valerate was observed for the Control group (Group × Period; P = 0.035) and in ruminal pH values (Group × Period; P = 0.046) in Period 2 relative to Period 1 (Table 3). During Period 2, the concentration of valerate (P = 0.039) and ruminal pH values (P = 0.049) were greater in BC than in Control (Table 3).
Table 3.
Rumen VFA, BUN, and rumen fluid pH after 13 (Period 1) and 21 days (Period 2) of feeding three groups of lambs (N = 8) with three different rations: (1) Control: alfalfa:barley (60:40), (2) BC: Control containing 2% of biochar, and (3) Choice: Lambs had a choice between Control and BC
| BC | Choice | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | Period 1 | Period 2 | Period 1 | Period 2 | Period 1 | Period 2 | SEM1 | P-value2 | P-value3 | P-value4 |
| VFA (mmol/L) | ||||||||||
| Acetate | 76.2a | 113.8bc | 107.2b | 83.9ab | 96.5ab | 92.4ab | 9.6 | 0.993 | 0.667 | 0.014 |
| Propionate | 31.5a | 42.6b | 42.2ab | 31.5ab | 33.1ab | 35.0ab | 4.1 | 0.830 | 0.809 | 0.031 |
| Acetate:propionate | 2.38 | 2.70 | 2.50 | 2.70 | 2.86 | 2.63 | 0.3 | 0.758 | 0.380 | 0.374 |
| Butyrate | 16.2 | 18.7 | 22.6 | 17.2 | 23.2 | 20.4 | 2.2 | 0.168 | 0.261 | 0.153 |
| Isobutyrate | 0.5 | 0.7 | 0.7 | 0.6 | 0.7 | 0.5 | 0.1 | 0.778 | 0.984 | 0.115 |
| Isovalerate | 0.1 | 0.4 | 0.3 | 0.4 | 0.3 | 0.4 | 0.1 | 0.517 | 0.116 | 0.397 |
| Valerate | 2.4ab | 3.1a | 3.2a | 2.4ab | 3.2a | 2.0b | 0.4 | 0.815 | 0.111 | 0.035 |
| Total | 127.0 | 179.1 | 176.0 | 135.7 | 157.0 | 150.5 | 14.5 | 0.980 | 0.880 | 0.020 |
| BUN (mg/dL) | 21.1 | 19.1 | 20.8 | 20.3 | 21.1 | 17.8 | 1.2 | 0.783 | 0.003 | 0.145 |
| pH | 5.8ab | 6.0a | 5.9ab | 5.9ab | 6.0a | 5.7b | 0.1 | 0.869 | 0.756 | 0.046 |
1Standard error of the group mean.
2Fixed effect of group.
3Fixed effect of period.
4Group × period effect.
a,b,cMeans within a row with different superscripts differ (P < 0.05).
Discussion
Intake and preference
The present study reveals that the addition of biochar (2%) to an alfalfa:barley ration (BC), or the simultaneous offer of rations with or without biochar (Choice) did not modify feed intake relative to the offer of a ration without biochar (Control). Previous studies using lesser or greater concentrations of biochar than those offered to the group BC yielded similar results. For instance, DMI was not affected when biochar was present in the diet of growing cattle (0.8% to 3%; Winders et al., 2019; 0.6% Leng et al., 2012b) or goat diets (1%, Silivong and Preston, 2015), although biochar added at a rate of 0.8% stimulated intake in a finishing cattle diet (Winders et al., 2019). Likewise, intake by dairy cows was enhanced with the addition of 20 or 40 g/d of activated carbon to poor-quality corn silage diets containing mycotoxins, as activated carbon bind to these toxins and attenuate their negative postingestive effects (Erickson et al., 2011). Beyond the potential actions as a toxin binder, the mechanism by which biochar may enhance feed intake in livestock is not completely understood but it may involve providing appropriate microenvironments for an improved microbial habitat, in particular by a substrate with a large surface area to weight ratio (Leng et al., 2012a, b). Increased microbial activity may entail an enhanced rate of passage through the gastrointestinal tract and thus greater levels of feed intake. Such effect may be more evident when diets are of lower nutritional quality than those offered in the present study (alfalfa, barley), where rates of passage are already high without the addition of biochar. Nevertheless, during some days in Period 2 of the study, it was evident that intake by the BC and Choice groups was greater than intake by the Control group.
Lambs offered a choice between the Control and BC rations preferred the feed without biochar (~60%). These results are consistent with previous research showing that dairy cows reduce their preference for rations that contain activated carbon (10 to 80 g/d; Erickson et al., 2011). Despite lower preference, lambs incorporated a significant proportion of BC (~40%) into their diets, representing 1.2% of biochar in the mixed ration ingested, a concentration closer to what has been typically used (i.e., 0.6% to 1%) in in vitro and in vivo studies. Thus, even when the biochar-containing ration was less preferred than the Control ration, lambs incorporated significant amounts of biochar into their diet, which were likely beneficial at those rates. Collectively, animals in the Choice group did not prefer the BC ration but their selection diluted the concentration of biochar in their diet, a behavior that led to some improvements in diet digestibility and that modified some ruminal parameters. Foraging preferences in ruminants are triggered by learning mechanisms that integrate the feeds’ oro-sensorial characteristics with their postingestive consequences (Provenza, 1995, 1996), such that those feeds that promote an homeostatic utility to the animal are ingested and those that reduce fitness are avoided. Nevertheless, no clear improvements in diet digestibility or performance were observed for lambs of the Choice group relative to lambs in the Control group (see below). However, potential aversive and stressful states triggered by ingesting the same ration and flavors too frequently or in excess might have been less prevalent in the Choice group, given that sheep are generalist herbivores that evolved ingesting diverse diets composed of different flavors and chemicals (Provenza, 1996). Sheep consuming a diversity of feeds had lower levels of stress than sheep exposed to monotonous rations (Catanese et al., 2013). Similarly, sheep in the Choice group experienced a greater diversity of flavors in their diet, and thus a greater degree of sensorial benefits, provided by the addition of charcoal to one of the rations on offer.
Animal weights, feed efficiency, and digestibility
Previous research has shown that biochar enhances BW gains and feed efficiency in cattle (Leng et al., 2012b) and goats (Silivong and Preston, 2015). Nevertheless, the present study did not find significant differences among groups of lambs for these parameters, an outcome that was probably influenced by a high variability among individuals, particularly during Period 1 of the study. During this period, ADG and feed conversion efficiencies were 23 and 28% greater, respectively, for the group that ingested the biochar-containing ration (BC) than for the group that received the ration without biochar (Control), although differences were non-significant. It is also possible that the type of biochar used in the current study presented different characteristics than those used in previous research where positive responses on efficiency and body weight gains were found (Leng, et al., 2012b; Silivong and Preston, 2015). For instance, different types of biochar of known and unknown sources significantly varied in their efficacy at reducing methane production during in vitro studies (Leng et al., 2013). The concentration of biochar in the ration might have also been a factor that constrained differences in ADG and efficiencies among groups. Silage mixtures containing 8.8% and 16.6% of biochar led to increments in total VFA production (Calvelo Pereira et al., 2014), which suggests increased microbial fermentation (Guan et al., 2008) at those higher concentrations of biochar. Finally, the length of exposure to the rations might have not been long enough to reveal performance differences among treatments. Nevertheless, growing sheep in other studies (e.g., Poli et al., 2018) change their performance responses to dietary treatments within the time frame of the combined periods assayed in this study (i.e., 34 d).
Findings on digestibility in the present study support the notion that biochar had a positive influence on lamb nutrition, as the group that had biochar in the ration (BC) had greater values of DMD and DDMI than the group not fed biochar (Control). Nevertheless, the greatest values of ADFD were observed for the Control diet, an outcome that could be attributed to lower fermentation rates of the ADF fraction present in biochar, which was produced from woody species that typically present lower levels of ADF digestibility.
The majority of studies exploring the effects of biochar on ruminant diets involve in vitro testing, which do not accurately replicate what happens inside the animal, as they do not include ingestive and other physiological processes occurring in vivo (Villalba and Provenza, 2010). Our study and others (e.g., Silivong and Preston, 2015; Winders et al., 2019) show that addition of biochars to rations can actually enhance digestibility in ruminant animals. Biochars may enhance growth of certain rumen microbial communities by providing solid surface areas where microorganisms could efficiently transfer substrate and improve the efficiency of ATP production, increasing feed digestibility and digestion efficiencies (Leng, 2013). In addition, biochar surfaces contribute to enhance populations of methanotrophic relative to methanogenic microbes (Feng et al., 2012), reducing the production of methane, which leads to improvements in feed efficiency (Leng et al., 2012a,b, 2013). Propionate to acetate ratios also increase with such improvements in efficiency (Shabat et al., 2016), but no significant differences among groups were detected for this parameter in the present study, although those ratios were ~13 to 17% greater for the Choice and BC groups than for the Control group during Period 1.
The concentration of acetate in Period 2 was greater for BC group than for Choice group, and it tended to be greater for BC group than for the Control group during the same feeding period, suggesting not only greater active bacterial fermentation and host rumen epithelial absorption for the BC group (Guan et al., 2008), but also greater production of methanogenic substrates (Danielsson et al., 2017). Concentrations of acetate and propionate were more variable across periods for groups that received biochar for BC and Choice groups than for the Control group.
During Period 2, the concentration of valerate was greater in BC than in Control (Table 3), and significantly greater concentrations of rumen butyrate and valerate have been found to be related to greater feed conversion efficiencies in steers (Guan et al., 2008). During the same period, ruminal pH values were greater in BC than in Control, suggesting that biochar may have prevented the decrease in pH due to several days of feeding with high concentration of grain, which was made evident in Period 2. This pattern could be attributed to the fact that most biochars are alkaline (Kammann et al., 2017), a benefit particularly relevant when diets contain high concentration of grain, such as those offered in the present study. Biochar alkalinity is defined as the capacity of biochar to accept protons without significantly altering its chemical structure (Fidel, 2012). Biochar alkalinities vary widely among biochars, mostly given by (1) organic functional groups (0.03 to 0.92 mEq protons accepted/g), (2) carbonates (0.02 to 1.5 mEq protons accepted/g), and (3) other inorganic groups (0.00 to 0.26 mEq protons accepted/g), and with pKa values > 5 (Fidel et al., 2017).
Finally, and in contrast to other studies (Silivong and Preston, 2015) where a positive effect of biochar was found on N retention, no effect was observed of biochar on nitrogen digestibility or BUN values. Previous in vitro studies also report no effects of different sources of biochar on ammonia production (Calvelo Pereira et al., 2014).
In conclusion, the addition of biochar to alfalfa:barley diets enhanced diet digestibility and influenced some ruminal parameters in sheep, some indicative of positive effects on lamb nutrition, although increments in the VFA acetate for the BC treatment may suggest increments in methane production and reductions in microbial efficiency (Johnson and Johnson, 1995). Biochar addition to the ration or self-selection of the biochar-containing ration did not improve ADG or feed conversion efficiencies, although these parameters were enhanced over 20% (Period 1) in animals fed the biochar-containing ration. Longer periods of exposure to biochar than those provided in this study may be needed for observing significant differences in performance responses.
Lambs offered a choice between rations with or without biochar preferred the later, although they incorporated substantial amounts of biochar-containing rations into their diets (40%), suggesting that these animals would tolerate biochar at 1.2% without reductions in ration palatability.
Acknowledgments
This research was supported by grants from the Utah Agricultural Experiment Station (grant numbers 1321 and 1068). This paper is published with the approval of the Director, Utah Agricultural Experiment Station, and Utah State University, as journal paper number UAES #9248. We thank R. Stott for veterinary services and E. Cromer, A. Baker, and J. Reynolds for technical support.
Glossary
Abbreviations
- ADF
acid detergent fiber
- ADFD
ADF digestibility
- ADL
acid detergent lignin
- ADG
average daily gain
- BUN
blood urea nitrogen
- BW
body weight
- CP
crude protein
- DE
digestible energy
- DM
dry matter
- DMI
dry matter intake
- DDMI
digestible dry matter intake
- DMD
dry matter digestibility
- FO
fecal DM output
- NDF
neutral detergent fiber
- NDFD
neutral detergent fiber digestibility
- LSMeans
least squares means
- VFA
volatile fatty acids
Conflict of interest statement
None declared.
Literature Cited
- AOAC 1990. Official methods of analysis. 15th ed. Arlington (VA): Association of Official Analytical Chemists. [Google Scholar]
- AOAC. 2000. Official methods of analysis. 17th ed. Gaithersburg (MD): Association of Official Analytical Chemists. [Google Scholar]
- ASTM D1762-84. 2013. Annual book of ASTM standards. D1762-84. West Conshohocken (PA): American Society of Medical Technologists; p. 281–282. [Google Scholar]
- Atkinson C. J., Fitzgerald J. D., and Hipps N. A.. . 2010. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil. 337:1–18. doi: 10.1007/s11104-010-0464-5 [DOI] [Google Scholar]
- Calvelo Pereira R., Muetzel S., Camps Arbestain M., Bishop P., Hina K., and Hedley M.. . 2014. Assessment of the influence of biochar on rumen and silage fermentation: A laboratory-scale experiment. Anim. Feed Sci. Technol. 196:22–31. doi: 10.1016/j.anifeedsci.2014.06.019 [DOI] [Google Scholar]
- Catanese F., Obelar M., Villalba J. J., and Distel R. A.. . 2013. The importance of diet choice on stress-related responses by lambs. Appl. Anim. Behav. Sci. 148:37–45. doi: 10.1016/j.applanim.2013.07.005 [DOI] [Google Scholar]
- Cochran R. C., and Galyean M. L.. . 1994. Measurement of in vivo forage digestion by ruminants. In: Fahey G. C., Jr., editor. Forage quality, evaluation, and utilization. Hoboken (NJ): Wiley; p. 613–643. doi: 10.2134/1994.foragequality [DOI] [Google Scholar]
- Damiran D., DelCurto T., Bohnert D. W., and Findholt S. L.. . 2008. Comparison of techniques and grinding size to estimate digestibility of forage based ruminant diets. Anim. Feed Sci. Technol. 141:15–35. doi: 10.1016/j.anifeedsci.2007.04.007 [DOI] [Google Scholar]
- Danielsson R., Dicksved J., Sun L., Gonda H., Müller B., Schnürer A., and Bertilsson J.. . 2017. Methane production in dairy cows correlates with rumen methanogenic and bacterial community structure. Front. Microbiol. 8:226. doi: 10.3389/fmicb.2017.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson P. S., Whitehouse N. L., and Dunn M. L.. . 2011. Activated carbon supplementation of dairy cow diets: Effects on apparent total-tract nutrient digestibility and taste preference. Prof. Anim. Sci. 27:428–434. doi: 10.15232/S1080-7446(15)30515-5 [DOI] [Google Scholar]
- Feng Y., Xu Y., Yu Y., Xie Z., and Lin X.. . 2012. Mechanisms of biochar decreasing methane emission from Chinese paddy soils. Soil Biol. Biochem. 46:80–88. doi: 10.1016/j.soilbio.2011.11.016 [DOI] [Google Scholar]
- Fidel R. B. 2012. Evaluation and implementation of methods for quantifying organic and inorganic components of biochar alkalinity. MS Thesis, Iowa State University. Graduate Theses and Dissertations. 12752. Available from https://lib.dr.iastate.edu/etd/12752.
- Fidel R. B., Laird D. A., Thompson M. L., and Lawrinenko M.. . 2017 Characterization and quantification of biochar alkalinity. Chemosphere 167:367–373. doi: 10.1016/j.chemosphere.2016.09.151 [DOI] [PubMed] [Google Scholar]
- Guan L. L., Nkrumah J. D., Basarab J. A., and Moore S. S.. . 2008. Linkage of microbial ecology to phenotype: Correlation of rumen microbial ecology to cattle’s feed efficiency. FEMS Microbiol. Lett. 288:85–91. doi: 10.1111/j.1574-6968.2008.01343.x [DOI] [PubMed] [Google Scholar]
- Hansen H. H., Storm I. M. L. D., and Sell A. M.. . 2012. Effect of biochar on in vitro rumen methane production. Acta Agric. Scand. A Anim. Sci. 62:305–309. doi: 10.1080/09064702.2013.789548 [DOI] [Google Scholar]
- Johnson K. A., and Johnson D. E.. . 1995. Methane emissions from cattle. J. Anim. Sci. 73:2483–2492. doi: 10.2527/1995.7382483x [DOI] [PubMed] [Google Scholar]
- Kammann C., Ippolito J., Hagemann N., Borchard N., Cayuela M. L., Estavillo J. M., Fuertes-Mendizabal T., Jeffery S., Kern J., Novak J., . et al. 2017. Biochar as a tool to reduce the agricultural greenhouse-gas burden – Knowns, unknowns and future research needs. J. Environ. Eng. Landsc. Manag. 25:114–139. doi: 10.3846/16486897.2017.1319375 [DOI] [Google Scholar]
- Knox O. G. G., Weitz H. J., Anderson P., Borlinghaus M., and Fountaine J.. . 2018. Improved screening of biochar compounds for potential toxic activity with microbial biosensors. Environ. Technol. Innov. 9:254–264. doi: 10.1016/j.eti.2017.12.007 [DOI] [Google Scholar]
- Leng R. A. 2014. Interactions between microbial consortia in biofilms: A paradigm shift in rumen microbial ecology and enteric methane mitigation. Anim. Prod. Sci. 54:519–543. doi: 10.1071/AN13381 [DOI] [Google Scholar]
- Leng R. A., Inthapanya S., and Preston T. R.. . 2012a. Biochar lowers net methane production from rumen fluid in vitro. Livest. Res. Rural Dev. 24:103. [Google Scholar]
- Leng R. A., Inthapanya S., and Preston T. R.. . 2013. All biochars are not equal in lowering methane production in in vitro rumen incubations. Livest. Res. Rural Dev. 12:12. [Google Scholar]
- Leng R. A., Preston T. R., and Inthapanya S.. . 2012b. Biochar reduces enteric methane and improves growth and feed conversion in local “Yellow” cattle fed cassava root chips and fresh cassava foliage. Livest. Res. Rural Dev. 24:11. [Google Scholar]
- Littell R. C., Henry P. R., and Ammerman C. B.. . 1998. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 76:1216–1231. doi: 10.2527/1998.7641216x [DOI] [PubMed] [Google Scholar]
- Manyà J. J. 2012. Pyrolysis for biochar purposes: A review to establish current knowledge gaps and research needs. Environ. Sci. Technol. 46:7939–7954. doi: 10.1021/es301029g [DOI] [PubMed] [Google Scholar]
- Mossé J. 1990. Nitrogen to protein conversion factor for ten cereals and six legumes or oilseeds. a reappraisal of its definition and determination. Variation according to species and to seed protein content. J. Agric. Food Chem. 38:18–24. doi: 10.1021/jf00091a004 [DOI] [Google Scholar]
- NRC 1985. Nutrient requirements of sheep. 6th ed. Washington (DC): The National Academies Press. [Google Scholar]
- Poli C. H. E C., Thornton-Kurth K. J., Legako J. F., Bremm C., Hampel V. S., Hall J., Ipharraguerre I. R., and Villalba J. J.. . 2018. Self-selection of plant bioactive compounds by sheep in response to challenge infection with Haemonchus contortus. Physiol. Behav. 194:302–310. doi: 10.1016/j.physbeh.2018.06.013 [DOI] [PubMed] [Google Scholar]
- Provenza F. D. 1995. Postingestive feedback as an elementary determinant of food preference and intake in ruminants. J. Range Manage. 48:2–17. doi: 10.2307/4002498 [DOI] [Google Scholar]
- Provenza F. D. 1996. Acquired aversions as the basis for varied diets of ruminants foraging on rangelands. J. Anim. Sci. 74:2010–2020. doi: 10.2527/1996.7482010x [DOI] [PubMed] [Google Scholar]
- Robertson J. B., and Van Soest P. J.. . 1981. The detergent system of analysis and its application to human foods. In: James W. P. T., and O. Theander, editors. The analysis of dietary fiber in food. New York (NY): Marcel Dekker; p. 123–158. [Google Scholar]
- Saleem A. M., Ribeiro G. O. Jr, Yang W. Z., Ran T., Beauchemin K. A., McGeough E. J., Ominski K. H., Okine E. K., and McAllister T. A.. . 2018. Effect of engineered biocarbon on rumen fermentation, microbial protein synthesis, and methane production in an artificial rumen (RUSITEC) fed a high forage diet. J. Anim. Sci. 96:3121–3130. doi: 10.1093/jas/sky204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabat S. K., Sasson G., Doron-Faigenboim A., Durman T., Yaacoby S., Berg Miller M. E., White B. A., Shterzer N., and Mizrahi I.. . 2016. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 10:2958–2972. doi: 10.1038/ismej.2016.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreve B., Thiex N., and Wolf M.. . 2006. National Forage Testing Association Reference: Dry matter by oven drying for 3 hr at 105°C. NFTA Ref. Methods. 1–4. doi: 10.1016/j.intermet.2015.07.010 [DOI] [Google Scholar]
- Silivong P., and Preston T. R.. . 2015. Growth performance of goats was improved when a basal diet of foliage of Bauhinia acuminata was supplemented with water spinach and biochar. Livest. Res. Rural Dev. 27:3. [Google Scholar]
- Thies J. E., and Rillig M. C.. . 2012. Characteristics of biochar: Biological properties. In: Lehmann J. and Joseph S., editors. Biochar for environmental management: Science and technology. Canandaigua (NY): International Biochar Initiative; p. 117–138. doi: 10.4324/9781849770552 [DOI] [Google Scholar]
- Toth J. D., and Dou Z.. . 2016. Use and impact of biochar and charcoal in animal production systems. In: Agricultural and Environmental Applications of Biochar: Advances and Barriers. 63: 199–224. doi: 10.2136/sssaspecpub63.2014.0043.5 [DOI] [Google Scholar]
- US EPA. 1996. “Method 3050B: Acid Digestion of Sediments, Sludges, and Soils,” Revision 2. Washington (DC). doi: 10.1117/12.528651 [DOI] [Google Scholar]
- US EPA. 1998a. Inductively coupled plasma-mass spectrometry. Method 6020A. Available from https://www.epa.gov/sites/production/files/2015-07/documents/epa-6020a.pdf.
- US EPA. 1998b. Mercury or solid or semisolid waste (manual cold-vapor technique). US EPA Region II data validation SOP for EPA method 7471, Revision B. Available from https://www.epa.gov/sites/production/files/2015-07/documents/epa-7471b.pdf.
- Van Soest P. J. 1994. Nutritional ecology of the ruminant. New York (NY): Cornell University Press. [Google Scholar]
- Van Soest P. J., Robertson J. B., and Lewis B. A.. . 1991. Symposium: Carbohydrate methodology, metabolism, and nutritional implications in dairy cattle. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Villalba J. J., and Provenza. F. D.. 2010. Challenges in extrapolating in vitro findings to in vivo evaluation of plant resources. In: Vercoe P., editor. In vitro screening of plant resources for extranutritional attributes in ruminants: Nuclear and related methodologies. International Atomic Energy Agency (IAEA), Vienna, Austria. p. 233–242. doi: 10.1007/978-90-481-3297-3_11 [DOI] [Google Scholar]
- Wang Z., and Goonewardene L. A.. . 2004. The use of MIXED models in the analysis of animal experiments with repeated measures data. Can. J. Anim. Sci. 84:1–11. doi: 10.4141/A03-123 [DOI] [Google Scholar]
- Winders T. M., Jolly-Breithaupt M. L., Wilson H. C., MacDonald J. C., Erickson G. E., and Watson A. K.. . 2019. Evaluation of the effects of biochar on diet digestibility and methane production from growing and finishing steers. Transl. Anim. Sci. 3:775–783. doi: 10.1093/tas/txz027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Yuan Y., Tang J., Wang Y., and Zhou S.. . 2015. Biochar as an electron shuttle for reductive dechlorination of pentachlorophenol by Geobacter sulfurreducens. Sci. Rep. 5:16221. doi: 10.1038/srep16221 [DOI] [PMC free article] [PubMed] [Google Scholar]