Abstract
Yellow horn (Xanthoceras sorbifolia) is an oil-rich woody plant cultivated for bio-energy production in China. Soil saline-alkalization is a prominent agricultural-related environmental problem limiting plant growth and productivity. In this study, we performed comparative physiological and transcriptomic analyses to examine the mechanisms of X. sorbifolia seedling responding to salt and alkaline-salt stress. With the exception of chlorophyll content, physiological experiments revealed significant increases in all assessed indices in response to salt and saline-alkali treatments. Notably, compared with salt stress, we observed more pronounced changes in electrolyte leakage (EL) and malondialdehyde (MDA) levels in response to saline-alkali stress, which may contribute to the greater toxicity of saline-alkali soils. In total, 3,087 and 2,715 genes were differentially expressed in response to salt and saline-alkali treatments, respectively, among which carbon metabolism, biosynthesis of amino acids, starch and sucrose metabolism, and reactive oxygen species signaling networks were extensively enriched, and transcription factor families of bHLH, C2H2, bZIP, NAC, and ERF were transcriptionally activated. Moreover, relative to salt stress, saline-alkali stress activated more significant upregulation of genes related to H+ transport, indicating that regulation of intracellular pH may play an important role in coping with saline-alkali stress. These findings provide new insights for investigating the physiological changes and molecular mechanisms underlying the responses of X. sorbifolia to salt and saline-alkali stress.
Introduction
Soil salinization and alkalization are important abiotic stress conditions that adversely affected growth and development processes in plants, such as seedling growth, tillering, metabolism and transcription [1–3]. Global human population expansion and inappropriate anthropogenic activities, such as overgrazing, deforestation, and improper soil and water management, are contributing to the salinization and alkalization of an ever-increasing expanse of land area [4]. Moreover, the formation and proportion of saline-alkali-affected land can be exacerbated by certain extreme weather/climatic events (i.e. droughts, floods, and heavy rainstorms) [5, 6]. Currently, approximately 950 million hectares of land are affected by salinization worldwide, and it is highly probable that the area adversely affected will undergo further expansion in the future [7–9]. To date, however, the majority of studies on saline-affected land have tended to focus on the neutral salt tolerance of plants, whereas comparatively less attention has been paid to plant tolerance to saline-alkali stress.
Salt stress can have a range of adverse effects on plants. Firstly, high salinity reduces the osmotic potential of the soil solution, and thereby inhibits water uptake by plants [10, 11]. Secondly, an excess accumulation of Cl- and Na+ ions can induce specific ionic toxicities and nutrient imbalances [12–14]. Thirdly, salt stress may also promote an increase in the generation of reactive oxygen species (ROS), the excessive amounts of which can cause damage to intracellular components [15, 16]. Compared with salt stress per se, saline-alkali (NaHCO3 or Na2CO3) stress is associated with more severe damage to plants, owing to the combined adverse effects of salt stress, attributable high salt ion concentrations, and alkaline stress, which is related to high pH levels [17]. Furthermore, elevated pH levels may also lead to a deficiency in external protons and inhibit Na+/H+ antiport systems, thereby contributing to an accumulation of Na+ in plants [18, 19]. As counter-measures, plants have, however, evolved an array of salt/saline-alkali tolerance mechanisms, at both molecular and physiological levels, to facilitate continued growth and development, including antioxidant defence systems and osmotic adjustment [20]. In this regard, transcriptome analysis has emerged as a powerful approach for elucidating gene regulatory networks, and this has made a significant contribution to identifying numerous genes that are differentially expressed in response salt or saline-alkali stress in many cultivated plant species, including sugarcane [21], rice [22], alfalfa [23], grapevine [24], peach [25], wheat [26], and flax [27].
Xanthoceras sorbifolia, commonly known as yellow horn, is the only validated species in the genus Xanthoceras within the family Sapindaceae [28]. It is a woody deciduous shrub or small tree that is widely distributed in North China [29]. Yellow horn is considered an important bio-energy feedstock plant on account of its abundant content of seed kernel oil (55%–65%), which is rich in unsaturated fatty acids (85%–93%) [30, 31]. The plant is characterized by a strong adaptability to nutrient-poor, drought, cold, and saline-alkali conditions, and is typically found growing on marginal land [32]. Recently, X. sorbifolia has been receiving increasing attention, owing to its potential economic and biological importance, and several studies have presented transcriptomic data for this species, including those relating to oil accumulation [33, 34], fertilized ovule development [35], and abiotic stresses (i.e. salt, abscisic acid, and low temperature) [36, 37]. To date, however, no evidence has emerged to enable a comparative analysis of the mechanisms underlying the tolerance of X. sorbifolia to salt and saline-alkali stress.
Comprehensive and systematic studies that focus on the responses of plants to multiple abiotic stresses will contribute to distinguish the different processes associated with the adaptions of plants to each stress. In the present study, we accordingly adopted such an approach to examine changes in a selection of physiological indices and the activities of antioxidant enzymes in X. sorbifolia in response to treatment with NaCl or Na2CO3. Illumina sequencing technology was used to analyze the comparative transcriptome of X. sorbifolia seedlings subjected to salt and saline-alkali treatments to identify differentially expressed genes (DEGs) associated with stress tolerance. The aims of this study were to characterize the physiological changes in X. sorbifolia under salt and saline-alkali stress conditions, and to elucidate the molecular features related to salt and saline-alkali stress tolerance in this plant. These findings could contribute to determining the distinct physiological effects and gene reprogramming in response to these two stresses, and provide novel insights for further enhancing the adaptivity of X. sorbifolia to saline- and saline-alkali-contaminated environments.
Materials and methods
Plant materials and stress treatments
The X. sorbifolia superior tree (accession number: A099) from the breeding base of Shanxi Agricultural University (37°25N, 112°34E), with high oil content and a certain resistance to saline-alkali soil, was used in this study. Seeds were germinated at 25°C using the sand-hiding method. Following germination, seedlings were cultivated in a greenhouse at 23°C ± 2°C under a 14 h light/10 h dark photoperiod at a relative humidity of 60%-70%. The seedlings were irrigated regularly under natural conditions for a month. Healthy and uniform seedlings (height, ~30 cm) were transferred to tanks filled with Hoagland’s liquid medium for 1 week. All solutions were renewed at 2-day intervals. The seedlings were divided into three groups, with the plants in one group being maintained in Hoagland’s solution as controls. Seedlings in the remaining two groups were exposed to 150 mmol/L NaCl or 150 mmol/L Na2CO3 (pH 9.5) as salt and saline-alkali stress treatments, respectively. Previous studies have shown that solutions with a pH value higher than 10.0 may cause severe and rapid damage, whereas solutions with an insufficient alkalinity may make it difficult to study the responses to alkaline stress. Accordingly, a pH of 9.5 was deemed to be an appropriate pH value for examining the effects of alkaline stress [38]. At 0 (control), 4, 12, 24, and 48 h after commencing treatment, the leaves of seedlings were harvested for physiological analyses, and whole seedlings collected at 0 (control), 4, and 24 h were used as samples for transcriptome sequencing. Three biological replicates of each sample were taken for physiological analyses and transcriptome sequencing. We selected 24 h as the final time point for the purposes of transcriptome sequencing based on observations of the phenotypic changes occurring in X. sorbifolia seedlings in response to stress treatment compared with the control. All samples were immediately frozen in liquid nitrogen and stored at -80°C until further use.
Physiological measurements
The electrolyte leakage (EL) value of leaves was measured using a digital conductivity meter as described in our previous study [37]. The contents of malondialdehyde (MDA) were measured using the thiobarbituric acid (TBA) colourimetric method with malondialdehyde assay kit (Solarbio, Beijing, China), and the contents of soluble sugars were detected using the thiobarbituric acid (TBA) colourimetric method with plant soluble sugar content assay kit (Solarbio, Beijing, China). Similarly, chlorophyll content was determined according to the protocol of a chlorophyll assay kit (Solarbio, Beijing, China). Chlorophyll was extracted using an anhydrous ethanol:acetone (1:2) solution, and the content was determined spectrophotometrically at 645 nm (for chlorophyll a) and 663 nm (for chlorophyll b). The soluble protein content of leaves was determined using Coomassie Brilliant Blue G-250 dye according to the Bradford method, with bovine serum albumin being used as the protein standard [39]. The content of MDA was expressed as micromoles of MDA per gramme fresh weight, whereas the contents of soluble sugars, soluble proteins, and chlorophyll were expressed in terms of milligrammes per gramme fresh weight.
Enzyme activity assays
The activities of SOD, POD, CAT, and APX were determined according to the instructions provided with commercially available kits (Solarbio, Beijing, China). Fresh leaves (0.5 g) were ground to a fine powder in liquid nitrogen, and enzymes were extracted using the provided extraction buffers. The extracts thus obtained were centrifuged at 8,000 × g for 10 min at 4°C, and the resultant supernatants were used for further experiments. The activities of SOD, POD, CAT, and APX in the corresponding reaction mixtures were determined spectrophotometrically at 560, 470, 240, and 290 nm, respectively, and expressed in terms of units of enzyme activity per gramme fresh weight. All measurements were performed according to the instructions of the antioxidant enzyme assay kit.
Statistical analysis was conducted with an analysis of variance (ANOVA) and Student’s t-test using Statistical Package for the Social Sciences (SPSS) software (version 21.0; IBM SPSS Statistics, Armonk, USA).
RNA extraction, library preparation, and RNA sequencing
Total RNA was extracted from plant samples using a TaKaRa MiniBEST Plant RNA Extraction Kit (TaKaRa, Dalian, China) according to the manufacturer’s protocol, and the integrity of the extracted RNA was monitored using 1% agarose gel electrophoresis. The purity and quality of RNA were assessed using a NanoDrop 2000C spectrophotometer (Thermo Fisher Scientific, Wilmington, USA) and an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, USA), respectively. Sequencing libraries were constructed using an NEB Next® Ultra™ RNA Library Prep Kit for Illumina® (NEB, USA) following the manufacturer’s instructions. The prepared libraries were sequenced using an Illumina Hiseq 2500 platform (Illumina Inc., San Diego, CA, USA) of BIOMARKER (Beijing, China) and paired-end reads were generated. The raw sequence reads have been submitted to the Short Read Archive (SRA) of NCBI with BioProject accession number PRJNA608707 (Biosample: SAMN15763440–SAMN15763454).
Transcriptome assembly and functional annotation
The X. sorbifolia transcriptome was assembled from 15 samples (three and 12 samples from control and stressed seedlings, respectively). Clean reads were obtained following the removal of reads containing adaptors and ploy-N sequences and low-quality reads from the raw data using the FastQC tool (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The high-quality reads were assembled de novo into transcripts using Trinity [40]. Trinity combines reads with a certain length of overlap into longer contig sequences without gaps [41]. All assembled unigenes were annotated based on BLAST searches (E-value ≤ 1.0 × 10−5) of the Kyoto Encyclopaedia of Genes and Genomes (KEGG), Gene Ontology (GO), Clusters of Orthologous Groups (COG), eggnog, Swiss-prot, NR, and euKaryotic Orthologous Groups (KOG) databases. The software KOBAS2.0 [42] was used to obtain the KEGG orthology of unigene. The predicted amino acid sequences for each Unigene were aligned against the Pfam database [43] using HMMER [44] (E-value < 1.0 × 10−10) to acquire the annotation information of unigene. The sequenced reads were compared with the unigene library by Bowtie [45], and then the expression level was estimated with RSEM [46]. Gene expression levels were evaluated based on the fragments per kilobase of exon per million fragments mapped (FPKM).
Differential expression analysis was performed using the DESeq R package, with a false discovery rate-adjusted P value (FDR) < 0.05 and an absolute value of log2 FC ≥ 1 being used as the empirical parameters for identifying differentially expressed genes (DEGs). All DEGs were annotated using the aforementioned databases and the gene number for each GO term was calculated. The enrichment analysis of GO terms was conducted using the R package TopGO. KEGG enrichment of DEGs was performed using KOBAS software 2.0 [42]. Transcription factors (TFs) were predicted using the PlantTFDB database (http://planttfdb.gao-lab.org/index.php).
Analysis of quantitative real-time PCR (qRT-PCR)
qRT-PCR experiments were performed using 20-μL reaction mixtures containing TB Green® Premix Ex Taq™ II (TaKaRa, Dalian, China) in an ABI 7500 (Applied Biosystems, Carlsbad, USA), with X. sorbifolia Actin (c225347.graph_c0) being used as a reference gene and three independent biological replicates being analysed for each sample. Specific primers were designed based on sequencing results using Primer 5.0 software, the sequences of which are listed in S1 Table. The relative expression levels of amplified genes were determined using ABI 7500 sequence detection system software V2.3, and quantified by measuring cycle threshold (Ct) values, normalized relative to the expression of the Actin gene, using the 2−ΔΔCt method.
Results
Physiological indices
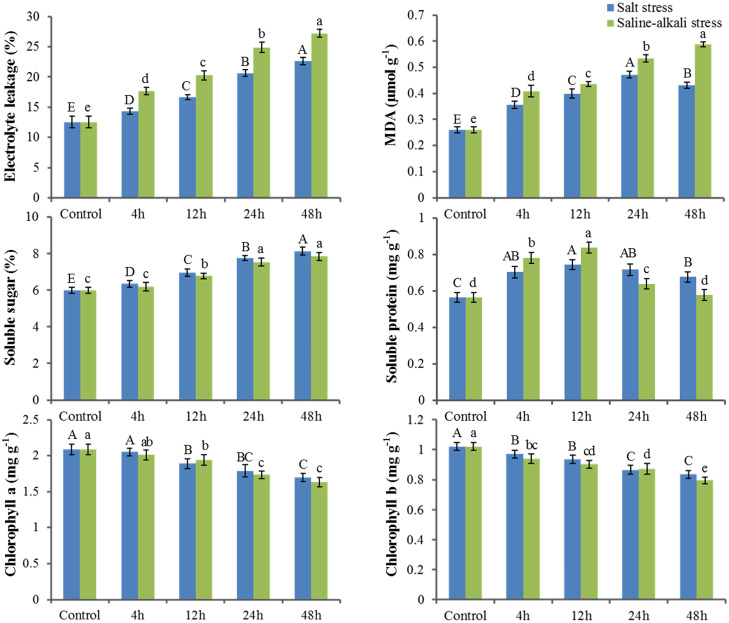
Compared with the control seedlings, salt and saline-alkali stress were found to promote increases in leaf EL and MDA, sugar, and protein contents, whereas leaf tissue chlorophyll content was observed to be reduced (Fig 1). The EL value increased almost linearly within 48 h of exposure to salt and saline-alkali stress, reaching levels 1.81- and 2.17-fold higher than the control, respectively. The content of MDA increased significantly in response to salt stress from 0 to 24 h, but subsequently underwent a gradual decline until the final measurement at 48 h. Under saline-alkali stress, MDA content increased rapidly within the initial 4 h, and thereafter gradually increased, peaking at 48 h. Notably, the levels of both EL and MDA in the saline-alkali treatment group were higher than those recorded for the control and salt stress groups. Leaf sugar content showed a slow increasing tendency with a prolongation of the time that seedlings had been exposed salt and saline-alkali stress. A similar tendency was observed for protein contents under the two stress treatments, with contents showing a marked increase at 12 h in treated leaves compared to with control leaves. Exposure to both salt and saline-alkali stress induced a continuous reduction in the contents of chlorophyll a and b until at least 48 h.
Fig 1. Changes in electrolyte leakage (EL), malondialdehyde (MDA), sugar, protein, and chlorophyll a/b in leaf tissues of X. sorbifolia under salt and saline-alkali stress.
4–48 h indicate different times of exposure to these two stresses. Error bars represent means ± SD (n = 3), and letters indicate significant statistical difference at p<0.05.
Antioxidant enzyme activity
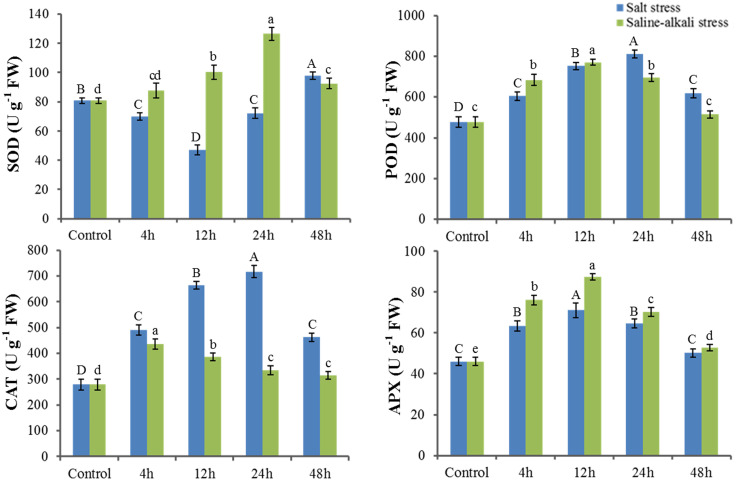
In response to salt stress, superoxide dismutase (SOD) activity showed an initial downward trend, followed by a subsequent increase, and at 48 h was significantly higher than that recorded in control seedlings. In contrast, under saline-alkali stress, SOD activity showed an initial slow increase within 4 h, then sharply increased until 24 h, but thereafter declined rapidly until 48 h. The peak value was, however, significantly higher than that recorded for the salt stress group (Fig 2). In response to both salt and saline-alkali stresses, the activities of peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) appeared to follow a ‘rise and fall’ pattern as time progressed, and under both stresses, the activities were invariably higher than those recorded in control seedlings (Fig 2). POD activity peaked at 24 h and 12 h in response to salt and saline-alkali stress, respectively, whereas with respect to CAT, salt stress induced a greater increase in activity than did saline-alkali stress, particularly at 24 h. APX activity showed a similar tendency in response to each of the two stresses, although the change was observed to be more pronounced in response to saline-alkali stress.
Fig 2. Changes in antioxidant enzyme activity in response to salt and saline-alkali stress.
SOD, superoxide dismutase; POD, peroxidase; CAT, catalase; APX, ascorbate peroxidase. 4–48 h indicate different times of exposure to these two stresses. Error bars represent means ± SD (n = 3), and letters indicate significant statistical difference at p<0.05.
Transcriptome sequencing, assembly, and functional annotation
Following the removal of adaptors and low-quality sequences, the 15 cDNA libraries yielded approximately 90 Gb of clean reads. Q30 values were used to evaluate sequencing quality, and our findings that for each library, there were 93.07%–93.55% of bases scoring Q30 indicated that the RNA-Seq datasets were of high quality (S2 Table). Following assembly and data analysis, a total of 45,380 unigenes were obtained with an average length of 1,500 bp and N50 of 2,204 bp, including 24,313 unigenes with lengths exceeding 1 kb (S3 Table). Among these unigenes, 37,945 (83.62%) were annotated in at least one database. Notably, 36,388 (80.19%) unigenes showed significant hits using the NR database, whereas 22,341 (49.23%) unigenes showed significant matches to proteins in the Swiss-Prot database (Table 1).
Table 1. Functional annotation of the X. sorbifolia transcriptome.
| Annotated Databases | Number of Unigenes | Percentage (%) |
|---|---|---|
| Annotated in COG | 15,779 | 34.77 |
| Annotated in GO | 17,505 | 38.57 |
| Annotated in KEGG | 15,866 | 34.96 |
| Annotated in KOG | 22,018 | 48.52 |
| Annotated in Pfam | 29,164 | 64.27 |
| Annotated in Swiss-Prot | 22,341 | 49.23 |
| Annotated in eggNOG | 33,941 | 74.80 |
| Annotated in NR | 36,388 | 80.19 |
| Annotated in at least one database | 37,945 | 83.62 |
| Total Unigenes | 45,380 | 100 |
Gene expression and identification of DEGs
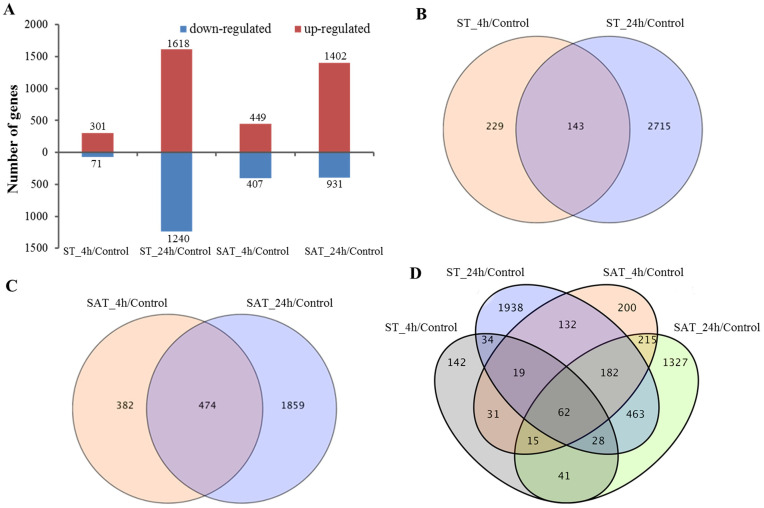
In order to identify DEGs, each of the treatment groups was compared with the control group. We accordingly identified 372 (301 up- and 71 downregulated) DEGs in the 4-h salt treatment group (ST_4h), 2858 (1,618 up- and 1,240 downregulated) DEGs in the 24-h salt treatment group (ST_24h), 856 (449 up- and 407 downregulated) DEGs in the 4-h saline-alkali treatment group (SAT_4h), and 2,333 (1,402 up- and 931 downregulated) DEGs in the 24-h saline-alkali treatment group (SAT_24h) (Fig 3A). These data indicate that the number of upregulated genes was greater than that of downregulated genes. Furthermore, we identified 62 DEGs showing response to both salt and saline-alkali stresses, 3,087 DEGs responding only to salt treatment, and 2,715 DEGs responding only to saline-alkali treatment (Fig 3B–3D). These stress-responsive genes detected at different sampling times under salt and saline-alkali stress were response- and time-specific, which may be related to the tolerance to different stresses.
Fig 3. DEGs in the four pairwise comparisons of the control and stress treatments.
(A) Bar chart showing the number of up- and downregulated genes in different comparisons. Venn diagram exposed the overlap of DEGs in two pairwise comparisons of salt stress (B), two pairwise comparisons of saline-alkali stress (C), and all four pairwise comparisons (D), respectively.
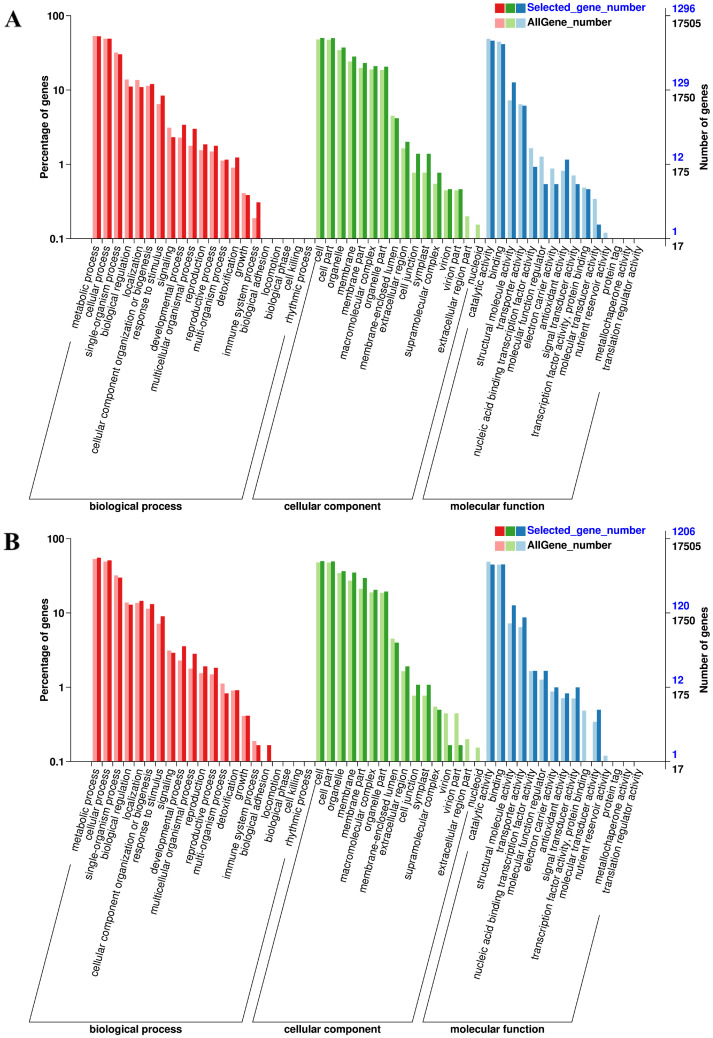
For the purposes of GO analysis, we compared the GO terms for DEGs with those of the entire transcriptome gene complement. GO functional classification revealed that the DEGs could be divided into 51 functional groups, belonging to the three main GO domains: biological processes, cellular components, and molecular functions. For salt treatment, a total of 1296 (of 3,087) DEGs were associated with certain important GO terms, including response to stimulus, developmental process, detoxification, membrane, cell junction, symplast, structural molecule activity, and antioxidant activity (Fig 4A). For saline-alkali treatment, 1206 (of 2,715) DEGs were over-represented in the categories response to stimulus, developmental process, biological adhesion, membrane, cell junction, symplast, structural molecule activity, transporter activity, antioxidant activity, and signal transducer activity (Fig 4B).
Fig 4. GO annotation of the DEGs, including the biological process, cell component and molecular function.
(A) Enriched GO terms from salt-responsive DEGs. (B) Enriched GO terms from saline-alkali-responsive DEGs. Bold colours indicate the whole transcriptome gene complement, the light colours indicate DEGs.
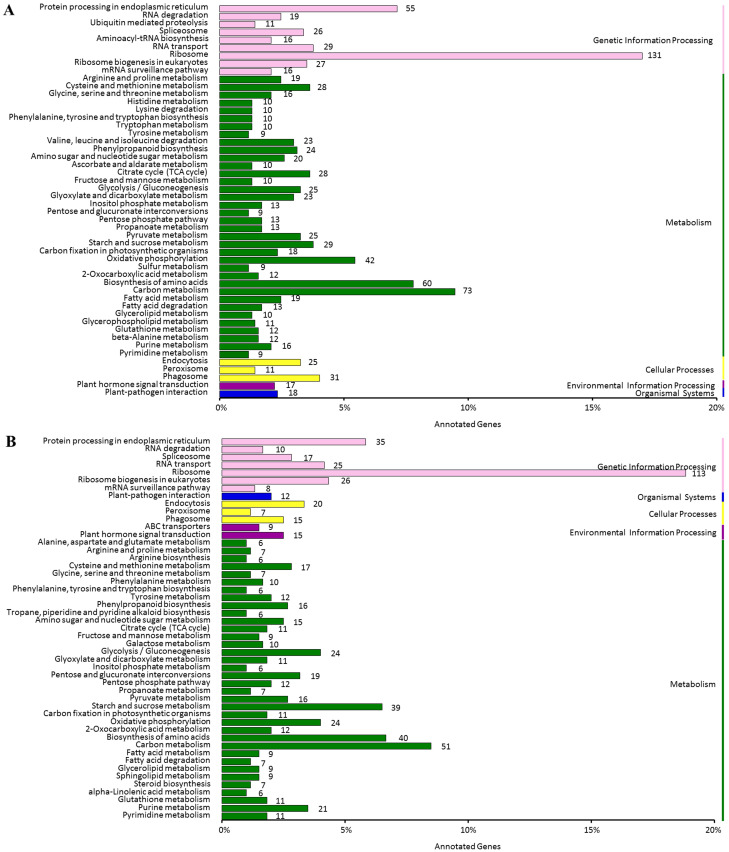
Enrichment analysis of the DEGs based on KEGG annotation revealed that both salt-responsive and saline-alkali-responsive DGEs were significantly enriched with respect to ribosomes, carbon metabolism, biosynthesis of amino acids, starch and sucrose metabolism, and oxidative phosphorylation (Fig 5). Moreover, salt-responsive DGEs were significantly enriched in RNA transport, and the citrate cycle (TCA cycle), whereas saline-alkali-responsive DGEs were significantly enriched in glycolysis and gluconeogenesis and ABC transporters.
Fig 5. KEGG pathways enrichment of the DEGs.
(A) The functional categories of the KEGG pathway enriched by salt-responsive DEGs are showed. (B) The functional categories of the KEGG pathway enriched by saline-alkali-responsive DEGs are showed.
It should be noted that approximately 73 and 51 genes in plants subjected to salt and saline-alkali stress, respectively, were grouped into carbon metabolism pathways, among which several genes related to abiotic stress responses were identified (S4 Table). Two genes (c254724.graph_c0 and c260317.graph_c0) encoding transketolase (TKT) were upregulated under salt stress. The TKT gene (c257495.graph_c0) and phosphoglycerate kinase (PGK) gene (c260510.graph_c0) were expressed at higher levels in response to saline-alkali stress. However, two genes (c249246.graph_c1, c256367.graph_c0) encoding ribose-5-phosphateisomerase (RPI2) were downregulated in response to these two stresses.
The accumulation of osmolyte (i.e. sugar and amino acid) is an important feature for the protection and survival of plants in coping with abiotic stress. Some pathways related to sugar metabolism and amino acid metabolism were enriched with DEGs under salt and saline-alkali stress, such as biosynthesis of amino acids and starch and sucrose metabolism. At least 25 salt and 26 saline-alkali tolerance genes involved in the synthesis of osmolytes were detected in these pathways. (S5 Table), and most of them were upregulated. Four genes (c258340.graph_c0, c262255.graph_c1, c238817.graph_c0, and c238817.graph_c1) encoding trehalose phosphate synthases (TPS) showed significant upregulation under two stress conditions, whereas two TPS (c253905.graph_c0 and c236826.graph_c3) were upregulated only under salt stress. Several DEGs related to the metabolism of sucrose, glucose, proline, arginine, alanine, and betaine were also upregulated under salt stress. Moreover, DEGs for metabolism of sucrose, glucose, fructose, threonine, alanine, arginine, and cysteine were upregulated by saline-alkali treatment.
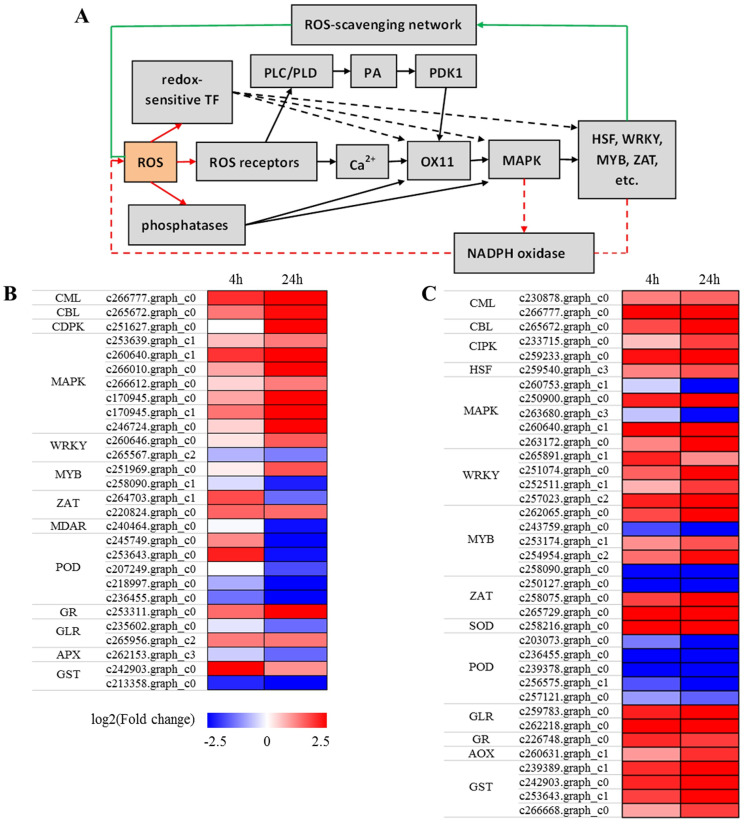
Genes related to the ROS signaling network
ROS have been identified as serving a signaling function in plants, wherein they activate defence-related genes via redox-sensitive signaling pathways and transcription factors [47]. Schematic diagrams of the ROS-mediated signaling network are presented in Fig 6A [48]. In those seedlings subjected to salt treatment, we detected a total of 28 salt tolerance genes that are involved in the ROS signaling network, among which, 15 genes were upregulated at 4 h and 24 h of salt treatment. These were mainly distributed in the CML, CBL, CDPK, MAPK, and WRKY gene families. In contrast, six genes, which mainly encode ROS-scavenging enzymes, were downregulated in the two salt treatment groups (Fig 6B; S6 Table). In response to saline-alkali treatment, we detected 37 saline-alkali tolerance genes involved in the ROS signaling network, belonging to the CML, CBL, CIPK, HSF, MAPK, MYB, WRKY, ZAT families, or encoding ROS-scavenging enzymes. Compared with the control, with the exception of five POD genes that were all downregulated, most of the other gene families were upregulated in the two saline-alkali treatment groups (Fig 6C; S6 Table).
Fig 6. Heatmap of DEGs involved in ROS signaling network.
(A) Overview of the ROS signal transduction pathway. (B) Expression patterns of salt tolerance DEGs are showed. (C) Expression patterns of saline-alkali tolerance DEGs are showed. CML, Calcium-binding protein; CBL, Calcineurin B-like protein; CIPK, CBL-interacting protein kinase; CDPK, Calcium-dependent protein kinase; HSF, Heat shock transcription factor; MAPK, Mitogen-activated protein kinase; WRKY, Wrky transcription factor; MYB, Myb transcription factor; ZAT, Zinc finger protein; MDAR, Monodehydroascorbate reductase; GLR, Glutaredoxin; GR, Glutathione reductase; AOX, Alternative oxidase; GST, Glutathione S-transferase.
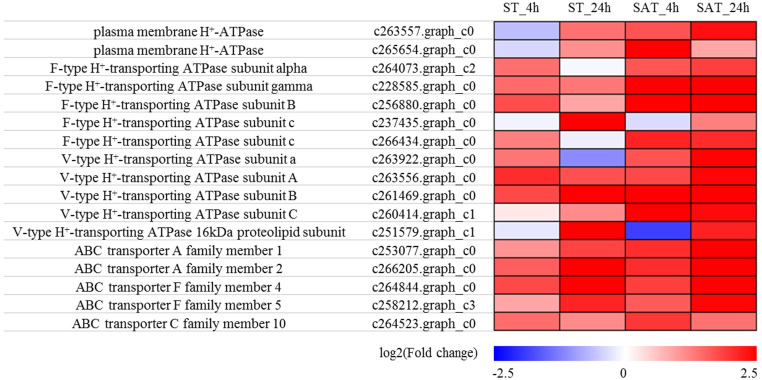
Genes involved in H+ transport
We identified a total of 17 genes involved in H+ transport that were differentially expressed in response to the two stress treatments, among which there were two genes encoding plasma membrane H+-ATPase, five genes encoding F-type H+-transporting ATPase, five genes encoding V-type H+-transporting ATPase, and five genes encoding ABC transporters (Fig 7; S7 Table). The majority of these genes were upregulated in the four stress groups compared with the control group, and all genes showed a higher level of expression at 24 h in response to saline-alkali treatment, whereas lower expression levels were detected after 4 h salt treatment. Moreover, exposure to saline-alkali stress resulted in a more significant upregulation of these genes than exposure to salt stress.
Fig 7. Expression patterns of DEGs involved in H+ transport in four pairwise comparisons of control and stress treatment groups.
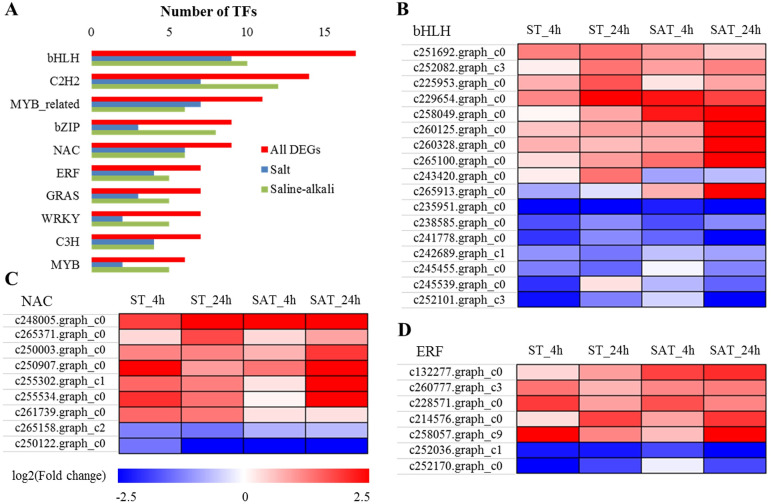
Dynamic expression of transcription factors
Based on all 81 families of TFs predicted from the Arabidopsis TF database [49], we detected 34 families with at least one gene matched to the DEG dataset. 90 and 99 DEGs encoding TFs were identified in X. sorbifolia in response to salt and saline-alkali stress, respectively (S8 Table). Among these TF families, bHLH, C2H2, bZIP, NAC, and ERF families showed more active, and 10 TF families included more than six differentially expressed TFs (Fig 8A). A total 17 bHLHs were differentially expressed, nine of which were upregulated by saline-alkali treatments, and eight of which were upregulated by both salt and saline-alkali stress treatments (Fig 8B). The NAC family, with nine DEGs, with the exception of two genes (c265158.graph_c2 and c250122.graph_c0), seven genes were upregulated following exposure to these two stresses (Fig 8C). Furthermore, a total of nine DEGs belonging to the ERF family were identified, and seven of which were upregulated, whereas two genes (c252036.graph_c1 and c252170.graph_c0) were downregulated under salt and saline-alkali conditions (Fig 8D).
Fig 8. Classification and expression of transcription factors (TFs).
(A) Distribution of DEGs into 10 major TF families that include more than six DEGs. (B) Heatmap of DEGs encoding the members of bHLH family. (C) Heatmap of DEGs encoding the members of NAC family. (D) Heatmap of DEGs encoding the members of ERF family.
To further verify the accuracy of the RNA-Seq results, we selected 10 representative genes for qRT-PCR analysis, seven of which are involved in the ROS signaling network, two are associated with carbon metabolism, and one plays a role in H+ transport. Correlation analysis of the qRT-PCR and RNA-Seq results, based on scatter plotting of log2 (fold change) data, indicated that the qRT-PCR results were consistent with the sequencing data (Pearson coefficient r2 = 0.82, n = 40; S1 Fig).
Discussion
Soil salinization and alkalinization often coincide in nature, inhibiting plant growth and leading to wilting or death [50]. Some salt-alkali soils have high salinity but low pH, while some have low salinity but high pH [51]. Therefore, neutral (NaCl or Na2SO4) and alkaline (NaHCO3 or Na2CO3) salts are usually defined as two distinct stresses. In this study, the physiological and RNA-Seq analyses were conducted on seedlings of X. sorbifolia exposed to salt (NaCl) and saline-alkali (Na2CO3) stress at different time point.
Environmental stresses can induce a range of physiological processes in plants, and in general, abiotic stresses have been found to activate oxidative responses and induce the production of ROS in plant cells [52]. ROS accumulation in turn enhances membrane lipid peroxidation due to a loss of cell membrane integrity, which negatively affects EL in response to abiotic stress [53–55]. Furthermore, the production of MDA, derived via lipid peroxidation, can exacerbate membrane damage [56]. Consequently, the extent of EL and amounts of MDA can reflect the degree of cell membrane damage incurred in response to different environmental stresses [57]. Indeed, MDA is commonly used as a marker of lipid peroxidation injury [58]. In the present study, we found that both salt and saline-alkali treatment resulted in increases in MDA concentrations and EL values in X. sorbifolia, indicating that the two stresses probably cause lipid peroxidation and disruption of the plasma membrane. Moreover, compared with the control seedlings, we found that the levels of MDA and EL were upregulated to a greater extent in response to saline-alkali stress than to salt stress, which we assume to be indicative of the greater harm caused by saline-alkali stress.
Soluble sugars, as effective osmoprotectants, can enhance the osmotic potential of plant cells to maintain ion homoeostasis [59]. Our study demonstrated that salt stress markedly increased the soluble sugar content of X. sorbifolia leaves, whereas the levels of these sugars were not significantly increased under conditions of saline-alkali stress. Interestingly, most genes involved in the metabolism of soluble sugars showed significant upregulation in our data (S5 Table). Protein synthesis has been considered a possible primary target of salt toxicity [60], and previous studies have shown that changes in soluble proteins in response to salinity tend to differ according to plant species and variety, plant developmental stage, and the duration and severity of salt exposure [61]. For example, a reduction in soluble protein content has been observed in tomato [62] and cabbage [59] under salt stress, whereas salt stress has been demonstrated to increase protein contents in maize [63] and Zoysia macrostachya [64]. In the current study, we found that the content of soluble proteins showed a ‘rise and fall’ tendency in response to the two imposed stresses. We suspect that this pattern could reflect an initial increase attributable to the expression of new stress-related proteins in X. sorbifolia, and a subsequent decrease in response to a marked decline in photosynthesis [65]. Notably, however, we found that the effects of saline-alkali treatment on protein content were more pronounced than those of salt treatment. Additionally, compared with the control, we detected gradual reductions in the contents of chlorophyll a/b, thereby indicating a decline in the rate of photosynthetic may be a common response to salt and saline-alkali stress in many plants [66–69].
SOD, POD, CAT, and APX are key protective enzymes that play vital roles in eliminating ROS and facilitating tolerance to abiotic stress. SOD functions by catalyzing the conversion of peroxide anions to H2O2 and O2, whereas POD, CAT, and APX catalyze the conversion of H2O2 to oxygen and water [70]. The physiological analyses conducted in the present study revealed certain differences in the antioxidant enzyme activities of X. sorbifolia seedlings exposed to salt and saline-alkali stress. The induction of the antioxidant system is dependent on the severity of the stress experienced by plants [71], and in this regard, our observations indicating that saline-alkali stress significantly induced SOD could imply that toxic superoxide might inflict greater damage in response to saline-alkali stress than when plants are exposed to salt stress. Our findings that the activities of POD, CAT, and APX appeared to show an initial increase, but then subsequently decreased, might reflect the fact that the initial slight stress stimulated the synthesis of oxidase enzyme, whereas later severe stress disrupted enzyme synthesis and perturbed enzyme degradation.
In plants, ROS are believed to play a key role in regulating signal transduction events during abiotic stress responses [72]. Plant cells are known to sense ROS through at least three different mechanisms, namely, unidentified receptor proteins, redox-sensitive transcription factors, and direct inhibition of phosphatases [48]. Downstream signaling events include calcium and phospholipid signaling pathways, and subsequent activation of serine/threonine protein kinase (OX11), MAPK cascades, NADPH oxidase, and transcription factors [48]. Furthermore, the generation of excess ROS can also trigger ROS-scavenging pathways and restricts the production of ROS in specific cellular locations or the entire cell. In the present study, the findings of our GO analysis revealed the significant enrichment of several ROS-related processes. A total of 28 and 37 differentially expressed genes related to the ROS signaling network were identified under salt and saline-alkali treatments, respectively. Previous studies have shown that numerous components of the ROS signaling network, including genes in the CML, CIPK, MAPK, MYB, WRKY, and HSF families, positively regulate the stress tolerance of plants [73–75]. Under salt and saline-alkali stresses, most genes in the CML, CBL, CDPK, MAPK, MYB, WRKY, and HSF families were upregulated. Additionally, we found that all almost of the identified genes encoding ROS-scavenging enzymes were downregulated to a greater extent in response to salt stress. In contrast, however, with the exception of POD genes, most genes encoding ROS-scavenging enzymes were upregulated following exposure to saline-alkali stress. We identified certain genes related to the ROS network that showed stress-specific expression, thereby indicating that in X. sorbifolia, different mechanisms regulate ROS homoeostasis in response to neutral salt and saline-alkali stress.
Saline-alkali stress affects plant nutrient absorption, growth, and photosynthesis via the combined effects of ion toxicity, osmotic stress, and high pH stress [13, 76, 77]. To counter the adverse effects of alkaline pH, plants can regulate intracellular pH through ion transport [19], and in this regard, several studies have shown that H+ transporter-related genes, such as plasma membrane H+-ATPase, F-type H+-transporting ATPase, and V-type H+-transporting ATPase, are positively regulated in response to salt or saline-alkali stress [78–80]. In the present study, we identified 17 H+ transporter-related DEGs, almost all of which were upregulated in response to salt and saline-alkali stress. Notably, a larger number of H+ transporter-related genes were upregulated under saline-alkali conditions, and the expression level of the upregulated genes tended to be higher than that of those genes upregulated in response to salt stress. These results are consistent with those reported by Zhang et al. [81], and we accordingly speculate that the higher expression of these genes may make an important contribution to maintaining intracellular ion balance and counteracting the negative effects of high pH associated with saline-alkali stress.
TFs are regulatory components in transcriptional networks and are involved in various processes, including plant development, hormone signaling, and stress response [82, 83]. In the present study, a number of TFs were differentially expressed under salt and saline-alkali stress, most of which belonged to bHLH, MYB, C2H2, bZIP, NAC, ERF, GRAS, WRKY, and C3H families, which is consistent with findings in certain plants [25, 67, 84]. It has been confirmed that members of the transcription factor family such as bHLH, NAC, ERF, MYB, and WRKY are involved in plant response to abiotic stress [85, 86]. A fraction of TFs, including 8 bHLHs, 7 NACs, and 5 ERFs, were upregulated under both salt and saline-alkali stress, implying their important roles in the regulation of X. sorbifolia responses to salt and saline-alkali stress.
In summary, this study is the first to report a comprehensive physiological and transcriptomic analysis of the responses of X. sorbifolia to salt and saline-alkali stress. We observed certain physiological changes in X. sorbifolia seedlings that had been subjected to salt and saline-alkali stress treatments, including ROS accumulation, membrane lipid peroxidation, and the reduction of chlorophyll content. Furthermore, on the basis of transcriptomic datasets, we identified a large amount of genes and pathways related to stress responses. These data enabled us to characterize the common and contrasting features of salt and saline-alkali stress tolerance in X. sorbifolia, will contribute to distinguish the response mechanisms of this species to these two stresses. Also, for the future, systemic investigation of candidate gene will be required to extend our results.
Supporting information
(TIF)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
Data Availability
The raw sequence reads have been submitted to the Short Read Archive (SRA) of NCBI with BioProject accession number PRJNA608707.
Funding Statement
This study was supported by Shanxi Key Laboratory of Northern Functional Oil Tree Cultivation and Research (201805D111010) and Science and Technology Research Project of Shanxi Province (20120311015-3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59(1):651–681. 10.1146/annurev.arplant.59.032607.092911 [DOI] [PubMed] [Google Scholar]
- 2.Yang CW, Chong JN, Li CY, Kim CM, Shi DC, Wang DL. Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant and Soil. 2007;294(1):263–276. 10.1007/s11104-007-9251-3 [DOI] [Google Scholar]
- 3.Li N, Liu Hl, Sun J, Zheng HL, Wang JG, Yang LM, et al. Transcriptome analysis of two contrasting rice cultivars during alkaline stress. Scientific Reports. 2018;8(1):9586 10.1038/s41598-018-27940-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Ke QB, Kim MD, Kim SH, Ji CY, Jeong JC, et al. Transgenic alfalfa plants expressing the sweetpotato orange gene exhibit enhanced abiotic stress tolerance. PLoS ONE. 2015;10(5):e0126050 10.1371/journal.pone.0126050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehner B, Doll P, Alcamo J, Henrichs T, Kaspar F. Estimating the impact of global change on flood and drought risks in Europe: a continental, integrated analysis. Climatic Change. 2006;75(3):273–299. 10.1007/s10584-006-6338-4 [DOI] [Google Scholar]
- 6.Wang SN, Tang J, Li ZY, Liu YQ, Zhou ZH, Wang JJ, et al. Carbon mineralization under different saline-alkali stress conditions in paddy fields of northeast China. Sustainability. 2020;12(7):2921 10.3390/su12072921 [DOI] [Google Scholar]
- 7.Latef AAHA, He CX. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Entia Horticulturae. 2011;127(3):228–233. 10.1016/j.scienta.2010.09.020 [DOI] [Google Scholar]
- 8.Hasegawa PM. Sodium (Na+) homeostasis and salt tolerance of plants. Environmental and Experimental Botany. 2013;92:19–31. 10.1016/j.envexpbot.2013.03.001 [DOI] [Google Scholar]
- 9.Geng G, Lv C, Stevanato P, Li R, Liu H, Yu L, et al. Transcriptome analysis of salt-sensitive and tolerant genotypes reveals salt-tolerance metabolic pathways in sugar beet. International Journal of Molecular Sciences. 2019;20(23):5910 10.3390/ijms20235910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawa D, Testerink C. Regulation of mRNA decay in plant responses to salt and osmotic stress. Cellular & Molecular Life Sciences. 2017;74:1165–1176. 10.1007/s00018-016-2376-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chauhan A, Abuamarah BA, Kumar A, Verma JS, Ghramh HA, Khan KA, et al. Influence of gibberellic acid and different salt concentrations on germination percentage and physiological parameters of oat cultivars. Saudi Journal of Biological Sciences. 2019;26(6):1298–1304. 10.1016/j.sjbs.2019.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rouphael Y, Raimondi G, Lucini L, Carillo P, Kyriacou MC, Colla G, et al. Physiological and metabolic responses triggered by omeprazole improve tomato plant tolerance to NaCl stress. Frontiers in Plant Science. 2018;9:249 10.3389/fpls.2018.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang EZ, Yi SZ, Bai F, Niu DW, Zhong JJ, Wu QH, et al. Cloning, characterization and expression pattern analysis of a Cytosolic Copper/Zinc Superoxide Dismutase (SaCSD1) in a highly Salt tolerant mangrove (Sonneratia alba). International Journal of Molecular Sciences. 2015;17(1):4 10.3390/ijms17010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamro GM, Chang SX, Naeth MA, Duan M, House J. Fine root dynamics in lodgepole pine and white spruce stands along productivity gradients in reclaimed oil sands sites. Ecology and Evolution. 2015;5(20):4655–4670. 10.1002/ece3.1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subramanian S, Ricci E, Souleimanov A, Smith DL. A proteomic approach to Lipo-Chitooligosaccharide and Thuricin 17 effects on soybean germination unstressed and salt stress. PLoS ONE. 2016;11(8):e0160660 10.1371/journal.pone.0160660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakamoto H, Matsuda O, Iba K. ITN1, a novel gene encoding an ankyrin-repeat protein that affects the ABA-mediated production of reactive oxygen species and is involved in salt-stress tolerance in Arabidopsis thaliana. Plant Journal. 2008;56(3):411–422. 10.1111/j.1365-313X.2008.03614.x [DOI] [PubMed] [Google Scholar]
- 17.Lin JX, Hua XY, Peng XY, Dong BL, Yan XF. Germination responses of ryegrass (annual vs. perennial) seed to the interactive effects of temperature and salt-alkali stress. Frontiers in Plant Science. 2018;9 10.3389/fpls.2018.01458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang CW, Wang P, Li CY, Shi DC, Wang DL. Comparison of effects of salt and alkali stresses on the growth and photosynthesis of wheat. Photosynthetica. 2008;46(1):107–114. 10.1007/s11099-008-0018-8 [DOI] [Google Scholar]
- 19.Guo R, Shi LX, Yan CR, Zhong XL, Gu FX, Liu Q, et al. Ionomic and metabolic responses to neutral salt or alkaline salt stresses in maize (Zea mays L.) seedlings. BMC Plant Biology. 2017;17(1):41–41. 10.1186/s12870-017-0994-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang BR, DaCosta M, Jiang YW. Research advances in mechanisms of turfgrass tolerance to abiotic stresses: from physiology to molecular biology. Critical Reviews in Plant Sciences. 2014;33(2–3):141–189. 10.1080/07352689.2014.870411 [DOI] [Google Scholar]
- 21.Sicilia A, Testa G, Santoro DF, Cosentino SL, Piero ARL. RNASeq analysis of giant cane reveals the leaf transcriptome dynamics under long-term salt stress. BMC Plant Biology. 2019;19(1):1–24. 10.1186/s12870-019-1964-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong W, Zhong H, Gong Z, Fang X, Sun T, Deng X, et al. Meta-analysis of salt stress transcriptome responses in different rice genotypes at the seedling stage. Plants 2019;8(3):64 10.3390/plants8030064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postnikova OA, Shao J, Nemchinov LG. Analysis of the alfalfa root transcriptome in response to salinity stress. Plant & Cell Physiology. 2013;54(7):1041–1055. 10.1093/pcp/pct056 [DOI] [PubMed] [Google Scholar]
- 24.Anuradha U, Tulsi G, Kumar UA, Satisha J, Shinde MP, Kadoo NY, et al. Global transcriptome analysis of grapevine (Vitis vinifera L.) leaves under salt stress reveals differential response at early and late stages of stress in table grape cv. thompson seedless. Plant Physiology & Biochemistry. 2018;129:168 10.1016/j.plaphy.2018.05.032 [DOI] [PubMed] [Google Scholar]
- 25.Sun SX, Song HY, Li J, Chen D, Zhou ZQ. Comparative transcriptome analysis reveals gene expression differences between two peach cultivars under saline-alkaline stress. Hereditas. 2020;157:9 10.1186/s41065-020-00122-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng C, Quan TY, Li ZY, Cui KL, Yan L, Liang Y, et al. Transcriptome profiling reveals the genetic basis of alkalinity tolerance in wheat. BMC Genomics. 2017;18:24 10.1186/s12864-016-3421-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu Y, Huang W, Chen HY, Wu GW, Yuan HM, Song XX, et al. Identification of differentially expressed genes in flax (Linum usitatissimum L.) under saline-alkaline stress by digital gene expression. Gene. 2014;549(1):113–122. 10.1016/j.gene.2014.07.053 [DOI] [PubMed] [Google Scholar]
- 28.Yang CY, Ha W, Lin Y, Jiang K, Yang JL, Shi YP. Polyphenols isolated from Xanthoceras sorbifolia husks and their anti-tumor and radical-scavenging activities. Molecules. 2016;21(12):1694 10.3390/molecules21121694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruan CJ, Yan R, Wang BX, Mopper S, Guan WK, Zhang J. The importance of yellow horn (Xanthoceras sorbifolia) for restoration of arid habitats and production of bioactive seed oils. Ecological Engineering. 2017;99:504–512. 10.1016/j.ecoleng.2016.11.073 [DOI] [Google Scholar]
- 30.Wang HY, Yao XD, Sui GX, Yin LM, Wang LH. Properties of Xanthoceras sorbifolia husk fibers with chemical treatment for applications in polymer composites. Journal of Materials Science & Technology. 2015;31(2):164–170. 10.1016/j.jmst.2014.07.004 [DOI] [Google Scholar]
- 31.Yao ZY, Qi JH, Yin LM. Biodiesel production from Xanthoceras sorbifolia in China: Opportunities and challenges. Renewable & Sustainable Energy Reviews. 2013;24:57–65. 10.1016/j.rser.2013.03.047 [DOI] [Google Scholar]
- 32.Shen Z, Zhang KQ, Ao Y, Ma LY, Duan J. Evaluation of biodiesel from Xanthoceras sorbifolia Bunge seed kernel oil from 13 areas in China. Journal of Forestry Research. 2019;30(3):869–877. 10.1007/s11676-018-0683-9 [DOI] [Google Scholar]
- 33.Liu YL, Huang ZD, Ao Y, Li W, Zhang ZX. Transcriptome analysis of yellow horn (Xanthoceras sorbifolia Bunge): a potential oil-rich seed tree for biodiesel in China. PLoS ONE. 2013;8(9):e74441 10.1371/journal.pone.0074441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Ruan CJ, Liu LY, Du W, Bao AM. Comparative RNA-Seq analysis of high- and low-oil yellow horn during embryonic development. International Journal of Molecular Sciences. 2018;19(10):3071 10.3390/ijms19103071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou QY, Zheng YR. Comparative de novo transcriptome analysis of fertilized ovules in Xanthoceras sorbifolium uncovered a pool of genes expressed specifically or preferentially in the selfed ovule that are potentially involved in late-acting self-incompatibility. PLoS ONE. 2015;10(10):e0140507 10.1371/journal.pone.0140507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lang YH, Liu Z, Zheng ZM. Investigation of yellow horn (Xanthoceras sorbifolia Bunge) transcriptome in response to different abiotic stresses: a comparative RNA-Seq study. Rsc Advances. 2020;10:6512 10.1039/c9ra09535g [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Wang J, Guo JP, Zhang YX, Yan XR. Integrated transcriptomic and metabolomic analyses of yellow horn (Xanthoceras sorbifolia) in response to cold stress. PLoS ONE. 2020;15(7):e0236588 10.1371/journal.pone.0236588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo MX, Li SP, Tian S, Wang B, Zhao XS, Zhang JS. Transcriptome analysis of genes involved in defense against alkaline stress in roots of wild jujube (Ziziphus acidojujuba). PLoS ONE. 2017;12(10):e0185732 10.1371/journal.pone.0185732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72(12):248–254. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 40.Grabherr M, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology. 2011;29(7):644–652. 10.1038/nbt.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mann A, Kumar N, Lata C, Kumar A, Kumar A, Meena BL. Functional annotation of differentially expressed genes under salt stress in Dichanthium annulatum. Plant Physiology Reports. 2019;24:104–111. 10.1007/s40502-019-0434-8 [DOI] [Google Scholar]
- 42.Xie C, Mao XZ, Huang JJ, Ding Y, Wu JM, Dong S, et al. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Research. 2011;39:W316–322. 10.1093/nar/gkr483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, et al. Pfam: the protein families database. Nucleic Acids Research. 2014;42:D222–D230. 10.1093/nar/gkt1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eddy SR. Profile hidden Markov models. Bioinformatics Italic. 1998;14(9):755–763. 10.1198/016214502388618870 [DOI] [PubMed] [Google Scholar]
- 45.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biology Italic. 2009;10(3):R25 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12(1):323 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hershkovitz V, Ben-Dayan C, Raphael G, Pasmanik-Chor M, Liu J, Belausov E, et al. Global changes in gene expression of grapefruit peel tissue in response to the yeast biocontrol agent Metschnikowia fructicola. Molecular Plant Pathology. 2012;13(4):338–349. 10.1111/j.1364-3703.2011.00750.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends in Plant Science. 2004;9(10):490–498. 10.1016/j.tplants.2004.08.009 [DOI] [PubMed] [Google Scholar]
- 49.Paulino PR, Mauricio RPD, Guedes CLG, Rensing SA, Birgit K, Bernd MR. PlnTFDB: updated content and new features of the plant transcription factor database. Nuclc Acids Research. 2010;38(Database issue):D822–D827. 10.1093/nar/gkp805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Zhang H, Zou ZR, Liu Y, Hu XH. Deciphering the protective role of spermidine against saline–alkaline stress at physiological and proteomic levels in tomato. Phytochemistry. 2015;110:13 10.1016/j.phytochem.2014.12.021 [DOI] [PubMed] [Google Scholar]
- 51.Mann A, Kumar A, Saha M, Lata C, Kumar A. Stress induced changes in osmoprotectants, ionic relations, antioxidants activities and protein profiling characterize Sporobolus marginatus Hochst. ex A. Rich. salt tolerance mechanism. Indian Journal of Experimental Biology. 2019;57(9):672–679. [Google Scholar]
- 52.Wang YP, Shen WZ, Chan ZL, Wu Y. Endogenous cytokinin overproduction modulates ROS homeostasis and decreases salt stress resistance in Arabidopsis Thaliana. Frontiers in Plant Science. 2015;6:1004 10.3389/fpls.2015.01004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lima JV, Lobato AKS. Brassinosteroids improve photosystem II efficiency, gas exchange, antioxidant enzymes and growth of cowpea plants exposed to water deficit. Physiology and Molecular Biology of Plants. 2017;23(1):59–72. 10.1007/s12298-016-0410-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vadim D, Darya S, Medvedev SS, Pozhvanov GA, Anatoliy S, Vladimir Y. Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. Journal of Experimental Botany. 2014;65(5):1259–1270. 10.1093/jxb/eru004 [DOI] [PubMed] [Google Scholar]
- 55.Sivankalyani V, Sela N, Feygenberg O, Zemach H, Maurer D, Alkan N. Transcriptome dynamics in mango fruit peel reveals mechanisms of chilling stress. Frontiers in Plant Science. 2016;7:1579–1579. 10.3389/fpls.2016.01579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmad P, Abd_Allah EF, Alyemeni MN, Wijaya L, Alam P, Bhardwaj R, et al. Exogenous application of calcium to 24-epibrassinosteroid pre-treated tomato seedlings mitigates NaCl toxicity by modifying ascorbate-glutathione cycle and secondary metabolites. Scientific Reports. 2018;8:13515 10.1038/s41598-018-31917-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao DQ, Zhang XY, Fang ZW, Wu YQ, Tao J. Physiological and transcriptomic analysis of tree peony (Paeonia section Moutan DC.) in response to drought stress. Forests. 2019;10(2): 135 10.3390/f10020135 [DOI] [Google Scholar]
- 58.Davey MW, Stals E, Panis B, Keulemans J, Swennen RL. High-throughput determination of malondialdehyde in plant tissues. Analytical Biochemistry. 2005;347(2):201–207. 10.1016/j.ab.2005.09.041 [DOI] [PubMed] [Google Scholar]
- 59.Qiu NW, Liu Q, Li JJ, Zhang YH, Wang FD, Gao JW. Physiological and transcriptomic responses of Chinese cabbage (Brassica rapa L. ssp. Pekinensis) to salt stress. International Journal of Molecular Sciences. 2017;18(9):1953-. 10.3390/ijms18091953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gulen H, Turhan E, Eris A. Changes in peroxidase activities and soluble proteins in strawberry varieties under salt-stress. Acta Physiologiae Plantarum. 2006;28(2):109–116. https://doi.org/0.1007/s11738-006-0037-7 [Google Scholar]
- 61.Doganlar ZB, Demir K, Basak H, Gul I. Effects of salt stress on pigment and total soluble protein contents of three different tomato cultivars. African Journal of Agricultural Research. 2010;5(15): 2056–2065. 10.1021/jf1012242 [DOI] [Google Scholar]
- 62.Fariduddin Q, Mir BA, Ahmad A. Physiological and biochemical traits as tools to screen sensitive and resistant varieties of tomatoes exposed to salt stress. Brazjplant Physiol. 2012;24(4):281–292. 10.1590/S1677-04202012000400007 [DOI] [Google Scholar]
- 63.Demir Y, Kocacaliskan I. Effects of proline on maize embryos cultured in salt stress. Fresenius Environmental Bulletin. 2008;17(5):536–542. [Google Scholar]
- 64.Wang R, Wang X, Liu K, Zhang XJ, Fan SJ. Comparative transcriptome analysis of Halophyte Zoysia macrostachya inresponse to salinity stress. Plants. 2020;9(4):458 10.3390/plants9040458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mohammadkhani N, Heidari R. Effects of drought stress on soluble proteins in two maize varieties. Turkish Journal of Biology. 2008;32(1):23–30. [Google Scholar]
- 66.Mohammad-Zaman Nouri, Ali Moumeni, Setsuko Komatsu. Abiotic stresses: insight into gene regulation and protein expression in photosynthetic pathways of plants. International Journal of Molecular Sciences. 2015;16: 20392–20416. 10.3390/ijms160920392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.An YM, Song LL, Liu YR, Shu YJ, Guo CH. De novo transcriptional analysis of alfalfa in response to saline-alkaline stress. Frontiers in Plant Science. 2016;7:931 10.3389/fpls.2016.00931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arias-Moreno DM, Jiménez-Bremont JF, Maruri-López I, Delgado-Sánchez P. Effects of catalase on chloroplast arrangement in Opuntia streptacantha chlorenchyma cells under salt stress. Scientific Reports. 2017;7(1):8656 10.1038/s41598-017-08744-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumar A, Kumar A, Kumar P, Lata C, Kumar S. Effect of individual and interactive alkalinity and salinity on physiological, biochemical and nutritional traits of Marvel grass. Indian Journal of Experimental Biology. 2018;56(8):573–581. [Google Scholar]
- 70.Meloni DA, Oliva MA, Martinez CA, Cambraia J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environmental and Experimental Botany. 2003;49(1):69–76. 10.1016/S0098-8472(02)00058-8 [DOI] [Google Scholar]
- 71.Harb A, Krishnan A, Ambavaram MMR, Pereira A. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiology. 2010;154(3):1254–1271. 10.1104/pp.110.161752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abdel-Moneim AM, Al-Kahtani MA, El-Kersh MA, Al-Omair MA, Avila MA. Free radical-scavenging, anti-inflammatory/anti-fibrotic and hepatoprotective actions of taurine and silymarin against CCl4 induced rat liver damage. PLoS ONE. 2015;10(12):e0144509 10.1371/journal.pone.0144509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rahaie M, Xue GP, Naghavi MR, Alizadeh H, Schenk PM. A MYB gene from wheat (Triticum aestivum L.) is up-regulated during salt and drought stresses and differentially regulated between salt-tolerant and sensitive genotypes. Plant Cell Reports. 2010;29(8):835–844. 10.1007/s00299-010-0868-y [DOI] [PubMed] [Google Scholar]
- 74.Ali MA, Azeem F, Nawaz MA, Acet T, Abbas A, Imran QM, et al. Transcription factors WRKY11 and WRKY17 are involved in abiotic stress responses in Arabidopsis. Journal of Plant Physiology. 2018;226:12 10.1016/j.jplph.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 75.Magnan F, Ranty B, Charpenteau M, Sotta B, Aldon D. Mutations in AtCML9, a calmodulin-like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant Journal. 2010;56(4):575–589. 10.1111/j.1365-313X.2008.03622.x [DOI] [PubMed] [Google Scholar]
- 76.Allakhverdiev SI. Salt stress inhibits the repair of photodamaged photosystem II by suppressing the transcription and translation of psbA genes in Synechocystis. Plant Physiology. 2002;130:1443–1453. 10.1104/pp.011114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yin ZP, Zhang H, Zhao Q, Yoo MJ, Zhu N, Yu JL, et al. Physiological and comparative proteomic analyses of saline-alkali NaHCO3-responses in leaves of halophyte Puccinellia tenuiflora. Plant and Soil. 2019;437:137–158. 10.1007/s11104-019-03955-9 [DOI] [Google Scholar]
- 78.Yu Y, Duan XB, Ding XD, Chen C, Zhu D, Yin KD, et al. A novel AP2/ERF family transcription factor from Glycine soja, GsERF71, is a DNA binding protein that positively regulates alkaline stress tolerance in Arabidopsis. Plant Molecular Biology. 2017;94(4–5):509–530. 10.1007/s11103-017-0623-7 [DOI] [PubMed] [Google Scholar]
- 79.Olfatmiri H, Alemzadeh A, Zakipour Z. Up-regulation of plasma membrane H+-ATPase under salt stress may enable Aeluropus littoralis to cope with stress. Molecular Biology Research Communications. 2014;3(1):67–75. [PMC free article] [PubMed] [Google Scholar]
- 80.Kirsch M, An Z, Viereck R, Lw R, Rausch T. Salt stress induces an increased expression of V-type H+-ATPase in mature sugar beet leaves. Plant Molecular Biology. 1996;32(3):543 10.1007/BF00019107 [DOI] [PubMed] [Google Scholar]
- 81.Zhang X, Wei LQ, Wang ZZ, Wang T. Physiological and molecular features of Puccinellia tenuiflora tolerating salt and alkaline-Salt Stress. Journal of Integrative Plant Biology. 2013;55(3):262–276. 10.1111/jipb.12013 [DOI] [PubMed] [Google Scholar]
- 82.Panchy NL, Azodi CB, Winship EF, O’Malley RC, Shiu SH. Expression and regulatory asymmetry of retained Arabidopsis thaliana transcription factor genes derived from whole genome duplication. BMC Evolutionary Biology. 2019;19(1):77 https://doi.org/0.1186/s12862-019-1398-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Golldack D, Li C, Mohan H, Probst N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Frontiers in Plant Science. 2014;22(5):151 10.3389/fpls.2014.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu C, Zhao Y, Zhao X, Wang J, Gu M, Yuan Z. Transcriptomic profiling of pomegranate provides insights into salt tolerance. Agronomy. 2019;10(1):44 10.3390/agronomy10010044 [DOI] [Google Scholar]
- 85.Aprile A, Sabella E, Vergine M, Genga A, Siciliano M, Nutricati E, et al. Activation of a gene network in durum wheat roots exposed to cadmium. BMC Plant Biology. 2018;18:238 10.1186/s12870-018-1473-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shinde H, Dudhate A, Tsugama D, Gupta SK, Liu S, Takano T. Pearl millet stress-responsive NAC transcription factor PgNAC21 enhances salinity stress tolerance in Arabidopsis. Plant Physiology and Biochemistry. 2019;135:546–553. 10.1016/j.plaphy.2018.11.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
Data Availability Statement
The raw sequence reads have been submitted to the Short Read Archive (SRA) of NCBI with BioProject accession number PRJNA608707.