Abstract
The new heart transplantation (HT) allocation policy was introduced on 10/18/2018. Using the UNOS registry, we examined early outcomes following HT for restrictive cardiomyopathy, hypertrophic cardiomyopathy, cardiac sarcoidosis or cardiac amyloidosis compared to the old system. Those listed who had an event (transplant, death or waitlist removal) prior to 10/17/2018 were in Era 1 and those listed on or after 10/18/2018 were in Era 2. The primary endpoint was death on the waitlist or delisting due to clinical deterioration. 1,232 HT candidates were included, 855 (69.4%) in Era 1 and 377 (30.6%) in Era 2. In Era 2, there was a significant increase in the use of temporary mechanical circulatory support and a reduction in the primary endpoint, (20.9 events per 100 PY (Era 1) versus 18.6 events per 100 PY (Era 2), OR 1.98, p=0.005). Median waitlist time decreased (91 vs. 58 days, p<0.001) and transplantation rate increased (119.0 to 204.7 transplants/100 PY for Era 1 vs Era 2). Under the new policy, there has been a decrease in waitlist time and waitlist mortality/delisting due to clinical deterioration, and an increase in transplantation rates for patients with infiltrative, hypertrophic and restrictive cardiomyopathies without any effect on post-transplant six-month survival.
Keywords: heart transplantation, restrictive cardiomyopathy, amyloidosis, cardiac sarcoidosis, waitlist
Introduction
Heart transplant (HT) allocation for patients with infiltrative, hypertrophic and restrictive cardiomyopathies has been debated extensively, particularly during the development of the recent Organ Procurement and Transplant Network (OPTN) heart allocation policy 1. Historically, under the three-tier system (status 1A,1B and status 2) which came into effect on January 19th, 1999, patients were allocated based on the level of support they required 2. Status 1A necessitated intensive care unit level support with intravenous medication, continuous hemodynamic monitoring, mechanical ventilation and/or mechanical circulatory support (MCS). Patients designated as Status 1B had intermediate level urgency for HT and were either in the hospital or at home on inotropic agents. Status 2 patients were those on oral medications and considered low urgency for HT. This 3-tier system did not adequately account for the needs of patients who are often less tolerant of inotropes or those with cardiomyopathies structurally incompatible with left ventricular assist devices or other MCS 3–7, such as patients with infiltrative, hypertrophic and restrictive cardiomyopathies. With this allocation system, status exceptions were needed to upgrade listing status for these individuals, in the event of clinical deterioration.
The new guidelines implemented on October 18th 2018 attempted to address these disparities by characterizing cardiac amyloidosis (CA), hypertrophic cardiomyopathy (HCM) and restrictive cardiomyopathy (RCM) as status 4, without specific hemodynamic or level of support criteria, with the ability to increase listing priority status based on clinical parameters 1, 8. These changes attempted to better prioritize those who were at a disadvantage under the historical 3-tier system. However, it remains to be determined whether this new allocation system has improved waitlist mortality, wait times and post HT survival for these unique patient populations. Furthermore, despite data suggesting that those with cardiac sarcoidosis (CS) were also disadvantaged under the historical system, this group was not similarly prioritized 9. In this retrospective cohort study, we compared outcomes of patients with infiltrative cardiomyopathy (specifically CA and CS), RCM and HCM who were transplanted at the time of the old versus the new allocation systems using the following metrics: (1) the proportion of patients supported by inotropes and/or temporary or durable MCS at time of HT; (2) waitlist mortality/delisting due to clinical deterioration; (3) time from listing to HT and (4) Six-month post HT survival.
Methods:
The United Network for Organ Sharing (UNOS) registry was analyzed for all adult (>18 years) candidates who underwent single organ primary HT between October 17, 2013 and March 31st, 2020. Patients were included if their listing diagnosis was restrictive cardiomyopathy (CS, CA, and RCM: UNOS Code 1050, 1051, 1052, 1053, 1054, 1099) or hypertrophic cardiomyopathy (UNOS Code 1201). Patients were classified into two groups centered on the October 18, 2018 Organ Procurement and Transplantation Network (OPTN) US adult heart allocation policy revision. Patients who were listed between October 17th, 2013 and October 17th, 2018 and had an event (transplant, death, or waitlist removal) prior to October 17th, 2018 were part of the Pre-Policy Change cohort (Era 1), and those listed on or after October 18th, 2018 were included in the Post-Policy Change cohort (Era 2). As patients listed in Era 1 had a greater possible waitlist time than those in Era 2 (5 years vs. 18 months), clinical follow-up time was limited to 18 months (or the maximum possible follow-up time in Era 2) after listing in an effort to control for time-window bias. Patients in Era 1 were censored at this 18-month time period if they remained on the waitlist. The primary endpoint was death on the waitlist or delisting due to clinical deterioration. The rate of waitlist mortality and delisting due to clinical deterioration were computed as the ratio of death or delisting to person years of exposure and displayed as events per 100 patient years. While there is no direct comparison between the old and new status designations, comparisons were made by grouping patients listed at a high priority status (Status 1A vs. Status 1-3), a moderate priority status (Status 1B vs. Status 4), and a low priority status (Status 2 vs. Status 5-6). Six-month post-transplant survival was assessed, restricting patients in Era 2 to those transplanted prior to September 17th, 2019 to provide at least six months of follow-up. Studies involving this dataset have been determined to be exempt from review by the Institutional Review Board of Columbia University Irving Medical Center.
Statistical Analysis
Demographic and clinical variables were expressed as median (with interquartile range) for continuous variables and count (with percentage) for categorical variables. Comparisons were made with the Kruskall-Wallis test and Chi-squared test as appropriate. Kaplan Meier survival analysis was used for post-transplant survival. Cumulative incidence was estimated using transplant, death and delisting as competing events. Two sensitivity analyses were performed. In the first, all patients listed in the 18 months before (Era 1) and after (Era 2) the October 18th, 2018 policy change were compared, censoring for clinical events that occurred after the policy change for patients listed in Era 1. A second sensitivity analysis was performed of all patients listed during Era 1 (i.e. including those who were removed from the waitlist at 18 months without an event), again censoring for events that occurred after 18 months on the waitlist. A two-tailed p-value of less than 0.05 was considered significant. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina) and R 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results:
1,232 patients were included in the study, 855 (69.4%) in Era 1 and 377 (30.6%) in Era 2. Age, sex, and body mass index did not significantly differ between groups. There was an increase in the proportion of Black and Hispanic patients listed in Era 2, but this did not meet statistical significance. In Era 2, there was an increased number of patients listed for amyloidosis or sarcoidosis (p=0.05). Other clinical variables, including comorbidities and blood type were similar between the two cohorts (Table 1).
Table 1:
Baseline demographics at time of listing for patients listed for heart transplant with restrictive, infiltrative and hypertrophic cardiomyopathies, pre- vs post-policy change
| Era 1 | Era 2 | p-value | |
|---|---|---|---|
| n | 855 | 377 | |
| Male (%) | 531 (62.1) | 245 (64.9) | 0.33 |
| Age | 56 (45-63) | 55 (45-63) | 0.39 |
| Ethnicity (%) | 0.15 | ||
| White | 614 (71.8) | 247 (65.5) | |
| Black | 163 (19.1) | 85 (22.5) | |
| Hispanic | 47 (5.5) | 28 (7.4) | |
| Other | 31 (3.6) | 17 (4.5) | |
| BMI | 26.7 (23.6-30.4) | 27.4 (23.6-31.8) | 0.11 |
| HF Etiology (%) | 0.05 | ||
| Amyloidosis | 211 (24.7) | 97 (25.7) | |
| HCM | 367 (42.9) | 161 (42.7) | |
| Sarcoidosis | 119 (13.9) | 69 (18.3) | |
| RCM | 158 (18.5) | 50 (13.3) | |
| Blood Type | 0.12 | ||
| A | 315 (36.8) | 138 (39.3) | |
| B | 133 (15.6) | 39 (10.3) | |
| AB | 38 (4.4) | 17 (4.5) | |
| O | 369 (43.2) | 173 (45.9) | |
| UNOS Status at Listing | <0.001 | ||
| Status 1A/Status 1 | 174 (20.4) | 11 (2.9) | |
| Status 2 | 53 (14.1) | ||
| Status 3 | 32 (8.5) | ||
| Status 1B/Status 4 | 296 (34.6) | 216 (57.3) | |
| Status 2/Status 5 | 375 (43.9) | 5 (1.3) | |
| Status 6 | 55 (14.6) | ||
| Temporarily Inactive | 10 (1.1) | 5 (1.3) | |
| ICD (%) | 577 (67.5) | 258 (68.4) | 0.84 |
| Amyloidosis | 87 (12.3) | 41 (15.9) | |
| HCM | 375 (53.1) | 133 (51.6) | |
| Sarcoidosis | 139 (19.7) | 60 (23.3) | |
| RCM | 105 (14.9) | 24 (9.3) | |
| Diabetes Mellitus (%) | 138 (16.2) | 69 (18.3) | 0.35 |
| Symptomatic Cerebrovascular Disease (%) | 59 (6.9) | 18 (4.8) | 0.33 |
| CKD (%) | 0.62 | ||
| GFR>60 | 496 (58.3) | 229 (61.2) | |
| GFR 30-60 | 229 (38.7) | 134 (35.8) | |
| GFR<30 | 26 (3.1) | 11 (2.9) | |
| MELD-Xi | 9.5 (5.9-13.5) | 9.6 (5.9-12.4) | 0.55 |
| Hemodynamics | |||
| PA Systolic | 42 (33-51) | 40 (30-50) | 0.04 |
| PA Diastolic | 21 (15-26) | 20 (14-25) | 0.02 |
| PA Mean | 29 (22-36) | 27 (20-35) | 0.01 |
| PCWP | 20 (15-25) | 18 (12-24) | 0.001 |
| Cardiac Index | 1.93 (1.62-2.30) | 1.95 (1.68-2.30) | 0.35 |
Legend: BMI, body mass index; CKD, chronic kidney disease; GFR, glomerular filtration rate; HCM, hypertrophic cardiomyopathy; HF, heart failure; ICD, implantable cardioverter defibrillator; MELD-Xi, model for end-stage liver disease excluding INR; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; RCM, restrictive cardiomyopathy.
Data are presented as n (%) or median (IQR)
In Era 2, pulmonary artery systolic, diastolic, and mean pressures as well as pulmonary capillary wedge pressures were significantly lower at listing compared to in Era 1 (p<0.05 for all), although it is unclear if these are clinically relevant differences. Cardiac index did not differ significantly between groups (p=0.35).
Following the allocation policy change, there was a significant increase in the use of both intra-aortic balloon pumps (6.4% [55 patients] in Era 1 vs. 18.0% [68 patients] in Era 2, p<0.001) and extracorporeal membrane oxygenation (0.7% [6 patients] in Era 1 vs. 3.4% [13 patients] in Era 2, p<0.001) (Figure 1). There was a corresponding decrease in the use of inotropes (37.6% [322 patients] in Era 1 vs. 24.1% [91 patients] in Era 2, p<0.001) and durable LVAD (8.1% [69 patients] in Era 1 vs. 2.9% [11 patients] in Era 2, P<0.001).
Fig. 1:
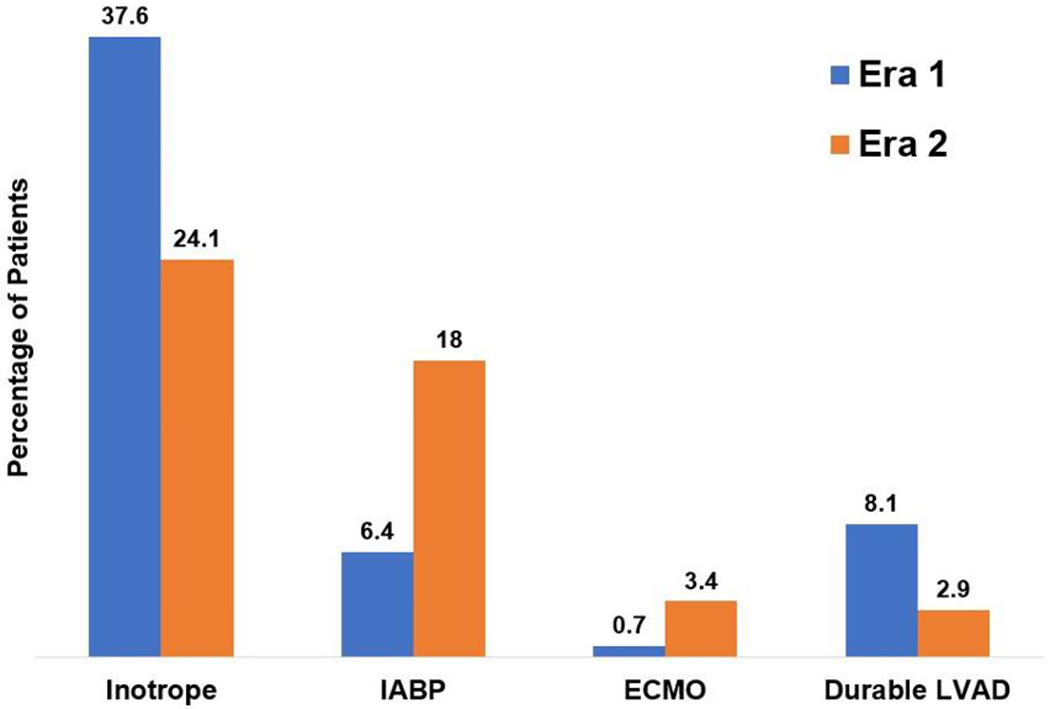
Use of advanced therapies while on the waitlist in patients with restrictive, infiltrative, and hypertrophic cardiomyopathies listed for heart transplant pre- and post-policy change. ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; LVAD, left ventricular assist device
Patients were generally listed at a higher priority status following the policy change: In Era 2, 25.5% (96 patients) were listed at a high priority status (Status 1-3) compared with 20.4% (174 patients Status 1A) in Era 1. Similarly, 57.3% (216 patients) were listed at a moderate priority status (Status 4) in Era 2 versus 34.6% (296 patients) initially listed as Status 1B in Era 1 (p<0.001). UNOS Status at the time of transplantation similarly differed. A greater proportion of patients were transplanted at a high priority status following the policy change (Status 1-3 70.6% [163 patients] vs. Status 1A 63.1% [385 patients], p=0.04) and numerically fewer were transplanted at a moderate priority status (Status 4 26.4% [61 patients] vs. Status 1B 31.5% [190 patients], p=0.18). In Era 2, the majority of patients were Status 1-3 at the time of transplantation, of which nearly half (47.8%-56.9%) utilized exceptions.
The combined endpoint of death on the waitlist or delisting due to clinical deterioration was more common in Era 1 (20.9 events per 100 person-years versus 18.6 events per 100 person-years, OR 1.98 95% CI 1.2-3.2, p=0.005). This was consistent for both endpoints individually: mortality (10.8 per 100 person-years [95% CI 8.3-14.0] vs. 8.9 per 100 person-years [95% CI 4.9-16.0]) and delisting (10.1 per 100 person-years [95% CI 7.7-13.2] vs. 9.7 per 100 person-years [95% CI 5.6-17.1]). Following the policy change, overall waitlist time decreased by a median of 33 days (91 days [IQR 31-310 days] vs. 58 days [IQR 18-166 days], p<0.001) and the rate of transplantation increased from 119.0 transplants per 100 patient years (95% CI 110.0-128.8) to 204.7 transplants per 100 patient years (95% CI 182.4-229.6). This difference was similar, but greater, to the rate of transplantation in non-infiltrative or restrictive patients: 95.1 (95% CI 94.6-95.7) in Era 1 vs. 162.2 (95% CI 156.5-168.0) in Era 2. Following the policy change, six-month post-transplant survival was 91.0% (95% CI 85.6-95.2) compared with 92.6% (95% CI 90.6-94.7, p=0.44) pre-policy change (Figure 2). Waitlist outcomes, rates of delisting, and rates of transplant by subtype of heart failure are included in the Supplemental Table.
Fig. 2:
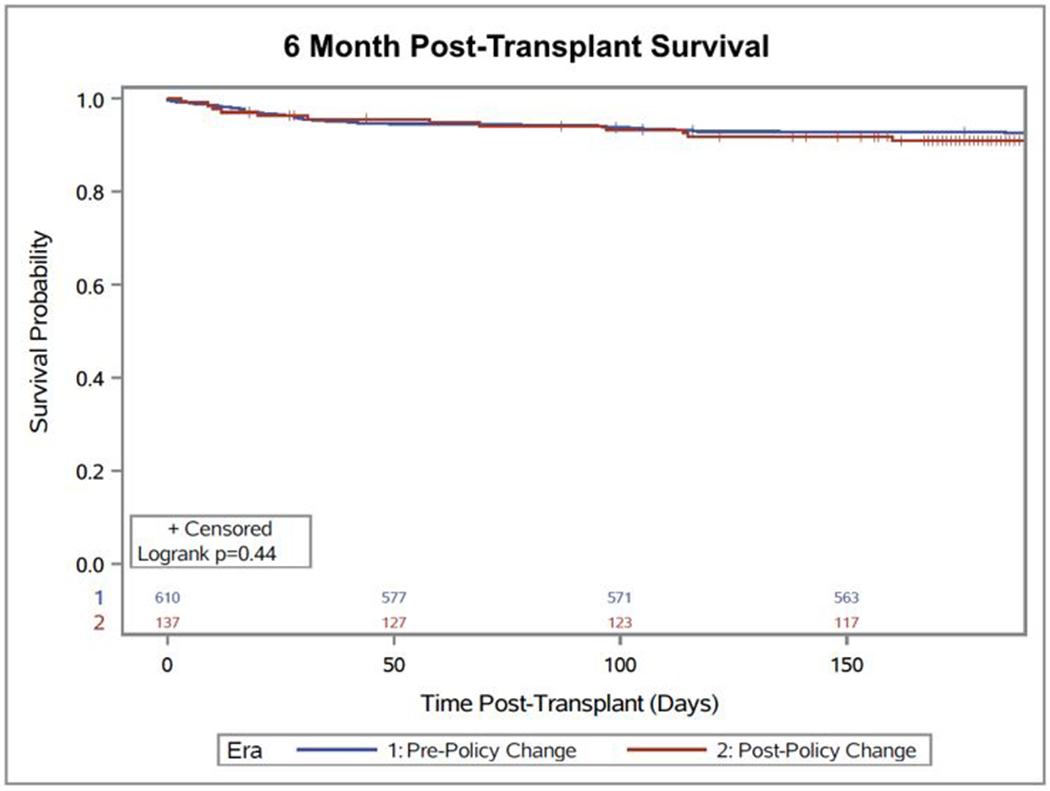
Six-month post-transplant survival pre- and post-policy change for restrictive, infiltrative, and hypertrophic cardiomyopathies
The sensitivity analyses demonstrated consistent results. The first sensitivity analysis comparing patients listed in the 18 months before and after the October 18th, 2018 policy change, censoring for clinical events that occurred after the policy change showed a trend towards a decrease in the combined endpoint of waitlist mortality and delisting due to clinical deterioration with 21.0 events per 100 person years (95% CI 14.9-29.5) in Era 1 compared with 18.6 events per 100 person years (95% CI 12.6-27.3) in Era 2 (p=0.08). This difference was predominantly driven by a decrease in waitlist mortality (12.9 deaths per 100 person years [95% CI 8.1-20.4] vs. 8.9 deaths per 100 person years [95% CI 4.9-16.0], p=0.03). Transplantation also occurred more frequently following the allocation policy change with 204.7 per 100 person years (95% CI 182.4-229.6) compared with 173.7 per 100 person years (95% CI 154.7-195.8, p<0.01) in the 18 months prior to the policy change. The second sensitivity analysis included 1,031 patients listed during Era 1, and the rates of events in Era 1 remained consistent: combined endpoint of 23.7 events per 100 person years (95% CI 20.1-27.9), deaths of 12.3 per 100 person years (95% CI 9.6-15.7), delisting due to clinical deterioration of 11.4 events per 100 person years (95% CI 8.8-14.7), and transplantation of 134.7 per 100 patient years (95% CI 125.2-145.0)
Discussion
In this analysis of the UNOS database, we compared outcomes of patients with infiltrative (specifically CA and CS), restrictive and hypertrophic cardiomyopathies, who underwent HT under the new allocation system compared to the prior 3-tier system. The main findings were the following: 1) The combined endpoint of death on the waitlist or delisting due to clinical deterioration occurred less frequently for patients listed under the new allocation system; 2) The use of temporary MCS while on the waitlist was higher under the new allocation system, despite having lower filling pressures; 3) Median time on the waitlist prior to HT decreased, with an observed higher priority status listing at time of transplant; and 4) The frequency of HT increased under the new allocation system.
Under the prior 3-tier allocation system, several patient groups were considered to be at a disadvantage when it came to priority at time of listing for HT and waitlist mortality 10. In patients with infiltrative, hypertrophic or restrictive cardiomyopathies, traditional heart failure testing (e.g. cardiopulmonary exercise testing or echocardiography) does not always reflect the severity of illness 6, 11. Traditionally, HT has typically been reserved for patients with an left ventricular ejection fraction (EF) below 50%, however, many in these subgroups may have progressive and advanced heart failure symptoms despite a preserved EF and peak VO2 consumption > 14ml/kg/min, meaning that alternative strategies must be available to ensure equitable listing priority. Furthermore, the prior policy did not take into account that the etiology of heart failure may have implications for which particular advanced therapy options may be appropriate for individual patients. CA and HCM typically lead to a thick walled, biventricular restrictive physiology, which is frequently intolerant of durable left ventricular assist devices (LVAD) due to small left ventricular chamber size and do not respond to treatment with standard heart failure therapies or inotropic medications 5. Since the widespread availability of continuous flow LVADs, waitlist mortality for ischemic and nonischemic cardiomyopathies has decreased by almost 50% to less than 9% at 1 year after listing 4. However, in HCM, waitlist mortality has remained relatively stable at 9.6%, partly due to the physiologic and structural intolerance of this group to MCS devices 4, similar to CA and RCM 7, 12.
Data from UNOS between 2008-2015 showed that patients with CA were least likely to be listed as status 1A, less likely to be placed on inotropes and less likely to have a durable LVAD compared to patients with dilated cardiomyopathy (DCM) or idiopathic RCM 13. Overall waitlist mortality was almost 2-fold higher than that of DCM 13. Similarly, under the previous allocation system patients with CS were less likely to be transplanted than those without CS 9. In addition, patients with CS are prone to life-threatening ventricular arrhythmias due to myocardial fibrosis or acute granulomatous inflammation, which may not be ameliorated by MCS and can be exacerbated by the use of inotropes. In addition, CS has been shown to involve the right ventricle in up to 16% of patients 14, which may also affect candidacy for durable LVAD 15. As such, HT may be the only life-prolonging treatment option available for end-stage heart failure for appropriate candidates in these unique cohorts. Given that the historical system prioritized patients requiring durable MCS, it could prove difficult to advance patients with RCM, HCM or infiltrative cardiomyopathies to a higher status without the addition of inotropes (which could initiate de-stablilizing arrhythmias) and without the option of durable MCS in many. As such, the new allocation system provided additional guidance on requesting exceptions for higher priority based on clinical and/or hemodynamic variables for patients with CA, HCM and RCM, however, no additional guidance was provided for the CS cohort 8.
The new OPTN allocation policy seems to be effective in addressing the shortfalls of the historical 3-tier system for these patient populations. It has resulted in a shortened waitlist time, decrease in waitlist mortality and increase in the rate of transplant, without affecting post-transplant survival (Figure 3 and Supplemental Figure). This appears to be partly driven by an increase in the use of temporary MCS, higher status at time of listing, and higher status at time of transplant; this finding is consistent with reports from the OPTN showing enhanced stratification of candidates by medical urgency 16, however, the similarity in post-transplant survival is contrary to earlier analyses of the new allocation system likely due to greater follow up time in our cohort 17. Our finding that patients under the new allocation system receive more MCS support (IABP and ECMO) despite having better hemodynamics is contrary to what would be expected. Whether this is due to a more aggressive medical approach in the modern era or adaptation to the new allocation system is unclear and warrants further investigation. Furthermore, the observed greater use of temporary MCS, lower inotrope use and high level of exceptions at time of transplant mirror that observed in the larger HT cohort, so although not specific to these groups do indicate greater equity compared with the larger transplant population 18.
Fig. 3:
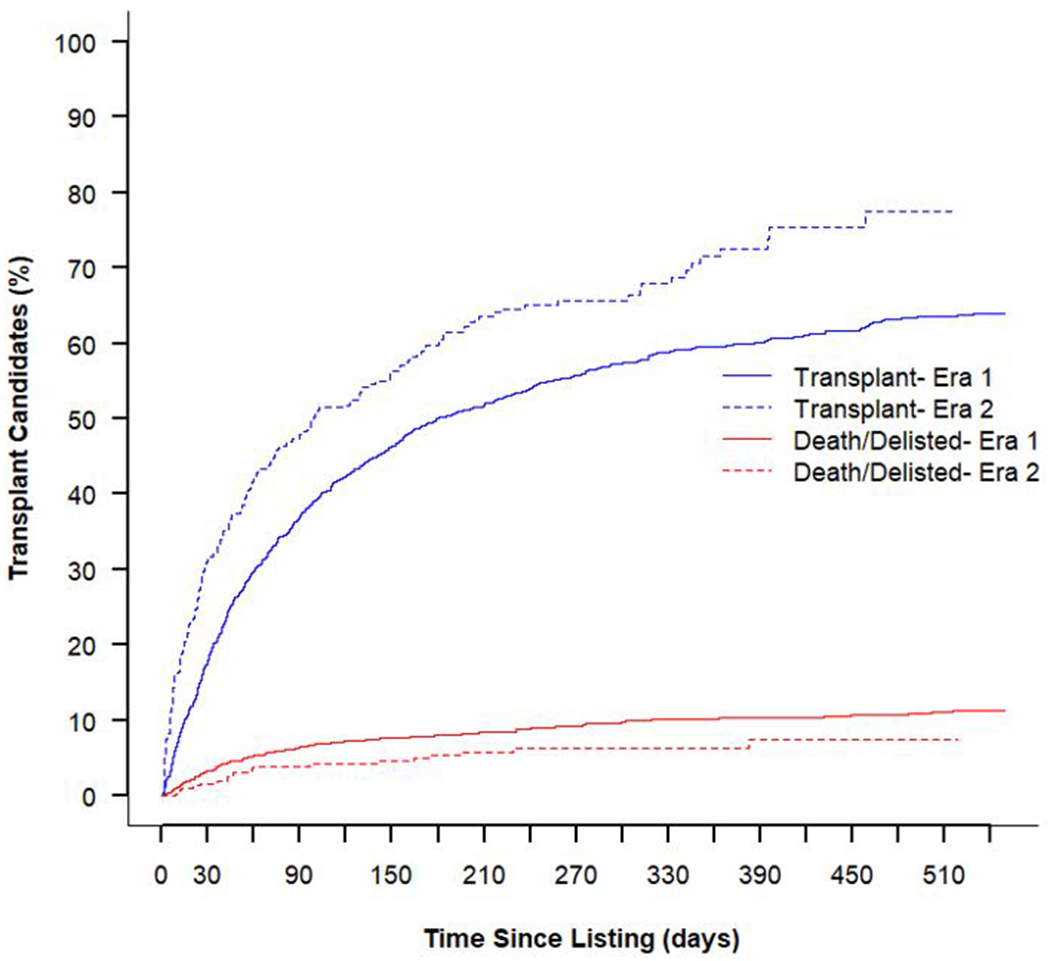
Cumulative incidence estimates from the competing risks of transplant and death/delisting pre- and post-policy change
CA and CS have been considered higher risk for HT, due to concerns for recurrence of the disease in the allograft and reduced post-transplant survival. In terms of CA, there were prior concerns regarding transplanting patients with immunoglobulin light-chain (AL) amyloidosis, which progresses more rapidly than transthyretin (ATTR) cardiac amyloidosis 7. However, post-transplant outcomes for patients with CA have improved significantly in both AL and ATTR cardiac amyloidosis in the current era, with post-transplant survival rates comparable to those of non-amyloid cardiomyopathies 19–22, likely related to advances in targeted amyloidosis therapy 23 in addition to more stringent selection criteria of transplant candidates. Reassuringly, the decrease in waitlist time can only serve to benefit this group of patients.
Similarly, patients with cardiac sarcoidosis, have often been considered higher risk for transplant due to concern for recurrent sarcoidosis in the allograft and graft rejection. Recent studies have shown that CS patients have outcomes comparable to those of non-CS patients with no difference in survival or graft rejection 9, 24, 25 highlighting the importance in bringing them successfully to transplant. Despite their omission from the guidance document for exception requests 8, those with CS seem to have similarly benefited under the new allocation policy (Supplemental table). In addition, outcomes in patients who undergo HT for HCM are comparable to, if not better than, non-HCM patients 26, 27. This underlines the importance of ensuring a level playing field when it comes to evaluating and listing these patients and ensuring they are not at a disadvantage while awaiting HT.
Limitations:
This retrospective analysis of the UNOS database has several limitations. First, in an effort to isolate the effects of the policy change, candidates listed in Era 1 who had events in Era 2 were excluded. Second, patients listed in Era 1 were included in Era 1 cohort provided they were removed from the waitlist within 18 months of listing (i.e. had an event – both transplant and waitlist death or delisting). Those who did not have an event within 18 months but had an event during Era 2 were excluded from the primary analysis, which raised the possibility of inflating waitlist events; however, a sensitivity analysis demonstrated that this did not alter the study’s conclusions. Additionally, patients listed in Era 1 had a greater possible waitlist time than those in Era 2 (5 years vs. 18 months) given that data for the new allocation period was only available up to March 31st, 2020. As a result, clinical follow-up time was limited to 18 months after listing in an effort to control for time-window bias. The consequence was that events (transplantation, waitlist death, or delisting) after 18 months were excluded among patients who were censored. Third, guideline-directed heart failure therapies for reduced ejection fraction heart failure have improved since the initiation of the historical 3-tier system. We addressed this limitation by confining our analysis to the 5 years prior to introduction of the new allocation policy during which time no significant advances in HF therapies occurred. However, given that the heart failure phenotypes included in this study typically have a preserved ejection fraction, this would likely not have impacted outcomes to any great extent. Lastly, post-transplant follow up is only available up to six months post policy change, which limits the conclusions regarding survival that can be drawn from this analysis.
Conclusion:
In this early analysis, the new allocation policy has been effective for patients with infiltrative, restrictive and hypertrophic cardiomyopathies, leading to increased transplantation rates in this cohort and reducing waitlist mortality, without affecting post-transplant outcomes. However, given that post HT follow-up is limited to 6-months, future studies are warranted to evaluate long-term survival and access to HT in these patient populations.
Supplementary Material
Acknowledgements:
Dr. Maurer is supported by a K24 grant from NIA (AG AG036778) entitled Midcareer Mentoring Award in Geriatric Cardiology.
Disclosure: Dr. Maurer reports grant support from National Institutes of Health [R01HL139671-01], [R21AG058348] and [K24AG036778], consulting income from Pfizer, GSK, EIdos, Prothena, Akcea and Alnylam, and institution received clinical trial funding from Pfizer, Prothena, Eidos and Alnylam. Dr. Clerkin is supported by the National Heart, Lung, and Blood Institute (Grant K23HL148528).
Abbreviations
- CA
cardiac amyloidosis
- CS
cardiac sarcoidosis
- HCM
hypertrophic cardiomyopathy
- HT
heart transplantation
- OPTN
Organ Procurement and Transplant Network
- RCM
restrictive cardiomyopathy
- UNOS
United Network for Organ Sharing
References:
- 1.Organ Procurement and Transplantation Network: Policy 6: Allocation of Hearts and Hearts-Lungs. October 2018. Available at: http://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf#nameddest=Policy_06 [Google Scholar]
- 2.Colvin-Adams M, Valapour M, Hertz M, et al. Lung and heart allocation in the United States. Am J Transplant. December 2012;12(12):3213–34. doi: 10.1111/j.1600-6143.2012.04258.x [DOI] [PubMed] [Google Scholar]
- 3.Sridharan L, Wayda B, Truby L, et al. Mechanical Circulatory Support Device Utilization and Heart Transplant Waitlist Outcomes in Patients With Restrictive and Hypertrophic Cardiomyopathy. Circ Heart Fail. 2018;11(3)::e004665. doi: 10.1161/CIRCHEARTFAILURE.117.004665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuñiga Cisneros J, Stehlik J, Selzman C, Drakos S, McKellar S, Wever-Pinzon O. Outcomes in Patients With Hypertrophic Cardiomyopathy Awaiting Heart Transplantation. Circ Heart Fail. 2018;11(3):e004378. doi: 10.1161/CIRCHEARTFAILURE.117.004378 [DOI] [PubMed] [Google Scholar]
- 5.Swiecicki PL, Edwards BS, Kushwaha SS, Dispenzieri A, Park SJ, Gertz MA. Left ventricular device implantation for advanced cardiac amyloidosis. J Heart Lung Transplant. May 2013;32(5):563–8. doi: 10.1016/j.healun.2013.01.987 [DOI] [PubMed] [Google Scholar]
- 6.Rowin EJ, Maron BJ, Kiernan MS, et al. Advanced heart failure with preserved systolic function in nonobstructive hypertrophic cardiomyopathy: under-recognized subset of candidates for heart transplant. Circ Heart Fail. November 2014;7(6):967–75. doi: 10.1161/CIRCHEARTFAILURE.114.001435 [DOI] [PubMed] [Google Scholar]
- 7.Gilstrap L, Niehaus E, Malhotra R, et al. Predictors of survival to orthotopic heart transplant in patients with light chain amyloidosis. J Heart Lung Transplant. February 2014;33(2):149–56. doi: 10.1016/j.healun.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thoracic Organ Transplant Committee. Review Board Guidance for Hypertrophic/Restrictive (HCM/RCM) Cardiomyopathy Exception Requests. October 2018. Available at: https://optn.transplant.hrsa.gov/media/2637/thoracic_guidance_review_board_hcm_rcm_201806.pdf. Accessed May 26th, 2020.
- 9.Crawford TC, Okada DR, Magruder JT, et al. A Contemporary Analysis of Heart Transplantation and Bridge-to-Transplant Mechanical Circulatory Support Outcomes in Cardiac Sarcoidosis. J Card Fail. June 2018;24(6):384–391. doi: 10.1016/j.cardfail.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 10.Hsich EM, Rogers JG, McNamara DM, et al. Does Survival on the Heart Transplant Waiting List Depend on the Underlying Heart Disease? JACC Heart Fail. September 2016;4(9):689–97. doi: 10.1016/j.jchf.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abt P, Rowin E, Maron B, et al. Heart Transplantation for Hypertrophic Cardiomyopathy: The Tufts Experience. Journal of Cardiac Failure. 2017;23(8):S23–24. doi: 10.1016/j.cardfail.2017.07.049 [DOI] [Google Scholar]
- 12.Topilsky Y, Pereira N, Shah D, et al. Left ventricular assist device therapy in patients with restrictive and hypertrophic cardiomyopathy. Circ Heart Fail. 2011;4(3):266–275. doi: 10.1161/CIRCHEARTFAILURE.110.959288 [DOI] [PubMed] [Google Scholar]
- 13.Panhwar MS, Al-Kindi SG, Tofovic D, Oliveira GH, Ginwalla M. Waitlist Mortality of Patients With Amyloid Cardiomyopathy who Are Listed for Heart Transplantation and Implications for Organ Allocation. J Card Fail. September 2019;25(9):767–771. doi: 10.1016/j.cardfail.2019.04.011 [DOI] [PubMed] [Google Scholar]
- 14.Smedema JP, van Geuns RJ, Ainslie G, Ector J, Heidbuchel H, Crijns H. Right ventricular involvement in cardiac sarcoidosis demonstrated with cardiac magnetic resonance. ESC Heart Fail. November 2017;4(4):535–544. doi: 10.1002/ehf2.12166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. February 2013;32(2):157–87. doi: 10.1016/j.healun.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 16.Goff R, Uccellini K, Lindblad K, et al. A Change of Heart: Preliminary Results of the US 2018 Adult Heart Allocation Revision. Am J Transplant. 2020;00:1–10. doi: 10.1111/AJT.16010 [DOI] [PubMed] [Google Scholar]
- 17.Cogswell R, John R, Estep JD, et al. An early investigation of outcomes with the new 2018 donor heart allocation system in the United States. J Heart Lung Transplant. January 2020;39(1):1–4. doi: 10.1016/j.healun.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 18.Parker WF, Chung K, Anderson AS, Siegler M, Huang ES, Churpek MM. Practice Changes at U.S. Transplant Centers After the New Adult Heart Allocation Policy. J Am Coll Cardiol. June 16 2020;75(23):2906–2916. doi: 10.1016/j.jacc.2020.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kristen A, Kreusser M, Blum P, et al. Improved outcomes after heart transplantation for cardiac amyloidosis in the modern era. J Heart Lung Transplant. 2018;37(5):611–618. doi: 10.1016/j.healun.2017.11.015 [DOI] [PubMed] [Google Scholar]
- 20.Barrett C, Alexander K, Zhao H, et al. Outcomes in Patients With Cardiac Amyloidosis Undergoing Heart Transplantation. J Am Coll Cardiol HF. 2020;8:461–8. doi: 10.1016/j.jchf.2019.12.013 [DOI] [PubMed] [Google Scholar]
- 21.Davis M, Kale P, Liedtke M, et al. Outcomes after heart transplantation for amyloid cardiomyopathy in the modern era. Am J Transplant. 2015;15(3):650–658. doi: 10.1111/ajt.13025 [DOI] [PubMed] [Google Scholar]
- 22.Griffin JM, Chiu L, Axsom KM, et al. Outcomes after heart transplantation for al compared to attr cardiac amyloidosis. Clin Transplant. July 5 2020:e14028. doi: 10.1111/ctr.14028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roussel M, Merlini G, Chevret S, et al. A prospective phase 2 trial of daratumumab in patients with previously treated systemic light-chain amyloidosis. Blood. 2020;135(18):1531–1540. [DOI] [PubMed] [Google Scholar]
- 24.Zaidi A, Zaidi A, Vaitkus P. Outcome of Heart Transplantation in Patients With Sarcoid Cardiomyopathy. J Heart Lung Transplant. 2007;26:714–7. doi: 10.1016/j.healun.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 25.Perkel D, Czer LS, Morrissey RP, et al. Heart transplantation for end-stage heart failure due to cardiac sarcoidosis. Transplantation proceedings. Jul-Aug 2013;45(6):2384–6. doi: 10.1016/j.transproceed.2013.02.116 [DOI] [PubMed] [Google Scholar]
- 26.Maron MS, Kalsmith BM, Udelson JE, Li W, DeNofrio D. Survival after cardiac transplantation in patients with hypertrophic cardiomyopathy. Circ Heart Fail. September 2010;3(5):574–9. doi: 10.1161/circheartfailure.109.922872 [DOI] [PubMed] [Google Scholar]
- 27.Coutu M, Perrault LP, White M, et al. Cardiac transplantation for hypertrophic cardiomyopathy: a valid therapeutic option. J Heart Lung Transplant. April 2004;23(4):413–7. doi: 10.1016/s1053-2498(03)00225-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


