Abstract
In the present article, we explore the risks of circadian disruptions and impact on the sleep-wake cycle of individuals with diabetes during COVID-19 pandemic. The association between the duration and quality of sleep and the stability of glucose levels is well-established. Therefore, during the pandemic with changes and limitations in the exposure to cyclic cues that entrain the circadian rhythms, such as light-dark and social interactions, we hypothesize that the power and stability of circadian rhythms decrease if measures are not taken to intentionally create a routine that includes zeitgebers. Knowing that sleep-wake cycle disruptions impair melatonin production, immune system response and glucose metabolism, and that individuals with diabetes are at higher risk for poor prognosis when infected by SARS-CoV-2 (especially if their blood glucose is out of target), we recommend monitoring and advising these individuals towards strategies to maintain adequate sleep quality and duration as part of their preventive and protective measures during the new pandemic routine.
Keywords: Diabetes Mellitus, Coronavirus Infections, Pandemics, Circadian Rhythm, Sleep Disorders, Circadian Rhythm, SARS Virus
INTRODUCTION
In early 2020, COVID-19 rapidly became the main global health concern due to its swift spreading pandemic characteristics. Reports from the different epicenters revealed severity and mortality of this novel virus more pronounced in men and elderly populations and in individuals with non-communicable diseases (NCDs), especially hypertension, diabetes, obesity, cancer and pulmonary chronic conditions1-3. Hygiene measures, social or physical distancing, and stay-at-home recommendations were established as main strategies to reduce transmission and to allow health systems preparedness in anticipation for the surge in demand in COVID-19 related services4,5,6. These interventions would also help to protect the population whilst allowing the recovery of those infected. While this seems reasonable from an epidemiological perspective, from a socio-economic-biological point of view, new challenges arose, including those with the potential to hamper the continuous self-care of individuals with diabetes and other NCDs1.
Whereas, in China, the overall case-fatality rate was 2.3%, it was 7.3% for individuals with diabetes2. In Italy, type 2 diabetes was the second most prevalent NCD associated with deaths (31.8%)1. Other countries reported similar trends; high severity and mortality of individuals with diabetes and other NCDs when infected by SARS-CoV-21,3,7. Recent studies revealed that more than diabetes, high blood glucose is a predictor of poor COVID- 19’s outcome7. Zhu et al.7 compared two groups of individuals with type 2 diabetes and COVID-19, one with blood glucose 70-180mg/dL and the other with blood glucose >180mg/dL, and found a lower all-cause mortality rate in the first group (HR=0.14; survival of 98.9% vs 89%)7. In hospitals in the US, while 41.7% of individuals with hyperglycemia (glycemia >180mg/dL) died, 85.2% of the ones with diabetes and glycemia below 180mg/dL survived (p<0.001)8. There seems to be various reasons for these outcomes associated with hyperglycemia, ranging from exacerbated expression of the coronavirus entry receptor (angiotensin-converting enzyme 2 - ACE2) to suboptimal immune response and induction of virus replication7,8,9.
Being aware of the delicate balance needed for individuals with diabetes to keep their blood glucose on target and knowing that in 62% of the countries services to treat diabetes and its complications were disrupted during the pandemic10, we intend in the present article to bring a contribution from chronobiology to potentially reduce metabolic consequences caused by alterations in these individuals’ routine. As widely studied, environmental cycles - especially the light-dark and the temperature - and the social interactions - from the alarm clock ringing, to meals, TV and exercise times - are zeitgebers, i.e., they entrain the endogenous rhythms in a period of approximately 24 hours, regulating the different body systems11. This circadian entrainment, leading to synchronized endogenous rhythms, is known for preventing diseases and promoting well-being6,11. For this reason, the impact of partial or full lockdown with restrictions in mobility changes the exposure to environmental and social cues, potentially leading to misalignment with environmental cycles and internal desynchrony6. Consequently, immune responses become impaired, and metabolic and mental disorders may develop or exacerbate12-17. In individuals with either main types 1 or 2 of diabetes, the described consequences of this misalignment, desynchrony or sleep impairment is insulin resistance, leading to increased glycemic levels and variability6,12,13,16,18,19.
The new routines varied widely during the pandemic. Few, if any, were able to maintain their pre-pandemic daily life pattern. While some lost their jobs or lacked access to schooling, others, including many healthcare professionals (HCP), worked day and night. Nevertheless, regarding the sleep-wake cycle, those who prior to the pandemic worked during hours that collided with their biopsychological timing might have appreciated waking up later or having a naptime after lunch. Whereas some may experience the feeling of “I can finally sleep the number of hours that I need and feel well”, many others might be “lost in time”20, sleeping or waking up at a different time each day, experiencing low sleep quality, fragmentation or insomnia, headaches and humor instability6,15,21,22.
Different aspects contribute towards this reduction in the sleep-wake cycle stability; some would be attributed to the psychological uncertainty of life during the pandemic, while others are associated with desynchrony due to limited or irregular exposure to zeitgebers6,21,22. In addition to the effect of desynchrony and circadian misalignment decreasing glucose tolerance and insulin sensitivity16,18, individuals with any of the main diabetes types present an additional concern related to reduced production of the night phase hormone, melatonin23,24. Melatonin is known for acting as an endogenous zeitgeber, entraining the different tissues in a circadian pattern, in addition to its powerful antioxidant and immunomodulatory characteristics25,26. As a result, it synchronizes the immune system through its cyclic release, and modulates the innate immunity, through its redox regulation and oxidative stress suppression14,25,26. This is understood as fundamental for an optimal response to viruses and other infections14,15,26. Therefore, while individuals with diabetes present a decrease in melatonin production associated with blood glucose levels23,24, it is hypothesized that melatonin would protect against COVID-1926.
Melatonin is intimately associated with the sleep-wake cycle regulation25. During the pandemic, certain factors may inhibit melatonin production and superficialize sleep. Among them are increased time watching TV and surfing the internet27 - screen time is negatively correlated with sleep duration and well-being, with screen light being a melatonin and sleep inhibitor28, and increased glycemia in individuals with diabetes27. Summing to this scenario of sleep-wake cycle instability the reduction in physical activity27, a known zeitgeber and traditional pillar for glycemic control in people with diabetes, and the inability of many nations to provide adequate health services and medicines to people with diabetes10,27,29, it is not surprising the consequences on their glycemic control12,27. In Brazil, 59.4% of the individuals with diabetes were unable to maintain their blood glucose at the same levels or stability as prior to the COVID-19 pandemic27. Thus, we see clear reasons for flagging the sleep-wake cycle as a point of attention with the potential to intervene, despite the fact that, to our knowledge, no study was conducted so far to evaluate it in individuals with diabetes during the COVID-19 pandemic.
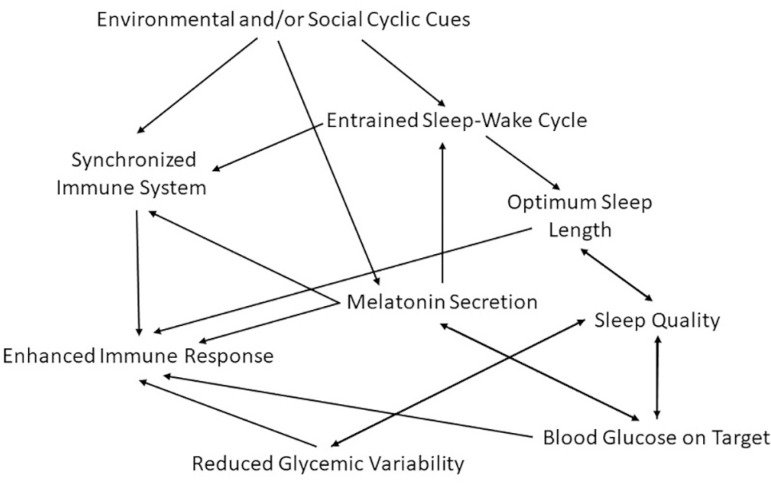
Taking into consideration that: 1) COVID-19 severity and mortality in people with diabetes are higher than in people without NCDs, and even higher when associated with increased blood glucose levels7-9; and, 2) misalignment with exogenous cycles during the pandemic may impair individuals’ sleep wake-cycle in different ways, contributing towards the reported inability to keep blood glucose control during the pandemic in people with diabetes12,13,16,18,19,27, we see opportunity for contributions from chronobiology and sleep sciences. While it is true that healthcare systems adjustments for access to medicines and healthcare services must be optimized1,3,10,27,29,30, strategies of exposure to zeitgebers and sleep hygiene can be widely adopted to assist in achieving the goal of bringing individuals’ blood glucose into target during the COVID-19 pandemic and, this way, improve their immune response and condition to fight SARS-CoV-2 and other potential infections7,8,11,14,25,26, as depicted in Figure 1.
Figure 1.
Map connecting environmental and social cyclic cues with the sleep- wake cycle to an enhanced immune response in individuals with diabetes.
CONCLUSION
Aspects discussed above delineate the consequences of circadian disruption in the population with diabetes, which would make them more vulnerable to severe SARS-CoV-2 infection. Our chronobiological recommendations include measures to entrain circadian rhythms of these individuals and their caretakers, and hence improve well-being, prevent sleep disorders, enhance immune response and aid in maintaining blood glucose levels on target (Figure 1). They should include strategies of exposure to zeitgebers and establishment of a routine that fits individual’s sleep needs including morningness/eveningness preferences, duration and quality. An option to identify the preferable routine would be by filling daily notes (diaries) in which the person captures the time of his/her activities along with evaluations of how he/she feels (mood, motivation and disposition). Doing that for a sequence of several days, would aid the subject to adopt the best routine for his/her well-being.
Strategies of exposure to environmental and social zeitgebers, whilst building a routine, should also be adopted by HCPs whom, in addition to close exposure to the virus, are at higher risk due to immune response impairment caused by shiftwork and exhaustive routines during the pandemic15,22. The recommendations do not necessarily mean a strict agenda, but rather improve the sleep-wake cycle power and stability, while reducing insomnia, short and fragmented sleep, and enhancing melatonin production. The plan must include cyclic elements such as: 1) regular schedule to go to bed and wake up; 2) meals together with home cohabitants; 3) regular online or phone interaction with friends and family; 4) exposure to sunlight in the morning; 5) limited screen time in the evenings (especially cellphone screens without night mode or blue light night filter); 6) regular exercise (preferably in the morning or afternoon); and 7) caffeinated beverages limited to the morning or early afternoon6,21,31,32,33. Moreover, we suggest that HCPs consider asking and guiding individuals with diabetes, as well as wider population and especially those with NCDs or in higher risk groups for severe COVID-19, about their sleep quality and routine.
In conclusion, we believe that there is ample evidence that strategies to keep the circadian rhythms, especially sleep-wake cycle, synchronized during the COVID-19 pandemic - improving sleep length and quality - can have significant effects on regulating the glycemia of individuals with diabetes, improving their immune response, well-being and, as a consequence, reducing the risk for severe consequences and mortality associated with SARS-CoV-2 infection.
REFERENCES
- 1.Kluge H, Wickramasinghe K, Rippin H, Mendes R, Peters DH, Kontsevaya A, et al. Prevention and control of non-communicable diseases in the COVID-19 response. Lancet. 2020;395(10238):1678–1680. doi: 10.1016/S0140-6736(20)31067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan J. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA Network. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Gentile S, Strollo F, Ceriello A. COVID-19 infection in Italian people with diabetes: Lessons learned for our future (an experience to be used) Diabetes Res Clin Pract. 2020 Apr;162:108137–108137. doi: 10.1016/j.diabres.2020.108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puig-Domingo M, Marazuela M, Giustina A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020 Apr;68(1):2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inglesby TV. Public health measures and the reproduction number of SARS-CoV-2. JAMA. 2020;323(21):2186–2187. doi: 10.1001/jama.2020.7878. [DOI] [PubMed] [Google Scholar]
- 6.Erren TC, Lewis P. SARS-CoV-2/COVID-19 and physical distancing: risk for circadian rhythm dysregulation, advice to alleviate it, and natural experiment research opportunities. Chronobiol Int. 2020 Jun 05; doi: 10.1080/07420528.2020.1772811. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Zhu L, She Z, Cheng X, Qin JJ, Zhang XJ, Cai J, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020 Jun;31(6):1068–77.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bode B, Garrett V, Messler J, McFarland R, Crowe J, Booth R, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Codo AC, Davanzo GG, Monteiro LB, Souza GF, Muraro SP, Virgilio-da-Silva JV, et al. Elevated glucose levels favor Sars-Cov-2 infection and monocyte response through a HIF-1a/glycolysis dependent axis. Cell Metabolism. 2020 Jul 15; doi: 10.1016/j.cmet.2020.07.007. [Epub ahead of print] [DOI] [Google Scholar]
- 10.World Health Organization (WHO) Rapid assessment of service delivery for NCDs during the COVID-19 pandemic [Internet] Geneva: WHO; 2020. [2020 Jul 02]. Available from: https://www.who.int/publications/m/item/rapid-assessment-of-service-delivery-for-ncds-during-the-covid-19-pandemic. [Google Scholar]
- 11.Roenneberg T, Merrow M. The circadian clock and human health. Curr Biol. 2016 May;26(10):R432–R443. doi: 10.1016/j.cub.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Barone MTU, Menna-Barreto L. Diabetes and sleep: a complex cause-and-effect relationship. Diabetes Res Clin Pract. 2011;91(2):129–137. doi: 10.1016/j.diabres.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2017 Feb;31:91–101. doi: 10.1016/j.smrv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Mazzoccoli G, Vinciguerra M, Carbone A, Relógio A. The circadian clock, the immune system, and viral infections: the intricate relationship between biological time and host-virus interaction. Pathogens. 2020 Jan;9(2):E83. doi: 10.3390/pathogens9020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva FR, Guerreiro RC, Andrade HA, Stieler E, Silva A, Mello MT. Does the compromised sleep and circadian disruption of night and shiftworkers make them highly vulnerable to 2019 coronavirus disease (COVID-19)? Chronobiol Int. 2020 May 20; doi: 10.1080/07420528.2020.1756841. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014 Jun;63(6):1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020 May 30; doi: 10.1016/j.bbi.2020.05.048. [Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason IC, Qian J, Adler GK, Scheer FAJL. Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia. 2020 Mar;63(3):462–472. doi: 10.1007/s00125-019-05059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barone MT, Wey D, Schorr F, Franco DR, Carra MK, Lorenzi Filho G, et al. Sleep and glycemic control in type 1 diabetes. Arch Endocrinol Metab. 2015 Feb;59(1):71–78. doi: 10.1590/2359-3997000000013. [DOI] [PubMed] [Google Scholar]
- 20.Cellini N, Canale N, Mioni G, Costa S. Changes in sleep pattern, sense of time and digital media use during COVID-19 lockdown in Italy. J Sleep Res. 2020 Apr;1:e13074. doi: 10.1111/jsr.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Society of Bipolar Disorders (ISBD) The COVID-19 pandemic and emotional well-being: tips for healthy routines and rhythms during unpredictable times. Psychiatric Times [Internet] Mar, 2020. [2020 Jul 02]. Available from: https://sltbr.org/wp-content/uploads/2020/04/Social-Rhythms-PSA_ISBD-Task- Force.pdf.
- 22.Zhang C, Yang L, Liu S, Ma S, Wang Y, Cai Z, et al. Survey of insomnia and related social psychological factors among medical staff involved in the 2019 novel coronavirus disease outbreak. Front Psychiatry. 2020 Apr;11:306–306. doi: 10.3389/fpsyt.2020.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karamitri A, Jockers R. Melatonin in type 2 diabetes mellitus and obesity. Nat Rev Endocrinol. 2019 Feb;15(2):105–125. doi: 10.1038/s41574-018-0130-1. [DOI] [PubMed] [Google Scholar]
- 24.Amaral FG, Turati AO, Barone M, Scialfa JH, Buonfiglio DC, Peres R. Melatonin synthesis impairment as a new deleterious outcome of diabetes-derived hyperglycemia. J Pineal Res. 2014 Aug;57(1):67–79. doi: 10.1111/jpi.12144. [DOI] [PubMed] [Google Scholar]
- 25.Amaral FGD, Cipolla-Neto J. A brief review about melatonin, a pineal hormone. Arch Endocrinol Metab. 2018 Aug;62(4):472–479. doi: 10.20945/2359-3997000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NaveenKumar SK, Hemshekhar M, Jagadish S, Manikanta K, Vishalakshi GJ, Kemparaju K, et al. Melatonin restores neutrophil functions and prevents apoptosis amid dysfunctional glutathione redox system. J Pineal Res. 2020 Jun;1:e12676. doi: 10.1111/jpi.12676. [DOI] [PubMed] [Google Scholar]
- 27.Barone MTU, Harnik SB, de Luca PV, Lima BLS, Wieselberg RJP, Ngongo B, et al. The impact of COVID-19 on people with diabetes in Brazil. Diabetes Res Clin Pract. 2020 Jul 03; doi: 10.1016/j.diabres.2020.108304. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hale L, Guan S. Screen time and sleep among school-aged children and adolescents: a systematic literature review. Sleep Med Rev. 2015 Jun;21:50–58. doi: 10.1016/j.smrv.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barone MTU, Villarroel D, de Luca PV, Harnik SB, Lima BLS, Wieselberg RJP, et al. COVID-19 impact on people with diabetes in South and Central America (SACA region) Diabetes Res Clin Pract. 2020 Jul 03; doi: 10.1016/j.diabres.2020.108301. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katulanda P, Dissanayake H, Ranathunga I, Ratnasamy V, Wijewickrama PSA, Yogendranathan N, et al. Prevention and management of COVID- 19 among patients with diabetes: an appraisal of the literature. Diabetologia. 2020;64:1440–1452. doi: 10.1007/s00125-020-05164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao H, Zhang Y, Kong D, Li S, Yang N. Social capital and sleep quality in individuals who self-isolated for 14 days during the coronavirus disease 2019 (COVID-19) outbreak in January 2020 in China. Med Sci Monit. 2020 Mar;26:e923921–e923928. doi: 10.12659/MSM.923921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altena E, Baglioni C, Espie CA, Ellis J, Gavriloff D, Holzinger B. Dealing with sleep problems during home confinement due to the COVID-19 outbreak: practical recommendations from a task force of the European CBT-I Academy. J Sleep Res. 2020 Apr;:e13052. doi: 10.1111/jsr.13052. [DOI] [PubMed] [Google Scholar]
- 33.Karthikeyan R, Spence DW, Pandi-Perumal SR. The contribution of modern 24-hour society to the development of type 2 diabetes mellitus: the role of insufficient sleep. 2019;12(3):227–227. doi: 10.5935/1984-0063.20190061. [DOI] [PMC free article] [PubMed] [Google Scholar]