Epidemiology
The number of reproductive age women with valvular heart disease (VHD) is rising given advancements in medicine and surgery, especially within the congenital heart disease (CHD) population.1,2 While only 1–2% of reproductive age women have VHD, it accounts for 1/3 of all heart disease among pregnant women.1,3 The most common etiology for valvular disease in the United States is CHD, however, rheumatic heart disease is most common among immigrant women from developing countries and worldwide.2,4 Heart failure and arrhythmia are frequent adverse maternal outcomes, depending on the type and severity of valvular disease, as well other concurrent cardiac abnormalities.3,5,6
Collaborative care with a Maternal Fetal Medicine (MFM) subspecialist and Cardiologist is recommended for women with valvular disease in pregnancy.4 Preconception and baseline cardiac function should be assessed prior to pregnancy, when possible, with transthoracic echocardiogram, stress testing, or both, depending on the clinical situation. In general, due to the hemodynamic and cardiovascular changes in pregnancy, repeat echocardiograms should be performed to evaluate valvular and overall cardiac function in the first trimester as well as at approximately 28–32 weeks when blood volume reaches its peak.
Evaluation of Valvular Heart Disease
Diagnosis of VHD should ideally occur prior to pregnancy with correction of severe, symptomatic lesions, with shared decision making on the part of patient and physician, as well as input of a heart team working together towards decreasing maternal and fetal risk. In practice, women with untreated VHD and inadequate medical care may not come to attention until they develop symptoms induced by the hemodynamic changes of pregnancy. Symptoms associated with VHD are similar to those of normal pregnancy, such as exertional dyspnea, limited exercise tolerance, fatigue, lightheadedness, syncope/pre-syncope or palpitations. Transthoracic echocardiography (TTE) should be performed for women with known VHD pre-conception or early in pregnant women with unexplained cardiac signs or symptoms.4,7 Severity of valvular lesions is graded by TTE, as a first line test. Of note, the American Heart Association/American College of Cardiology guidelines use more descriptive VHD categories.8 Instead of mild, moderate or severe VHD, individuals are described at being at risk, having progressive VHD, with asymptomatic, severe or severe, symptomatic VHD. The medical literature and European Society of Cardiology (ESC) guidelines on VHD in pregnancy use mild, moderate, severe categories and we refer to this classification henceforth.4
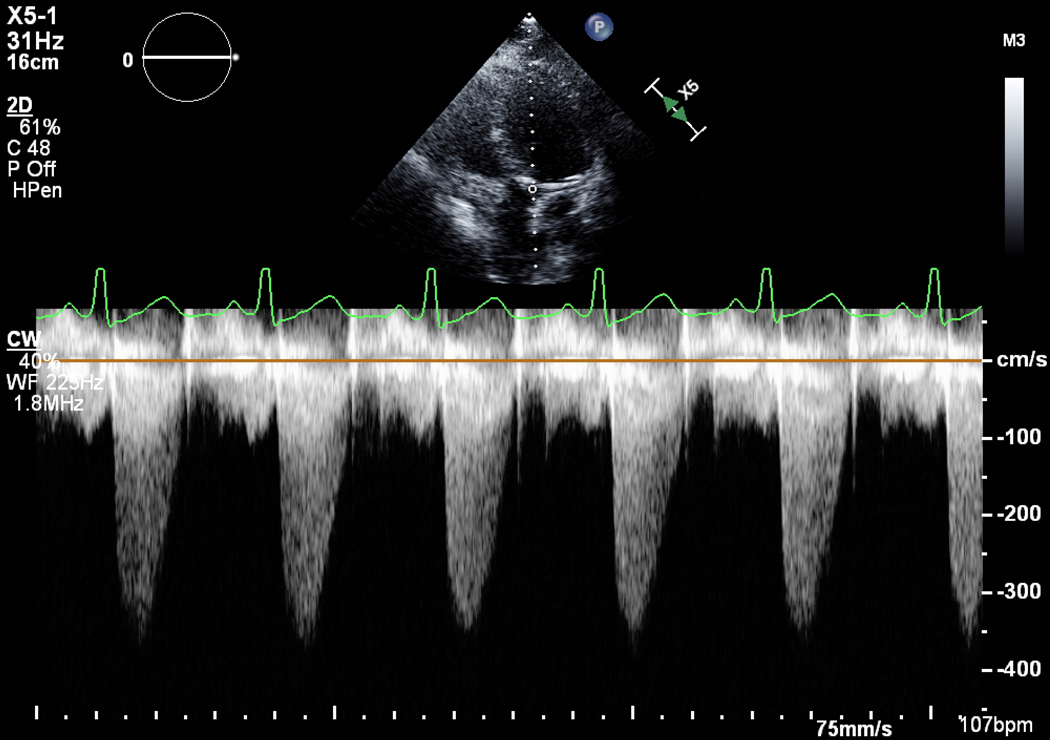
Serial imaging may be useful in progressive trimesters, as hemodynamics, intravascular volume, physiologic anemia and systemic vascular resistance influence valve gradients. Serial TTE is also used to measure pulmonary artery pressure and to detect decreased left ventricular ejection fraction (LVEF). For aortic and mitral stenosis (AS and MS, respectively), direct planimetry of the valve is a flow-independent measure of valve area. However, planimetry is highly dependent on image quality which can be improved with transesophageal echocardiography (TEE), if 2D TTE is limited (Fig. 1 A, B). Planimetry is more accurate than flow-dependent measures in pregnancy, since higher cardiac output and tachycardia will increase the measured gradient across the valve. Importantly, the calculated valve area by the continuity equation will remain unaffected because of conservation of flow and should be part of the evaluation of severity.
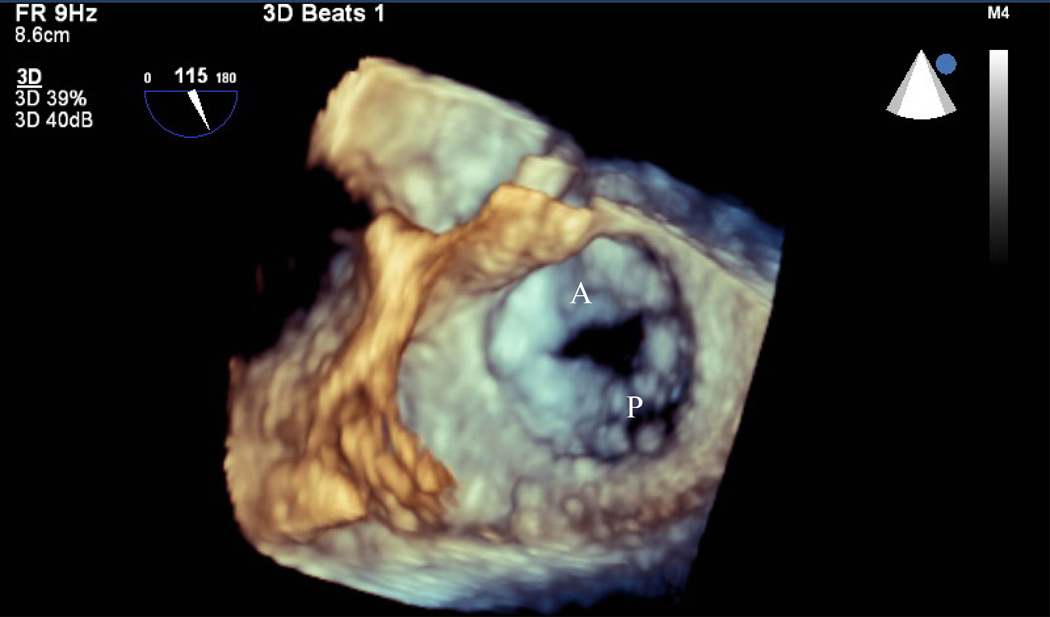
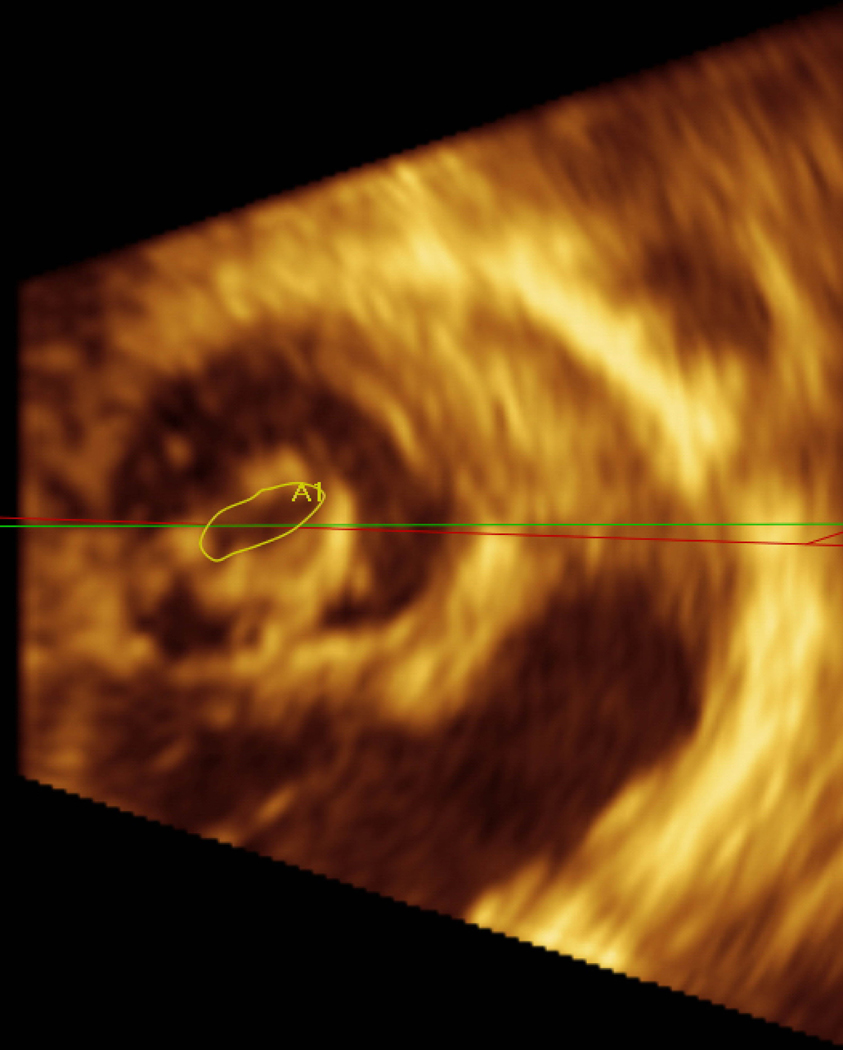
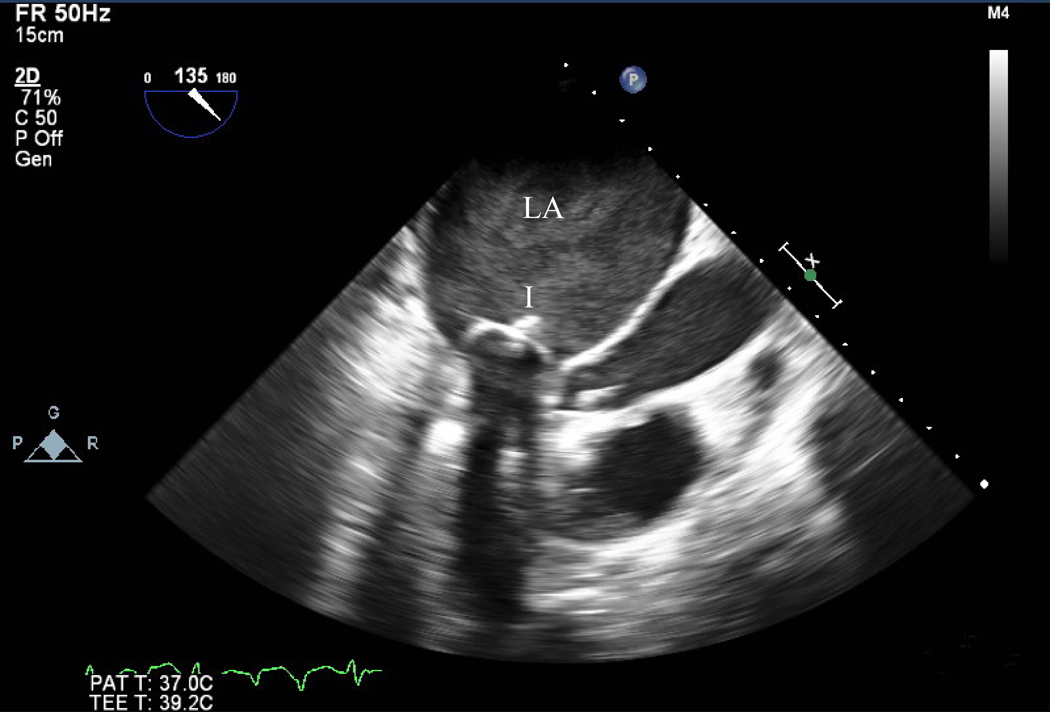
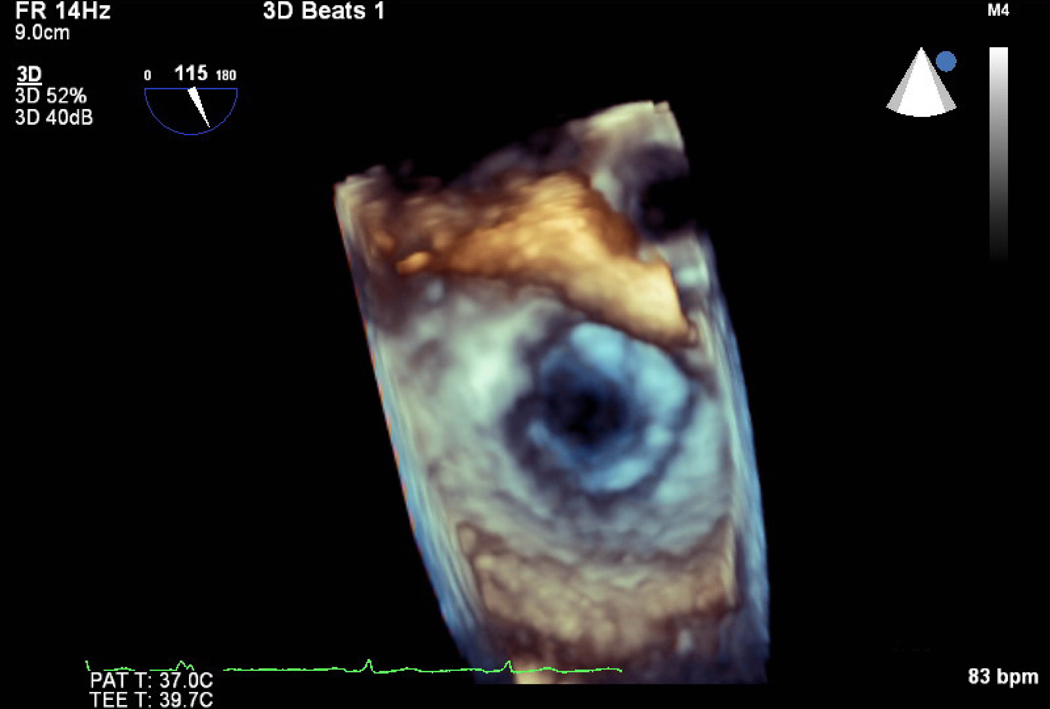
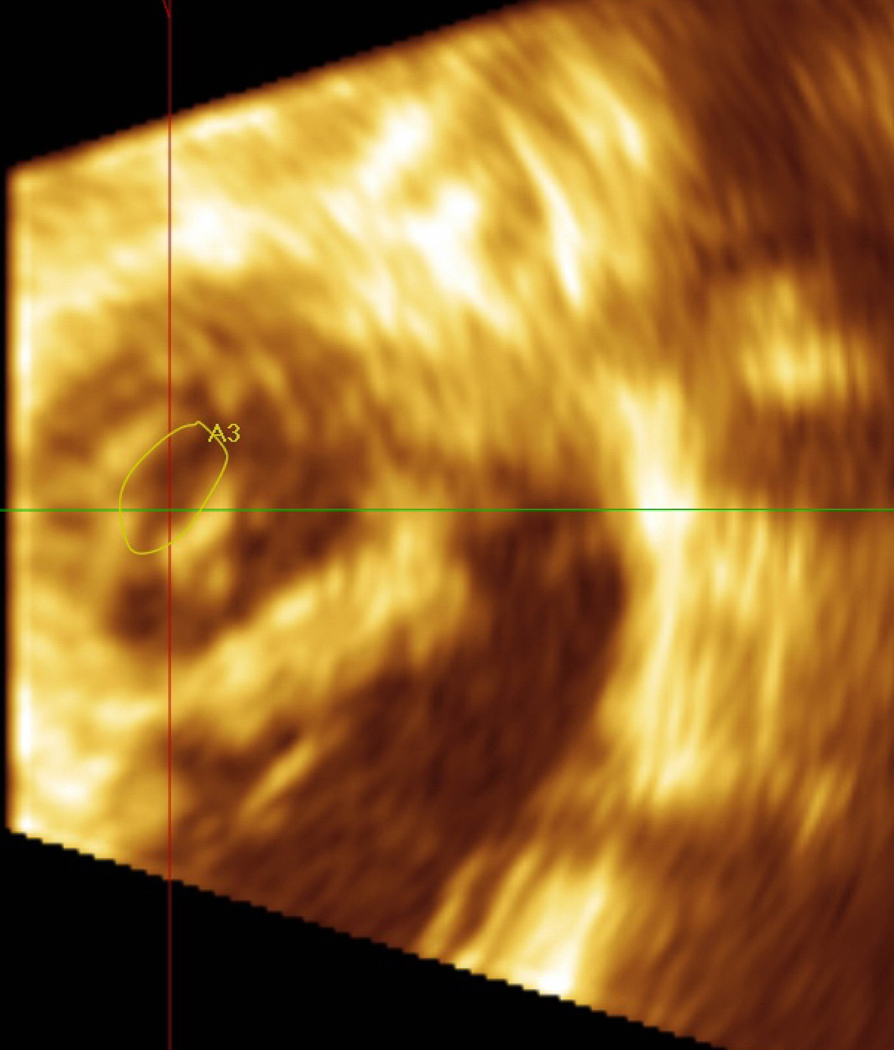
Figure 1.
Pre mitral balloon valvuloplasty for rheumatic mitral stenosis (MS)—A) 3D transesophageal echocardiogram of severe MS, A=Anterior leaflet P=Posterior leaflet. B) Planimetry of valve area (A1=0.79 cm2). C) Inflation of an Inoue balloon (I) in the stenotic mitral valve during mitral balloon valvuloplasty (MBV). Note spontaneous echocardiographic contrast or “smoke” in the left atrium (LA) indicating stasis of flow during MBV. Post MBV—D) 3D of the mitral valve. E) Planimetry of the valve area post MBV (A3=1.32 cm2). The patient presented for MBV in the 2nd trimester, the preferred time for intervention.
Left Sided Valvular Heart Disease
Left sided stenotic lesions, MS and AS, are usually more poorly tolerated in pregnancy as compared to regurgitant ones (mitral and aortic regurgitation, MR and AR, respectively). The risk of acute congestive heart failure (CHF) with either stenotic or regurgitant lesions is worsened by increased plasma blood volume (which doubles in pregnancy) and increased afterload, stemming from chronic or gestational hypertension or preeclampsia, for example. Cardiac output, afterload, and preload change markedly peripartum, which makes women with left sided lesions especially vulnerable to CHF in the first 1–3 days after delivery.9 Tachycardia is particularly problematic in MS because a longer diastolic filling period is needed across the valve, while less problematic for AR, in which longer diastolic filling is undesirable as it can overload the left ventricle. Pulmonary edema can result from severe stenotic or regurgitant lesions, particularly when coupled with low LVEF and/or arrhythmia, or acute valvular regurgitation, from a ruptured mitral valve chord with flail leaflet, for example.
Mitral Stenosis
MS is the most common valvular lesion in pregnancy, largely due to rheumatic heart disease in the developing world.10,11 The valve takes on a characteristic “fish mouth” appearance as the anterior and posterior leaflets become scarred at the commissures (Fig. 1 A, B). Poor leaflet mobility is also associated with MR for some. The gradient across the valve is highly dependently on heart rate, with less severe gradients at a slower rate. Thus, the cornerstone of medical management is rate control with beta blockers and volume management with judicious use of diuretics when congestive symptoms are present.4 While beta blockers and diuretics can theoretically impact fetal growth and amniotic fluid volume status, maternal benefits in preventing decompensation and mortality outweigh theoretical risks. Mild MS is often well tolerated in pregnancy and vaginal delivery recommended. However, even mild MS has been associated with a risk of small for gestational age fetuses and premature birth.12–14
Due to the physiologic changes of pregnancy, women with MS are at increased risk for both maternal and fetal complications. Medical management is often inadequate for severe MS in pregnancy. For women with severe MS, pregnancy should be avoided until the valve is treated or replaced.4 Maternal mortality has been reported as 0–2% and fetal mortality as 1–4%, with roughly 35% of fetuses born pre-term, small for gestational age, and 50% low birth weight.12–14 Data indicate that outcomes vary in severity by degree of MS.14 CHF is frequent; nearly 50% experiencing this adverse outcome in severe MS, in some reports.12–14 Studies of women with mild MS demonstrate a 10–20% risk of arrhythmia (usually atrial fibrillation or flutter) or heart failure/pulmonary edema and 4–10% risk of pre-term delivery and small for gestational age.12–14 Women with moderate MS have up to a 45% chance of arrhythmia or heart failure/pulmonary edema, 10–30% risk of preterm delivery and/or small for gestational age.12–14 Lastly, women with severe MS have an up to 65% chance of arrhythmia and heart failure/pulmonary edema and 20–40% chance of preterm delivery.12–14
If significant MS is diagnosed in pregnancy, then the general preference is for percutaneous mitral balloon valvuloplasty (MBV) if the valve is suitable on transthoracic or transesophageal echocardiogram (TTE or TEE, respectively). MBV is a catheter based procedure performed under echocardiographic and fluoroscopic guidance, in which the goal is to open the fused commissures of the mitral valve to improve orifice area (Fig. 1C-E).8,15,16 In severe MS, the subvalvular apparatus is often involved, with leaflet and/or chordal thickening and calcification. The Wilkin’s score rates leaflet mobility, calcification, valve and subvalvular thickening, with increasing scores portending a worse result with MBV.17 MBV is usually performed in the 2nd trimester and considered in those with New York Heart Association (NYHA) class III/IV congestive heart failure or pulmonary artery systolic pressures of ≥50 mmHg.4,15 It carries the risk of severe, acute MR which can necessitate emergent surgical repair. Open surgical mitral valve replacement is associated with maternal and fetal morbidity and mortality (see “Valve Replacement”) and there is presently no approved percutaneous valve replacement for the mitral valve.
L&D Considerations in MS
L&D (L&D) poses a specific challenge for patients with MS.4,18 Each contraction increases circulating blood volume by 300–500cc, subsequently increasing venous return, preload and cardiac output. Systolic and diastolic blood pressure values (afterload) increase with each contraction. There is auto-transfusion of uteroplacental blood (~500cc) immediately after delivery from decompression of the inferior vena cava. Tachycardia, doubling of plasma volume and a 50% rise in cardiac output are all physiologic changes which are not well tolerated during L&D, particularly for women with severe MS who are at high risk of acute left systolic CHF.12,18 The main hemodynamic goal during L&D is to maintain euvolemia to net negative fluid balance. Intrathoracic pressure rises and venous return declines, thus, decreasing stroke volume when a woman is performing Valsalva to push in the second stage of labor. Heart rate increases in an attempt to maintain cardiac output. With a prolonged and repeated Valsalva, compensatory mechanisms can become exhausted with less ability to maintain cardiac output. Therefore, an assisted second stage, with forceps or vacuum, should be considered. MS is not an absolute indication for Cesarean delivery under the care of experienced Anesthesiologists, Obstetricians, MFM subspecialists and Cardiologists. While Cesarean delivery avoids the increase in cardiac output with contractions, it does not avoid the large auto-transfusion that occurs after delivery nor the larger blood loss that accompanies Cesarean as compared to a vaginal delivery.
Other L&D considerations for MS include the avoidance of terbutaline, a tocolytic, as it can cause tachycardia. Routine regional anesthesia should be employed early in the labor process to prevent tachycardia associated with labor pain. Importantly, women with MS remain at an increased risk for CHF 1–2 weeks postpartum as there is mobilization of extravascular fluid into the vascular system. Therefore, women with MS should be seen in the outpatient setting shortly after delivery.
Aortic Stenosis
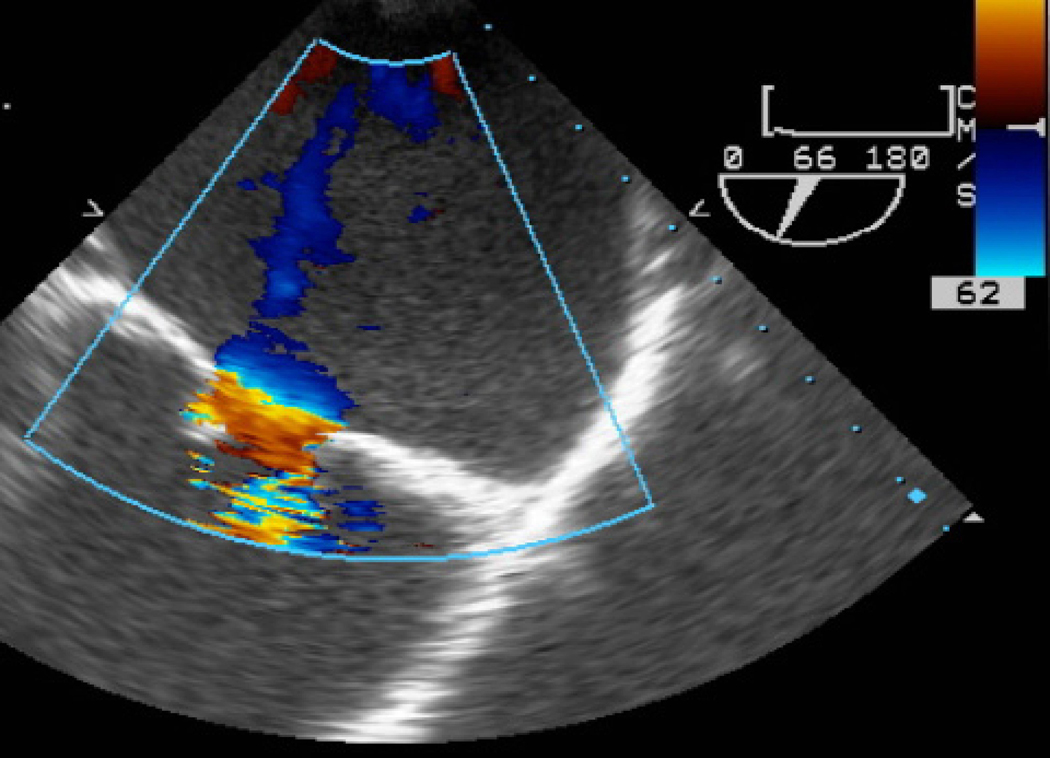
Etiologies of AS in pregnancy include congenital uni- or bicuspid aortic valve and rheumatic heart disease (Fig. 2).19 Bicuspid AS is associated with dilation of the ascending aorta in some cases due to underlying mutations which affect both the valve and collagen fibril organization. Ideally, a pre-pregnancy (baseline) diameter should be measured with repeat assessment during the first and third trimester of pregnancy as well as postpartum to rule out progressive enlargement. Pregnancy is contraindicated in women with bicuspid aortic valve when aortic dilation is >50 mm and Cesarean delivery should be considered with progressive enlargement to avoid increased pressure associated with Valsalva.4,20,21
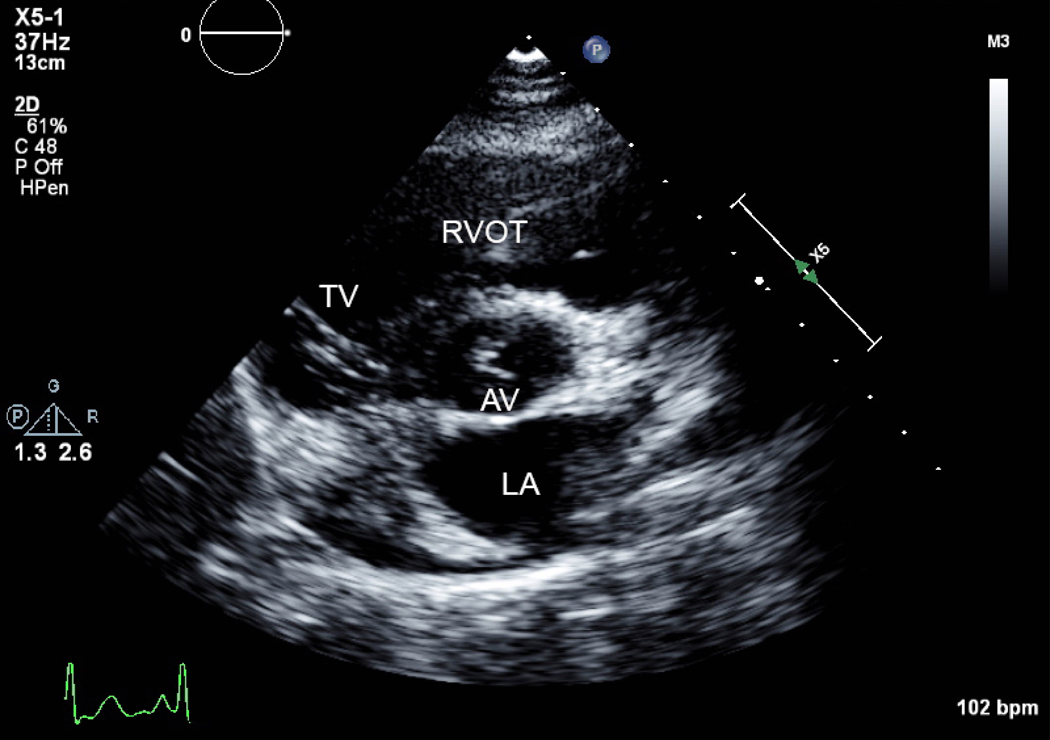
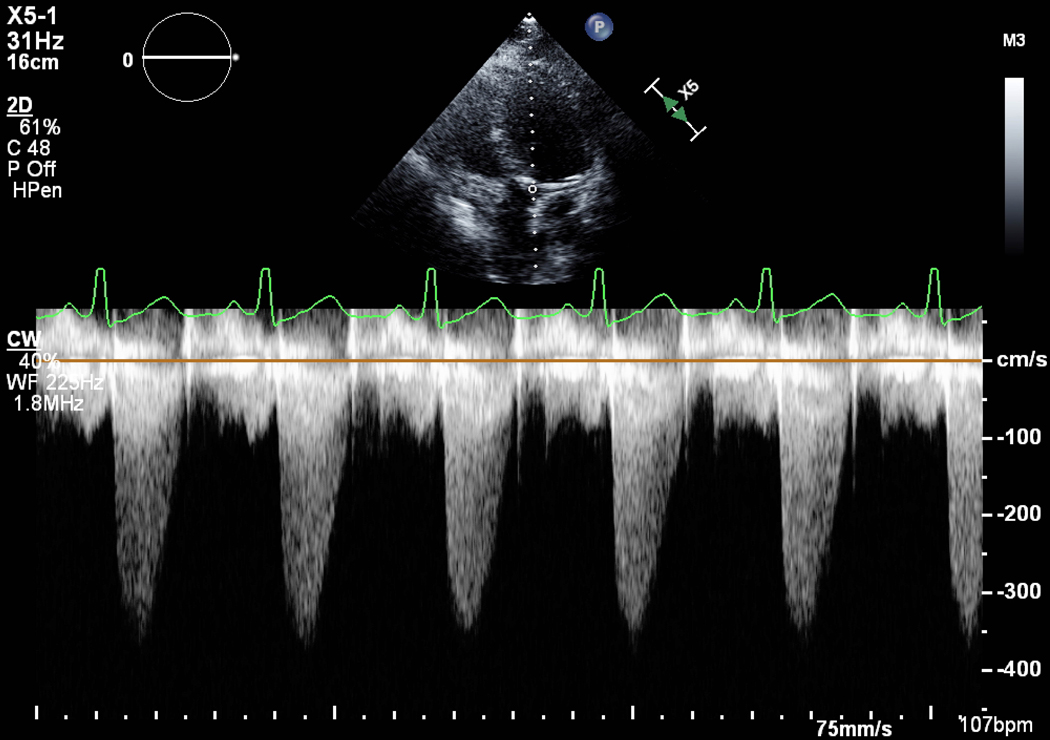
Figure 2.
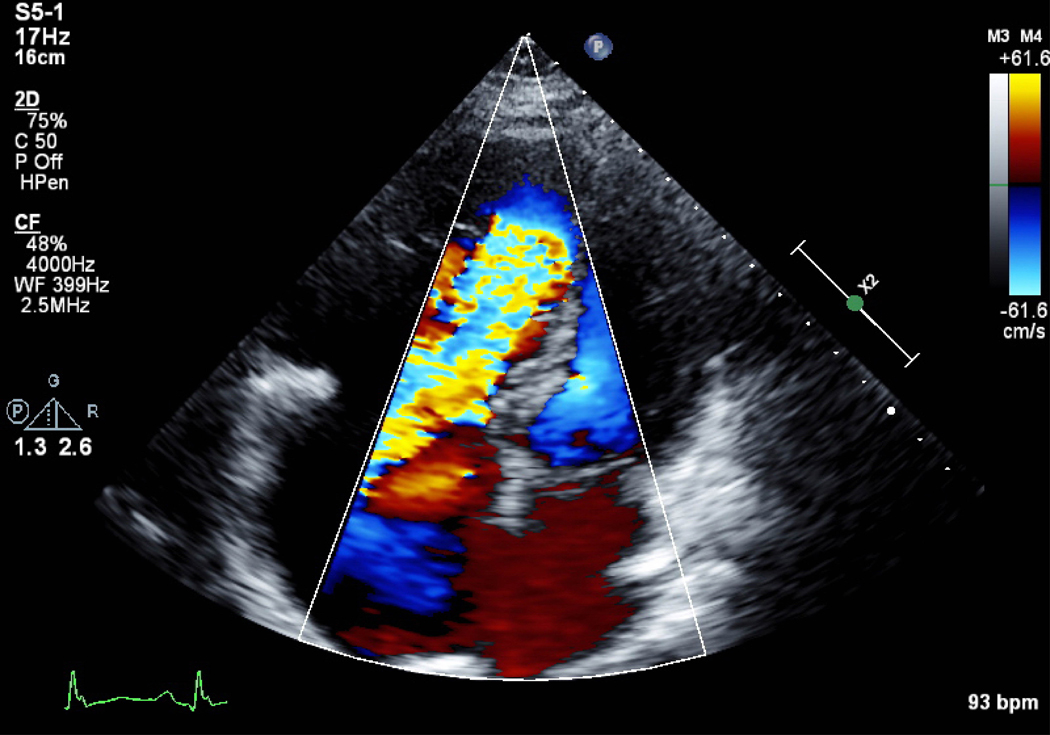
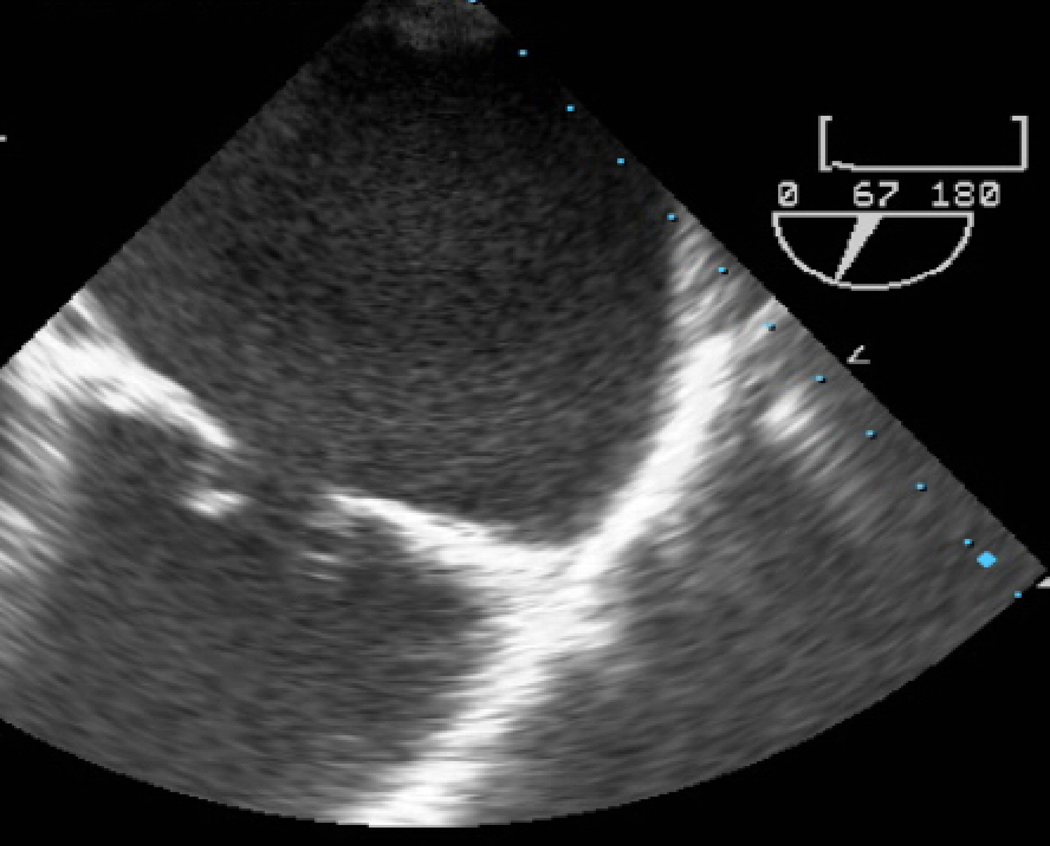
A) Short axis view of a unicuspid vs. bicuspid aortic valve (AV), with combined aortic stenosis and regurgitation. B) Continuous wave Doppler across the AV, indicating a mild gradient. AV=aortic valve, LA=left atrium, RVOT= right ventricular outflow tract, TV=tricuspid valve.
Mild or moderate AS is usually well tolerated in pregnancy. Conversely, severe symptomatic AS has been associated with significant adverse maternal and fetal outcomes.8,15 Women with severe AS, and particularly those with low LVEF, should avoid pregnancy until the valve is treated or consider termination, per guidelines.4 Interventions are recommended prior to pregnancy in women with severe AS who are symptomatic, have LV dysfunction with LVEF <50%, or in those who are symptomatic during exercise testing.4 This is due, in part, to the fact that women with AS have a fixed stroke volume. Therefore, they are only able to increase their cardiac output by increasing the heart rate in order to accommodate the increasing blood volume and demand from pregnancy. Increased heart rate leads to decreased time in diastole and therefore decreased time for ventricular filling and coronary perfusion that can lead to ischemia, hypoperfusion of the coronary arteries, hypotension and syncope. In contrast to MS, in which beta blockers are useful to decrease heart rate and improve filling time, beta blockers are not recommended in patients with AS, unless treating an arrhythmia, because increased heart rate is an important compensatory mechanism for fixed stroke volume.
Women are at risk of acute CHF and death, with over 1/3 of women with severe, symptomatic AS requiring hospitalization during pregnancy.21 Despite frequent CHF, no maternal mortalities up to 1 week postpartum were reported in a recent study of the global Registry on Pregnancy and Cardiac Disease (ROPAC), highlighting the importance of medical heart failure management intrapartum.21 However, follow-up time was limited and valve gradients were obtained during pregnancy for 2/3 of the cohort which may have overestimated the severity of AS.21 Approximately 1/4 of fetuses born to women with moderate to severe AS exhibit growth restriction or are premature, and over 1/3 have been reported as low birth weight, with most delivered by Cesarean.21,22
Some individuals are asymptomatic at rest despite severe anatomic AS by TTE. Treadmill stress echocardiography can be considered to evaluate women with asymptomatic, severe AS prior to pregnancy as favorable maternal and fetal outcomes were reported for those who remained asymptomatic during stress testing and elevated brain natriuretic peptide levels can detect subclinical heart failure.21 Symptoms revealed during exercise, like a decrease in systolic blood pressure or pre-syncope, are related to the hemodynamic limitation imposed by the valve and should prompt pre-pregnancy valve replacement. Antepartum balloon aortic valvuloplasty for rheumatic AS and percutaneous transcatheter aortic valve replacement (TAVR) for bicuspid severe AS, have been reported.23–25 Open surgical aortic valve replacement increases maternal and fetal morbidity and mortality (see “Valve Replacement”).
L&D considerations in AS
Patients with AS rely on normal to high preload and normal or low afterload to prevent hemodynamic decompensation. Compromise of preload (e.g. large blood loss, hypotension) or increased afterload (pressors) should be avoided and patients should be in a euvolemic state to net positive fluid balance. Physicians should be cautious of the second stage of L&D, when Valsalva increases intrathoracic pressure and decreases preload. Cesarean delivery is typically recommended for severe AS. In women with moderate AS, an operative vaginal delivery with forceps or vacuum can be considered, in conjunction with MFM subspecialists and Cardiologists, depending on the length of the second stage, patient preference, gestational age, parity, and other clinical factors.4,18 Regional anesthesia is useful to avoid tachycardia due to pain, however, epidural anesthetics should be infused slowly to minimize peripheral vasodilation, which could drop preload.
Mitral and Aortic Regurgitation
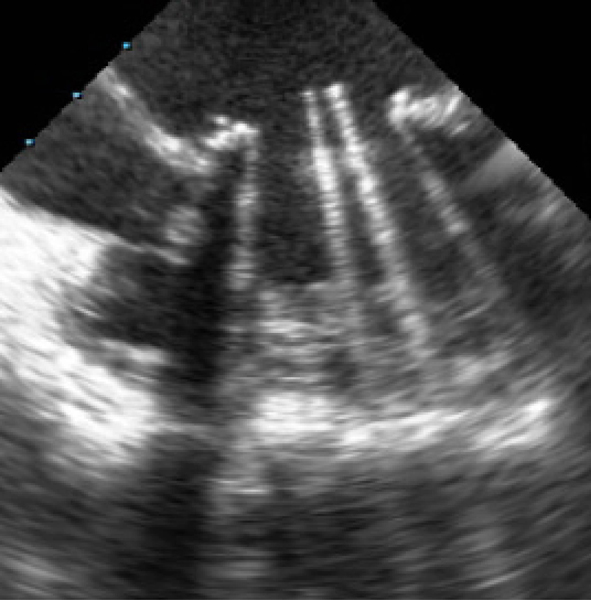
Mitral valve prolapse (MVP) and rheumatic mitral regurgitation (MR) are the most common reasons for MR in pregnancy. MVP is often progressive, leading to redundancy and floppiness of the mitral valve, and sometimes involves the chordae (Fig. 3). MR results from a leaflet scallop sliding past its counterpart. Mitral valve repair for severe MR, involving trimming of the redundant leaflet, and/or restructuring of the chordae, is generally recommended over replacement, where anatomically feasible, and should be undertaken pre-pregnancy. For those presenting with severe symptomatic MR in pregnancy, CHF can occur in nearly 1/4 of patients, hence, managing volume, afterload and arrhythmia are important components of care, particularly peripartum.14
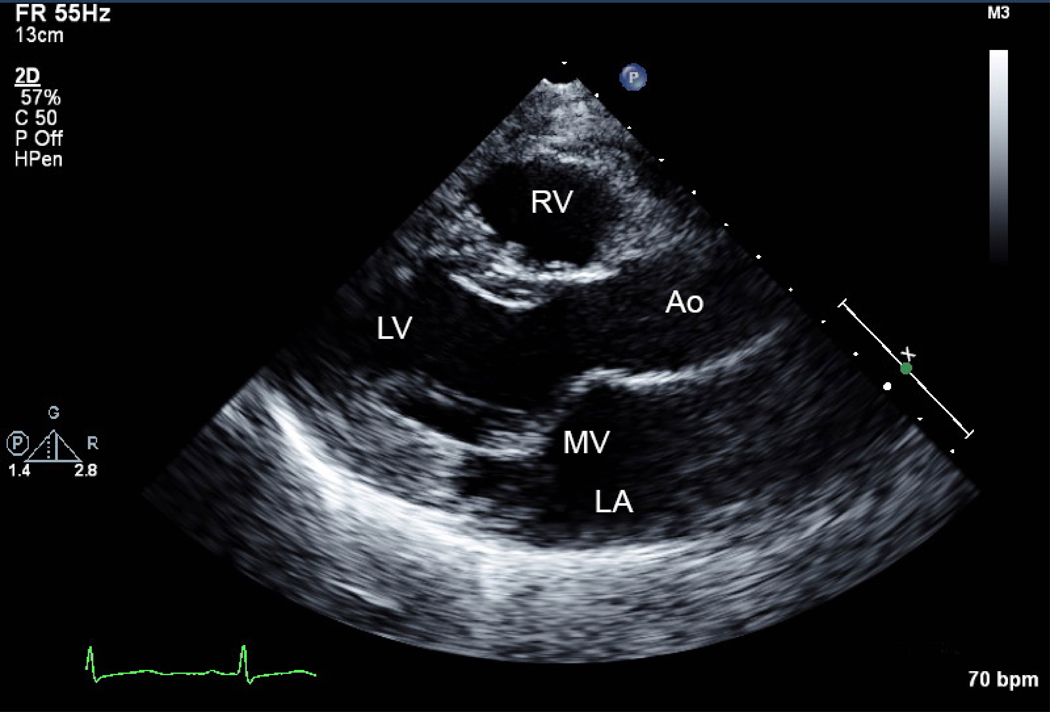
Figure 3.
A) Parasternal long axis view of mitral valve (MV) prolapse. B) Mild mitral regurgitation indicated by Doppler color flow backwards into the left atrium (LA). Ao=aorta, LV=left ventricle, RV=right ventricle.
Aortic regurgitation (AR) occurs with bicuspid aortic valves, aortopathies, infective endocarditis and rheumatic heart disease, and the various etiologies thus necessitate different approaches to therapy or management. For women contemplating pregnancy, evaluation of severe MR and AR is based on evaluation of exercise-induced symptoms, LVEF and left ventricular dilation. TTE and referral to cardiology for evaluation is essential. Surgical intervention should be considered before pregnancy in patients with severe AR or MR when symptoms of ventricular dysfunction are present.4 Beta-blockers are recommended with MR, but not for AR, as they increase the diastolic filling period, worsen AR, and increase left sided filling pressures. Vaginal delivery is favored for both MR and AR, with epidural analgesia for its vasodilatory effects, and immediate diuresis once delivery has occurred.
Tricuspid and Pulmonic Regurgitation
Regurgitant lesions are generally well tolerated during pregnancy because the decrease in systemic vascular resistance helps increase forward flow. Right sided CHF with severely regurgitant lesions leads to hepatic congestion, ascites, and lower extremity edema. Under-filling of the left ventricle portends systemic hypoperfusion and shock. Primary TR may be due to rheumatic heart disease, endocarditis, or congenital heart disease (Ebstein’s anomaly, Fig. 4). Congenital displacement of the tricuspid valve results in atrialization of the right ventricle (RV), which in turn, can lead to supraventricular tachycardia. The functional RV is small in relation to the size of the atrialized RV. Tethering of valve leaflets leads to severe TR and potentially to right-sided CHF. Ebstein’s is associated with right-to-left shunting through an atrial septal defect or patent foramen ovale, causing hypoxemia. Filtered intravenous lines are often used to reduce the risk of air embolism.4,26,27 Correction may be undertaken pre-pregnancy.28
Figure 4.
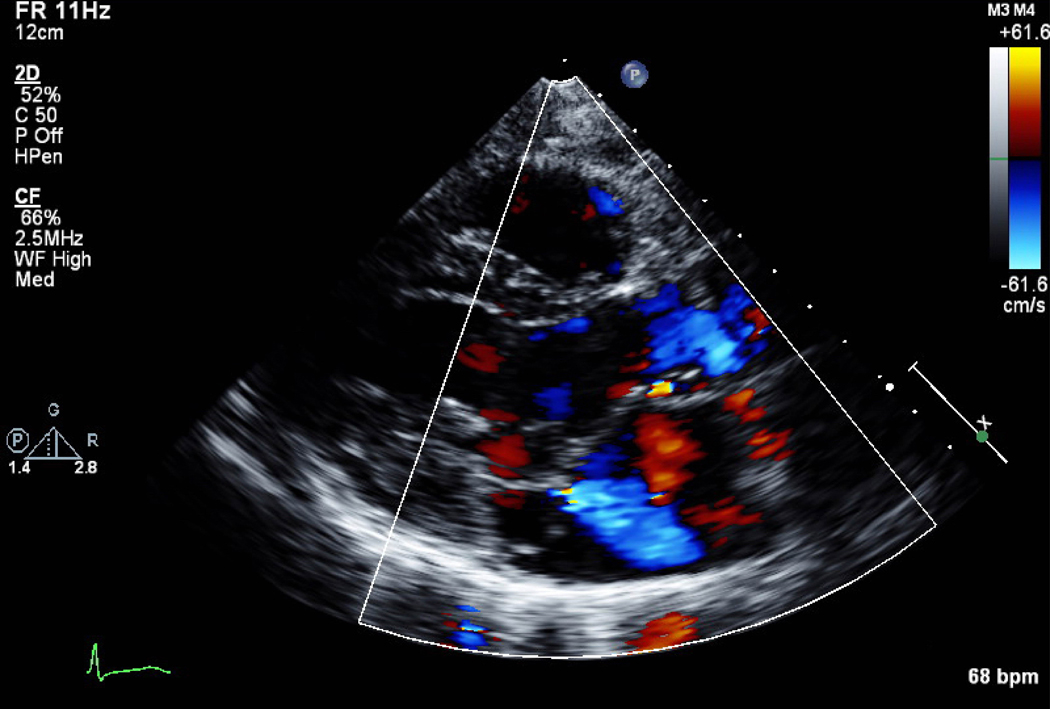
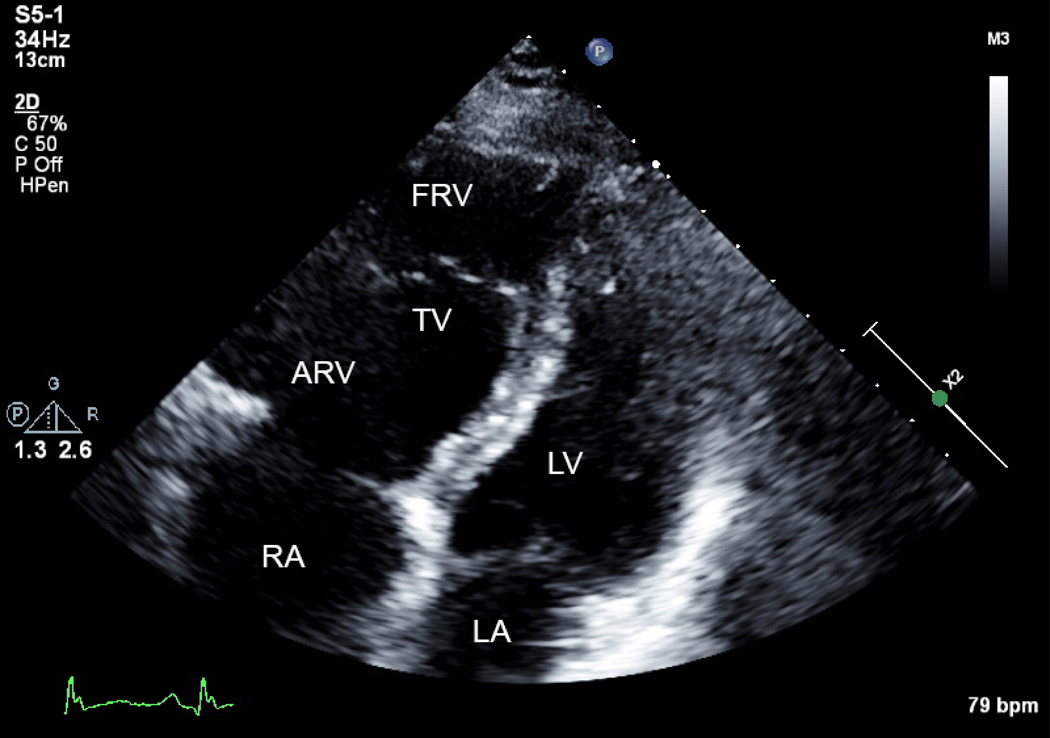
A) Apical 4 chamber view of Ebstein’s anomaly. The true annulus of the tricuspid valve (TV) is dilated, the valve is apically displaced leading to atrialization of the right ventricle (ARV) and the functional right ventricle (FRV) is small. The left ventricle (LV) is flattened. RA=right atrium, LA=left atrium. B) Severe tricuspid regurgitation indicated by Doppler color flow backwards into the ARV and RA is due to tethering of the septal and mural leaflet, in combination with decreased anterior leaflet mobility. The patient had a murmur and developed shortness of breath in pregnancy with chronic hypoxemia. Ebstein’s anomaly is frequently associated with right-to-left shunting.
Secondary TR is more common than primary, usually caused by annular dilatation from chronic left sided CHF. This in turn, creates elevated RV pressures and volume.2 In the absence of RV dysfunction or RV failure, severe secondary TR is often well-tolerated during pregnancy and managed medically with diuresis.4,8,15 Generally, tricuspid annuloplasty, valvular repair or replacement for severe TR is performed in the context of other cardiac surgery. Patients with severe, symptomatic PR and RV failure should consider surgical replacement before pregnancy as severe PR has been identified as an independent predictor of maternal complications in women with an impaired RV.4,26 Severe PR is seen post-balloon valvuloplasty, with failed Ross homografts, or after operative repair for Tetralogy of Fallot, and predicts adverse maternal outcomes.2 Successful pregnancy outcomes post transcatheter pulmonic valve replacement with Melody (Medtronic, Minneapolis, MN and Edwards Sapien (Edwards Lifesciences, Irvine, CA) valves have been reported.29
Pulmonic Stenosis
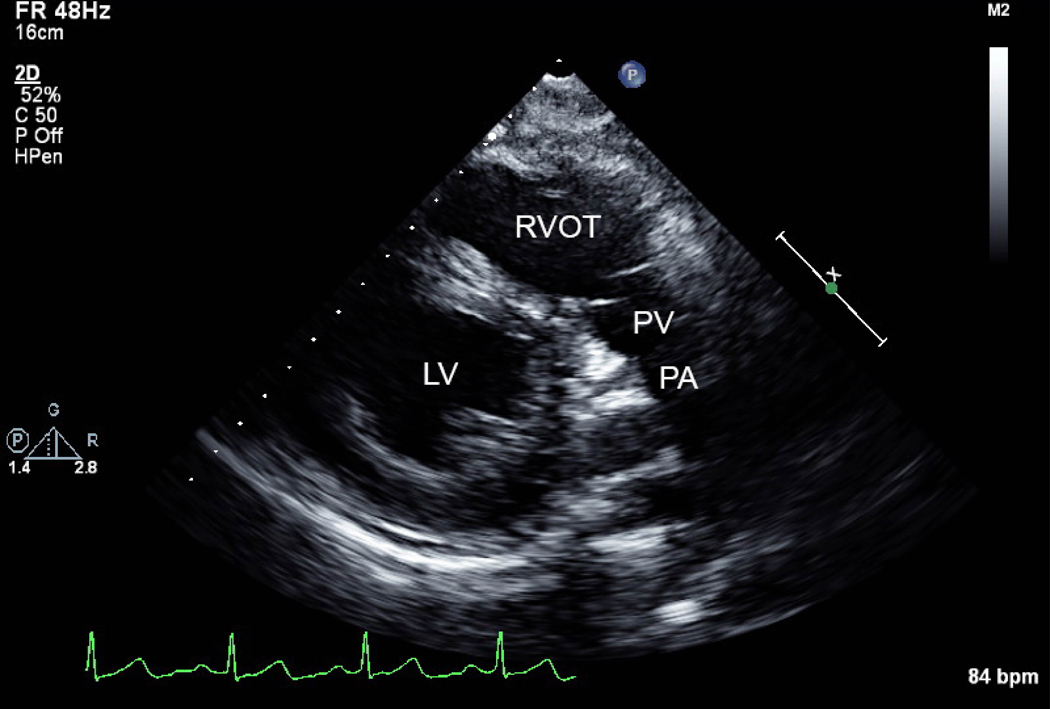
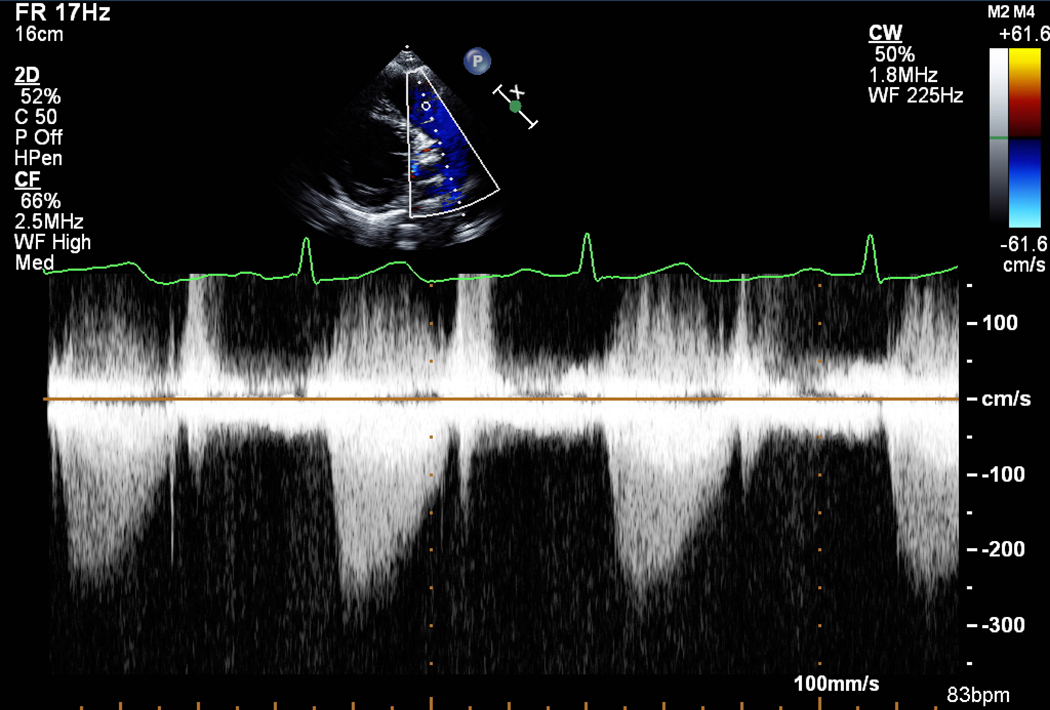
Tricuspid stenosis, either congenital or a consequence of rheumatic heart disease, is uncommon and not therefore, not discussed. Pulmonic stenosis (PS), when mild, is well tolerated. Severe PS is congenital or occurs as a consequence of the Ross procedure, a childhood surgery in which the pulmonic valve is placed into the aortic position to treat severe AS or AR and a cadaver valve is placed in the pulmonic position.2 Unlike severe AS or MS, mild or moderate PS is well-tolerated in pregnancy (Fig. 5A, B). In the absence of severe pulmonary arterial hypertension, women are often delivered vaginally. Severe PS, even if asymptomatic, should be considered for balloon pulmonary valvuloplasty prior to pregnancy. Severe stenosis with obstruction to right ventricular outflow can lead to the development of right ventricular hypertrophy and the inability to augment cardiac output during pregnancy, leading to right sided heart failure. As such, severe symptomatic PS or PS with associated cardiac dysfunction in pregnancy is associated with an increased risk of complications and pre-pregnancy interventions should be considered.4
Figure 5.
A) Pulmonic stenosis (PS) on 2D transthoracic echocardiogram with B) a mild gradient by continuous wave Doppler flow. PV=pulmonic valve, PA=pulmonary artery, RVOT=right ventricular outflow tract, LV=left ventricle.
Valve Replacement
Pre- or post-pregnancy valve replacement is preferable for severe valvular lesions, rather than antepartum cardiothoracic surgery, which increases morbidity and mortality for both the pregnant woman and fetus. In a meta-analysis of publications spanning 1990 to 2016, MS, prosthetic valve dysfunction and AS were the most frequent indications for antepartum cardiac surgery.30 Maternal mortality was 11.2 per 100 pregnancies (95% CI, 6.8 to 17.8), and maternal morbidity 8.8 per 100 pregnancies (95% CI 2.8 to 24.2), mostly due to CHF, arrhythmia and bleeding, while fetal loss was 33.1 per 100 pregnancies (95% CI, 25.1 to 41.2).30
Percutaneous valve replacement is of uncertain utility in pregnant women with severe valvular heart disease. Successful transcatheter aortic valve replacement with a self-expanding CoreValve (Medtronic, Minneapolis, MN) implant, guided by peripheral intravascular ultrasound, transesophageal echocardiography, and fluoroscopy, has been reported for bicuspid severe AS.24 Percutaneous valve-in-valve replacement, in which a second bioprosthetic valve is deployed inside the first, has been used for AS and MS in older adults.31 This requires a large enough valve size so as to avoid patient-prosthetic mismatch, which can lead to a high gradient across the stented valve. Successful double valve-in-valve Edwards Sapien XT (Edwards Lifesciences, Irvine, CA) implantation for severe bioprosthetic AS and MS in pregnancy via the left ventricular apex has been reported.25
Valve replacement in women who may choose to experience pregnancy over the course of their reproductive lifetime should address bioprosthetic versus mechanical valve choices and there should be shared decision-making between patients and physicians. Mechanical valves offer extended durability, but necessitate lifelong therapeutic anticoagulation (Fig. 6). Mechanical mitral valves pose a higher risk of thrombosis than aortic valves due to the low pressure flow from the left atrium to left ventricle. Valve thrombosis is high in pregnancy, estimated at 3–9%, and thrombolysis or surgery to treat it can be complicated by bleeding or stroke.32,33 Women with mechanical valves have an increased risk of both maternal and fetal complications, and their live-birth rates are significantly lower than for women with bioprosthetic valves (58% vs. 79%).3,32 Risks stem from anticoagulation, ventricular and valvular dysfunction.
Figure 6.
A) Pre-operative transesophageal echocardiogram (TEE) demonstrating severe stenosis of a congenitally cleft mitral valve with B) increased Doppler color flow across the valve. C) Post-operative TEE of the mechanical mitral valve in the open position. Valve replacement occurred 10 years prior to pregnancy. She was successfully managed in pregnancy; transitioned from warfarin to low molecular weight heparin titrated to peak anti-Xa levels, then to intravenous unfractionated heparin just prior to delivery.
There are various regimens that have been proposed for anticoagulation and randomized controlled trials to guide use in pregnancy are lacking. The most common treatments of choice are warfarin and low molecular weight heparin; each has advantages and disadvantages.32,34–37 Warfarin exposure in the first trimester is associated with up to a 10% risk of fetal loss and toxicity including intracranial hemorrhage, warfarin embryopathy (characterized by nasal hypoplasia, stippled epiphyses, choanal atresia), and central nervous system (CNS) and ocular abnormalities.38 Effects may be dose dependent; >5 mg daily has been associated with more frequent fetal abnormalities. Warfarin exposure in the second and third trimester has also been associated with fetal hemorrhage/intracranial bleeding and CNS abnormalities like schizencephaly and microcephaly. Up to a 40% risk of spontaneous abortion, fetal death and congenital defects have been reported with warfarin and similarly high proportions were observed when exposure was limited to only the second and third trimester.37
The risk of valve thrombosis, however, appears to be lower in pregnant women who use oral anticoagulation throughout pregnancy, 3.9%, as compared to up to a 30% risk with unfractionated heparin (UFH). UFH is therefore not recommended for pregnant women with mechanical valves.4 Low molecular weight heparin (LMWH) appears to have less risk of valve thrombosis than UFH, however some studies report up to a 9–10% risk of valve thrombosis in women who use LMWH throughout pregnancy.2,4 LMWH has a greater fetal safety profile with no association with congenital anomalies as compared to warfarin, although higher risk of bleeding complications.34 The concern about valve thrombosis with LMWH however, makes the selecting the best approach for anticoagulation a challenge when weighing both maternal and fetal risks with warfarin.2 Case series suggest routinely assessing peak anti-Xa levels and ensuring therapeutic trough levels.36,39 Importantly, LMWH is weight based regimen that should be administered every 12 hours during pregnancy, with dose adjustment given more rapid renal clearance and volume of distribution in pregnancy. Undertreatment could explain previous reports of higher thrombosis with LMWH since a significant number of trough levels were subtherapeutic when targeting a peak anti-Xa level of 1.0–1.2.36 Suggested targets are: trough ≥0.6 international units (IU)/mL for low risk patients and ≥0.7 IU/mL for high risk patients, with an anti-Xa peak level not to exceed 1.5 IU/mL, assessed 4–8 hours after ≥4 doses.36
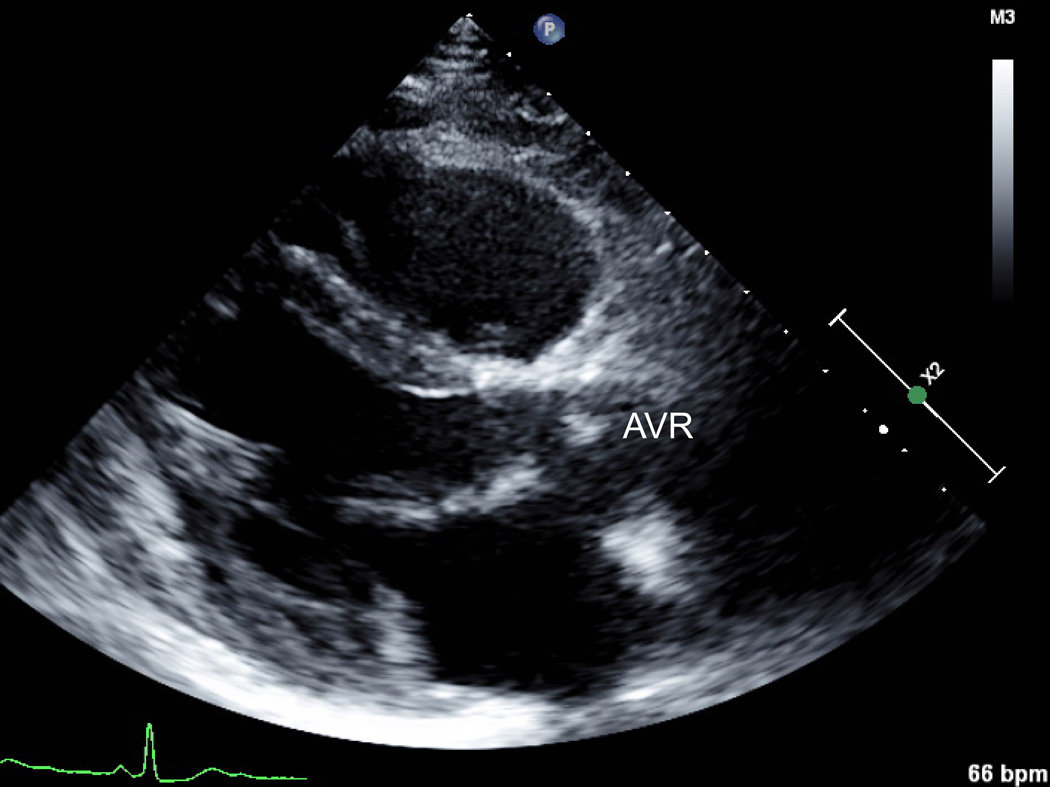
Aspirin is used for bioprosthetic valves instead of anticoagulation and this may be preferable if warfarin is undesirable or contraindicated. Bioprosthetic valves typically last 10–15 years and will require replacement if implanted in a young woman. The advantage of selecting a bioprosthetic valve may be to avoid warfarin exposure during pregnancy, as well as the risk of anticoagulation transitions or treatment gaps. Some individuals plan for future mechanical valve replacement once the bioprosthetic has deteriorated, ideally after their reproductive years. Elevated gradients across bioprosthetic valves can result from naturally increased flow and decreased afterload in pregnancy (Fig. 7), but whether this has an impact on the longevity of the valve is unclear.
Figure 7.
A) Parasternal long axis view of a bioprosthetic aortic valve replacement performed pre-pregnancy for infectious endocarditis of a congenitally bicuspid aortic valve. B) Elevated gradients across the valve by continuous wave Doppler flow were noted during pregnancy, but the patient was clinically asymptomatic. AVR=aortic valve replacement.
Conclusion
Outside of severe AS or MS, severe pulmonary arterial hypertension, or significant right or left ventricular failure, pregnancy in the context of mild to moderate valvular heart disease is often well-tolerated. Comprehensive evaluation by an interdisciplinary team of physicians and nurses can provide guidance to women both prior to conception and during pregnancy.40,41 Pre-pregnancy treatment of severe valvular heart disease must be considered, with different percutaneous and surgical options depending on the lesion. Family planning and contraception should be discussed with women who have valvular disease. The severity of the lesion and pregnancy risk must be evaluated by her physicians. Obstetricians, MFM subspecialists and cardiologists must collaborate to improve maternal and neonatal outcomes for women with valvular heart disease.
Acknowledgements
AEB recognizes support from an American Heart Association Mentored and Clinical Population Research Award 17MCPRP33630098 and K23 HL146982 from the National Heart, Lung and Blood Institute
References:
- 1.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 2.Nanna M, Stergiopoulos K. Pregnancy complicated by valvular heart disease: an update. Journal of the American Heart Association. 2014;3(3):e000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roos-Hesselink J, Baris L, Johnson M, et al. Pregnancy outcomes in women with cardiovascular disease: evolving trends over 10 years in the ESC Registry Of Pregnancy And Cardiac disease (ROPAC). Eur Heart J. 2019;40(47):3848–3855. [DOI] [PubMed] [Google Scholar]
- 4.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. European heart journal. 2018;39(34):3165–3241. [DOI] [PubMed] [Google Scholar]
- 5.Silversides CK, Grewal J, Mason J, et al. Pregnancy Outcomes in Women With Heart Disease: The CARPREG II Study. J Am Coll Cardiol 201871(21):2419–2430. doi: 2410.1016/j.jacc.2018.2402.2076. [DOI] [PubMed] [Google Scholar]
- 6.Siu SC, Sermer M, Colman JM, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104(5):515–521. [DOI] [PubMed] [Google Scholar]
- 7.Hameed AB, Rahimtoola SH. Congenital Aortic Stenosis: Pregnancy Is Another Dimension. Journal of the American College of Cardiology. 2016;68(16):1738–1740. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63(22):2438–2488. [DOI] [PubMed] [Google Scholar]
- 9.Robson SC, Dunlop W, Moore M, Hunter S. Combined Doppler and echocardiographic measurement of cardiac output: theory and application in pregnancy. Br J Obstet Gynaecol. 1987;94(11):1014–1027. [DOI] [PubMed] [Google Scholar]
- 10.Elkayam U, Goland S, Pieper PG, Silverside CK. High-Risk Cardiac Disease in Pregnancy: Part I. Journal of the American College of Cardiology. 2016;68(4):396–410. [DOI] [PubMed] [Google Scholar]
- 11.Stout KK, Otto CM. Pregnancy in women with valvular heart disease. Heart (British Cardiac Society). 2007;93(5):552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silversides CK, Colman JM, Sermer M, Siu SC. Cardiac risk in pregnant women with rheumatic mitral stenosis. The American journal of cardiology. 2003;91(11):1382–1385. [DOI] [PubMed] [Google Scholar]
- 13.Hameed A, Karaalp IS, Tummala PP, et al. The effect of valvular heart disease on maternal and fetal outcome of pregnancy. Journal of the American College of Cardiology. 2001;37(3):893–899. [DOI] [PubMed] [Google Scholar]
- 14.van Hagen IM, Thorne SA, Taha N, et al. Pregnancy Outcomes in Women With Rheumatic Mitral Valve Disease: Results From the Registry of Pregnancy and Cardiac Disease. Circulation. 2018;137(8):806–816. [DOI] [PubMed] [Google Scholar]
- 15.Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. European heart journal. 2017;38(36):2739–2791. [DOI] [PubMed] [Google Scholar]
- 16.Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10(1):1–25. [DOI] [PubMed] [Google Scholar]
- 17.Wilkins GT, Weyman AE, Abascal VM, Block PC, Palacios IF. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60(4):299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canobbio MM, Warnes CA, Aboulhosn J, et al. Management of Pregnancy in Patients With Complex Congenital Heart Disease: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2017;135(8):e50–e87. [DOI] [PubMed] [Google Scholar]
- 19.Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). European heart journal. 2010;31(23):2915–2957. [DOI] [PubMed] [Google Scholar]
- 20.De Martino A, Morganti R, Falcetta G, et al. Acute aortic dissection and pregnancy: Review and meta-analysis of incidence, presentation, and pathologic substrates. J Card Surg. 2019;34(12):1591–1597. [DOI] [PubMed] [Google Scholar]
- 21.Orwat S, Diller GP, van Hagen IM, et al. Risk of Pregnancy in Moderate and Severe Aortic Stenosis: From the Multinational ROPAC Registry. Journal of the American College of Cardiology. 2016;68(16):1727–1737. [DOI] [PubMed] [Google Scholar]
- 22.Yap SC, Drenthen W, Pieper PG, et al. Risk of complications during pregnancy in women with congenital aortic stenosis. International journal of cardiology. 2008;126(2):240–246. [DOI] [PubMed] [Google Scholar]
- 23.Dawson J, Rodriguez Y, De Marchena E, Alfonso CE. Aortic balloon valvuloplasty in pregnancy for symptomatic severe aortic stenosis. International journal of cardiology. 2012;162(1):e12–13. [DOI] [PubMed] [Google Scholar]
- 24.Hodson R, Kirker E, Swanson J, Walsh C, Korngold EC, Ramelli S. Transcatheter Aortic Valve Replacement During Pregnancy. Circulation. Cardiovascular interventions. 2016;9(10). [DOI] [PubMed] [Google Scholar]
- 25.Chengode S, Shabadi RV, Rao RN, Alkemyani N, Alsabti H. Perioperative management of transcatheter, aortic and mitral, double valve-in-valve implantation during pregnancy through left ventricular apical approach. Annals of cardiac anaesthesia. 2018;21(2):185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khairy P, Ouyang DW, Fernandes SM, Lee-Parritz A, Economy KE, Landzberg MJ. Pregnancy outcomes in women with congenital heart disease. Circulation. 2006;113(4):517–524. [DOI] [PubMed] [Google Scholar]
- 27.Drenthen W, Boersma E, Balci A, et al. Predictors of pregnancy complications in women with congenital heart disease. European heart journal. 2010;31(17):2124–2132. [DOI] [PubMed] [Google Scholar]
- 28.Donnelly JE, Brown JM, Radford DJ. Pregnancy outcome and Ebstein’s anomaly. Br Heart J. 1991;66(5):368–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozicka U, Weronski K, Ruzyllo W, et al. Pregnancy After Transcatheter Pulmonary Valve Implantation. The Canadian journal of cardiology. 2017;33(12):1737.e1735–1737.e1737. [DOI] [PubMed] [Google Scholar]
- 30.Jha N, Jha AK, Chand Chauhan R, Chauhan NS. Maternal and Fetal Outcome After Cardiac Operations During Pregnancy: A Meta-Analysis. The Annals of thoracic surgery. 2018;106(2):618–626. [DOI] [PubMed] [Google Scholar]
- 31.Murdoch DJ, Webb JG. Transcatheter valve-in-valve implantation for degenerated surgical bioprostheses. Journal of thoracic disease. 2018;10(Suppl 30):S3573–s3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Hagen IM, Roos-Hesselink JW, Ruys TP, et al. Pregnancy in Women With a Mechanical Heart Valve: Data of the European Society of Cardiology Registry of Pregnancy and Cardiac Disease (ROPAC). Circulation. 2015;132(2):132–142. [DOI] [PubMed] [Google Scholar]
- 33.Vause S, Clarke B, Tower CL, Hay C, Knight M. Pregnancy outcomes in women with mechanical prosthetic heart valves: a prospective descriptive population based study using the United Kingdom Obstetric Surveillance System (UKOSS) data collection system. BJOG : an international journal of obstetrics and gynaecology. 2017;124(9):1411–1419. [DOI] [PubMed] [Google Scholar]
- 34.D’Souza R, Ostro J, Shah PS, et al. Anticoagulation for pregnant women with mechanical heart valves: a systematic review and meta-analysis. Eur Heart J 201738(19):1509–1516. doi: 1510.1093/eurheartj/ehx1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sareli P, England MJ, Berk MR, et al. Maternal and fetal sequelae of anticoagulation during pregnancy in patients with mechanical heart valve prostheses. Am J Cardiol. 1989;63(20):1462–1465. [DOI] [PubMed] [Google Scholar]
- 36.Elkayam U. Anticoagulation Therapy for Pregnant Women With Mechanical Prosthetic Heart Valves: How to Improve Safety? J Am Coll Cardiol. 2017;69(22):2692–2695. [DOI] [PubMed] [Google Scholar]
- 37.Steinberg ZL, Dominguez-Islas CP, Otto CM, Stout KK, Krieger EV. Maternal and Fetal Outcomes of Anticoagulation in Pregnant Women With Mechanical Heart Valves. J Am Coll Cardiol. 2017;69(22):2681–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elkayam U, Goland S. The search for a safe and effective anticoagulation regimen in pregnant women with mechanical prosthetic heart valves. J Am Coll Cardiol. 2012;59(12):1116–1118. [DOI] [PubMed] [Google Scholar]
- 39.Goland S, Schwartzenberg S, Fan J, Kozak N, Khatri N, Elkayam U. Monitoring of anti-Xa in pregnant patients with mechanical prosthetic valves receiving low-molecular-weight heparin: peak or trough levels? J Cardiovasc Pharmacol Ther. 2014;19(5):451–456. [DOI] [PubMed] [Google Scholar]
- 40.Bredy C, Ministeri M, Kempny A, et al. New York Heart Association (NYHA) classification in adults with congenital heart disease: relation to objective measures of exercise and outcome. Eur Heart J Qual Care Clin Outcomes. 2018;4(1):51–58. [DOI] [PubMed] [Google Scholar]
- 41.Holland R, Rechel B, Stepien K, Harvey I, Brooksby I. Patients’ self-assessed functional status in heart failure by New York Heart Association class: a prognostic predictor of hospitalizations, quality of life and death. J Card Fail. 2010;16(2):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]