Abstract
Objectives:
There is a paucity of knowledge in the literature relating to the extent of clot burden and stroke etiology. In this study, we measured the area of the Extracted Clot Area (ECA) retrieved during endovascular treatment (EVT) and investigated relationships with suspected etiology, administration of intravenous thrombolysis and recanalization.
Methods:
As part of the multi-institutional RESTORE registry, the area of the ECA retrieved during mechanical thrombectomy was quantified using ImageJ. The effect of stroke etiology (Large-artery atherosclerosis (LAA), Cardioembolism, Cryptogenic and other) and recombinant tissue plasminogen activator (rtPA) on ECA and recanalization outcome (mTICI) was assessed. Successful recanalization was described as mTICI 2c-3.
Results:
A total of 550 patients who underwent EVT with any clot retrieved were included in the study. The ECA was significantly larger in the LAA group compared to all other etiologies. The average ECA size of each etiology was; LAA=109mm2, Cardioembolic=52mm2, Cryptogenic=47mm2 and Other=52mm2 (p=0.014*). LAA patients also had a significantly poorer rate of successful recanalization (mTICI 2c-3) compared to all other etiologies (p=0.003*). The administration of tPA was associated with a smaller ECA in both LAA (p=0.007*) and cardioembolic (p=0.035*) groups.
Conclusion:
The ECA of LAA clots was double the size of all other etiologies and this is associated with a lower rate of successful recanalization in LAA stroke subtype. rtPA administration prior to thrombectomy was associated with reduced ECA in LAA and CE clots.
Keywords: Acute Ischemic Stroke, Etiology, Thrombectomy, Thrombolysis
Introduction
Recombinant tissue plasminogen activator (tPA) and mechanical thrombectomy are currently FDA approved treatments for Acute Ischemic Stroke (AIS)1, 2. Studies have suggested that both longer clot length and a large clot volume are associated with worse functional outcome following both intravenous thrombolysis and endovascular treatment3, 4. The Clot Burden Score system was proposed to assess the intracranial thrombus extent using diagnostic imaging prior to treatment and predicts both functional outcome and final infarct size5. The cellular composition of acute ischemic stroke clots vary considerably with suspected stroke etiology6–8, however, there is a paucity of knowledge in the literature investigating the relationship between clot burden and suspected stroke etiology. There is also a lack of clinical studies investigating the influence of rtPA on the clot burden in non-responsive patients subsequently treated with mechanical thrombectomy.
We describe a post-procedural approach to the assessment of clot burden by measuring the area of extracted clot retrieved during mechanical thrombectomy procedures. In this study we measure the area of the clot retrieved following mechanical thrombectomy, termed the Extracted Clot Area (ECA), in a series of patients with large vessel occlusion (LVO) and investigate relationships between suspected stroke etiology, ECA, rtPA and recanalization rate.
Methods
Patient Selection and Clinical Data
The RESTORE registry is a European Registry of AIS clinical, procedural, imaging and histopathologic data compiled by investigators at CÚRAM, National University of Ireland Galway. This study included AIS patients from four hospitals treated between March 2018 and November 2019 and was approved by the regional hospital ethics committees (Beaumont Hospital, Dublin; Sahlgrenska Hospital, Gothenburg; NICN, Budapest; Metropolitan Hospital, Athens) and National University of Ireland Galway (NUIG) research ethics committee (16-SEPT-08). A waiver of informed consent was granted in accordance with the ethical standards of the Declaration of Helsinki. The inclusion criteria were patients’ ≥18 years with LVO, having undergone EVT following relevant diagnostic procedures and with clot material successfully retrieved for clot area analysis. EVT was performed according to individual institution’s own routine procedures.
Clot processing and Area Analysis
Clots removed during endovascular procedures were collected and placed in 10% phosphate-buffered formalin before being shipped to NUI Galway for analysis. Upon receipt, a gross photograph of the clot fragments removed was taken using a Canon EOS 1300D Camera. ImageJ software (https://imagej.nih.gov/ij/) was used to analyze the area of each fragment of clot individually. First the scale was set (Figure 1A) and then the Polygon tool was used to draw a Region of Interest around a fragment of the clot and the area of that fragment was measured (Figure 1B). Each of the fragments of clot was measured individually, an example is shown in Figure 1A&B. The total ECA for each case is defined as the sum of the clot area from all clot fragments within a case. A small ECA was defined as ≤ Median ECA of all cases.
Figure 1: Measurement of Extracted Clot Area using ImageJ.
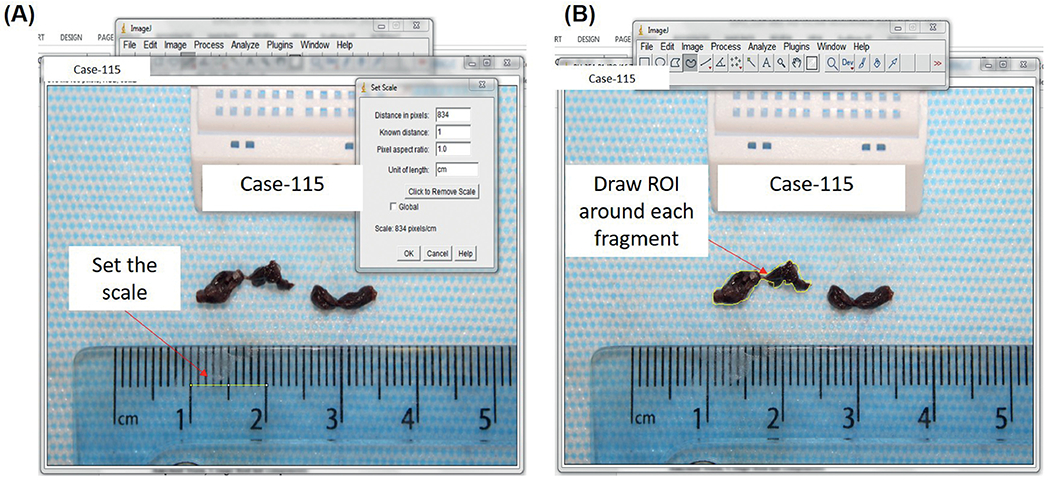
(A) The scale of the image is set using the ruler. (B) A Region of Interest (ROI) is drawn around each fragment and the area is measured.
Data Collection
Data regarding recanalization outcome and stroke etiology were collected using a data abstraction form completed by the treating physician on completion of the interventional procedure. Stroke etiology was classified using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) system; 1) large-artery atherosclerosis (LAA), 2) cardioembolism, 4) stroke of other determined etiology and 5) cryptogenic. All data were self-reported at the included centers.
Statistical analyses
All statistics were completed using Graphpad Prism 8. A Shapiro-Wilk test indicated that quantitative variables did not follow a standard normal distribution. The non-parametric unpaired t test and Kruskal-Wallis tests was used to assess statistically significant difference among the groups. Statistical correlations were assessed by Chi-squared analysis.
Results
Baseline Characteristics
A total of 550 patients were included. Forty-six-percent of patients were treated with systemic thrombolysis using recombinant tissue Plasminogen Activator. Successful recanalization (mTICI = 2c/3) was achieved in 67.7% of patients and good recanalization (mTICI ≥2b) was achieved in 91.3%. A complete dataset was not available for 67 (12%) patients and these patients were excluded from further analysis. Stroke etiologies were Large Artery Atherosclerotic (n=110, 20%), Cardioembolic (n=197, 36%), Cryptogenic (n=143, 26%) and Other etiologies (n=33, 6%).
Etiology and Extracted Clot Area
A significant correlation between ECA as measured by ImageJ and Clot Weight was found (n=80, R2=0.898, p>0.05*, data not shown). The mean ECA area for all cases was 64mm2 and the median number of fragments per case was 3. The average ECA size of each etiology was; LAA = 108.95mm2, Cardioembolic = 52.41mm2, Cryptogenic = 46.84mm2 and Other = 51.75mm2 (Figure 1C). The Kruskal-Wallis test identified that there was a significant difference in both the ECA (H(4) = 24.62, p<0.001*) and the number of fragments (H(4) = 10.62, p=0.014*) between the four reported etiologic groups (Supplementary table 1). LAA Clots are twice as big as clots of all other etiologies and are associated with a greater number of fragments compared to cardioembolic group (figure 2). Cardioembolic, cryptogenic and other clots are all similar in size to each other with a similar number of fragments (Figure 2D).
Figure 2: Results of Extracted Clot Area and No of Fragments per Suspected Etiology.
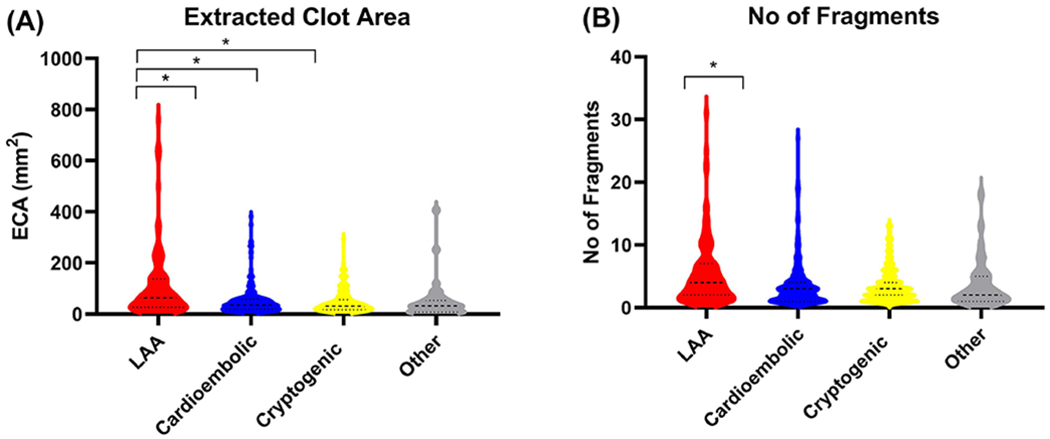
(A) The extracted clots area (mm2) of each suspected etiology is shown in the violin plots. (B) The number of fragments of each suspected etiology in the violin plots. (LAA=Red, Cardioembolic=Blue, Cryptogenic=Yellow and Other=Grey).
Etiology, tPA and Extracted Clot Area
The unpaired t test demonstrated that patient that received tPA has a significantly smaller ECA than those that did not receive tPA (50.27 vs 74.73mm2, p=0.002*). The proportion of clots that had a small ECA, defined as less than the median ECA of all clots, was significantly greater in the cohort that received tPA when compared to the cohort of patients that did not receive tPA (X2(1, n=549) =5.941, p=0.015*). The administration of tPA was associated with a smaller ECA in of both LAA (p=0.007*) and cardioembolic (p=0.035*) groups (table 1). A strong similar trend of reduced clot size was observed in patients with other determined stroke etiologies, but not in cryptogenic cases (table 1). tPA did not have a significant effect on the number of procedural passes and final mTICI score.
Table 1:
ECA, Fragments, Passes and Outcome of each stroke etiology.
| Suspected Etiology | Total Mean ECA (mm2) | tPA – Yes Mean ECA (mm2) | tPA – No Mean ECA (mm2) | Median Number of Fragments | Median Number of Passes | Final mTICI Score | |
|---|---|---|---|---|---|---|---|
| (0-2b) | 2c-3 | ||||||
| Cardioembolic (n=197) | 52.41 ± 60.02 | 39.94 (n=90) | 62.89 (n=107) | 3 [1-4] | 2 [1-4] | 57 (29%) | 140 (71%) |
| LAA (n=110) | 108.95 ± 139.9 | 76.65 (n=54) | 140.09 (n=56) | 4 [2-7] | 2 [1-3] | 54 (49%) | 56 (51%) |
| Cryptogenic (n=143) | 46.84 ± 45.96 | 47.59 (n=60) | 46.31 (n=83) | 3 [2-4] | 2 [1-3] | 44 (31%) | 99 (69%) |
| Other Determined (n=33) | 51.75 ± 79.33 | 29.46 (n=16) | 72.74 (n=17) | 2 [1-5] | 2 [1-4] | 11 (33%) | 22 (67%) |
Etiology and Recanalization Outcome
The Kruskal-Wallis test identified that there was a significant difference in mTICI score (H(4) = 10.66, p=0.014*) between the four reported etiologic groups (Supplementary table 1). A significantly higher proportion of LAA cases had a poorer recanalization outcome (mTICI 0–2b) compared to cardioembolic (X2(1, n=307) = 12.424, p<0.001*) and cryptogenic (X2(1, n=253) = 8.795, p=0.003*) cases (Figure 3). Recanalization success was not significantly different between LAA cases and cases of other determined etiology, although a similar trend was observed (X2(1, n=145) =2.849, p=0.092).
Figure 3: Revascularization Outcome per Suspected Etiology.
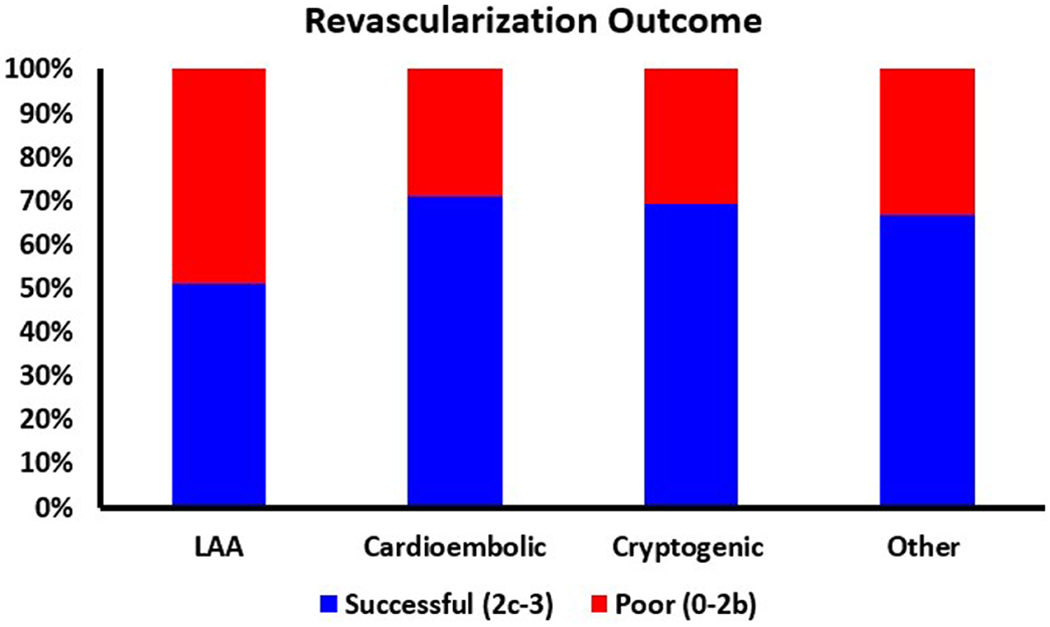
The revascularization outcome defined as Bad (0-2b, Red) and Good (2c-3, Green) of each suspected etiology.
Discussion
Our study of 550 retrieved clots in LVO patients found that patients with an LAA etiology had double the Extracted Clot Area of all other stroke etiologies. Despite having a larger ECA, LAA cases had significantly lower levels of successful recanalization (mTICI 2c/3) than other reported etiologies. The administration of tPA prior to mechanical thrombectomy was found to be associated with an overall reduced ECA following removal, which was significant in both the LAA and Cardioembolic cases.
The findings from this study have implications for the treatment of LVOs. The ultimate goal of newer generation thrombectomy devices is to achieve first pass mTICI 39, 10 and therefore, in this study, we dichotomized revascularization outcome into mTICI 0-2b and mTICI 2c-3. Given the expanding range of thrombectomy devices and techniques available to the clinicians11–15, a device capable of retrieving a large clot burden in one procedural pass may be preferred as a first option device in the treatment of suspected LAA stroke cases in order to optimize the chances of achieving a successful First Pass Effect14. The findings also suggest that the administration of tPA prior to endovascular treatment helps to reduce the clot burden in LAA and cardioembolic patients.
Previous studies have used various imaging modalities to assess the clot burden in acute ischemic stroke prior to treatment and demonstrated that a higher clot burden is associated with longer procedural times, larger final infarct size and a poorer functional outcome3–5. We describe a post-endovascular treatment approach to assessment of clot burden, by measuring the area of extracted clot removed by thrombectomy. This study demonstrated that the area of occlusive clot removed during endovascular procedure, termed the Extracted Clot Area, is associated with stroke etiology. The ECA from LAA patients was found to be twice the size of all other stroke etiologies and LAA clots were also associated with a significantly larger number of fragments than cardioembolic clots. LAA clots have previously been shown to have a more RBC-Rich composition than Cardioembolic and cryptogenic clots6, 16 and previous studies have shown that RBC-Rich clots are softer, more friable clots than more fibrin and platelet-rich clots17. Despite the fact that a larger ECA was typically retrieved from LAA patients, a significantly higher proportion of LAA patients had a poorer recanalization (0-2b) outcome following endovascular treatment compared to all other stroke etiologies. This suggests that LAA cases are more prone to distal embolization, likely due to their large, soft, friable composition, and that in cardioembolic and cryptogenic patients, the clots are smaller and likely stiffer, fibrin and platelet-rich clots that are less prone to fragmentation.
Recombinant tissue plasminogen activator (tPA) is the current FDA approved thrombolytic treatment for AIS and has been demonstrated to reduce the clot burden in responsive patients, however, the length and volume of the clot are critical to the effectiveness of tPA18, 19. This study demonstrates that the administration of tPA was associated with a smaller extracted clot Area. This effect might be due in part to the propagation of the thrombus in patients that did not receive tPA due to being outside of the time window for administration. tPA was associated with a reduced ECA in of both LAA and Cardioembolic etiologies, suggesting that tPA administration in eligible LAA and Cardioembolic patients’ might result in a reduced clot burden.
Our study has limitations. First, the method described to assess the volume of the Extracted Clot Area is a 3D extrapolation from a 2D image which may lead to some slight inaccuracies. Second, the determination of stroke etiology was self-reported at each site and therefore there may have been some site-to-site variability in the implementation of the TOAST criteria. However we feel that the number of cases in this large cohort minimizes these effects.
Conclusion
The Extracted Clot Area of Large Artery Atherosclerotic clots was double the size of all other etiologies and this is associated with poorer rate of successful recanalization following mechanical thrombectomy. rtPA administration prior to thrombectomy was associated with reduced ECA in LAA and CE clots.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge the contributions made by the Interventional, Nursing and Clinical coordination teams at each of the RESTORE registry sites.
SOURCES OF FUNDING
This work was supported by the European Regional Development Fund and Science Foundation Ireland grant number (13/RC/2073). R01 funding to the Brinjikji lab is also acknowledged (1R01NS105853).
CONFLICT OF INTEREST STATEMENT
Dr Karen Doyle received Research Funding support from Science Foundation Ireland that is co-funded by Cerenovus. All other authors declare no competing interests in relation to work described.
References
- 1.Powers William J, Rabinstein Alejandro A, Ackerson T, Adeoye Opeolu M, Bambakidis Nicholas C, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2019;50:e344–e418 [DOI] [PubMed] [Google Scholar]
- 2.Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, et al. European stroke organisation (eso) - european society for minimally invasive neurological therapy (esmint) guidelines on mechanical thrombectomy in acute ischemic stroke. Journal of NeuroInterventional Surgery. 2019:neurintsurg-2018–014569 [DOI] [PubMed] [Google Scholar]
- 3.Dutra Bruna G, Tolhuisen Manon L, Alves Heitor CBR, Treurniet Kilian M, Kappelhof M, Yoo Albert J, et al. Thrombus imaging characteristics and outcomes in acute ischemic stroke patients undergoing endovascular treatment. Stroke. 2019;50:2057–2064 [DOI] [PubMed] [Google Scholar]
- 4.Riedel Christian H, Jensen U, Rohr A, Tietke M, Alfke K, Ulmer S, et al. Assessment of thrombus in acute middle cerebral artery occlusion using thin-slice nonenhanced computed tomography reconstructions. Stroke. 2010;41:1659–1664 [DOI] [PubMed] [Google Scholar]
- 5.Derraz I, Bourcier R, Soudant M, Soize S, Hassen WB, Hossu G, et al. Does clot burden score on baseline t2*-mri impact clinical outcome in acute ischemic stroke treated with mechanical thrombectomy? J Stroke. 2019;21:91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sporns PB, Hanning U, Schwindt W, Velasco A, Minnerup J, Zoubi T, et al. Ischemic stroke: What does the histological composition tell us about the origin of the thrombus? Stroke. 2017;48:2206–2210 [DOI] [PubMed] [Google Scholar]
- 7.Simons N, Mitchell P, Dowling R, Gonzales M, Yan B. Thrombus composition in acute ischemic stroke: A histopathological study of thrombus extracted by endovascular retrieval. Journal of Neuroradiology. 2015;42:86–92 [DOI] [PubMed] [Google Scholar]
- 8.Boeckh-Behrens T, Kleine Justus F, Zimmer C, Neff F, Scheipl F, Pelisek J, et al. Thrombus histology suggests cardioembolic cause in cryptogenic stroke. Stroke. 2016;47:1864–1871 [DOI] [PubMed] [Google Scholar]
- 9.Zaidat OO, Castonguay AC, Linfante I, Gupta R, Martin CO, Holloway WE, et al. First pass effect. Stroke. 2018;49:660–666 [DOI] [PubMed] [Google Scholar]
- 10.Nikoubashman O, Dekeyzer S, Riabikin A, Keulers A, Reich A, Mpotsaris A, et al. True first-pass effect. Stroke. 2019;50:2140–2146 [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald S, Ryan D, Mullins L, Thornton J, Nogueira R. E-018 a novel 8fr aspiration catheter significantly increases the first pass effect in comparison with industry standard 6fr devices in an in-vitro human vasculature model. 2020 [Google Scholar]
- 12.Arslanian RA, Marosfoi M, Caroff J, King RM, Raskett C, Puri AS, et al. Complete clot ingestion with cyclical adapt increases first-pass recanalization and reduces distal embolization. Journal of NeuroInterventional Surgery. 2019;11:931. [DOI] [PubMed] [Google Scholar]
- 13.Delgado Almandoz JE, Kayan Y, Wallace AN, Tarrel RM, Fease JL, Scholz JM, et al. Larger ace 68 aspiration catheter increases first-pass efficacy of adapt technique. Journal of NeuroInterventional Surgery. 2019;11:141. [DOI] [PubMed] [Google Scholar]
- 14.Ducroux C, Piotin M, Gory B, Labreuche J, Blanc R, Ben Maacha M, et al. First pass effect with contact aspiration and stent retrievers in the aspiration versus stent retriever (aster) trial. Journal of NeuroInterventional Surgery. 2020;12:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chueh J- Y, Puri AS, Gounis MJ. An in vitro evaluation of distal emboli following lazarus cover-assisted stent retriever thrombectomy. Journal of NeuroInterventional Surgery. 2017;9:183. [DOI] [PubMed] [Google Scholar]
- 16.Niesten JM, van der Schaaf IC, van Dam L, Vink A, Vos JA, Schonewille WJ, et al. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS One. 2014;9:e88882–e88882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson S, Chueh J, Gounis MJ, McCarthy R, McGarry JP, McHugh PE, et al. Mechanical behavior of in vitro blood clots and the implications for acute ischemic stroke treatment. Journal of NeuroInterventional Surgery. 2019:neurintsurg-2019-015489 [DOI] [PubMed] [Google Scholar]
- 18.Bilgic AB, Gocmen R, Arsava EM, Topcuoglu MA. The effect of clot volume and permeability on response to intravenous tissue plasminogen activator in acute ischemic stroke. Journal of Stroke and Cerebrovascular Diseases. 2020;29:104541. [DOI] [PubMed] [Google Scholar]
- 19.Mishra SM, Dykeman J, Sajobi TT, Trivedi A, Almekhlafi M, Sohn SI, et al. Early reperfusion rates with iv tpa are determined by cta clot characteristics. American Journal of Neuroradiology. 2014;35:2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


